Abstract
Cardiac arrest (CA) affects >550,000 people annually in the United States whereas 80–90% of survivors suffer from a comatose state. Arousal from coma is critical for recovery, but mechanisms of arousal are undefined. Orexin-A, a hypothalamic excitatory neuropeptide, has been linked to arousal deficits in various brain injuries. We investigated the orexinergic system's role in recovery from CA-related neurological impairments, including arousal deficits. Using an asphyxial CA and resuscitation model in rats, we examine neurological recovery post-resuscitation in conjunction with changes in orexin-A levels in cerebrospinal fluid (CSF) and orexin-expressing neurons. We also conduct pharmacological inhibition of orexin post-resuscitation. We show that recovery from neurological deficits begins between 4 and 24 h post-resuscitation, with additional recovery by 72 h post-resuscitation. Orexin-A levels in the CSF are lowest during periods of poorest arousal post-resuscitation (4 h) and recover to control levels by 24 h. Immunostaining revealed that the number of orexin-A immunoreactive neurons declined at 4 h post-resuscitation, but increased to near normal levels by 24 h. There were no significant changes in the number of neurons expressing melanin-concentrating hormone, another neuropeptide localized in similar hypothalamus regions. Last, administration of the dual orexin receptor antagonist, suvorexant, during the initial 24 h post-resuscitation, led to sustained neurological deficits. The orexin pathway is critical during early phases of neurological recovery post-CA. Blocking this early action leads to persistent neurological deficits. This is of considerable clinical interest given that suvorexant recently received U.S. Food and Drug Administration approval for insomnia treatment.
Keywords: : arousal, cardiac arrest, coma, neurological recovery, orexin
Introduction
Cardiac arrest (CA) is a life-threatening condition affecting over half a million people in the United States with less than 10% survival rate for people who suffer CA out of the hospital.1 A vast majority (80–90%) of survivors emerge in a state of coma and suffer severe long-term neurological deficits.1 Arousal from coma is the number one predictor of post-CA outcome, with quicker arousal strongly linked to improved long-term outcome.2,3 However, the mechanisms of post-CA arousal are unclear, and it is unknown whether faster arousal is a cause or correlate of improved neurological outcome. If it is a cause, it may be possible to develop interventions to accelerate arousal. Beyond targeting 36°C core body temperature and supportive therapy, there is currently no treatment for post-CA survivors to improve neurological recovery.4
In recent years, a close relationship has been found between orexin levels and severe brain injury that affect levels of consciousness. Orexin is a neuropeptide that exists as two isoforms, orexin-A and -B (hypocretin-1 and -2), which can bind the orexin-1 and/or orexin-2 receptor. Orexin is only produced in a restricted group of neurons in the hypothalamus5 that project widely throughout the brain to the cortex, thalamus, brainstem, and spinal cord. This wide projection pattern suggests that the orexinergic system plays a role in the regulation of multiple brain functions, and recent studies implicate orexin in arousal.6 Indeed, studies have revealed that narcolepsy results from the failure of signaling pathways that are mediated by orexin neuropeptides7 and that orexin levels in the cerebrospinal fluid (CSF) are reduced or undetected in narcolepsy patients.8 Orexin's critical role in wakefulness has made it a novel target for the treatment of insomnia, with suvorexant, a potent dual orexin receptor antagonist, being approved in 2014 by the U.S. Food and Drug Administration as treatment for insomnia.
Though orexin's role is well known to be critical in the sleep-wake cycle, recent studies demonstrate significant changes also occurring in the orexin system after various types of acute brain injury. For example, orexin levels in the CSF are reduced after traumatic brain injury (TBI), acute ischemic and hemorrhagic stroke, and subarachnoid hemorrhage, all of which exhibit deficits in arousal, including coma, the complete lack of arousal, in the severest forms of brain injury.9–15 In a series of patients who suffered from post-CA coma, some of the patients exhibited electroencephalography patterns similar to particular stages of sleep,16 raising the possibility that one reason for arousal deficits post-CA may include deficits in the orexin pathway. In a rat model of transient global ischemia post-CA, orexin levels were unchanged at 12 h post-CA, elevated at 24 h, decreased at 48 and 72 h, and recovered to baseline at 7 days.17 However, orexin levels have not been investigated within the first 12 h post-CA when rats are beginning to arouse from coma. Here, we induced global ischemia by CA in rats and measured CSF orexin levels along with the number of neurons expressing orexin at 4 h post-CA and beyond to determine the relationship between orexin and post-CA neurological recovery. To test whether the orexinergic pathway is necessary for neurological recovery post-CA, we tested whether blocking orexin's effects in the early stage of post-CA recovery would impair long-term outcome.
Methods
Animals
All animal procedures approved by the University of California Animal Care Committee (Irvine, CA) and conformed to the recommendations of the American Veterinary Medical Association Panel on Euthanasia. Male Wistar rats (Charles River Laboratories, Wilmington, MA) weighing 300–350 (approximately 8–12 weeks), were maintained in a 12-h light/12-h dark (6:00 am/6:00 pm) cycle and fed standard rat chow. Animals typically arrive 2 weeks before experiments and are handled daily for 5 min to allow for habituation and reduction of stress levels.
Cardiac arrest
After 6:00 pm the night preceding the CA experiment, rats were fasted with 25% of their normal dietary intake. On the day of CA, rats were anesthetized with isoflurane, intubated, and connected to a TOPO™ mechanical ventilator (Kent Scientific, Torrington, CT) to allow delivery of 2% isoflurane and 50% O2 and 50% N2 gas during surgical preparation for CA. Operative procedures typically began by 9:00 am. The femoral artery and vein were cannulated to monitor blood pressure (BP) and heart rate and to allow for the intravenous (i.v.) administration of medications. While intubated and mechanically ventilated, positive end expiratory pressure was maintained at 3 cmH2O and body temperature was monitored with a rectal thermometer and maintained at 37°C. The CA experiment occurs in sequence beginning at “minute 0” when isoflurane level was reduced to 1.0–1.5% to lighten the level of anesthesia while inhaled gas was switched to 100% O2. Two minutes later, isoflurane was stopped to wash out residual anesthesia and a neuromuscular blocker paralyzer (Vecuronium 2 mg/kg i.v.) was administered. At this time, the ventilator inlet was disconnected from oxygen gas to allow room air to be mechanically delivered to the animal. Asphyxia was initiated at minute 5 of the experiment by turning the ventilator off and clamping the ventilator tubing, leading to asphyxial CA. CA time was defined as systolic BP less than 30 and pulse pressure of 10 or less. We decided to choose an 8-min duration of asphyxia to induce CA after our preliminary experiments showed that 7-min asphyxia duration led to milder and more transient neurological deficits that are less representative of human CA outcome whereas 9-min asphyxia duration led to significant mortality in rats (inability to resuscitate). After 8 min of total asphyxia time, cardiopulmonary resuscitation (CPR) was performed (manual sternal compressions at 180–240 per minute), mechanical ventilation was resumed at 100% O2 (4 liters per minute flow rate), tidal volume ∼8 mL/kg (by adjusting peak inspiratory pressures) with respiratory rate 70–80 per min, and epinephrine (0.01 mg/kg) was administered with NaHCO3 (1.0 mmol/kg). CPR continued for 1 min or until return of spontaneous circulation (ROSC). No isoflurane anesthesia was administered during or after CPR for the remainder of the experiment. Arterial blood gases were obtained 10 min before asphyxia and 10 min after ROSC to allow optimal adjustment of the ventilator. Over the next 1–2 h after ROSC, vessels were decannulated, and when spontaneous respirations were adequate, rats were extubated. The entire CA procedure from initial anesthesia to extubation typically took approximately 5 h.
