Abstract
Tocopherols, the major forms of vitamin E, are a family of fat-soluble compounds that exist in alpha (α-T), beta (β-T), gamma (γ-T) and delta (δ-T) variants. A cancer preventive effect of vitamin E is suggested by epidemiological studies. However, past animal studies and human intervention trials with α-T, the most active vitamin E form, have yielded disappointing results. A possible explanation is that the cancer preventive activity of α-T is weak compared to other tocopherol forms. In the present study, we investigated the effects of δ-T, γ-T and α-T (0.2% in diet) in a novel colon cancer model induced by the meat-derived dietary carcinogen, 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) and promoted by dextran sodium sulfate (DSS)-induced colitis in CYP1A-humanized (hCYP1A) mice. PhIP/DSS treatments induced multiple polypoid tumors, mainly tubular adenocarcinomas, in the middle to distal colon of the hCYP1A mice after 10 weeks. Dietary supplementation with δ-T and γ-T significantly reduced colon tumor formation and suppressed markers of oxidative and nitrosative stress (i.e., 8-oxo-dG and nitrotyrosine) as well as pro-inflammatory mediators (i.e., NF-κB p65 and p-STAT3) in tumors and adjacent tissues. By administering δ-T at different time periods, we obtained results suggesting that the inhibitory effect of δ-T against colon carcinogenesis is mainly due to protection against early cellular and DNA damages caused by PhIP. α-T was found to be ineffective in inhibiting colon tumors and less effective in attenuating the molecular changes. Altogether, we demonstrated strong cancer preventive effects of δ-T and γ-T in a physiologically relevant model of human colon cancer.
Keywords: Tocopherols, colon carcinogenesis, chemoprevention, PhIP, DSS
INTRODUCTION
Colorectal cancer (CRC) is one of the most common cancer in both men and women in the United States, and its incidence is rapidly increasing in developing countries, where populations are adopting Western-style diets [1,2]. While significant progress has been made in elucidating the genetic events underlining CRC, the etiology of the disease is still not well understood. In addition to age and family history, risk factors for sporadic CRC include chronic inflammatory bowel diseases (i.e., ulcerative colitis and Crohn disease), obesity, sedentary lifestyle, alcohol consumption, long-term smoking, and a diet high in red or processed meat [3]. Although the survival rate has improved substantially in the past decades, CRC remains a major public health priority, highlighting the need for improved preventive and therapeutic interventions against the disease.
In studying CRC development and prevention, a model relevant to the human disease is important. Currently, the most commonly used models for CRC studies are the genetic ApcMin mutants and the azoxymethane (AOM) ± dextran sodium sulfate (DSS) chemical-induced colon carcinogenesis [4]. While these models have proven to be valuable, the utilization of genetic manipulation or synthetic chemicals is nonetheless imperfect in mimicking actual human CRC initiation and development. Recently, a novel colon carcinogenesis model was developed in our laboratory using the meat-derived dietary carcinogen, 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) in combination with DSS in cytochrome P450 1A-humanized (hCYP1A) mice [5]. PhIP is the most abundant heterocyclic amine generated from high-temperature cooking of meat, and its consumption has been associated with elevated risk of CRC in epidemiologic studies [6,7]. Because PhIP is preferentially activated by human CYP1A2 but detoxified by the murine Cyp1a2, the hCYP1A transgenic mice, which replaced murine Cyp1a2 with the human CYP1A2 gene, was employed to mimic human metabolism of PhIP [8]. With DSS, which is used extensively to produce ulcerative colitis, PhIP/DSS-induced colon carcinogenesis in hCYP1A mice is a physiologically relevant model for studying CRC and cancer prevention.
Tocopherols, the major forms of vitamin E, are a family of fat-soluble phenolic compounds widely found in vegetable oils and nuts [9]. A cancer preventive activity of vitamin E is suggested by several epidemiological studies, and tocopherols have been extensively studied due of their antioxidant and anti-inflammatory effects [10–13]. These lipophilic compounds, which are comprised of a chromanol ring system and a 16-carbon phytyl tail, exist in alpha (α-T), beta (β-T), gamma (γ-T) and delta (δ-T) variants, depending on the number and position of the methyl group on the chromanol ring. Because of the higher levels in human tissues and superior activity in a classic fertility restoration assay, α-T is recommended as vitamin E [14]. Consequently, past animal studies and human intervention trials on vitamin E have focused on α-T, but the results have been inconsistent and disappointing, particularly in relation to CRC [10]. Of the most recent epidemiologic studies, only 1 of 3 case-control studies and 2 of 7 cohort studies demonstrated an inverse association between supplementary α-T vitamin E intake and CRC risk [15-20]. In addition, a mere 1 of 12 studies in animal models showed a protective effect of α-T on colon tumorigenesis and aberrant crypt foci formation [12]. A possible explanation is that the cancer preventive activity of α-T is weak compared to other tocopherol forms, such as δ-T and γ-T. Dietary supplementation with δ-T and γ-T, but not α-T, have been shown to inhibit the growth of mammary tumors induced by N-methyl-N-nitrosourea (NMU) and human lung cancer cells transplanted in nude mice [21,22]. Our previous study in an AOM-induced colon carcinogenesis model in rats showed that δ-T was most effective in inhibiting the formation of aberrant crypt foci, whereas γ-T and γ-T-rich mixture of tocopherols (γ-TmT) were less effective and α-T was ineffective [23]. The study was the first clear demonstration of the higher cancer preventive activity of δ-T and the ineffectiveness of α-T in animal models of colon cancer. However, cancer was not the endpoint of the study.
In the present study, we investigated the cancer preventive effects of δ-T, γ-T and α-T (0.2% in diet) in the PhIP-induced and colitis-promoted colon carcinogenesis in hCYP1A mice. The dosage of tocopherols used was based on the optimal doses from previous studies [21–24] and corresponded to the intake of approximately 1 gram of tocopherol for a person consuming 500g of diet per day. Colon tumor multiplicity and volume were evaluated in mice at 10 weeks after the start of PhIP/DSS treatments to assess the effects of different tocopherols. Markers of oxidative and nitrosative stress as well as pro-inflammatory mediators were analyzed in the colon tumors and their adjacent tissues. Oxidative and nitrosative stress, DNA damage, and apoptosis markers were also analyzed in the early stage of colon carcinogenesis.
MATERIALS AND METHODS
Chemicals and Diets
PhIP was obtained from Wako Pure Chemicals (Osaka, Japan) and dissolved in deionized water to 20 mg/ml concentration. DSS (molecular weight 35,000–44,000) was purchased from MP Biomedicals (Solon, OH) and dissolved in deionized water to 1.5% (wt/vol) before given to mice. AIN-93M diet (used as control) and tocopherol-enriched diet were prepared by Research Diets (New Brunswick, NJ). The diets were prepared by adding 0.2% δ-T, γ-T or α-T to the AIN-93M diet using highly purified d-α-forms of δ-T, γ-T and α-T. δ-T and α-T were purified to ≥97% pure from the commercial grade δ-tocopherol and α-tocopherol, respectively, from Sigma-Aldrich (St. Louis, MO). γ-T was purified similarly from γ-T-rich mixture of tocopherols (Covi-ox T-90 containing 56.1% γ-T, 22.3% δ-T, 11.5% α-T, and 1.2% β-T) obtained from BASF Corporation (Florham Park, NJ) to ≥97% pure with no detectable α-T and δ-T impurities. A CombiFlash Companion XL automated flash chromatographic system (Teledyne ISCO, Lincoln, NE) with a RediSep Rf Gold high performance flash silica gel column (20–40 μm in particle size) was used for the purifications. All diets were stored in sealed plastic bags in boxes at around 4°C and warmed to room temperature before use.
