Key Points
Question
What is the comparison between survival rates and causes of death among patients with incident, clinically diagnosed synucleinopathies and age- and sex-matched referent participants?
Findings
In this population-based study, individuals with multiple system atrophy with parkinsonism, dementia with Lewy bodies, Parkinson disease, and Parkinson disease dementia had increased mortality compared with the general population, and patients with multiple system atrophy with predominant parkinsonism had the highest risk followed by dementia with Lewy bodies, Parkinson disease dementia, and Parkinson disease.
Meaning
Because survival rates and the causes of death vary across the clinically diagnosed synucleinopathies, an individualized approach to prognosing the different diseases is warranted.
Abstract
Importance
To our knowledge, a comprehensive study of the survival and causes of death of persons with synucleinopathies compared with the general population has not been conducted. Understanding the long-term outcomes of these conditions may inform patients and caregivers of the expected disease duration and may help with care planning.
Objective
To compare survival rates and causes of death among patients with incident, clinically diagnosed synucleinopathies and age- and sex-matched referent participants.
Design, Setting, and Participants
This population-based study used the Rochester Epidemiology Project medical records–linkage system to identify all residents in Olmsted County, Minnesota, who received a diagnostic code of parkinsonism from 1991 through 2010. A movement-disorders specialist reviewed the medical records of each individual to confirm the presence of parkinsonism and determine the type of synucleinopathy. For each confirmed patient, an age- and sex-matched Olmsted County resident without parkinsonism was also identified.
Main Outcomes and Measures
We determined the age- and sex-adjusted risk of death for each type of synucleinopathy, the median time from diagnosis to death, and the causes of death.
Results
Of the 461 patients with synucleinopathies, 279 (60.5%) were men, and of the 452 referent participants, 272 (60.2%) were men. From 1991 through 2010, 461 individuals received a diagnosis of a synucleinopathy (309 [67%] of Parkinson disease, 81 [17.6%] of dementia with Lewy bodies, 55 [11.9%] of Parkinson disease dementia, and 16 [3.5%] of multiple system atrophy with parkinsonism). During follow-up, 68.6% (n = 316) of the patients with synucleinopathies and 48.7% (n = 220) of the referent participants died. Patients with any synucleinopathy died a median of 2 years earlier than referent participants. Patients with multiple system atrophy with parkinsonism (hazard ratio, 10.51; 95% CI, 2.92-37.82) had the highest risk of death compared with referent participants, followed by those with dementia with Lewy bodies (hazard ratio, 3.94; 95% CI, 2.61-5.94), Parkinson disease with dementia (hazard ratio, 3.86; 95% CI, 2.36-6.30), and Parkinson disease (hazard ratio, 1.75; 95% CI, 1.39-2.21). Neurodegenerative disease was the most frequent cause of death listed on the death certificate for patients, and cardiovascular disease was the most frequent cause of death among referent participants.
Conclusions and Relevance
Individuals with multiple system atrophy with parkinsonism, dementia with Lewy bodies, and Parkinson disease dementia have increased mortality compared with the general population. The mortality among persons with Parkinson disease is only moderately increased compared with the general population.
This population-based study compares patients with synucleinopathies with sex- and age-matched participants without parkinsonism to determine the risk of death for each type of synucleinopathy, the median time from diagnosis to death, and the causes of death.
Introduction
The aggregation of α-synuclein, a ubiquitous protein in the nervous system, contributes to Lewy body formation. Diseases defined as synucleinopathies, including Parkinson disease (PD), dementia with Lewy bodies (DLB), PD dementia (PDD), and multiple system atrophy (MSA), share the presence of α-synuclein as the pathological hallmark. Several studies have determined the incidence and prevalence of these diseases. However, few studies have examined survival rates among patients with synucleinopathies after symptom onset compared with the general population.
A recent study showed decreased survival rates among patients with MSA and parkinsonism compared with patients with PD. To our knowledge, however, no comprehensive comparison of the survival rates and causes of death of persons with different types of synucleinopathies (PD, DLB, PDD, and MSA) in the general population has been performed. Understanding the long-term outcomes of these conditions may inform patients and caregivers of the expected disease duration and facilitate care planning.
To address questions of survival and causes of death in these diseases, we used an incident study of synucleinopathy–associated clinical syndromes (PD, DLB, PDD, and MSA-p [MSA with parkinsonism]) occurring from 1991 to 2010 and age- and sex-matched referent participants living in Olmsted County, Minnesota. We compared survival rates of patients with incident disease with those of referent participants and described their causes of deaths.