Post–cardiac arrest care
Normal saline (5 mL) and lactated Ringer's (5 mL) was injected subcutaneously (s.c.) 5 h after CPR to limit dehydration until the rat was able to drink water over the subsequent hours. At the same time, animals received prophylactic cefazolin (45 mg/kg) to limit risk of infection. One cup of HydroGel (ClearH2O, Portland, ME) and 10 pellets pre-soaked in water were placed near the rat's mouth and throughout the cage until the rat recovered and resumed eating normal rat chow. The rat was re-examined 12–18 h post-CA and every 24 h post-CA to ensure proper hydration and food consumption. Rats that were unable to drink water or eat pre-soaked pellets (e.g., suvorexant-treated rats) were hydrated daily with s.c. saline and lactated Ringer's injections.
Neurological evaluation
Arousal and neurological recovery were quantified using the Neurological Deficit Scale (NDS; Table 1), as described previously.18 The NDS consists of components that measure arousal, brainstem function, motor, and sensory activities. The NDS was determined at 4, 24, and 72 h post-ROSC by at least two well-trained personnel who were blind to treatments. Table 2 shows the number rats undergoing NDS evaluations at the various time points post-ROSC. Sample sizes were determined based on a preliminary power analysis. All rats were successfully resuscitated and survived until 72 h post-CA, when euthanasia occurred.
Table 1.
Neurological Deficit Scale (NDS)
| Assessment | Component | Subscore | Total |
|---|---|---|---|
| A] Arousal | Consciousness | Normal (10) | Lethargic (5) | Comatose (0) | —/19 |
| Eyes | Open Independently (3) | Open to Pain (1) | Absent (0) | ||
| Respiration | Normal (6) | Abnormal (3) | Absent (0) | ||
| B] Brainstem | Olfaction | Present (3) | Absent (0) | —/21 |
| Vision Reflex | Present (3) | Absent (0) | ||
| Pupillary Reflex | Present (3) | Absent (0) | ||
| Corneal Reflex | Present (3) | Absent (0) | ||
| Startle Reflex | Present (3) | Absent (0) | ||
| Whisker Stimulation | Present (3) | Absent (0) | ||
| Swallowing | Present (3) | Absent (0) | ||
| C] Motor | Limbsa | Normal (3) | Weak (1) | No Movement (0) | —/6 |
| D] Sensory | Pain Responsea | Brisk (3) | Weak (1) | No Movement (0) | —/6 |
| E] Motor Behavioral | Gait | Normal (3) | Abnormal (1) | Absent (0) | —/6 |
| Balance on Beam | Normal (3) | Abnormal (1) | Absent (0) | ||
| F] Behavioral | Righting Reflex | Normal (3) | Abnormal (1) | Absent (0) | —/12 |
| Negative Reflex | Normal (3) | Abnormal (1) | Absent (0) | ||
| Spatial Awareness | Normal (3) | Abnormal (1) | Absent (0) | ||
| Turning Alley | Normal (3) | Abnormal (1) | Absent (0) | ||
| G] Seizures | Seizures | None (10) | Focal (5) | Generalized (0) | —/10 |
| Outcome: Best = 80, Worst = 0 Total Score = | —/80 |
tested and scored separately, arms only
Table 2.
Number of Rats Used For NDS, CSF OXA RIA Assay, and Immunochemistry
| Time points measured/no. of ratsa | ||||
|---|---|---|---|---|
| Endpoint measured | Control | 4 h | 24 h | 72 h |
| NDS | 8 | 8 | 5 | 5 |
| CSF | 5 | 8 | 5 | 5 |
| Histology | 6 | 5 | 4 | 5 |
All rats euthanized after endpoint listed; no attrition.
NDS, Neurological Deficit Scale; CSF, cerebrospinal fluid.
Drug administration
Suvorexant (MK4305, catalog no.: 10352; CAS#: 1030377-33-3; Advanced ChemBlocks Inc, Burlingame, CA) was dissolved in vehicle (10% polyethylene glycol 400, 10% Tween-80, and 80% saline) at a concentration of 10 mg/mL. Rats received intraperitoneal (i.p.) injections supplemented with suvorexant (30 mg/kg) immediately (i.e., within 5 min) after resuscitation and 10 and 20 h post-CA. Control rats were injected with 1 mL of vehicle at the same time intervals. Personnel were blinded to the treatment.
Because of the complexity of the experimental procedures, only 1 rat can be prepared for CA on a given day. Because of the need for animal care and behavioral tests on subsequent days, only 1–2 rats undergoing suvorexant or vehicle treatments were conducted per week. Accordingly, suvorexant and saline groups were comprised of rats prepared in the following time sequence. A total of 10 experiments (five drug and five vehicle) were planned from the beginning. A separate group of lab personnel who were not involved with the animal experiments randomly assigned rats into either drug or vehicle groups and prepared three syringes for i.p. injection at the three separate time points: immediately after resuscitation; at 10 h post-CA; and at 20 h post-CA. Syringes were covered with aluminum foil and labeled in a masked fashion to maintain blindness for staff conducting the injection. Table 3 shows the treatment versus vehicle animal numbers undergoing NDS testing at the various time points. All animals were successfully resuscitated and survived until 72 h post-CA, when euthanasia occurred.
Table 3.
Number of Rats Used For Suvorexant Treatment
| NDS Time points measured/no. of ratsa | ||||
|---|---|---|---|---|
| Treatment group | Control | 4 h | 24 h | 72 h |
| Drug (suvorexant) | 5 | 5 | 5 | 5 |
| Control (vehicle) | 5 | 5 | 5 | 5 |
All experiments terminated at 72 h; no attrition.
NDS, Neurological Deficit Scale.
Cerebrospinal fluid and brain tissue collections
Rats were anesthetized with Euthasal (catalog no.: NDC 66794-013-25, a dose of 1 mL/kg by i.p. injection; Pramal/Healthcare, Mumbai, India). After rats lost withdrawal to paw pinch, approximately 50–100 μL of CSF were collected by puncture into the cisterna magna using a glass capillary. Rats were then immediately perfused with phosphate-buffered saline (PBS; 137 mM of NaCl, 2.7 mM of KCl, 10 mM of Na2HPO4, and 1.8 mM of KH2PO4; pH 7.4), and brains were dissected out and fixed in 4% paraformaldehyde for 20 h at 4°C. The fixed brain was transferred and maintained in 30% sucrose for 4 days and subsequently frozen in optimal cutting temperature (OCT) embedding medium and kept in −80°C. CSF and brains from control rats were collected in a similar manner without undergoing the CA procedure. Table 2 shows all rats successfully undergoing cisternal tap and CSF isolation. Technical difficulties prevented successful isolation of CSF from some rats.
