Abstract
Successfully expanding hematopoietic stem cells (HSCs) is advantageous for clinical HSC transplantation. The present study investigated the influence of testosterone on the proliferation, antigen phenotype and expression of hematopoiesis-related genes in umbilical cord blood-derived cluster of differentiation (CD)34+ cells under normoxic or hypoxia conditions. Cord blood (CB) CD34+ cells were separated using magnetic activated cell sorting. A cytokine cocktail and feeder cells were used to stimulate the expansion of CD34+ cells under normoxic (20% O2) and hypoxic (1% O2) conditions for 7 days and testosterone was added accordingly. Cells were identified using flow cytometry and reconstruction capacity was determined using a colony-forming unit (CFU) assay. The effects of oxygen concentration and testosterone on the expression of hematopoietic-related genes, including homeobox (HOX)A9, HOXB2, HOXB4, HOXC4 and BMI-1, were measured using reverse transcription-quantitative polymerase chain reaction. The results indicated that the number of CFUs and total cells in the testosterone group increased under normoxic and hypoxic conditions compared with the corresponding control groups. Furthermore, the presence of testosterone increased the number of CFU-erythroid colonies. In liquid culture, the growth of CD34+ cells was rapid under normoxic conditions compared with under hypoxic conditions, however CD34+ cells were maintained in an undifferentiated state under hypoxic conditions. The addition of testosterone under hypoxia promoted the differentiation of CD34+ cells into CD34+CD38+CD71+ erythroid progenitor cells. Furthermore, it was determined that the expression of hematopoietic-related genes was significantly increased (P<0.05) in the hypoxia testosterone group compared with the other groups. Therefore, the results of the current study indicate that a combination of hypoxia and testosterone may be a promising cultivation condition for HSC/hemopoietic progenitor cell expansion ex vivo.
Keywords: hematopoietic stem cells, expansion, testosterone, hypoxia, umbilical cord blood
Introduction
Hematopoietic stem cell (HSC) transplantation is a potentially life-saving procedure used to treat a broad spectrum of disorders, including hematological, immune and genetic diseases (1). It has been demonstrated that bone marrow reconstituting HSCs reside within a small subpopulation of bone marrow or blood-derived mononuclear cells that express the surface antigen cluster of differentiation (CD)34. The efficacy of cord blood (CB) transplantation is limited by the low cell dose available. Low cell doses at transplant are correlated with delayed engraftment, prolonged neutropenia and thrombocytopenia and elevated risk of graft failure. The successful ex vivo culture and amplification of blood-derived CD34+ progenitor cells offers the possibility of HSC transplantation (2). CB is used as an alternative for bone marrow or mobilized peripheral blood grafts, particularly when no matched human leukocyte antigen-related or unrelated donors are available (3). Under similar conditions, recipients of CB transplants exhibit a lower incidence of acute and chronic graft-versus-host disease compared with recipients of bone marrow transplants (4). The use of CB as a source of HSCs utilized for transplantation has increased and >3,000 CB transplants are conducted annually (5).
Allogeneic transplantation with human umbilical cord blood (hUCB) in adult recipients is mainly limited by a low CD34+ cell dose (6). Thus, CBT is generally only used in children and low-weight adults. Multiple strategies have been investigated to try to overcome these limitations, one of which involves the ex vivo expansion of CB units prior to transplantation (7). Previous studies have demonstrated that HSCs may expand, suggesting that in vitro HSCs should be exposed to specific factors and signals that promote self-renewal and amplification (8–10). Furthermore, it has been demonstrated that cell survival and proliferation in vitro may be efficiently stimulated by several cytokines, particularly stem cell growth factor and thrombopoietin (TPO) (11). It has also been indicated that the fate of HSCs may be chemically modulated by adding small biological and chemical molecules to the culture media in vitro to induce cell survival and division, while simultaneously preventing stem cell differentiation (1). Small molecules, including all-trans retinoic acid copper chelator, tetraethylenepentamine, prostaglandin E2 and 6-bromoindirubin-3′-oxime (BIO) all serve a role in HSC (12); for instance, all-trans retinoic acid serves a role in stem cell differentiation to several lineages, including myeloid differentiation, and BIO is the first pharmacological agent demonstrated to maintain self-renewal in embryonic stem cells (12).
The present study evaluated the effects of three small-molecule steroid hormones, testosterone, norepinephrine and epinephrine, on HSCs. As HSCs all express the surface antigen CD34, CD34+ cells were selected for subsequent experiments.
Oxygen concentration is an important influence on the growth of HSCs. In vivo, HSCs are found in microenvironments, known as niches, in the bone marrow. Throughout the bone marrow, physiological oxygen concentrations are <4% and almost 0% in certain areas (13). It has been hypothesized that the hypoxic environment maintains the characteristics of HSCs and numerous in vitro studies investigating the cultivation of HSCs under hypoxic conditions have been performed (14–17). Thus, the present study included hypoxia as a condition in the study design, in order to determine the effects of the three small molecules on CD34+ cell amplification under hypoxic conditions.
Materials and methods
Collection and purification of CD34+ cells
Human CB (n=12; male newborns) was obtained from mothers undergoing full-term deliveries between January and May 2015 in the Department of Obstetrics in Qilu Hospital of Shandong University (Jinan, China) after informed written consent was obtained. Maternal age was between 20 to 40 years old (mean, 26±2), and there was no history of acute, chronic or infectious disease, neonatal apnea, edema or jaundice. The present study was approved by the Ethics Committee of Qilu Hospital of Shandong University. Within 12 h of harvesting the CB. Mononuclear cells (MNCs) were separated using Ficollpaque medium (density 1.077±0.001 g/ml; HaoYang company, Tianjin, China) and centrifuging at 1,726 × g at 12°C for 25 min. Isolated MNCs were collected and washed twice in RPMI 1640 (Gibco, Los Angeles, USA) plus 5% fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA). Cord blood MNCs were incubated with 100 µl of CD34+ micro beads (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany; cat. no. 130046703) at 4°C for 30 min. Cells were subsequently passed through an LS MACS column (Miltenyi Biotec GmbH) and enriched CD34+ cells were collected in 15 ml tubes by flushing the column. Cells were subsequently suspended in 0.1 M phosphate buffered saline (PBS; pH 7.4). The purity of CD34+ cells was detected using a fluorescence-activated cell sorting system (Guava easyCyte8HT; EMD Millipore, Billerica, MA, USA) and data were analyzed with Guava Incyte version 2.8 (EMD Millipore).
