SUMMARY
Although Zika virus (ZIKV)-induced congenital disease occurs more frequently during early stages of pregnancy, its basis remains undefined. Using established type I interferon (IFN)-deficient mouse models of ZIKV transmission in utero, we found that the placenta and fetus were more susceptible to ZIKV infection at earlier gestational stages. Whereas ZIKV infection at embryonic day 6 (E6) resulted in placental insufficiency and fetal demise, infections at midstage (E9) resulted in reduced cranial dimensions, and infection later in pregnancy (E12) caused no apparent fetal disease. In addition, we found that fetuses lacking type III IFN-λ signaling had increased ZIKV replication in the placenta and fetus when infected at E12, and reciprocally, treatment of pregnant mice with IFN-λ2 reduced ZIKV infection. IFN-λ treatment analogously diminished ZIKV infection in human midgestation fetal- and maternal-derived tissue explants. Our data establish a model of gestational stage-dependence of ZIKV pathogenesis and IFN-λ-mediated immunity at the maternal-fetal interface.
eTOC Blurb
Jagger et al report that severity of Zika virus (ZIKV)-induced adverse fetal outcomes correlates with placentation and gestational stage at the time of infection. IFN-λ signaling in maternal and fetal tissues restricted placental and fetal ZIKV infection. IFN-λ treatment limited ZIKV transmission in pregnant mice and in human mid-gestation tissues.
INTRODUCTION
Zika virus (ZIKV) is an emerging mosquito-transmitted flavivirus that is responsible for ongoing epidemics in the Western Hemisphere of severe disease in developing fetuses and immunocompromised adults (Lazear and Diamond, 2016). Although ZIKV in the Americas initially was recognized due to an increased incidence of congenital microcephaly in northeast Brazil, further analysis has broadened the spectrum of ZIKV-disease. Most symptomatic adults infected with ZIKV experience a self-limiting influenza-like illness consisting of fever, myalgia, arthralgia, conjunctivitis, and rash. However, a minority develop more serious clinical syndromes, including Guillain-Barré syndrome (GBS) and potentially fatal encephalitis (Cao-Lormeau et al., 2016). ZIKV infection in pregnancy is particularly concerning because of its capacity for trans-placental transmission to the fetus. Recent studies suggest that ~40% of symptomatic ZIKV infections during pregnancy results in abnormal gestational development, manifesting as congenital malformations, including microcephaly, ocular anomalies, and postnatal neurodevelopmental deficits (Brasil et al., 2016).
Observational data from human studies suggest that ZIKV-associated congenital microcephaly is most common when pregnant women are infected during the first and early second trimesters (Honein et al., 2017). This likely reflects the greater vulnerability of the fetal brain to infection and injury during these early stages of development (Coyne and Lazear, 2016). Beyond this, the placenta, which serves as a physical and immunological barrier, changes dramatically during gestation, particularly between the early (first trimester) and later (second and third trimester) stages of human pregnancy when the intervillous space becomes filled with maternal blood and the placental villi have fully developed (Arora et al., 2017). Isolated human fetal-derived chorionic villi or trophoblast cells obtained later during gestation support less ZIKV infection compared to many non-placental cell types, which likely reflects stage-dependent effects of trophoblast differentiation (Bayer et al., 2016; Corry et al., 2017; Sheridan et al., 2017; Weisblum et al., 2016). The reduced susceptibility to ZIKV infection at later stages of gestation could result from distinct innate immune profiles including production of type III interferon (IFN)-λ (Bayer et al., 2016) or differential expression of putative entry receptors (Tabata et al., 2016). With increasing evidence suggesting that gestational age modulates fetal outcomes in the context of ZIKV infection of humans during pregnancy, there is a need to define the underlying mechanisms of stage-dependent susceptibility and disease. Although several mouse models of ZIKV infection and disease in pregnancy have been described (Cugola et al., 2016; Li et al., 2016a; Miner et al., 2016; Yockey et al., 2016), only one reported isolated fetal microcephaly (Li et al., 2016), and it required direct injection of ZIKV into the fetal brain.
Here, we performed longitudinal infection experiments in pregnant dams with genetic or acquired deficiencies of type I IFN. Whereas the maternal tissues were equally susceptible to ZIKV infection at different gestational time points (embryonic days E6, E9, or E12), the placenta and fetus were more vulnerable to infection at E6 compared to later stages. We observed a relationship between gestational age, ZIKV infection, and fetal outcome: infection at E6 resulted in placental insufficiency and fetal demise at E11; infection at E9 resulted in placental and fetal morphologic abnormalities at E14 consistent with isolated microcephaly; and infection at E12 resulted in viable-appearing, normal-sized fetuses at E17 even though viral RNA was detected in fetal heads. In support of a proposed role for IFN-λ in restricting ZIKV infecton in pregnancy, we show that Ifnlr1−/− dams and fetuses lacking a functional IFN-λ receptor were more susceptible to ZIKV infection, especially at later gestational stages that follow complete placentation at E10. Consistent with this observation, treatment of pregnant dams with pegylated IFN-λ2 decreased ZIKV replication at late, but not early, gestational timepoints. Moreover, explanted human mid-gestation decidual and placental tissues including chorionic villi and fetal membranes responded to exogenous IFN-λ treatment, which inhibited ZIKV infection. Our experiments establish a model of fetal microcephaly in the context of trans-placental transmission in mice, suggest that gestational age and the state of placentation influence the levels and outcome of fetal ZIKV infection, and show that restriction of ZIKV infection and transmission is at least partially modulated by the actions of IFN-λ at the maternal-fetal interface.
RESULTS
ZIKV replication in the placenta is greatest during early gestation
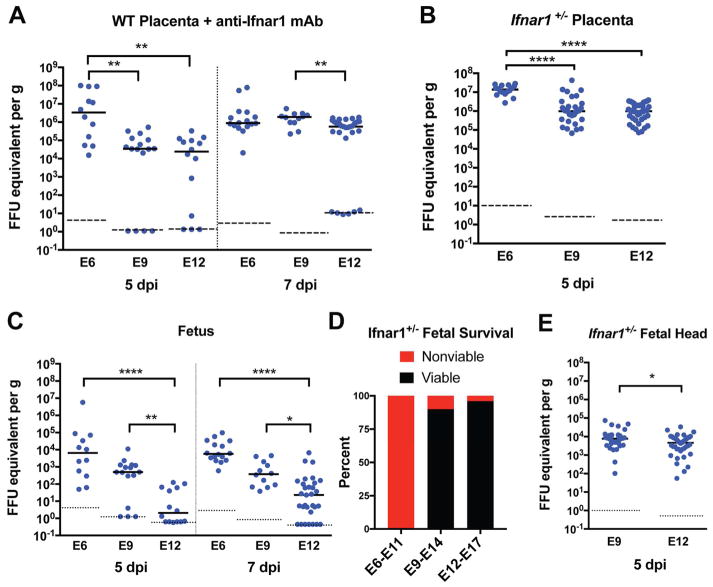
We recently established a trans-placental transmission model of fetal infection with ZIKV in mice deficient in type I IFN signaling (Miner et al., 2016). A deficiency of type I IFN signaling in the dam was required to allow sufficient viremia to seed the maternal decidua and fetal placenta after subcutaneous inoculation. To begin to assess how the timing of ZIKV infection affects pathogenesis at the maternal-fetal interface, we inoculated wild-type C57BL/6 pregnant dams with a Brazilian ZIKV strain (Paraíba, 2015) at E6, E9, or E12 after pre-treatment with a type I IFN receptor (Ifnar1) blocking antibody (2 mg, administered at day -1 relative to infection) and measured viral infection in the placenta 5 or 7 days later (Fig 1A); for reference, C57BL/6 mice have a gestation period of ~19.3 days (Murray et al., 2010). ZIKV replicated to higher titers at 5 days post-infection (98-fold, P < 0.005) in placentas from dams infected at E6 compared to those infected at E9; infection at E12 resulted in similar placental viral burden when compared to E9. Even when infected at E12, high levels of ZIKV RNA were present in the majority of placentas one week later (E19), which corresponds to term in mice; however, a subset (21%, 6 of 29) of placentas from E12-infected dams had ZIKV RNA levels at or below the limit of detection and did not appear to become infected (Fig 1A, right panel).
Figure 1. Timecourse of Placental and Fetal Susceptibility to ZIKV infection.
A, C. WT C57BL/6J pregnant dams were treated with 2 mg of anti-Ifnar1-blocking antibody (MAR1-5A3) on the day prior to infection and then subcutaneously inoculated on E6, E9, or E12 with 103 FFU of ZIKV-Brazil. ZIKV replication in placenta and whole fetuses (E6, 5 days post-infection (dpi)) or fetal heads (all other timepoints) was quantified by RT-qPCR 5 or 7 days after infection. B, E. Ifnar1−/− dams were mated with WT sires and inoculated with ZIKV-Brazil on E6, E9, or E12. Tissues were harvested 5 dpi for viral quantification by RT-qPCR. P values in A–C were calculated by Kruskal-Wallis test with Dunn’s multiple comparisons test: *, P < 0.05; **, P > 0.01; ***, P > 0.001; ****, P < 0.0001. Statistical significance in E was determined by an unpaired two-tailed Student’s t-test: *, P < 0.05. Dots indicate data from individual fetuses or placentas, and bars and dashed lines indicate median values and limits of detection, respectively. D. Ifnar1+/− fetal viability at the time of harvest was assessed 5 dpi at the timepoints shown (0% for E6–E11, 90% for E9–E14, and 96% for E12–E17, P < 0.0001 by the Chi-Square test; n = 44 fetuses for E6–E11; n = 70 for E9–E14; and n = 68 for E12–E17). Data in this figure is pooled from at least three independent experiments. See also Figure S1.