Immunofluorescence
Frozen brains in OCT were coronally sectioned at 30 μm using a cryostat (Microtome HM 505N). Sections were stored in serial order in a 96-well plate in 1 × PBS with NaN3 at 4°C. For immunohistochemistry (IHC), brain sections were blocked with 5% donkey serum in 0.3% PBST (0.3% Triton X-100 in 1 × PBS) for 1 h at room temperature, incubated with 1% donkey serum in 0.3% PBST with primary antibodies for 72 h at 4°C, washed, and then incubated with secondary antibodies and 4′,6-diamidino-2-phenylindole for 2 h at room temperature, followed by final washes and cover-slipped onto microscope slides using Vectashield Mounting Medium (H-1000; Vector Laboratories, Burlingame, CA). The following primary and secondary antibodies were used: goat anti-orexin-A C-19 (OXA, SC-8070; Santa Cruz Biotechnology, Santa Cruz, CA); rabbit antimelanin concentrating hormone (MCH); Alexa Fluor 488 donkey antirabbit immunoglobulin G (IgG; A21206; Life Technologies, Carlsbad, CA); and Alexa Fluor 555 donkey antigoat IgG (A21432; Life Technologies).
Measurement of cerebrospinal fluid orexin-A concentration
OXA concentration in CSF was analyzed using a radioimmunoassay kit (Phoenix Pharmaceuticals, Belmont, CA). For each rat, approximately 2–10 μL of CSF sample was subjected to measurement, and the average of duplicated sets was used for statistical analysis.
Cell counting
Quantification of OXA and MCH neurons was performed as described elsewhere.19–22 Briefly, based on the rat brain atlas (The Rat Brain in Stereotaxic Coordinates, 6th Edition) from figure 54 (bregma, −2.52 mm) to figure 65 (bregma, −3.84 mm), 12 sections were chosen at a 90-μm interval (one of every four sections) covering a total 1350 μm of the hypothalamus area where OXA and MCH are expressed. Sections were prepared for double immunofluorescence for OXA and MCH as described above. All of the brain sections were screened under the microscope and five sections (between Figures 58 and 62), which included the highest number of OXA and MCH neurons, were selected. Large images (3.0 mm dorsoventral × 1.8 mm mediolateral) that encompassed the hypothalamic area containing all neurons expressing either OXA or MCH were obtained using the large scan acquisition tool on a Nikon Eclipse Ti-E microscope (Nikon Corporation, Tokyo, Japan). For cell counts, images were imported into ImageJ (NIH, Bethesda, MD) and the numbers of OXA- and MCH-immunoreactive neurons were counted by three trained personnel using ImageJ Plugin “Cell Counter.” Cells with nuclei were included in counts. To avoid cell-counting errors, brains that were cut in oblique angles were excluded from the analysis. Total number of rat brains that were included in histology and cell counting after taking into account these technical limitations are shown in Table 2.
Semiquantitative image analysis by using CellProfiler
One brain section that contained the highest number of OXA neurons (typically the area shown in Figure 61, bregma −3.36 mm of The Rat Brain in Stereotaxic Coordinates, 6th edition) was selected per animal. These selected sections from each rat were simultaneously stained according to the IHC protocol described above. Large scan images of the hypothalamus (∼1.5 × 1.2 mm) were acquired using Nikon Eclipse Ti-E. The same imaging conditions applied to each section, including exposure time, gain, and resolution. For unbiased, high-throughput computational image analysis, CellProfiler Cell Image Analysis Software (version 2.1.1) was used.23,24 OXA- and MCH-immunoreactive neurons were identified under the same algorithms of image analysis, image detection thresholds, parameters, and filter settings. All images were blindly analyzed to acquire 1) sizes of neurons, 2) immunofluorescent pixel intensities per neuron, and 3) mean pixel intensities of OXA- and MCH-immunoreactive neurons.
Statistical analysis
Data analysis was performed using IBM SPSS Statistics software (V21; IBM Corp., Armonk, NY). Statistical pair-wise comparisons to determine significance were made either by unpaired two-tailed t-test or by one-way analysis of variance (ANOVA) with a post-hoc Tukey test. Data in Figure 4A were analyzed by repeated-measure ANOVA general linear model; data in Figure 4B were analyzed by two-way ANOVA and post-hoc t-tests using Prism software (Version 6.0; GraphPad Software Inc., La Jolla, CA). Correlation analyses were done using bivariate correlations and Spearman coefficients were reported. A p value <0.05 was considered statistically significant.
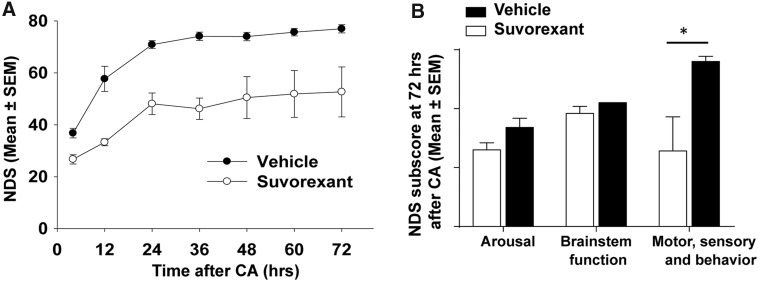
FIG. 4.
Dual orexin receptor antagonist (suvorexant) impairs neurological recovery post-CA. Rats subjected to CA were administrated either suvorexant or vehicle three separate times during the initial 24 h post-CA (i.e., immediately after resuscitation and at 8 and 20 h post-CA) and were monitored for NDS at serial intervals post-CA (4, 12, 24, 36, 48, 60, and 72 h). (A) Suvorexant-injected rats (n = 5) demonstrated persistently lower NDS scores than vehicle-injected rats (n = 5) at all time points (p < 0.01, determined by general linear model for repeated measure). (B) Component subscores of NDS at 72 h post-CA. “Arousal” was total score of assessment A in Table 1, “brainstem function” was the total score of assessment B, and “motor, sensory and behavior” were the sum of assessment C, D, E, and F. Two-way analysis of variance using Prism software (version 6.0; GraphPad Software Inc., La Jolla, CA) revealed a significant difference between treatment groups (p < 0.003); post-hoc comparisons by t-test revealed significant differences between treatment groups on motor, sensory, and behavior (*p = 0.032) and no significant differences on the other subscores (arousal, p = 0.094; brainstem function, p = 0.172). CA, cardiac arrest; NDS, Neurological Deficit Scale; SEM, standard error of the mean.
Results
Post–cardiac arrest rats have severe arousal deficits at 4 h after return of spontaneous circulation
To evaluate the level of arousal and neurological deficit post-CA in rats, we measured NDS at 4, 24, and 72 h post-ROSC. As shown in Table 1, NDS testing assesses arousal, brainstem reflexes, basic motor strength and sensation, gait, and primitive behaviors. None of the rats in this study exhibited seizures. Control rats have perfect NDS scores (mean ± standard deviation [SD], 80.0 ± 0.0; n = 8), whereas post-CA all rats exhibited deficits in NDS. At 4 h post-CA, there were severe deficits in NDS scores, mostly in categories assessing arousal, brainstem function, and sensory/motor behavior (27.0 ± 4.5; n = 8; p < 0.001 in comparison to control rats; Fig. 1).
