Abstract
The aim of the present study was to explore the protective effects of Berberine (BBR) against non-alcoholic steatohepatitis (NASH). Male 4-week-old C57BL/6J Apolipoprotein E-deficient (ApoE−/−) mice were divided into the following three groups, which were given different diets: Normal chow diet (SC group); high-fat high-cholesterol diet (HFHC group); and HFHC diet supplemented with BBR (BBR group). Serum biochemical indicators of hepatic function and histological liver tissue changes were evaluated. The expression of neutrophil elastase (NE) and genes involved in the inflammatory response was measured. ApoE−/− mice fed a HFHC diet for 12 weeks developed NASH, characterized by steatosis and liver inflammation. Body weight, and serum triglyceride and cholesterol levels were markedly reduced by BBR. BBR supplementation significantly lowered serum alanine aminotransferase and aspartate aminotransferase levels in mice with HFHC diet-induced NASH, and significantly downregulated hepatic expression and activity of NE, whereas α1-antitrypsin (α1-AT) expression was significantly recovered by BBR (all P<0.05 vs. the HFHC group). Furthermore, treatment with BBR induced a significant reduction in the expression of key genes, including phospoinositide 3-kinase, nuclear factor-κB and interleukin-8, in the C-X-C chemokine receptor type 4 (CXCR4) signaling pathway (all P<0.05 vs. the HFHC group). These results suggest that BBR alleviates NASH in ApoE−/− mice fed a HFHC diet. Restoration of the balance of NE and α1-AT levels, which in turn facilitate the inhibition of the CXCR4 signaling pathways, may be involved in the hepatoprotective effect of BBR. These results indicate that BBR may be a candidate therapeutic agent for the treatment of NASH.
Keywords: berberine, neutrophil elastase, non-alcoholic steatohepatitis, C-X-C chemokine receptor type 4, C-X-C motif chemokine 12
Introduction
Nonalcoholic fatty liver disease (NAFLD) occurs in a wide range of histopathological conditions, from asymptomatic steatosis to severe nonalcoholic steatohepatitis (NASH) (1). The transition from steatosis to steatohepatitis represents an important step in liver damage progression, which eventually culminates in hepatic fibrosis and cirrhosis (2). At present, the underlying molecular mechanism that promotes this transition remains unknown and no effective therapy for NASH is available.
A growing body of evidence suggests that neutrophil infiltration into tissues is associated with the pathogenesis of inflammatory conditions, including NASH (3,4). A proteolytic enzyme that is produced by neutrophils, neutrophil elastase (NE), was recognized as a factor contributing to liver injury, and a potential target for treatment (5). A previous study by our group demonstrated that neutrophils serve an essential role in the early stages of NASH development via the production NE (6). Correspondingly, the serine protease inhibitor α1-antitrypsin (α1-AT), which is an endogenous inhibitor of NE, was identified to be dramatically reduced in patients with NASH (7). A previous study also identified that an imbalance between levels of NE and α1-AT in obesity affects insulin sensitivity, inflammation and energy expenditure (8).
Berberine (BBR) is a natural supplement that is popular for its potent antimicrobial, antiprotozoal and antitrachoma effects (9). Previous studies have suggested that BBR exerts notable anticancer, antioxidant and anti-inflammatory activities (10), in addition to hepatoprotective effects in liver fibrosis (11,12).
The present study investigated whether BBR was able to alleviate the development of NASH development in apolipoprotein E-deficient (ApoE−/−) mice with experimentally induced NASH. In addition, the underlying molecular mechanisms of the effects of BBR in this model were examined.