To corroborate these findings, we used a second established model of ZIKV-induced maternal-fetal disease in which Ifnar1−/− dams were mated with WT sires so that fetal-derived placentas were heterozygous for Ifnar1 expression (Miner et al., 2016). Pregnant Ifnar1−/− dams were inoculated with ZIKV at E6, E9, or E12, and placentas were analyzed five days later, at E11, E14, and E17, respectively (Fig 1B). Ifnar1+/− placentas also sustained higher levels of ZIKV replication when dams were infected at E6 compared to E9 or E12 (10-fold for E6 versus E9, P < 0.0001; 65-fold for E6 versus E12, P < 0.0001). These findings suggest that although the gestational stage of the fetus impacts the extent of ZIKV replication in the placenta, with highest levels observed at earlier stages, vertical transmission and sustained ZIKV replication in the placenta can occur throughout pregnacy. Additionally, the integrity of the type I IFN signaling pathway in the mother does not appear to be a key determinant of this time-dependent phenotype.
Because differential infection of the placenta at different gestational times could reflect variation in maternal infection, we assessed the levels of ZIKV in tissues of the pregnant dams five days after infection using the Ifnar1−/− pregancy model. Maternal viremia or tissue viral burden in the spleen and brain were not substantially different 5 days after inoculation at E6, E9, or E12 (Fig S1A). Similarly, there were no consistent differences in maternal viral burden at five or seven days post-infection at E6, E9, or E12 in anti-Ifnar1-treated, WT dams (Fig S1B). Together, these data suggest that gestation stage-dependent factors in the maternal decidua and/or fetal-derived placenta are responsible for the observed virological phenotypes rather than differences in systemic infection of the pregnant dam.
ZIKV replication in the fetus declines with advancing gestational age
We next assessed whether the higher levels of ZIKV replication in placentas observed earlier in gestation correlated with infection trends in the fetus. Fetal ZIKV infection was assessed five or seven days after inoculation of Ifnar1-blocking antibody-treated WT pregnant dams at E6, E9, or E12. Due to the small size of the fetus at E11, ZIKV RNA from whole fetuses were quantified for this timepoint, whereas for the remaining timepoints, fetal heads were dissected for viral RNA extraction and quantification. Consistent with the placental data, ZIKV RNA levels were higher after inoculation at pre-placentation (E6) versus post-placentation (E12) time points when assayed at 5 (~3,000-fold, P < 0.0001) or 7 days (250-fold higher, P < 0.0001) after infection (Fig 1C). Infection at E9 resulted in intermediate levels of viral infection at both timepoints. When the corresponding experiment was performed in Ifnar1−/− pregnant dams mated to WT sires, the clinical phenotype was more pronounced; ZIKV inoculation at E6 uniformly resulted in fetal demise (Fig 1D), as seen previously (Miner et al., 2016; Yockey et al., 2016). A reduced frequency of fetal demise was observed when ZIKV inoculation was performed at E9 (P < 0.0001), and no additional fetal demise occurred with inoculation at E12 beyond that seen in WT uninfected mice. Although viral quantification could not be performed after infection at E6 due to fetal resorption, ZIKV RNA levels in the fetal head 5 days after inoculation at E9 were slightly higher (2-fold, P < 0.05) than corresponding ones from E12 inoculated dams (Fig 1E). Thus, in mice, earlier gestational age at the time of ZIKV infection results in higher levels of viral replication in the placenta (Fig 1A–B), which faciliates more viral replication in the fetus and severe clinical outcomes.
Ultrasound analysis of ZIKV-infected pregnant dams reveals gestational age-dependent placental and fetal pathology
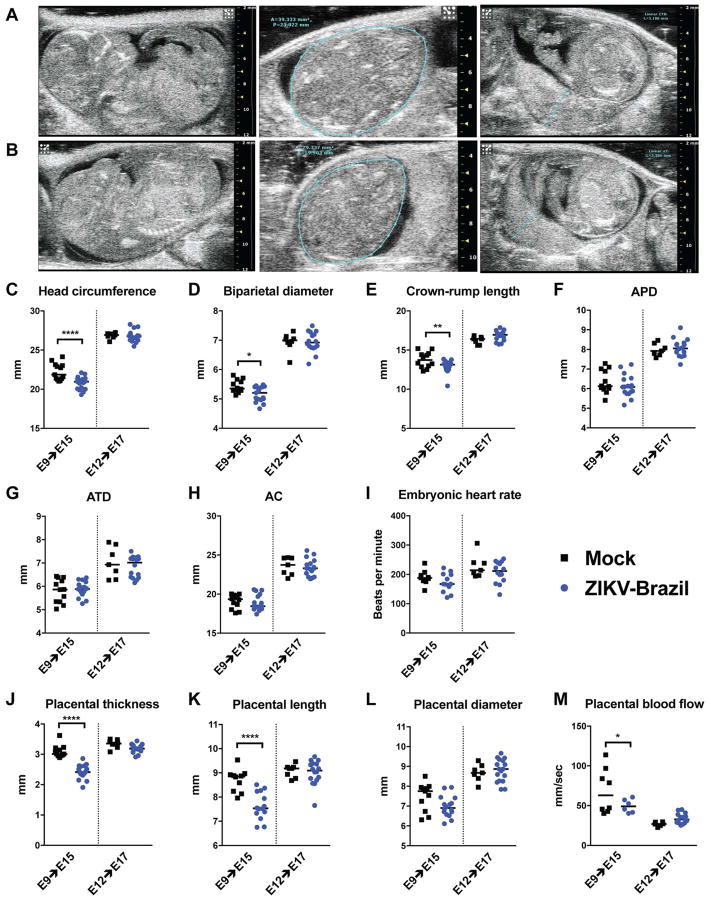
Several fetal and neonatal pathological sequelae of ZIKV infection have been reported in humans, including microcephaly, intrauterine growth retardation, defects in neuronal development of hearing and vision, and cerebral cortical thinning (de Paula Freitas et al., 2016; Driggers et al., 2016; Honein et al., 2017; Leal et al., 2016; Martines et al., 2016). To gain insight into the basis for these phenotypes, we examined Ifnar1+/− fetuses by ultrasound analysis after Ifnar1−/− pregnant dams were inoculated with ZIKV-Brazil at E6, E9 or E12, in comparison to mock (PBS)-infected dams (Fig 2, Movie S1 and Movie S2). This diagnostic modality allows for a detailed characterization of the maternal-fetal interface and permits accurate measurements of fetal and placental sizes in situ (Mu et al., 2008).
Figure 2. Ultrasound assessment of fetuses from mock- and ZIKV-infected pregnant mice.
Ifnar1−/− dams were mated with WT sires and then inoculated with PBS (row A) or 103 FFU of ZIKV-Brazil (row B) on E9. Representative ultrasound images taken on E15 including midsagittal sections (left panels), transverse sections at the level of the lateral ventricles with teal-colored outline of head circumference (middle panels), and placental sections at insertion of the umbilical cord with teal-colored outline showing placental thickness (right panels). C–M. Ifnar1−/− dams mated to WT sires and inoculated with ZIKV-Brazil or PBS (mock-infected) at E9 or E12 were evaluated by ultrasound on E15 or 17, respectively. APD = anteroposterior abdominal diameter; ATD = abdominal transverse diameter; AC = abdominal circumference. P values were determined using a one-way ANOVA: *, P < 0.05; **, P < 0.01; ****, P < 0.0001. Bars indicate median values. Data is pooled from independent experiments with placentas and fetuses from 2 mock-infected and 4 ZIKV-infected dams at E9–E15 and 2 mock-infected and 3 ZIKV-infected dams at E12–E17. See also Movie S1.
Ultrasound images were captured and feto-placental dimensions recorded from multiple Ifnar1+/− fetuses in ZIKV- and mock-infected Ifnar1−/− pregnant dams (Fig 2). As noted previously (Miner et al., 2016), infection at E6 in this model resulted in universal fetal demise by E11. In contrast, fetuses from dams inoculated at E9 and interrogated at E15 were viable, but exhibited impaired head growth as measured by diminished head circumference (0.9 mm reduction, P < 0.0001), bi-parietal diameter (0.15 mm reduction, P < 0.05), and crown-rump length (0.61 mm reduction, P < 0.01; Fig 2C–E) as compared to mock-infected controls. However, abdominal measurements in Ifnar1+/− fetuses inoculated with ZIKV at E9 and measured at E15 were unaffected; these included the anteroposterior abdominal diameter (APD), abdominal transverse diameter (ATD), and abdominal circumference (AC) (Fig 2F–H). Embryonic heart rate similarly was not affected by ZIKV infection at this timepoint (Fig 2I). In contrast, placental thickness and length were reduced (by 0.6 and 1.3 mm, respectively; P < 0.0001) in fetuses from dams inoculated with ZIKV at E9 and evaluated at E15 compared to mock-infected animals; placental diameter, however, was unaffected (Fig 2J–L). Consistent with the observed placental abnormalities, peak flow velocity in chorionic villus arterioles, a surrogate marker for placental blood flow, was slightly reduced in dams infected with ZIKV at E9 as compared to mock-infected dams (Fig 2M); this phenotype could be due to damage to the placental vasculature caused by ZIKV infection (Miner et al., 2016). In comparison, none of these ultrasound-measured differences were apparent in fetuses or placentas from Ifnar1−/− dams inoculated at E12 and measured at E17 compared to gestational age-matched, mock-infected animals. These ultrasound findings support the virological analysis showing decreasing susceptibility of the placenta and fetus to ZIKV replication with infection occurring at advanced gestational age. Moreover, these data establish divergent disease phenotypes (fetal demise, fetal microcephaly, and no apparent disease) depending on the stage of gestation at which the pregnant dam is infected.
