Abstract
Endothelial colony-forming cells (ECFCs) are a population of endothelial progenitor cells (EPCs) that display robust proliferative potential and vessel-forming capability. Previous studies have demonstrated that a limited number of ECFCs may be obtained from adult bone marrow, peripheral blood and umbilical cord (UC) blood. The present study describes an effective method for isolating ECFCs from human UC. The ECFCs derived from human UC displayed the full properties of EPCs. Analysis of the growth kinetics, cell cycle and colony-forming ability of the isolated human UC-ECFCs indicated that the cells demonstrated properties of stem cells, including relative stability and rapid proliferation in vitro. Gene expression of Fms related tyrosine kinase 1, kinase insert domain receptor, vascular endothelial cadherin, cluster of differentiation (CD)31, CD34, epidermal growth factor homology domains-2, von Willebrand factor and endothelial nitric oxide synthase was assessed by reverse transcription-polymerase chain reaction. The cells were positive for CD34, CD31, CD73, CD105 and vascular endothelial growth factor receptor-2, and negative for CD45, CD90 and human leukocyte antigen-antigen D related protein according to flow cytometry. 1,1′-dioctadecyl-3,3,3′,3′-tetra-methyl-indocarbocyanine perchlorate-labeled acetylated low-density lipoprotein and fluorescein isothiocyanate-Ulex europaeus-l were used to verify the identity of the UC-ECFCs. Matrigel was used to investigate tube formation capability. The results demonstrated that the reported technique is a valuable method for isolating human UC-ECFCs, which have potential for use in vascular regeneration.
Keywords: endothelial colony-forming cells, umbilical cord, proliferative potential, vessel forming capability
Introduction
Endothelial progenitor cells (EPCs) are a population of cells that participate in vessel formation in both physiological and pathological processes, and demonstrate characteristics of both endothelial and progenitor cells (1). EPCs have great potential as a source of cells for the repair of vasculogenic injuries (2). Endothelial colony-forming cells (ECFCs), which typically possess a cobblestone appearance and are also referred to as blood outgrowth endothelial cells or late outgrowth cells, are a rare putative type of EPC that have the ability to produce endothelial cells and contribute to the functional endothelial lining of injured vascular structures in vivo at the colony level (3). EPCs were first isolated from adult human peripheral blood in 1997 (3,4). Since then, they have been successfully isolated from bone marrow (5), peripheral blood (6) and umbilical cord (UC) blood (7). Bone marrow is considered the original source of EPCs. Depletion of or increases in the number of EPCs in peripheral blood has been reported in various pathologic conditions, such as cardiovascular diseases, hypertension, type 2 diabetes mellitus, rheumatoid arthritis, aging and hematological diseases (8,9). EPCs have been used to repair ischemic or damaged cardiac tissue in animal models (8,9), and these cells have also been explored for the creation of living blood vessels (10,11). The promising results obtained in these studies suggest that these cells have the potential to be employed in clinical trials (12).
Although transplantation of autologous bone marrow-derived or peripheral blood-derived ECFCs has been demonstrated to be safe, the utility of these cells is limited due to the significant drop in cell number and proliferative/differentiation capacity with age (13). A study by Mandraffino et al (14) reported that, in elderly patients, the peripheral cell count is not necessarily weakened and the cluster of differentiation (CD)34+ cells maintain their role in predicting mortality. CD34+ cells may therefore be considered as a biomarker of longevity, not EPCs. Two possible ideal sources of ECFCs are cord blood and UC, which were discarded as medical waste in the past (15). However, the number of nucleated cells in cord blood is limited, which is thought to be a serious limitation to their utility for transplantation (16). In addition, a previous study demonstrated that human placenta-derived ECFCs have greater vasculogenic potential than cord blood-derived ECFCs in vivo (9).
Numerous previous studies that have aimed to identify EPCs have focused on simultaneous expression of CD34, CD117, CD133, CD105, CD144, CD184, CD309 [kinase insert domain receptor (KDR) or vascular endothelial growth factor receptor 2 (VEGFR2)], acetylated low-density lipoprotein and various plant lectins (14,17–19).
Isolating sufficient EPCs is a major limitation to clinical applications, as the number of EPCs obtained from bone marrow, peripheral blood, adipose tissue and cord blood is limited. To the best of our knowledge, there are no published studies aimed at obtaining EPCs from the umbilical vein by direct enzymatic digestion. The purpose of the present investigation was to isolate and characterize the population of resident ECFCs from UCs and explore it as an optimized source of ECFCs.
Materials and methods
Isolation and culture of human UC-ECFCs
A total of 10 human UCs were obtained between January 2012 and June 2015 from the General Hospital of the Chinese People's Liberation Army (Beijing, China). The present study was conducted in accordance with the Declaration of Helsinki, with approval from the Ethics Committee of the Affiliated Hospital of Academy of Military Medical Sciences (Beijing, China). All newborns' mothers provided written informed consent.
