Abstract
The function of the molecular chaperone Hsp90 depends on large conformational changes, the rearrangement of local motifs, and the binding and hydrolysis of ATP. The size and complexity of the Hsp90 system impedes the detailed investigation of their interplay using standard methods. To overcome this limitation, we developed a three-color single-molecule FRET assay to study the interaction of Hsp90 with a fluorescently labeled reporter nucleotide in detail. It allows us to directly observe the cooperativity between the two nucleotide binding pockets in the protein dimer. Furthermore, our approach disentangles the protein conformation and the nucleotide binding state of Hsp90 and extracts the kinetics of the state transitions. Thereby, we can identify the kinetic causes mediating the cooperativity. We find that the presence of the first nucleotide prolongs the binding of the second nucleotide to Hsp90. In addition, we observe changes in the kinetics for both the open and the closed conformation of Hsp90 in dependence on the number of occupied nucleotide binding sites. Our analysis also reveals how the co-chaperone Aha1, known to accelerate Hsp90’s ATPase activity, affects those transitions in a nucleotide-dependent and independent manner, thereby adding another layer of regulation to Hsp90.
Introduction
The molecular chaperone heat shock protein 90 (Hsp90) is one of the most abundant proteins found in cells, accounting for up to 2% of all cytosolic protein under physiological conditions (1). It is highly conserved and essential for viability in all eukaryotes (2). Hsp90 is a homodimer with each subunit consisting of three domains, the N-, the middle-, and the C-terminal domain (NTD, MD, and CTD). It can bind and hydrolyze ATP in its NTDs. Hydrolysis is slow in the class of Hsp90s, e.g., ∼1 ATP/min for the yeast homolog (3). While Hsp90 is stably dimerized at the CTD (4), the dimer exhibits transitions between globally closed and N-terminal open conformations (5, 6, 7). A set of more than 20 co-chaperones is known to affect Hsp90 by constituting a network of transiently interacting proteins (1). This Hsp90 machinery is essential for the stabilization or correct folding of many cellular proteins as well as the maturation of kinases and steroid hormone receptors, and thereby affects important cellular pathways. A prominent cochaperone is the Activator of the Hsp90 ATPase 1 (Aha1) that strongly stimulates the hydrolysis rate (8). The interaction of Aha1 with Hsp90 involves both domains of Aha1 and the NTD as well as the MD of the Hsp90 dimer (9). Aha1 causes conformational changes in local motifs of Hsp90 participating in nucleotide binding and hydrolysis (10). Therefore, Aha1 is an important coordinate in understanding Hsp90’s ATPase.
Despite the fact that Hsp90 has become a popular drug target (11), many mechanistic details of Hsp90’s function are unknown. In particular, the interplay between nucleotide binding, hydrolysis, and conformational changes of Hsp90 remains enigmatic (12), and several experiments have provided controversial results. On the one hand, ATP binding and hydrolysis weakly affect the conformational equilibrium between the globally open and closed conformations of Hsp90 (8, 13), and bulk experiments studying the nucleotide binding or hydrolysis could not reveal any cooperativity (14, 15). Both findings hint toward a weak intramolecular communication between the two subunits of the dimer, or even its absence. On the other hand, a stable closed structure of the otherwise predominantly open Hsp90 dimer is observed upon the incubation with the nonhydrolyzable ATP transition state analog AMP-PNP (6). Recent studies suggest a coordination of the kinetics of different local rearrangements linked to nucleotide binding within one subunit (16). Moreover, mutational studies have demonstrated that the hydrolysis activity is affected differently in dimers lacking the ability to either bind or hydrolyze ATP in one NTD (4, 17, 18). These results imply a communication within each Hsp90 subunit, as well as between the two nucleotide binding pockets in the dimer, despite the lack of direct evidence.
The standard procedure to assess the coordination between the two binding sites for a ligand (i.e., cooperativity) in a protein is the evaluation of Hill plots from bulk experiments, where the binding site occupation is measured as a function of ligand concentration. Cooperativity leads to a deviation from a slope of one in a log-log plot. However, this analysis is limited by the simplifications of the underlying models (19) and weak cooperativity is hardly detectable (Fig. S1). In the case of Hsp90, no strong cooperativity can be expected, because most interactions with clients, co-chaperones, and nucleotides do not exhibit strong effects either on the conformation or on the kinetics of the conformational changes. Possible cooperative effects are therefore unlikely to be detected by the application of standard approaches.
Studying the interaction of Hsp90 and a labeled reporter nucleotide directly and in real time by the application of three-color single-molecule Förster resonance energy transfer (smFRET) (13, 20, 21, 22) enables us to resolve the states of the interaction between Hsp90 and nucleotides. We track Hsp90’s global conformation by measuring FRET between two fluorophores attached to the opposing subunits in the Hsp90 dimer (5). At the same time, the presence of the reporter nucleotide in one of the two nucleotide binding pockets is probed by FRET (13). This experimental setup, together with the kinetic description derived from hidden Markov models (HMMs), enables us to study the effects from additional, unlabeled nucleotide and/or co-chaperones with high sensitivity. Those effects have previously been hidden by the averaging inherent to bulk experiments. The direct observation of the influence of the nucleotide state of one binding pocket on the other eliminates the need for an indirect deduction via titration experiments. Our data reveals cooperativity between the two nucleotide binding pockets in the Hsp90 dimer. We can quantify the effects of nucleotide on the state transitions of Hsp90. Thereby, we identify the kinetic causes of the cooperativity, which together with existing structural data allows us to suggest a mechanism for this cooperativity. Moreover, we find that the co-chaperone Aha1 modulates the interplay of the two nucleotide binding pockets by accelerating and inhibiting at least two transitions.
Materials and Methods
All chemicals were purchased from Sigma-Aldrich (St. Louis, MO), if not stated otherwise.