Materials and methods
Experimental animals and diets
The present study utilized a total of 26 4-week-old male C57BL/6 J ApoE−/− mice, weighing 16.30±0.45 g (Model Animal Research Center of Nanjing University, Nanjing, China). All mice were bred in a specific pathogen-free facility and maintained in a 12-h light-dark cycle at a controlled temperature (22±2°C) and humidity (55±5%) and fed ad libitum. The mice were divided into the following three groups, which were given different diets: Standard chow diet (SC group, n=8); high-fat high-cholesterol diet (HFHC group, n=8); and HFHC diet supplemented with BBR (BBR group, n=10). C57BL/6J ApoE−/− mice receiving a HFHC diet serves as a typical model of obesity-induced NASH (13). The chow diet from Research Diets, Inc. (New Brunswick, NJ, USA; cat. no. D10012 G) contained 15.8% fat, 20.3% protein, and 63.9% carbohydrate. The HFHC diet feed (cat. no. D12079B; Research Diets, Inc.) contained 41% fat, 17% protein, 43% carbohydrate and 0.21% cholesterol. BBR (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) was administered at a dosage of 0.2 g/kg daily, starting from the sixth week to the end of the experiment. Food intake was measured 3 times/week and individual body weight was measured weekly over a 12-week period. At the end of the experiments, the animals fasted for 12 h, and following anesthesia with 3% pentobarbital (Sigma-Aldrich; Merck KGaA) and sacrifice via cervical dislocation. Blood samples were rapidly obtained by retro-orbital sampling, as previously described (14). Liver tissues were harvested and samples were fixed with 4% formaldehyde at room temperature for 24 h, whilst the remaining liver was snap-frozen in liquid nitrogen until required. All animal protocols were reviewed and approved by the Ethics Committee of Hangzhou Normal University Affiliated Hospital (Hangzhou, China).
Serum biochemical analyses
Serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), triglyceride and cholesterol levels were measured using a Hitachi 7180 automatic biochemical analyzer (Hitachi Ltd., Tokyo, Japan) according to the manufacturer's protocol. Serum NE and α1-AT levels were examined using a Mouse Neutrophil Elastase/ELA2 DuoSet ELISA or α 1 Antitrypsin ELISA kit following the manufacturers' instructions (DY4517-05, R&D Systems, Inc., Minneapolis, MN, USA; ab205088, Abcam, Cambridge, UK).
Histopathological analysis
Formalin-fixed liver tissues were embedded in paraffin, sectioned (5 µm thickness), and stained with hematoxylin and eosin as previously described (15). Frozen liver sections were cut to 10 µm thickness, fixed with 10% paraformaldehyde for 5 min at room temperature and stained with Oil Red O for 10 min at room temperature. Histological scoring was performed by two specialists blinded to the experimental design and data. Scoring ranges were as follows: Degree of steatosis (0–3), lobular inflammation (0–3), hepatocyte ballooning (0–2). In addition, the NAFLD activity score (NAS) was scored according to the NASH clinical research network scoring system (16).
Immunohistochemical staining