The median cell yield was 4.2×105 cells/cm of UC [number of isolated cells (mean ± standard deviation (SD)): 5.22×105±2.09×105 cells/10 cm; n=10; length of UC (mean ± SD): 20.67±2.75 cm; n=10]. The characteristics and functions, including growth kinetics, cell cycle, colony-forming ability and tube formation capability, of the isolated cells were similar among all samples. UCs were obtained by cesarean section after normal deliveries and were flushed repeatedly with phosphate-buffered saline (PBS; pH=7.0) containing 2% gentamicin (Thermo Fisher Scientific, Inc., Waltham MA, USA). Following the removal of the residual blood from the UC (particularly the umbilical vein cavity), the umbilical vein was injected with 5–10 ml 0.1% collagenase type II (Gibco; Thermo Fisher Scientific, Inc.) with dual-port ligation. Subsequently, the UCs were placed in containers with PBS and incubated for 1 h at 37°C. The digested umbilical vein was washed five times, for 2 min with PBS and the digested cells were collected by centrifugation at 256 × g at 4°C for 10 min. The resuspended cells were plated at a density of 2×104 cells/cm2 in fibronectin-coated T75 culture flasks containing complete endothelial cell growth medium (EGM)-2 medium supplemented with 10% fetal bovine serum (FBS; Lonza, Basel, Switzerland) and incubated in a humidified incubator at 37°C under 5% CO2. After 6 days, the medium was replaced to remove non-adherent cells and debris. The EPCs may be further purified by attachment-changing culture methods and subculturing for three passages, which eliminates contamination with digested smooth muscle cells for the following two reasons: First, the smooth muscle cells do not adhere to the culture container; and second, the markers detected by flow cytometry and reverse transcription-polymerase chain reaction in the present study (RT-PCR) are only expressed in EPCs. Adherent cells were passaged at a ratio of 1:3. ECFCs clustered as colonies after 5–22 days. ECFCs from passage three were used for the following experiments. Four replicates were performed.
RT-PCR
Total RNA was extracted from 5×106 ECFCs using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.) and reverse transcribed using a RevertAid First-Strand cDNA Synthesis kit (Thermo Fisher Scientific, Inc.), according to the manufacturer's protocol. To evaluate the gene expression of human endothelial-nitric oxide synthase (ec-NOS), Fms related tyrosine kinase 1 (Flt-1), KDR, vascular endothelial (VE)-cadherin, CD31, CD34, tyrosine kinase with immunoglobulin (Ig) and epidermal growth factor homology domains-2 (Tie-2) and von Willebrand factor (vWF) in ECFCs, cDNA was amplified using Premix Ex Taq (Shanghai Sangon Biological Engineering Co., Ltd., Shanghai, China) and sequence-specific primers (Table I) that were previously described (1–3,13). GAPDH was used as a control. The PCR program was as follows: 95°C for 3 min, followed by 35 cycles of 94°C for 30 sec, 55°C for 30 sec and 72°C for 45 sec, and a final step of 72°C for 5 min. The amplified samples were run on a 1% agarose gel and images were captured using the Q550CW image acquisition and analysis system (Leica Microsystems, Inc., Buffalo Grove, IL, USA). Four replicates were performed.
Table I.
Primers for human endothelial colony-forming cells.
| Gene | Primer direction | Primer sequence | Amplicon, bp | (Refs.) |
|---|---|---|---|---|
| CD31 | Forward | 5′-GCTGTTGGTGGAAGGAGTGC-3′ | 645 | (1) |
| Reverse | 5′-GAAGTTGGCTGGAGGTGCTC-3′ | |||
| CD34 | Forward | 5′-CTAGCCTTGCAACATCTCCC-3′ | 409 | (3) |
| Reverse | 5′-GAATAGCTCTGGTGGCTTGC-3′ | |||
| KDR | Forward | 5′-CAACAAAGTCGGGAGAGGAG-3′ | 819 | (1) |
| Reverse | 5′-ATGACGATGGACAAGTAGCC-3′ | |||
| Flt-1 | Forward | 5′-AGCAAGTGGGAGTTTGC-3′ | 617 | (2) |
| Reverse | 5′-AGGTCCCGATGAATGC-3′ | |||
| VE-cadherin | Forward | 5′-AAGACATCAATGACAACTTCC-3′ | 594 | (2) |
| Reverse | 5′-CCTCCACAGTCAGGTTATACC-3′ | |||
| vWF | Forward | 5′-GAGGCTGAGTTTGAAGTGC-3′ | 477 | (2) |
| Reverse | 5′-CTGCTCCAGCTCATCCAC-3′ | |||
| Tie-2 | Forward | 5′-TGTTCCTGTGCCACAGGCTG-3′ | 312 | (13) |
| Reverse | 5′-CACTGTCCCATCCGGCTTCA-3′ | |||
| ec-NOS | Forward | 5′-AAGACATTTTCGGGCTCACGCTGCGCACCC-3′ | 548 | (1) |
| Reverse | 5′-TGGGGTAGGCACTTTAGTAGTTCTCCTAAC-3′ | |||
| GAPDH | Forward | 5′-TGAAGGTCGGAGTCAACGGATTTG-3′ | 983 | (1) |
| Reverse | 5′-CATGTGGGCCATGAGGTCCACCAC-3′ |
CD, cluster of differentiation; KDR, kinase insert domain receptor; Flt-1, Fms related tyrosine kinase 1; ec-NOS, endothelial-nitric oxide synthase; VE, vascular endothelial; vWF, von Willebrand factor; Tie-2, epidermal growth factor homology domains-2.
