Abstract
The article reviews the major challenges related to the principles of the correct technique of musculoskeletal ultrasound (MSK US). All the crucial aspects of correct MSK soft tissue diagnosis have been discussed, including equipment settings, use of recent image software innovations and ultrasound standoff pads, and correct transducer positioning. The importance of the fundamental principles of MSK US, facilitating good quality image and limiting the occurrence of artifacts, has been highlighted. The most common artifacts of the musculoskeletal system have been described, including those that diagnostically helpful, such as the presence of echo enhancement deep to a fluid-filled structure, or an acoustic shadow behind a calcification. The presence of acoustic shadow in the context of lesions of a different type has also been discussed. The common anisotropy-related artifacts, frequently leading to diagnosis of a pathological condition where none is present, have been elaborated on. The frequently encountered mirror reflection artifact has been described. Special attention has been paid to the means of either eliminating, or taking advantage of artifacts for the correct diagnosis of musculoskeletal lesions. The possibilities and technique of correct differentiation of hypoechoic or anechoic foci, commonly found in the pathological conditions of the musculoskeletal system, have been analysed. Non-typical ultrasound findings leading to misdiagnosis of given pathological conditions have been discussed.
Keywords: ultrasound, musculoskeletal system, artifacts, diagnostic mistakes
Over the past 20 years, musculoskeletal ultrasound (MSK US) has emerged as an essential diagnostic tool for such medical specialties as orthopedics, sports medicine, rheumatology and other fields of medicine where musculoskeletal lesions are encountered. Its merits consist in the wide availability, safety, ease of use in various clinical settings, and a vast diagnostic potential, including the possibility of real-time functional evaluation and instant results.
Despite all its advantages and the inclusion of ultrasound findings in an increasing number of diagnostic algorithms, it should be remembered that musculoskeletal ultrasound is not an easy examination to perform due to the complex MSK anatomy and pathophysiology, including image variability related to movement, its key feature. A comprehensive knowledge of functional anatomy is essential for the correct functional assessment which usually is an integral part of MSK US. Another challenge are the commonly encountered artifacts, especially seen when scanning curved, uneven tissues and small, superficially located tendon and joint structures. On the other hand, the deep location of large muscles and a thick layer of fat tissue require the use of a low frequency transducer, sometimes a convex (curvilinear) one, typically used for abdominal scans. This results in a loss of resolution, especially spatial resolution.
Rapidly advancing ultrasound technology continue to improve image quality, including reduction of artifacts, wider range of a single transducer utility, and making equipment easier to use, thus decreasing the time necessary to learn the correct scanning technique and apply it in everyday work.
Like each imaging modality, ultrasound still has its limitations and its unique artifacts, potentially leading to misdiagnosis. Multiple factors affect the correct performance and interpretation of MSK US, including:
the quality of a US machine,
the choice of an appropriate transducer,
the correct machine settings,
the correct scanning technique, including proper positioning of the transducer or use of an ultrasound standoff pad where necessary,
knowledge of the capabilities and limitations of the modality, including knowledge of typical artifacts,
knowledge of normal MSK anatomy, functional MSK anatomy and MSK pathophysiology.
The choice of ultrasound machine and transducers depends on economic factors, and, partially, the extent of the performed examination. Recent ultrasound machines, even basic ones, generate and process broadband ultrasound waves, have a wide range of applications with a wide choice of transducers. Nearly all new machines may be used for basic MSK US. Sonography with Doppler techniques has been emerging as an important tool, as it allows to show the activity of synovial inflammation, formation of abnormal connective tissue at sites of tissue healing, inflammatory reactions and overuse symptoms, yet it is by no means the only modality able to detect pathologies. The lesions may also be seen without the use of Doppler ultrasound, but their proper differentiation may be difficult or prove impossible. A comprehensive examination with the assessment of blood vessels requires the use of high quality machines with sensitive Doppler options. Basic MSK US may complement the clinical exam, and then may be extended for further diagnostics with Doppler US, depending on the results of basic ultrasound and clinical findings.