Methods
Ascertaining Patients With Synucleinopathy
Details about ascertaining patients with parkinsonism are reported elsewhere. Briefly, we used the medical records–linkage system of the Rochester Epidemiology Project to identify all individuals in Olmsted County, Minnesota, with a clinically diagnosed synucleinopathy with parkinsonism between 1991 and 2010. The Rochester Epidemiology Project infrastructure indexes and links all medical information of the individuals in the county population. All medical diagnoses, surgical interventions, and other procedures are abstracted and entered into computerized indexes using the Hospital Adaptation of the International Classification of Diseases, Eighth Revision (H-ICDA) and the International Classification of Diseases, Ninth Revision (ICD-9).
We ascertained potential cases of parkinsonism using a computerized screening phase and a subsequent clinical-confirmation phase. The complete medical records of all people receiving at least 1 screening diagnostic code for parkinsonism were reviewed by a movement disorders specialist (R.S.) using specifically designed abstracting forms and instruction manuals. The movement disorders specialist established the onset year and type of parkinsonism using specified diagnostic criteria. We included patients residing in Olmsted County at the time of parkinsonian-symptom onset but excluded people who denied permission to use their medical records for research purposes. Further details about case-finding procedures are published elsewhere. The institutional review boards of the Mayo Clinic and the Olmsted Medical Center approved the study. Written informed consent was waived because it was a passive medical record review.
Diagnostic Criteria
Our diagnostic adjudication included 2 steps: defining parkinsonism as a syndrome and defining different types of parkinsonism within the syndrome. We defined parkinsonism as the presence of at least 2 of 4 cardinal signs: rest tremor, bradykinesia, rigidity, and impaired postural reflexes. Among persons fulfilling the parkinsonism criteria, we applied diagnostic criteria for specific types of parkinsonism and grouped patients with parkinsonism into presumed synucleinopathies.
Parkinson disease was defined as parkinsonism with all 3 of the following: no other cause (eg, repeated stroke with stepwise progression, repeated head injury, a history of encephalitis, neuroleptic treatment within 6 months before onset, hydrocephalus, or a brain tumor), no documented unresponsiveness to levodopa at doses of at least 1 g/d in combination with carbidopa (applicable only to treated patients), and no prominent or early (within 1 year of onset) signs of more extensive nervous system involvement (eg, dysautonomia) that was not explained otherwise. We used previously published consensus criteria to diagnose DLB, PDD, and MSA.
Our case-finding procedures have proven reliable. We also reviewed available autopsy reports of all patients who died during the study to validate our classification of presumed synucleinopathies and tauopathies and found an 81.5% clinicopathological agreement.
Ascertaining Referent Participants
Each patient with clinically defined synucleinopathy was individually matched by age (±1 year) and sex to a general population referent participant residing in Olmsted County, Minnesota, who was free of parkinsonism and tremor of any type in the year of onset of the synucleinopathy. The medical records–linkage system provided a list of all the county residents from whom potential referent participants were randomly drawn. This list was compared with a random-digit-dialing telephone sample and with the US census data and was shown to be complete.
A nurse abstractor reviewed the records of potential referent participants and flagged any possible parkinsonism, tremor, or related diagnoses. A neurologist (R.S.) then reviewed the record of the potential referent participant to exclude the presence of PD, other types of parkinsonism, or tremor before or during the year of synucleinopathy onset in the matched patient with parkinsonism. A panel of movement disorders specialists (R.S., J.H.B.) adjudicated any individual with diagnostic uncertainties. Potential referent participants with confirmed parkinsonism or a tremor before the index year of the synucleinopathy were excluded and potential replacement participants were randomly selected and screened. Referent participants with other neurologic diseases were not excluded.
Data Analysis
Patients with presumed synucleinopathies and the age- and sex-matched referent participants included in this study were originally identified for a case-control study nested within a cohort. Therefore, the cases were sampled conditionally based on being alive at the onset of the synucleinopathy (onset year) and on the date of diagnosis (diagnosis date). Onset was defined as the first identification of parkinsonian symptoms in the medical records. By contrast, controls were selected conditionally only based on being alive in the year of onset of the synucleinopathy in the matched case. To avoid an immortality bias (people who died before diagnosis), we started survival analyses for both patients and referent participants at the date of the diagnosis rather than the year of onset. We assigned referent participants the same index date (date of diagnosis) as their matched cases and excluded from analyses referent participants who died between the onset year and the diagnosis date (n = 9). The original matching of cases and referent participants was not considered in the survival analyses, and age at diagnosis and sex were included as covariates in the models to remove residual confounding.
We followed-up patients and referent participants until they were lost to follow-up, until June 30, 2015 (the end of the study), or until death (whichever occurred first). Persons who were alive on June 30, 2015, or who were lost to follow-up, were censored at the date that they were last known to be alive. We constructed Kaplan-Meier survival curves with death from any cause as the event of interest. We used Cox proportional hazards models, with age as the time scale, to calculate hazard ratios (HR) and 95% confidence intervals of death among patients with synucleinopathies (overall and separately by type) compared with referent participants. All statistical testing was done at 2-tailed α levels of .05. The proportionality assumption was tested visually and by including a time-dependent coefficient in the Cox proportional hazards models. All analyses were completed using Stata, version 12.0 (StataCorp).