Immunohistochemical staining was performed as previously described (6). The primary antibodies used were as follows: Anti-NE (1:200; cat. no. orb1614; Biorbyt Ltd., Cambridge, UK), anti-C-X-C chemokine receptor type 4 (CXCR4; 1:50; cat. no. ab124824, Abcam) and anti-C-X-C motif chemokine 12 (CXCL12; 1:50; cat. no. sc-28876; Santa Cruz Biotechnology, Inc., Dallas, TX, USA). Samples were then incubated with the anti-rabbit (1:2,000; cat. no. ab97051; Abcam) secondary antibody for 1 h at room temperature under gentle agitation. Samples were subsequently visualized under an Eclipse 80i light microscope (Nikon Corporation, Tokyo, Japan) and representative images were captured for analysis.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR) analysis
Hepatic mRNA levels were analyzed by RT-qPCR using an ABI Prism 7900 HT Fast Real-Time PCR system (Applied Biosystems; Thermo Fisher Scientific, Inc., Waltham, MA, USA). Total RNA was isolated from liver tissues using TRIzol (Invitrogen; Thermo Fisher Scientific, Inc.) followed by DNAse treatment to remove genomic DNA. cDNA was synthesized using 2 µg of total RNA with Superscript® II Reverse Transcriptase (Invitrogen; Themo Fisher Scientific, Inc.) at 42°C for 50 min. Amplification reactions were performed using a SYBR Select Master Mix (AmpliTaq® Fast DNA Polymerase, Thermo Fisher Scientific, Inc.) according to the manufacturer's protocol. PCR conditions were as follows: 95°C for 10 min, followed by 40 cycles at 95°C for 10 sec and 60°C for 1 min. PCR products were verified by melt curve analysis (17). Mouse β-actin or GAPDH were used as the reference genes to normalize for differences in the amount of total RNA in each sample. Relative gene expression levels were analyzed using the 2−ΔΔCq method (18). PCR murine primers were obtained from Invitrogen (Thermo Fisher Scientific, Inc.) and their sequences were as follows: β-actin forward (F), 5′-CTGGCTCCTAGCACCATGAA-3′ and reverse (R), 5′-CGCAGCTCAGTAACAGTCCG-3′; GAPDH F, 5′-AACAGCAACTCCCACTCTTC-3′ and R, 5′-CCTGTTGCTGTAGCCGTATT-3′; α1-AT F, 5′-GAGCATTGGCACAGCGTTTG-3′ and R, 5′-AAGCGATGGTTGGATGTCAGC-3′; CXCR4 F, 5′-CTACAGCAGCGTTCTCATCC-3′ and R, 5′-TTTCAGCCAGCAGTTTCCTT-3′; CXCL12 F, 5′-CGACTTCTCAGGCAGGTGA-3′ and R, 5′-GCCATTCCCATAGCATTCAT-3′; phosphatidylinositol-3 kinase (PI3K) F, 5′-AATGTGCCCTCTTTCGTTGT-3′ and R, 5′-TGAATGGTGACTGGCTGACT-3′; interleukin (IL)-1 F, 5′-CTCTTGCCTGTCATCCCAAC-3′ and R, 5′-ACCATCTGTTCCCAATACGG-3′; IL-6 F, 5′-CGGAGAGGAGACTTCACAGAG-3′ and R, 5′-CATTTCCACGATTTCCCAGA-3′; IL-8 F, 5′-GGATGGGAACAACGATAGGA-3′ and R, 5′-AGAACGTGGCGGTATCTCTG-3′; protein kinase B (AKT) F, 5′-ACTCATTCCAGACCCACGAC-3′ and R, 5′-ACAATCTCCGCACCATAGA-3′; nuclear factor-κB (NF-κB) F, 5′-GCTCAAGATCTGCCGAGTAAA-3′ and R, 5′-GTCCCGTGAAATACACCTCAA-3′; tumor necrosis factor (TNF)-α F, 5′-AAGGGAGAGTGGTCAGGTTG-3′ and R, 5′-TCTGTGAGGAAGGCTGTGC-3′; phospholipase C-β (PLC-β) F, 5′-GCAGGTCCAAGTGTTGATTG-3′ and R, 5′-TTCTTCTCCGCTCAGGTAGC-3′; and monocyte chemoattractant protein-1 (MCP-1) F, 5′-TCTCTCTTCCTCCACCACCAT-3′ and R, 5′-GCTCTCCAGCCTACTCATTGG-3′. Each experiment was performed in duplicate and repeated three times.