Sample preparation
Hsp90 from Saccharomyces cerevisiae (UniProt: P02829) is expressed in the form of two single cysteine point mutants at the positions D61 or Q385, with a cleavable N-terminal His-SUMO-tag and a C-terminal tag that prevents dimer dissociation at picomolar concentrations (a coiled-coil motif from the kinesin neck region of Drosophila melanogaster (DmKHC) (5)). In the case of the Q385C mutant, the construct contains an additional AviTag (Avidity, Aurora, CO) at the extreme C-terminus for in vivo biotinylation (23). Hsp90 expression and purification is detailed in the Supporting Material. Hsp90 D61C is labeled in 1× PBS pH 6.7 with Atto488-Maleimide, and Hsp90 Q385C with Atto550-Maleimide (AttoTec, Siegen, Germany), following a previously published protocol (13). In the case of Atto488, additional 0.5 mg/mL BSA is present.
Aha1 from S. cerevisiae (UniProt: Q12449) is expressed and purified as wild-type construct with a cleavable N-terminal His-SUMO tag from pET28a. Transformed Escherichia coli BL21Star (DE3) (Thermo Fisher Scientific, Waltham, MA) is grown at 37°C in TB medium with 50 μg/mL Kanamycin to an OD600 of 0.6 and induced with 1 mM IPTG for 4 h. Cells are harvested by centrifugation, washed with PBS, and lysed in a Cell Disruptor (Constant Systems, Low March, UK) at 1.6 kbar. Cell debris is pelleted by centrifugation at 25,000 × g at 4°C for 30 min (Avanti JXN-26; Beckman Coulter, Brea, CA). After filtration (Filtropur S 0.45; Sarstedt, Nümbrecht, Germany), the cell lysate is applied to a 5 mL HisTrap HP column (GE Healthcare, Chalfont St. Giles, UK) and eluted by a linear gradient from 0 to 500 mM imidazole in 100 mM sodium phosphate, 300 mM NaCl pH 8.0 at 8°C. Protein-containing fractions are pooled and dialyzed into the imidazole-free buffer overnight in the presence of 1:100 equivalent SENP protease to cleave the His-tag. The solution is again applied to the HisTrap column and the flow-through is collected and diluted 1:3 in 40 mM MES, 40 mM NaCl pH 6 and subsequently applied to a HiTrapSP 5 mL column (GE Healthcare). The protein is eluted with a linear gradient to 40 mM MES, 1 M NaCl pH 6. Protein is reduced with 1 mM DTT and concentrated. Finally, it is applied to a HiLoad 16/600 Superdex200 (GE Healthcare) and eluted with 40 mM HEPES, 200 mM KCl pH 7.5. Sample purity is checked by sodium dodecyl sulfate-polyacrylamide gel electrophoresis.
Three-color smFRET measurements
All experiments are conducted in measurement buffer (40 mM HEPES, 150 mM KCl, 10 mM MgCl2, pH 7.5 and 0.5 mg/mL BSA). Heterodimers of Hsp90 D61C-Atto488 and Hsp90Biotin Q385C-Atto550 are formed by the incubation of an overall 1 μM 5:1 mix for 20 min at 47°C. Aggregates are subsequently removed by extensive centrifugation (>30 min 17,000 × g, 4°C).
Measurements are performed in a custom-built flow chamber, which is a sandwich of a PEG/biotin-PEG passivated quartz slide, a thin film that is adhesive on both sides, and a coverslip (13). NeutrAvidin (Thermo Fisher Scientific) is incubated and washed out again before the heterodimers are flushed into the flow chamber at an appropriate concentration (∼10 pM) to reach sufficient surface density. Unbound protein is flushed out with measurement buffer. A quantity of 25 nM γ-[(6-Aminohexyl)-imido]-AMP-PNP-Atto647N (Jena Bioscience, Jena, Germany), here called AMP-PNP∗, and, in the respective experiments, 250 μM unlabeled nucleotide (ATP or AMP-PNP) and/or 10 μM co-chaperone Aha1 in measurement buffer, are flushed into the chamber. This step is repeated after 5 min of incubation.
smFRET traces are recorded with alternating laser excitation on a multicolor smFRET prism-type total internal reflection fluorescence setup (13). Atto488 and Atto550 are excited with a blue (50 mW Cobolt Blues; Cobolt, Solna, Sweden) and a green (75 mW Compass 215M; Coherent, Santa Clara, CA) laser at 473 and 532 nm, and fluorescence in the respective blue, green, and red (for Atto647N) emission channels is detected by two electron-multiplying charge-coupled device cameras (iXon Ultra 897; Andor Technology, Belfast, UK). Shutters, acousto-optic tunable filters, and cameras are synchronized by a digital I/O card (PCIe-6535; National Instruments, Austin, TX). The overall time resolution of our experiments is 200 ms per excitation cycle, with each excitation lasting 70 ms (+30 ms read-out time).
Extraction of time traces and relative state population
All data analysis is done with in-house-written scripts in the software Igor Pro (WaveMetrics, Tigard, OR). The code is available upon request.
Fluorescence intensity time traces for single molecules are extracted from the recorded movies as detailed in the Supporting Material and reported elsewhere (13). Initial criteria for the manual selection process are a single bleaching step in the channels for the dyes coupled to Hsp90 (Atto 488 and Atto 550) and at least one binding event of AMP-PNP∗. Plateaus of the fluorescence intensities are required to be roughly flat and the appropriate channel intensities have to show anticorrelated behavior.
We calculate the partial fluorescence from the five intensity traces analogous to the FRET efficiency of a two-color experiment (13). The intensities are corrected for instrumental and dye parameters as described in detail in the Supporting Material. E.g., the partial fluorescence of AMP-PNP∗ (identifier red) after excitation of Atto488 (blue) is given by
| (1) |
In a second round of selection, molecules with a poor trace quality (i.e., >10% of the time points show |PF – 0.5| > 1.5) are discarded.