Results
We identified 461 patients who had an onset of a clinically presumed synucleinopathy manifesting as parkinsonism from 1991 through 2010. Of these, 309 (67%) received a diagnosis of PD, 81 (18%) of DLB, 55 (125) of PDD, and 16 (3%) of MSA-p. One referent participant was initially age- and sex-matched to each patient; however, 9 referent participants died between the onset year and diagnosis date. Thus, our final analyses included 461 patients with incident synucleinopathies and 452 referent participants.
The median (interquartile range [IQR]) lag time between the date of onset and the date of the diagnosis of all synucleinopathies was 0.50 (0.01-1.22) years. Table 1 shows the characteristics of the patients and the referent participants stratified by the type of synucleinopathy. Of the 461 patients, 279 (60.5%) were men. The median age at parkinsonian symptom onset for all cases was 75.8 years (IQR, 67.4-81.7 years), and the median age at death was 84.7 years (IQR, 79.3-89.7). The median age at symptom onset did not differ by sex (P = .17, rank sum test). Among the 452 referent participants, 272 (60.2%) were men. Their median age at the index date was 75.6 years (IQR, 67.2-81.3), and the median age of death was 87.8 (IQR, 83.0-92.2). Women lived significantly longer than men among patients (median difference, 2.2 years; P = .02) and referent participants (median difference, 2.3 years; P = .02).
Table 1. Survival Analyses Comparing Patients With Synucleinopathies With Referent Participants, Olmsted County, Minnesota, 1991 to 2005.
| Total No. | Men, No. (%) | Age, Median (IQR) | Age at Death | |||
|---|---|---|---|---|---|---|
| Symptom Onset or Index | Diagnosis or Index | No. | Median (IQR) | |||
| Patients | ||||||
| All | 461 | 279 (60.5) | 75.8 (67.4-81.7) | 76.5 (69.2-82.3) | 316 | 84.7 (79.3-89.7) |
| PD | 309 | 184 (59.6) | 73.9 (65.6-80.8) | 74.9 (67.2-81.8) | 175 | 86.2 (79.7-90.8) |
| DLB | 81 | 56 (69.1) | 77.1 (72.9-80.4) | 77.9 (73.5-81.2) | 72 | 83.4 (79.2-87.2) |
| MSA-p | 16 | 12 (75.0) | 70.7 (63.7-76.9) | 71.5 (64.3-77.4) | 16 | 79.8 (67.1-84.9) |
| PDD | 55 | 27 (49.1) | 81.8 (75.3-86.7) | 82.3 (75.3-87.6) | 53 | 84.7 (79.7-90.8) |
| Referent Participants | ||||||
| All | 452 | 272 (60.2) | 75.6 (67.2-81.3) | 76.2 (68.9-82.1) | 220 | 87.8 (83.0-92.2) |
| PD | 303 | 178 (58.8) | 73.9 (65.1-80.8) | 75.0 (66.3-81.6) | 132 | 87.3 (82.4-92.0) |
| DLB | 80 | 56 (70.0) | 76.3 (72.2-80.2) | 77.4 (73.3-81.0) | 41 | 85.2 (82.8-90.8) |
| MSA-p | 16 | 12 (75.0) | 70.1 (63.9-76.1) | 71.2 (64.5-76.6) | 7 | 92.1 (78.7-92.5) |
| PDD | 53 | 26 (49.1) | 80.7 (75.7-86.1) | 81.6 (76.0-87.3) | 40 | 90.0 (87.2-93.6) |
Abbreviations: DLB, dementia with Lewy bodies; IQR, interquartile range; MSA-p, multiple system atrophy; PD, Parkinson disease; PDD, Parkinson disease dementia.
