Abstract
Evidence suggests that both 14-3-3σ and long non-coding RNA HOX transcript antisense RNA (HOTAIR) are involved in the tumorigenesis and progression of lung cancer. In the present study, the potential association between 14-3-3σ and HOTAIR in non-small cell lung cancer (NSCLC) was investigated. In tissue samples collected from 54 patients with NSCLC, expression of HOTAIR and 14-3-3σ was analyzed by reverse transcription-quantitative polymerase chain reaction (RT-qPCR). After stable ectopic expression of HOTAIR and stable HOTAIR knockdown in PC9 cancer cells, the effect of HOTAIR on levels of mRNA and protein 14-3-3σ expression levels were detected using RT-qPCR and western blotting, respectively. Expression of HOTAIR and 14-3-3σ in NSCLC tissues was significantly higher than in adjacent non-cancerous lung tissue (P<0.05). Correlation analysis also identified a correlation between levels of HOTAIR and 14-3-3σ expression in NSCLC tissues (r=0.725, P=0.0005). In addition, overexpression and knockdown of HOTAIR in the human NSCLC cell line PC9 led to the upregulation and downregulation of 14-3-3σ, respectively, at both the mRNA and protein levels (all P<0.05). To the best of our knowledge, the present study provides the first in vivo and in vitro evidence to suggest that HOTAIR promotes the expression of 14-3-3σ in NSCLC. The potential association between HOTAIR and 14-3-3σ indicates that both biomolecules may be viable targets in anticancer therapy.
Keywords: long non-coding RNA, HOX transcript antisense RNA, 14-3-3σ, non-small cell lung cancer
Introduction
Lung cancer is the most commonly diagnosed cancer and the leading cause of cancer-associated mortality worldwide (1). Non-small cell lung cancer (NSCLC) is the predominant form of lung cancer, accounting for >80% of all lung cancer cases (2). There have been numerous improvements in surgical treatment and chemotherapy; however there has no corresponding improvement in the survival rate of NSCLC patients over the past 10 years (3). Therefore, studies into novel therapeutic targets and strategies to treat patients with NSCLC are warranted. The 14-3-3 protein family consists of at least seven isoforms, namely β, ε, γ, η, σ, τ/θ and ξ, which are found in mammalian cells (4). A number of important signaling events, including cell proliferation and survival are regulated by 14-3-3 proteins (5,6). This is important, as enhanced cell proliferation and survival are key characteristics of cancer cells (7). Overexpression of 14-3-3 proteins has been detected in many types of human cancer such as pancreatic cancer (8), lung adenocarcinoma (9), breast tumor (10) and is correlated with more aggressive tumors and poor prognosis (10,11). In lung cancer, it has been demonstrated that the 14-3-3 isoforms β, γ, σ and θ are overexpressed in tumor tissue compared with normal tissues (4,12). Among these, 14-3-3σ has been indicated to be elevated in NSCLC as a result of 14-3-3σ DNA hypomethylation (13). The results of a recent study also suggested that 14-3-3σ expression was correlated with cisplatin resistance in NSCLC cells (14).
Long non-coding RNAs (lncRNAs) serve key regulatory roles in cellular and biochemical processes (15). Regarding lung cancer, the lncRNA HOX transcript antisense RNA (HOTAIR) has been demonstrated to repress gene expression and promote proliferation, survival, metastasis, invasion and drug resistance in lung cancer cells (15). The expression of HOTAIR may also be elevated in lung cancer and correlate with cancer metastasis and poor patient prognosis (16). Furthermore, a recent study observed that HOTAIR was overexpressed in metastatic lung cancer tissue, suggesting that HOTAIR may be associated with the invasion and progression of lung cancer (17).
Collectively, the results from these previous studies suggest that both 14-3-3σ and HOTAIR are involved in the tumorigenesis and progression of lung cancer. Therefore, the current study, investigated the association between 14-3-3σ and HOTAIR in NSCLC.