IFN-λ contributes to an antiviral state following placentation in mice
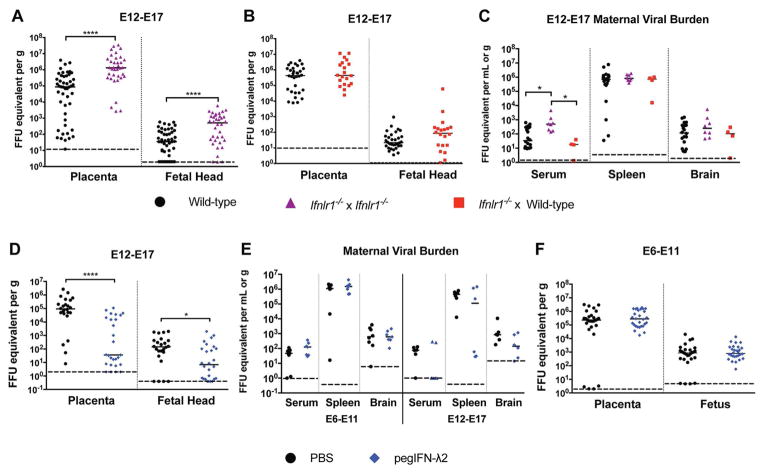
IFN-λ has been implicated in the protection of the human placenta from ZIKV infection through its constitutive release from full-term human trophoblasts (Bayer et al., 2016). In addition, maternal decidua isolated from early and mid-gestation human pregnancy induce IFN-λ in response to ZIKV infection (Weisblum et al., 2016). Given these studies, we examined the contribution of IFN-λ to ZIKV protection in pregnant mice. We mated mice genetically deficient in one (Ifnlr1) of the subunits of the IFN-λ receptor (Ifnlr1−/− ♂ x Ifnlr1−/− ♀) and inoculated pregnant dams on E12 with ZIKV-Brazil, one day after treatment with Ifnar1-blocking antibody. Placental and fetal tissues were harvested 5 days later (E17) for viral RNA quantification. ZIKV infection was greater in Ifnlr1−/− placentas (~15-fold, P < 0.0001) and fetal heads (14-fold, P < 0.0001) than in control WT mice (Fig 3A). In contrast, when Ifnlr1−/− females were mated with WT males (decidua is Ifnlr1−/− but placenta and fetus are Ifnlr1+/−), ZIKV replication was not different than in WT placentas and fetal heads (Fig 3B); this data suggests that feto-placental unit has to be fully deficient in IFN-λ signaling to be affected. Of note, we observed an increase in the amount of ZIKV circulating in maternal serum in Ifnlr1−/− x Ifnlr1−/− pregnant dams at E17 (14-fold, P < 0.05) (Fig 3C), whereas viral RNA levels in maternal serum from Ifnlr1−/− x WT matings at the same timepoint were not different from WT mice (P > 0.8). This suggests that the increase in viremia observed in Ifnlr1−/− x Ifnlr1−/− dams is not due to the loss of IFN-λ signalling within maternal tissues, but rather may reflect reverse spread of virus from the more susceptible placenta, as has been postulated to explain persistent maternal viremia in humans (Suy et al., 2016). In comparison, there were no differences in ZIKV burden in the maternal spleen and brain derived from Ifnlr1−/− x Ifnlr1−/−, Ifnlr1−/− x WT, and WT x WT matings at E12–17 (Fig 3C).
Figure 3. ZIKV replication in Ifnlr1−/− and IFN-λ-treated mice.
A–C. WT or Ifnlr1−/− female C57BL6J mice were mated with WT or Ifnlr1−/− male C57BL6J mice as indicated, treated with anti-Ifnar1-blocking antibody at E11, and inoculated subcutaneously with 103 FFU of ZIKV-Brazil on E12. Fetal, placental, and maternal tissues were collected 5 days later and virus quantified by RT-qPCR. A. ****, P < 0.0001 by the Mann-Whitney test. C. *, P < 0.05 by Kruskal-Wallis test with Dunn’s multiple comparisons. D–F. WT C57BL/6J mice were mated, treated with anti-Ifnar1-blocking antibody on the day before inoculation with ZIKV-Brazil (E6 or E12 as indicated) and treated with PBS or pegIFNλ-2 at the time of infection and every 48 h thereafter. Fetal, placental, and maternal tissues were harvested 5 days after infection and virus quantified by RT-qPCR. D. *, P < 0.05; ****, P < 0.0001 by the Mann-Whitney test. Bars and dashed lines indicate median values and limits of detection, respectively. Data in this figure is pooled from three independent experiments.
To corroborate these findings, we evaluated whether systemic administration of exogenous IFN-λ could inhibt ZIKV infection in the placenta. WT female and male mice were mated, and Ifnar1-blocking antibody was administered on E11 followed by ZIKV inoculation on E12. Pegylated mouse IFN-λ2 (pegIFN-λ2) or PBS was administered intravenously at the time of virus inoculation and at days +2 and +4, with tissue harvests occurring five days after infection. Treatment with pegIFN-λ2 reduced ZIKV infection in the placenta (2,500-fold median reduction, P < 0.0001) and fetal head (20-fold median reduction, P < 0.05) (Fig 3D), even though viral RNA levels in maternal tissues were not appreciably altered (Fig 3E, right panel). Given our findings showing gestational stage as a key determinant of ZIKV replication in fetal and placental tissues, we hypothesized that IFN-λ might contribute to ZIKV restriction in a stage-dependent manner. To assess this, anti-Ifnar1-treated WT pregnant dams were inoculated with ZIKV and treated with pegIFN-λ2 at E6, followed by harvest at E11. In this case, pegIFN-λ2 treatment at E6 did not reduce ZIKV infection in the placenta or fetal head (Fig 3F), nor did it influence ZIKV replication in maternal tissues (Fig 3E, left panel). As placentation in mice is completed at ~E10.5 (Mu et al., 2008), the lack of antiviral effect of pegIFN-λ2 when administered beginning at E6 likely reflects an incompletely formed placental barrier and lower expression of IFN-responsive genes, as observed in human trophoblasts derived from the earliest stages of human pregnancy (Sheridan et al., 2017).
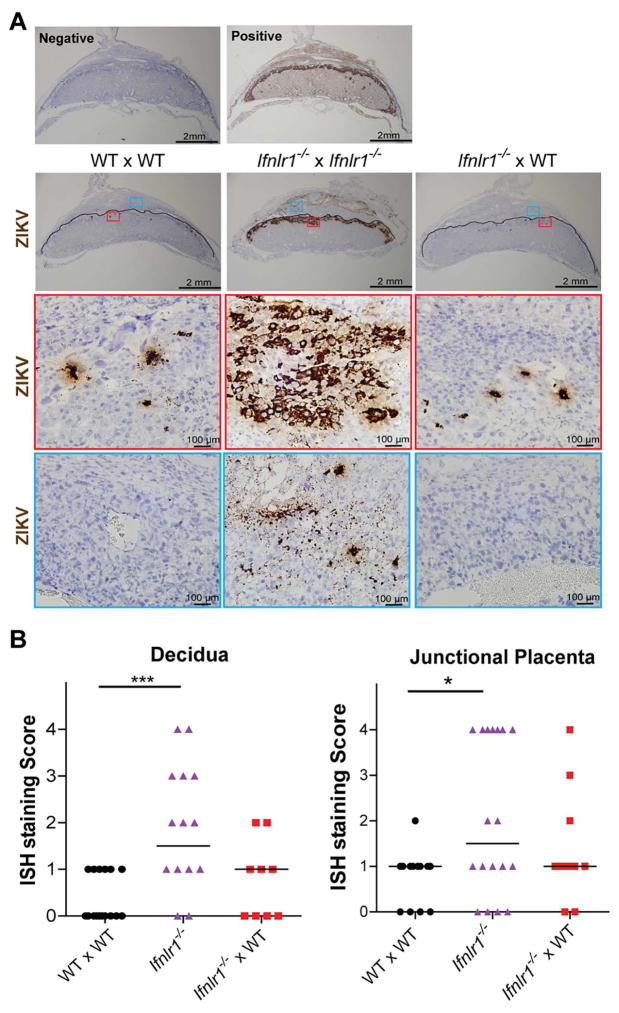
IFN-λ restricts ZIKV replication in the junctional zone of the placenta and the maternal decidua
To further define the antiviral properties of IFN-λ on ZIKV at a late gestational stage, the placentas from anti-Ifnar1 mAb-treated Ifnlr1−/− x Ifnlr1−/−, Ifnlr1−/− x WT, and WT x WT dams inoculated at E12 and harvested at E17 were analyzed by in situ hybridization for ZIKV RNA (Fig 4). Consistent with previous studies, isolated, scattered ZIKV RNA-positive cells were observed in anti-Ifnar1 mAb-treated WT x WT placentas, predominantly localizing to the junctional zone. A similar pattern was observed in the placentas from Ifnlr1−/− x WT dams, although in two specimens, slightly higher levels of decidual and junctional zone staining was seen (Fig S2). These results contrasted with the placentas from Ifnlr1−/− x Ifnlr1−/− dams, which exhibited more extensive ZIKV RNA staining, both in the maternal decidua, and especially in the junctional zone of the placenta. Indeed, in many placentas from Ifnlr1−/− x Ifnlr1−/− dams, ZIKV RNA was present in a contiguous band across the junctional zone, from the decidua to the labyrinth (Fig 4A). This pattern of ZIKV infection was never seen in any of the placentas from Ifnlr1−/− x WT or WT x WT dams. When the degree of ZIKV RNA staining was assessed using semi-quantitative scoring strategy (see Methods), placentas from Ifnlr1−/− x Ifnlr1−/− dams exhibited greater ZIKV RNA staining in the decidua (P < 0.001) and junctional zone (P < 0.05) compared to the others, whereas those from Ifnlr1−/− x WT dams did not differ significantly from WT x WT dams (Fig 4B). At E17, little ZIKV RNA was detected in the labyrinth zone of the placenta in any of the genotypes tested, as judged by ISH.