Immunoblotting
Liver tissue (60 mg) was homogenized in RIPA buffer (P0013B; Beyotime Institute of Biotechnology, Haimen, China), supplemented with protease inhibitor cocktail (Sigma-Aldrich; Merck KGaA). Samples (50 µg protein per lane) were separated by 10% SDS-PAGE and electrotransferred onto polyvinylidene difluoride membranes. Following blocking with 5% nonfat milk, membranes were probed with anti-NE antibody (1:200) overnight at 4°C. The membranes were then incubated with the secondary antibody for 1 h at room temperature, followed by SuperSignal™ West Pico Chemiluminescent detection (34077; Thermo Fisher Scientific, Inc.). Protein band intensities were quantified using Quantity One software 4.62 (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
NE imaging
The Neutrophil Elastase 680 FAST imaging agent (PerkinElmer, Inc, Waltham, MA, USA) was used to detect NE in vivo at the end of the experiment according to the manufacturer's protocol. This agent is based on a peptide (PMAVVQSVP) that is specifically recognized by NE. Mice were administered with 4 nmol of the NE imaging agent intravenously via the tail vein, and 4 h later an in vivo imaging system (Molecular Imaging software version 5.3.5; Carestream, CA, USA) was used to capture the fluorescence and detect NE activity.
Statistical analyses
Statistical analysis was conducted using GraphPad Prism (version 5; GraphPad Software, Inc., La Jolla, CA, USA). All data are presented as the mean ± standard deviation. The statistical significance of differences between groups was assessed using an unpaired Student's t-test. P<0.05 was considered to indicate a statistically significant difference.
Results
BBR has a hepatoprotective role
The effect of BBR on weight gain and liver metabolic features in C57BL/6J ApoE−/− mice receiving a HFHC diet was observed (Fig. 1). Mice fed a HFHC diet had significantly increased body weights, associated with hypertriglyceridemia and hypercholesterolemia, compared with regular chow-fed mice in the SC group (P<0.05; Fig. 1A). This weight gain was also accompanied by a significantly higher relative liver:body weight ratio (liver index) in the HFHC group compared with the SC group (P<0.01; Fig. 1B). Furthermore, mice in the HFHC group had significantly enhanced increased ALT and AST levels, indicative of liver injury, compared with the SC group (P<0.01; Fig. 1E and F).
Figure 1.
BBR treatment protects against liver injury. (A) Body weight, (B) liver-to-body weight ratio, (C) total cholesterol, (D) serum triglyceride, (E) ALT and (F) AST levels from the three experimental groups at the end of the study. *P<0.05, **P<0.01 vs. the HFHC group; ∆P<0.05, ∆∆P<0.01 vs. the BBR group. BBR, berberine; ALT, alanine transaminase; AST, aspartate transaminase; SC, standard chow; HFHC, high-fat high-cholesterol.
BBR supplementation markedly reduced body weight, serum total cholesterol and triglyceride levels in ApoE−/− mice, though the results were not statistically significant compared with the SC group (Fig. 1A, C and D). Thus, treatment with BBR did not notably impact the global metabolic profile of the mice. However, the liver index and serum ALT and AST levels were significantly lower in the BBR group compared with the HFHC group, demonstrating a hepatoprotective role of BBR against liver injury (P<0.05; Fig. 1E and F).
BBR alleviates hepatic steatosis and inflammation in ApoE−/− mice with NASH
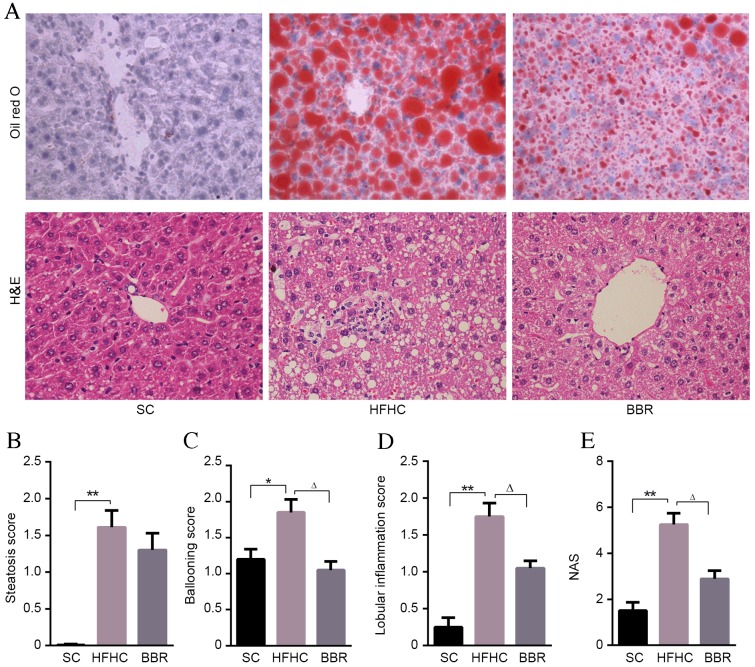
Compared with mice in the SC group, significant pathological findings of NASH, including marked steatosis (P<0.01), ballooning (P<0.05), lobular inflammation (P<0.01) and NAS (P<0.01) were observed in the HFHC group (Fig. 2). However, no significant fibrosis was observed in the mouse liver tissues.