Animal studies and evaluations
All animal experiments were conducted in accordance to the protocol (#02-027) approved by the Institutional Animal Care and Use Committee of Rutgers, the State University of New Jersey. The hCYP1A mice [Cyp1a2/Cyp1a1tm2Dwn Tg (CYP1A1, CYP1A2)1Dwn/DwnJ and C57BL/6J] were purchased from Jackson Laboratories (Bar Harbor, ME) and used as founders to establish homozygous breeding colonies in-house. All mice were maintained at room temperature and on an alternating 12 h light/dark cycle with water and diet provided ad libitum. For the experiments, mice (~5 weeks old) were given the control diet or diet supplemented with 0.2% δ-T, γ-T, or α-T starting 1 week before PhIP administration and continuing until sacrifice or otherwise specified (Supplementary Figure S1A). An optimized treatment regimen from a previous study [5] was used to induce colon carcinogenesis. Two doses of PhIP (100 mg/kg b.w.) were administered intragastrically to mice at 3 days apart. At 1 week after the initial PhIP administration, the mice were given DSS treatment (1.5% in drinking water) for 4 days (Figure S1A). The general health and body weight of mice were monitored daily during PhIP and DSS treatments, and then on a weekly basis until sacrifice at 10 weeks after the first PhIP administration. To investigate the effects of tocopherols in early stages of carcinogenesis, male hCYP1A mice on the control or tocopherol-supplemented diets were administered a single dose of PhIP (100mg/kg b.w.) and sacrificed at 1, 3 and 7 days after dosing. Mice were euthanized by CO2 asphyxiation. Blood was taken by cardiac puncture for serum extraction. Entire mouse colon was excised and cleansed with phosphate-buffered saline before thorough evaluation for tumors. Visible tumors were carefully measured under a dissecting microscope with a digital caliper, and tumor volumes (mm3) were calculated using the formula V = 4/3πr3, where r is average radius of tumor. After evaluation, the colons were frozen or fixed in 10% buffered formalin before processed and embedded in paraffin.
Immunohistochemistry
Immunohistochemical analysis was carried out using 4 μm paraffin-embedded sections of mouse colon. A standard avidin–biotin peroxidase complex method was employed. Briefly, deparaffinized sections on glass slides were heated in a microwave oven for antigen retrieval, endogenous peroxidase was quenched using 3% H2O2, and tissue sections were incubated at 4°C overnight with primary antibodies. Biotinylated secondary antibody and Vectastain Elite ABC reagent (Vector Laboratories, Burlingame, CA) were applied with 3, 3′-diaminobenzidine. Sections were then counterstained with hematoxylin. Primary antibodies used in the study included: anti-8-oxo-deoxyguanosine (8-oxo-dG) (mouse monoclonal; JalCA), anti-nitrotyrosine (rabbit polyclonal; Millipore), anti-Nuclear Factor Kappa B p65 subunit (NF-κB p65) (rabbit polyclonal, Abcam), anti-phospho-STAT3 (p-STAT3) (rabbit monoclonal, Cell Signaling Technology), anti-phospho-Histone H2AX (γH2AX) (rabbit monoclonal, Cell Signaling Technology), and anti-cleaved caspase-3 (rabbit polyclonal, Cell Signaling Technology). The number of positively stained cells and total number of cells were quantified using the Aperio ScanScope GL system (Vista, CA). About 10,000–30,000 cells from colon tumors or adjacent tissues were analyzed per mouse.
Tocopherol analysis
For the analysis of tocopherol levels in serum and colon tissues, a modification of our previous method was used [22]. Briefly, each sample (20 μL) was mixed with 130 μL of 0.1% ascorbic acid and 150 μL of 100% ethanol. The mixture was extracted twice with 1 mL of hexane and the supernatant dried in a Savant Speedvac SC110 centrifugal vacuum concentrator (Thermo Scientific, Waltham, MA). The residue was reconstituted in 100 μL methanol, injected onto high-performance liquid chromatography equipped with a Supelcosil C18 reversed-phase column, and eluted with a mobile phase consisting of Solvent A (acetonitrile/methanol/water, 280/40/680, v/v/v, containing 30 mmol/L of lithium acetate and adjusted to pH 4.0 with trifluroacetic acid) and Solvent B (acetonitrile/methanol/water, 840/130/30, v/v/v, containing 15 mmol/L of lithium acetate and adjusted to pH 4.0 with trifluroacetic acid). The eluent was monitored with an ESA 5600A Coulochem electrode array system (Bedford, MA). The concentration of the analyte in each of the elution peak was determined by comparing the peak height with that of the standard sample.
Statistical analysis
Results were analyzed using the GraphPad Prism (GraphPad, CA) software. Two-tailed Student’s t-test was used to determine the difference between two groups in the tocopherol concentrations, tumor multiplicity, and immunostaining in the PhIP/DSS-treated hCYP1A mice. One-way ANOVA followed by Dunnett’s post hoc test was used to compare the differences in tumor multiplicity and immunostainings of tumors and adjacent tissues between the controls and tocopherol-supplemented groups. One-way ANOVA followed by Tukey’s post hoc test was used to compare the differences in immunostainings between the controls and tocopherol-supplemented groups in the early time points. Differences were considered statistically significant when P < 0.05.
RESULTS
Effects of PhIP/DSS treatments and different tocopherols on the health of hCYP1A mice
Male hCYP1A mice on the control or tocopherol-supplemented diets appeared healthy with normal body weight gain. PhIP administrations and DSS treatment caused an overall ~10% decrease in mouse body weight (Supplementary Figure S1B). Compared to the control diet group, lesser reductions in body weight were observed in the tocopherol-supplemented groups. Treatment with only PhIP or DSS resulted in ~5% or ~10% body weight reduction, respectively (data not shown). No death occurred as a result of PhIP and/or DSS toxicity, contrary to previous studies in which PhIP/DSS treatments resulted in death of a small percentage of mice [5]. Rectal bleeding was observed at 3 or 4 days of DSS treatment, but ceased a few days after DSS treatment was stopped. The body weight in all groups recovered after DSS cessation and assumed normal weight gain until sacrifice (Supplementary Figure S1B). The body weight trend is consistent with our previous study and other carcinogenesis models with PhIP and DSS [5,25,26].