Figure 4. In situ hybridization of placentas from ZIKV-infected Ifnlr1−/− and pegIFN-λ2-treated mice.
A. Representative ISH staining. Top panels. ISH staining with negative (bacterial gene dapB) and positive (mouse Ppib gene) probe controls. Middle panels. Images showing ZIKV RNA localization in placental and decidual tissues from dams after matings (dam and sire genoypes are listed in order, respectively). Low and high power images are presented in vertical sequence. In low power images, solid black lines outline the boundary between maternal decidua and fetal placenta. Images of decidua and placenta within the red and blue frames (top boxes) are shown at higher magnification (two lower boxes). The images are representative of several sections from independent experiments. B. Grading of ISH staining intensity. ISH staining intensities were assessed in a blinded manner on a scale of 0 to 4, and data analyzed by Kruskal-Wallis with Dunn’s post-test: *, P < 0.05; ***, P < 0.001. See also Figure S2.
IFN-λ inhibits ZIKV infection in midgestation explanted human maternal and fetal tissue
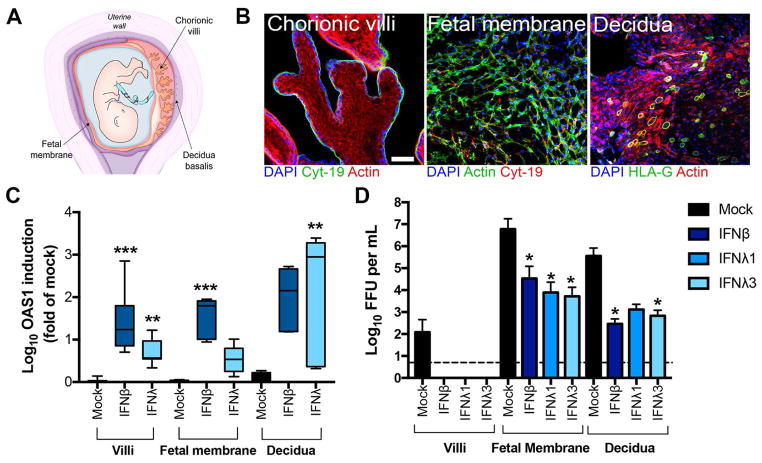
To establish the relevance of our findings, we evaluated the significance of IFN-λ signaling on ZIKV infection in explant models of maternal and fetal tissues derived from discarded, healthy second trimester human samples. We isolated chorionic villi, fetal amniotic and chorionic membranes, and maternal decidua basalis from mid-gestation human pregnancies (~19 to 23 weeks) (scheme, Fig 5A). Isolated chorionic villi retain the morphology of human placental villi in vivo, including a continuous syncytiotrophoblast layer covering the villi surfaces that express epithelial-specific cytokeratin-19 (Fig 5B, left panel). The fetal membrane is a multilayered avascular tissue composed of cytokeratin-19+ trophoblasts and cytokeratin-19− fibroblasts and mesenchymal cells (Fig 5B, middle panel). The maternal decidua basalis was confirmed by the staining for presence of HLA-G+ extravillous trophoblasts (EVTs), which invade the decidua from the anchoring villi (Fig 5B, right panel). We also confirmed the expression of tissue type-specific markers: human placental lactogen, which is expressed exclusively in the syncytiotrophoblast layer, was abundant in isolated chorionic villi, and the decidua-associated transcripts prolactin and IGFBP1, were expressed in isolated decidua but not appreciably in villi or fetal membranes (Fig S3). Isolated second trimester chorionic villi, fetal membranes, and decidua responded to treatment with recombinant IFN-λ, as judged by induction of the IFN-stimulated genes OAS1 or IFI44L (Fig 5C and Fig S4). Notably, treatment of the decidua and fetal membranes with IFN-λ1 and IFN-λ3 (100 ng/ml) inhibited ZIKV infection in a manner comparable to recombinant type I IFN-β treatment (100 ng/ml) (Fig 5D). IFN-λ also appeared to inhibit ZIKV infection in the chorionic villi, although only two preparations (of seven total) supported detectable replication. Overall, these data support the hypothesis that IFN-λ exerts antiviral effects against ZIKV in human tissues and cell types at the maternal-fetal interface that are relevant to vertical transmission.
Figure 5. IFN-λ protects human mid-gestation maternal and fetal explant tissues from ZIKV infection.
A. Schematic of the intrauterine compartment during human pregnancy denoting chorionic villi, fetal membranes (amnion and chorion), and decidua basalis. B. Confocal micrographs of human chorionic villi, fetal membrane, or decidua stained for cytokeratin-19 (green, left panel; red, middle panel), HLA-G (green, right panel), or β-actin (red, left of right panels; green, middle panel). DAPI-stained nuclei are shown in blue. Scale bar, 50 μm. C. IFN-λ induction of OAS1 in midgestation chorionic villi (**, P < 0.01; n = 6), fetal membranes (P = 0.07; n = 6), or decidua (**, P < 0.01; n = 3) after treatment with 100 ng/mL of IFN-λ (λ1 or λ3) for 16 h as assessed by RT-qPCR. Experiments with recombinant IFN-β (100 ng/ml) were performed in parallel (***, P < 0.001). Data are normalized to mock-treated controls and are shown as mean ± standard deviation. D. Infectious ZIKV-Brazil titers from midgestation chorionic villi (n = 7), fetal membranes (n = 6), or decidua (n = 3) preparations pre-treated with medium (Mock) or 100 ng/ml of IFN-β, IFN-λ1, or IFN-λ3 overnight (~16 h) and then infected with ZIKV-Brazil for 72 h. Data are shown as mean ± standard deviations. Statistical analyses in C and D were peformed using a Kruskal-Wallis ANOVA (*, P < 0.05). See also Figures S3, S4, and S5.
DISCUSSION
Our experiments defined the effect of gestational stage on fetal outcome of ZIKV infection in pregnant mice, demonstrated the utility of ultrasound analysis in measuring impacts on placental and fetal development in mice, and established an important role for IFN-λ signaling in the control of ZIKV infection in utero. In the background of a deficiency of type I IFN signaling, whch was required to allow dissemination to the placenta (Miner et al., 2016; Yockey et al., 2016), ZIKV infection at an early stage (E6) resulted in fetal demise, infection at mid-stages of development (E9) caused placental abnormalities and smaller cranial dimensions, and infection at a later stage (E12) resulted in no gross anatomic abnormalities compared to mock-infected animals. This pathological progression was mirrored by the virological analysis, which showed higher viral burdens in the placenta and fetus at early time points. Our resuts are consistent with observational studies in ZIKV-infected pregnant women, in which most (Honein et al., 2017; Kleber de Oliveira et al., 2016; Shapiro-Mendoza et al., 2017) but not all (Brasil et al., 2016) reports have shown a greater risk of fetal anomalies when infection occurs in the first or early second trimester. These finding also are consistent with data from other TORCH pathogens (e.g., toxoplasma, rubella, cytomegalovirus, and herpes simplex virus), where the risk of severe congenital malformations and fetal demise is greatest in early stages of human pregnancy (Arora et al., 2017; Coyne and Lazear, 2016). Although microcephaly is a dramatic manifestation of fetal ZIKV infection, the spectrum of adverse outcomes in fetuses and infants attributable to ZIKV continues to grow (van der Linden et al., 2016).