Growth kinetics assay of human UC-ECFCs
Growth of ECFCs during passage three was plotted. Adherent cells were harvested at 80% confluence by digestion with 0.25% trypsin/EDTA (Thermo Fisher Scientific, Inc.). Subsequently, mononuclear cells were seeded in 24-well tissue culture plates at 2×104 cells/cm2 in 0.5 ml complete EGM-2 supplemented with 10% FBS, which was replaced every 4 days. The experiment continued for 7 days, and the number of living cells in three wells was evaluated by 0.4% trypan blue staining (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) after trypsinization at 4°C for 24 h, followed by observation under BX61 light microscope (Olympus Corp., Tokyo, Japan). Subsequently, the mean cell number was calculated and growth curves were generated. Four replicates were performed.
Cell cycle analysis
Human UC-ECFCs in the logarithmic phase were fixed with precooled 75% ethanol at 4°C for 1 h after trypsinization and digested with RNase A (10 µg/ml; Fuzhou Maixin Biotechnology Development Co., Ltd., Fuzhou, China) at 37°C for 30 min. Treated cells were incubated with propidium iodide (50 µg/ml) for 5 min at 4°C in the dark. Cells were analyzed by flow cytometry using a FACSCalibur flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA). The procedure was performed as previously described (20). Four replicates were performed.
Flow cytometry
All monoclonal antibodies used in the present experiment were purchased from BD Pharmingen (San Diego, CA, USA). ECFCs (1×105 cells/well) were separately labeled with eight antibodies, including fluorescein isothiocyanate (FITC)-conjugated mouse anti-human CD90 (cat no. 555595), FITC-mouse anti-human human leukocyte antigen-antigen D related protein (HLA-DR; cat no. 555560), phycoerythrin (PE)-conjugated mouse anti-human CD31 (cat no. 560983), PE-mouse anti-human CD34 (cat no. 555822), PE-mouse anti-human CD73 (cat no. 550257), PE-mouse anti-human CD105 (cat no. 560839), PE-mouse anti-human VEGRF2 (cat no. 560872) and a peridinin-chlorophyll-protein complex-conjugated mouse anti-human CD45 (cat no. 564106). Each antibody used at a dilution of 1:10. Incubation with antibodies was performed for 30 min at room temperature in the dark. After washing 2–3 times (5 min each time) with PBS, cells were analyzed by flow cytometry using a FACSCalibur flow cytometer. Data were quantified using CELLQuest software (version no. 5.1; BD Biosciences). The procedure was performed as previously described (21). Four replicates were performed.
Colony-forming assay
Colony-forming assays were performed in triplicate as previously described (22). Cells (1×103 cells/plate) were incubated in 6-cm cell culture dishes with 2 ml DF-12 culture medium (Lonza) for 14 days at 37°C and 5% CO2. After fixing in methanol at 37°C for 5 min, cells were stained with 0.5% crystal violet (Sigma-Aldrich; Merck KGaA) and observed under a BX61 microscope. A cell cluster that contained >50 cells was considered a colony. Four replicates were performed.
Matrigel assay
In vitro tube formation was assayed with BD Matrigel basement membrane matrix (BD Biosciences), according to the manufacturer's instructions. Matrigel basement membrane matrix (300 µl/well) was added to a precooled 24-well plate and then incubated at 37°C for 30 min. Subsequently, 300 µl UC-ECFCs from a monolayer (at 70–80% confluence, as recommended) was added to each well. After 24 h of culture at 37°C, capillary tube formation was assessed by BX61 microscope.
1,1′-dioctadecyl-3,3,3′,3′-tetra-methyl-indocarbocyanine perchlorate-labeled acetylated low-density lipoprotein (DiL-ac-LDL) uptake and FITC-Ulex europaeus (UEA)-1 binding assays
To assess the uptake of DiL-ac-LDL (Molecular Probes; Thermo Fisher Scientific, Inc.) by EPCs, the cells were seeded in 24-well tissue culture plates at 1×105 cells/cm2 in 1 ml of DF-12 culture medium containing DiL-ac-LDL (10 µg/ml) at 37°C for 24 h. Following analysis by fluorescence microscopy, cells were fixed with 4% paraformaldehyde at 4°C for 20 min, washed twice (2 min each time) with PBS and incubated in PBS containing 10 µg/ml plant lectin from U. europaeus conjugated to FITC (FITC-UEA-1; Sigma-Aldrich; Merck KGaA) at room temperature for 1 h. Four replicates were performed.