In PhIP/DSS-treated hCYP1A mice, dietary tocopherol supplementations resulted in significant changes in their levels in the serum and colon tissues (Table 1). Supplementation with δ-T increased δ-T concentrations by 2.5-fold (P = 0.009) in the serum and 14.0-fold (P = 0.001) in the colon tissues. γ-T supplementation increased γ-T concentration by 2.7-fold (P = 0.003) in the serum and 5.8-fold (P < 0.001) in the colon tissues, whereas α-T supplementation increased α-T concentration by 3.9-fold (P < 0.001) in the serum and 3.4-fold (P < 0.001) in the colon tissues. A significant difference in γ-T concentrations were observed between the two control diet groups. However, the exact reason for the difference is unclear. δ-T (and γ-T) is known to be extensively metabolized by side-chain degradation [10]. The serum and colon tissue levels of the two major metabolites, carboxyethyl hydroxychroman (CEHC) and carboxymethylbutyl hydroxychroman (CMBHC), were measured in the set of experiments with δ-T. In δ-T-supplemented mice, serum concentrations of δ-CEHC (1.41 μmol/L) and δ-CMBHC (3.28 μmol/L) were 2 and 4.7 times that of the δ-T concentration (0.70 μmol/L), respectively. In the colon, much higher levels of δ-CEHC (26.81 μmol/kg) and δ-CMBHC (49.02 μmol/kg) were detected, which were 5.5 and 9.4 times that of the δ-T level (5.03 μmol/kg), respectively. The high levels of δ-T and its metabolites may be important for the colon cancer preventive activity.
Table 1.
Tocopherols and metabolites levels in serum and colon tissues of male hCYP1A mice.
| Diet Group | α-T | γ-T | δ-T | α-CEHC | γ-CEHC | δ-CEHC | α-CMBHC | γ-CMBHC | δ-CMBHC |
|---|---|---|---|---|---|---|---|---|---|
| Serum Concentrations (μmol/L)† | |||||||||
| Control | 9.40±2.36 | 0.17±0.09 | 0.28±0.14 | 0.24±0.03 | 0.05±0.00 | 0.04±0.00 | 0.97±0.20 | 0.06±0.00 | 0.06±0.00 |
| 0.2% δ-T | 8.80±5.32 | 0.15±0.02 | 0.70±0.02a | 0.07±0.00b | 0.05±0.00 | 1.41±0.87a | 1.21±0.85 | 0.06±0.00 | 3.28±2.60a |
| Control | 8.93±1.95 | 1.05±0.18 | 0.22±0.06 | – | – | – | – | – | – |
| 0.2% γ-T | 8.37±0.92 | 2.83±0.66a | 0.10±0.03b | – | – | – | – | – | – |
| 0.2% α-T | 34.93±6.24a | 0.64±0.29 | 0.16±0.04 | – | – | – | – | – | – |
| Colon Tissues Concentrations (μmol/kg)† | |||||||||
| Control | 16.12±3.04 | 0.24±0.04 | 0.36±0.08 | 0.10±0.06 | 0.59±0.50 | 1.24±0.33 | 0.80±0.20 | 1.03±1.00 | 0.29±0.26 |
| 0.2% δ-T | 18.85±2.87 | 0.26±0.03 | 5.03±1.32a | 0.84±0.12a | 0.62±0.14 | 26.8±6.3a | 0.80±0.24 | 1.31±0.50 | 49.0±20.6a |
| Control | 22.51±2.55 | 0.54±0.10 | 0.36±0.03 | – | – | – | – | – | – |
| 0.2% γ-T | 21.09±1.82 | 8.90±1.48a | 0.20±0.06b | – | – | – | – | – | – |
| 0.2% α-T | 76.86±8.45a | 1.05±0.44 | 0.40±0.06 | – | – | – | – | – | – |
Value expressed as mean ± SD (n=4–5).
Significantly increased from the control diet group (two-tailed Student’s t-test, p<0.05).
Significantly decreased from the control diet group (two-tailed Student’s t-test, p<0.05).
, not determined; CEHC, carboxyethyl hydroxychroman; CMBHC, carboxymethylbutyl hydroxychroman.
Dietary tocopherols inhibit PhIP/DSS-induced colon tumorigenesis in male hCYP1A mice
PhIP/DSS treatments induced multiple polypoid tumors, mainly tubular adenocarcinomas, in the middle to distal colon of the hCYP1A mice at 10 weeks after administration of PhIP (Supplementary Figure S1C & S1D). To study the effects of different tocopherols on PhIP/DSS-induced colon carcinogenesis, male hCYP1A mice were given the control diet or 0.2% tocopherol-supplemented diet at one week before PhIP administration, and the development of colon tumors was evaluated at 10 weeks after PhIP administration (Supplementary Figure S1A, Experiment #1). Due to limited numbers of hCYP1A mice from our breeding colony, δ-T diet was examined first and then γ-T and α-T were studied. Significant reduction in colon tumor multiplicity was found in the δ-T and γ-T diet groups, but not in the α-T diet group (Figure 1A). The δ-T-supplemented diet reduced colon tumor numbers by 64% (P < 0.001). Supplementation with γ-T produced a moderate 45% reduction (P < 0.040), whereas α-T produced a 21% reduction that was not statistically significant. No statistical difference in tumor volume was observed in all tocopherols-supplemented groups in comparison with their respective control (Figure 1B).
Figure 1. Inhibitory effect of different tocopherols on PhIP/DSS-induced colon tumorigenesis in male hCYP1A mice.
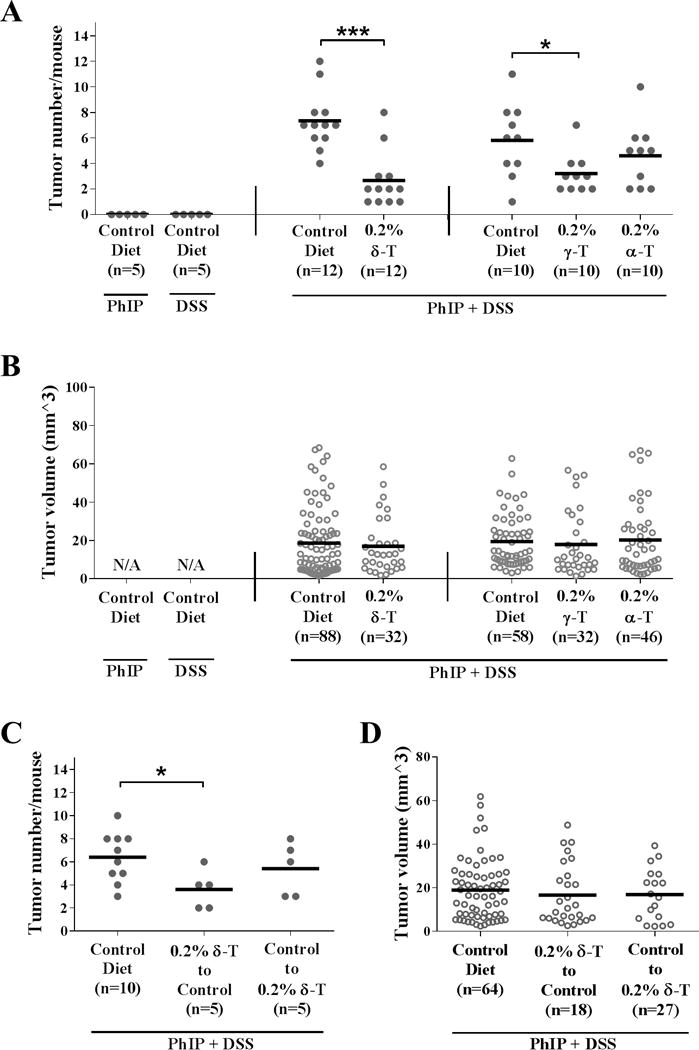
A, dietary supplementation with δ-T and γ-T significantly reduced tumor multiplicity in male mice (Means ± SD for groups from left to right: 0, 0, 7.3±2.3, 2.7±2.2, 5.8±2.9, 3.2±1.6, and 4.6±2.5, respectively). B, dietary tocopherol supplementation did not alter tumor volumes (Means ± SD for groups from left to right: N/A, N/A, 18.7±17.6, 17.0±14.7, 19.5±13.7, 17.9±16.6, and 20.2±18.4, respectively). C, δ-T supplementation before (and not after) PhIP/DSS treatment significantly reduced tumor multiplicity in male mice (Means ± SD for groups from left to right: 6.4±2.2, 3.6 ±1.7, and 5.4±2.3, respectively). D, δ-T supplementation before or after PhIP/DSS treatment did not alter tumor volumes (Means ± SD for groups from left to right: 18.9±14.0, 16.8±12.0, and 16.7±13.9, respectively). Statistical analysis was done using two-tailed Student’s t-test or ANOVA-Dunnett (***P<0.001, **P<0.01, *P<0.05).