Hormone screening test
CD34+ cells at a density of 2×103 cells/well were seeded into 24-well plates with methylcellulose semisolid medium (MethoCult™ GF H4434) supplemented with stem cell factor (SCF), granulocyte-macrophage colony-stimulating factor, erythropoietin and interleukin (IL)-3 (all Stem Cell Technologies, Inc., Vancouver, BC, Canada) and used to perform colony-forming unit (CFU) assays following the manufacturer's instructions. Cells were cultured under either normoxic or hypoxic conditions. CD34+ cells were divided into control, testosterone (4.6×10−8 mol/l; XianJu company, Zhejiang, China), norepinephrine (5.9×10−5 mol/l; ShuangHe Company, Beijing, China) and epinephrine groups (2.7×10−6 mol/l; YongKang company, Beijing, China). The control groups were treated without hormones and at the same oxygen concentration as other groups. The concentrations of these hormones were determined as previously described (18–23). Cells were incubated in an atmosphere containing 20% O2 (normoxic conditions) using a Heal Force Tris-gas incubator (HF240; Heal Force Bio-meditech Holdings Limited, Shanghai, China) or 1% O2 (hypoxic conditions; HF100; Heal Force Bio-meditech Holdings Limited) containing 5% CO2 at 37°C. Following 2 weeks culture, the number and type of CFUs were determined using an inverted microscope (IX71 Olympus Inverted Microscope; Olympus Corporation; Tokyo, Japan). The types of colonies identified included colony-forming units-erythroid (CFU-E), burst-forming unit-erythroid (BFU-E), colony-forming unit-granulocyte/macrophage (CFU-GM) and colony forming units-mixed (CFU-Mix; >50 cells) as previously described (24).
Preparation of feeder
Umbilical cord tissue was also obtained from the healthy donor mothers who donated CB for the present study. Informed written consent was received. Umbilical cords were dissected following thorough washing and blood vessels were removed. Small fragments (1–2 mm3) were cut and placed in plates containing low glucose-Dulbecco's modified Eagle's medium (L-DMEM) supplemented with 10% fetal bovine serum, 100 U/ml penicillin and 100 µg/ml streptomycin (All Gibco; Thermo Fisher Scientific, Inc.). Cultures were maintained at 37°C in a humidified atmosphere containing 5% CO2, as previously described (25,26). The medium was replenished every 3–4 days. Following 7 days culture, adherent cells were observed growing from the individual tissue explants. Adherent fibroblast-like cells became confluent following 2–3 weeks culture. Subsequently, cells were treated with 0.25% trypsin (Gibco; Thermo Fisher Scientific, Inc.) and passaged at 1×104 cells/cm2 in L-DMEM. Cells at the 5 and 7th passage were used following γ-irradiation at a dose of 15 Gy following a previously described protocol (25). A total of 5×105 umbilical cord-mesenchymal stem cells were seeded in a 25-cm2 culture flask and served as the feeder layer for subsequent experiments.
Co-cultivation of CD34+ cells with feeder
CB CD34+ cells (1.1×105 cells/ml) were co-cultured with feeder in HSC expansion medium (Stem Cell Technologies, Inc.) supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.), 70 ng/ml SCF, 30 ng/ml IL-3, 30 ng/ml FMS-like tyrosine kinase 3 ligand, 20 ng/ml of IL-6, 20 ng/ml bone morphogenetic protein-2 and 20 ng/ml TPO (all R&D Systems, Inc., Minneapolis, MN, USA). Cultures were maintained at 37°C in an atmosphere containing 5% CO2 for 7 days. Cytokine concentrations were determined as described previously (11,27). CD34+ cells were divided into 4 groups: i) A normoxia testosterone group, consisting of a co-culture of CD34+ cells and feeder plus testosterone undecanoate (4.6×10−8 mol/l) in 20% O2; ii) a normoxia control group, consisting of co-culture of CD34+ cells and feeder without hormone in 20% O2; iii) a hypoxia testosterone group, consisting of a co-culture of CD34+ cells and feeder plus testosterone undecanoate in 1% O2 and iv) a hypoxia control group, consisting of a co-culture of CD34+ cells and feeder without hormone in 1% O2. The medium was replenished every 3–4 days. On day 7, the total cell suspensions were harvested for use in subsequent experiments.
CFU assay following liquid culture
CD34+ cells were amplified 7 days after co-cultivation with feeder and were seeded into 24-well plates in the MethoCult GF H4434 medium. The cells were seeded at a density of 2×103 cells/well following the manufacturer's instructions for the CFU assay. Each cell group was plated in 6 replicate wells and cultures were maintained at 37°C in a humidified atmosphere with 5% CO2 and 20% O2. Following 2 weeks culture, the number and type of CFUs were determined using an inverted microscope (magnification, ×20; IX71 Olympus Inverted Microscope; Olympus Corporation).
Immunophenotypic analysis
Following co-culture of CD34+ cells for 7 days, cells were confirmed by four-color flow cytometry using a fluorescence-activated cell sorting Calibur analyzer (Guava Cyte 8HT; EMD Millipore). The cells (1×106) were suspended in 100 µl PBS containing fluorescein isothiocyanate-conjugated anti-CD34 antibody 10 µl (BioLegend, San Diego, USA; cat. no. 343604), phycoerythrin-conjugated anti-CD71 (BD Biosciences, San Jose, CA, USA; cat. no. 560981), and phycoerythrin-71-conjugated anti-CD38 antibodies (BD Biosciences; cat. no. 555537; 1:10) for 15 min at room temperature, following the manufacturer's instructions. Cells were subsequently washed with PBS and the data was examined using Guava Incyte (Version 2.8, EMD Millipore).
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
CD34+ cells were underwent RT-qPCR following 7 days co-culture in vitro. Total RNA was extracted using TRIzol reagent (Ambion; Thermo Fisher Scientific, Inc.) and cDNA was synthesized using the ReverTra Ace QPCR RT Master mix kit (Toyobo Co., Ltd., Osaka, Japan) according to the manufacturer's protocol. Levels of homeobox (HOX)A9, HOXB2, HOXB4, HOXC4, BMI1, GATA-1, C-MYB, HOXB6, NFE2 and hypoxia inducible factor α (HIF-1α) were analyzed using qPCR (95°C for 1 min, 95°C for 15 sec, and 60°C for 1 min for a total of 40 cycles) on an ABI 500 PCR system (Applied Biosystems; Thermo Fisher Scientific, Inc.) with SYBR green I dye (Toyobo Co., Ltd.). Table I presents the primer sequences used in RT-qPCR and all primers were purchased from BioSun Technology Co. Ltd. (Shanghai, China). GAPDH was used as an internal control. The expression of each gene was determined using the 2−ΔΔCq method (28) and data were analyzed using Sequence Detection software (version 1.4; Applied Biosystems; Thermo Fisher Scientific, Inc.). The expression of mRNA is presented as the fold difference with respect to the untreated control groups and the control group values were set at a fold change equal to one.
Table I.