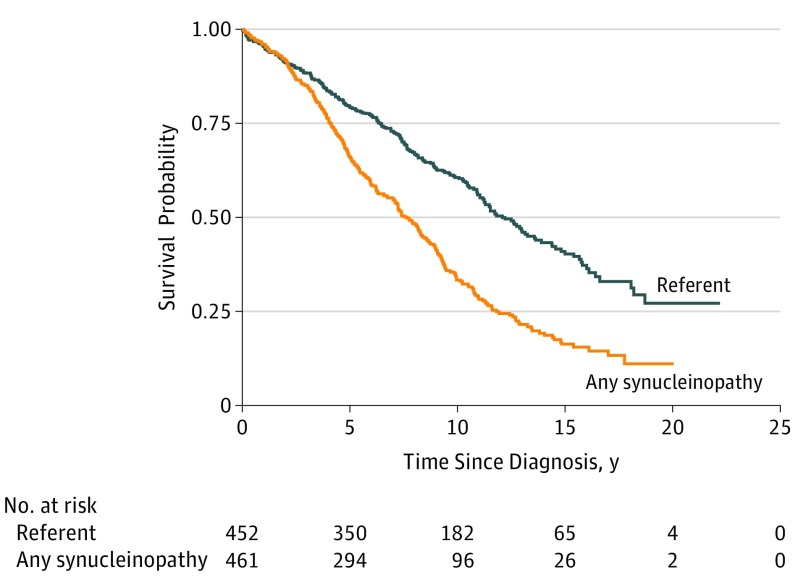
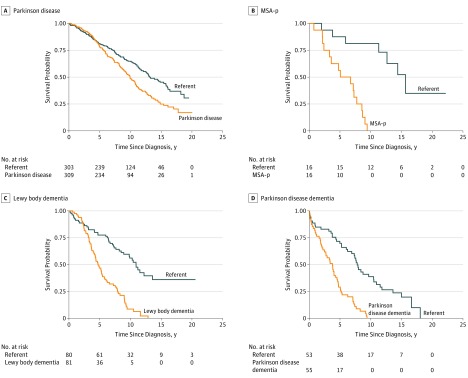
Of the 461 patients with synucleinopathies, 316 (68.6%) died during follow-up and 311 had a known cause of death (98.4%). Of the 452 referent participants, 220 (48.7%) died during follow-up and 216 had a known cause of death (98.2%). Figure 1 displays Kaplan-Meier survival curves for all synucleinopathies vs referent participants. The overall curves for all patients and referent participants started to diverge approximately 2 years after the diagnosis date. Compared with referent participants, patients had more than a 2-fold increased risk of death (HR, 2.26; 95% CI, 1.89-2.69) (Table 2). Survival curves by synucleinopathy type are shown in Figure 2. The highest risk of death, compared with referent participants, was observed among patients with MSA-p (HR, 10.51; 95% CI, 2.92-37.82), followed by patients with DLB (HR, 3.94; 95% CI, 2.61-5.94), PDD (HR, 3.86; 95% CI, 2.36-6.30), and PD (HR, 1.75; 95% CI, 1.39-2.21). As a sensitivity analysis, we generated Kaplan-Meier survival curves using the year of onset rather than the date of the diagnosis and found no noticeable differences compared with the primary analyses. However, there was an inversion of the curves in the first 1 to 2 years because of the immortality bias. In additional analyses, we searched for an interaction with sex and age in predicting survival rates but did not find a significant interaction for any type of synucleinopathy.
Figure 1. Kaplan-Meier Survival Curves Among Patients With Synucleinopathies and Referent Participants.
Table 2. Survival Analyses Stratified by Type of Synucleinopathy, Olmsted County, Minnesota, 1991 to 2005a.
| Type of Synucleinopathy | No. | Survival, Median (IQR), y | Hazard Ratio (95% CI) | |
|---|---|---|---|---|
| Sample Size | Deaths | |||
| All synucleinopathies | 461 | 316 | 6.3 (4.1-9.3) | 2.26 (1.89-2.69) |
| Referent participants | 452 | 220 | 8.3 (5.3-12.1) | NA |
| PD | 309 | 175 | 7.4 (5.0-10.8) | 1.75 (1.39-2.21) |
| Referent participants | 303 | 132 | 8.2 (5.5-12.3) | NA |
| DLB | 81 | 72 | 4.7 (3.3-7.5) | 3.94 (2.61-5.94) |
| Referent participants | 80 | 41 | 8.7 (5.4-11.1) | NA |
| MSA-p | 16 | 16 | 5.9 (2.8-8.2) | 10.51 (2.92-37.82) |
| Referent participants | 16 | 7 | 11.9 (9.3-15.3) | NA |
| PDD | 55 | 53 | 3.8 (1.6-5.2) | 3.86 (2.36-6.30) |
| Referent participants | 53 | 40 | 7.4 (4.3-10.9) | NA |
Abbreviations: DLB, dementia with Lewy bodies; IQR, interquartile range; MSA-p, multiple system atrophy; NA. not applicable; PD, Parkinson disease; PDD, Parkinson disease dementia.
All models adjusted for age at diagnosis and sex.
Figure 2. Kaplan-Meier Survival Curves By Type of Synucleinopathy.
MSA-p indicates multiple system atrophy.
Lastly, we compared the causes of death between the patients and the referent participants (Table 3). We categorized the causes of death into respiratory failure/ pneumonia, acute and chronic cardiovascular events (pooled), stroke/hemorrhages, cancers (of all types), renal failure, aspiration, neurodegenerative diseases (dementia, PD, Alzheimer disease, etc), traumas (including falls), sepsis, and other causes of death (Table 3). Neurodegenerative disease was the most frequent cause of death among patients for all synucleinopathies (31.5%) and in PD alone (25.6%). Cardiovascular events were the second most common cause of death (15.7%). These results were consistent with the causes of death observed among patients with DLB, PDD, and MSA-p. However, the sample size was limited to observe a sufficient number of events. Among referent participants, cardiovascular events were the most common cause of death (25.5%).