Materials and methods
Tissue samples
Non-cancerous lung and NSCLC tissues were collected from 54 NSCLC patients (mean age, 59.46±10.05) undergoing surgical treatment at the Second Xiangya Hospital of Central South University (Changsha, China) from June 2010 to April 2014. All patients had been diagnosed with NSCLC (stage I, II and III) based on histopathological evaluation (18). Clinicopathological characteristics including tumor-node-metastasis (TNM) staging were collected (19). No local or systemic treatment was conducted in these patients prior to surgery. All tissue samples were immediately frozen in liquid nitrogen prior to use in the current experiments. The clinicopathological characteristics of patients in the current study are presented in Table I. The present study was approved by the Ethics Committee of the Second Xiangya Hospital and all patients provided written informed consent.
Table I.
Patient characteristics.
| Variable | Number | % |
|---|---|---|
| Sex | ||
| Male | 44 | 81.5 |
| Female | 10 | 18.5 |
| Smoker | ||
| Yes | 34 | 63.0 |
| No | 20 | 37.0 |
| Cancer type | ||
| Adenocarcinoma | 21 | 38.9 |
| Squamous cell carcinoma | 33 | 61.1 |
| Differentiation | ||
| Low-Moderate | 49 | 90.7 |
| High | 5 | 9.3 |
| Tumor T stage | ||
| T1 | 5 | 9.3 |
| T2 | 45 | 83.3 |
| T3 | 4 | 7.4 |
| Tumor N stage | ||
| N0 | 15 | 27.8 |
| N1-N3 | 39 | 72.2 |
| Clinical stage | ||
| I–II | 33 | 61.1 |
| III | 21 | 38.9 |
T, tumor size; N, lymph node metastasis.
Cell culture
The human NSCLC cell line PC9 was purchased from the Chinese Academy of Sciences (Shanghai, China). PC9 cells were cultured in Dulbecco's modified Eagle's medium (Invitrogen; Thermo Fisher Scientific, Inc.) supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.), 100 U/ml penicillin (Sigma-Aldrich; Merck KGaA; Darmstadt, Germany) and 100 µg/ml streptomycin (Sigma-Aldrich; Merck KGaA). Cell cultures were incubated in a humidified atmosphere of 5% CO2 at 37°C. For transfection experiments, PC9 cells were seeded 1×104 per well in 6-wells plate.
Plasmids and transient transfection
The plasmids were described in our previous paper (20). Briefly, the HOTAIR expression plasmid pLZRS-HOTAIR was provided by Dr Howard Chang (Stanford University, Stanford, CA, USA) (16) and the HOTAIR coding region was subcloned into the retroviral vector pLVX-EF1α-IRES-Puro (pLZRS; Clontech Laboratories, Inc., Mountainview, CA, USA). HOTAIR shRNA vectors (GV248; HOTAIR-shRNA 1/2) and control shRNA (NC-sh) were purchased from GeneChem Co., Ltd., Shanghai, China. Transfection of plasmids was performed using Lipofectamine® 2000 (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) according to the manufacturer's protocol. Stable shRNA expressing colonies were selected using puromycin. The experiments included 5 groups: Overexpressed control (pLZRS), HOTAIR (pLZRS-HOTAIR), downregulated control (NC-shRNA), shHOT-1 and shHOT-2.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was isolated from PC9 cells using TRIzol reagent (Thermo Fisher Scientific, Inc.). A total of 1 µg mRNA was reverse transcribed into cDNA using SuperScript II reverse transcriptase (Thermo Fisher Scientific, Inc.) according to the manufacturer's protocol, and the reaction product was treated with ezDNase (Thermo Fisher Scientific, Inc.). qPCR was performed on an Abi-Prism 7700 Sequence Detection System using the SYBR-Green Master Mix (Applied Biosystems; Thermo Fisher Scientific, Inc.) to determine the relative expression levels of target genes, according to the manufacturer's protocol. β-actin was used as the reference and normalization control. The following cycling conditions were performed: Holding stage, 1 cycle of 50°C for 2 min and 95°C for 10 min; cycling stage, 40 cycles of 95°C for 15 sec and 60°C for 60 sec; and melt curve stage, 95°C for 15 sec, 60°C for 60 sec, 95°C for 30 sec and 60°C for 15 sec. The primers used were as follows: HOTAIR, forward 5′-GCAGTGGAATGGAACGGATT-3′ and reverse 5′-CGTGGCATTTCTGGTCTTGTA-3′; 14-3-3σ, forward 5′-TCCGTCTTCCACTACGAGAT-3′ and reverse 5′-TGATGAGGGTGCTGTCTTTG-3′; and β-actin, forward 5′-GCACCACACCTTCTACAATGAG-3′ and reverse 5′-GATAGCACAGCCTGGATAGCA-3′. Levels of target mRNA were quantified using the ΔΔCq method (21) and normalized against that of β-actin in the same sample. RT-qPCR was repeated in duplicate in three independent experiments.