The relative state populations are determined from 2D projections of the PF histogram on the and planes. Then populations are selected by drawing free-hand polygons that represent the location of each state (13). This procedure is repeated three times to minimize the effect of manual selection.
Ensemble HMM
The 3D histogram of the observed PFs reveals five different populations that are fitted by the sum of five 3D Gaussians with means and covariance matrix as fitting parameters. A global HMM is optimized for each data set with the emission probabilities for each state fixed to the 3D Gaussians determined by the fit. The resulting transition matrix is multiplied with the frame rate to yield the rates of the transitions. Rates for transitions from functionally equal states (the two O∗ states, Fig. 1 c) are summed for clarity. Dwell times of the AMP-PNP∗ bound fraction are extracted from the Viterbi path. Dwells without recorded start and/or end are also included.
Figure 1.
Data acquisition, data analysis, and state allocation. (a) Pictogram of the studied system consisting of an Hsp90 dimer with the labels Atto488 (blue) and Atto550 (green) attached and the reporter nucleotide AMP-PNP∗ in solution, labeled with Atto647N (red). The protein is immobilized by NeutrAvidin/biotin interaction on the top of the flow chamber and excited by an evanescent field using alternating laser excitation with a blue and a green laser in a prism-type total internal reflection fluorescence geometry. The fluorescence light is collected by an objective, separated by dichroic mirrors, and detected with electron-multiplying charge-coupled device cameras. (b) Fluorescence intensity traces of a single particle after the excitation of the blue (top) and the green (center) dye measured in the absence of additional unlabeled nucleotide or co-chaperone. The partial fluorescence traces (, with excitation of the ex dye and emission of the em dye) calculated from the intensity traces are shown below. (c) Pictograms of the distinguishable states and the respective identifiers used in this work. The first two populations represent the same functional state, namely open Hsp90 with nucleotide bound. (d) 3D representation of the Gaussians (isosurface at FWHM) fitted to the partial fluorescence data, which represent the five different populations. The same color code as in (c) is used. (e) The resulting state allocation for the fluorescence intensity traces is shown in (b).
Error estimates
Dwell time distributions follow exponential functions. The mean of random samples from a dwell time distribution follows approximately a normal distribution for a large number of samples (central limit theorem). The variance of a normal distributed quantity that has been sampled n times can be estimated by the jackknife-1 method, i.e., calculation from all possible combinations of n−1 dwells. It is then estimated by (24)
| (2) |
The error of the population size was estimated by the SD of 10 random subsets containing 75% of the frames in the full data set.
Confidence intervals
The 99% confidence interval (CI) for each transition is determined according to (25, 26) to get a measure for the uncertainty of the extracted transition probabilities. The likelihood profile of the parameter space around the maximum likelihood estimate is calculated for each transition probability. The test model λ′ is obtained by variation of the parameter of interest with all other parameters being fixed to the maximum likelihood estimate. A likelihood ratio test of the likelihood L(λ′|O) given the data set O is performed:
| (3) |
The confidence bound for the parameter is reached when LR exceeds 6.64 (the 99% quantile of a χ2-distribution with one degree of freedom).
Statistical tests
The dwell time distributions were posthoc tested pairwise for significant differences by a one-sided Wilcoxon-Mann-Whitney two-sample rank test as implemented in Igor Pro. Test results are given in Table S1.
The determined populations were tested for normal distribution by a Shapiro-Wilk test (as implemented in Igor Pro) on the subset distributions; results are given in Table S2. The populations from different subsets were tested for significant differences posthoc by unpaired pairwise t-tests on the C∗ population. Results are given in Table S3.
Results
The nucleotide binding pockets of Hsp90 act cooperatively
We study the effect of a nucleotide bound to one nucleotide binding pocket of the Hsp90 dimer on its other pocket using fluorescently labeled AMP-PNP as reporter nucleotide (subsequently denoted as AMP-PNP∗). To follow the nucleotide binding state of Hsp90 while tracking the conformation of Hsp90 at the same time, we perform three-color smFRET measurements on labeled Hsp90 (Fig. 1 a). The Hsp90 dimers are attached to the surface of a flow chamber and studied in the presence of 25 nM AMP-PNP∗ (13). We ensure that only one AMP-PNP∗ is bound at a time and that the second nucleotide binding site is accessible to the unlabeled nucleotide for all molecules subjected to further analysis in two ways. First, we apply AMP-PNP∗ at a concentration well below the KD (see Fig. S2), and second, we exclude molecules that may show binding of two AMP-PNP∗ in the trace selection process. The spatial interdye distance is approximated by the partial fluorescence (PF, the extension of the FRET efficiency for multicolor experiments; Fig. 1 b) calculated from the recorded fluorescence intensities. To obtain the full information necessary to resolve the three distances between the three dyes, alternating laser excitation (20) is used.
We are able to distinguish and allocate five different states to this system, as depicted in Fig. 1 c, of which four are functionally different. Namely, open nucleotide-free (O), open AMP-PNP∗ bound (O∗), closed nucleotide-free (C), and closed AMP-PNP∗ bound (C∗). The data spans a 3D space of the different PFs, which we subsequently use for state separation (Fig. 1 d). We collect traces from more than 400 single molecules that exhibited binding of a single AMP-PNP∗ under each experimental condition (see Fig. S3–S8). A 3D HMM is used to allocate states in the individual smFRET time traces and extract a kinetic model from the data (13).
