Abstract
Study objectives
To examine the resting-state functional connectivity (FC) between subcortical regions in relation to whole-brain activity in patients with psychophysiological insomnia (PI) and changes following cognitive–behavioral therapy for insomnia (CBTi).
Methods
The FC between subcortical seed regions (caudate, putamen, pallidum, amygdala, thalamus, and hippocampus) and whole-brain voxels were compared between the PI group (n = 13, mean age: 51.0 ± 10.2 years) and good sleepers (GS, n = 18, mean age: 42.7 ± 12.3 years). Also, in the PI group, FC was compared before and after 5 weeks of CBTi.
Results
Compared to the GS group, the PI group exhibited stronger FC between the thalamus and prefrontal cortex and between the pallidum and precuneus but weaker FC between the pallidum and angular gyrus, the caudate and orbitofrontal cortex, and the hippocampus and fusiform gyrus. After CBTi, the PI group exhibited decreased FC between the thalamus and parietal cortex, the putamen and motor cortices, and the amygdala and lingual gyrus, but increased FC between the caudate and supramarginal gyrus, the pallidum and orbitofrontal cortex, and the hippocampus and frontal/parietal gyri.
Conclusions
The present findings demonstrate different FC in PI patients compared to GS and provide insight into the neurobiological rationale for CBTi.
Keywords: Psychophysiological insomnia, Insomnia, Resting state, Functional magnetic resonance imaging, Cognitive–behavioral therapy, Functional connectivity
Highlights
-
•
The functional connectivity (FC) of psychophysiological insomnia patients differed from that of good sleepers.
-
•
The FC of psychophysiological insomnia patients changed after cognitive-behavioral therapy for insomnia.
-
•
The neural basis for insomnia and cognitive-behavioral therapy may be demonstrated by changes in subcortical FC.
1. Introduction
Almost half of the general population has reported experiencing insomnia, making it one of the most common sleep disorders (Riemann et al., 2010). Insomnia is diagnosed based on subjective clinical features because its pathogenesis is a complex interplay of psychological, behavioral, and physiological elements. As insomnia symptoms warrant independent attention along with the associated mental or physical condition, primary insomnia has been removed from the 5th edition of the Diagnostic and Statistical Manual of Mental Disorders (Association AP, 2013), and the condition has been incorporated into insomnia disorder, which is now specified by comorbidity with other mental, medical, and sleep disorders. The International Classification of Sleep Disorders (ICSD-2) defines psychophysiological insomnia (PI) as a state of “heightened arousal and learned sleep-preventing associations that result in a complaint of insomnia and associated decreased function during wakefulness” (AASM, 2005). Despite the removal of various insomnia subdiagnoses, including PI in ICSD-3 (AASM, 2014), the term PI is notable for encompassing the diverse aspects of pathogenesis in insomnia.
The most widely accepted model of the pathophysiology of PI is the hyperarousal theory, which states that difficulties with initiating and/or maintaining sleep are due to global increases in cortical and physiological arousal across the sleep–wake cycle (Perlis et al., 1997). Spielman's 3-P model encompasses the hyperarousal theory by describing an individual's hyper-arousability with regard to constitutional predisposing factors or to perpetuating factors such as cognitive distortions and maladaptive behaviors, which cause insomnia to persist even after the acute precipitating factors (such as stressful life events) have disappeared (Spielman et al., 2011). However, the identification of trait-like neurobiological factors that act as predisposing factors in patients with insomnia has proven elusive (Spielman et al., 2011). Thus, cognitive–behavioral therapy for insomnia (CBTi), which addresses the maladaptive behaviors and cognitive distortions of insomnia patients, is considered the first-line treatment for this chronic disorder (Qaseem et al., 2016).
There has been a recent increase in neuroimaging studies attempting to reveal the underlying neurobiological bases of insomnia disorder. A previous report supporting hyperarousal was a PET study showing altered brain metabolism in patients with insomnia (Nofzinger et al., 2004). More recently, altered glucose metabolism has been localized to areas related to cognition and the DMN, which affect patients with primary insomnia (Kay et al., 2016). Structural magnetic resonance imaging (MRI) studies have identified volume changes in the frontal cortex (Joo et al., 2013, Altena et al., 2010, Stoffers et al., 2012, Winkelman et al., 2013) and the hippocampus (Riemann et al., 2007, Joo et al., 2014). Another measure of brain activity, functional connectivity (FC), is the temporal dependence of neuronal activity across anatomically separate brain regions. Insomnia patients have been shown to exhibit alterations in FC during specific cognitive tasks (Drummond et al., 2013, Altena et al., 2008, Stoffers et al., 2014), and two previous studies investigated the effects of non-pharmacological therapies for insomnia (CBTi and/or light therapy) on altered FC during specific tasks (Altena et al., 2008, Stoffers et al., 2014).
More recently, technical improvements in studying FC have allowed whole-brain analyses to identify networks of highly correlated regions, such as the default-mode network (DMN), that are exclusively activated during a resting state (Buckner et al., 2008). Resting-state studies may be able to significantly contribute to the field of insomnia research because the neurobiology of this disorder is becoming increasingly recognized as a 24-hour process that continues throughout the sleep-wake cycle. Previous studies observed disruptions in FC within the DMN and in regions associated with executive function (Li et al., 2014, Nie et al., 2015), sensorimotor functions, and limbic regions (Chen et al., 2014, Killgore et al., 2013, Huang et al., 2012), supporting previous physiological and emotional arousal findings associated with insomnia patients. A recent study found the resting-state FC between the amygdala and rostral anterior cingulate cortex to be intermediate in patients with primary insomnia compared to those with generalized anxiety disorder and controls, indicating that the emotional circuit is disrupted by insomnia (Pace-Schott et al., 2017). Intrinsic resting-state activity, identified by brain entropy or regional homogeneity analyses, has also been introduced as a variable in insomnia studies, resulting in consistent evidence for hyperarousal in related structures such as the hippocampus, DMN, basal ganglia (BG) (Zhou et al., 2016), and temporal cortex (Dai et al., 2014). If the therapeutic effects of CBTi are, in fact, related to the recovery of intrinsic FC, then the current understanding of the neurobiology of insomnia will be broadened. However, to date, no studies have explored the effects of CBTi on the intrinsic resting-state FC of insomnia patients.
Recently, the involvement of the BG in emotional and cognitive functioning through connections with the frontal cortex and thalamus has been highlighted (Arsalidou et al., 2013). In particular, the striatum and pallidum play important roles in emotional processing via input from the amygdala and hippocampus, which then relay signals to the thalamus. Interconnected with the prefrontal cortex, the cortico-striato-thalamo-cortical circuit regulates cortical arousal by filtering sensory input of the thalamus (Alexander and Crutcher, 1990). Marked hypoperfusion in the BG was demonstrated earlier by single-photon emission computed tomography (SPECT) in insomnia patients (Smith et al., 2002), and a recent whole-brain FC analysis showed increased FC among regions including the putamen and amygdala (Li et al., 2017). However, because the traditional view of the BG is limited to motor processing functions, studies focusing on BG-related resting-state networks in insomnia patients are limited.