Western blot analysis
PC9 cells were lysed with radioimmunoprecipitation assay buffer (Beyotime Biotechnology, Haimen, China) added with protease inhibitor cocktail (cOmplete ULTRA tablets; Roche Diagnostics GmbH, Mannheim, Germany) then incubated on ice for 30 min prior to removal of cell debris by centrifugation at 2,000 × g for 15 min at 4°C. The protein concentration of resulting lysates was determined using a BCA Protein Assay kit (Thermo Fisher Scientific, Inc.) according to the manufacturer's instructions. Equal amounts of protein (20 µg) from each sample were loaded and separated using 10% SDS-PAGE and blotted onto polyvinylidene difluoride microporous membranes (EMD Millipore, Billerica, MA, USA). The membranes were blocked with 5% non-fat milk in TBS Tween-20 (TBST; 25 mM Tris, pH 7.5, 150 mM NaCl and 0.1% Tween-20) for 2 h at room temperature, then incubated for overnight at 4°C with a 1:500 dilution of mouse anti-human 14-3-3σ monoclonal antibody (cat. no. ab76532; Abcam, Cambridge, MA, USA) and 1:5,000 dilution of mouse anti-human β-actin (cat. no. 60008-1-Ig; Wuhan Sanying Biotechnology, Wuhan, China). After washing three times (each time for 10 min) with TBST, membranes were then incubated with a 1:5,000 dilution of bovine anti-mouse secondary antibody conjugated to horseradish peroxidase (cat. no. sc-2371; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) for 1 h at room temperature. Peroxidase labeling was detected using a GE Healthcare enhanced chemiluminescence kit (GE Healthcare Life Sciences, Shanghai, China) and quantified by using the Moecular Imager ChemiDoc XRS System (Bio-Rad Laboratories, Inc., Hercules, CA, USA). β-actin was used for loading control. The band intensities were semiquantified by densitometry using Quantity-One software v4.62 (Bio-Rad Laboratories, Inc.) and a ChemiDoc XRS System. Three independent experiments were performed.
Statistical analysis
Statistical analyses were performed using GraphPad Prism 6.0 software (GraphPad Software, Inc., La Jolla, CA, USA). Student's t-tests were used to evaluate significant difference between any two groups of data. All data are represented as mean ± standard deviation. Correlation analyses were performed with Spearman's correlation analysis test. P<0.05 was considered to indicate a statistically significant difference.