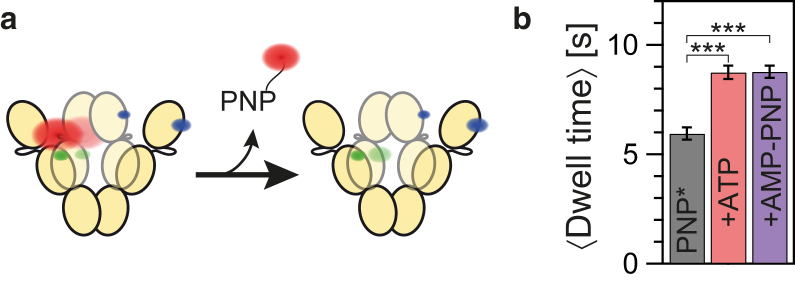
With our single-molecule approach we are able to measure the time for a single AMP-PNP∗ to stay bound to Hsp90 (Fig. 2 a). Analyzing more than 800 dissociation events, we find that the average dwell time of AMP-PNP∗ on Hsp90 is 5.9 ± 0.3 s (Fig. 2 b). If no cooperativity between the two binding pockets of Hsp90 existed, the addition of unlabeled nucleotide would not affect the average dwell time of the reporter already bound to Hsp90. Surprisingly, under conditions with additional, unlabeled ATP or AMP-PNP, the average dwell time is increased significantly by almost 50% to 8.8 ± 0.3 s. Thus, the binding of a second nucleotide to the Hsp90 dimer decreases the apparent overall dissociation probability of the reporter nucleotide AMP-PNP∗ (averaged over all conformations of Hsp90), i.e., the two nucleotide binding pockets are not independent of each other. In other words, there exists cooperativity between the two binding pockets of Hsp90. Please note that under the assumption of a concentration-independent mechanism for the cooperativity, which is also assumed in all bulk titration experiments, no titration is necessary in the single-molecule experiments to detect cooperativity. The identification of the state-specific effects of additional nucleotide allows us to further characterize the kinetic causes for our finding.
Figure 2.
The average dwell time of the reporter nucleotide AMP-PNP∗ bound to Hsp90 is prolonged by additional nucleotide. (a) Pictogram of the observed dissociation of labeled AMP-PNP∗ (PNP) from the Hsp90 dimer. (b) Average dwell time of AMP-PNP∗ bound to Hsp90 in the absence of additional nucleotide (PNP∗) and in the presence of 250 μM unlabeled ATP or unlabeled AMP-PNP. The underlying dwell time distributions are shown in Fig. S9. Error bars represent the SD estimated from jackknife resampling. Differences between the dwell time distributions are significant with ∗∗∗p < 0.001. To see this figure in color, go online.
Nucleotides have effects on multiple steps in the interaction between Hsp90 and nucleotide
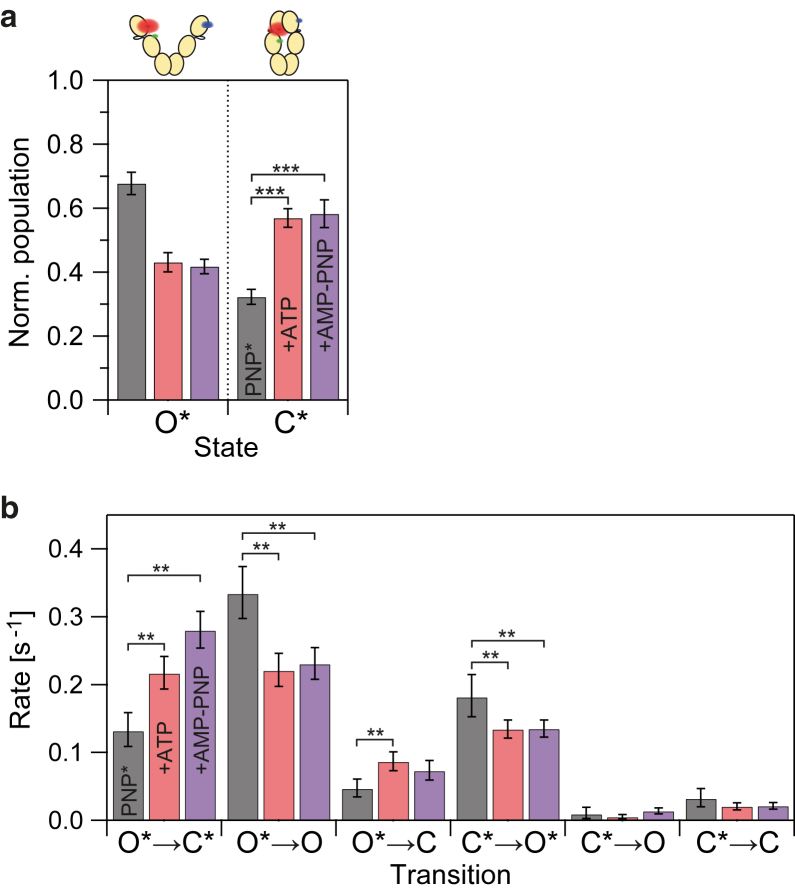
Fig. 3 a shows that the ratio of Hsp90 with AMP-PNP∗ bound in the open and the closed state (O∗/C∗) is shifted toward the closed state in the presence of additional nucleotide. Hence, ATP and AMP-PNP have a similar effect on both the mean dwell time of AMP-PNP∗ and the population shift between O∗ and C∗. However, the underlying cause of this effect remains unclear at this stage. The increase in the population of C∗ could be due to an increase in the rates leading to this state or due to a decrease in the rates depopulating this state. Accordingly, the increase in the average dwell time of bound AMP-PNP∗ may be caused by a population shift (from O∗ to C∗), because the closed state has a slower dissociation rate of the nucleotide, or by a decrease in the dissociation rate from the open state (O∗→O)—or by a combination of both. The actual mechanism mediating the cooperativity in Hsp90 requires a complete kinetic description. For such a multistate system, this is currently only possible by a single-molecule approach.
Figure 3.