To further explore the cancer preventive effect of δ-T in PhIP/DSS-induced colon carcinogenesis, male hCYP1A mice were divided into 3 groups and were given: Group 1, the control diet only; Group 2, the 0.2% δ-T-supplemented diet at first before switching to the control diets immediately after finishing the DSS treatment; and Group 3, the control diet at first before switching to the 0.2% δ-T-supplemented diet immediately after finishing the DSS treatment (Supplementary Figure S1A, Experiment #2). At 10 weeks after PhIP administration, the mice in Group 2 demonstrated a modest 44% (P < 0.048) reduction in tumor multiplicity, compared to mice in Group 1 (Figure 1C). A lesser reduction of 22% was observed in mice in Group 3. No statistical difference in tumor volume was found (Figure 1D). These results demonstrate the effectiveness of δ-T and γ-T and suggest that tocopherol supplementation is more effective when given before and during PhIP/DSS treatments.
Dietary tocopherols suppress oxidative and nitrosative stress and pro-inflammatory markers in colon tumors and adjacent tissues
Oxidative stress and inflammation are widely known to be key contributing factors to colon carcinogenesis [27–30]. Here, we assessed markers of oxidative and nitrosative stress and inflammation by measuring levels of 8-oxo-dG and nitrotyrosine as well as the pro-inflammatory mediators, NF-κB p65 and p-STAT3, in the colon tumors and adjacent tissues.
Both 8-oxo-dG and nitrotyrosine were found to be highly elevated in the PhIP/DSS-induced colon tumors and adjacent tissues, and dietary tocopherols supplementation with significantly suppressed the levels of 8-oxo-dG and nitrotyrosine (Figure 2A & C, also Supplementary Figure S2). Supplementation with δ-T, γ-T and α-T attenuated the 8-oxo-dG staining by a respective 49% (P < 0.001), 35% (P < 0.001) and 25% (P = 0.003) in the tumors and 50% (P < 0.001), 42% (P < 0.002) and 28% (P = 0.003) in the adjacent tissues (Figure 2B). Similarly, δ-T, γ-T and α-T supplementation attenuated the nitrotyrosine staining by a respective 47% (P < 0.001), 41% (P < 0.001), and 30% (P = 0.005) in the tumors and 50% (P < 0.001), 45% (P < 0.001) and 31% (P = 0.005) in the adjacent tissues (Figure 2D). The results indicate that dietary δ-T and γ-T were more effective than α-T in inhibiting oxidative and nitrosative stress in the colon.
Figure 2. Dietary tocopherols suppress oxidative and nitrosative stress and pro-inflammatory mediators in the PhIP/DSS-induced colon tumors and adjacent tissues.
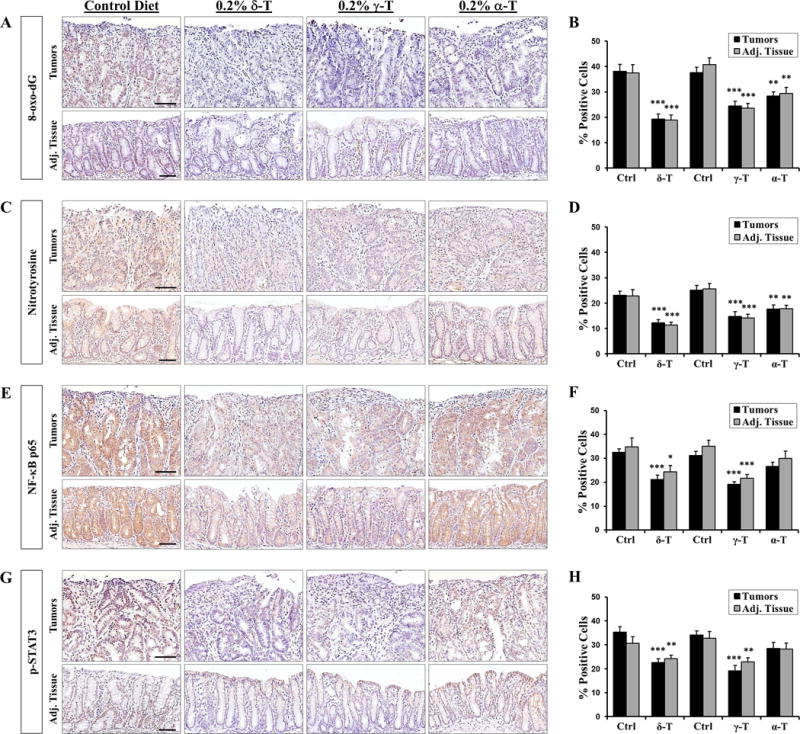
A & C, representative micrographs showing that δ-T and γ-T supplementations were more effective than α-T in attenuating the levels of 8-oxo-dG and nitrotyrosine, respectively, in the colon tumors and adjacent tissues. B & D, quantitative analysis of 8-oxo-dG and nitrotyrosine immunostaining, respectively, in the colon tumors and adjacent tissues. E & G, representative micrographs showing that δ-T and γ-T supplementation, but not α-T, significantly attenuated the levels of NF-κB and p-STAT3, respectively, in the colon tumors and adjacent tissues. F & H, quantitative analysis of NF-κB and p-STAT3 immunostaining, respectively, in the colon tumors and adjacent tissues. Scale bar represents 50μm. Data presented as mean ± SD (n=5). Statistical analysis was done using two-tailed Student’s t-test or ANOVA-Dunnett (***P<0.001, **P<0.01, *P<0.05).
NF-κB p65 and p-STAT3 were also found to be highly elevated in colon tumors and tissues of mice, and dietary supplementation with δ-T and γ-T, but not α-T, significantly suppressed the elevated levels of both NF-κB p65 and p-STAT3 (Figure 2E & G, also Supplementary Figure S2). δ-T and γ-T supplementation attenuated of levels of NF-κB p65 by a respective 35% (P < 0.001) and 39% (P < 0.001) in the tumors and 30% (P = 0.034) and 38% (P < 0.001) in the adjacent tissues (Figure 2F & H). Similarly, δ-T and γ-T supplementation attenuated of levels of p-STAT3 by a respective 36% (P < 0.001) and 44% (P < 0.001) in the tumors and 21% (P = 0.010) and 30% (P = 0.003) in the adjacent tissues (Figure 2F & H). In contrast, α-T attenuated levels of NF-κB p65 and p-STAT3 levels by a mere 14–16% in the tumors and adjacent tissues and was not statistically significant. These results indicate that δ-T and γ-T, but not α-T, are effective in suppressing inflammatory markers in the colon.