The mechanisms underlying the gestational-stage dependent variation in fetal injury have not been fully elucidated. Beyond the inherent greater vulnerability of the fetal brain to infection and injury during early stages of development (Coyne and Lazear, 2016), the maturation of the placental barrier over time may independently restrict fetal infection and mitigate outcome. This is particularly true after the first trimester of human pregnancy, when the placenta undergoes dramatic morphologic changes, the most notable of which is the establishment of a hemochorial placenta (i.e., fetal-derived placenta in direct contact with maternal blood). The detection of ZIKV RNA in placental trophoblasts (most commonly cytotrophoblasts), Hofbauer fetal-derived macrophages, and fetal endothelial cells (Jurado et al., 2016; Miner et al., 2016; Quicke et al., 2016; Richard et al., 2017; Simoni et al., 2017; Tabata et al., 2016) is strongly suggestive of a transplacental route of transmission. Given that fetal-derived syncytiotrophoblasts form a key cellular barrier to the hematogenous spread of ZIKV at a variety of gestational stages, ZIKV likely has evolved a mechanism to bypass the placental villous barrier for vertical transmission. Indeed, the detection of ZIKV RNA in Hofbauer macrophage cells, with a corresponding lack of detection in syncytiotrophoblasts, in human cases of ZIKV-induced fetal loss highlights the lack of susceptibility of these cells to infection in vivo (Ritter et al., 2017). Routes of ZIKV vertical transmission could include infection of permissive extravillous trophoblasts (Tabata et al., 2016) and/or the maternal decidua, which would allow ZIKV to circumvent many of the most robust placental barrier functions, including those presented by syncytiotrophoblasts. In addition, primary human umbilical vein endothelial cells (HUVEC), uterine microvascular endothelial cells (hUtMEC), and primary full-term placental fibroblasts all are non-responsive to IFN-λ ((Fig S5) and (Corry et al., 2017)); cells that are non-responsive to IFN-λ could provide portals to facilitate ZIKV transplacental transmission.
Fetal outcomes correlated with the timing of infection and the relative level of ZIKV infection in the placenta and fetal head. The placenta in the Ifnar1−/− dam x WT sire pregnancy model is heterozygous for Ifnar1 gene expression and likely functionally competent for IFN signaling. Nonetheless, stage-dependent developmental changes (e.g., at the epigenetic or transcriptional level) could modulate the effectiveness of this signaling response. A recent report showed that third trimester primary human syncytialized trophoblasts were resistant to ZIKV infection, and conditioned medium from these cells protected against ZIKV infection, a property ascribed to the actions of constitutively released IFN-λ (Bayer et al., 2016). Consistent with this observation, first trimester syncytialized trophoblasts appear resistant to ZIKV infection (Bhatnagar et al., 2017; Ritter et al., 2017), and the syncytium in second trimester human explants constitutively release IFN-λ and do not support ZIKV infection (Corry et al., 2017). In comparison, first- and second-trimester cytotrophoblasts in cell culture are susceptible to ZIKV infection (El Costa et al., 2016; Weisblum et al., 2016). These cells, however, were not evaluated for their ability to produce or respond to IFN-λ. We found that at later stages in pregnancy in the setting of deficient type I IFN signaling, IFN-λ inhibited ZIKV replication in the placenta, which correlated with lower viral burden in the fetal head. Experiments comparing ZIKV RNA levels in placentas derived from Ifnlr1−/− ♂ x Ifnlr1−/− ♀ and Ifnlr1−/− ♂ x WT ♀ matings suggest that the effect of IFN-λ on maternal-fetal infection occurs primarily in the fetal placenta rather than the maternal decidua. Furthermore, the relative responsiveness of the late gestation placenta to IFN-λ treatment, as contrasted to the lack of inhibitory effect in early gestation, suggests that IFN-λ may contribute to the stage-dependent susceptibility of the placenta and fetus to ZIKV infection observed here and described elsewhere (Honein et al., 2017; Shapiro-Mendoza et al., 2017). However, RNASeq-based transcpritonal profiling of mid- and late-gestation human placental explants or cells did not identify substantive differences in mRNA expression of the type I (IFNAR) or III (IFNLR1 and IL10R-β) IFN receptors with increasing gestational age that would impact the responsiveness of the human placenta to IFNs during gestation (Corry et al., 2017). Although treatment of WT dams with pegIFN-λ2 comfirmed an inhibitory effect aganst ZIKV infection in the placenta at later gestational times, at present, it remains uncertain if its actions were on the maternal decidua or the adjacent junctional layer of the placenta. Further studies also are needed to determine the magnitude of the antiviral effect IFN-λ in the setting of an intact type I IFN response at the maternal-fetal interface. Additionally, IFN-λ treatment experiments of pregnant dams derived from matings of conditional Ifnlr1 or Ifnar1 deletions (e.g., LysM Cre+ Ifnar1fl/fl mice (Tang et al., 2016)) may help identify the key IFN-responding cell types (e.g., trophoblasts, endothelial cells, and/or Hofbauer macrophages) in the placenta. Finally, despite similar ZIKV RNA levels in the placenta seven days after infection at E6, E9, or E13, we observed different clinical outcomes ranging from fetal demise to complete viability; thus, in addition to stage-dependent effects on transmission to the fetus and actions of IFN-λ, the placenta may be more vulnernable to ZIKV-induced cytopathic damage at earlier gestational stages.
The treatment studies with pegIFN-λ2 suggest a possible therapeutic option in humans for ZIKV infections once placentation has occurred and syncytialized trophoblasts are present, which occurs very early following conception. In support of this, mid-gestation human fetal-derived placental and maternal-derived decidua explant tissues were responsive to exogenous IFN-λ treatment, with induction of ISGs and inhibition of ZIKV replication. These experiments also suggest an antiviral role for IFN-λ in cells of the maternal-fetal interface with intact type I IFN responses, which could occur in the earlier stages of human pregnancy. As ZIKV can degrade human STAT2 but not mouse Stat2 (Grant et al., 2016; Kumar et al., 2016), and type I and III interferon both signal through this transcription factor, the mouse models could overestimate the impact of IFN-λ in humans. Notwithstanding this caveat, our experiments in primary placental explants suggest that IFN-λ may be relevant for restricting in utero transmission of ZIKV in humans, as the human placenta constutively produces IFN-λ at both mid- (Corry et al., 2017) and late- (Bayer et al., 2016) stages of gestation. Thus, type I and III IFN may act in parallel to enhance the antiviral capacity of the placental barrier against vertical transmission of ZIKV and other pathogens.
Our study demonstrated the utility of in vivo ultrasound of ZIKV-inoculated Ifnar−/− pregnant mice in modeling deficits in cranial size (i.e., microcephaly) after mid-gestational infection. Although a prior study produced microcephaly by direct injection of ZIKV into the fetal mouse brain (Li et al., 2016b), our model incorporates vertical transmission of ZIKV from mother to fetus. Another report described global intrauterine growth restriction, including proportionate reductions in body and head dimensions, after an extraordinarily high-dose (1011 PFU) intravenous inoculation of ZIKV in the relatively immunocompetent SJL mice (Cugola et al., 2016). Although the model demonstrated high levels of ZIKV RNA in the fetal brain and neuroanatomical abnormalities, it is limited by the absence of requirement for antecedent viral replication in peripheral organs or analysis of the placenta, which likely is the primary site for ZIKV transmission in utero. Our model of microcephaly after midgestation infection with a contemporary Brazilian strain of ZIKV may have utility for future studies of disease pathogenesis, vaccine efficacy, and therapeutic counter-measures. One caveat of our studies is that while fetal or infant microcephaly in humans is diagnosed when the head circumference is >2 (Leviton et al., 2002) or 3 (Kleber de Oliveira et al., 2016) standard deviations below the mean for age, analogous definitions for mice do not exist in part because of the disparity in neocortical mass between mice and humans (Sun and Hevner, 2014), as well as the significant differences in the duration of gestation. Thus, the utility of mice in modeling human congenital microcephaly requires further invesitgation.
Although there are important anatomical differences between human and mouse placentas (reviewed in (Rossant and Cross, 2001)), the trophoblast layers in both function as a physical and immunological barrier between the maternal and fetal circulation to prevent the hematogenous spread of microbial agents. Our experiments help define the consequences of ZIKV infection as a function of the gestational age of the fetus, and provide evidence across species for another important role for IFN-λ at barrier surfaces (Lazear et al., 2015b). While our findings are most directly applicable to the understanding of ZIKV congenital disease, and may provide the basis for promising immunomodulatory therapeutics against it, IFN-λ might modulate vertical transmission of other human pathogens, particularly viruses. Future clinical and laboratory studies are necessary to fully understand the potential of IFN-λ-directed treatments and define their maternal-fetal cellular targets, both in the setting of ZIKV infection, as well as the broader context of other intrauterine pathogens.
STAR Methods
Contact for reagent and resource sharing
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Michael S. Diamond (diamond@wusm.wustl.edu).
Experimental model and subject details
Mouse experiments
Ifnar1−/− mice (Hwang et al., 1995) were backcrossed (>99%) onto a C57BL/6J background, and bred in a specific-pathogen-free facility at Washington University. WT animals were purchased from Jackson Laboratories. Ifnlr1−/− mice (originally called IL28Rα−/−, obtained from Bristol-Myers Squibb) lack the entire Ifnlr1 coding sequence (Ank et al., 2008) and were backcrossed onto a C57BL/6J background (Lazear et al., 2015a). Timed matings were set up, and breeding females were checked daily with the day of vaginal plugging designated as E0. For infection studies with WT mice, animals were treated once with 2 mg of anti-Ifnar1 blocking mouse mAb (MAR1-5A3, Leinco Technologies) (Sheehan et al., 2006) by intraperitoneal injection on the day prior to infection. ZIKV (103 FFU) was inoculated subcutaneously in the footpad in 50 μl of PBS under anesthesia. Mice were sacrificed, and maternal and fetal tissues were harvested as previously described (Miner et al., 2016).
Animal studies were carried in accordance with the recommendations of the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health, and were approved by the Institutional Animal Care and Use Committee at the Washington University School of Medicine (Assurance number A3381-01). Mice were inoculated with ZIKV after induction of anesthesia using ketamine hydrochloride and xylazine, and all efforts were made to minimize pain and suffering.