Primer sequences used for reverse transcription-quantitative polymerase chain reaction.
| Gene | Direction | Primer sequence (5′-3′) |
|---|---|---|
| HOXB4 | Forward | AGCACGGTAAACCCCAATTACG |
| Reverse | GTGTCAGGTAGCGGTTGTAGTG | |
| HOXB6 | Forward | TCGTGCAACAGTTCCTCCTT |
| Reverse | CGCGTCAGGTAGCGATTGTA | |
| HOXA9 | Forward | CCACGCTTGACACTCACACT |
| Reverse | GGGTTATTGGGATCGATGGGG | |
| GATA1 | Forward | GACACTCCCCAGTCTTTCAGG |
| Reverse | CAGTTGAGGCAGGGTAGAGC | |
| NFE2 | Forward | ACTCTGGCCCAGTAGGATGT |
| Reverse | TTGGAGCATTCAGACCCTGC | |
| HIF-1α | Forward | TTCCTTCTCTTCTCCGCGTG |
| Reverse | AACTTATCTTTTTCTTGTCGTTCGC | |
| HOXB2 | Forward | CTAGCCTACAGGGTTCTCTC |
| Reverse | CACAGAGCGTACTGGTGAAAAA | |
| BMI-1 | Forward | TGGACTGACAAATGCTGGAGA |
| Reverse | GAAGATTGGTGGTTACCGCTG | |
| C-MYB | Forward | GAGGTGGCATAACCACTTGAA |
| Reverse | AGGCAGTAGCTTTGCGATTTC | |
| HOXC4 | Forward | GCACCGTCAAGGCTGAGAAC |
| Reverse | TGGTGAAGACGCCAGTGGA | |
| GAPDH | Forward | GCACCGTCAAGGCTGAGAAC |
| Reverse | TGGTGAAGACGCCAGTGGA |
HOX, homeobox; HIF-1α, hypoxia inducible factor α.
Statistical analysis
Data were analyzed using SPSS software version 14.0 (SPSS Inc., Chicago, IL, USA). Quantitative data are presented as the mean ± standard deviation. Two-way analysis of variance with Fisher's least significant difference as a post hoc analysis was used for comparisons among multiple groups. P<0.05 was considered to indicate a statistically significant difference.
Results
Effects of hormone and oxygen concentrations on CFU
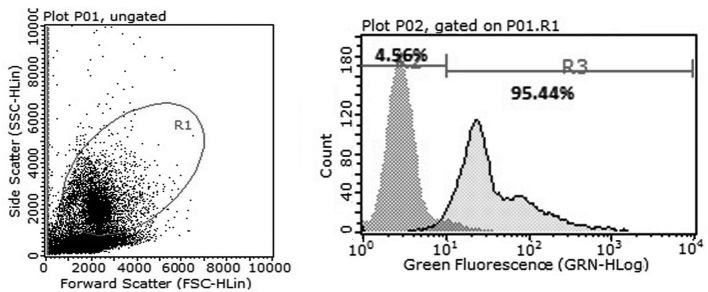
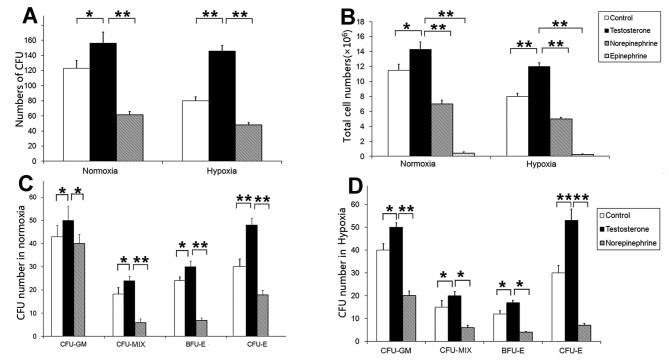
Magnetic activated cell sorting and flow cytometric analysis demonstrated that >95% cells expressed CD34+ (Fig. 1). Subsequently, the effects of testosterone, norepinephrine and epinephrine on the colony formation function of CD34+cells were investigated. The colony number of the testosterone groups were significantly increased compared with the control and norepinephrine groups under normoxia (P<0.05 and P<0.01, respectively) and hypoxia (both P<0.01; Fig. 2A). There was no evidence of colony formation in the epinephrine group under normoxic and hypoxic conditions, thus the epinephrine group was excluded from the analysis. In addition, the total number of cells in the testosterone group was significantly increased compared with the control, norepinephrine and epinephrine groups under normoxia (P<0.05, P<0.01 and P<0.01 respectively) and hypoxia (all P<0.01; Fig. 2B).
Figure 1.
Flow cytometric analysis determined the magnetic activated cell sorting-enriched CD34+ cell purity. Flow cytometry indicated that >95% magnetic activated cell sorting-enriched cells expressed CD34+. CD, cluster of differentiation.
Figure 2.
Effects of different hormones and different oxygen concentrations on CFU formation (A) Comparison of the number of CFUs in different groups. (B) Comparison of the total number of cells in different groups. (C) Comparison of the colony types in different normoxia groups. The presence of testosterone significantly increased the numbers of CFU, particularly CFU-Es. (D) Comparison of the colony types among different hypoxia groups. The presence of testosterone significantly increased CFU numbers, particularly CFU-Es. Norepinephrine significantly inhibited colony formation and cell amplification under normoxic and hypoxic conditions. Data are presented as the mean + standard deviation. *P<0.05 and **P<0.01. CFU, Colony-forming unit; CFU-GM, colony-forming unit-granulocyte/macrophage; CFU-MIX, colony forming units-mixed; BFU-E, burst-forming unit-erythroid; CFU-E, colony-forming units-erythroid.
Regarding colony types, the presence of testosterone significantly increased the number of different CFUs (Fig. 2C). In the testosterone groups, under normoxic conditions the number of CFU-GMs was significantly increased (50±8) compared with the control group (43±6; P<0.05) and the norepinephrine group (39±4; P<0.05). Furthermore, the number of CFU-MIXs was significantly increased (22±2) compared with the control group (18±3; P<0.05) and the norepinephrine group (5±1; P<0.01). In addition, the number of BFU-Es was significantly increased (30±4) compared with the control group (26±5; P<0.05) and the norepinephrine group (6±1; P<0.01). The number of CFU-Es was significantly increased (48±5) compared with the control group (30±6; P<0.01) and the norepinephrine group (18±2; P<0.01; Fig. 2C). In the testosterone groups under hypoxic conditions, the number of CFU-GM colonies was significantly increased (50±2) compared with the control group (40±4; P<0.05) and the norepinephrine group (20±2; P<0.01). The number of CFU-MIX colonies was significantly increased (19±2) compared with the control group (18±3; P<0.05) and the norepinephrine group (5±2; P<0.05), and the number of BFU-E colonies was significantly increased (17±2) compared with the control group (14±3; P<0.05) and the norepinephrine group (3±1; P<0.05). Similarly, the number of CFU-E colonies was significantly increased (54±8) compared with the control group (28±5; P<0.01) and the norepinephrine group (5±1; P<0.01; Fig. 2D). The results demonstrated that norepinephrine and epinephrine may inhibit cell amplification under normoxic and hypoxic conditions and were therefore not used in subsequent experiments.