Results
HOTAIR and 14-3-3σ are upregulated in NSCLC tissues
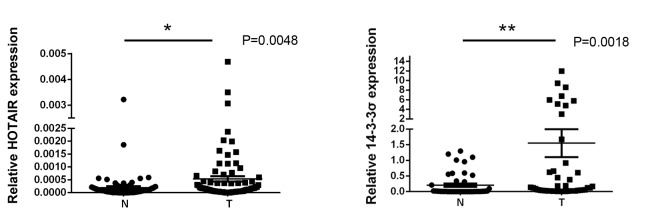
NSCLC tumor and adjacent non-cancerous lung tissues were isolated from 54 NSCLC patients, and levels of HOTAIR and 14-3-3σ expression were measured using RT-qPCR. Expression of HOTAIR and 14-3-3σ in NSCLC tissues was significantly higher than in adjacent non-cancerous lung tissues (0.0047±0.0011 vs. 0.00018±0.00003 and 1.32±0.56 vs. 0.12±0.0035, respectively; both P<0.05; Fig. 1). Spearman's correlation analysis also demonstrated that levels of HOTAIR and 14-3-3σ in NSCLC tissues were significantly correlated (r=0.3735; P=0.0005; Fig. 2), indicating a regulatory relationship between HOTAIR and 14-3-3σ expression.
Figure 1.
Expression of HOTAIR and 14-3-3σ in NSCLC and non-cancerous lung tissues. (A) Levels of HOTAIR mRNA were measured in NSCLC tumor (T) and adjacent non-cancerous lung tissues (N) from 54 patients using reverse transcription-quantitative polymerase chain reaction (P=0.0048). (B) Levels of 14-3-3σ expression were measured by reverse transcription-quantitative polymerase chain reaction in NSCLC (T) and adjacent non-cancerous lung (N) tissues from the same 54 patients (P=0.0018). The results are presented in scatter plots and the horizontal bars represent the mean ± standard deviation. *P<0.05. **P<0.01. HOTAIR, HOX transcript antisense RNA; NSCLC, non-small cell lung cancer; T, NSCLC lung tissue; N, adjacent non-cancerous lung tissue.
Figure 2.
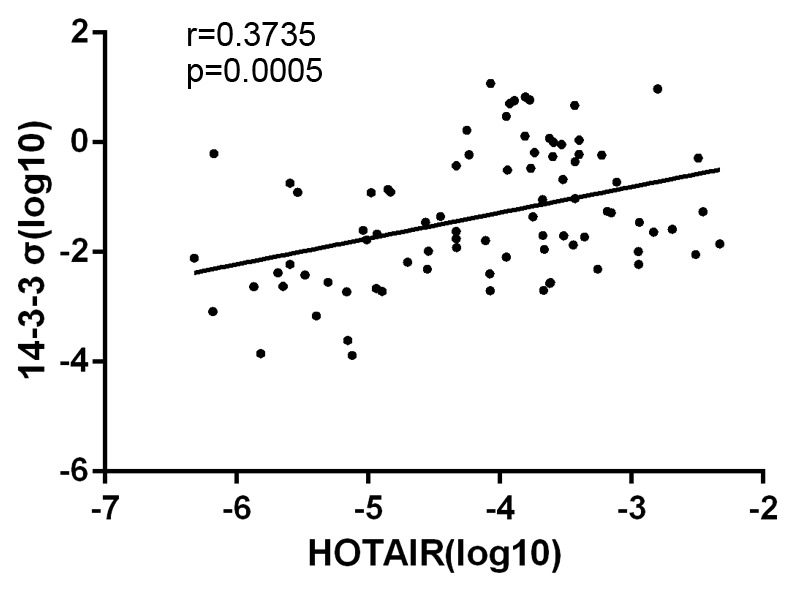
Correlation between HOTAIR and 14-3-3σ expression in NSCLC tissues. Levels of HOTAIR and 14-3-3σ expression in NSCLC tissues from 54 patients were determined to be significantly correlated by Spearman's correlation analysis (r=0.3735, P=0.0005). HOTAIR, HOX transcript antisense RNA; NSCLC, non-small cell lung cancer.