The effects of nucleotide on Hsp90’s conformation and its state transitions. (a) Populations of open and closed conformation for Hsp90 bound to AMP-PNP∗ (normalized to unity) in the absence of additional nucleotide (PNP∗) and in the presence of 250 μM ATP or AMP-PNP. Error bars represent the SD within 10 subsets, each comprising 75% of the full dataset. The addition of nucleotide results in a significant population shift with ∗∗∗p < 0.001. (b) Transition rates for Hsp90 bound to labeled AMP-PNP∗ in dependence on additional nucleotide. Error bars represent the 99% CI; differences with ∗∗p < 0.01 (CIs do not overlap) are highlighted. To see this figure in color, go online.
Our three-color smFRET data enables us to simultaneously resolve the rate constants for conformational changes and AMP-PNP∗ unbinding for Hsp90. As shown in Fig. 3 b, additional nucleotide affects the nucleotide dissociation and the conformational transitions differently. On the one hand, additional nucleotide decreases the rates for AMP-PNP∗ dissociation from both conformational states of Hsp90 (O∗ → O and to a lesser extent, C∗ → C). On the other hand, the equilibrium between the labeled nucleotide-bound populations of Hsp90 is shifted by both increasing the rate for closing (O∗ → C∗) and decreasing the rate for opening (C∗ → O∗). Because the bleaching rates in all data sets are similar, this effect is not introduced into our data by different trace lengths (Fig. S10). Thus, cooperativity is not caused by the nucleotide affecting one single rate, but by the combined effects of the nucleotide on four rates (indicated by ∗∗ in Fig. 3 b) in the studied system.
Aha1 and nucleotide binding affect independent but interfering transitions
The ATPase activity of Hsp90 is known to be affected by regulatory co-chaperones. The strongest stimulating effect has been found for Aha1 so far, with a >10-fold acceleration of the ATPase activity (27). Because nucleotide binding is a prerequisite for hydrolysis, the question arises whether Aha1 directly interferes with nucleotide binding (i.e., by affecting its cooperativity) or by N-terminal closing, which is another prerequisite for hydrolysis.
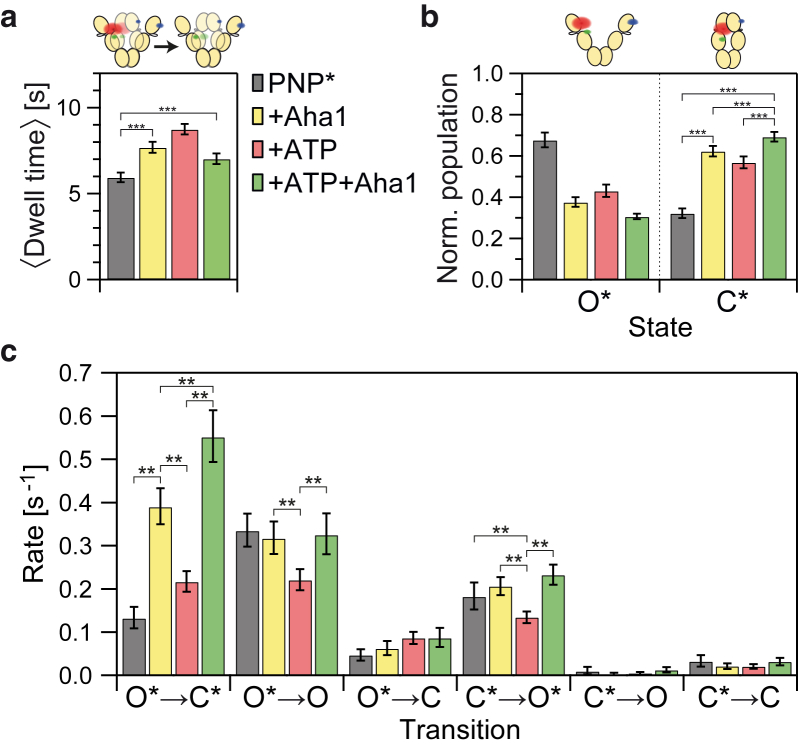
Therefore, we test the effect of Aha1 on the cooperativity in nucleotide binding by the addition of 10 μM of wild-type Aha1 to our assay. In absence of additional ATP, Aha1 alone already increases the mean dwell time of the reporter nucleotide bound to Hsp90 from 5.9 ± 0.3 to 7.7 ± 0.3 s (Fig. 4 a). Surprisingly, the combination of Aha1 and ATP increases the dwell time of AMP-PNP∗ (7.0 ± 0.3 s) to a lesser degree than either Aha1 or ATP. Thus, the effects of ATP and Aha1 are not additive. Fig. 4 b shows how the O∗/C∗ ratio of nucleotide-bound Hsp90 is shifted toward the C∗ state by the addition of Aha1. In contrast to our findings for the dwell times, the combination of ATP and Aha1 leads to a more pronounced effect on the O∗/C∗ ratio than either of them separately. Hence, Aha1 and ATP affect independent processes of Hsp90 and these processes interfere with each other.
Figure 4.
The effects of Aha1 (10 μM), ATP (250 μM), and Aha1 combined with ATP on Hsp90 and AMP-PNP∗. (a) The mean dwell time of AMP-PNP∗ on Hsp90 is significantly (∗∗∗p < 0.001) increased by Aha1. The effect observed for Aha1+ATP is smaller than for Aha1 or ATP alone. Error bars are calculated as in Fig. 2. (b) Effects on the normalized population of open and closed conformation for Hsp90 bound to AMP-PNP∗. Error bars are calculated as in Fig. 3a. (c) Transition rates and the effects of nucleotide, co-chaperone, and their combination. Errors bars represent the 99% CI; differences with ∗∗p < 0.01 are highlighted. To see this figure in color, go online.