Expansion of CD34+ cells and CFU assay
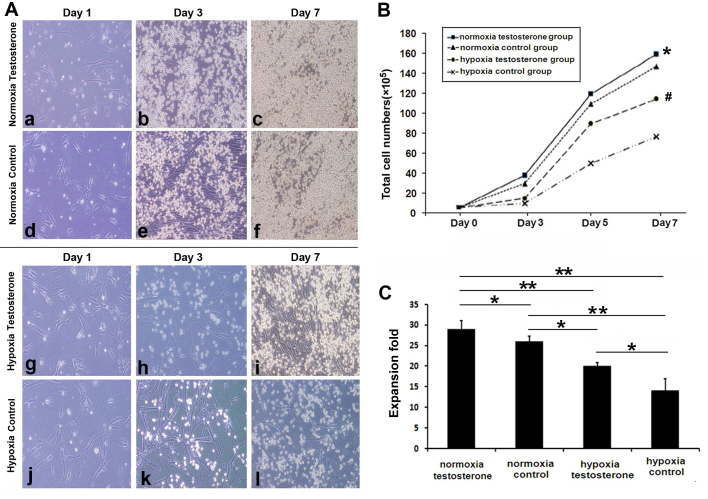
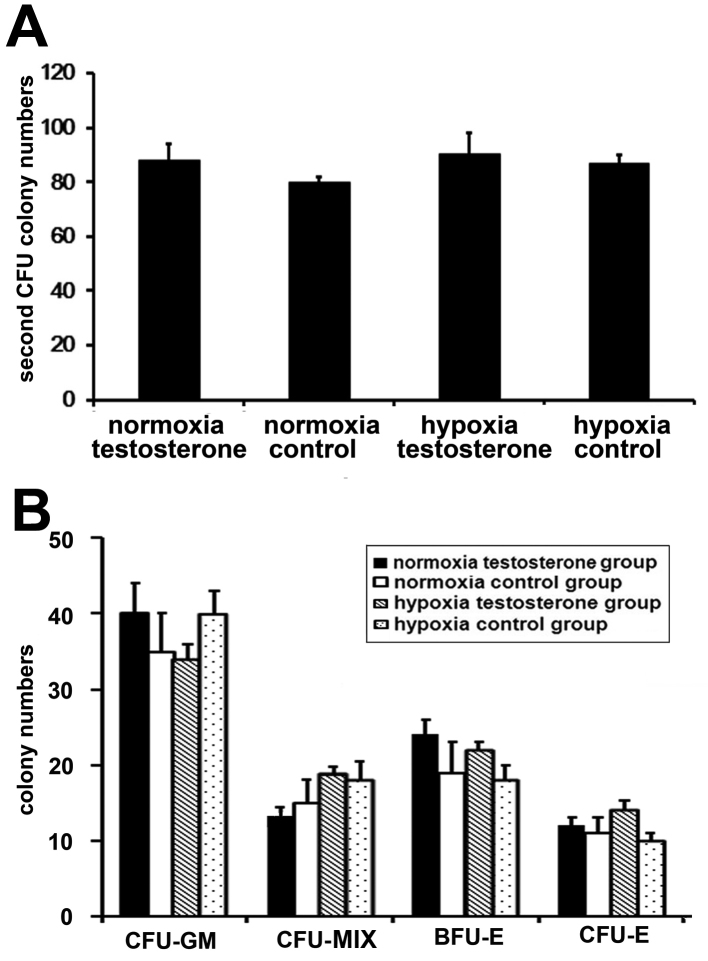
Fresh enriched CB CD34+ cells were co-cultured for 7 days with feeder. Under normoxic conditions, during the first 3 days the cells rapidly entered the logarithmic growth period (testosterone group, 41.53±4.31×105; control group, 30.65±2.74×105; P>0.05), whereas cells under hypoxia grew more slowly (testosterone group, 15.22±3.41×105; control group, 13.37±2.10×105; P>0.05; Fig. 3A and B). However, there was an acceleration in the growth of cells in the hypoxia groups after the first 3 days (testosterone group, 118.44±17.72×105; control group, 79.04±10.54×105; P<0.05 Fig. 3B). Following 7 days culture, total cell numbers in the two normoxia groups were significantly increased compared with the two hypoxia groups (P<0.05; Fig. 3C). In addition, the highest amplification efficiency of the cells was observed in the normoxia testosterone group. Subsequently, the expanded CD34+ cells were cultured in methylcellulose medium for the CFU assay to observe the differential potential of HPCs. No significant differences were observed in the number (P>0.05; Fig. 4A) and type (P>0.05; Fig. 4B) of CFUs among the 4 groups.
Figure 3.
Cluster of differentiation CD34+ cell expansion with cytokine cocktail and feeder (magnification, ×200). (A) Cells in the (a-f) normoxia or (g-l) hypoxia testosterone and control groups were cultured for (a,d,g,l) 1, (b,e,h,j) 3 and (c,f,i,l) 7 days. Cell proliferation was markedly higher in the normoxia groups compared with the hypoxia groups and addition of testosterone promoted cell expansion in the normoxia and hypoxia groups. (B) Comparison of the total cell growth rate among all groups. The amplification efficiency was significantly increased in the normoxia groups compared with the hypoxia groups and the presence of testosterone increased cell expansion, particularly during the final 4 days under hypoxia. *P<0.05 vs. normoxia control, hypoxia testosterone and hypoxia control groups. #P<0.05 vs. hypoxia control group. (C) Comparison of total cell numbers. Cell numbers were significantly increased in the normoxia groups compared with the hypoxia groups. The addition of testosterone increased amplification in normoxia and hypoxia groups. Data are presented as the mean + standard deviation. *P<0.05 and **P<0.01.
Figure 4.
CD34+ cells were amplified 7 days later and transferred into the methylcellulose medium for CFU assay. Similar CFU colony numbers were observed among all groups, indicating that the ability of CD34+ cells to differentiate was not affected. (A) Comparison of CFU numbers in all groups indicated that there was no significant difference among any of the groups. (B) Comparison of CFU types in all groups. No significant differences were observed among groups. Data are presented as the mean + standard deviation. CD, cluster of differentiation; CFU, colony-forming unit; CFU-GM, colony-forming unit-granulocyte/macrophage; CFU-MIX, colony forming units-mixed; BFU-E, burst-forming unit-erythroid; CFU-E, colony-forming units-erythroid.