The primary aim of the present study was to determine whether patients with PI would exhibit different resting-state FC, using the caudate, putamen, pallidum, thalamus, amygdala, and hippocampus as seed regions in relation to whole-brain neural activity. The secondary purpose of the present study was to evaluate the therapeutic effects of CBTi on resting-state FC in insomnia patients.
2. Methods
2.1. Participants
This study included 25 patients recruited from the Center for Sleep and Chronobiology at Seoul National University Hospital who were diagnosed with PI based on the criteria of the International Classification of Sleep Disorders, version 2 (ICSD-2). Additionally, 23 good sleepers (GS) were enrolled in the study via advertisements. The study protocol was approved by the Institutional Review Board of Seoul National University Hospital, and written informed consent was obtained from the participants after a complete description of the study was given. Individuals who had 1) a past history of serious medical or neurological illness, 2) a current medical or neurological illness, 3) an Axis I psychiatric disorder other than primary insomnia based on the criteria of the Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV), 4) a sleep disorder other than PI (based on ICSD-2 criteria), 5) insomnia duration < 6 months, 6) shift-work employment, 7) borderline or antisocial personality disorder, or 9) any contraindications for magnetic resonance imaging (MRI) scans, and 10) those who were pregnant were not eligible for enrollment.
To screen out those with psychiatric disorders, the Structural and Clinical Interview for the DSM-IV (SCID-IV) was administered to all participants by trained psychologists. To screen out participants with common sleep disorders such as obstructive sleep apnea, in lab nocturnal polysomnography (PSG; Profusion PSG3; Compumedics, Abbotsford, VIC Australia) was performed. Additionally, participants were asked not to take any medications that could potentially affect sleep, including hypnotics, sedatives, antipsychotics, antidepressants, and mood stabilizers.
PSG and functional MRI (fMRI) were conducted 15.8 ± 12.4 and 6.9 ± 4.5 days before the first CBTi session, respectively. Of the 25 PI patients, six were excluded prior to the initiation of CBTi due to brain lesions identified on the MRI scan (n = 2), the presence of obstructive sleep apnea on the nocturnal PSG (n = 1), the inability to discontinue hypnotics (n = 2), or withdrawal of consent during screening (n = 1). Of the 19 remaining PI patients, six who began CBTi withdrew from the study due to refusal to undergo a second fMRI scan after the CBTi sessions (n = 2), missing fMRI data (n = 1), and incomplete CBTi sessions (n = 3). Of the 23 GS, five were excluded at screening due to the presence of obstructive sleep apnea on the nocturnal PSG (n = 2) or withdrawal of consent during screening (n = 3). Thus, 13 PI patients and 18 GS were included in the final analyses of the present study. Among the 13 patients with PI, four were taking zolpidem at the time of recruitment. No patient was taking any other psychotropic medication. Those taking zolpidem participated in the study after a washout period ranging from 7 to 30 days. The included and excluded participants did not significantly differ in terms of their demographic and clinical characteristics. The PI group underwent a second fMRI scan after five sessions of CBTi were completed.
2.2. Baseline clinical assessments of sleep
All participants completed several sleep-related questionnaires, including the Pittsburgh Sleep Quality Index (PSQI), Dysfunctional Beliefs and Attitudes about Sleep Scale (DBAS-16), and the Beck Depression Inventory (BDI); the PI group also completed the Insomnia Severity Index (ISI). The PSQI is a self-report questionnaire assessing overall sleep quality including, but not limited to, insomnia (Buysse et al., 1989). The ISI is a self-report questionnaire that rates distress related to insomnia and sleep quality (Bastien et al., 2001). The DBAS-16 is a self-report questionnaire that assesses sleep-related cognitive dysfunction, such as unrealistic expectations, faulty beliefs, and excessive worry regarding sleep (Morin et al., 2007). The BDI (Beck et al., 1996) was administered to control for and exclude the risk of depressive symptoms, which may independently affect imaging findings.
2.3. CBTi
Five sessions of individual CBT-I were delivered face to face by two certified psychologists; the sessions were approximately 90 min long and were conducted weekly. Any medications that could potentially affect sleep were prohibited during the entire study procedure. A modified CBTi protocol (Edinger and Carney, 2008) that included behavioral, cognitive, and educational interventions was used in the present study. Patients were asked to go to bed only when sleepy, to get out of bed whenever they were unable to fall asleep, to wake up at the same time every morning, and to limit naps. They were also asked to restrict their sleep time according to their individual time-in-bed (TIB) window, which was prescribed based on the sleep efficiency (SE) during the previous week. Initially, the TIB window was 30 min more than TST. In the next session, the TIB window was titrated based on the SE of the previous week. If the SE in the sleep diary was < 85%, the prescribed TIB was decreased by 15 min. If the SE was over 90%, TIB was increased by 15 min. The sleep diary was collected for at least 7 days to provide baseline data before CBT-I was initiated. Sleep diaries for each CBT-I session were collected continuously. Additionally, cognitive interventions were performed to address dysfunctional thoughts and beliefs.
To track changes in sleep over time, the participants kept a sleep diary that recorded actual TIB, sleep latency (SL), total sleep time (TST), wake time after sleep onset (WASO), and SE. In the PI group, the ISI, PSQI, DBAS-16, and BDI were also administered after the 5-week CBTi period (post-CBTi). For each questionnaire, the pre-CBTi scores were subtracted from the post-CBTi sleep scores (Δ ISI, Δ PSQI, Δ DBAS-16, and Δ BDI, respectively).
2.4. MRI data acquisition
Resting-state fMRI data were acquired with a 3 T whole-body Siemens Tim Trio scanner (Siemens AG; Erlangen, Germany) using a 12-channel birdcage head coil and interleaved T2*-weighted echo planar imaging with the following characteristics: TR = 3500 ms, TE = 30 ms, flip angle = 90°, slice thickness = 3.5 mm, in-plane resolution = 1.9 × 1.9 mm, no gap, 35 axial slices, FOV = 240 mm, 116 volumes, and a scan duration of 6 min and 58 s for each subject. Following the fMRI scanning, high-resolution structural images were acquired with a T1-weighted 3D gradient echo pulse sequence with magnetization-prepared rapid gradient-echo sequencing using the following characteristics: TR = 1670 ms, TE = 1.89 ms, flip angle = 9°, slice thickness = 1.0 mm, in-plane resolution = 1.0 × 1.0 mm, and FOV = 250 mm.
2.5. Data pre-processing
Pre-processing of the resting-state fMRI data was done using SPM12 (Wellcome Trust Centre for Neuroimaging; London, UK), and all images were checked to ensure that the data were not corrupted by artifacts. The DICOM format of the data was converted to the NIfTi format, head motion was corrected by realigning the data to the first image, and differences in slice timing were corrected. The functional images were co-registered with anatomical images and then spatially normalized to the Montreal Neurological Institute (MNI) space using a transformation matrix derived from the T1 anatomical image segmentation. The obtained data were then resliced to 3 × 3 × 3 mm. Finally, the data were spatially smoothed using a Gaussian kernel with full-width at half-maximum of 6 mm.