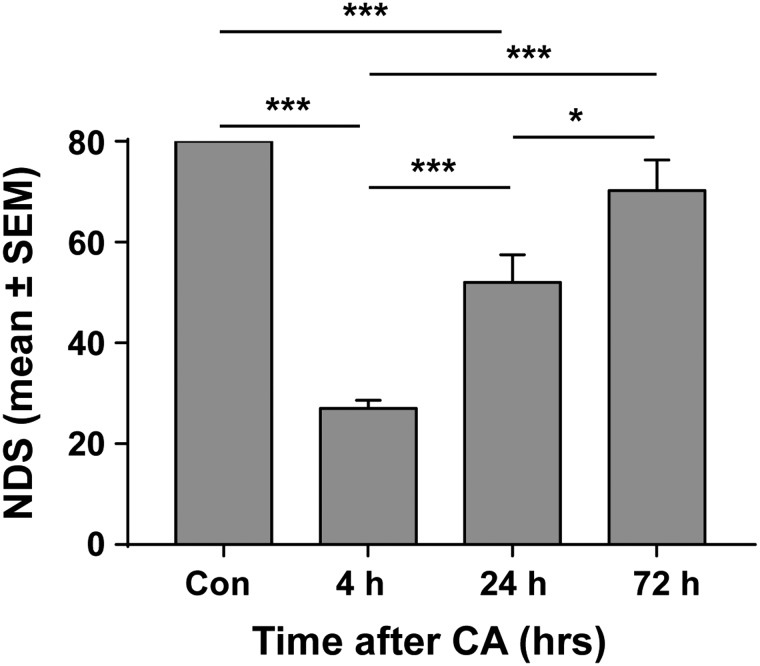
FIG. 1.
Neurological recovery of post-CA rats. For those rats subjected to cardiac arrest, NDS was measured at 4, 24, and 72 h after resuscitation. Rats without CA were used as control. At 4 h post-CA, NDS was lowest and gradually went up at 24 and 72 h. ***p < 0.001; *p < 0.05 by one-way analysis of variance. CA, cardiac arrest; Con, control; NDS, Neurological Deficit Scale; SEM, standard error of the mean.
Neurological deficits recover progressively post-CA with the greatest recovery in NDS scores occurring between 4 and 24 h post-CA (27 ± 4.5 at 4 h [n = 8] vs. 52.0 ± 12.2 at 24 h [n = 5]; p < 0.001). During these 20 h, NDS scores recovered to 65% of control (Fig. 1). By 72 h post-CA, neurological outcomes further improved and reached 87.5% of control (NDS = 70.2 ± 13.7 at 72 h [n = 5], compared to control values of 80 ± 0.0; p = 0.187). NDS scores at 72 h were significantly higher than at 4 and 24 h post-CA (p < 0.001 and p < 0.05, respectively).
Neurological recovery post–cardiac arrest is correlated with orexin-A levels in cerebrospinal fluid
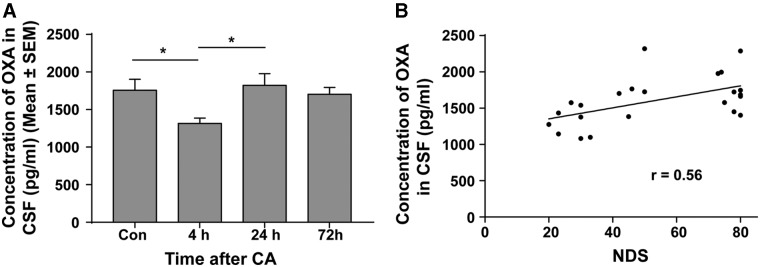
To examine whether the orexinergic pathway is linked to neurological recovery post-CA, we examined the level of CSF OXA, which reflects levels secreted from neurons into the CSF. At 4 h post-CA, when NDS scores were lowest, CSF OXA level was also lowest among the groups tested (74% of the control). Values were 1316.4 ± 195.8 pg/mL (mean ± SD) at 4 h (n = 8) versus 1757.1 ± 324.5 pg/mL in controls (n = 5; p < 0.05; Fig. 2A). By 24 h, when NDS scores had recovered, the amount of OXA in CSF had recovered back to control levels (1822.5 ± 348.9 pg/mL at 24 h [p = 0.980] in comparison to controls). Similar values were observed at 72 h post-CA (1702.5 ± 206.2 [p = 0.988] in comparison to control; Fig. 2A). There was a significant monotonic relationship between CSF OXA levels and NDS scores (Fig. 2B; Spearman's Rho correlation coefficient, 0.555; p < 0.006). The significant concurrent upregulation of OXA level in CSF between 4 and 24 h post-CA, and improvement of NDS during the same period, suggests the hypothesis that secreted orexin is linked with neurological recovery post-CA.
FIG. 2.
Fluctuation of OXA concentration in CSF post-CA. (A) Secreted OXA in CSF was analyzed by radioimmunoassay. At 4 h post-CA, the level of OXA protein in CSF was significantly lower than that of control and 24 h. (B) Change of OXA concentration in CSF was correlated to NDS. *p < 0.05 by one-way analysis of variance. CA, cardiac arrest; Con, control; CSF, cerebrospinal fluid; OXA, orexin-A; SEM, standard error of the mean.
Dynamic changes in the number of orexin-A immunoreactive neurons are linked to neurological outcome during post–cardiac arrest recovery
The changes in CSF levels of orexin could occur in two ways that modify expression and release: 1) Orexin expression may be regulated in a graded fashion in individual neurons. In this case, decreases and then increases in orexin in CSF would be correlated with decreases and increases in average orexin levels in hypothalamic neurons; 2) orexin levels may be determined by turning orexin expression off and then on in individual neurons. In this case, decreases and then increases in orexin in CSF would be correlated with decreases and increases in the number of orexin-positive hypothalamic neurons.
To examine whether intracellular OXA levels follow similar dynamic patterns as OXA levels in CSF, we measured integrated OXA signal intensity in OXA-IR (immunoreactivity) neurons using CellProfiler. Images of the region of the hypothalamus that contain neurons expressing OXA and MCH were captured (as indicated by the red rectangle in Fig. 3A and the representative images for each time point in Fig. 3B). Unlike CSF OXA levels, intracellular OXA protein measured by integrated OXA signal intensity appeared lower in all tested post-CA time points compared to control. At 4 h post-CA, integrated OXA signal intensity was 87% of control (97.2 ± 7.6 [n = 6]; mean ± standard error of the mean [SEM] vs. control values of 111.8 ± 10.7 [n = 6]; p = 0.10; Fig. 3C, OXA). At 24 and 72 h post-CA, integrated OXA signal intensity was 81.5% and 73.2% of controls, respectively (91.2 ± 13.0 at 24 h [n = 4; p < 0.05] and 81.8 ± 10.1 at 72 h [n = 4; p < 0.01). Because CA can have global effects on gene and protein expression, we compared OXA expression to another protein, MCH, which is also expressed in similar regions of the hypothalamus. In contrast to OXA, intracellular integrated MCH signal intensity at all post-CA time points remained similar to controls (Fig. 3E, MCH).