Table 3. Causes of Death for Patients With Any Synucleinopathy, and for PD, Compared With Referent Participants.
| Primary Cause of Death | No. (%) | |
|---|---|---|
| Patients (n = 311)a |
Referent Participants (n = 216)a |
|
| Synucleinopathies | ||
| Neurodegenerative disease | 98 (31.5) | 25 (11.6) |
| Cardiovascular events | 49 (15.8) | 55 (25.5) |
| Respiratory failure/pneumonia | 48 (15.4) | 32 (14.8) |
| Others | 29 (9.3) | 19 (8.8) |
| Aspiration | 25 (8.1) | 7 (3.2) |
| Cancers | 22 (7.1) | 39 (18.1) |
| Sepsis | 17 (5.5) | 9 (4.2) |
| Stroke/hemorrhages | 11 (3.5) | 16 (7.4) |
| Traumas (including falls) | 7 (2.3) | 8 (3.7) |
| Renal failure | 5 (1.6) | 6 (2.8) |
|
Patients (n = 172) |
Referent Participants (n = 130) |
|
| Parkinson Disease | ||
| Neurodegenerative disease | 44 (25.6) | 10 (7.7) |
| Cardiovascular events | 28 (16.3) | 34 (26.2) |
| Respiratory failure/pneumonia | 23 (13.4) | 17 (13.1) |
| Cancers | 20 (11.6) | 29 (22.3) |
| Others | 19 (11.1) | 11 (8.5) |
| Aspiration | 15 (8.7) | 4 (3.1) |
| Stroke/hemorrhages | 9 (5.2) | 11 (8.5) |
| Traumas (including falls) | 5 (2.9) | 6 (4.6) |
| Sepsis | 5 (2.9) | 5 (3.8) |
| Renal failure | 4 (2.3) | 3 (2.3) |
Abbreviation: PD, Parkinson disease.
Death certificates were not available for 5 patients and 4 referent participants. These persons are not included in this table.
Discussion
Our study suggests that an increased risk of mortality exists for individuals with clinically defined synucleinopathies compared with age- and sex-matched referent participants. Overall, patients with synucleinopathies died 2 years earlier than referent participants. When stratified by synucleinopathy type, patients with MSA-p died 6 years earlier, patients with DLB died 4 years earlier, patients with PDD died 3.5 years earlier, and patients with PD died 1 year earlier than the respective referent participants. In additional analyses, we compared the causes of death as listed in the death certificates of patients and participants. Neurodegenerative disease appeared most frequently among patients with clinically diagnosed synucleinopathies, whereas cardiovascular diseases were the most frequent cause of death among referent participants.
Our findings are consistent with the findings from a previous report in the Olmsted County population that examined patients with incident PD and matched referent participants from 1976 to 1995. The relative risk of death for PD was 1.6 (95% CI, 1.2-2.1), which is consistent with the 1.75-fold increased risk (95% CI, 1.39-2.21) observed for the period from 1991 to 2010. We avoided overlapping the 2 studies by removing the patients with PD from the 1991 to 2010 period who were already included in the 1976 to 1995 period. Thus, the risk of death after a PD diagnosis seems relatively stable across a 30-year time span. Another cohort study also reported a similar 2-fold increased mortality risk for patients with PD compared with matched referents. By contrast, other studies have reported a decreasedrisk of mortality among patients with PD. However, these studies were based mostly on prevalent cases of PD (rather than incident cases) and included small numbers of patients with PD.
Few studies have examined the risk of death after a diagnosis of other types of synucleinopathies, and those that have are primarily based on clinical series and are not population-based. A pathology-confirmed series of patients with DLB and PDD combined showed a median survival of 5 years from symptom onset. The results of a large prospective clinical series of MSA showed that the presence of parkinsonism in MSA was associated with shorter survival and a higher risk of death. By contrast, a meta-analysis on pathologically proven cases of MSA did not confirm shorter survival in MSA. Few studies have compared survival across synucleinopathies. One study recently reported a 6-fold increased risk of mortality among patients with MSA-p compared with patients with PD. The patients with PD lived approximately 8 years longer than the patients with MSA-p (7.5 years in MSA vs 15.8 years in PD). These results are consistent with our study showing that patients with MSA-p have a 10-fold greater risk of death compared with referent participants and the shortest survival rates after symptom onset when compared with all other types of synucleinopathies.