2.6. FC analysis
Resting-state FC analyses were performed using CONN functional connectivity toolbox v16b (http://www.nitrc.org/projects/conn) (Whitfield-Gabrieli and Nieto-Castanon, 2012). All data were band-pass filtered (0.008–0.09 Hz), and physiological and other spurious noise sources in the blood oxygenation level-dependent (BOLD) signal were removed using the anatomical component-based noise correction (CompCor) strategy implemented in CONN (Behzadi et al., 2007). White matter signals, cerebrospinal fluid signals, and six motion correction parameters obtained from the pre-processing procedure were also removed. The seed-to-voxel analysis was performed with 12 subcortical seed regions (the thalamus, caudate, putamen, pallidum, amygdala, and hippocampus for both hemispheres) that were predefined by the Harvard-Oxford atlas (FSL, [fMRIB, Oxford, UK]) (Smith et al., 2004) (Fig. S1). The mean time series for each seed region was calculated and then correlated with the time courses of all other voxels in the brain for each participant. Pearson's correlation coefficients were converted to normally distributed scores using the Fisher's r- to -z transformation. Group-level analyses for the second-level general linear model were carried out using an independent t-test between the z–scores of the PI and GS groups and paired t-test between the pre-and post-CBTi groups. The reported results of the seed-to-voxel correlation analyses were thresholded at a false discovery rate (FDR)-corrected cluster level of q < 0.05 and an uncorrected peak level of P < 0.001 to correct for false positive rates.
Supplementary Fig. 1.
The six subcortical structures that were used as seed regions in the present study were predefined by the Harvard-Oxford Atlas. The Harvard–Oxford Atlas covers 48 cortical and 21 subcortical structural areas based on structural data and segmentations provided by the Harvard Center for Morphometric Analysis.
2.7. Statistical analyses
First, the baseline demographic and clinical data were compared using independent t-tests for continuous values and Fisher's exact test for categorical values. The pre-CBTi and post-CBTi clinical data were compared using paired t-tests. Next, the z-scores of the baseline FC maps for the PI and GS groups were compared using independent t-tests, with age, gender, and BDI scores with the insomnia-related item excluded ([insomnia excluded]-BDI) as nuisance covariates. Additionally, the z-scores of the FC maps from the PI group before and after CBTi were compared using paired t-tests with Δ (insomnia excluded)-BDI score as a nuisance covariate. Third, correlations of baseline sleep questionnaire scores and PSG parameters with the pre-CBTi z-scores, and the correlations of Δ sleep questionnaire scores with the pre-CBTi z-scores subtracted from the post-CBTi z-scores (i.e., Δ z-scores) in the PI group were examined using Pearson's correlation analyses. Data were analyzed using SPSS for Windows software (v21; SPSS, Inc., Chicago, IL, USA), and P-values < 0.05 were considered to indicate statistical significance.
3. Results
3.1. Demographic and clinical data
Table 1 shows the demographic characteristics and PSG sleep parameters of the PI and GS groups; the two groups did not significantly differ in terms of age, gender distribution, or BDI scores. Compared to the GS group, the PI group had significantly higher scores on PSQI and DBAS, shorter TST, and greater WASO. In the PI group, scores on all post-CBTi sleep questionnaires were significantly lower than the pre-CBTi scores. Additionally, the post-CBTi sleep parameters assessed by sleep diaries showed significant improvement in WASO and SL compared to pre-CBTi levels (Table 2).
Table 1.
Comparisons of the demographic and clinical variables between the PI and GS groups.
| PI (n = 13) | GS (n = 18) | T | P | |
|---|---|---|---|---|
| Age | 51.0 ± 10.2 | 42.7 ± 12.3 | 1.994 | 0.056 |
| Gendera | 3 M, 10F | 4 M, 14F | N/A | 0.642 |
| PSQI† | 12.9 ± 3.76 | 4.8 ± 2.5 | 6.836 | < 0.001 |
| DABS† | 92.0 ± 16.8 | 59.7 ± 25.4 | 3.990 | < 0.001 |
| BDIb | 8.3 ± 7.4 | 5.1 ± 5.4 | 0.883 | 0.385 |
| Nocturnal PSG | ||||
| TIB (min) | 477.9 ± 25.3 | 486.1 ± 11.9 | − 1.650 | 0.110 |
| TST (min)⁎ | 407.1 ± 49.7 | 443.7 ± 19.1 | − 2.512 | 0.024 |
| SE (%) | 85.8 ± 9.0 | 91.3 ± 3.9 | − 2.082 | 0.054 |
| SL (min) | 11.8 ± 12.2 | 11.5 ± 10.3 | 0.085 | 0.932 |
| WASO (min)⁎ | 55.8 ± 38.0 | 31.2 ± 16.9 | 2.190 | 0.044 |
| REML (min) | 90.5 ± 40.9 | 92.8 ± 25.0 | − 0.189 | 0.851 |
| N1 (%) | 13.8 ± 5.3 | 10.3 ± 5.5 | 1.800 | 0.082 |
| N2 (%) | 58.1 ± 9.4 | 61.0 ± 7.0 | − 1.000 | 0.325 |
| N3 (%) | 5.0 ± 4.7 | 6.5 ± 5.8 | − 0.791 | 0.435 |
| REM (%) | 23.1 ± 7.4 | 21.8 ± 3.8 | 0.658 | 0.516 |
Independent t-test; aFisher's exact test; bInsomnia-excluded BDI scores; ⁎P < 0.05; †P < 0.001. Abbreviations: PI, psychophysiological insomnia; GS, good sleepers; PSQI, Pittsburgh Sleep Quality Index; DBAS, Dysfunctional Beliefs and Attitudes about Sleep; BDI, Beck Depression Inventory; PSG, polysomnography; TIB, time in bed, TST, total sleep time; SE, sleep efficiency; SL, sleep latency; WASO, wake after sleep onset; REM, rapid eye movement sleep; REML, REM latency; N/A, not available.
Table 2.
Changes in clinical after CBTi in the PI group (n = 13).
| Pre-CBTi | Post-CBTi | T | P | |
|---|---|---|---|---|
| ISI⁎ | 14.3 ± 4.5 | 7.0 ± 5.4 | 3.724 | 0.003 |
| PSQI† | 12.9 ± 3.8 | 7.0 ± 3.0 | 5.272 | < 0.001 |
| DABS† | 92.0 ± 16.8 | 50.8 ± 33.3 | 6.178 | < 0.001 |
| BDIa | 7.0 ± 7.3 | 5.8 ± 7.9 | 1.505 | 0.158 |
| Sleep diary | ||||
| TST (hr) | 5.6 ± 1.2 | 5.9 ± 1.9 | − 0.442 | 0.666 |
| SL (min)⁎ | 39.3 ± 34.7 | 14.0 ± 16.2 | 3.701 | 0.003 |
| WASO (min)⁎ | 68.2 ± 50.8 | 30.8 ± 33.9 | 3.193 | 0.008 |
| SE (%) | 75.7 ± 13.8 | 82.1 ± 26.5 | − 0.806 | 0.436 |
Paired t-test; aInsomnia-excluded BDI scores; ⁎P < 0.05; †P < 0.001. Abbreviations: PI, psychophysiological insomnia; GS, good sleepers; PSQI, Pittsburgh Sleep Quality Index; DBAS, Dysfunctional Beliefs and Attitudes about Sleep; BDI, Beck Depression Inventory; TST, total sleep time; SL, sleep latency; SE, sleep efficiency; WASO, wake after sleep onset.