Assessment of cell-surface vWF expression
Immunofluorescence was used to detect cell-surface expression of vWF, which is an endothelial cell-specific gene. Cells (1×103 cells/well) were seeded on coverslips for 3 days, and then fixed in 1% paraformaldehyde at 4°C for at least 10 min. After being washed twice (2 min each time) with PBS, cells were permeabilized with 0.1% Triton X-100 (Sigma-Aldrich; Merck KGaA) four times, 5 min each time. Membranes were blocked with goat serum (cat no. SL038; dilution 1:50; Beijing Solarbio Science & Technology Co., Ltd., Beijing, China) at 37°C for 10 min. Subsequently, cells were incubated with 100 µl mouse anti-human vWF antibody (cat no. sc-516102; dilution 1:50; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) for 1 h at 37°C. After washing three times (2 min each time) with PBS, the cells were incubated with the secondary antibody, FITC-conjugated AffiniPure goat anti-mouse IgG (H+L) antibody (cat no. 127-095-099; dilution 1:50; Jackson Laboratory, Ben Harbor, ME, USA), at 37°C for 1 h. Nuclear counterstaining was performed with 1.5 µg/ml DAPI (Biotium, Inc., Hayward, CA, USA) for 30 min at 4°C. Immunofluorescence was observed under a fluorescence microscope. Four replicates were performed.
Statistical analysis
All experiments were repeated at least three times. Data were presented as the mean ± standard deviation. SPSS 13.0 software (SPSS, Inc., Chicago, IL, USA) was used for all statistical analyses. The significance of differences between mean values was assessed using Student's t-tests. P<0.05 was considered to indicate a statistically significant difference.
Results
Morphology of human UC-ECFCs
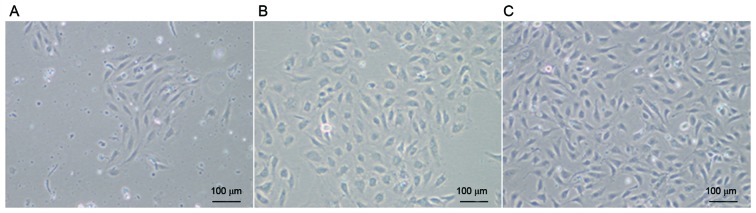
After culturing for 3–7 days, some cells started to adhere to the wall (Fig. 1A). After continuous culture for 7–10 days, cells became confluent and formed colonies (Fig. 1B), with a cobblestone appearance, which is typical of ECFCs. There were no marked changes in cell morphology during passage for 10 generations (Fig. 1C).
Figure 1.
Mononuclear cells isolated from human UCs were cultivated in complete endothelial cell growth medium-2. Cells demonstrated a typical cobblestone appearance. The human UC-derived ECFCs were (A) cultured for 72 h, (B) passaged once and (C) passaged 10 times. Scale bar, 100 µm (magnification, ×100). UC, umbilical cord; ECFCs, endothelial colony-forming cells.
Human UC-ECFC proliferation
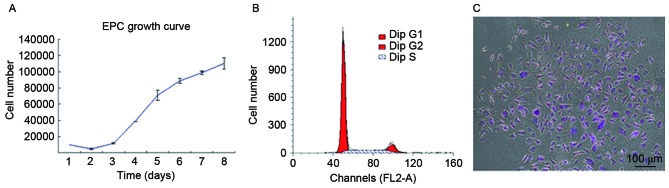
To quantitatively assess proliferative potential, the growth of UC-derived ECFCs was plotted (Fig. 2A). The results demonstrated a basic cell growth curve, strikingly divided into an incubation period, logarithmic phase and plateau phase, and all cells maintained their normal growth state. For UC-ECFCs, the incubation period was on days 1–2, which was followed by a logarithmic phase on days 3–7. After the logarithmic phase, the cells entered a plateau phase. The cell doubling time in the logarithmic growth phase was 43.53±5.38 h. This indicated that, in vitro, ECFCs demonstrated relatively stable and rapid proliferation.
Figure 2.
Proliferative potential of human UC-ECFCs was quantitatively assessed. (A) Growth curve of cultured UC-ECFCs. The cell doubling time was calculated to be ~43.53±5.38 h. Data are presented as the mean ± standard deviation. (B) The cell cycle of human UC-ECFCs was analyzed using flow cytometry. The majority of cells were in the G0/G1 phase (75.58±1.7%) with only ~24.42% in the S and G2 phases. (C) In the colony-forming study, cell colonies emerged as well-circumscribed monolayers under the microscope. Cells were stained with 0.5% crystal violet and demonstrated noticeable colony-forming potential. A total of 103 single cells were seeded onto the cell culture dish and ~36 colonies were scored. Numbers were counted in triplicate. Scale bar, 100 µm. UC-ECFCs, umbilical cord-derived endothelial colony-forming cells; EPC, endothelial progenitor cells.