Analysis of cell phenotypes
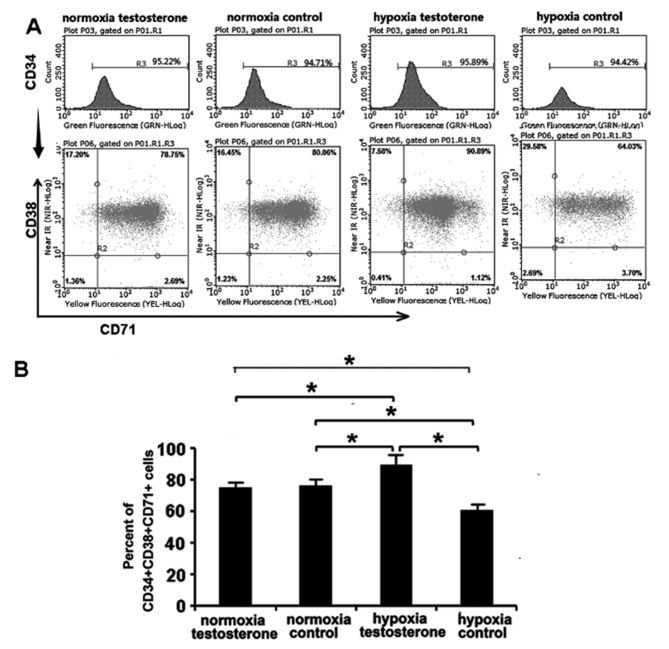
Following culture of CD34+ cells for 7 days, CD34, CD38 and CD71 antigen phenotypes were analyzed using flow cytometry (Fig. 5). CD34 and CD38 are the surface markers currently used to identify HSC/HPC and CD71 is the surface marker of erythroid progenitor cells (29,30). The results demonstrated that the proportion of CD34+CD38+CD71+ cells in the normoxia testosterone and control groups were similar (74.98±8.79% vs. 75.82±9.50%, respectively; P>0.05; Fig. 5). However, there were significant differences between the hypoxia testosterone and control groups (87.15±10.13% vs. 60.45±6.58%; P<0.05; Fig. 5). Furthermore, the proportion of CD34+CD38+CD71+ cells in the hypoxia testosterone group was significantly higher compared with the other groups (87.15±10.13% vs. 74.98±8.79%, 75.82±9.50% and 60.45±6.58%; P<0.05). The proportion of CD34+CD38+CD71+ cells in the hypoxia control group was lowest compared with the other groups (60.45±6.58%; P<0.05; Fig. 5). These results suggest that hypoxia may be beneficial in maintaining CD34+ cells in an undifferentiated state and the addition of testosterone may promote the differentiation of CD34+ cells into erythroid hematopoietic progenitor cells (HPCs).
Figure 5.
Detection of the hematopoietic stem cell antigen phenotype. (A) Flow cytometry graphs of the normoxia testosterone, normoxia control, hypoxia testosterone and hypoxia control groups. (B) The proportion of CD34+CD38+CD71+ cells in the groups were compared and it was determined that the hypoxic testosterone group exhibited a significantly increased percentage of such cells compared with the other groups. The proportion of CD34+CD38+CD71+ cells was lowest in the hypoxic control group. Data are presented as the mean + standard deviation. *P<0.05. CD, cluster of differentiation.
Expression of hematopoiesis-related genes
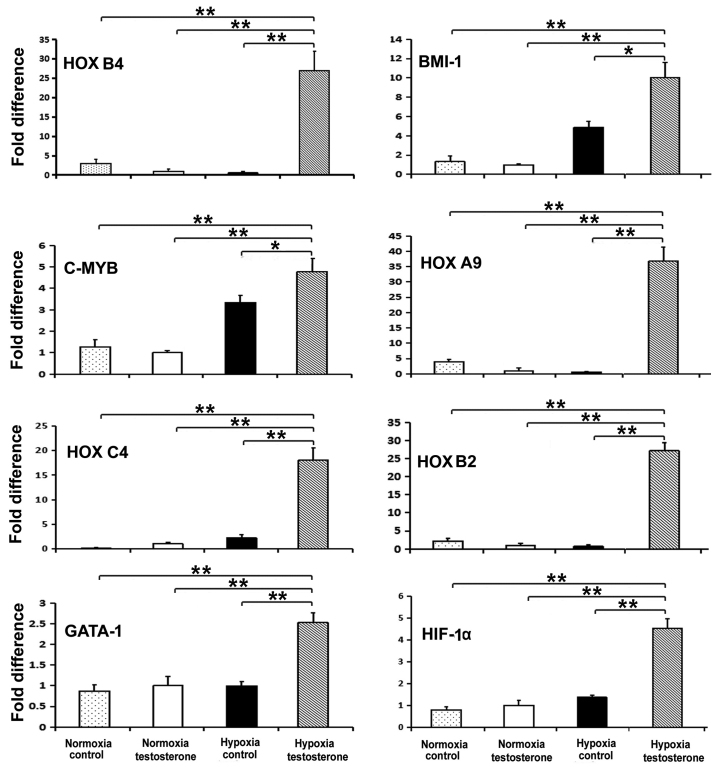
RT-qPCR was performed to detect the expression of the stem cell-specific genes HOXB4, BMI-1 and C-MYB, the erythroid-specific genes HOXB2 and HOXB6, the lymphocyte lineage-related gene HOXC4, the granulocyte lineage-related gene HOXA9, the megakaryocyte lineage-related genes GATA-1 and NFE2, and the hypoxia-related gene HIF-α to determine the properties of self-renewal and multi-differentiation of CD34+ cells. Following 7 days culture, the expression of the HOXA9, HOXB2, HOXB4, BMI1, HOXC4, GATA-1, HIF-1α and C-MYB genes were significantly increased in the hypoxia testosterone group compared with all other groups (P<0.05; Fig. 6). However, the expression of HOXB6 and NFE2 did not significantly differ among the hypoxia control group, normoxia testosterone group and normoxia control group (data not shown).
Figure 6.
The expression of genes in co-cultured CD34+ cells and feeder after 1 week. The results demonstrated that the expression of the HOXA9, HOXB2, HOXB4, BMI1, HOXC4, GATA-1, HIF-1α, and C-MYB genes was significantly higher in the hypoxia testosterone group compared with all other groups. Data are presented as the mean + standard deviation. *P<0.05 and **P<0.01. HOX, homeobox; HIF-1α, hypoxia inducible factor α.
Discussion
To promote efficient HSC proliferation and to mimic the microenvironment in the bone marrow, small molecules were introduced in the culture medium. In the present study, testosterone, norepinephrine and epinephrine small-molecule steroid hormones were selected and the effects of these hormones on CD34+ cells were investigated. The results demonstrated that the number of CFUs and total cells in the testosterone group were significantly increased under normoxic and hypoxic conditions compared with the corresponding control groups. Furthermore, the results indicated that norepinephrine and epinephrine significantly inhibited colony formation and cell amplification under normoxic and hypoxic conditions. As a result, testosterone was selected for use in subsequent experiments.