Dietary δ-T and γ-T protect against cellular and DNA damages, and enhance apoptosis in early stage of colon carcinogenesis
The results of the above experiments suggest that the effects of tocopherols in the early stages of PhIP/DSS carcinogenesis may be vital to their cancer preventive effects. To investigate possible protective role of tocopherols in the initiation stage of colon carcinogenesis, control or tocopherol-supplemented mice were administered with a single dose of PhIP and sacrificed at 1, 3 and 7 days after PhIP administration. In addition to 8-oxo-dG and nitrotyrosine, levels of γH2AX and cleaved caspase-3 were measured in the colon tissues.
Compared to vehicle-treated mice, highly elevated levels of 8-oxo-dG and nitrotyrosine stainings were found in the colon mucosa of PhIP-treated mice on the control diet, and dietary supplementation with δ-T and γ-T, but not α-T, significantly lowered the levels of 8-oxo-dG and nitrotyrosine (Figure 3A & B). δ-T supplementation decreased 8-oxo-dG staining by 35% (P < 0.001) at day 1, 53% (P = 0.002) at day 3, and 51% (P = 0.003) at day 7, while γ-T supplementation decreased 8-oxo-dG staining by 32% (P = 0.001) at day 1, 47% (P = 0.005) at day 3, and 49% (P = 0.004) at day 7 (Figure 3C). Likewise, δ-T supplementation decreased nitrotyrosine staining by 42% (P = 0.002) at day 1, 45% (P = 0.004) at day 3, and 41% at day 7, while γ-T supplementation decreased nitrotyrosine staining by 47% (P < 0.001) at day 1, 51% (P = 0.001) at day 3, and 47% at day 7 (Figure 3D). α-T supplementation, on the other hand, decreased both 8-oxo-dG and nitrotyrosine staining by 21% or less and was not statistically significant. In comparison to α-T, δ-T was significantly different in reducing 8-oxo-dG staining (P = 0.043) and γ-T was significantly different in reducing nitrotyrosine staining (P = 0.035) on day 3.
Figure 3. Dietary δ-T and γ-T reduce 8-oxo-dG and nitrotyrosine in colon mucosa of PhIP-treated mice at the early time points.
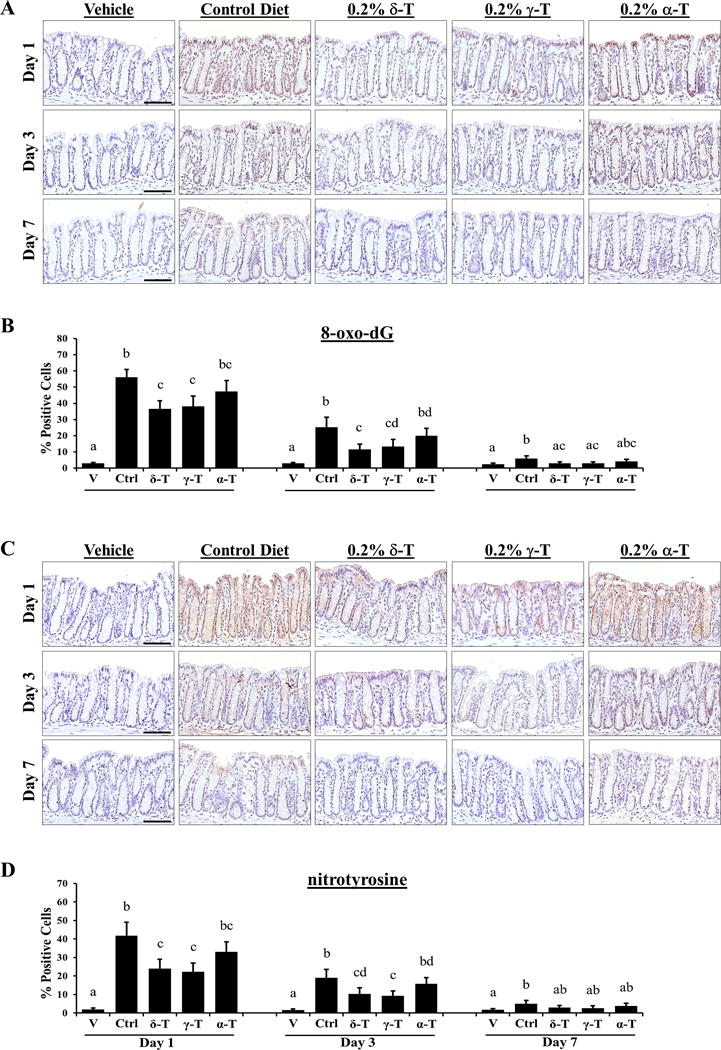
A and C, representative micrographs of 8-oxo-dG and nitrotyrosine immunostaining, respectively, showing weak nuclear-positive staining in vehicle-treated mice on control diet, strong staining in PhIP-treated mice on control diet, and lowered levels of staining by δ-T or γ-T in PhIP-treated mice at 1, 3 and 7 days after PhIP administration. B and D, quantitative analysis of the 8-oxo-dG and nitrotyrosine immunostaining, respectively, of vehicle-treated mice and PhIP-treated mice on control (Ctrl), δ-T-, γ-T- and α-T-supplemented diets. Scale bar represents 50μm. Data presented as mean ± SD (n=4–5). Statistical analysis was done using ANOVA-Tukey’s test.
γH2AX, a sensitive marker of DNA damage and subsequent repair, was elevated in the colon mucosa of PhIP-treated mice with strong nuclear-positive staining in the luminal and basal epithelia of colonic crypts (Figure 4A). Dietary supplementation with δ-T and γ-T, but not α-T, significantly lowered the level of γH2AX. δ-T supplementation decreased γH2AX staining by 25% at day 1, 53% (P < 0.001) at day 3, and 60% (P < 0.001) at day 7, while γ-T supplementation decreased staining by 26% at day 1, 46% (P < 0.001) at day 3, and 51% (P = 0.001) at day 7 (Figure 4B). In contrast, α-T supplementation decreased γH2AX staining by less than 19% in the early time points and was not statistically significant. Compare to α-T, δ-T was significantly different in reducing γH2AX staining on day 3 (P = 0.036) and 7 (P = 0.008).
Figure 4. Dietary δ-T and γ-T reduce γH2AX and enhance cleaved caspase-3 in colon mucosa of PhIP-treated mice at the early time points.