3.2. Between-group FC findings
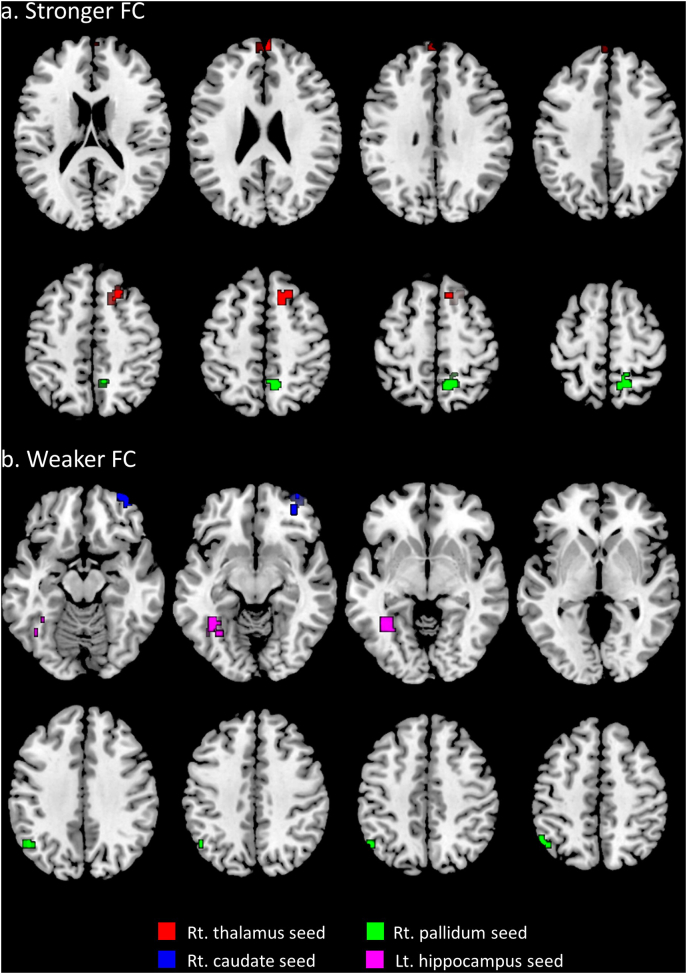
Compared to the GS group, the PI group exhibited stronger FC between the right thalamus and right superior frontal gyrus and bilateral frontal poles, and between the right pallidum and bilateral precuneus (Fig. 1a and Table 3). FC was significantly weaker between the right caudate and right orbitofrontal cortex (OFC), the right pallidum and left angular gyrus, and the left hippocampus and left fusiform gyrus (Fig. 1b and Table 3). FC between the putamen and amygdala and other brain regions was not significantly different.
Fig. 1.
Differences in FC in the PI group compared to the GS group. a) Areas where FC was stronger in the PI group compared to the GS group. Red areas: right superior frontal gyrus and frontal poles; green area: precuneus. b) Areas where FC was weaker in the PI group compared to the GS group. Blue area: right orbitofrontal cortex; green area: left angular gyrus; pink area: left fusiform. The colored areas indicate differences in FC in relation to the same-colored seed region. Abbreviations: FC, functional connectivity; PI, psychophysiological insomnia; GS, good sleepers; rt., right; lt., left. Thresholded at a whole-brain false discovery rate-corrected cluster level of q < 0.05 and an uncorrected peak level of P < 0.001.
Table 3.
Significant differences in FC in the PI group compared to the GS group.
| Seed | Brain region | BA | FC increased Vs. decreased | MNI coordinate (x, y, z) | Cluster size (number of voxels) | T-score |
|---|---|---|---|---|---|---|
| Rt. thalamus | Rt. superior frontal gyrus | 32, 8 | Increased | 16, 16, 50 | 101 | 5.71 |
| Bilateral frontal poles | 10 | Increased | 0, 58, 32 | 73 | 4.23 | |
| Rt. caudate | Rt. orbitofrontal cortex | 47 | Decreased | 42, 58, − 16 | 86 | 4.84 |
| Rt. pallidum | Bilateral precuneus | 5 | Increased | 10, − 52, 50 | 118 | 5.52 |
| Lt. angular gyrus | 39 | Decreased | − 48, − 58, 52 | 113 | 4.54 | |
| Lt. hippocampus | Lt. fusiform gyrus | 37 | Decreased | − 36, − 52, − 8 | 100 | 6.47 |
The threshold was set at uncorrected peak-level of P < 0.001 and a whole-brain, false discovery rate-corrected cluster level of q < 0.05. FC: functional connectivity; BA: Brodmann area; MNI: Montreal Neurological Institute; Rt.: right, Lt.: left.
3.3. FC in the PI group before and after CBTi
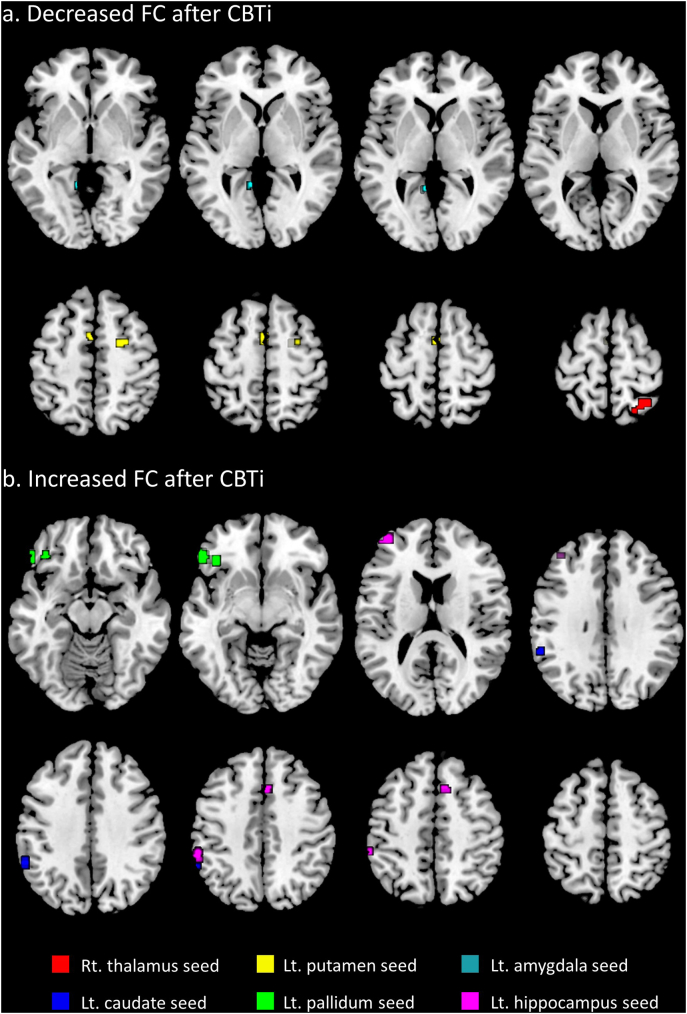
Compared to pre-CBTi FC, post-CBTi FC significantly increased between the left caudate and left supramarginal gyrus, between the left pallidum and left OFC, and between the left hippocampus and the left frontal pole, left supramarginal gyrus, and right paracingulate gyrus (Fig. 2a and Table 4). FC significantly decreased between the right thalamus and right superior parietal gyrus, between the left amygdala and left lingual gyrus, and between the left putamen and the right superior frontal gyrus and left supplementary motor area (Fig. 2b and Table 4).