It has been reported that androgens significantly reduce the quiescence ratio and promote HSC proliferation (31). Previous studies have also demonstrated that testosterone significantly enhances colony formation and the expansion of HSCs (32–34). Kim et al (20) revealed that androgens exhibit a modest growth- and survival-enhancing effect on CFU-E, but not on CFU-GM or BFU-E. Similarly, the present study demonstrated that testosterone significantly increased the number of different types of CFUs, particularly CFU-E, under normoxic and hypoxic conditions. These results indicate that androgens have an effect on HPC that is restricted to mature erythroid progenitors. Norepinephrine and epinephrine also affected HSCs. Norepinephrine signaling controls HSC/HPC mobilization and the sympathetic nervous system to regulate the attraction of stem cells to their niche (35). However, increasing the concentrations of norepinephrine and epinephrine may reduce HSC cloning, thereby inhibiting hematopoiesis (18,23,30).
Previous studies have reported that hypoxia is beneficial in maintaining the self-renewal properties of HSCs (36–38). Furthermore, a number of studies have suggested that hypoxia may promote HSC amplification (14,39,40). Ivanovic et al (14) identified that 3% was the lowest O2 concentration that resulted in the same rate of colony-forming cell expansion as when O2 concentration was 20%. In the present study, the number of CFUs and total cells in all hypoxia groups were significantly decreased compared with those in the normoxia groups. These results were consistent with the results of a study by Eliasson et al (16). However, in the present study, the addition of testosterone promoted CD34+ cell amplification in hypoxia and normoxia groups, indicating that hypoxia and testosterone may be important factors regulating the growth of CD34+ cells.
The results of the present study suggested that in the normoxia groups, CD34+ cells rapidly entered the logarithmic growth period within the first 3 days in liquid culture. Cell amplification in the normoxia testosterone group was increased, whereas cells grew slowly in the hypoxia groups. Notably, cell amplification in the hypoxia testosterone groups gradually accelerated between days 3 and 7 in culture. Although hypoxia was not beneficial to CD34+ cell proliferation, treatment with testosterone promoted CD34+ cell proliferation in normoxic and hypoxic conditions. Flow cytometry determined that the proportion of CD34+CD38+CD71+cells was lowest in the hypoxic control group, indicating that hypoxic conditions were beneficial in maintaining the characteristics of CD34+ cells and delaying differentiation. Additionally, the highest proportion of CD34+CD38+CD71+ cells was detected in the hypoxic testosterone group. These results suggest that a combination of hypoxia and testosterone may promote the differentiation of CD34+ cells into erythroid HPCs. To further identify the ability of hematopoietic reconstitution, a CFU assay was performed. A variety of colonies formed, demonstrating that the multi-differentiation ability of the cells was unaffected by hypoxia or testosterone.
The expression of hematopoiesis-related genes in CD34+ cells was detected using RT-qPCR and it was determined that there were significant differences in the levels of gene expression between the hypoxia testosterone group and the other groups. In the hypoxia testosterone group, the expression of hematopoiesis-related genes, including HSC-specific and differentiated, erythroid-specific, lymphocyte lineage-related granulocyte lineage-related, megakaryocyte lineage-related and hypoxia-related genes, were significantly higher compared with all other groups. This increase in the expression of genes may have been caused in part by the differentiation of CD34+ cells into HPCs. The results of the secondary CFU assay and flow cytometry suggested that an increased number of CD34+cells were differentiated into erythroid HPCs under the combined effects of testosterone and hypoxia. This indicates that the combination of hypoxia and testosterone was advantageous in promoting the expression of hematopoietic genes.
Androgens promote the amplification of HSCs but cannot effectively maintain the self-renewal characteristics of stem cells. Huang et al (41) demonstrated that bone marrow mesenchymal stem cells in androgen receptor-knockdown mice exhibited enhanced self-renewal ability. Nilutamidie is an anti-androgen agent that blocks the effects of androgen and promotes the self-renewal of ESCs (42). Given the common characteristics of stem cells, it was speculated that androgens may promote HSC proliferation rather than maintain the self-renewal of HSCs. Hypoxia (1%) is conducive to maintaining the undifferentiated HSC state but does not promote amplification (16). The results of the present study demonstrated that a combination of hypoxia and testosterone in vitro may increase CD34+ cell differentiation and prolong the HPC stage. This phenomenon may be caused by a number of mechanisms. Firstly, hypoxia may not be conducive to HSC expansion, potentially due to the action of HIF-1α, which may decrease HSC proliferation and block cells in the G0 phase (16,32). The results of the present study indicated that there was a significant increase in the expression of HIF-1α in the hypoxia testosterone compared with the control groups. Another potential mechanism to consider is that hypoxia may directly suppress the proliferation and differentiation of erythroid progenitor/precursor cells. These effects may be reduced or counter-balanced by increasing erythropoietin (EPO) levels (43) and one acknowledged mechanism involved in the hematological effects of androgens is the promotion of erythroid progenitor expansion by increasing EPO levels (44). Furthermore, androgen and androgen receptor signals may promote the proliferation of HSCs/HPCs and stimulate hematopoietic lineage differentiation (45). Another potential mechanism may be that the addition of testosterone may enhance the G1-S transition rate in the cell cycle and the survival of HSCs (32). Additionally, androgens may stimulate telomerase-related gene expression and enzymatic activity in bone marrow CD34+ cells and extend the lifespan of the CD34+ stem/progenitor cells (46).
In conclusion, CB ex vivo expansion is a promising approach to deliver high doses of cells and improve the outcomes of CBT. Careful selection of optimal CB units for transplantation may improve the efficiency of the source of HSCs and HPCs in adult transplantation and reduce the cost of processing (47). The present results may provide a means to improve HSC/HPC culture conditions in vitro. Future studies investigating this technique may require a larger sample size, however the present study demonstrated that the combination of hypoxia and androgen in vitro may be a promising condition of cultivation.
Acknowledgements
The present study was supported by grants from the Major State Basic Research Development Program (grant no. 2012CB966504), Jinan Natural Science Foundation (grant no. 201403010) and the Basic Scientific Fund of Shandong University (grant no. 2014QLKY02).