HOTAIR promotes the expression of 14-3-3σ in NSCLC cells
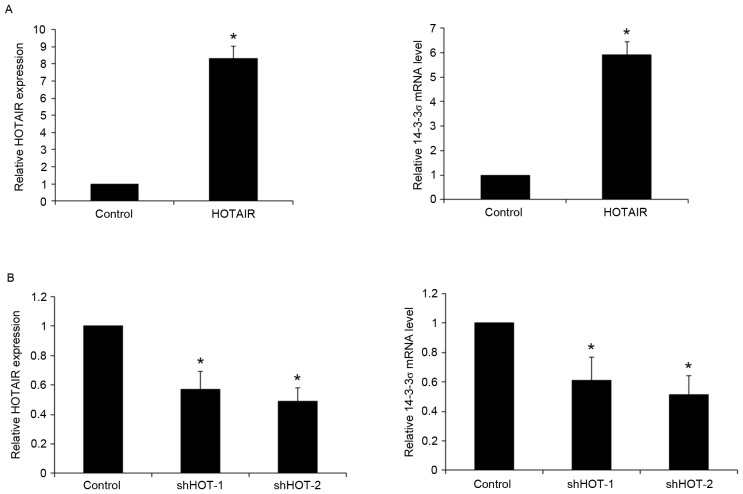
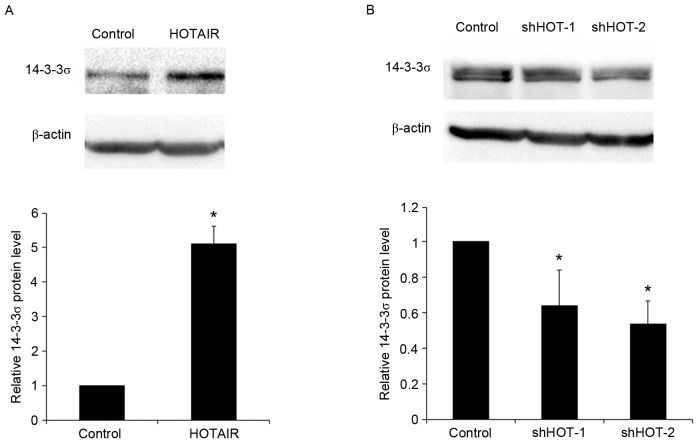
To determine the potential regulatory effects of HOTAIR on 14-3-3σ expression, HOTAIR was overexpressed and knocked-down in human NSCLC PC9 cells. Transfection of PC9 cells with a HOTAIR expression vector significantly increased the expression of HOTAIR by >8-fold, relative to blank plasmid control (P<0.05). This overexpression of HOTAIR led to a significant 5.9-fold increase in 14-3-3σ mRNA expression in PC9 cells (P<0.05; Fig. 3A). Compared with shRNA control, transfection of PC9 cells with two HOTAIR shRNA expression vectors (HOTAIR-shRNA 1/2) significantly decreased the expression of HOTAIR by 0.43 and 0.51-fold respectively (both P<0.05), which led to a 0.39- and 0.48-fold significant decrease in 14-3-3σ mRNA expression (both P<0.05; Fig. 3B). These results were confirmed by western blot analysis. Overexpression of HOTAIR significantly increased the levels of 14-3-3σ protein by 5.1-fold in PC9 cells (P<0.05), while knockdown of HOTAIR with HOTAIR shRNA 1/2 vectors significantly reduced 14-3-3σ levels by approximately 0.36- and 0.46-fold, respectively (both P<0.05; Fig. 4). The results indicate that HOTAIR promotes the expression of 14-3-3σ mRNA and protein in NSCLC cells.
Figure 3.