Correct adjustments of the ultrasound machine allow to optimize the image so that tissues situated at different depths and subtle echogenicity differences are visible. First, the right settings for a given type of exam need to be selected. Most of the available devices have general or more detailed presets for MSK US, including image quality, size and focal depth. Selecting them is typically enough to correctly perform the examination. Nonetheless, the image may sometimes require modifications to match the sonographer’s individual preferences. The adjustments involve the gray scale, dynamic range, edge enhancements, gamma curve. The image adapted to individual requirements can be easily saved in the memory of every machine as an individual imaging preset.
After the initial settings, further optimization of the following features may be necessary:
gain,
time gain compensation (TGC),
focal depth,
use of additional image-improving software
Recent middle- and high-end ultrasound machines all have an automatic image optimalization button, making the sonographer’s work easier and faster. Yet, such auto setting is not always sufficient.
The next important step involves careful adjustment of the ultrasound beam focus (position, sometimes multiple focal depths). Reducing beam width and thickness has a dramatic effect on spatial and contrast resolution. The currently used systems of dynamic beam focusing involving alternate activation of different transducer segments at given time intervals or special Hanafy lenses placed in front of converters are available in high-end “premium” US machines, allowing to modify the focus of the emitted wave and the received echo(1–3). These more complex systems in some machines allow adjusting the width of the focal zone. The improved focusing quality is, however, linked to the higher cost of the device.
The focus should be adjusted at the level of or slightly below to the examined structures. The scanning of thin, superficially located tissues (wrist, dorsal aspect of the foot, fingers or toes) requires a single focus, adjusted at the highest level. When examining thicker layers of tissues, additional focal zones should be added, leaving the first focal zone at the uppermost level (Fig. 1). If deeper-located tissues are to be evaluated, and a thick superficial layer of fat tissue is present, the uppermost focal zone may be moved to a deeper layer.
Fig. 1.
The effect of the focal depth (arrow) setting on the image of tissues situated at different depths. Median nerve (MN) in the inferior one third of the forearm, between flexor digitorum superficialis and flexor digitorum profundus: A. focal point set low, the structure of the nerve and superficially situated muscles less visible; B. focal point moved up results in better visualization of the nerve and superficially situated tissues
The basic transducer used in MSK US is a linear array transducer of average frequency 7–8 MHz. The broader the band of transducer, the wider its application range. Transducers typically included in middle- and high-end machines have a frequency of 5–12 MHz, whilst in lowerend devices – of up to 10 MHz.
The presence of thick layers of superficial tissues, especially a thick layer of subcutaneous fatty tissue within lower extremities or thicker muscles in the shoulder region necessitates the use of a linear transducer with a lower frequency range (for vascular applications). A convex transducer, typically used for abdominal ultrasound may also be used as long as it has a frequency range up to 5–6 MHz (Fig. 2). Additionally, for scanning thin and small tissues located superficially (fingers and toes, especially in children), a smaller, hockey-stick transducer is helpful.
Fig. 2.
Sonogram of the posterior cruciate ligament in a patient with a thick layer of tissues in the popliteal fossa: A. 3–9 MHz linear transducer, very weak US beam, nondiagnostic image; B. 3–6 MHz convex transducer, image of the same area, the posterior cruciate ligament and posteriorly situated tissues better visible
According to the principles of ultrasound image formation, the angle of the insonating beam should be perpendicular to the scanned tissues for the optimal image. Musculoskeletal tissues often include thin, narrow or curved structures. The correct positioning of the transducer is one of the key prerequisites for avoiding artifacts and diagnostic errors. Frequently, the perpendicular positioning of the transducer is challenging, requiring considerable effort. The primary principle of handling the transducer is moving it gradually over a scanned region whilst keeping its perpendicular orientation, and avoiding any movement resulting in its rotation to the sides, or back and forth. Some ultrasound machines have the beam steering feature allowing to improve the image without changing the probe’s position. Exerting strong pressure on tissues should be avoided, as in the case of hard bone background, some pathologies may not be visualized or may be distorted, and vascular flow may not be visible. In our experience, the transducer held like a pen between the thumb and the index finger, with a slightly protruding little finger and sometimes ring finger works best. This permits to stabilize the transducer on the scanned area, and control the pressure strength. A similar way of holding the transducer is described in the textbook edited by Bianchi et Martinolli(2).