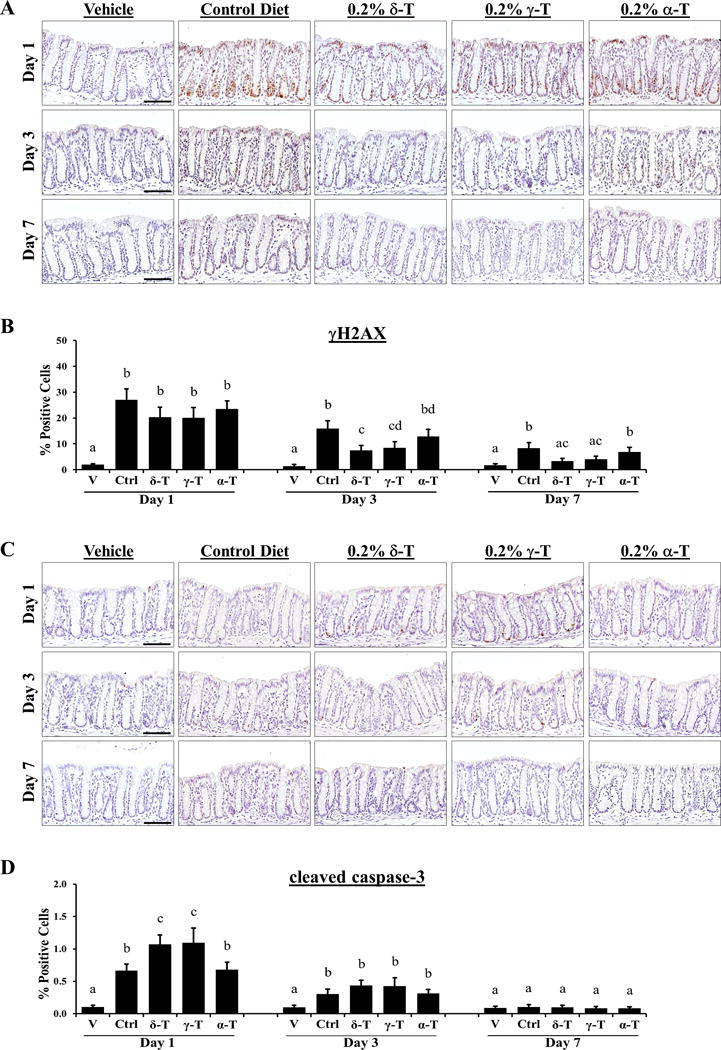
A, representative micrographs of γH2AX immunostaining showing weak nuclear-positive staining in vehicle-treated mice on control diet, strong staining in PhIP-treated mice on control diet, and lowered levels of staining in PhIP-treated mice on δ-T or γ-T diet at 1, 3 and 7 days after PhIP administration. B, quantitative analysis of γH2AX immunostaining of vehicle-treated mice (V) and PhIP-treated mice on control (Ctrl), δ-T-, γ-T- and α-T-supplemented diets. C, representative micrographs of cleaved caspase-3 immunostaining showing negligible staining in vehicle-treated mice on control diet, mild staining in PhIP-treated mice on control diet, and increased levels of staining in PhIP-treated mice on 0.2% δ-T or γ-T-supplemented diet at day 1 PhIP administration. D, quantitative analysis of cleaved caspase-3 immunostaining, respectively, of vehicle-treated mice (V) and PhIP-treated mice on control (Ctrl), δ-T-, γ-T- and α-T-supplemented diets. Scale bar represents 50μm. Data presented as mean ± SD (n=4–5). Statistical analysis was done using ANOVA-Tukey’s test.
Cleaved caspase-3, a marker of apoptosis, was detected in the basal epithelial cells of colonic crypts of PhIP-treated mice at day 1 and day 3 (Figure 4C). Dietary supplementation with δ-T and γ-T increased the level of cleaved caspase-3 significantly by a respective 61% (P = 0.005) and 65% (P = 0.008) at day 1 and marginally by a respective 43% and 40% at day 3, before diminishing to baseline levels at day 7 (Figure 4D). α-T supplementation failed to change the cleaved caspase-3 levels in the early time points. Compare to α-T, δ-T and γ-T was significantly different in increasing cleaved caspase-3 on day 1 (P = 0.010 and P = 0.006, respectively). Together, these results suggest that δ-T and γ-T inhibit PhIP/DSS-induced colon carcinogenesis involves protection against early PhIP-induced cellular and DNA damages on the colon mucosa.
DISCUSSION
In studying cancer prevention, a relevant model to the human disease is important. In the present study, we evaluated the effects of different tocopherols using a novel colon carcinogenesis model induced by a meat-derived carcinogen and promoted by colitis, both of which are well-recognized risk factors for human CRC. The PhIP/DSS colon carcinogenesis model in hCYP1A mice provides a valuable experimental system for studying CRC and evaluating possible cancer preventive agents. The current model is further optimized for colon tumorigenesis from the previous publication [5]. Instead of one high dose (200mg/kg) of PhIP and 7 days of DSS treatments, hCYP1A mice in this study were administered two lower doses (100mg/kg) of PhIP at 3 days apart and treated with DSS for four days. These treatment changes not only improved the health of the mice considerably, but also increased colon tumor incidence to 100% (from previous ~86%) and multiplicity to ~6 tumors per mouse (from previous ~4 tumors). The increased tumor incidence in mice may be attributed to longer exposure to the PhIP carcinogen, which leads to greater likelihood of cells acquiring key mutations that drive the PhIP/DSS colon carcinogenesis [31].
In our study, dietary supplementation with δ-T and γ-T, but not α-T, significantly inhibited colon tumor multiplicity in the male hCYP1A mice. To our knowledge, this is the first demonstration of the strong inhibitory effects of δ-T and γ-T in an in vivo mouse model of colon cancer. The potency of δ-T and γ-T is consistent with previous studies using different tocopherols in AOM-induced colon carcinogenesis in male F344 rats, NMU-induced mammary carcinogenesis in female Sprague-Dawley rats, and lung cancer H1299 cell xenograft model in nude mice [21–23]. Our study also revealed that δ-T works effectively when given to mice before and during PhIP/DSS treatments, but not after. This finding suggests that tocopherol supplementation inhibits early stages of colon carcinogenesis, which has not been explored in previous studies with tocopherols.
In addition to males, we also evaluated the effects of different tocopherols in the female hCYP1A mice and found no statistically significant difference between the control and tocopherol-supplemented groups (Supplementary Figure S3). The PhIP/DSS-treated female mice also exhibited slightly fewer colon tumors (~4 tumors per mouse) than the male mice (~6.5 tumor per mouse) on the control diet. While the null effect of tocopherols in female mice is unclear, studies have shown that females produced less colon tumors than males in the ApcPirc/+ mutant rats and chemical-induced mouse models, suggesting the disparity may be the result of indirect tumor-promoting effects of testosterone [32–34]. Estrogen has also been purported to exert a protective role in CRC based on several lines of experimental, epidemiologic and clinical evidences [35,36]. In humans, an overall 30–40% lower CRC incidence rate in women have been attributed to gender-related differences in exposure to hormones and risk factors [37,38]. To explore the dissimilar effect between the sexes, further studies are warranted.
The antioxidant and anti-inflammatory activities of tocopherols have been the most studied mechanisms of their cancer preventive effect [10,11]. In the PhIP/DSS-induced colon tumors and adjacent tissues, dietary δ-T and γ-T supplementations attenuated the levels of 8-oxo-dG and nitrotyrosine as well as NF-κB p65 and p-STAT3, while α-T was less or not effective. These results are consistent with the previous findings that δ-T and γ-T significantly suppressed oxidative and nitrosative stress in lung, mammary and colon cancer models and inflammation in a colon cancer model [21–23]. As effective antioxidants, tocopherol inhibition on 8-oxo-dG and nitrotyrosine are primarily due to their ability to quench reactive oxygen and nitrogen species (RONS) generated from PhIP/DSS treatments. These activities likely also contribute to their anti-inflammatory activities as RONS are pro-inflammatory [39]. However, the exact mechanisms of NF-κB p65 and p-STAT3 inhibition by tocopherols remain to be elucidated. Nevertheless, this is the first demonstration of inhibitory activities of tocopherols on NF-κB p65 and p-STAT3 pro-inflammatory mediators in an animal model of colon cancer.