Viruses
ZIKV-Brazil (strain Paraíba 2015) was provided by S. Whitehead (Bethesda, MD) (Tsetsarkin et al., 2016). ZIKV stocks were prepared by propagation in Vero cells and titration utilizing focus-forming assays (FFA) as previously described (Miner et al., 2016).
Cells
Vero cells were cultured at 37°C in Dulbecco’s Modi fied Eagle Medium (DMEM) supplemented with 10% fetal bovine serum (FBS). Vero cells were originally acquired from American Type Culture Collection and were tested and judged free of mycoplasma contamination.
Human placental explants
Human fetal placental tissue (<24 weeks gestation) was obtained from the University of Pittsburgh Health Sciences Tissue Bank through an honest broker system after approval from the University of Pittsburgh Institutional Review Board and in accordance with the University of Pittsburgh anatomical tissue procurement guidelines. Chorionic villi, fetal membrane, or decidua were dissected and cultured in DMEM/F12 (1:1) supplemented with 10% FBS, penicillin/streptomycin, and amphotericin B (1 μg/mL) for between 24 and 96 h.
Method details
Quantification of viral burden in vivo
Mouse tissues were weighed and homogenized using a MagNA Lyser (Roche LifeScience), and RNA was extracted using the RNeasy Mini Kit (tissues) or the Viral RNA Mini Kit (serum) (Qiagen). ZIKV RNA quantification was performed by one step qRT-PCR on an ABI 7500 Fast instrument, and RNA quantity expressed as viral RNA equivalents per gram (tissue) or ml (serum) after interpolation onto a standard curve of 10-fold dilutions of ZIKV RNA. The primer set has been previously published (Lanciotti et al., 2008; Lazear et al., 2016) (Integrated DNA Technologies).
Histology
Fetuses and placentas were removed by Caesarian section on the indicated days of gestation and immediately fixed for 24 to 30 h in 4% paraformaldehyde (PFA) in PBS followed by paraffin-embedding, sectioning, and mounting (Digestive Diseases Research Cores Center and Tissue Histology Core at Center for Reproductive Health Sciences, Washington University).
In situ viral RNA hybridization
RNA ISH was performed using RNAscope 2.5 HD (Brown) (Advanced Cell Diagnostics) according to the manufacturer’s instructions and as previously described (Govero et al., 2016). PFA-fixed, paraffin-embedded tissue sections were deparaffinized by incubating for 60 min at 60°C and endogenous peroxidases were quenched with H2O2 for 10 min at room temperature. Slides were then boiled for 15 min in RNAscope Target Retrieval Reagents and incubated for 30 min in RNAscope Protease Plus reagent prior to ZIKV RNA (Advanced Cell Diagnostics, catalog #467771), positive control Mus musculus Ppib gene (#313911), or negative control bacterial gene dapB (#310043) probe hybridization and signal amplification. Sections were counterstained with Gill’s hematoxylin and visualized by brightfield microscopy. Slides stained with ZIKV RNA ISH were scored in a blinded manner with a semi-quantitative score ranging from 0 to 4 based on the number of ZIKV positive cells per section. A score of “0” was assigned if no positive signal as compared to negative control slide; 1 for <10 positive cells; 2 for 10–25 positive cells; 3 for 26–50 positive cells; and 4 for >50 positive cells per slide.
Ultrasound analysis
Ultrasound imaging of the pregnant uterus was conducted according to a modification of previously described methods (Mu and Adamson, 2006). Briefly, non-invasive trans-abdominal ultrasound studies were performed using a Vevo2100 Ultrasound System (VisualSonics, Toronto, ON, Canada) equipped with a 30 MHz linear array transducer (MS400). Pregnant mice were lightly anesthetized with 1.5% gaseous isoflurane administered through a specialized nose cone. Hair was removed from the abdomen by chemical hair remover. Maternal heart rate and rectal temperature were monitored, and body temperature was maintained at 36–38°C with the use of a heating pad and lamp. A series of long- and short-axis 2-dimensional images, and color Doppler guided spectral Doppler tracings of 5–7 embryos per pregnant mouse were acquired digitally. To ensure stable hemodynamic conditions, imaging time was limited to less than 1 h. Image analysis was performed using off-line computer workstation equipped with Vevo2100 analysis software. Embryonic crown-rump length was quantified from longitudinal images as the maximum distance between the cephalic and caudal poles. Biparietal diameter and head circumference were measured from transverse sections at the level of the lateral ventricles. Abdominal circumference (AC) was calculated from the abdominal anteroposterior diameter (APD) and abdominal transverse diameter (ATD) measured from a transverse section of the fetal abdomen at the level of the liver just below the diaphragm, where AC = π (ATD + APD)/2. For placental measurements (placental diameter, placental length, placental thickness), orthogonal transverse images of the placenta were obtained at the insertion site of the umbilical cord. Doppler velocity waveforms were obtained in the umbilical artery in the umbilical cord near the placental surface, and in the embryonic intraplacental arteries of the labyrinth near the midpoint between the chorionic and basal plates. Doppler waveforms were obtained in the uterine artery near the lateral-inferior margin of the uterocervical junction close to the iliac artery. Peak systolic velocity and end-diastolic velocity were measured from three consecutive cardiac cycles that were not affected by motion, and the results were averaged.
Immunofluorescence microscopy
Explants were fixed in 4% paraformaldhehyde and permeabilized with 0.25% Triton X-100. Following washing in PBS, tissues were incubated with primary antibody diluted in PBS for 1 to 2 h at room temperature, washed in PBS, and then incubated with Alexa Fluor conjugated secondary antibodies for 30 to 60 min in PBS at room temperature. Alexa Fluor-conjugated phalloidin was purchased from Invitrogen (A12379 or A12381). Rabbit anti-cytokeratin 19 (ab52625) was purchased from Abcam. Mouse anti-HLA-G (sc-21799) was purchased from Santa Cruz Biotechnology. Tissue was mounted with VectaShield (Vector Laboratories) containing 4′,6-diamidino-2-phenylindole (DAPI) and images captured using an Olympus FV1000 laser scanning confocal microscope. Images were adjusted for brightness/contrast and/or pseudcolored using Adobe Photoshop (Adobe).
RNA Isolation and RT-qPCR in explant infections
Total RNA was isolated using a GenElute Total RNA Miniprep Kit (Sigma). Following isolation and treatment with deoxyribonuclease (Sigma), RNA was reverse transcribed using an iScript cDNA synthesis kit (BioRad) containing 0.1 to 0.5 μg of sample RNA per reaction. RT-qPCR was conducted using IQ SYBR Green Supermix (BioRad) in a BioRad CFX96 touch real time PCR detection system. A modified ΔCt method was used to calculate gene expression using human actin for normalization. Primer sequences for IFNλ1, IFNλ2, OAS1, hPL, actin, and ZIKV have been described (Bayer et al., 2016; McConkey et al., 2016). Additional primer sequences used are as follows: IGFBP1 TTTTATCACAGCAGACAGTG and AATATATCTGGCAGTTGGGG; PRL GGTTCATCCTGAAACCAAAG and CTTCAGGAGCTTGAGATAATTG.
Human placental explants
For infections, placental, decidual, or fetal membrane explants were infected immediately with 5 x 105 FFU/ml of ZIKV for 72 h following isolation and treatment (~16 h) with 100 ng/mL of recombinant IFN-β, IFN-λ1 or IFN-λ3 (R&D Systems; 1598-IL-025, 5259-IL-025, 8499-IF-010). For imaging, explants were fixed and imaging performed as detailed below. Explant infections were performed with ZIKV-Brazil propagated on Vero cells, and viral output was quantified by fluorescent focus assay as previously described (Payne et al., 2006) using an anti-double-stranded RNA monoclonal antibody provided by Abraham Brass (University of Massachusetts).
Primary endothelial cultures
HUVEC and hUtMEC were purchased from Lonza and cultured as described previously (Rausch et al., 2017). Cells were inoculated with ZIKV (Paraiba 2015; MOI of 3) for 48 h following a 16 h treatment with 100 ng/mL of recombinant IFN-β, IFN-λ1 or IFN-λ3 (R&D Systems). Viral titers were determined by fluorescent focus assays, as previously described (Payne et al., 2006).
Quantification and Statistical Analysis
Data were analyzed using GraphPad Prism software. Viral burden quantification analysis was analyzed by Kruskal-Wallis or one way ANOVA with multiple comparisons testing. Ultrasound dimensions were analyzed by one-way ANOVA. P values of < 0.05 were considered statistically significant.
Supplementary Material
Videos taken at E15.5 after infection at E9.5 showing cardiac motion in mock-infected (left panel) and ZIKV-Brazil infected (middle panel) fetuses, as well as fetal demise after ZIKV infection (right panel).
The same fetuses from Movie S1 were imaged with color Doppler to show umbilical artery flow (left and middle panels) or lack thereof (right panel). Data in this Figure is representative of three independent experiments.
HIGHLIGHTS.