Direct application of the probe to the thin structures located just beneath the skin and the thin subcutaneous tissue, to uneven, projecting tissue contours leads to the occurrence of artifacts at the interface of the skin and the transducer, hence difficulties in the imaging of superficial tissues. Dynamic evaluation may also be difficult under such circumstances. An ultrasound standoff pad is then useful, enabling to accurately visualize the dermis, subcutaneous tissue, fascia and tendon’s contour (Fig. 3), and facilitating dynamic evaluation. The use of a standoff pad is also recommended in the case of externally projecting nodules, and in the rare circumstances where a convex or sector transducer must be used for scanning superficial tissues. It is indispensible when scanning through a wound or skin lesions. We have used a standoff pad to examine areas with clearly pronounced bone contours (such as the knee or medial and lateral malleolus), where correct positioning of the transducer is difficult, and obtaining a reliable image is time- and effort-consuming.
Fig. 3.
Sonogram of the dorsal aspect of the wrist, transverse plane: A. without ultrasound standoff pad; B. with ultrasound standoff pad. Examination with standoff pad gives a clear view of all skin layers, better defined cyst margins, and contour of extensor carpi radialis brevis tendon (arrow). Examination without standoff pad shows tissue compression due to transducer-related pressure, with fluid dislocated from the tendon area, its edges poorly visible
Another prerequisite for the correct performance of ultrasound is the knowledge of the principles of ultrasound image formation, and hence the knowledge when the image may be distorted. The principles of MSK US, are the same as in ultrasound diagnostics of other organs. For the majority of examinations high frequency waves are used, which on the one hand permit a high spatial resolution, but on the other facilitate artifacts, and impedes the visualization of deeper situated structures, both in terms of anatomy and evaluation of vascular flow.
Recent ultrasound techniques such as tissue harmonic imaging, compound imaging (cross beam imaging), beam steering and other complementary software introduced under various names by equipment producers attempt to limit or eliminate some of these problems and primarily improve contrast resolution. New techniques utilizing various types of impulses and dedicated software analyzing the returning signal allow to increase the penetration depth without compromising axial resolution(2,3).
Traditionally, ultrasound artifacts are divided into diagnostically helpful and adverse(4,5).
The list of helpful artifacts facilitating a correct diagnosis includes the following:
an acoustic shadow that arises posterior to calcifications,
enhanced through-transmission commonly encountered deep to a fluid-filled structure,
comet tail artifact deep to a metallic object or large piece of glass.
An acoustic shadow typically arises deep to a strong reflector. A classic example is the strong echo (ultrasound wave reflection) of a calcified tissue (such as cortical bone or calcification) producing an acoustic shadow (Fig. 4). A shadow posterior to a strong echo allows a definitive diagnosis of calcification, whereas a strong echo not producing a shadow may only be associated with small calcifications. Additionally, in musculoskeletal tissues, a shadow caused by a strong echo may occur posterior to larger foreign bodies (Fig. 5). The shadow may also form deep to a larger collection of gas (e.g. in the joint), yet owing to its unstable structure the shadow’s image is also variable, and as such the artifact may not be visible (Fig. 6).
Fig. 4.
Shoulder joint, supraspinatus muscle tendon. Characteristic image of calcification in tendon as strong echo (CAL) and acoustic shadow (arrow). ACR – acromion, SS – supraspinatus muscle tendon
Fig. 5.
Strong echo generated by a foreign body – a shrapnel embedded in the inguinal region, vicinity of the hip joint, similar to calcification (arrow). B – piece of bullet, IL – iliac bone
Fig. 6.