The HMM analysis of the three-color smFRET data (Fig. 4 c) provides a more detailed understanding of the mechanisms causing our observations. Aha1 alone accelerates the transition from open, AMP-PNP∗-bound Hsp90 to the closed state (O∗→C∗), even more strongly than ATP. In combination with ATP, the effects of both on this transition actually add up. In contrast, the rates for the transitions from closed Hsp90 with AMP-PNP∗ bound to the open state as well as the dissociation of labeled nucleotide from open Hsp90 are increased to values similar to the ones observed without ATP and Aha1. Therefore, Aha1 attenuates the additional effects of ATP.
Discussion
Our assay is based on the observation of the binding of a labeled reporter nucleotide (AMP-PNP∗) to one subunit of Hsp90. It allows us to track the nucleotide binding state of Hsp90 while at the same time monitoring the large conformational changes of the dimer. Our results demonstrate that the nucleotide release from one subunit depends on the nucleotide state of the other. This cooperativity has been missed in Hill plots so far, because it is not very strong (Fig. S1), but it explains several previous findings—for example, the effects of point mutants that impair nucleotide binding (D79N) or hydrolysis (E33A) on the ATPase activity of the dimer (4, 8, 17, 18). On the one hand, an Hsp90 able to bind ATP in only one subunit exhibits a decreased apparent nucleotide affinity, because no cooperativity can occur here. On the other hand, an Hsp90 dimer able to bind two ATPs, but with the ability to hydrolyze only one, displays an increased apparent nucleotide affinity (17). The presence of a second binding site, but absence of the potential for hydrolysis in one subunit, mirrors our experiment with AMP-PMP∗ and ATP.
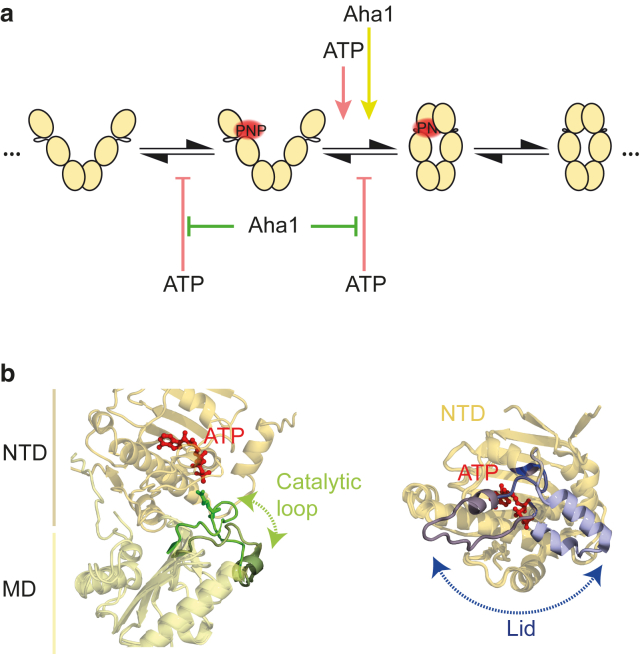
Our data analysis allows a deeper insight into the kinetic causes for the cooperativity (see Fig. 5 a). The addition of ATP accelerates the closing of AMP-PNP∗-bound Hsp90 (O∗→C∗), and it decelerates the opening of AMP-PNP∗-bound Hsp90 (C∗→O∗) and the dissociation of AMP-PNP∗ from open Hsp90 (O∗→O). The structural origins of these effects could either be a communication between the two nucleotide binding sites through the whole dimer (18, 28) or a direct interaction between the NTDs. Direct steric contacts of these two domains in the open state are possible, because the open conformation of Hsp90 represents a flexible and highly dynamic ensemble (6). Additionally, the presence of ATP in one binding pocket and AMP-PNP∗ in the other results in a larger population of the closed state, an effect that could be observed previously only in a large excess of AMP-PNP (5, 6, 23). Thus, the binding of one AMP-PNP results in a conformational change that is the prerequisite for a more stable N-terminal dimerization. We speculate that this might be mediated through the nucleotide-dependent lid dynamics of Hsp90 (29), which can then facilitate the NTD/MD arrangement necessary for the transition into the closed Hsp90 conformation.
Figure 5.
The effects of ATP and Aha1 on the state transitions of Hsp90 and their possible structural origins. (a) A minimal model for the state transitions of Hsp90 in the presence of AMP-PNP∗ and the effects of ATP and Aha1 on these transitions. Only the most frequent transitions are shown. ATP increases the closing rate of Hsp90 with AMP-PNP∗ bound, and decelerates the reverse reaction, as well as the dissociation of AMP-PNP∗. Aha1 also accelerates the closing of AMP-PNP∗-bound Hsp90. In a combination of Aha1 and ATP, their effects on the closing of AMP-PNP∗-bound Hsp90 add up, whereas Aha1 prevents the decelerating effects of ATP. (b) Our observations could be caused by the depicted local rearrangements, which have been proposed to be affected by Aha1 and nucleotide binding. (Left) Reported rearrangement of the catalytic loop upon binding of Aha1 (green and dark green, superposition of the crystal structures PDB: 2CG9 (35) and 1USV (10)). (Right) Reported conformation of the nucleotide lid in the AMP-PNP or the ADP-bound crystal structures (dark and light blue, superposition of the crystal structures PDB: 2CG9 and 2WEP (36)).
Furthermore, our data reflect the role of Aha1 as an accelerator of Hsp90’s conformational rearrangements and ATPase activity (27, 30). We find that Aha1 accelerates the transition from the open nucleotide bound to the closed nucleotide-bound state (O∗→C∗) in the absence of additional nucleotide. Aha1 has been reported to modulate the catalytic loop of Hsp90 to facilitate the conformational transition from open to closed Hsp90 (Fig. 5 b) (10). We speculate that this is the structural cause for the kinetic effect we observe.