Flow cytometric analysis demonstrated that 75.58% of cells were in G0/G1 phase, while 24.42% were in the S and G2/M phases (Fig. 2B). The results of the cell cycle analysis were consistent with the stemness potency of the UC-ECFCs (3).
The colony-forming ability of these cells was also measured. In the colony-forming assay, cell colonies appeared as well-circumscribed monolayers under the microscope (Fig. 2C). The colonies were counted in triplicate, and there were ~36 colonies per 103 single cells seeded in a cell culture dish.
mRNA expression of EPC-specific genes in human UC-ECFCs
By RT-PCR, the mRNA expression of Flt-1, KDR, VE-cadherin, CD31, CD34, Tie-2, vWF and ec-NOS was assessed (Fig. 3). All of these EPC-specific genes were expressed in the UC-ECFCs.
Figure 3.
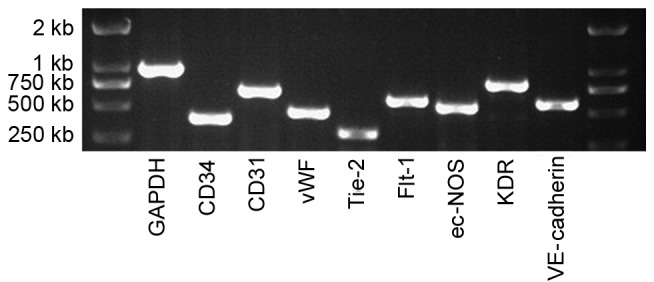
mRNA expression of human UC-ECFCs at passage three. The endothelial cell genes, CD34, CD31, vWF, Tie-2, Flt-1, ec-NOS, KDR and VE-cadherin were all expressed by these cells, suggesting that these cells were EPCs. GAPDH was used as a positive control. UC-ECFCs, umbilical cord-derived endothelial colony-forming cells; EPC, endothelial progenitor cells; CD, cluster of differentiation; vWF, von Willebrand factor; Tie-2, epidermal growth factor homology domains-2; Flt-1, Fms related tyrosine kinase 1; ec-NOS, endothelial-nitric oxide synthase; KDR, kinase insert domain receptor; VE, vascular endothelial.
Cell-surface marker expression on UC-ECFCs
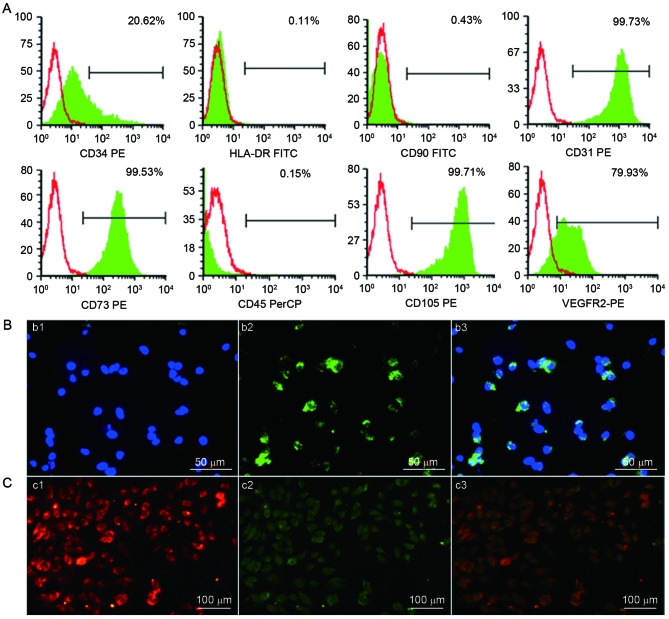
Flow cytometric analysis demonstrated that adherent cells in the human UC-ECFC cultures were positive for CD31 (99.73%), CD34 (20.62%), CD73 (99.53%), CD105 (99.71%) and VEGFR2 (79.93%). The adherent UC-ECFCs were negative for the hematopoietic marker CD45 (0.15%), mesenchymal marker CD90 (0.43%) and HLA-DR (a major histocompatibility complex-II molecule; 0.11%). These results indicated that the cultures were not contaminated with mesenchymal cells (Fig. 4A). These cells weakly expressed the hematopoietic lineage marker CD34, which decreased with passaging (data not published).
Figure 4.