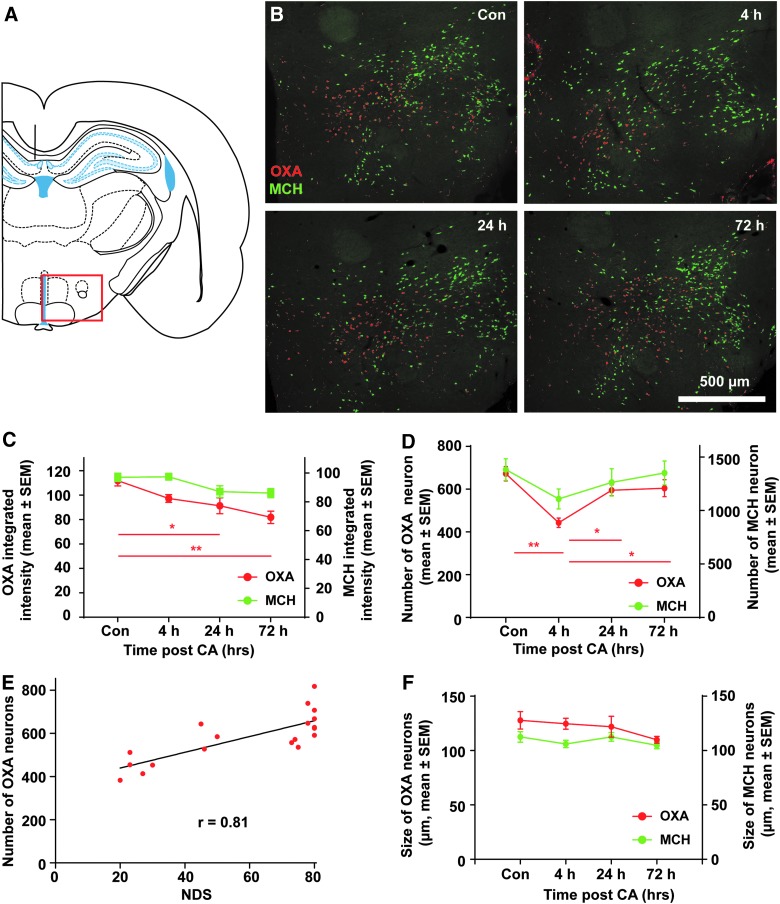
FIG. 3.
Regulation of OXA expression post-CA. (A) Brain atlas. The region within the red rectangle displays the hypothalamic region where orexin and MCH are expressed as shown in (B). (B) Brain sections were co-labeled with OXA (red) and MCH (green). Images were cropped and signal intensities were adjusted linearly to be optimal for demonstration. (C) The integrated intensity of OXA and MCH neurons were obtained by CellProfiller. OXA integrated intensity at 24 hrs and 72 hrs was significantly lower than controls, whereas MCH neuron integrated intensity was not significantly different than controls. (D) Number of OXA neurons and MCH neurons were counted from 5 brain sections with the highest number of neurons in each animal. The number of OXA neurons at 4 hrs post-CA was significantly lower than control rats and rats at 72 hrs post-CA, however, there was no significant difference in MCH neuron number at different time points. ** p < 0.01, * p < 0.05 by one-way analysis of variance. (E) NDS was correlated to the number of OXA neurons (p < 0.001). No correlation was found between MCH neurons and NDS. (F) The size of OXA and MCH neurons were obtained by CellProfiller. There was no difference in the size of either OXA or MCH neurons. CA, cardiac arrest; Con, control; MCH, melanin-concentrating hormone; NDS, Neurological Deficit Scale; OXA, orexin-A; SEM, standard error of the mean.
Next, we assessed whether there were changes in the number of orexin-positive neurons as detected by OXA-IR that might correlate with changes in OXA CSF levels. Consistent with the dynamic change of CSF OXA level, the number of OXA-IR neurons in 4 h post-CA rats was the lowest among tested groups, decreasing to 64% of control (443.1 ± 48.8 at 4 h [n = 5] vs. 672.4 ± 81.6 in control [n = 6] mean ± SEM; p < 0.001; Fig. 3E, OXA). By 24 h post-CA, the number of OXA-IR neurons increased (595.1 ± 44.1 at 24 h [n = 3] compared with 443.1 ± 48.8 at 4 h; p < 0.05); this value was not significantly different from control (p = 0.454). The number of OXA-IR neurons remained consistent up to 72 h post-CA (604.4 ± 88.9 at 72 h [n = 5] compared with 672.4 ± 81.6 of control; p = 0.433). There was a strong positive correlation between the number of OXA-IR neurons and NDS (Spearman's Rho correlation coefficient = 0.813; p < 0.001; Fig. 3F). There was no significant difference in the number of neurons expressing MCH among all the tested groups and no correlation between the number of MCH neurons and NDS (Spearman's rho correlation coefficient = 0.362; p = 0.128).
An important issue for the cell counts is that we did not use stereological techniques that correct for possible changes in the size of the elements being counted. For example, if there were decreases in cell size at 4 h post-CA, this could account for the decreases in the number of cells that were counted. To control for this, we measured the size of OXA- and MCH-positive neurons at the different time points. As illustrated in Fig. 3D, there were no significant changes in the size of orexin-positive neurons that would account for the decreases in cell number. Taken together, our results suggest that changes in orexin levels in CSF, and especially the increases in orexin with recovery, are attributed largely to changes in the number of neurons that are actively expressing orexin at the different time periods.
Blocking orexin function early during post–cardiac arrest phase impairs neurological recovery and leads to sustained impairments
Because we found a reduction, followed by a rise, in CSF orexin levels and the number of orexin-IR cells between 4 and 24 h post-CA, when recovery of NDS scores occurs, we hypothesized that the first 24 h of orexin expression may be critical for neurological recovery post-CA. To test this, we treated in a separate cohort of rats with the dual orexin receptor (Orexin-1 and -2) antagonist, suvorexant, to block orexinergic function post-CA. Each animal received i.p. suvorexant at three time points: 1) immediately (within 5 min) after resuscitation from CA; 2) 8 h post-CA; and 3) 20 h post-CA. These times were chosen based on the half-life and pharmacological profile of suvorexant. Neurological recovery was monitored by measuring NDS at 12-h intervals until 72 h post-CA.
Remarkably, rats treated with suvorexant remained severely impaired at all post-CA time points compared with rats injected with vehicle (n = 5 for suvorexant and n = 5 for vehicle [p < 0.01] by general linear model for repeated measure; Fig. 4A). It is important to note that suvorexant was not administered beyond 24 h post-CA, and the sedative effect of suvorexant lasts approximately 6 h,25,26 but the neurological impairments persisted even at 72 h post-CA. Indeed, suvorexant-treated rats had to be euthanized at 72 h as an animal welfare issue because of the severe neurological impairments. Analysis of the subscores of the NDS test at 72 h, as indicated in Table 1, revealed that the overall neurological impairments of suvorexant-treated rats mainly came from dysfunction in the motor, sensory, and behavior assessments (Fig. 4B, 12.8 ± 5.8 for suvorexant, 28.0 ± 0.9 for vehicle) and not from arousal scores (Fig. 4B, 13.0 ± 1.2 for suvorexant, 16.8 ± 1.6 for vehicle), or brainstem function scores (Fig. 4B, 19.2 ± 1.2 for suvorexant, 21.0 ± 0.0 for vehicle). Analysis by two-way ANOVA revealed an overall group difference (p < 0.003), and significant differences between vehicle and suvorexant groups on the motor, sensory, and behavior assessments by post-hoc t-test (p = 0.032). There were no significant differences for the other subscores, however. These results strongly support the interpretation that early treatment with suvorexant leads to persistent severe neurological deficits that are not related to any residual sedative effect of suvorexant. These results strongly support an early critical period after survival from CA when a functional orexinergic pathway is necessary for optimal neurological recovery.