ZIKV-induced fetal microcephaly in mice is gestation stage-dependent
Placentas lacking IFN-λ receptor expression sustain high levels of ZIKV infection
IFN-λ treatment of pregnant mice protects against ZIKV infection in utero
IFN-λ inhibits ZIKV in human mid-gestation fetal and maternal tissue explants
Acknowledgments
This work was supported by grants from the NIH (R01 AI073755, R01 AI104972, and U19 AI083019 to M.S.D; R01 HD091218 to I.U.M and M.S.D.; T32 AI007172 to B.W.J.; and R01 HD075665 to C.B.C) and the Burroughs Wellcome Investigators in the Pathogenesis of Infectious Disease Award (to C.B.C.). M.S.D. is a consultant for Inbios, Visterra, Aviana, and Sanofi-Pasteur, and is on the Scientific Advisory Boards of Moderna and OvaGene. We thank Jacqueline Corry (University of Pittsburgh) for technical support, Stephanie Worrell (Magee Women’s Hospital) for assistance with placental tissue pathology, and the UPCI Tissue and Research Pathology/Health Sciences Tissue Bank, which receives funding from P30CA047904, for fetal tissue.
Footnotes
AUTHOR CONTRIBUTIONS.
B.W.J., J.J.M., A.K., I.U.M., C.B.C. and M.S.D. designed the experiments. B.W.J., A.M.S., N.A., A.K., B.C., and J.J.M. performed the experiments. B.W.J., A.M.S., A.K., B.C., I.U.M, J.J.M., C.B.C., and M.S.D analyzed the data. B.W.J. and M.S.D. wrote the first draft of the paper. All authors participated in editing the final version of the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ank N, Iversen MB, Bartholdy C, Staeheli P, Hartmann R, Jensen UB, Dagnaes-Hansen F, Thomsen AR, Chen Z, Haugen H, et al. An important role for type III interferon (IFN-lambda/IL-28) in TLR-induced antiviral activity. J Immunol. 2008;180:2474–2485. doi: 10.4049/jimmunol.180.4.2474. [DOI] [PubMed] [Google Scholar]
- Arora N, Sadovsky Y, Dermody TS, Coyne CB. Microbial Vertical Transmission during Human Pregnancy. Cell Host Microbe. 2017;21:561–567. doi: 10.1016/j.chom.2017.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer A, Lennemann NJ, Ouyang Y, Bramley JC, Morosky S, Marques ET, Jr, Cherry S, Sadovsky Y, Coyne CB. Type III Interferons Produced by Human Placental Trophoblasts Confer Protection against Zika Virus Infection. Cell Host Microbe. 2016;19:705–712. doi: 10.1016/j.chom.2016.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatnagar J, Rabeneck DB, Martines RB, Reagan-Steiner S, Ermias Y, Estetter LB, Suzuki T, Ritter J, Keating MK, Hale G, et al. Zika Virus RNA Replication and Persistence in Brain and Placental Tissue. Emerg Infect Dis. 2017;23:405–414. doi: 10.3201/eid2303.161499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasil P, Pereira JP, Jr, Moreira ME, Ribeiro Nogueira RM, Damasceno L, Wakimoto M, Rabello RS, Valderramos SG, Halai UA, Salles TS, et al. Zika Virus Infection in Pregnant Women in Rio de Janeiro. N Engl J Med. 2016;375:2321–2334. doi: 10.1056/NEJMoa1602412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao-Lormeau VM, Blake A, Mons S, Lastere S, Roche C, Vanhomwegen J, Dub T, Baudouin L, Teissier A, Larre P, et al. Guillain-Barre Syndrome outbreak associated with Zika virus infection in French Polynesia: a case-control study. Lancet. 2016;387:1531–1539. doi: 10.1016/S0140-6736(16)00562-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corry J, Arora N, Good C, Sadovsky Y, Coyne CB. Organotypic models of type III interferon-mediated protection from Zika virus infections at the maternal-fetal interface. Proceedings of the National Academy of Sciences of the United States of America; 2017; 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyne CB, Lazear HM. Zika virus - reigniting the TORCH. Nat Rev Microbiol. 2016;14:707–715. doi: 10.1038/nrmicro.2016.125. [DOI] [PubMed] [Google Scholar]
- Cugola FR, Fernandes IR, Russo FB, Freitas BC, Dias JL, Guimaraes KP, Benazzato C, Almeida N, Pignatari GC, Romero S, et al. The Brazilian Zika virus strain causes birth defects in experimental models. Nature. 2016;534:267–271. doi: 10.1038/nature18296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Paula Freitas B, de Oliveira Dias JR, Prazeres J, Sacramento GA, Ko AI, Maia M, Belfort R., Jr Ocular Findings in Infants With Microcephaly Associated With Presumed Zika Virus Congenital Infection in Salvador, Brazil. JAMA ophthalmology. 2016 doi: 10.1001/jamaophthalmol.2016.0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driggers RW, Ho CY, Korhonen EM, Kuivanen S, Jaaskelainen AJ, Smura T, Rosenberg A, Hill DA, DeBiasi RL, Vezina G, et al. Zika Virus Infection with Prolonged Maternal Viremia and Fetal Brain Abnormalities. The New England journal of medicine. 2016;374:2142–2151. doi: 10.1056/NEJMoa1601824. [DOI] [PubMed] [Google Scholar]
- El Costa H, Gouilly J, Mansuy JM, Chen Q, Levy C, Cartron G, Veas F, Al-Daccak R, Izopet J, Jabrane-Ferrat N. ZIKA virus reveals broad tissue and cell tropism during the first trimester of pregnancy. Sci Rep. 2016;6:35296. doi: 10.1038/srep35296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govero J, Esakky P, Scheaffer SM, Fernandez E, Drury A, Platt DJ, Gorman MJ, Richner JM, Caine EA, Salazar V, et al. Zika virus infection damages the testes in mice. Nature. 2016;540:438–442. doi: 10.1038/nature20556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant A, Ponia SS, Tripathi S, Balasubramaniam V, Miorin L, Sourisseau M, Schwarz MC, Sanchez-Seco MP, Evans MJ, Best SM, et al. Zika Virus Targets Human STAT2 to Inhibit Type I Interferon Signaling. Cell Host Microbe. 2016;19:882–890. doi: 10.1016/j.chom.2016.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honein MA, Dawson AL, Petersen EE, Jones AM, Lee EH, Yazdy MM, Ahmad N, Macdonald J, Evert N, Bingham A, et al. Birth Defects Among Fetuses and Infants of US Women With Evidence of Possible Zika Virus Infection During Pregnancy. Jama. 2017;317:59–68. doi: 10.1001/jama.2016.19006. [DOI] [PubMed] [Google Scholar]
- Hwang SY, Hertzog PJ, Holland KA, Sumarsono SH, Tymms MJ, Hamilton JA, Whitty G, Bertoncello I, Kola I. A null mutation in the gene encoding a type I interferon receptor component eliminates antiproliferative and antiviral responses to interferons alpha and beta and alters macrophage responses. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:11284–11288. doi: 10.1073/pnas.92.24.11284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurado KA, Simoni MK, Tang Z, Uraki R, Hwang J, Householder S, Wu M, Lindenbach BD, Abrahams VM, Guller S, et al. Zika virus productively infects primary human placenta-specific macrophages. JCI Insight. 2016;1 doi: 10.1172/jci.insight.88461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleber de Oliveira W, Cortez-Escalante J, De Oliveira WT, do Carmo GM, Henriques CM, Coelho GE, Araujo de Franca GV. Increase in Reported Prevalence of Microcephaly in Infants Born to Women Living in Areas with Confirmed Zika Virus Transmission During the First Trimester of Pregnancy - Brazil, 2015. MMWR Morbidity and mortality weekly report. 2016;65:242–247. doi: 10.15585/mmwr.mm6509e2. [DOI] [PubMed] [Google Scholar]
- Kumar A, Hou S, Airo AM, Limonta D, Mancinelli V, Branton W, Power C, Hobman TC. Zika virus inhibits type-I interferon production and downstream signaling. EMBO reports. 2016;17:1766–1775. doi: 10.15252/embr.201642627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanciotti RS, Kosoy OL, Laven JJ, Velez JO, Lambert AJ, Johnson AJ, Stanfield SM, Duffy MR. Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg Infect Dis. 2008;14:1232–1239. doi: 10.3201/eid1408.080287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazear HM, Daniels BP, Pinto AK, Huang AC, Vick SC, Doyle SE, Gale M, Jr, Klein RS, Diamond MS. Interferon-lambda restricts West Nile virus neuroinvasion by tightening the blood-brain barrier. Sci Transl Med. 2015;7:284ra259. doi: 10.1126/scitranslmed.aaa4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazear HM, Diamond MS. Zika Virus: New Clinical Syndromes and Its Emergence in the Western Hemisphere. J Virol. 2016;90:4864–4875. doi: 10.1128/JVI.00252-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazear HM, Govero J, Smith AM, Platt DJ, Fernandez E, Miner JJ, Diamond MS. A Mouse Model of Zika Virus Pathogenesis. Cell Host Microbe. 2016;19:720–730. doi: 10.1016/j.chom.2016.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazear HM, Nice TJ, Diamond MS. Interferon-lambda: Immune Functions at Barrier Surfaces and Beyond. Immunity. 2015b;43:15–28. doi: 10.1016/j.immuni.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leal MC, Muniz LF, Ferreira TS, Santos CM, Almeida LC, Van Der Linden V, Ramos RC, Rodrigues LC, Neto SS. Hearing Loss in Infants with Microcephaly and Evidence of Congenital Zika Virus Infection - Brazil, November 2015–May 2016. MMWR Morbidity and mortality weekly report. 2016;65:917–919. doi: 10.15585/mmwr.mm6534e3. [DOI] [PubMed] [Google Scholar]