Effect of HOTAIR on the expression of 14-3-3σ mRNA in human NSCLC cells. (A) The human NSCLC cell line PC9 was transfected with the HOTAIR expression vector LZRS-HOTAIR. Levels of HOTAIR (left) and 14-3-3σ mRNA (right) were measured using RT-qPCR. PC9 cells transfected with an empty expression vector were used as a control. (B) PC9 cells were transfected with HOTAIR shRNA expression vectors. Levels of HOTAIR (left) and 14-3-3σ mRNA (right) were measured using RT-qPCR. PC9 cells transfected with a scramble-shRNA expression vector were used as a control. Levels of 14-3-3σ expression were measured relative to that of β-actin. Data are presented as the mean ± standard deviation of three independent experiments. *P<0.05. HOTAIR, HOX transcript antisense RNA; NSCLC, non-small cell lung cancer; shRNA, short hairpin RNA; shHOT-1/2, HOTAIR shRNA expression vectors; RT-qPCR, reverse transcription-quantitative polymerase chain reaction.
Figure 4.
Effect of HOTAIR on the expression of 14-3-3σ protein in human NSCLC cells. (A) The human NSCLC cell line PC9 was transfected with the HOTAIR expression vector pLZRS-HOTAIR and levels of 14-3-3σ protein expression were determined by western blot analysis. PC9 cells transfected with pLZRS vector were used as a control. (B) PC9 cells were transfected with HOTAIR shRNA expression vectors and levels of 14-3-3σ protein expression were determined by western blot analysis. PC9 cells transfected with a NC-shRNA expression vector were used as a control. Levels of 14-3-3σ expression were measured relative to that of β-actin. Representative gel images of three independent experiments were semiquantified by densitometry using Quantity-One software and data are presented as the mean ± standard deviation. *P<0.05. HOTAIR, HOX transcript antisense RNA; NSCLC, non-small cell lung cancer; shRNA, short hairpin RNA; shHOT-1/2, HOTAIR shRNA expression vectors.
Discussion
The present study provided the first evidence to suggest that HOTAIR promotes the expression of 14-3-3σ in NSCLC. 14-3-3 proteins bind to numerous phospho-client proteins within cells, thus regulating key signaling events. In particular, 14-3-3 proteins regulate the Raf mitogen-activated protein kinase and phosphoinositide-3-kinase-Akt signaling pathways (5,6), which are considered to be critical mediators of tumorigenesis and cancer progression (7). Recent studies have demonstrated that 14-3-3σ is overexpressed in lung cancer compared with adjacent normal lung tissue, and may be involved in determining the malignancy of lung cancer (12,13). Analogous to previous results, the present study detected that expression of 14-3-3σ in NSCLC tissues was significantly higher than in adjacent normal lung tissues. Previous studies have demonstrated that HOTAIR promotes proliferation, survival, invasion, metastasis and drug resistance in lung cancer cells (15,17,20,22). In addition, it has been determined that HOTAIR is elevated in lung cancer (15). Similarly, the present study observed that expression of HOTAIR in NSCLC was significantly higher than in adjacent normal lung tissues.
As confirmation that both 14-3-3σ and HOTAIR were elevated in NSCLC compared with adjacent normal lung tissues, the present in vivo assays identified a correlation between levels of 14-3-3σ and HOTAIR expression in NSCLC tissues, indicating a regulatory relationship between HOTAIR and 14-3-3σ expression in NSCLC. Analogous to these in vivo findings, in vitro assays involving overexpression and knockdown of HOTAIR in a human NSCLC cell line indicated that HOTAIR was a potent upstream enhancer of 14-3-3σ expression in NSCLC cells. HOTAIR represses gene expression through the recruitment of chromatin modifiers, which may involve binding of HOTAIR to the transcriptional co-repressor polycomb repressive complex 2 (PRC2) and subsequent recruitment of PRC2, leading to the silencing of target genes (23). The present results indicate that HOTAIR promotes 14-3-3σ mRNA and protein expression, thus it is unlikely that HOTAIR directly effects the 14-3-3σ gene but may act by inhibiting 14-3-3σ gene repressors.