Given the diagnostic challenges, few studies to our knowledge have examined mortality in DLB and PDD. However, patients with PD with dementia have a faster progression than those without. Patients with DLB have a faster progression than Alzheimer disease as well as a shorter time to disability and death. A previous study listed the median survival time of patients with DLB as approximately 7 years from diagnosis, which is longer than the observed median survival in our study of about 4.7 years for patients with DLB and 3.8 years for patients with PDD. A possible explanation for the difference is that the previous study defined disease duration as from cognitive-symptom onset rather than from the date of the clinical diagnosis.
In additional analyses, we compared the underlying causes of death found among patients with synucleinopathies and referent participants as listed in their death certificates. Despite recognizing the limitations of death certificates, we found neurodegeneration to be the most common underlying cause of death in all clinically defined synucleinopathies, including patients with PD. Cardiovascular events were the second most frequent underlying cause of death. The frequencies of underlying causes of death across the different types of synucleinopathies appear similar. Interestingly, respiratory diseases, infection, cardiovascular disease, and neurodegenerative diseases are the most common causes of death among elderly people in the United States. Among referent participants, the most frequent cause of death was a cardiovascular event (25.5%), followed by cancer (18.1%). Our findings on causes of death are consistent with the growing evidence of possible inverse comorbidities. Among patients with neurodegenerative diseases, some studies have reported a lower than expected frequency of cancer. A proposed explanation is that neurodegeneration—in this case, synuclein-related neurodegeneration—may be associated with a reduced risk of cancer. However, other studies reported an increased risk of nonmelanoma cancers after the diagnosis of PD; and melanoma has also been associated with PD and with the long-term use of L-Dopa. Additional studies are needed to understand the relationship between cancer and neurodegenerative diseases. Notably, we observed a higher number of falls among the referent group who were not affected by synucleinopathies. While additional research is needed, a possible explanation is that the patients with synucleinopathies were more aware and more educated on the risk of falls and therefore more cautious and less likely to have major consequences of falls compared with referent participants.
Strengths and Limitations
Our study has several strengths. First, we included all incident cases of synucleinopathy from a defined population over a defined time window and age- and sex-matched referent participants who were derived from the same population and time window. Thus, the referral bias should be minimal. Most previous studies were hospital-based and more likely to recruit advanced or atypical cases. Additionally, other studies examining mortality among patients with synucleinopathies included prevalent cases, had smaller sample sizes, and included only some clinical subtypes. One study only included men. Second, previous studies primarily evaluated survival from the study enrollment date rather than from the date of the diagnosis, a design that may overestimate relative mortality. However, we performed analyses using the year of onset rather than date of the diagnosis and found no noticeable differences. Third, our investigation can be interpreted as a long-term, prognostic study of synucleinopathies. We followed up 68.6% of patients and 48.7% of referents through death. We obtained survival estimates from diagnosis, which is longer than in most previous studies (6.3 years for patients; 8.3 years for referent participants). Fifth, our study explored synucleinopathies as a group and also as separate types including PD, PDD, DLB, and MSA-p. Therefore, we established mortality risks for all 4 clinically presumed synucleinopathies. Finally, we studied a relatively stable population using a population-based records-linkage system that encompassed all medical facilities in Olmsted County, Minnesota. A movement disorders specialist adjudicated all of the patients at the time of abstraction to reduce diagnostic-criteria differences over time or among different specialists.
Our study also has limitations. First, assessing the precise chronology of symptoms, time of onset of clinical features, severity of parkinsonian symptoms, and treatment history from an historical review of medical records can be difficult. Second, cases of DLB presenting without parkinsonism were not identified by our methods. However, given that parkinsonism is present among most patients with DLB, especially after some years into the disease process, we most likely did not overlook a large number of patients. Third, our study population was relatively small, which limits the stability of estimates, particularly for less common diseases such as MSA-p.
Conclusions
We found that an increased mortality risk exists for all types of synucleinopathies compared with the general population. Our findings contribute important new evidence about the natural history and survival of people affected by synucleinopathies of various types. Our results may be helpful to guide clinicians counseling patients and caregivers.