The addition of both ATP and Aha1 to our assay allows further insights into the distinct effects of nucleotide and co-chaperone on Hsp90’s conformational dynamics. Their common and strongest effects, accelerating the closing of open AMP-PNP∗-bound Hsp90 (O∗→C∗), add up. Because the concentration of Aha1 in our experiment is close to saturation (9, 27, 31), we assume that the co-chaperone is already exerting its full activation potential. Nevertheless, the transition can be further accelerated by the addition of ATP. Therefore, we can infer that ATP and Aha1 affect different structural rearrangements independently, both resulting in an accelerated closing of Hsp90. The decelerating effect of ATP on the opening of AMP-PNP∗-bound Hsp90 and dissociation of AMP-PNP∗ from open Hsp90 is abolished by Aha1. Thus, a structural arrangement leading to a tighter binding of nucleotide is impeded by the co-chaperone when both binding pockets are occupied. This means that, on the one hand, ATP and Aha1 work to the same aim (namely the closure of the dimer). On the other hand, they act antagonistic on two different state transitions (i.e., the opening of dimer and the dissociation of nucleotide). These effects are summarized in Fig. 5 a.
Conclusion
Switching from two-color to three-color smFRET experiments adds another dimension to the analysis of biomolecular machines. Now the correlation between molecular interactions and conformational changes can be directly accessed on the single-molecule level and in real time. This is essential to fully understand biological multistate and multicomponent systems.
Here we uncover the previously overlooked cooperativity between the two nucleotide binding pockets of the Hsp90 dimer and identify the kinetic causes of this cooperativity. The direct observation of the influence of one subunit of Hsp90 on the other abolishes the need for indirect conclusions from titration experiments to detect cooperativity and allows us to quantify the effects of the co-chaperone Aha1.
Our results demonstrate that cross effects of different interaction partners (in this case, nucleotide binding and Aha1) have to be taken into account to understand the Hsp90 chaperone machinery to its full extent and that co-chaperones most likely affect more than one single step in the functional cycle. Both ideas might also apply to many other catalytic systems with more than one interaction partner.
Author Contributions
T.H., P.W., and M.G. designed research. P.W. and M.G. performed experiments and analyzed the data. P.W., M.G., and T.H. interpreted the data and wrote the manuscript.
Acknowledgments
We thank Dieter Hauschke and Jens Timmer for advice on data statistics. We thank Bizan Balzer, Björn Hellenkamp, Markus Jahn, Sonja Schmid, Katarzyna Tych, and Matthias Rief for helpful discussions.
This work is funded by the European Research Council (ERC) through ERC grant agreement No. 681891.
Editor: Gijs Wuite.
Footnotes
Philipp Wortmann and Markus Götz contributed equally to this work.
Supporting Materials and Methods, Supporting Results, fourteen figures, and four tables are available at http://www.biophysj.org/biophysj/supplemental/S0006-3495(17)30926-8.
Supporting Citations
References (32, 33, 34) appear in the Supporting Material.
Supporting Material
References
- 1.Taipale M., Jarosz D.F., Lindquist S. HSP90 at the hub of protein homeostasis: emerging mechanistic insights. Nat. Rev. Mol. Cell Biol. 2010;11:515–528. doi: 10.1038/nrm2918. [DOI] [PubMed] [Google Scholar]
- 2.Eckl J.M., Richter K. Functions of the Hsp90 chaperone system: lifting client proteins to new heights. Int. J. Biochem. Mol. Biol. 2013;4:157–165. [PMC free article] [PubMed] [Google Scholar]
- 3.Panaretou B., Prodromou C., Pearl L.H. ATP binding and hydrolysis are essential to the function of the Hsp90 molecular chaperone in vivo. EMBO J. 1998;17:4829–4836. doi: 10.1093/emboj/17.16.4829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Richter K., Muschler P., Buchner J. Coordinated ATP hydrolysis by the Hsp90 dimer. J. Biol. Chem. 2001;276:33689–33696. doi: 10.1074/jbc.M103832200. [DOI] [PubMed] [Google Scholar]
- 5.Mickler M., Hessling M., Hugel T. The large conformational changes of Hsp90 are only weakly coupled to ATP hydrolysis. Nat. Struct. Mol. Biol. 2009;16:281–286. doi: 10.1038/nsmb.1557. [DOI] [PubMed] [Google Scholar]
- 6.Hellenkamp B., Wortmann P., Hugel T. Multidomain structure and correlated dynamics determined by self-consistent FRET networks. Nat. Methods. 2017;14:174–180. doi: 10.1038/nmeth.4081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krukenberg K.A., Förster F., Agard D.A. Multiple conformations of E. coli Hsp90 in solution: insights into the conformational dynamics of Hsp90. Structure. 2008;16:755–765. doi: 10.1016/j.str.2008.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zierer B.K., Rübbelke M., Buchner J. Importance of cycle timing for the function of the molecular chaperone Hsp90. Nat. Struct. Mol. Biol. 2016;23:1020–1028. doi: 10.1038/nsmb.3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Retzlaff M., Hagn F., Buchner J. Asymmetric activation of the Hsp90 dimer by its co-chaperone Aha1. Mol. Cell. 2010;37:344–354. doi: 10.1016/j.molcel.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 10.Meyer P., Prodromou C., Pearl L.H. Structural basis for recruitment of the ATPase activator Aha1 to the Hsp90 chaperone machinery. EMBO J. 2004;23:511–519. doi: 10.1038/sj.emboj.7600060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jhaveri K., Ochiana S.O., Chiosis G. Heat shock protein 90 inhibitors in the treatment of cancer: current status and future directions. Expert Opin. Investig. Drugs. 2014;23:611–628. doi: 10.1517/13543784.2014.902442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pearl L.H. Review: the Hsp90 molecular chaperone-an enigmatic ATPase. Biopolymers. 2016;105:594–607. doi: 10.1002/bip.22835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Götz M., Wortmann P., Hugel T. A multicolor single-molecule FRET approach to study protein dynamics and interactions simultaneously. Methods Enzymol. 2016;581:487–516. doi: 10.1016/bs.mie.2016.08.024. [DOI] [PubMed] [Google Scholar]