14-3-3 proteins are a family of highly conserved proteins comprised of at least seven isoforms in mammalian cells (4). Overexpression of the 14-3-3 isoforms β, γ, σ and θ has been identified in lung cancer (12). The present study focused on the regulatory effect of HOTAIR on 14-3-3σ expression in NSCLC. Future studies by our group will focus on the potential effects of HOTAIR on the expression of 14-3-3 β, γ and θ, as inhibition of HOTAIR may be an effective way of inhibiting the expression of 14-3-3σ and other 14-3-3 proteins. In addition, as overexpression of HOTAIR and 14-3-3 protein has been detected in pancreatic cancer, lung adenocarcinoma and breast tumor (8,9,10) and correlates with more aggressive tumors and poor prognosis (11,24,25), further studies investigating the association between HOTAIR and 14-3-3 proteins in other types of cancer are warranted.
In conclusion, the present results suggest that HOTAIR promotes the expression of 14-3-3σ in NSCLC in vivo and in vitro. To the best of our knowledge, the current study is the first to identify a link between HOTAIR and 14-3-3σ expression and further studies are now warranted to identify the underlying mechanisms regarding the effects of HOTAIR on 14-3-3σ expression.
Acknowledgements
The present study was supported by the National Natural Science Foundation of China (grant nos. 81272584 and 81602030), China Postdoctoral Science Foundation (grant no. 2014RS4006) and The Hunan Province Natural Sciences Foundation of China (grant no. 13JJ3039).
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Chang A. Chemotherapy, chemoresistance and the changing treatment landscape for NSCLC. Lung Cancer. 2011;71:3–10. doi: 10.1016/j.lungcan.2010.08.022. [DOI] [PubMed] [Google Scholar]
- 3.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 4.Raungrut P, Wongkotsila A, Lirdprapamongkol K, Svasti J, Geater SL, Phukaoloun M, Suwiwat S, Thongsuksai P. Prognostic significance of 14-3-3γ overexpression in advanced non-small cell lung cancer. Asian Pac J Cancer Prev. 2014;15:3513–3518. doi: 10.7314/APJCP.2014.15.8.3513. [DOI] [PubMed] [Google Scholar]
- 5.Porter GW, Khuri FR, Fu H. Dynamic 14-3-3/client protein interactions integrate survival and apoptotic pathways. Sem Cancer Biol. 2006;16:193–202. doi: 10.1016/j.semcancer.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 6.Morrison D. The 14-3-3 proteins: Integrators of diverse signaling cues that impact cell fate and cancer development. Trends Cell Biol. 2008;19:16–23. doi: 10.1016/j.tcb.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 8.Okada T, Masuda N, Fukai Y, Shimura T, Nishida Y, Hosouchi Y, Kashiwabara K, Nakajima T, Kuwano H. Immunohistochemical expression of 14-3-3 sigma protein in intraductal papillary-mucinous tumor and invasive ductal carcinoma of the pancreas. Anticancer Res. 2006;26:3105–3110. [PubMed] [Google Scholar]
- 9.Shiba-Ishii A, Kano J, Morishita Y, Sato Y, Minami Y, Noguchi M. High expression of stratifin is a universal abnormality during the course of malignant progression of early-stage lung adenocarcinoma. Int J Cancer. 2011;129:2445–2453. doi: 10.1002/ijc.25907. [DOI] [PubMed] [Google Scholar]