References
- 1.Jellinger KA. Formation and development of Lewy pathology: a critical update. J Neurol. 2009;256(suppl 3):270-279. [DOI] [PubMed] [Google Scholar]
- 2.Savica R, Grossardt BR, Bower JH, Ahlskog JE, Rocca WA. Incidence and pathology of synucleinopathies and tauopathies related to parkinsonism. JAMA Neurol. 2013;70(7):859-866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Twelves D, Perkins KS, Counsell C. Systematic review of incidence studies of Parkinson’s disease. Mov Disord. 2003;18(1):19-31. [DOI] [PubMed] [Google Scholar]
- 4.de Lau LM, Breteler MM. Epidemiology of Parkinson’s disease. Lancet Neurol. 2006;5(6):525-535. [DOI] [PubMed] [Google Scholar]
- 5.de Lau LM, Giesbergen PC, de Rijk MC, Hofman A, Koudstaal PJ, Breteler MM. Incidence of parkinsonism and Parkinson disease in a general population: the Rotterdam Study. Neurology. 2004;63(7):1240-1244. [DOI] [PubMed] [Google Scholar]
- 6.Goldstein DS, Holmes C, Sharabi Y, Wu T. Survival in synucleinopathies: a prospective cohort study. Neurology. 2015;85(18):1554-1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Savica R, Grossardt BR, Bower JH, Boeve BF, Ahlskog JE, Rocca WA. Incidence of dementia with Lewy bodies and Parkinson disease dementia. JAMA Neurol. 2013;70(11):1396-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.St Sauver JL, Grossardt BR, Yawn BP, Melton LJ III, Rocca WA. Use of a medical records linkage system to enumerate a dynamic population over time: the Rochester epidemiology project. Am J Epidemiol. 2011;173(9):1059-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.St Sauver JL, Grossardt BR, Leibson CL, Yawn BP, Melton LJ III, Rocca WA. Generalizability of epidemiological findings and public health decisions: an illustration from the Rochester Epidemiology Project. Mayo Clin Proc. 2012;87(2):151-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.St Sauver JL, Grossardt BR, Yawn BP, et al. . Data resource profile: the Rochester Epidemiology Project (REP) medical records-linkage system. Int J Epidemiol. 2012;41(6):1614-1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rocca WA, Yawn BP, St Sauver JL, Grossardt BR, Melton LJ III. History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clin Proc. 2012;87(12):1202-1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Commission on Professional and Hospital Activities H-ICDA, Hospital Adaptation of ICDA. 2nd ed Ann Arbor, MI: Commission on Professional and Hospital Activities; 1973. [Google Scholar]
- 13.World Health Organization Manual of the International Classification of Diseases, Injuries, and Causes of Death, Based on the Recommendations of the Ninth Revision Conference, 1975, and Adopted by the Twenty-Ninth World Health Assembly World Health Organization: Switzerland, Geneva; 1977. [Google Scholar]
- 14.Collins SJ, Ahlskog JE, Parisi JE, Maraganore DM. Progressive supranuclear palsy: neuropathologically based diagnostic clinical criteria. J Neurol Neurosurg Psychiatry. 1995;58(2):167-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gilman S, Low PA, Quinn N, et al. . Consensus statement on the diagnosis of multiple system atrophy. J Neurol Sci. 1999;163(1):94-98. [DOI] [PubMed] [Google Scholar]
- 16.Bower JH, Maraganore DM, McDonnell SK, Rocca WA. Incidence and distribution of parkinsonism in Olmsted County, Minnesota, 1976-1990. Neurology. 1999;52(6):1214-1220. [DOI] [PubMed] [Google Scholar]
- 17.McKeith IG. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the Consortium on DLB International Workshop. J Alzheimers Dis. 2006;9(3)(suppl):417-423. [DOI] [PubMed] [Google Scholar]
- 18.Savica R, Grossardt BR, Bower JH, Ahlskog JE, Rocca WA. Risk factors for Parkinson’s disease may differ in men and women: an exploratory study. Horm Behav. 2013;63(2):308-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McKeith IG, Dickson DW, Lowe J, et al. ; Consortium on DLB . Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65(12):1863-1872. [DOI] [PubMed] [Google Scholar]
- 20.Gilman S, Wenning GK, Low PA, et al. . Second consensus statement on the diagnosis of multiple system atrophy. Neurology. 2008;71(9):670-676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elbaz A, Peterson BJ, Yang P, et al. . Nonfatal cancer preceding Parkinson’s disease: a case-control study. Epidemiology. 2002;13(2):157-164. [DOI] [PubMed] [Google Scholar]
- 22.Benedetti MD, Bower JH, Maraganore DM, et al. . Smoking, alcohol, and coffee consumption preceding Parkinson’s disease: a case-control study. Neurology. 2000;55(9):1350-1358. [DOI] [PubMed] [Google Scholar]
- 23.Therneau TM, Grambsch PM. Modeling Survival Data: Extending the Cox Model. New York, NY: Springer-Verlag; 2000. [Google Scholar]