Identification of human UC-ECFCs. (A) Immunophenotype of human UC-ECFCs by FACSCalibur flow cytometry. Human UC-ECFCs were positive for CD31 (99.73%), CD34 (20.62%), CD73 (99.53%), VEGFR-2 (79.93%), CD105 (99.71%) and negative for CD45 (0.15%), CD90 (0.43%) and HLA-DR (0.11%). Filled white histograms represent antigen staining with negative isotype, and the green part represents positive isotype. (B) Human UC-ECFCs expressed the endothelial cell-surface marker vWF. The immunofluorescence detection results demonstrated that the cells could be strained by (b1) DAPI (blue) and (b2) mouse anti-human vWF antibody (green). (b3) Double stained cells (merged b1 and b2) Scale bar, 50 µm. (C) Following 24 h of culture, the adherent cells were identified by the uptake of (c1) 1,1′-dioctadecyl-3,3,3′,3′-tetra-methyl-indocarbocyanine perchlorate-labeled acetylated low-density lipoprotein (red fluorescence) and binding of (c2) FITC-Ulex europaeus lectin-l (green fluorescence). (c3) Double-positive stained cells showed in yellow. Scale bar, 100 µm. UC-ECFCs, umbilical cord-derived endothelial colony-forming cells; CD, cluster of differentiation; HLA-DR, human leukocyte antigen-antigen D related protein; VEGFR, vascular endothelial growth factor receptor; PE, phycoerythrin; FITC, fluorescein isothiocyanate; PerCP, peridin-chlorophyll-protein.
The results of indirect immunofluorescent stainingwas performed to examine the expression of endothelial colony (EC) markers. The results demonstrated that the isolated ECFCs expressed vWF in a punctate pattern within the cytoplasm, which is characteristic of ECs (23,24) (Fig. 4B).
UC-ECFC DiL-ac-LDL uptake and FITC-UEA-l binding
Cellular immunostaining demonstrated that all adherent cells were capable of taking up DiL-ac-LDL and binding to FITC-UEA-l (Fig. 4C). This was characteristic of EPCs (23,24).
Tube formation capability of UC-ECFCs
The results demonstrated that human UC-ECFCs had true endothelial cell potential. Cells seeded on Matrigel and incubated for 24 h assembled into vascular tube-like structures (Fig. 5).
Figure 5.
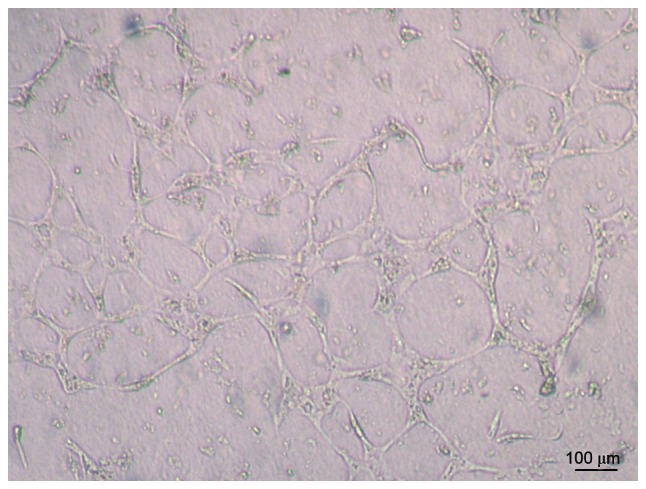
Human UC-ECFCs were plated in 24-well plates on Matrigel to analyze their tube forming capability. Cells readily formed tube-like structures. Scale bar, 100 µm. UC-ECFCs, umbilical cord-derived endothelial colony-forming cells.
Discussion
A series of studies have indicated that ECFCs, as EPCs, demonstrated a proliferative hierarchy of clones and the capability to form blood vessels in vivo (25). In the present study, human UCs were selected as a source of ECFCs as newborn UCs may be easily obtained. To isolate human UC-ECFCs, a single enzyme approach was applied, as a previously reported approach using magnetic activated cell sorting was expensive and complicated, and the cells obtained using this method demonstrated low functional activity (24,26). In the present method, collagenase type II was injected into the umbilical veins to liberate the cells. The isolation procedure was simple and economical, and sufficient amounts of ECFCs were successfully obtained through sequential flushing, digesting, flushing and collecting. Furthermore, compared with mechanical dissection methods, this single enzyme approach may better prevent cell damage and contamination.
The results of the present study demonstrated that all human UC-ECFCs obtained from the 10 UCs were stable. Compared to the limited expansion ability of EPCs isolated from other sources (24–26), UC-ECFCs exhibited a markedly higher growth rate when cultured in vitro. The cell doubling time was calculated as 43.53±5.38 h; the majority of cells were quiescent (in G0/G1 phase); and a few cells (24.42%) were in the M and S phase. The results of the cell cycle analysis were consistent with the reported stemness potencies of EPCs (23,24).
The results of the present study suggested that human UC-ECFCs may be readily expanded for clinical-scale production in a short culture period. The results of gene expression analysis demonstrated that ECFCs expressed markers identical to those in EPCs from bone marrow and cord blood, such as Flt-1, KDR, VE-cadherin, CD31, CD34, Tie-2, vWF and ec-NOS (23). In addition, the flow cytometry results demonstrated the presence of CD73, CD31, CD105 and VEGFR-2, and the absence of CD45, CD90 and HLA-DR, consistent with previously reported data (27). The present flow cytometry assay results met the aim of the present experiments.