- 10.Boudreau A, Tanner K, Wang D, Geyer FC, Reis-Filho JS, Bissell MJ. 14-3-3σ stabilizes a complex of soluble actin and intermediate filament to enable breast tumor invasion. Proc Natl Acad Sci USA. 2013;110:E3937–E3944. doi: 10.1073/pnas.1315022110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neal CL, Yu D. 14-3-3ζ as a prognostic marker and therapeutic target for cancer. Expert Opin Ther Targets. 2010;14:1343–1354. doi: 10.1517/14728222.2010.531011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qi W, Liu X, Qiao D, Martinez JD. Isoform-specific expression of 14-3-3 proteins in human lung cancer tissues. Int J Cancer. 2005;113:359–363. doi: 10.1002/ijc.20492. [DOI] [PubMed] [Google Scholar]
- 13.Radhakrishnan VM, Jensen TJ, Cui H, Futscher BW, Martinez JD. Hypomethylation of the 14-3-3σ promoter leads to increased expression in non-small cell lung cancer. Genes Chromosomes Cancer. 2011;50:830–836. doi: 10.1002/gcc.20904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cetintas VB, Tetik A, Cok G, Kucukaslan AS, Kosova B, Gunduz C, Veral A, Eroglu Z. Role of 14-3-3σ in resistance to cisplatin in non-small cell lung cancer cells. Cell Biol Int. 2013;37:78–86. doi: 10.1002/cbin.10006. [DOI] [PubMed] [Google Scholar]
- 15.Zhao W, An Y, Liang Y, Xie XW. Role of HOTAIR long noncoding RNA in metastatic progression of lung cancer. Eur Rev Med Pharmacol Sci. 2014;18:1930–1936. [PubMed] [Google Scholar]
- 16.Gupta RA, Shah N, Wang KC, Kim J, Horlings HM, Wong DJ, Tsai MC, Hung T, Argani P, Rinn JL, et al. Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature. 2010;464:1071–1076. doi: 10.1038/nature08975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu XH, Liu ZL, Sun M, Liu J, Wang ZX, De W. The long non-coding RNA HOTAIR indicates a poor prognosis and promotes metastasis in non-small cell lung cancer. BMC Cancer. 2013;13:464. doi: 10.1186/1471-2407-13-464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greene FL, Sobin LH. The staging of cancer: A retrospective and prospective appraisal. CA Cancer J Clin. 2008;58:180–190. doi: 10.3322/CA.2008.0001. [DOI] [PubMed] [Google Scholar]
- 19.Gospodarowicz MK, Miller D, Groome PA, Greene FL, Logan PA, Sobin LH. The process for continuous improvement of the TNM classification. Cancer. 2004;100:1–5. doi: 10.1002/cncr.11898. [DOI] [PubMed] [Google Scholar]
- 20.Wang R, Shi Y, Chen L, Jiang Y, Mao C, Yan B, Liu S, Shan B, Tao Y, Wang X. The ratio of FoxA1 to FoxA2 in lung adenocarcinoma is regulated by LncRNA HOTAIR andchromatin remodeling factor LSH. Sci Rep. 2015;5:17826. doi: 10.1038/srep17826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 22.Zhuang Y, Wang X, Nguyen HT, Zhuo Y, Cui X, Fewell C, Flemington EK, Shan B. Induction of long intergenic non-coding RNA HOTAIR in lung cancer cells by type I collagen. J Hematol Oncol. 2013;6:35. doi: 10.1186/1756-8722-6-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rinn JL, Kertesz M, Wang JK, Squazzo SL, Xu X, Brugmann SA, Goodnough LH, Helms JA, Farnham PJ, Segal E, Chang HY. Functional demarcation of active and silent chromatin domains in human HOX loci by noncoding RNAs. Cell. 2007;129:1311–1323. doi: 10.1016/j.cell.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim K, Jutooru I, Chadalapaka G, Johnson G, Frank J, Burghardt R, Kim S, Safe S. HOTAIR is a negative prognostic factor and exhibits pro-oncogenic activity in pancreatic cancer. Oncogene. 2013;32:1616–1625. doi: 10.1038/onc.2012.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Niinuma T, Suzuki H, Nojima M, Nosho K, Yamamoto H, Takamaru H, Yamamoto E, Maruyama R, Nobuoka T, Miyazaki Y, et al. Upregulation of miR-196a and HOTAIR drive malignant character in gastrointestinal stromal tumors. Cancer Res. 2012;72:1126–1136. doi: 10.1158/0008-5472.CAN-11-1803. [DOI] [PubMed] [Google Scholar]