- 24.Elbaz A, Bower JH, Peterson BJ, et al. . Survival study of Parkinson disease in Olmsted County, Minnesota. Arch Neurol. 2003;60(1):91-96. [DOI] [PubMed] [Google Scholar]
- 25.Driver JA, Kurth T, Buring JE, Gaziano JM, Logroscino G. Parkinson disease and risk of mortality: a prospective comorbidity-matched cohort study. Neurology. 2008;70(16, pt 2):1423-1430. [DOI] [PubMed] [Google Scholar]
- 26.Darweesh SK, Koudstaal PJ, Stricker BH, Hofman A, Ikram MA. Trends in the incidence of Parkinson disease in the general population: the Rotterdam study. Am J Epidemiol. 2016;183(11):1018-1026. [DOI] [PubMed] [Google Scholar]
- 27.Chen RC, Chang SF, Su CL, et al. . Prevalence, incidence, and mortality of PD: a door-to-door survey in Ilan county, Taiwan. Neurology. 2001;57(9):1679-1686. [DOI] [PubMed] [Google Scholar]
- 28.Jellinger KA, Wenning GK, Seppi K. Predictors of survival in dementia with lewy bodies and Parkinson dementia. Neurodegener Dis. 2007;4(6):428-430. [DOI] [PubMed] [Google Scholar]
- 29.Wenning GK, Geser F, Krismer F, et al. ; European Multiple System Atrophy Study Group . The natural history of multiple system atrophy: a prospective European cohort study. Lancet Neurol. 2013;12(3):264-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ben-Shlomo Y, Wenning GK, Tison F, Quinn NP. Survival of patients with pathologically proven multiple system atrophy: a meta-analysis. Neurology. 1997;48(2):384-393. [DOI] [PubMed] [Google Scholar]
- 31.Aarsland D, Ballard C, McKeith I, Perry RH, Larsen JP. Comparison of extrapyramidal signs in dementia with Lewy bodies and Parkinson’s disease. J Neuropsychiatry Clin Neurosci. 2001;13(3):374-379. [DOI] [PubMed] [Google Scholar]
- 32.Alves G, Müller B, Herlofson K, et al. ; Norwegian ParkWest study group . Incidence of Parkinson’s disease in Norway: the Norwegian ParkWest study. J Neurol Neurosurg Psychiatry. 2009;80(8):851-857. [DOI] [PubMed] [Google Scholar]
- 33.Breitve MH, Chwiszczuk LJ, Hynninen MJ, et al. . A systematic review of cognitive decline in dementia with Lewy bodies versus Alzheimer’s disease. Alzheimers Res Ther. 2014;6(5-8):53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Olichney JM, Galasko D, Salmon DP, et al. . Cognitive decline is faster in Lewy body variant than in Alzheimer’s disease. Neurology. 1998;51(2):351-357. [DOI] [PubMed] [Google Scholar]
- 35.Josephs KA, Ahlskog JE, Parisi JE, et al. . Rapidly progressive neurodegenerative dementias. Arch Neurol. 2009;66(2):201-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rongve A, Vossius C, Nore S, Testad I, Aarsland D. Time until nursing home admission in people with mild dementia: comparison of dementia with Lewy bodies and Alzheimer’s dementia. Int J Geriatr Psychiatry. 2014;29(4):392-398. [DOI] [PubMed] [Google Scholar]
- 37.Oesterhus R, Soennesyn H, Rongve A, Ballard C, Aarsland D, Vossius C. Long-term mortality in a cohort of home-dwelling elderly with mild Alzheimer’s disease and Lewy body dementia. Dement Geriatr Cogn Disord. 2014;38(3-4):161-169. [DOI] [PubMed] [Google Scholar]
- 38.Graff-Radford J, Lesnick TG, Boeve BF, et al. . Predicting survival in dementia with Lewy bodies with hippocampal volumetry. Mov Disord. 2016;31(7):989-994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sahyoun NR, et al. . Trends in Causes of Death Among the Elderly. Aging Trends; No. 1. Hyattsville, MD: National Center for Health Statistics; 2001. [DOI] [PubMed] [Google Scholar]
- 40.Tabarés-Seisdedos R, Rubenstein JL. Inverse cancer comorbidity: a serendipitous opportunity to gain insight into CNS disorders. Nat Rev Neurosci. 2013;14(4):293-304. [DOI] [PubMed] [Google Scholar]
- 41.Elbaz A, Peterson BJ, Bower JH, et al. . Risk of cancer after the diagnosis of Parkinson’s disease: a historical cohort study. Mov Disord. 2005;20(6):719-725. [DOI] [PubMed] [Google Scholar]
- 42.Huang P, Yang XD, Chen SD, Xiao Q. The association between Parkinson’s disease and melanoma: a systematic review and meta-analysis. Transl Neurodegener. 2015;4:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sackett DL. Bias in analytic research. J Chronic Dis. 1979;32(1-2):51-63. [DOI] [PubMed] [Google Scholar]
- 44.Wermuth L, Stenager EN, Stenager E, Boldsen J. Mortality in patients with Parkinson’s disease. Acta Neurol Scand. 1995;92(1):55-58. [DOI] [PubMed] [Google Scholar]
- 45.Morens DM, Grandinetti A, Davis JW, Ross GW, White LR, Reed D. Evidence against the operation of selective mortality in explaining the association between cigarette smoking and reduced occurrence of idiopathic Parkinson disease. Am J Epidemiol. 1996;144(4):400-404. [DOI] [PubMed] [Google Scholar]