References
- 1.Walasek MA, van Os R, de Haan G. Hematopoietic stem cell expansion: Challenges and opportunities. Ann N Y Acad Sci. 2012;1266:138–150. doi: 10.1111/j.1749-6632.2012.06549.x. [DOI] [PubMed] [Google Scholar]
- 2.Möbest D, Mertelsmann R, Henschler R. Serum-free ex vivo expansion of CD34(+) hematopoietic progenitor cells. Biotechnol Bioeng. 1998;60:341–347. doi: 10.1002/(SICI)1097-0290(19981106)60:3<341::AID-BIT10>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 3.Ballen KK, Gluckman E, Broxmeyer HE. Umbilical cord blood transplantation: The first 25 years and beyond. Blood. 2013;122:491–498. doi: 10.1182/blood-2013-02-453175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rocha V, Wagner JE, Jr, Sobocinski KA, Klein JP, Zhang MJ, Horowitz MM, Gluckman E. Graft-versus-host disease in children who have received a cord-blood or bone marrow transplant from an HLA-identical sibling. Eurocord and International Bone Marrow Transplant Registry Working Committee on Alternative Donor and Stem Cell Sources. N Engl J Med. 2000;342:1846–1854. doi: 10.1056/NEJM200006223422501. [DOI] [PubMed] [Google Scholar]
- 5.Foeken LM, Green A, Hurley CK, Marry E, Wiegand T, Oudshoorn M. Monitoring the international use of unrelated donors for transplantation: The WMDA annual reports. Bone Marrow Transplant. 2010;45:811–818. doi: 10.1038/bmt.2010.9. [DOI] [PubMed] [Google Scholar]
- 6.Gao L, Chen X, Zhang X, Liu Y, Kong P, Peng X, Liu L, Liu H, Zeng D. Human umbilical cord blood-derived stromal cell, a new resource of feeder layer to expand human umbilical cord blood CD34+ cells in vitro. Blood Cells, Molecules and Diseases. 2006;36:322–328. doi: 10.1016/j.bcmd.2005.12.036. [DOI] [PubMed] [Google Scholar]
- 7.Horwitz ME, Frassoni F. Improving the outcome of umbilical cord blood transplantation through ex vivo expansion or graft manipulation. Cytotherapy. 2015;17:730–738. doi: 10.1016/j.jcyt.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 8.Pawliuk R, Eaves C, Humphries RK. Evidence of both ontogeny and transplant dose-regulated expansion of hematopoietic stem cells in vivo. Blood. 1996;88:2852–2858. [PubMed] [Google Scholar]
- 9.Iscove NN, Nawa K. Hematopoietic stem cells expand during serial transplantation in vivo without apparent exhaustion. Curr Biol. 1997;7:805–808. doi: 10.1016/S0960-9822(06)00341-1. [DOI] [PubMed] [Google Scholar]
- 10.Osawa M, Hanada K, Hamada H, Nakauchi H. Long term lymp hematopoietic reconstitution by a single CD34-low/negative hematopoietic stem cell. Science. 1996;273:242–245. doi: 10.1126/science.273.5272.242. [DOI] [PubMed] [Google Scholar]
- 11.Sauvageau G, Iscove NN, Humphries RK. In vitro and in vivo expansion of hematopoietic stem cells. Oncogene. 2004;23:7223–7232. doi: 10.1038/sj.onc.1207942. [DOI] [PubMed] [Google Scholar]
- 12.Schugar RC, Robbins PD, Deasy BM. Small molecules in stem cell self-renewal and differentiation. Gene Therapy. 2008;15:126–135. doi: 10.1038/sj.gt.3303062. [DOI] [PubMed] [Google Scholar]
- 13.Hermitte F, de la Grange Brunet P, Belloc F, Praloran V, Ivanovic Z. Very low O2 concentration (0. 1%) favors G0 return of dividing CD34+ cells. Stem Cells. 2006;24:65–73. doi: 10.1634/stemcells.2004-0351. [DOI] [PubMed] [Google Scholar]
- 14.Ivanovic Z, Hermitte F, de la Grange Brunet P, Dazey B, Belloc F, Lacombe F, Vezon G, Praloran V. Simultaneous maintenance of human cord blood SCID-repopulating cells and expansion of committed progenitors at low O2 concentration (3%) Stem Cells. 2004;22:716–724. doi: 10.1634/stemcells.22-5-716. [DOI] [PubMed] [Google Scholar]
- 15.Ivanovic Z, Dello Sbarba P, Trimoreau F, Faucher JL, Praloran V. Primitive human HPCs are better maintained and expanded in vitro at 1 percent oxygen than at 20 percent. Transfusion. 2000;12:1482–1488. doi: 10.1046/j.1537-2995.2000.40121482.x. [DOI] [PubMed] [Google Scholar]
- 16.Eliasson P, Rehn M, Hammar P, Larsson P, Sirenko O, Flippin LA, Cammenga J, Jönsson JI. Hypoxia mediates low cell-cycle activity and increases the proportion of long-term reconstituting hematopoietic stem cells during in vitro culture. Experimental Hematology. 2010;38:301–310. doi: 10.1016/j.exphem.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 17.Rehn M, Olsson A, Reckzeh K, Diffner E, Carmeliet P, Landberg G, Cammenga J. Hypoxic induction of vascular endothelial growth factor regulates murine hematopoietic stem cell function in the low-oxygenic niche. Blood. 2011;118:1534–1543. doi: 10.1182/blood-2011-01-332890. [DOI] [PubMed] [Google Scholar]
- 18.Penn A, Mohr AM, Shah SG, Sifri ZC, Kaiser VL, Rameshwar P, Livingston DH. Dose-response relationship between norepinephrine and erythropoiesis: Evidence for a critical threshold. J Surg Res. 2010;163:85–90. doi: 10.1016/j.jss.2010.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kröpfl JM, Stelzer I, Mangge H, Pekovits K, Fuchs R, Allard N. Exercise-induced norepinephrine decreases circulating hematopoietic stem and progenitor cell colony-forming capacity. PLoS One. 2014;9:106–120. doi: 10.1371/journal.pone.0106120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim SW, Hwang JH, Cheon JM, Park NS, Park SE, Park SJ, Yun HJ, Kim S, Jo DY. Direct and indirect effects of androgens on survival of hematopoietic progenitor cells in vitro. J Korean Med Sci. 2005;20:409–416. doi: 10.3346/jkms.2005.20.3.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beckman B, Fisher JW. Decreased erythroidcolony-forming cell response of XTfm/Y mice to testosterone and 5 betadihydrotestosterone. Endocrinology. 1980;107:1587–1592. doi: 10.1210/endo-107-5-1587. [DOI] [PubMed] [Google Scholar]
- 22.Huang CK, Luo J, Lee SO, Chang C. Concise review: Androgen receptor differential roles in stem/progenitor cells including prostate, embryonic, stromal and hematopoietic lineages. Stem Cells. 2014;32:2299–2308. doi: 10.1002/stem.1722. [DOI] [PubMed] [Google Scholar]
- 23.Chen C, Cao J, Song X, Zeng L, Li Z, Li Y, Xu K. Adrenaline administration promotes the efficiency of granulocyte colony stimulating factor-mediated hematopoietic stem and progenitor cell mobilization in mice. Int J Hematol. 2013;97:50–57. doi: 10.1007/s12185-012-1228-1. [DOI] [PubMed] [Google Scholar]