- 14.McLaughlin S.H., Ventouras L.-A., Jackson S.E. Independent ATPase activity of Hsp90 subunits creates a flexible assembly platform. J. Mol. Biol. 2004;344:813–826. doi: 10.1016/j.jmb.2004.09.055. [DOI] [PubMed] [Google Scholar]
- 15.Cunningham C.N., Krukenberg K.A., Agard D.A. Intra- and intermonomer interactions are required to synergistically facilitate ATP hydrolysis in Hsp90. J. Biol. Chem. 2008;283:21170–21178. doi: 10.1074/jbc.M800046200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schulze A., Beliu G., Neuweiler H. Cooperation of local motions in the Hsp90 molecular chaperone ATPase mechanism. Nat. Chem. Biol. 2016;12:628–635. doi: 10.1038/nchembio.2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mishra P., Bolon D.N.A. Designed Hsp90 heterodimers reveal an asymmetric ATPase-driven mechanism in vivo. Mol. Cell. 2014;53:344–350. doi: 10.1016/j.molcel.2013.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Obermann W.M.J., Sondermann H., Hartl F.U. In vivo function of Hsp90 is dependent on ATP binding and ATP hydrolysis. J. Cell Biol. 1998;143:901–910. doi: 10.1083/jcb.143.4.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stefan M.I., Le Novère N. Cooperative binding. PLOS Comput. Biol. 2013;9:e1003106. doi: 10.1371/journal.pcbi.1003106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee N.K., Kapanidis A.N., Weiss S. Three-color alternating-laser excitation of single molecules: monitoring multiple interactions and distances. Biophys. J. 2007;92:303–312. doi: 10.1529/biophysj.106.093211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Person B., Stein I.H., Tinnefeld P. Correlated movement and bending of nucleic acid structures visualized by multicolor single-molecule spectroscopy. ChemPhysChem. 2009;10:1455–1460. doi: 10.1002/cphc.200900109. [DOI] [PubMed] [Google Scholar]
- 22.Hohng S., Joo C., Ha T. Single-molecule three-color FRET. Biophys. J. 2004;87:1328–1337. doi: 10.1529/biophysj.104.043935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schmid S., Götz M., Hugel T. Single-molecule analysis beyond dwell times: demonstration and assessment in and out of equilibrium. Biophys. J. 2016;111:1375–1384. doi: 10.1016/j.bpj.2016.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shao J., Wu C.F.J. A general theory for jackknife variance estimation. Ann. Stat. 1989;17:1176–1197. [Google Scholar]
- 25.Greenfeld M., Pavlichin D.S., Herschlag D. Single molecule analysis research tool (SMART): an integrated approach for analyzing single molecule data. PLoS One. 2012;7:e30024. doi: 10.1371/journal.pone.0030024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Giudici P., Rydén T., Vandekerkhove P. Likelihood-ratio tests for hidden Markov models. Biometrics. 2000;56:742–747. doi: 10.1111/j.0006-341x.2000.00742.x. [DOI] [PubMed] [Google Scholar]
- 27.Panaretou B., Siligardi G., Prodromou C. Activation of the ATPase activity of Hsp90 by the stress-regulated co-chaperone Aha1. Mol. Cell. 2002;10:1307–1318. doi: 10.1016/s1097-2765(02)00785-2. [DOI] [PubMed] [Google Scholar]
- 28.Morra G., Verkhivker G., Colombo G. Modeling signal propagation mechanisms and ligand-based conformational dynamics of the Hsp90 molecular chaperone full-length dimer. PLOS Comput. Biol. 2009;5:e1000323. doi: 10.1371/journal.pcbi.1000323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prodromou C., Panaretou B., Pearl L.H. The ATPase cycle of Hsp90 drives a molecular ‘clamp’ via transient dimerization of the N-terminal domains. EMBO J. 2000;19:4383–4392. doi: 10.1093/emboj/19.16.4383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prodromou C. Mechanisms of Hsp90 regulation. Biochem. J. 2016;473:2439–2452. doi: 10.1042/BCJ20160005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Siligardi G., Hu B., Prodromou C. Co-chaperone regulation of conformational switching in the Hsp90 ATPase cycle. J. Biol. Chem. 2004;279:51989–51998. doi: 10.1074/jbc.M410562200. [DOI] [PubMed] [Google Scholar]
- 32.Panavas T., Sanders C., Butt T.R. SUMO fusion technology for enhanced protein production in prokaryotic and eukaryotic expression systems. Methods Mol. Biol. 2009;497:303–317. doi: 10.1007/978-1-59745-566-4_20. [DOI] [PubMed] [Google Scholar]
- 33.Hessling M., Richter K., Buchner J. Dissection of the ATP-induced conformational cycle of the molecular chaperone Hsp90. Nat. Struct. Mol. Biol. 2009;16:287–293. doi: 10.1038/nsmb.1565. [DOI] [PubMed] [Google Scholar]
- 34.Monod J., Wyman J., Changeux J.P. On the nature of allosteric transitions: a plausible model. J. Mol. Biol. 1965;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- 35.Ali M.M.U., Roe S.M., Pearl L.H. Crystal structure of an Hsp90-nucleotide-p23/Sba1 closed chaperone complex. Nature. 2006;440:1013–1017. doi: 10.1038/nature04716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prodromou C., Nuttall J.M., Piper P.W. Structural basis of the radicicol resistance displayed by a fungal Hsp90. ACS Chem. Biol. 2009;4:289–297. doi: 10.1021/cb9000316. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.