To elucidate the mechanism of inhibition by tocopherols on PhIP/DSS-induced colon carcinogenesis, we investigated the effects of tocopherols on early molecular changes caused by the PhIP administration. Our study showed that dietary δ-T and γ-T, but not α-T, significantly reduced levels of 8-oxo-dG, nitrotyrosine and γH2AX, and enhanced the pro-apoptotic cleaved caspase-3 during the early time points after PhIP administration. These results correspond to the extent of inhibition by the different tocopherols on PhIP/DSS-induced colon tumorigenesis. Apparently, large amounts of RONS were produced after PhIP treatment. The protective effects of δ-T and γ-T were manifested in the reductions of 8-oxo-dG, nitrotyrosine and γH2AX. These inhibitory activities at the early stages of carcinogenesis could significantly decrease colon tumor formation observed at the later stage. The ineffectiveness of α-T could be due to the presence of 5-methyl group on the chromanol ring, which is less active in quenching RONS than δ-T and γ-T with their 5-position unmethylated. In addition to the action of δ-T and γ-T, their side-chain degradation metabolites, CEHC and CMBHC, which retain the intact chromanol ring structure and can get into the cytosol and nucleus, could also play a role in quenching RONS in the colon. Indeed, high levels of δ-T and even higher levels of its metabolites were found in colon tissues. Additionally, other molecular mechanisms, such as inhibition of Akt cell survival pathway and activation of PPAR-γ, Nrf2 or apoptotic pathways, could also contribute to the inhibition of tumorigenesis at the tumor promotion stage [21,40].
In summary, we demonstrated that δ-T and γ-T supplementation at 0.2% in diet significantly inhibited PhIP-induced and colitis-promoted colon carcinogenesis in a relevant model of human colon cancer. Dietary δ-T and γ-T significantly reduced colon tumor formation and suppressed oxidative and nitrosative stress as well as pro-inflammatory mediators in tumors and adjacent tissues. The inhibitory effect of δ-T and γ-T against colon carcinogenesis appears to be mainly due to the protection against early cellular and DNA damages caused by PhIP at the initiation stage of carcinogenesis. γ-T is the most abundant vitamin E form in our diet, mostly from vegetable oils and nuts. δ-T is also abundant in soybean and other oils. The strong cancer preventive activities of δ-T and γ-T suggest that these compounds have a potential of daily use for the prevention of CRC, and this possibly remains to be further studied.
Supplementary Material
Acknowledgments
The authors thank the personnel of Laboratory Animal Service in the Laboratory for Cancer Research for excellent animal care.
Funding
This work was supported by the US NIH grants (RO1 CA133021 & RO1 AT007036) and the John L. Colaizzi Chair endowment as well as shared facilities funded by NCI Cancer Center support grant (P30 CA72720) and NIEHS Center grant (P30 ES005022). Jayson X. Chen was supported by NIEHS training grant (T32 ES007148) and NIH-NCI fellowship grant (F31 CA168333).
Abbreviations
- α-T
alpha-tocopherol
- β-T
beta-tocopherol
- γ -T
gamma-tocopherol
- δ-T
delta-tocopherol
- PhIP
2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine
- DSS
dextran sodium sulfate
- hCYP1A
cytochrome P450 1A-humanized
- CRC
colorectal cancer
- AOM
azoxymethane
- γ-TmT
a γ-T rich mixture of tocopherols
- NMU
N-methyl-N-nitrosourea
- 8-oxo-dG
8-oxo-deoxyguanosine
- NF-κB p65
Nuclear Factor Kappa B p65 subunit
- p-STAT3
phospho-STAT3
- γH2AX
phospho-Histone H2AX
- CEHC
carboxyethyl hydroxychroman
- CMBHC
carboxymethylbutyl hydroxychroman
- RONS
reactive oxygen and nitrogen species
Footnotes
Conflicts of Interest:
No potential conflicts of interest were disclosed.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Center MM, Jemal A, Smith RA, Ward E. Worldwide variations in colorectal cancer. CA Cancer J Clin. 2009;59(6):366–378. doi: 10.3322/caac.20038. [DOI] [PubMed] [Google Scholar]
- 3.Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin. 2014;64(2):104–117. doi: 10.3322/caac.21220. [DOI] [PubMed] [Google Scholar]
- 4.Rosenberg DW, Giardina C, Tanaka T. Mouse models for the study of colon carcinogenesis. Carcinogenesis. 2009;30(2):183–196. doi: 10.1093/carcin/bgn267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheung C, Loy S, Li GX, Liu AB, Yang CS. Rapid induction of colon carcinogenesis in CYP1A-humanized mice by 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine and dextran sodium sulfate. Carcinogenesis. 2011;32(2):233–239. doi: 10.1093/carcin/bgq235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferrucci LM, Sinha R, Huang WY, et al. Meat consumption and the risk of incident distal colon and rectal adenoma. Br J Cancer. 2012;106(3):608–616. doi: 10.1038/bjc.2011.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miller PE, Lazarus P, Lesko SM, et al. Meat-related compounds and colorectal cancer risk by anatomical subsite. Nutr Cancer. 2013;65(2):202–226. doi: 10.1080/01635581.2013.756534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheung C, Ma X, Krausz KW, et al. Differential metabolism of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) in mice humanized for CYP1A1 and CYP1A2. Chem Res Toxicol. 2005;18(9):1471–1478. doi: 10.1021/tx050136g. [DOI] [PubMed] [Google Scholar]
- 9.Eitenmiller RR, Lee J, Vitamin E. food chemistry, composition, and analysis. CRC Press; 2005. [Google Scholar]
- 10.Ju J, Picinich SC, Yang Z, et al. Cancer-preventive activities of tocopherols and tocotrienols. Carcinogenesis. 2010;31(4):533–542. doi: 10.1093/carcin/bgp205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiang Q. Natural forms of vitamin E: metabolism, antioxidant, and anti-inflammatory activities and their role in disease prevention and therapy. Free Radic Biol Med. 2014;72:76–90. doi: 10.1016/j.freeradbiomed.2014.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang CS, Suh N. Cancer prevention by different forms of tocopherols. Top Curr Chem. 2013;329:21–33. doi: 10.1007/128_2012_345. [DOI] [PubMed] [Google Scholar]
- 13.Yang CS, Suh N, Kong AN. Does vitamin E prevent or promote cancer? Cancer Prev Res (Phila) 2012;5(5):701–705. doi: 10.1158/1940-6207.CAPR-12-0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Medicine Io. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids. Washington, DC: The National Academies Press; 2000. p. 529. [PubMed] [Google Scholar]
- 15.White E, Shannon JS, Patterson RE. Relationship between vitamin and calcium supplement use and colon cancer. Cancer Epidemiol Biomarkers Prev. 1997;6(10):769–774. [PubMed] [Google Scholar]