Strong echo generated by gas (G) in the knee joint over the contour of the femoral condyle (C) with reverberations and irregular shadow (arrow)
An acoustic shadow is not a conclusive symptom for the presence of calcifications, as it also occurs as a result of refraction (a change in direction of wave propagation, scattering of the ultrasound bean on a curved, uneven tissue) and significant echo intensity decline at this place. It may occur in the case of injured and curled fibrous tissue, such as a torn piece of ligament or tendon, in the location of a large fibrous scar. It is worth noting that opposed to calcifications, no hyperechoic focus is then visible (Fig. 7). It should also be noted that the use of high frequency/resolution transducers leads to the amplification of this artifact. Careful assessment of reflections in the shadow’s area allows for differentiation of these lesions and a conclusive calcification diagnosis. On the whole, it should be remembered that not every acoustic shadow is consistent with the presence of calcification, and the lack of a shadow does not exclude the presence of small calcifications.
Fig. 7.
Acoustic shadow (arrow) deep to a scar resulting from a partial muscle tear. Fibrous scar (B) without the strong echo characteristic for calcification
Enhanced through-transmission deep to a fluid-filled structure occurs due to weak sound wave attenuation within simple fluid, gelatinous structure, as well as to some degree wave bending at the interface of two media, resulting with a localized area of increased echo posteriorly to the interface. The wave that passes deeper has higher energy and is more strongly reflected from deeper tissue layers, resulting with a stronger echo compared to the adjacent tissues. Based on the presence of this artifact, a hypoechoic or anechoic lesion may with more certainty be assumed to be a fluid collection (Fig. 8). In rare cases, enhancement may also occur posterior to hypoechoic or nearly anechoic foci consistent with the presence of a richly vascular, loose soft tissue. This symptom is, however, seldom found in musculoskeletal structures.
Fig. 8.
OImage of echo enhancement behind a fluid-filled structure (arrow), visible deep to a small gelatinous cyst situated next to the flexor digitorum tendon (FD)
Comet tail artifact typically occurs deep to a metallic object(5,6). It may also be seen posterior to a large piece of glass. It is visualized as dense, strong linear reflections deep to the reflecting surface. The echo’s intensity tapers, hence the shape of a comet’s tail (Fig. 9). Visualization of this artifact typically prompts the diagnosis of a metallic object embedded in the tissue.
Fig. 9.
Fixation screw (S) in the humerus bone. Comet tail artifact deep to a metallic object (arrow)
Diagnostically adverse artifacts include:
a broad shadow deep to a calcified structure, superimposed on the posterior tissues
lateral (edge) shadowing
anisotropy
reverberations
beam width artifact.
Even though shadowing posterior to a calcification is helpful, the shadow, when too large, may cover the tissues situated beneath, thus hampering the visualization of structures such as a medullary cavity, tissues within a joint, or tissues deep to large calcifications.
Lateral shadows form on the flanks of curved (rounded) structures, where there are no large differences in acoustic impedance at the interface of tissues, yet the insonating angle is nearly adherent to the tissue’s curvature, or different than 90°. Such structures abound in musculoskeletal system, including e.g. tendons or cysts. A lateral shadow may cover or sometimes imitate small lesions in the tendon sheath or paratenon, or post-injury lesions. In dubious cases, the transducer should be moved over the area, altering the insonating angle, to verify if the lesions will remain visible (Fig. 10). Such a maneuver is not in all such locations. The use of cross beam imaging or beam steering allows to reduce this artifact, even though it typically does not resolve completely.
Fig. 10.