Fig. 2.
Changes in post-CBTi FC relative to pre-CBTi FC in the PI group. a) Areas of significant decreases in post-CBTi FC compared to pre-CBTi FC in the PI group. Red area: right superior parietal gyrus; yellow areas: right superior frontal gyrus and left supplementary motor cortex; blue area: left lingual gyrus. b) Areas of significant increases in post-CBTi FC compared to pre-CBTi FC in the PI group. Blue area: left supramarginal gyrus; green area: left orbitofrontal cortex; pink areas: left frontal pole, left supramarginal gyrus, and right paracingulate gyrus. The colored areas indicate altered FC in relation to the same-colored seed region. Abbreviations: FC, functional connectivity; CBTi, cognitive-behavioral therapy for insomnia; PI, psychophysiological insomnia; rt., right; lt., left. Thresholded at a whole brain false discovery rate-corrected cluster-level of q < 0.05 and an uncorrected peak-level of P < 0.001.
Table 4.
Significant differences in FC in the PI group after CBTi compared to baseline.
| Seed | Brain region | BA | FC increased or decreased | MNI coordinate (x, y, z) | Cluster size (number of voxels) | T-score |
|---|---|---|---|---|---|---|
| Rt. thalamus | Rt. superior parietal gyrus | 7 | Decreased | 34, − 56, 68 | 61 | 5.44 |
| Lt. caudate | Lt. supramarginal gyrus | 40 | Increased | − 60, − 52, 40 | 58 | 6.33 |
| Lt. putamen | Rt. superior frontal gyrus | 6 | Decreased | 28, − 2, 50 | 56 | 7.54 |
| Lt. supplementary motor area | 6 | Decreased | − 2, 2, 58 | 54 | 6.18 | |
| Lt. pallidum | Lt. orbitofrontal cortex | 47 | Increased | − 42, 32, − 10 | 146 | 6.67 |
| Lt. hippocampus | Lt. frontal pole | 46 | Increased | − 38, 52, 14 | 113 | 7.01 |
| Lt. supramarginal gyrus | 40 | Increased | − 56, − 38, 44 | 51 | 6.77 | |
| Rt. Paracingulate | 32 | Increased | 6, 20, 50 | 50 | 4.83 | |
| Lt. amygdala | Lt. lingual gyrus | 18 | Decreased | − 12, − 50, − 4 | 61 | 5.83 |
The threshold was set at an uncorrected peak-level of P < 0.001 and a whole-brain, false discovery rate-corrected cluster level of q < 0.05. FC: functional connectivity; BA: Brodmann area; MNI: Montreal Neurological Institute; Rt.: right, Lt.: left.
3.4. Correlations between FC and clinical sleep parameters
In the PI group, the pre-CBTi ISI score was correlated with FC between the left hippocampus and left fusiform gyrus (r = − 0.770, P = 0.009; Fig. 3a), and the pre-CBTi PSQI score was significantly correlated with FC between the right pallidum and precuneus (r = 0.673, P = 0.033). After the CBTi sessions, the Δ FC between the right thalamus and right superior parietal gyrus was significantly correlated with the Δ SE (r = − 0.678, P = 0.015; Fig. 3b) and approached near-significant correlation with the Δ WASO (r = 0.570, P = 0.053). The correlation of the Δ ISI scores with the Δ FC between the left hippocampus and left supramarginal gyrus and the Δ FC between the left caudate nucleus and the left supramarginal gyrus approached, but did not reach, significance (r = 0.551, P = 0. 063; r = 0.545, P = 0.067, respectively). However, after a Bonferroni correction for multiple comparisons, no significant results were observed.
Fig. 3.
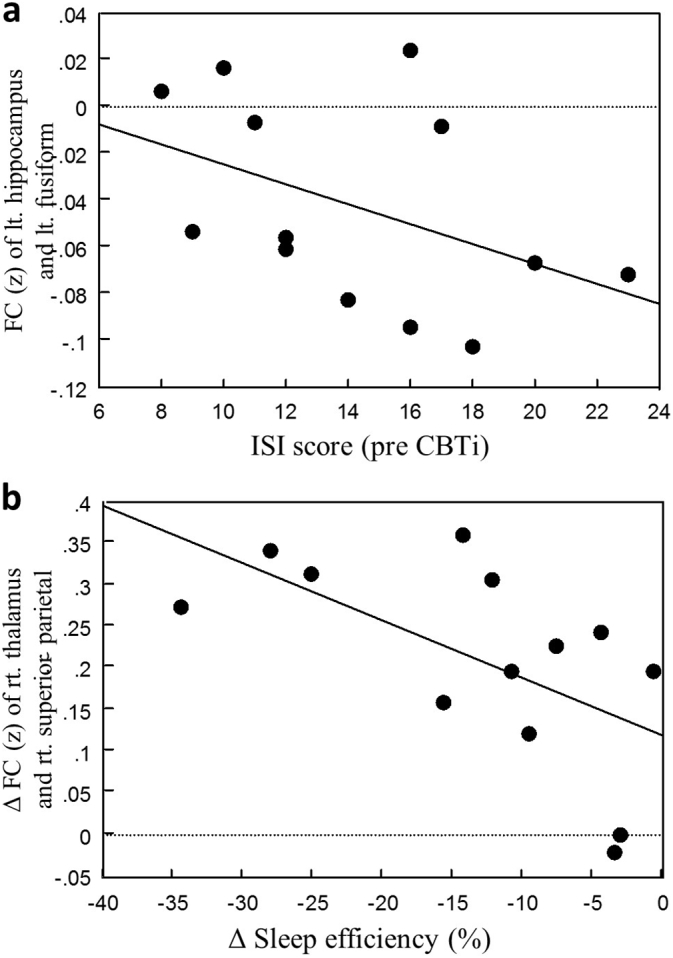
Correlations between FC and clinical parameters. a) Baseline ISI scores were significantly correlated with FC between the left hippocampus and left fusiform gyrus in the PI group (r = − 0.770, P = 0.009). b) Increases in SE (assessed by sleep diaries) were significantly correlated with increased FC between the right thalamus and right superior parietal gyrus after CBTi in the PI group (r = − 0.678, P = 0.015). Correlation results are not corrected for multiple comparisons. Abbreviations: FC, functional connectivity; ISI, insomnia severity index; PI, psychophysiological insomnia; CBTi, cognitive-behavioral therapy for insomnia; SE, sleep efficiency.
4. Discussion
The present study investigated resting-state FC in relation to subcortical nuclei seed regions, including the BG and was the first to evaluate the effects of CBTi on intrinsic resting-state FC in PI patients. The present results showed significantly different FC of the BG, thalamus, amygdala, and hippocampus with various cortical regions in patients with PI. Additionally, the intrinsic FC of the insomnia patients exhibited significant changes after a 5-week CBTi treatment program.