While complex antigen phenotypes may be more specific, they are difficult to reproduce, and the complexity of the antigenic combination detected does not necessarily improve the performance of EPCs as clinical biomarkers. Thus, research should be aimed at making their isolation, identification and quantification more simple and reproducible. Furthermore, tube formation, uptake of DiL-ac-LDL and binding of FITC-UEA-l by the isolated human UC-ECFCs was observed in the present study. It was demonstrated that cultured ECFCs had all the characteristics of EPCs, and the capacity of human UC-ECFCs to form tubes was greater than that of EPCs isolated from cord blood (data not published). The above findings suggested that the cells obtained in the present study were true EPCs. In addition, one generation of cells from one 10-cm cord may be passaged for 7–15 days, and it was possible to obtain ~1×107 cells by culturing for another 2–3 days. A clinical trial demonstrated that the amount of EPCs required for one autologous EPC transplantation is 2.5 million cells per kg (19,27). Thus, using the UC-ECFC isolation method from the present study, it may be possible to obtain sufficient EPCs for clinical applications in a short time. However, further studies are required to confirm the characteristics of UC-derived ECFCs and their potential clinical applications.
In conclusion, the present study described a simple and rapid method for isolating ECFCs from human UCs. The ability of these cells to proliferate, take up DiL-ac-LDL, bind FITC-UEA-1, express endothelial/progenitor cell-specific antigens and form tubules in vitro was also evaluated. These results suggest that human UCs may be a feasible and efficient source of ECFCs for vascular injury.
Acknowledgements
The present study was supported by the ‘863 Projects’ of Ministry of Science and Technology of China (grant no. 2011AA020114), Military Clinical High-Tech Key Program (grant no. 2010gxjs100), Clinical Feature and Application Research of Capital (grant no. Z111107058811107), Science and Technology Development Projects of Shandong Province (grant no. 2012YD18066), Shandong Province Commission for Population and Family Planning (grant no. 201309) and Jining Science and Technology Bureau (grant no. 2012jnnk03).
References
- 1.Khakoo AY, Finkel T. Endothelial progenitor cells. Annu Rev Med. 2005;56:79–101. doi: 10.1146/annurev.med.56.090203.104149. [DOI] [PubMed] [Google Scholar]
- 2.Zwaginga JJ, Doevendans P. Stem cell-derived angiogenic/vasculogenic cells: Possible therapies for tissue repair and tissue engineering. Clin Exp Pharmacol Physiol. 2003;30:900–908. doi: 10.1046/j.1440-1681.2003.03931.x. [DOI] [PubMed] [Google Scholar]
- 3.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 4.Ingram DA, Mead LE, Moore DB, Woodard W, Fenoglio A, Yoder MC. Vessel wall-derived endothelial cells rapidly proliferate because they contain a complete hierarchy of endothelial progenitor cells. Blood. 2005;105:2783–2786. doi: 10.1182/blood-2004-08-3057. [DOI] [PubMed] [Google Scholar]
- 5.Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, Kearne M, Magner M, Isner JM. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999;85:221–228. doi: 10.1161/01.RES.85.3.221. [DOI] [PubMed] [Google Scholar]
- 6.Lin Y, Weisdorf DJ, Solovey A, Hebbel RP. Origins of circulating endothelial cells and endothelial outgrowth from blood. J Clin Invest. 2000;105:71–77. doi: 10.1172/JCI8071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahrens I, Domeij H, Topcic D, Haviv I, Merivirta RM, Agrotis A, Leitner E, Jowett JB, Bode C, Lappas M, Peter K. Successful in vitro expansion and differentiation of cord blood derived CD34+ cells into early endothelial progenitor cells reveals highly differential gene expression. PLoS One. 2011;6:e23210. doi: 10.1371/journal.pone.0023210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu CH, Li ZM, DU ZM, Zhang AX, Yang DY, Wu GF. Human umbilical cord-derived endothelial progenitor cells promote growth cytokines-mediated neorevascularization in rat myocardial infarction. Chin Med J (Engl) 2009;122:548–555. [PubMed] [Google Scholar]
- 9.Rapp BM, Saadatzedeh MR, Ofstein RH, Bhavsar JR, Tempel ZS, Moreno O, Morone P, Booth DA, Traktuev DO, Dalsing MC, et al. Resident endothelial progenitor cells from human placenta have greater vasculogenic potential than circulating endothelial progenitor cells from umbilical cord blood. Cell Med. 2011;2:85–96. doi: 10.3727/215517911X617888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matsumura G, Miyagawa-Tomita S, Shin'oka T, Ikada Y, Kurosawa H. First evidence that bone marrow cells contribute to the construction of tissue-engineered vascular autografts in vivo. Circulation. 2003;108:1729–1734. doi: 10.1161/01.CIR.0000092165.32213.61. [DOI] [PubMed] [Google Scholar]