Lateral shadow (arrows) next to the Achilles tendon (T): A. perpendicular positioning of the transducer; B. oblique positioning of the transducer, reducing the shadow. Note the use of the standoff pad facilitating the correct positioning of the transducer
The anisotropic effect in ultrasound is when tissues show abnormal echogenicity, typically loss of echogenicity, due to an oblique insonating angle, suggesting the presence of a pathological condition(7). In the musculoskeletal system, this symptom is commonly encountered, potentially prompting misdiagnosis. The structures most affected by anisotropy are tendons and muscles. A slight rotation of the transducer without changing the course of its adherence to the surface results in abrupt decline of the tendon’s or muscle’s echogenicity. This artifact is pronounced at curved tendon and ligament insertions (Fig. 11). Anisotropy of nerves is a similar, yet less intensive, effect. In muscles, it is also possible to see artifacts in the form hyperechoic foci imitating edematous or inflammatory lesions. Currently, many machines are equipped with beam steering or cross beam imaging features, allowing to reduce, if not eliminate, anisotropy related artifacts. To completely overcome anisotropy, the transducer should be held in a strictly perpendicular position in relation to the anatomy in question, and the potential lesion ruled out or confirmed in the second, perpendicular plane. Keeping anisotropy in mind (especially since it is not fully resolved by corrective software) during MSK US helps to prevent misdiagnosis.
Fig. 11.
Anisotropy-related artifact seen at the insertion of the quadriceps femoris tendon (T) next to the basis of patella (P): A. insertion with a hypoechoic focus following the use of crossed beam imaging (arrow); B. correct image of the tendon following a slight move of the transducer and flexing of the quadriceps femoris
Refraction occurs at the interface of two media of different ultrasound propagation speeds, such as fat tissue and muscle. The wave direction changes on passing from one medium to another, causing lesions deep to the interface appear displaced. The artifact is partially overcome by steadily keeping the transducer in a perpendicular position to the examined structures. In some, newest machines, it is possible to calculate the correct ultrasound wave speed values, and correct the image by incorporating the measurements.
Reverberations are seen when the ultrasound beam encounters two strong parallel reflectors, and is reflected back and forth between them, taking different time to return to the transducer. It is one of the causes of linear echoes forming in fluid-filled structures, posterior to a bone contour, or a mirror image (Fig. 12). In musculoskeletal tissues, this effect typically occurs due to the presence of a curved cortical bone tissue strongly reflecting ultrasound.
Fig. 12.
Mirror reflection artifact next to the anterior aspect of the tibia (TIB). Superficial to the bone contour, a post-traumatic hematoma (HEM) is visible in the subcutaneous tissue. The hypoechoic focus visible deep to the bone contour is a mirror reflection artifact (arrow) mimicking a pathological condition within the bone
Ultrasound beam width or volume averaging artifacts, occurs when the machine registers echoes from a given tissue volume depending on the transducer’s design and the thickness of the examined tissues. If the scanned structure is smaller than the beam’s width, its image is obtained from the echoes reflected from the structure and the adjacent tissues. This may result in the elimination of a shadow posterior to a small calcification, displaying echo within a fluid-filled structure or showing tissue abnormalities. Newest ultrasound devices have additional possibility to focus US beam in its transverse plane (narrowing the beam) to decrease this effect.
In ultrasound imaging, especially MSK US, various normal tissues and pathological conditions may look similar, requiring adequate differential workup. Such images include anechoic and hypoechoic foci and spaces that may represent the following tissues and lesions:
hyaline cartilage;
various fluid-filled structures, such as synovial bursa, fluid-filled sheath, hematoma, cyst, infected fluid (purulent);
inflammatory foci, edema;
mucoid or hyaline soft tissue degeneration at site of injury;
necrotic tissue;
inflammatory lesions with increased vascularity, involving e.g. the synovium (joints, tendon sheaths, bursae), tendon insertions, and muscles;
angiofibroblastic hyperplasia;
compact fibrous scar tissue with an irregular pattern of thick collagen fibers strongly scattering the ultrasound wave.