4.1. Thalamus and prefrontal cortex
In the present study, the PI group exhibited stronger FC between the thalamus and prefrontal cortex. The thalamus and cortex are strongly connected by neuronal fibers radiating from the thalamus to the cortex (Sherman, 2016). Accordingly, the present results provide a neural basis for the sensory related hyperarousal based on the observed hyperactivity of the thalamus in relation to cortical excitability. After CBTi, there was a decrease in connectivity between the thalamus and parietal cortex, rather than the frontal cortex, and this change was correlated with an increase in SE and nearly significantly correlated with a decrease in WASO, as assessed by the sleep diaries of the participants. Patients with insomnia are reported to show weaker FC between the parietal and frontal cortices (Li et al., 2014). Perhaps after CBTi, FC in the frontoparietal network improved and hyperarousal was reduced by the decrease in thalamus activity.
4.2. BG and OFC
The striatum is innervated from the OFC, dorsolateral prefrontal cortex, and posterior (inferior) parietal cortex, and the caudate is more likely receive input from the OFC and parietal cortex, while the putamen receives input from the somatosensory, primary motor, and premotor cortices in paralleled circuits (Arsalidou et al., 2013). Reductions in orbitofrontal volume have been identified in several studies of insomnia patients (Joo et al., 2013, Altena et al., 2010, Stoffers et al., 2012), and more recently, Stoffers et al. (Stoffers et al., 2014) observed a smaller BOLD response in the left caudate during executive tasks in insomnia patients compared to controls. Through additional analyses, the investigators interpreted the findings to be unrelated to increased baseline perfusion and possibly due to decreased FC from the OFC. These authors also emphasized the role that aberrant caudate activation plays in cortical activity and hyperarousal and showed that the reduced caudate activity was not recovered after CBTi with light therapy; thus, it was suggested that this pattern of activity may be an endophenotype for insomnia.
The present findings showing weaker FC between the caudate and OFC during the resting state strengthen prior claims that attenuated inhibition from the OFC may account for weaker caudate activity (Stoffers et al., 2014). The cortico-striato-thalamo-corticol circuit has multiple neurocognitive functions including regulating arousals along with cognitive and affective functions. The cortex connects to the striatum (caudate and putamen) and via the pallidum to the thalamus and back to the cortex (Alexander and Crutcher, 1990). After CBTi, the caudate did not show changes in FC and this may also be interpreted as supporting the notion that weak caudate activity is a trait-like marker of insomnia persisting despite therapy (Stoffers et al., 2014).
4.3. BG and DMN
The inferior parietal cortex comprises the angular gyrus and the supramarginal gyrus, which, along with the precuneus, are hubs of the DMN. The present results revealed different resting-state FC activities between the pallidum and various DMN regions, particularly the inferior parietal cortex (IPC) and precuneus in the PI group compared to GS. FC was weaker between the lt. pallidum and lt. angular gyrus, but it was stronger between the rt. pallidum and precuneus. After CBTi, the FC between the lt. caudate and lt. supramarginal gyrus increased. Altered DMN function is thought to be related to the hyperarousal symptoms of insomnia patients, who may evidence higher levels of activity in DMN areas during the day and possibly during sleep stages (Marques et al., 2015). Activation of the DMN specifically prior to bedtime is thought to contribute to increased rumination and worries concerning sleep (cognitive distortions), which in turn may hinder the progression from wakefulness to sleep (Marques et al., 2015). Two prior studies that specifically investigated DMN region-to-region FC in insomnia patients produced ambiguous results (Li et al., 2014, Nie et al., 2015).
4.4. BG and motor cortex
After the PI group underwent CBTi, FC between the left putamen and the supplementary motor area (SMA) decreased. The putamen is involved in motor regulation via connections with the primary motor cortex/premotor area (Arsalidou et al., 2013). Specifically, complex and voluntary movements, in contrast to automated and well-learned movements, are suggested to be lateralized in the lt. hemisphere and more related to the putamen (Arsalidou et al., 2013). If abnormal FC between the putamen and motor cortex is related to motor restlessness, a manifestation of physiological arousal, then the present findings imply that CBTi may be an effective therapy for physiological arousal.
4.5. Hippocampus, amygdala, and fronto–parietal cortex
The PI group exhibited weaker FC between the left hippocampus and left fusiform gyrus compared to the GS group. After CBTi, FC between the left hippocampus and the frontal cortex and left supramarginal gyrus increased. Previous studies have also reported decreased hippocampal volume and abnormal FC between the hippocampus and other DMN regions in patients with insomnia disorders (Riemann et al., 2007, Joo et al., 2014, Regen et al., 2016).
The hippocampus plays an important role in memory, along with its being in part of the limbic system and the DMN. In conjunction with the amygdala via the limbic system, the hippocampus sends signals to the striatum via cortico–striato–thalamic and limbic circuits. The present results showing increased FC between the hippocampus and the fronto-parietal regions after CBTi suggest that this may be a neurobiological basis for the recovery of reduced cognitive functioning, which is a common finding in insomnia patients (Nissen et al., 2011).
The amygdala is a central aspect of the emotional circuit and has bidirectional connections with the prefrontal cortex and limbic structures (Roy et al., 2009). An amygdala seed-based study in insomnia patients suggested the involvement of a compensatory response to disrupted emotional functioning in which decreased FC between the amygdala and other subcortical regions, but increased FC with the sensorimotor cortices including the occipital cortex, were observed (Huang et al., 2012). However, findings regarding the relationship of sleep-related stimuli to the emotional circuits, including the amygdala, have been inconsistent (Spiegelhalder et al., 2016). The present study did not identify significant FC changes in the amygdala of the PI group, but after CBTi, there was a significant decrease in FC between the amygdala and the lingual gyrus, which is linked to visual processing. This suggests that there may be disrupted FC within the emotional circuit that is related to sensory hyperarousal.
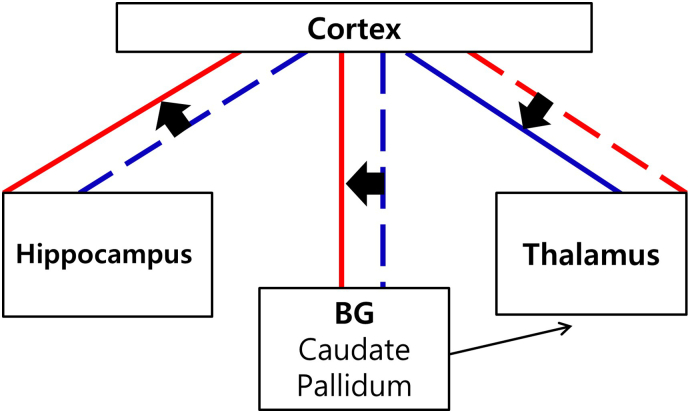
The present study looked into FC of subcortical structures in relation to the whole-brain cortex and arrived at several findings related to the hyperarousal and cognition of chronic insomnia (Fig. 4). However, the differences in FC between the PI and GS groups were not reflected consistently in post-CBTi changes in the PI group. The regions related to cognitive, emotional, and sensory arousal are interrelated and have overlapping functions in the salience, somatosensory motor, DMN, and limbic networks; other compensatory networks are yet to be revealed. With limited knowledge of the complexity of brain functioning, our findings are insufficient to demonstrate an integrative explanation of the pathogenesis of insomnia, and our attempt to link the significant changes in FC after CBTi with differences between the PI and GS at baseline are, at this point, speculative.