- Leviton A, Holmes LB, Allred EN, Vargas J. Methodologic issues in epidemiologic studies of congenital microcephaly. Early Hum Dev. 2002;69:91–105. doi: 10.1016/s0378-3782(02)00065-8. [DOI] [PubMed] [Google Scholar]
- Li C, Xu D, Ye Q, Hong S, Jiang Y, Liu X, Zhang N, Shi L, Qin CF, Xu Z. Zika Virus Disrupts Neural Progenitor Development and Leads to Microcephaly in Mice. Cell stem cell. 2016a doi: 10.1016/j.stem.2016.04.017. [DOI] [PubMed] [Google Scholar]
- Li C, Xu D, Ye Q, Hong S, Jiang Y, Liu X, Zhang N, Shi L, Qin CF, Xu Z. Zika Virus Disrupts Neural Progenitor Development and Leads to Microcephaly in Mice. Cell Stem Cell. 2016b;19:672. doi: 10.1016/j.stem.2016.10.017. [DOI] [PubMed] [Google Scholar]
- Martines RB, Bhatnagar J, de Oliveira Ramos AM, Davi HP, Iglezias SD, Kanamura CT, Keating MK, Hale G, Silva-Flannery L, Muehlenbachs A, et al. Pathology of congenital Zika syndrome in Brazil: a case series. Lancet (London, England) 2016;388:898–904. doi: 10.1016/S0140-6736(16)30883-2. [DOI] [PubMed] [Google Scholar]
- McConkey CA, Delorme-Axford E, Nickerson CA, Kim KS, Sadovsky Y, Boyle JP, Coyne CB. A three-dimensional culture system recapitulates placental syncytiotrophoblast development and microbial resistance. Sci Adv. 2016;2:e1501462. doi: 10.1126/sciadv.1501462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miner JJ, Cao B, Govero J, Smith AM, Fernandez E, Cabrera OH, Garber C, Noll M, Klein RS, Noguchi KK, et al. Zika Virus Infection during Pregnancy in Mice Causes Placental Damage and Fetal Demise. Cell. 2016;165:1081–1091. doi: 10.1016/j.cell.2016.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu J, Adamson SL. Developmental changes in hemodynamics of uterine artery, utero- and umbilicoplacental, and vitelline circulations in mouse throughout gestation. Am J Physiol Heart Circ Physiol. 2006;291:H1421–1428. doi: 10.1152/ajpheart.00031.2006. [DOI] [PubMed] [Google Scholar]
- Mu J, Slevin JC, Qu D, McCormick S, Adamson SL. In vivo quantification of embryonic and placental growth during gestation in mice using micro-ultrasound. Reprod Biol Endocrinol. 2008;6:34. doi: 10.1186/1477-7827-6-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray SA, Morgan JL, Kane C, Sharma Y, Heffner CS, Lake J, Donahue LR. Mouse gestation length is genetically determined. PLoS One. 2010;5:e12418. doi: 10.1371/journal.pone.0012418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne AF, Binduga-Gajewska I, Kauffman EB, Kramer LD. Quantitation of flaviviruses by fluorescent focus assay. J Virol Methods. 2006;134:183–189. doi: 10.1016/j.jviromet.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Quicke KM, Bowen JR, Johnson EL, McDonald CE, Ma H, O’Neal JT, Rajakumar A, Wrammert J, Rimawi BH, Pulendran B, et al. Zika Virus Infects Human Placental Macrophages. Cell Host Microbe. 2016 doi: 10.1016/j.chom.2016.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rausch K, Hackett BA, Weinbren NL, Reeder SM, Sadovsky Y, Hunter CA, Schultz DC, Coyne CB, Cherry S. Screening Bioactives Reveals Nanchangmycin as a Broad Spectrum Antiviral Active against Zika Virus. Cell Rep. 2017;18:804–815. doi: 10.1016/j.celrep.2016.12.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard AS, Shim BS, Kwon YC, Zhang R, Otsuka Y, Schmitt K, Berri F, Diamond MS, Choe H. AXL-dependent infection of human fetal endothelial cells distinguishes Zika virus from other pathogenic flaviviruses. Proceedings of the National Academy of Sciences of the United States of America. 2017;114:2024–2029. doi: 10.1073/pnas.1620558114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter JM, Martines RB, Zaki SR. Zika Virus: Pathology From the Pandemic. Archives of pathology & laboratory medicine. 2017;141:49–59. doi: 10.5858/arpa.2016-0397-SA. [DOI] [PubMed] [Google Scholar]
- Rossant J, Cross JC. Placental development: lessons from mouse mutants. Nat Rev Genet. 2001;2:538–548. doi: 10.1038/35080570. [DOI] [PubMed] [Google Scholar]
- Shapiro-Mendoza CK, Rice ME, Galang RR, Fulton AC, VanMaldeghem K, Prado MV, Ellis E, Anesi MS, Simeone RM, Petersen EE, et al. Pregnancy Outcomes After Maternal Zika Virus Infection During Pregnancy - U.S. Territories, January 1, 2016–April 25, 2017. MMWR Morbidity and mortality weekly report. 2017;66:615–621. doi: 10.15585/mmwr.mm6623e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan KC, Lai KS, Dunn GP, Bruce AT, Diamond MS, Heutel JD, Dungo-Arthur C, Carrero JA, White JM, Hertzog PJ, et al. Blocking monoclonal antibodies specific for mouse IFN-alpha/beta receptor subunit 1 (IFNAR-1) from mice immunized by in vivo hydrodynamic transfection. J Interferon Cytokine Res. 2006;26:804–819. doi: 10.1089/jir.2006.26.804. [DOI] [PubMed] [Google Scholar]
- Sheridan MA, Yunusov D, Balaraman V, Alexenko AP, Yabe S, Verjovski-Almeida S, Schust DJ, Franz AW, Sadovsky Y, Ezashi T, et al. Vulnerability of primitive human placental trophoblast to Zika virus. Proc Natl Acad Sci U S A. 2017;114:E1587–e1596. doi: 10.1073/pnas.1616097114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoni MK, Jurado KA, Abrahams VM, Fikrig E, Guller S. Zika virus infection of Hofbauer cells. Am J Reprod Immunol. 2017;77 doi: 10.1111/aji.12613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun T, Hevner RF. Growth and folding of the mammalian cerebral cortex: from molecules to malformations. Nat Rev Neurosci. 2014;15:217–232. doi: 10.1038/nrn3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suy A, Sulleiro E, Rodo C, Vazquez E, Bocanegra C, Molina I, Esperalba J, Sanchez-Seco MP, Boix H, Pumarola T, et al. Prolonged Zika Virus Viremia during Pregnancy. N Engl J Med. 2016;375:2611–2613. doi: 10.1056/NEJMc1607580. [DOI] [PubMed] [Google Scholar]
- Tabata T, Petitt M, Puerta-Guardo H, Michlmayr D, Wang C, Fang-Hoover J, Harris E, Pereira L. Zika Virus Targets Different Primary Human Placental Cells, Suggesting Two Routes for Vertical Transmission. Cell Host Microbe. 2016;20:155–166. doi: 10.1016/j.chom.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang WW, Young MP, Mamidi A, Regla-Nava JA, Kim K, Shresta S. A Mouse Model of Zika Virus Sexual Transmission and Vaginal Viral Replication. Cell Rep. 2016;17:3091–3098. doi: 10.1016/j.celrep.2016.11.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsetsarkin KA, Kenney H, Chen R, Liu G, Manukyan H, Whitehead SS, Laassri M, Chumakov K, Pletnev AG. A Full-Length Infectious cDNA Clone of Zika Virus from the 2015 Epidemic in Brazil as a Genetic Platform for Studies of Virus-Host Interactions and Vaccine Development. MBio. 2016;7 doi: 10.1128/mBio.01114-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Linden V, Pessoa A, Dobyns W, Barkovich AJ, Junior HV, Filho EL, Ribeiro EM, Leal MC, Coimbra PP, Aragao MF, et al. Description of 13 Infants Born During October 2015–January 2016 With Congenital Zika Virus Infection Without Microcephaly at Birth - Brazil. MMWR Morbidity and mortality weekly report. 2016;65:1343–1348. doi: 10.15585/mmwr.mm6547e2. [DOI] [PubMed] [Google Scholar]
- Weisblum Y, Oiknine-Djian E, Vorontsov OM, Haimov-Kochman R, Zakay-Rones Z, Meir K, Shveiky D, Elgavish S, Nevo Y, Roseman M, et al. Zika virus infects early- and mid-gestation human maternal-decidual tissues, inducing distinct innate tissue responses in the maternal-fetal interface. Journal of virology. 2016 doi: 10.1128/JVI.01905-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yockey LJ, Varela L, Rakib T, Khoury-Hanold W, Fink SL, Stutz B, Szigeti-Buck K, Van den Pol A, Lindenbach BD, Horvath TL, et al. Vaginal Exposure to Zika Virus during Pregnancy Leads to Fetal Brain Infection. Cell. 2016;166:1247–1256. e1244. doi: 10.1016/j.cell.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Videos taken at E15.5 after infection at E9.5 showing cardiac motion in mock-infected (left panel) and ZIKV-Brazil infected (middle panel) fetuses, as well as fetal demise after ZIKV infection (right panel).
The same fetuses from Movie S1 were imaged with color Doppler to show umbilical artery flow (left and middle panels) or lack thereof (right panel). Data in this Figure is representative of three independent experiments.