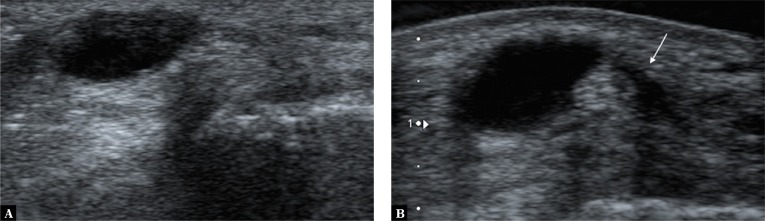
The first step of the differential workup involves identifying the structure’s location as for cartilage superficial to the bone contour or fluid layer situated in a synovial recess, bursa or sheath. Anechoic focus seen at site of injury may be consistent with a fluid-filled structure of various types, as well as a whole range of degenerative foci. A simple compression test helps to further distinguish between fluid-filled structures and other lesions. When pressure is applied with the transducer, fluid-filled structures change shape, with the fluid sometimes changing location or entirely disappearing from view. The test, however, may come out negative, if the fluid collection has high pressure, and the change of shape may only be very slight. To differentiate a structure filled with high-pressure fluid from other lesions the Doppler option may be used, as it shows fluid fluctuation. The fluid-filled area will fill with color, Doppler signal (image of fluid movement), especially while pressure is being released (Fig. 13).
Fig. 13.
Additional symptom facilitating differentiation of fluid-filled structures in equivocal findings: A. a typical fluid-filled structure in the popliteal fossa, consistent with an enlarged synovial bursa of the gastrocnemius muscle; B. the fluctuation symptom showed by the power Doppler option, visible as colour filled the fluid space due to the fluid motion caused by the pressure applied with the transducer
The compression test also helps to distinguish softer connective tissue (mucoid degeneration, necrosis, granulation tissue), which is somewhat compressible and flattens under pressure as opposed to stiff, non-compressible irregular scars composed of collagen fibers, or degenerative hyaline lesions.
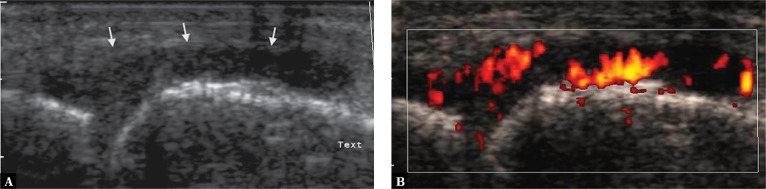
Color or power Doppler option should be used as the next step of the diagnostic workup. The presence of a vascular network within the lesion allows to differentiate inflammatory lesions and evaluate the inflammatory activity (Fig. 14), as well as identify abnormally healing lesions with a history of trauma or chronic mechanical overuse (Fig. 15 A)(8). Whenever the presence of such lesions is suspected, a sensitive Doppler option should be used.
Fig. 14.
Metacarpophalangeal joint: A. thickened joint capsule with hypoechoic edema of the synovium (arrows,) resembling fluid; B. power Doppler scan showed numerous vessels consistent with highly active inflammatory lesions (Grade 3 vascularity)
Fig. 15.
Enthesopathy at the proximal insertion of the patellar ligament, lesions with the history of overuse injury in A professional athlete: A. power Doppler option shows multiple vessels at the proximal insertion of the patellar ligament, consistent with abnormal healing with angiofibroblastic hyperplasia; B. vessels not visible when stronger pressure is applied with the transducer
Imaging blood vessels of the musculoskeletal system is aimed at identifying increased tissue vascularization (hyperemia) or any vascular pathologies, that is finding out whether blood vessels are visible, what is their number and location. It is necessary to try to visualize even the smallest vessels in the smallest structures, such as the nerves. Hence, searching for few, small vessels, the Doppler mode requires maximum gain at the level of small motion artifacts. The correct technique requires proper immobilization of the transducer without compressing the tissues (Fig. 15 B). Artifacts typically show as random color flashes; in the vicinity of larger vessels perivascular tissue pulsation may be detected.
It is essential for examiners to be aware of THE technical capabilities of the equipment they use(8,9). Recent “premium” ultrasound machines feature additional options improving the sensitivity of detection of the vascular flow in small vessels. It should be remembered that despite these additional features, the sensitivity significantly decreases with THE increasing depth of the scanned tissues. A low frequency transducer, e.g. a convex one, provides an increased depth of penetration, yet it is not always enough TO rule out the presence of small vessels. It should also be noted that the presence of increased, abnormal vascularity is not necessarily consistent with a diagnosis of an inflammatory condition. The assessment requires a careful analysis of the tissue morphology (2D image), vessel location and the clinical data. Apart from inflammatory conditions, increased vascularity is found in early stages of a normal healing process, in fibroangioblastic hyperplasia(10), in the nerve compression syndrome(11), tumors and vascular malformations(12).