- 16.Ingles SA, Bird CL, Shikany JM, Frankl HD, Lee ER, Haile RW. Plasma tocopherol and prevalence of colorectal adenomas in a multiethnic population. Cancer Res. 1998;58(4):661–666. [PubMed] [Google Scholar]
- 17.Leenders M, Leufkens AM, Siersema PD, et al. Plasma and dietary carotenoids and vitamins A, C and E and risk of colon and rectal cancer in the European Prospective Investigation into Cancer and Nutrition. Int J Cancer. 2014;135(12):2930–2939. doi: 10.1002/ijc.28938. [DOI] [PubMed] [Google Scholar]
- 18.Bostick RM, Potter JD, McKenzie DR, et al. Reduced risk of colon cancer with high intake of vitamin E: the Iowa Women’s Health Study. Cancer Res. 1993;53(18):4230–4237. [PubMed] [Google Scholar]
- 19.Ghadirian P, Lacroix A, Maisonneuve P, et al. Nutritional factors and colon carcinoma: a case-control study involving French Canadians in Montreal, Quebec, Canada. Cancer. 1997;80(5):858–864. doi: 10.1002/(sici)1097-0142(19970901)80:5<858::aid-cncr5>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 20.Kabat GC, Kim MY, Sarto GE, Shikany JM, Rohan TE. Repeated measurements of serum carotenoid, retinol and tocopherol levels in relation to colorectal cancer risk in the Women’s Health Initiative. Eur J Clin Nutr. 2012;66(5):549–554. doi: 10.1038/ejcn.2011.207. [DOI] [PubMed] [Google Scholar]
- 21.Smolarek AK, So JY, Burgess B, et al. Dietary administration of delta- and gamma-tocopherol inhibits tumorigenesis in the animal model of estrogen receptor-positive, but not HER-2 breast cancer. Cancer Prev Res (Phila) 2012;5(11):1310–1320. doi: 10.1158/1940-6207.CAPR-12-0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li GX, Lee MJ, Liu AB, et al. delta-tocopherol is more active than alpha - or gamma -tocopherol in inhibiting lung tumorigenesis in vivo. Cancer Prev Res (Phila) 2011;4(3):404–413. doi: 10.1158/1940-6207.CAPR-10-0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guan F, Li G, Liu AB, et al. delta- and gamma-tocopherols, but not alpha-tocopherol, inhibit colon carcinogenesis in azoxymethane-treated F344 rats. Cancer Prev Res (Phila) 2012;5(4):644–654. doi: 10.1158/1940-6207.CAPR-11-0521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zheng X, Cui XX, Khor TO, et al. Inhibitory effect of a gamma-tocopherol-rich mixture of tocopherols on the formation and growth of LNCaP prostate tumors in immunodeficient mice. Cancers (Basel) 2012;3(4):3762–3772. doi: 10.3390/cancers3043762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tanaka T, Suzuki R, Kohno H, Sugie S, Takahashi M, Wakabayashi K. Colonic adenocarcinomas rapidly induced by the combined treatment with 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine and dextran sodium sulfate in male ICR mice possess beta-catenin gene mutations and increases immunoreactivity for beta-catenin, cyclooxygenase-2 and inducible nitric oxide synthase. Carcinogenesis. 2005;26(1):229–238. doi: 10.1093/carcin/bgh292. [DOI] [PubMed] [Google Scholar]
- 26.Nakanishi M, Tazawa H, Tsuchiya N, Sugimura T, Tanaka T, Nakagama H. Mouse strain differences in inflammatory responses of colonic mucosa induced by dextran sulfate sodium cause differential susceptibility to PhIP-induced large bowel carcinogenesis. Cancer Sci. 2007;98(8):1157–1163. doi: 10.1111/j.1349-7006.2007.00528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Itzkowitz SH, Yio X. Inflammation and cancer IV. Colorectal cancer in inflammatory bowel disease: the role of inflammation. Am J Physiol Gastrointest Liver Physiol. 2004;287(1):G7–17. doi: 10.1152/ajpgi.00079.2004. [DOI] [PubMed] [Google Scholar]
- 28.Hussain SP, Hofseth LJ, Harris CC. Radical causes of cancer. Nat Rev Cancer. 2003;3(4):276–285. doi: 10.1038/nrc1046. [DOI] [PubMed] [Google Scholar]
- 29.Hussain SP, Harris CC. Inflammation and cancer: an ancient link with novel potentials. Int J Cancer. 2007;121(11):2373–2380. doi: 10.1002/ijc.23173. [DOI] [PubMed] [Google Scholar]
- 30.Seril DN, Liao J, Yang GY, Yang CS. Oxidative stress and ulcerative colitis-associated carcinogenesis: studies in humans and animal models. Carcinogenesis. 2003;24(3):353–362. doi: 10.1093/carcin/24.3.353. [DOI] [PubMed] [Google Scholar]
- 31.Wang H, Zhou H, Liu A, Guo X, Yang CS. Genetic analysis of colon tumors induced by a dietary carcinogen PhIP in CYP1A humanized mice: Identification of mutation of beta-catenin/Ctnnb1 as the driver gene for the carcinogenesis. Mol Carcinog. 2014;2014(17):22199. doi: 10.1002/mc.22199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Amos-Landgraf JM, Heijmans J, Wielenga MC, et al. Sex disparity in colonic adenomagenesis involves promotion by male hormones, not protection by female hormones. Proc Natl Acad Sci U S A. 2014;111(46):16514–16519. doi: 10.1073/pnas.1323064111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moon RC, Fricks CM. Influence of gonadal hormones and age on 1,2-dimethylhydrazine-induced colon carcinogenesis. Cancer. 1977;40(5 Suppl):2502–2508. doi: 10.1002/1097-0142(197711)40:5+<2502::aid-cncr2820400917>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 34.Izbicki JR, Schmitz R, Kamran D, Izbicki W. Androgens as promoters of colon carcinogenesis. Cancer Detect Prev. 1983;6(3):355–362. [PubMed] [Google Scholar]
- 35.Kennelly R, Kavanagh DO, Hogan AM, Winter DC. Oestrogen and the colon: potential mechanisms for cancer prevention. Lancet Oncol. 2008;9(4):385–391. doi: 10.1016/S1470-2045(08)70100-1. [DOI] [PubMed] [Google Scholar]
- 36.Singh S, Langman MJ. Oestrogen and colonic epithelial cell growth. Gut. 1995;37(6):737–739. doi: 10.1136/gut.37.6.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Society AC. Colorectal Cancer Facts & Figures 2014–2016. American Cancer Society; Atlanta, GA: 2014. [Google Scholar]
- 38.Murphy G, Devesa SS, Cross AJ, Inskip PD, McGlynn KA, Cook MB. Sex disparities in colorectal cancer incidence by anatomic subsite, race and age. Int J Cancer. 2010;128(7):1668–1675. doi: 10.1002/ijc.25481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kidane D, Chae WJ, Czochor J, et al. Interplay between DNA repair and inflammation, and the link to cancer. Crit Rev Biochem Mol Biol. 2014;49(2):116–139. doi: 10.3109/10409238.2013.875514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen JX, Li G, Wang H, et al. Dietary tocopherols inhibit PhIP-induced prostate carcinogenesis in CYP1A-humanized mice. Cancer Lett. 2015;3835(15):00684–00689. doi: 10.1016/j.canlet.2015.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