Fig. 4.
A hypothetical illustration of altered subcortical FC with the cortex and the changes after CBTi in patients with PI. Between the cortex and the BG, weaker FC compared to GS, was increased after CBTi. Between the cortex and the thalamus, stronger FC compared to GS, was decreased after CBTi. Between the cortex and the hippocampus, weaker FC compared to GS, was increased after CBTi. The connection between the BG and the thalamus was not explored in the present study, but presented in the illustration based on the cortico-striato-thalamo-cortical circuit. Broken lines represent weaker or decreased FC compared to GS or pre-CBTi, respectively. Thick solid lines represent stronger or increased FC compared to GS or pre-CBTi, respectively. Abbrevations: FC, functional connectivity; CBTi, cognitive-behavioral therapy for insomnia; BG, basal ganglia; GS, good sleepers.
The present study has several limitations. First, the sample size of the PI group was small. Second, a wait list comparison was not possible due to the lack of a second fMRI scan and the absence of follow-up in the GS group after 5 weeks; thus, time or placebo effects on the FC changes between pre- and post-CBTi cannot be excluded. However, correlations between changes in clinical sleep measurements and changes in FC after CBTi do support the association of CBTi per se with FC changes. Also, the PI group showed a reduction in the ISI score of > 7 points, which is considered to represent modest improvement, and they subsequently reached a score of below 8 points, which is the cutoff score for an absence of insomnia (Morin et al., 2011); these findings cannot be explained by time or placebo effects alone. Third, follow-up PSG analyses were not conducted after the CBTi sessions and no objective clinical measures related to the alterations in FC were administered to the PI group. However, an attempt was made to link the observed neural mechanisms with the clinical features using data from the sleep questionnaires and sleep diaries, which were comparable to objective PSG measures (Morin et al., 1999). Fourth, the correlation between the FC and clinical sleep parameters did not remain significant after correction for multiple comparisons. Also, non-significant difference in the ages of the PI and GS groups were noted, but controlled for in the between-group comparisons. Fifth, the regions implicated with FC change by CBTi were different from baseline group comparisons. However, many of our discussion points attempting to provide an integrative interpretation are at a speculative level and are therefore inconclusive. Nevertheless, the present study provides the first prospective resting-state data of PI patients who underwent CBTi without medication, and these data can be used as grounds for future research.
In conclusion, the present study found significant differences in the resting-state FC of the BG, amygdala, hippocampus, and thalamus with various cortical regions in insomnia patients compared to GS. Additionally, a 5-week CBTi program without medication modified the resting-state FC of these patients. These findings suggest the involvement of subcortical structures in the pathogenesis of insomnia disorder and provide insights into the neurobiological basis for the effectiveness of CBTi.
The following is the supplementary data related to this article.
Acknowledgments
Acknowledgements
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea, funded by the Ministry of Education (Study No. 2013R1A1A2062517, Dr. Yu Jin Lee) and the Brain Research Program through the National Research Foundation of Korea, funded by the Ministry of Science, ICT and Future Planning (Study No. NRF-2016M3C7A1904336, Dr. Seog Ju Kim).
Disclosure statement
Financial disclosure
None.
Non-financial disclosure
None.
References
- AASM . 2nd ed. American Academy of Sleep Medicine; Westchester, IL: 2005. International Classification of Sleep Disorders: Diagnostic and Coding Manual, 2nd Ed. [Google Scholar]
- AASM . 3rd ed. American Academy of Sleep Medicine; Darien, IL: 2014. The International Classification of Sleep Disorders. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander G.E., Crutcher M.D. Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci. 1990;13(7):266–271. doi: 10.1016/0166-2236(90)90107-l. [DOI] [PubMed] [Google Scholar]
- Altena E., Van Der Werf Y.D., Sanz-Arigita E.J. Prefrontal hypoactivation and recovery in insomnia. Sleep. 2008;31(9):1271–1276. [PMC free article] [PubMed] [Google Scholar]
- Altena E., Vrenken H., Van Der Werf Y.D., van den Heuvel O.A., Van Someren E.J. Reduced orbitofrontal and parietal gray matter in chronic insomnia: a voxel-based morphometric study. Biol. Psychiatry. 2010;67(2):182–185. doi: 10.1016/j.biopsych.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Arsalidou M., Duerden E.G., Taylor M.J. The centre of the brain: topographical model of motor, cognitive, affective, and somatosensory functions of the basal ganglia. Hum. Brain Mapp. 2013;34(11):3031–3054. doi: 10.1002/hbm.22124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Association AP . 5th edition. American Psychiatric Publishing; Arlington, VA: 2013. Diagnostic and Statistical Manual of Mental Disorders. [Google Scholar]
- Bastien C.H., Vallieres A., Morin C.M. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2(4):297–307. doi: 10.1016/s1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]
- Beck A.T., Steer R.A., Brown G.K. 2nd ed. Psychological Corp; San Antonio, Texas: 1996. Beck Depression Inventory, Manual. [Google Scholar]
- Behzadi Y., Restom K., Liau J., Liu T.T. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. NeuroImage. 2007;37(1):90–101. doi: 10.1016/j.neuroimage.2007.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner R.L., Andrews-Hanna J.R., Schacter D.L. The brain's default network: anatomy, function, and relevance to disease. Ann. N. Y. Acad. Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Buysse D.J., Reynolds C.F., 3rd, Monk T.H., Berman S.R., Kupfer D.J. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Chen M.C., Chang C., Glover G.H., Gotlib I.H. Increased insula coactivation with salience networks in insomnia. Biol. Psychol. 2014;97:1–8. doi: 10.1016/j.biopsycho.2013.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai X.J., Peng D.C., Gong H.H. Altered intrinsic regional brain spontaneous activity and subjective sleep quality in patients with chronic primary insomnia: a resting-state fMRI study. Neuropsychiatr. Dis. Treat. 2014;10:2163–2175. doi: 10.2147/NDT.S69681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond S.P., Walker M., Almklov E., Campos M., Anderson D.E., Straus L.D. Neural correlates of working memory performance in primary insomnia. Sleep. 2013;36(9):1307–1316. doi: 10.5665/sleep.2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edinger J.D., Carneyt C.E. Oxford University Press; NY: 2008. Overcoming Insomnia: A Cognitive-Behavioral Therapy Approach-Therapist Guide. [Google Scholar]
- Huang Z., Liang P., Jia X. Abnormal amygdala connectivity in patients with primary insomnia: evidence from resting state fMRI. Eur. J. Radiol. 2012;81(6):1288–1295. doi: 10.1016/j.ejrad.2011.03.029. [DOI] [PubMed] [Google Scholar]