Discussion
In this study, we investigated the role of the orexin pathway in neurological recovery after asphyxial CA, the second-most common type of cardiac arrest in adults.27 Our results reveal dynamic changes in the orexinergic pathway that correlate with neurological recovery post-CA. Specifically, OXA levels in CSF and the number of OXA-IR neurons in the hypothalamus are reduced in rats 4 h after resuscitation from CA, preceding arousal from coma. By 24 h post-CA, when rats exhibited improvements in neurological function, we found a significant recovery of CSF OXA levels and increases in the number of OXA-IR neurons in the hypothalamus. Most important, transiently blocking orexin function during these 24 h post-CA with a dual orexin receptor antagonist led to profound and sustained deficits in neurological function. Our results suggest that the orexin system plays a key role in early recovery post-CA, and that blocking orexin action during the early phase of recovery causes profoundly impaired long-term recovery.
Post–cardiac arrest regulation of orexin levels
Our results document substantial decreases in orexin levels in CSF at early time points post-CA and then recovery toward control levels during the period of neurological recovery. Changes in CSF levels of orexin could occur as a result of decreases and increases in expression of orexin by hypothalamic neurons or decreases and increases in the number of neurons expressing orexin. Our results indicate that changes in CSF levels of orexin are more closely related to changes in the number of neurons expressing OXA rather than average orexin levels in individual neurons.
One caveat of our results is that cell counts were not done using stereological techniques. Ours are profile counts per section and do not accurately indicate the total number of orexin-expressing neurons in the hypothalamus. We did control for one of the primary errors that can skew profile counts; there were no changes in the size of orexin-positive neurons that could account for the changes in cell number. Accordingly, our counts can be taken as reliable indicators of changes in the number of orexin-positive neurons that are detected by IHC at the different time points.
Other studies have also reported changes in the number of orexin-expressing neurons in many different paradigms.28–30 Importantly, there were no changes in the number of neurons expressing another neuropeptide, MCH, indicating that CA-related effects may be specific to orexin neurons rather than a global reduction in neuropeptide expression. Although orexin and MCH neurons are known to play reciprocal roles in the sleep-awake cycle,31 reciprocal changes in the number of MCH neurons were not observed in our study, suggesting a potentially unique role for orexin in arousal and neurological recovery post-CA.
Changes in CSF OXA levels are most closely related to changes in the number of OXA-positive neurons, whereas histochemical measures of OXA levels were slightly, but not significantly, decreased at all time points post-CA. One caveat is that this is, at best, a semiquantitative measure and, at best, indicates OXA levels in the neuron. Changes in CSF OXA levels could also be attributed to altered release or altered degradation or metabolism of the OXA in CSF, though such mechanisms affecting CSF OXA levels have not been elucidated.32 Whatever the mechanism of regulation, our results reveal a close relationship between CSF levels and neurological function, which could be important for predicting outcome in patients recovering from CA.
Transient blockade of orexin leads to persistent neurological deficits
Remarkably, blockade of orexin with a dual orexin receptor antagonist, suvorexant, during what would otherwise be the recovery period post-CA led to sustained neurological deficits that persisted long after suvorexant action would have terminated. These neurological deficits cannot be explained by arousal level given that NDS subscores measuring arousal were not different between suvorexant- and vehicle-treated rats. These results suggest that orexin action during the early post-CA interval is critical for neurological recovery. In this regard, a previous study reported that a single injection of OXA into the ventricular space immediately after resuscitation from CA led to accelerated neurological recovery of rodents.18 It remains to be determined whether delayed, instead of early, blockade of orexin would also impair recovery. Defining this time window would help to elucidate the relationships between orexin, arousal, and the final level of neurological recovery.
Implications for clinical practice
The vast majority of CA survivors emerge in a comatose condition, and arousal from coma (defined as a state of unresponsiveness where both arousal and awareness are absent33) remains the best predictor for outcome.1 Leading causes of coma, including TBI and nontraumatic conditions, such as strokes, immune disorders, and toxic-metabolic insults, are accompanied by deficits in the orexin pathway.10,14,15,34–39 The upstream role of orexin in primary disorders of sleep and arousal, such as narcolepsy, is well established.40 However, the role of orexinergic dysfunction after acute brain injury is unclear. One important question is whether it is orexin per se or instead orexin-induced arousal that contributes to recovery. If it is arousal rather than a specific action of orexin, there may be other ways to intervene to enhance arousal. Whatever the cause, our results add to the story that manipulations of orexin during the early period post-CA can profoundly alter recovery. This is of considerable clinical relevance because early acceleration of neurological recovery post-CA can reduce comorbidities, such as deep vein thrombosis, ventilator-associated pneumonia, and critical illness neuromyopathy, ultimately improving outcome.41–43
Another important implication of our findings is the possible consequence of inadvertent blockade of orexin for people who suffer cardiac arrest. For example, suvorexant has recently been approved for treatment of insomnia. Because many people suffer cardiac arrest during the early morning hours upon awakening, when suvorexant may still be active in individuals consuming this medication for insomnia, it may be important to consider risk factors for coma-inducing insults, such as CA, before prescribing suvorexant.
Other caveats
Our study has several limitations. First, our measure of neurological recovery was the NDS test, which predominantly measures arousal, brainstem reflexes, gross sensory/motor function, and primitive behavior. Although this may be adequate for assessment of an arousal-related neuropeptide such as orexin, additional functional tests need to be done to better understand the role of orexin in post-CA recovery. Second, we limited our study to OXA; our data only assessed OXA levels secreted in the CSF and the number of OXA-IR neurons, leaving the possibility that OXB may also have an important role for post-CA recovery. Given that suvorexant is a dual-receptor antagonist, and OXA (in comparison to OXB) has higher affinity for OX-1 receptor and equal affinity for OX-2 receptor,44 it is likely that OXA plays a more important role in post-CA recovery.
Conclusion
Our results suggest that post-CA recovery is dependent on a functional orexin system, and that orexin may play a key role in determining the final extent of neurological recovery post-CA. These findings motivate future studies to assess the role of orexin as a therapeutic target for improving post-CA recovery.
Acknowledgments
Thanks to Nayna Sanathara and Dr. Olivier Civelli, University of California (UC) in Irvine, for the gift of the rabbit anti MCH antibody; and to Ramin Badiyan, Caleb Lou, Juan Alcocer, Tiffany Nguyen, Shuhab M. Zaher, Tin Dinh, Angeli Bernardo, Ashar Ahmed Khan, Tameena Wais, and Yusuf Suri for experimental assistance. This work was supported by 5KL2TR000147 to Yama Akbari through UC Irvine's NIH CATS UL1 TR001414, as well as funds from the UC Irvine Department of Neurology and School of Medicine.
Author Discolsure Statement
No competing financial interests exist.