- 24.Duchez P, Chevaleyre J, de la Grange Brunet P, Vlaski M, Boiron JM, Wouters G, Ivanovic Z. Cryopreservation of hematopoietic stem and progenitor cells amplified ex vivo from cord blood CD34+ cells. Transfusion. 2013;53:2012–2019. doi: 10.1111/trf.12015. [DOI] [PubMed] [Google Scholar]
- 25.Dumont N, Boyer L, Émond H, Celebi-Saltik B, Pasha R, Bazin R, Mantovani D, Roy DC, Pineault N. Medium conditioned with mesenchymal stromal cell-derived osteoblasts improves the expansion and engraftment properties of cord blood progenitors. Exp Hematol. 2014;42:741–752. doi: 10.1016/j.exphem.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 26.Zhuang Y, Li D, Fu J, Shi Q, Lu Y, Ju X. Comparison of biological properties of umbilical cord-derived mesenchymal stem cells from early and late passages: Immunomodulatory ability is enhanced in aged cells. Mol Med Rep. 2015;11:166–174. doi: 10.3892/mmr.2014.2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robinson S, Niu T, de Lima M, Ng J, Yang H, McMannis J, Karandish S, Sadeghi T, Fu P, del Angel M. Ex vivo expansion of umbilical cord blood. Cytotherapy. 2005;3:243–250. doi: 10.1080/14653240510027172. [DOI] [PubMed] [Google Scholar]
- 28.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2 (-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 29.Bock TA, Ziegler BL, Bühring HJ, Scheding S, Brugger W, Kanz L. Characterization of purified and ex vivo manipulated human hematopoietic progenitor and stem cells in xenograft recipients. Ann N Y Acad Sci. 1999;872:200–207. doi: 10.1111/j.1749-6632.1999.tb08465.x. [DOI] [PubMed] [Google Scholar]
- 30.Frascoli M, Proietti M, Grassi F. Phenotypic analysis and isolation of murine hematopoietic stem cells and lineage-committed progenitors. J Vis Exp. 2012;65 doi: 10.3791/3736. pii: 3736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang QS, Benedetti E, Deater M, Schubert K, Major A, Pelz C, Impey S, Marquez-Loza L, Rathbun RK, Kato S, et al. Oxymetholone therapy of fanconi anemia suppresses osteopontin transcription and induces hematopoietic stem cell cycling. Stem Cell Reports. 2015;4:90–102. doi: 10.1016/j.stemcr.2014.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gallien-Lartigue O. Differential effects of external agents on the G1-S transit rate of murine pluripotent hemopoietic stem cells (CFUs) after their release from G0. Stem Cells. 1982;2:218–228. [PubMed] [Google Scholar]
- 33.Freedman MH, Saunders EF. Factors affecting erythroid colony growth (CFU-E) from human marrow. Exp Hematol. 1977;5:250–253. [PubMed] [Google Scholar]
- 34.Reissmann KR, Udupa KB, Kawada K. Effects of erythropoietin and androgens on erythroid stem cells after their selective suppression by BCNU. Blood. 1974;44:649–657. [PubMed] [Google Scholar]
- 35.Katayama Y, Battista M, Kao WM, Hidalgo A, Peired AJ, Thomas SA, Frenette PS. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell. 2006;124:407–421. doi: 10.1016/j.cell.2005.10.041. [DOI] [PubMed] [Google Scholar]
- 36.Ivanović Z, Bartolozzi B, Bernabei PA, Cipolleschi MG, Rovida E, Milenković P, Praloran V, Dello Sbarba P. Incubation of murine bone marrow cells in hypoxia ensures the maintenance of marrow-repopulating ability together with the expansion of committed progenitors. Br J Haematol. 2000;108:424–429. doi: 10.1046/j.1365-2141.2000.01842.x. [DOI] [PubMed] [Google Scholar]
- 37.Ivanović Z, Dello Sbarba P, Trimoreau F, Faucher JL, Praloran V. Primitive human HPCs are better maintained and expanded in vitro at 1 percent oxygen than at 20 percent. Transfusion. 2000;40:1482–1488. doi: 10.1046/j.1537-2995.2000.40121482.x. [DOI] [PubMed] [Google Scholar]
- 38.Ivanovic Z, Belloc F, Faucher JL, Cipolleschi MG, Praloran V, Dello Sbarba P. Hypoxia maintains and interleukin-3 reduces the pre-colony-forming cell potential of dividing CD34(+) murine bone marrow cells. Experimental Hematology. 2002;30:67–73. doi: 10.1016/S0301-472X(01)00765-2. [DOI] [PubMed] [Google Scholar]
- 39.Ishikawa Y, Ito T. Kinetics of hemopoietic stem cells in a hypoxic culture. Eur J Haematol. 1988;40:126–129. doi: 10.1111/j.1600-0609.1988.tb00808.x. [DOI] [PubMed] [Google Scholar]
- 40.Lu L, Broxmeyer HE. Comparative influences of phytohemagglutinin-stimulated leukocyte conditioned medium, hemin, prostaglandin E and low oxygen tension on colony formation by erythroid progenitor cells in normal human bone marrow. Exp Hematol. 1985;13:989–993. [PubMed] [Google Scholar]
- 41.Huang CK, Tsai MY, Luo J, Kang HY, Lee SO, Chang C. Suppression of androgen receptor enhances the self renewal of mesenchymal stem cells through helevated expression of EGFR. Biochim Biophys Acta. 2013;1833:1222–1234. doi: 10.1016/j.bbamcr.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chang CY, Hsuuw YD, Huang FJ, Shyr CR, Chang SY, Huang CK, Kang HY, Huang KE. Androgenic and antiandrogenic effects and expression of androgen receptor in mouse embryonic stem cells. FertilSteril. 2006;85:1195–1203. doi: 10.1016/j.fertnstert.2005.11.031. [DOI] [PubMed] [Google Scholar]
- 43.Rogers HM, Yu X, Wen J, Smith R, Fibach E, Noguchi CT. Hypoxia alters progression of the erythroid program. Exp Hematol. 2008;1:17–27. doi: 10.1016/j.exphem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fried W, Morley C. Effects of androgenic steroids on erythropoiesis. Steroids. 1985;46:799–826. doi: 10.1016/0039-128X(85)90031-5. [DOI] [PubMed] [Google Scholar]
- 45.Huang CK, Luo J, Lee SO, Chang C. Concise review: Androgen receptor differential roles in stem/progenitor cells including prostate, embryonic, stromal and hematopoietic lineages. Stem Cells. 2014;9:2299–2308. doi: 10.1002/stem.1722. [DOI] [PubMed] [Google Scholar]
- 46.Calado RT, Yewdell WT, Wilkerson KL, Regal JA, Kajigaya S, Stratakis CA, Young NS. Sex hormones, acting on the TERT gene, increase telomerase activity in human primary hematopoietic cells. Blood. 2009;114:2236–2243. doi: 10.1182/blood-2008-09-178871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lane TA. Umbilical cord blood grafts for hematopoietic transplantation in adults: A cup half empty or half full? Transfusion. 2005;45:1027–1034. doi: 10.1111/j.1537-2995.2005.05120.x. [DOI] [PubMed] [Google Scholar]