All things considered, sonography of the musculoskeletal system is a highly sensitive examination, allowing to visualize even very small lesions in musculoskeletal tissues. At the same time, the image of the visualized lesions frequently has low specificity. Multiple factors affect correct US diagnosis. Apart from understanding MSK US principles, taking full advantage of its technical capabilities, and knowing the pitfalls discussed above, there is a need for a reliable correlation of ultrasound findings with the clinical symptoms and results of additional tests when necessary. All these elements combined warrant a comprehensive interpretation of the symptoms found on ultrasound.
Conflict of interest
The authors do not declare any financial or personal links to other people or organizations that could adversely affect the content of this publication or claim rights hereto.
References
- 1.Nowicki A. Ultrasonografia: wprowadzenie do obrazowania i metod dopplerowskich. Warszawa: Wydawnictwo IPPT PAN; 2016. [Google Scholar]
- 2.Derci LE, Rizzatto G. Technical requirements. In: Bianchi S, Martinoli C, editors. Ultrasound of the Musculoskeletal System. Berlin: Springer; 2007. pp. 1–16. [Google Scholar]
- 3.Claudon M, Tranquart F, Evans DH, Lefèvre F, Correas M. Advances in ultrasound. Eur Radiol. 2002;12:7–18. doi: 10.1007/s00330-001-1185-1. [DOI] [PubMed] [Google Scholar]
- 4.Laing FC. Commonly encountered artifacts in clinical ultrasound. Semin Ultrasound CT MR. 1983;4:27–43. [Google Scholar]
- 5.Thickman DI, Ziskin MC, Goldenberg NJ, Linder BE. Clinical manifestations of the comet-tail artifacts. J Ultrasound Med. 1983;2:225–230. doi: 10.7863/jum.1983.2.5.225. [DOI] [PubMed] [Google Scholar]
- 6.Ziskin MC, Thickman DI, Goldenberg NJ, Lapayowker MS, Becker JM. The comet-tail artifact. J Ultrasound Med. 1982;1:1–7. doi: 10.7863/jum.1982.1.1.1. [DOI] [PubMed] [Google Scholar]
- 7.Fornage BD. The hypoechoic normal tendon. A pitfall. J Ultrasound Med. 1987;6:19–22. doi: 10.7863/jum.1987.6.1.19. [DOI] [PubMed] [Google Scholar]
- 8.Koski JM, Saarakkala S, Helle M, Hakulinen U, Heikkinen JO, Hermunen H. Power Doppler ultrasonography and synovitis: correlating ultrasound imaging with histopathological findings and evaluating the performance of ultrasound equipements. Ann Rheum Dis. 2006;65:1590–1595. doi: 10.1136/ard.2005.051235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Torp-Pedersen ST, Terslev L. Settings and artifacts in colour/power Doppler ultrasound in rheumatology. Ann Rheum Dis. 2008;67:143, 149. doi: 10.1136/ard.2007.078451. [DOI] [PubMed] [Google Scholar]
- 10.Kraushaar BS, Nirschl RP. Tendinosis of the elbow (tennis elbow). Clinical features and findings histological and electron microscopy studies. J Bone Joint Surg Am. 1999;81:259–278. [PubMed] [Google Scholar]
- 11.Kowalska B. Assessment of the utility of ultrasonography with high-frequency transducers in the diagnosis of entrapment neuropathies. J Ultrason. 2014;14:371–392. doi: 10.15557/JoU.2014.0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Teh J. Ultrasound of soft tissue masses of the hand. J Ultrason. 2012;12:381–401. doi: 10.15557/JoU.2012.0028. [DOI] [PMC free article] [PubMed] [Google Scholar]