- Joo E.Y., Noh H.J., Kim J.S. Brain gray matter deficits in patients with chronic primary insomnia. Sleep. 2013;36(7):999–1007. doi: 10.5665/sleep.2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo E.Y., Kim H., Suh S., Hong S.B. Hippocampal substructural vulnerability to sleep disturbance and cognitive impairment in patients with chronic primary insomnia: magnetic resonance imaging morphometry. Sleep. 2014;37(7):1189–1198. doi: 10.5665/sleep.3836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay D.B., Karim H.T., Soehner A.M. Sleep-wake differences in relative regional cerebral metabolic rate for glucose among patients with insomnia compared with good sleepers. Sleep. 2016;39(10):1779–1794. doi: 10.5665/sleep.6154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killgore W.D., Schwab Z.J., Kipman M., Deldonno S.R., Weber M. Insomnia-related complaints correlate with functional connectivity between sensory-motor regions. Neuroreport. 2013;24(5):233–240. doi: 10.1097/WNR.0b013e32835edbdd. [DOI] [PubMed] [Google Scholar]
- Li Y., Wang E., Zhang H. Functional connectivity changes between parietal and prefrontal cortices in primary insomnia patients: evidence from resting-state fMRI. Eur. J. Med. Res. 2014;19:32. doi: 10.1186/2047-783X-19-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Dong M., Yin Y., Hua K., Fu S., Jiang G. Abnormal whole-brain functional connectivity in patients with primary insomnia. Neuropsychiatr. Dis. Treat. 2017;13:427–435. doi: 10.2147/NDT.S128811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques D.R., Gomes A.A., Clemente V., Moutinho dos Santos J., Castelo-Branco M. Default-mode network activity and its role in comprehension and management of psychophysiological insomnia: a new perspective. New Ideas Psychol. 2015;36:30–37. [Google Scholar]
- Morin C.M., Colecchi C., Stone J., Sood R., Brink D. Behavioral and pharmacological therapies for late-life insomnia: a randomized controlled trial. JAMA. 1999;281(11):991–999. doi: 10.1001/jama.281.11.991. [DOI] [PubMed] [Google Scholar]
- Morin C.M., Vallieres A., Ivers H. Dysfunctional beliefs and attitudes about sleep (DBAS): validation of a brief version (DBAS-16) Sleep. 2007;30(11):1547–1554. doi: 10.1093/sleep/30.11.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin C.M., Belleville G., Belanger L., Ivers H. The insomnia severity index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011;34(5):601–608. doi: 10.1093/sleep/34.5.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie X., Shao Y., Liu S.Y. Functional connectivity of paired default mode network subregions in primary insomnia. Neuropsychiatr. Dis. Treat. 2015;11:3085–3093. doi: 10.2147/NDT.S95224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nissen C., Kloepfer C., Feige B. Sleep-related memory consolidation in primary insomnia. J. Sleep Res. 2011;20(1 Pt 2):129–136. doi: 10.1111/j.1365-2869.2010.00872.x. [DOI] [PubMed] [Google Scholar]
- Nofzinger E.A., Buysse D.J., Germain A., Price J.C., Miewald J.M., Kupfer D.J. Functional neuroimaging evidence for hyperarousal in insomnia. Am. J. Psychiatry. 2004;161(11):2126–2128. doi: 10.1176/appi.ajp.161.11.2126. [DOI] [PubMed] [Google Scholar]
- Pace-Schott E.F., Zimmerman J.P., Bottary R.M., Lee E.G., Milad M.R., Camprodon J.A. Resting state functional connectivity in primary insomnia, generalized anxiety disorder and controls. Psychiatry Res. 2017;265:26–34. doi: 10.1016/j.pscychresns.2017.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlis M.L., Giles D.E., Mendelson W.B., Bootzin R.R., Wyatt J.K. Psychophysiological insomnia: the behavioural model and a neurocognitive perspective. J. Sleep Res. 1997;6(3):179–188. doi: 10.1046/j.1365-2869.1997.00045.x. [DOI] [PubMed] [Google Scholar]
- Qaseem A., Kansagara D., Forciea M.A., Cooke M., Denberg T.D. Clinical guidelines committee of the American College of P. Management of Chronic Insomnia Disorder in adults: a clinical practice guideline from the American College of Physicians. Ann. Intern. Med. 2016;165(2):125–133. doi: 10.7326/M15-2175. [DOI] [PubMed] [Google Scholar]
- Regen W., Kyle S.D., Nissen C. Objective sleep disturbances are associated with greater waking resting-state connectivity between the retrosplenial cortex/hippocampus and various nodes of the default mode network. J. Psychiatry Neurosci. 2016;41(5):295–303. doi: 10.1503/jpn.140290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riemann D., Voderholzer U., Spiegelhalder K. Chronic insomnia and MRI-measured hippocampal volumes: a pilot study. Sleep. 2007;30(8):955–958. doi: 10.1093/sleep/30.8.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riemann D., Spiegelhalder K., Feige B. The hyperarousal model of insomnia: a review of the concept and its evidence. Sleep Med. Rev. 2010;14(1):19–31. doi: 10.1016/j.smrv.2009.04.002. [DOI] [PubMed] [Google Scholar]
- Roy A.K., Shehzad Z., Margulies D.S. Functional connectivity of the human amygdala using resting state fMRI. NeuroImage. 2009;45(2):614–626. doi: 10.1016/j.neuroimage.2008.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman S.M. Thalamus plays a central role in ongoing cortical functioning. Nat. Neurosci. 2016;19(4):533–541. doi: 10.1038/nn.4269. [DOI] [PubMed] [Google Scholar]
- Smith M.T., Perlis M.L., Chengazi V.U. Neuroimaging of NREM sleep in primary insomnia: a Tc-99-HMPAO single photon emission computed tomography study. Sleep. 2002;25(3):325–335. [PubMed] [Google Scholar]
- Smith S.M., Jenkinson M., Woolrich M.W. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 2004;23(Suppl. 1):S208–219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Spiegelhalder K., Baglioni C., Regen W. Brain reactivity and selective attention to sleep-related words in patients with chronic insomnia. Behav. Sleep Med. 2016:1–15. doi: 10.1080/15402002.2016.1253014. [DOI] [PubMed] [Google Scholar]
- Spielman A., Yang C.M., Glovinsky P.B. Principles and Practice of Sleep Medicine. 5th ed. WB Saunders; Philadelphia: 2011. Assessment techniques for insomnia. [Google Scholar]
- Stoffers D., Moens S., Benjamins J. Orbitofrontal gray matter relates to early morning awakening: a neural correlate of insomnia complaints? Front. Neurol. 2012;3:105. doi: 10.3389/fneur.2012.00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoffers D., Altena E., van der Werf Y.D. The caudate: a key node in the neuronal network imbalance of insomnia? Brain. 2014;137(Pt 2):610–620. doi: 10.1093/brain/awt329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S., Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012;2(3):125–141. doi: 10.1089/brain.2012.0073. [DOI] [PubMed] [Google Scholar]
- Winkelman J.W., Plante D.T., Schoerning L. Increased rostral anterior cingulate cortex volume in chronic primary insomnia. Sleep. 2013;36(7):991–998. doi: 10.5665/sleep.2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F., Huang S., Gao L., Zhuang Y., Ding S., Gong H. Temporal regularity of intrinsic cerebral activity in patients with chronic primary insomnia: a brain entropy study using resting-state fMRI. Brain Behav. 2016;6(10) doi: 10.1002/brb3.529. [DOI] [PMC free article] [PubMed] [Google Scholar]