- 11.Kaushal S, Amiel GE, Guleserian KJ, Shapira OM, Perry T, Sutherland FW, Rabkin E, Moran AM, Schoen FJ, Atala A, et al. Functional small diameter neovessels created using endothelial progenitor cells expanded ex vivo. Nat Med. 2001;7:1035–1040. doi: 10.1038/nm0901-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun XT, Yuan XW, Zhu HT, Deng ZM, Yu DC, Zhou X, Ding YT. Endothelial precursor cells promote angiogenesis in hepatocellular carcinoma. World J Gastroenterol. 2012;18:4925–4933. doi: 10.3748/wjg.v18.i35.4925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scheubel RJ, Zorn H, Silber RE, Kuss O, Morawietz H, Holtz J, Simm A. Age-dependent depression in circulating endothelial progenitor cells in patients undergoing coronary artery bypass grafting. J Am Coll Cardiol. 2003;42:2073–2080. doi: 10.1016/j.jacc.2003.07.025. [DOI] [PubMed] [Google Scholar]
- 14.Mandraffino G, Sardo MA, Riggio S, D'Ascola A, Alibrandi A, Saitta C, Versace A, Castaldo M, Mormina E, Imbalzano E, et al. Circulating progenitor cells and the elderly: A seven-year observational study. Exp Gerontol. 2012;47:394–400. doi: 10.1016/j.exger.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 15.O'Brien TA, Tiedemann K, Vowels MR. No longer a biological waste product: Umbilical cord blood. Med J Aus. 2006;184:407–410. doi: 10.5694/j.1326-5377.2006.tb00292.x. [DOI] [PubMed] [Google Scholar]
- 16.Tse W, Laughlin MJ. Umbilical cord blood transplantation: A new alternative option. Hematology Am Soc Hematol Educ Program. 2005:377–383. doi: 10.1182/asheducation-2005.1.377. [DOI] [PubMed] [Google Scholar]
- 17.Yoder MC. Endothelial progenitor cell: A blood cell by many other names may serve similar functions. J Mol Med (Berl) 2013;91:285–295. doi: 10.1007/s00109-013-1002-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aragona CO, Imbalzano E, Mamone F, Cairo V, Lo Gullo A, D'Ascola A, Sardo MA, Scuruchi M, Basile G, Saitta A, Mandraffino G. Endothelial progenitor cells for diagnosis and prognosis in cardiovascular disease. Stem Cells Int. 2016;2016:8043792. doi: 10.1155/2016/8043792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sidney LE, Branch MJ, Dunphy SE, Dua HS, Hopkinson A. Concise review: Evidence for CD34 as a common marker for diverse progenitors. Stem Cells. 2014;32:1380–1389. doi: 10.1002/stem.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reinisch A, Strunk D. Isolation and animal serum free expansion of human umbilical cord derived mesenchymal stromal cells (MSCs) and endothelial colony forming progenitor cells (ECFCs) J Vis Exp. 2009 doi: 10.3791/1525. pii: 1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zeisberger SM, Zoller S, Riegel M, Chen S, Krenning G, Harmsen MC, Sachinidis A, Zisch AH. Optimization of the culturing conditions of human umbilical cord blood-derived endothelial colony-forming cells under xeno-free conditions applying a transcriptomic approach. Genes Cells. 2010;15:671–687. doi: 10.1111/j.1365-2443.2010.01409.x. [DOI] [PubMed] [Google Scholar]
- 22.Shintani S, Murohara T, Ikeda H, Ueno T, Sasaki K, Duan J, Imaizumi T. Augmentation of postnatal neovascularization with autologous bone marrow transplantation. Circulation. 2001;103:897–903. doi: 10.1161/01.CIR.103.6.897. [DOI] [PubMed] [Google Scholar]
- 23.Dome B, Timar J, Dobos J, Meszaros L, Raso E, Paku S, Kenessey I, Ostoros G, Magyar M, Ladanyi A, et al. Identification and clinical significance of circulating endothelial progenitor cells in human non-small cell lung cancer. Cancer Res. 2006;66:7341–7347. doi: 10.1158/0008-5472.CAN-05-4654. [DOI] [PubMed] [Google Scholar]
- 24.Resch T, Pircher A, Kähler CM, Pratschke J, Hilbe W. Endothelial progenitor cells: Current issues on characterization and challenging clinical applications. Stem Cell Rev. 2012;8:926–939. doi: 10.1007/s12015-011-9332-9. [DOI] [PubMed] [Google Scholar]
- 25.Zhang H, Zhang B, Tao Y, Cheng M, Hu J, Xu M, Chen H. Isolation and characterization of mesenchymal stem cells from whole human umbilical cord applying a single enzyme approach. Cell Biochem Funct. 2012;30:643–649. doi: 10.1002/cbf.2843. [DOI] [PubMed] [Google Scholar]
- 26.Yoder MC, Mead LE, Prater D, Krier TR, Mroueh KN, Li F, Krasich R, Temm CJ, Prchal JT, Ingram DA. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood. 2007;109:1801–1809. doi: 10.1182/blood-2006-08-043471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao YH, Yuan B, Chen J, Feng DH, Zhao B, Qin C, Chen YF. Endothelial progenitor cells: Therapeutic perspective for ischemic stroke. CNS Neurosci Ther. 2013;19:67–75. doi: 10.1111/cns.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]