References
- 1.Mozaffarian D., Benjamin E.J., Go A.S., Arnett D.K., Blaha M.J., Cushman M., Das S.R., Ferranti S. De, Després J.P., Fullerton H.J., Howard V.J., Huffman M.D., Isasi C.R., Jiménez M.C., Judd S.E., Kissela B.M., Lichtman J.H., Lisabeth L.D., Liu S., MacKey R.H., Magid D.J., McGuire D.K., Mohler E.R., Moy C.S., Muntner P., Mussolino M.E., Nasir K., Neumar R.W., Nichol G., Palaniappan L., Pandey D.K., Reeves M.J., Rodriguez C.J., Rosamond W., Sorlie P.D., Stein J., Towfighi A., Turan T.N., Virani S.S., Woo D., Yeh R.W., and Turner M.B. (2016). Heart disease and stroke statistics—2016 update a report from the American Heart Association. Circulation 133, e38–e48 [DOI] [PubMed] [Google Scholar]
- 2.Schefold J.C., Storm C., Krüger A., Ploner C.J., and Hasper D. (2009). The Glasgow Coma Score is a predictor of good outcome in cardiac arrest patients treated with therapeutic hypothermia. Resuscitation 80, 658–661 [DOI] [PubMed] [Google Scholar]
- 3.Berek K., Jeschow M., and Aichner F. (1997). The prognostication of cerebral hypoxia after out-of-hospital cardiac arrest in adults. Eur. Neurol. 37, 135–145 [DOI] [PubMed] [Google Scholar]
- 4.Nielsen N., Wetterslev J., Cronberg T., Erlinge D., Gasche Y., Hassager C., Horn J., Hovdenes J., Kjaergaard J., Kuiper M., Pellis T., Stammet P., Wanscher M., Wise M.P., Åneman A., Al-Subaie N., Boesgaard S., Bro-Jeppesen J., Brunetti I., Bugge J.F., Hingston C.D., Juffermans N.P., Koopmans M., Køber L., Langørgen J., Lilja G., Møller J.E., Rundgren M., Rylander C., Smid O., Werer C., Winkel P., and Friberg H. (2013). Targeted temperature management at 33°C versus 36°C after cardiac arrest. N. Engl. J. Med. 369, 2197–2206 [DOI] [PubMed] [Google Scholar]
- 5.Nambu T., Sakurai T., Mizukami K., Hosoya Y., Yanagisawa M., and Goto K. (1999). Distribution of orexin neurons in the adult rat brain. Brain Res. 827, 243–260 [DOI] [PubMed] [Google Scholar]
- 6.Alexandre C., Andermann M.L., and Scammell T.E. (2013). Control of arousal by the orexin neurons. Curr. Opin. Neurobiol. 23, 752–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peyron C., Faraco J., Rogers W., Ripley B., Overeem S., Charnay Y., Nevsimalova S., Aldrich M., Reynolds D., Albin R., Li R., Hungs M., Pedrazzoli M., Padigaru M., Kucherlapati M., Fan J., Maki R., Lammers G.J., Bouras C., Kucherlapati R., Nishino S., and Mignot E. (2000). A mutation in a case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brains. Nat. Med. 6, 991–997 [DOI] [PubMed] [Google Scholar]
- 8.Dauvilliers Y., Baumann C.R., Carlander B., Bischof M., Blatter T., Lecendreux M., Maly F., Besset A., Touchon J., Billiard M., Tafti M., and Bassetti C.L. (2003). CSF hypocretin-1 levels in narcolepsy, Kleine-Levin syndrome, and other hypersomnias and neurological conditions. J. Neurol. Neurosurg. Psychiatry 74, 1667–1673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kotan D., Deniz O., Aygul R., and Yildirim A. (2013). Acute cerebral ischaemia: relationship between serum and cerebrospinal fluid orexin-A concentration and infarct volume. J. Int. Med. Res. 41, 404–409 [DOI] [PubMed] [Google Scholar]
- 10.Baumann C.R., Stocker R., Imhof H.-G., Trentz O., Hersberger M., Mignot E., and Bassetti C.L. (2005). Hypocretin-1 (orexin A) deficiency in acute traumatic brain injury. Neurology 65, 147–149 [DOI] [PubMed] [Google Scholar]
- 11.Ang B.T., Tan W.L., Lim J., and Ng I. (2005). Cerebrospinal fluid orexin in aneurysmal subarachnoid haemorrhage—a pilot study. J. Clin. Neurosci. 12, 758–762 [DOI] [PubMed] [Google Scholar]
- 12.Dohi K., Ripley B., Fujiki N., Ohtaki H., Yamamoto T., Goto Y., Nakamachi T., Shioda S., Aruga T., and Nishino S. (2008). CSF orexin-A/hypocretin-1 concentrations in patients with intracerebral hemorrhage (ICH). Regul. Pept. 145, 60–64 [DOI] [PubMed] [Google Scholar]
- 13.Dohi K., Ripley B., Fujiki N., Ohtaki H., Shioda S., Aruga T., and Nishino S. (2005). CSF hypocretin-1/orexin-A concentrations in patients with subarachnoid hemorrhage (SAH). Peptides 26, 2339–2343 [DOI] [PubMed] [Google Scholar]
- 14.Rejdak K., Petzold A., Lin L., Smith M., Kitchen N., and Thompson E.J. (2005). Decreased CSF hypocretin-1 (orexin-A) after acute haemorrhagic brain injury. J. Neurol. Neurosurg. Psychiatry 76, 597–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suzuki K., Miyamoto T., Miyamoto M., Maeda H., Nokura K., Tohyama J., Hirata K., Shimizu T., and Kanbayashi T. (2016). Hypocretin-1 levels in the cerebrospinal fluid of patients with Percheron artery infarction with or without midbrain involvement. Medicine (Baltimore) 95, e4281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chokroverty S. (1975). “Alpha-like” rhythms in electroencephalograms in coma after cardiac arrest. Neurology 25, 655–663 [DOI] [PubMed] [Google Scholar]
- 17.Dohi K., Nishino S., Nakamachi T., Ohtaki H., Morikawa K., Takeda T., Shioda S., and Aruga T. (2006). CSF orexin A concentrations and expressions of the orexin-1 receptor in rat hippocampus after cardiac arrest. Neuropeptides 40, 245–250 [DOI] [PubMed] [Google Scholar]
- 18.Koenig M. a., Jia X., Kang X., Velasquez A., Thakor N. V., and Geocadin R.G. (2009). Intraventricular orexin-A improves arousal and early EEG entropy in rats after cardiac arrest. Brain Res. 1255, 153–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Porkka-Heiskanen T., Alanko L., Kalinchuk A., Heiskanen S., and Stenberg D. (2004). The effect of age on prepro-orexin gene expression and contents of orexin A and B in the rat brain. Neurobiol. Aging 25, 231–238 [DOI] [PubMed] [Google Scholar]
- 20.Hahn J.D. (2010). Comparison of melanin-concentrating hormone and hypocretin/orexin peptide expression patterns in a current parceling scheme of the lateral hypothalamic zone. Neurosci. Lett. 468, 12–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sawai N., Ueta Y., Nakazato M., and Ozawa H. (2010). Developmental and aging change of orexin-A and -B immunoreactive neurons in the male rat hypothalamus. Neurosci. Lett. 468, 51–55 [DOI] [PubMed] [Google Scholar]
- 22.Kitamura E., Hamada J., Kanazawa N., Yonekura J., Masuda R., Sakai F., and Mochizuki H. (2010). The effect of orexin-A on the pathological mechanism in the rat focal cerebral ischemia. Neurosci. Res. 68, 154–157 [DOI] [PubMed] [Google Scholar]
- 23.Carpenter A.E., Jones T.R., Lamprecht M.R., Clarke C., Kang I.H., Friman O., Guertin D.A., Chang J.H., Lindquist R. a, Moffat J., Golland P., and Sabatini D.M. (2006). CellProfiler: image analysis software for identifying and quantifying cell phenotypes. Genome Biol. 7, R100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kamentsky L., Jones T.R., Fraser A., Bray M.A., Logan D.J., Madden K.L., Ljosa V., Rueden C., Eliceiri K.W., and Carpenter A.E. (2011). Improved structure, function and compatibility for cellprofiler: modular high-throughput image analysis software. Bioinformatics 27, 1179–1180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cox C.D., Breslin M.J., Whitman D.B., Schreier J.D., McGaughey G.B., Bogusky M.J., Roecker A.J., Mercer S.P., Bednar R.A., Lemaire W., Bruno J.G., Reiss D.R., Harrell C.M., Murphy K.L., Garson S.L., Doran S.M., Prueksaritanont T., Anderson W.B., Tang C., Roller S., Cabalu T.D., Cui D., Hartman G.D., Young S.D., Koblan K.S., Winrow C.J., Renger J.J., and Coleman P.J. (2010). Discovery of the dual orexin receptor antagonist [(7 R)-4-(5-chloro-1,3- benzoxazol-2-yl)-7-methyl-1,4-diazepan-1-yl][5-methyl-2-(2 H − 1,2,3-triazol-2-yl)phenyl]methanone (MK-4305) for the treatment of insomnia. J. Med. Chem. 53, 5320–5332 [DOI] [PubMed] [Google Scholar]
- 26.Winrow C.J., Gotter A.L., Cox C.D., Doran S.M., Tannenbaum P.L., Breslin M.J., Garson S.L., Fox S. V, Harrell C.M., Stevens J., Reiss D.R., Cui D., Coleman P.J., and Renger J.J. (2011). Promotion of sleep by suvorexant-a novel dual orexin receptor antagonist. J. Neurogenet. 25, 52–61 [DOI] [PubMed] [Google Scholar]
- 27.Katz L., Ebmeyer U., Safar P., Radovsky A., and Neumar R. (1995). Outcome model of asphyxial cardiac arrest in rats. J. Cereb. Blood Flow Metab. 15, 1032–1039 [DOI] [PubMed] [Google Scholar]
- 28.Mikrouli E., Wörtwein G., Soylu R., Mathé A.A., and Petersén Å. (2011). Increased numbers of orexin/hypocretin neurons in a genetic rat depression model. Neuropeptides 45, 401–406 [DOI] [PubMed] [Google Scholar]
- 29.Jalewa J., Wong-Lin K., McGinnity T.M., Prasad G., and Hölscher C. (2014). Increased number of orexin/hypocretin neurons with high and prolonged external stress-induced depression. Behav. Brain Res. 272, 196–204 [DOI] [PubMed] [Google Scholar]
- 30.Michinaga S., Hisatsune A., Isohama Y., and Katsuki H. (2011). Orexin neurons in hypothalamic slice cultures are vulnerable to endoplasmic reticulum stress. Neuroscience 190, 289–300 [DOI] [PubMed] [Google Scholar]
- 31.Hassani O.K., Lee M.G., and Jones B.E. (2009). Melanin-concentrating hormone neurons discharge in a reciprocal manner to orexin neurons across the sleep-wake cycle. Proc. Natl. Acad. Sci. U. S. A. 106, 2418–2422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zink A.N., Perez-Leighton C.E., and Kotz C.M. (2014). The orexin neuropeptide system: physical activity and hypothalamic function throughout the aging process. Front. Syst. Neurosci. 8, 211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Laureys S., Owen A.M., and Schiff N.D. (2004). Brain function in coma, vegetative state, and related disorders. Lancet Neurol. 3, 537–546 [DOI] [PubMed] [Google Scholar]
- 34.Scammell T.E., Nishino S., Mignot E., and Saper C.B. (2001). Narcolepsy and low CSF orexin (hypocretin) concentration after a diencephalic stroke. Neurology 56, 1751–1753 [DOI] [PubMed] [Google Scholar]
- 35.Baumann C.R., Bassetti C.L., Valko P.O., Haybaeck J., Keller M., Clark E., Stocker R., Tolnay M., and Scammell T.E. (2009). Loss of hypocretin (orexin) neurons with traumatic brain injury. Ann. Neurol. 66, 555–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kanbayashi T., Ishiguro H., Aizawa R., Saito Y., Ogawa Y., Abe M., Hirota K., Nishino S., and Shimizu T. (2002). Hypocretin-1 (orexin-A) concentrations in cerebrospinal fluid are low in patients with Guillain-Barre syndrome. Psychiatry Clin. Neurosci. 56, 273–274 [DOI] [PubMed] [Google Scholar]
- 37.Ripley B., Overeem S., Fujiki N., Nevsimalova S., and Uchino M. (2001). CSF hypocretin / orexin levels in narcolepsy and other neurological. Neurology 57, 2253–2258 [DOI] [PubMed] [Google Scholar]
- 38.Saji N., Kawarai T., Tadano M., Shimizu H., Kita Y., Susuki K., and Kanbayashi T. (2007). Does CSF hypocretin-1 decrease in Bickerstaff's brainstem encephalitis? Clin. Neurol. Neurosurg. 109, 547–548 [DOI] [PubMed] [Google Scholar]
- 39.Horsting M.W., Franken M.D., Meulenbelt J., van Klei W.A., and de Lange D.W. (2015). The etiology and outcome of non-traumatic coma in critical care: a systematic review. BMC Anesthesiol. 15, 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang X.-Y., Yu L., Zhuang Q.-X., Zhu J.-N., and Wang J.-J. (2013). Central functions of the orexinergic system. Neurosci. Bull. 29, 355–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Davis K.A. (2006). Ventilator-associated pneumonia: a review. J. Intensive Care Med. 21, 211–26 [DOI] [PubMed] [Google Scholar]
- 42.Patel R., Cook D.J., Meade M.O., Griffith L.E., Mehta G., Rocker G.M., Marshall J.C., Hodder R., Martin C.M., Heyland D.K., Peters S., Muscedere J., Soth M., Campbell N., and Guyatt G.H. (2005). Burden of illness in venous thromboembolism in critical care: a multicenter observational study. J. Crit. Care 20, 341–347 [DOI] [PubMed] [Google Scholar]
- 43.Desai S. V, Law T.J., and Needham D.M. (2011). Long-term complications of critical care. Crit. Care Med. 39, 371–379 [DOI] [PubMed] [Google Scholar]
- 44.Sakurai T., Amemiya A., Ishii M., Matsuzaki I., Chemelli R.M., Tanaka H., Williams S.C., Richardson J.A., Kozlowski G.P., Wilson S., Arch J.R.S., Buckingham R.E., Haynes A.C., Carr S.A., Annan R.S., Mcnulty D.E., Liu W.-S., Terrett J.A., Elshourbagy N.A., and Bergsma D.J. (1998). Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell 92, 573–585 [DOI] [PubMed] [Google Scholar]