Figure 2.
BBR alleviates liver injury. (A) Representative Oil Red O and HE staining of liver tissue sections. Magnification, ×400. Scores for (B) steatosis, (C) ballooning, (D) lobular inflammation and (E) NAS for the three experimental groups. *P<0.05, **P<0.01 vs. the HFHC group; ∆P<0.05 vs. the BBR group. BBR, berberine; HFHC, high-fat high-cholesterol; H&E, hematoxylin and eosin; NAS, non-alcoholic fatty liver disease activity score; SC, standard chow.
BBR-treated mice had a lower steatosis score compared with HFHC-fed mice, although this difference was not statistically significant (Fig. 2B). Lobular inflammation and ballooning scores were significantly lower in the BBR group compared with the HFHC group (P<0.05; Fig. 2C and D). Indeed the overall NAS score indicated that BBR treatment had a protective effect, being significantly lower in the BBR group compared with the HFHC group (P<0.05; Fig. 2E). These data indicate that inflammation is significantly associated with HFHC diet-induced liver damage, and that treatment with BBR significantly ameliorates this inflammation and liver injury.
An imbalance between NE and α1-AT is associated with NASH development in ApoE−/− mice fed a HFHC diet
Feeding a high-fat diet to mice induces an increase in neutrophil recruitment to tissues (19), which may initiate inflammatory signaling pathway cascades. Particularly, NE serves an important role in host defense and inflammation (20), whereas its counterpart, α1-AT, protects tissues from serine protease-induced damage (8). To validate whether the expressions of α1-AT and NE were altered in the present model, ELISA assays were performed. The results demonstrated that serum α1-AT levels were significantly lower in the HFHC group compared with the SC or BBR groups (P<0.05; Fig. 3A), whereas NE levels and the NE/α1-AT ratio were significantly higher in the HFHC group compared with the SC and BBR groups (P<0.05; Fig. 3B and C).
Figure 3.
NE/α1-AT imbalance is associated with NASH progression. Serum (A) α1-AT, (B) NE and (C) NE/α1-AT levels were determined by ELISA. (D) Immunohistochemical staining for NE in the liver tissue from different groups. Black arrows indicate NE staining. (E) Hepatic NE protein levels were determined by immunoblotting, and normalized to β-actin and quantified using densitometry. (F) NE activity was measured using in vivo imaging. (G) Relative hepatic α1-AT mRNA levels were determined by reverse transcription-quantitative polymerase chain reaction analysis and were normalized to β-actin. *P<0.05 vs. the HFHC group; ∆P<0.05 vs. the BBR group. NE, neutrophil elastase; α1-AT, α1-antitrypsin; NASH, non-alcoholic steatohepatitis; SC, standard chow; HFHC, high-fat high-cholesterol; BBR, berberine.
Histopathological and immunoblot examinations of liver specimens demonstrated that NE protein expression was significantly elevated in the HFHC group compared with the SC group (P<0.05), and BBR treatment significantly reduced NE expression in liver tissues (P<0.05) (Fig. 3D and E). It was also observed that NE enzymatic activity was markedly associated with NASH (the HFHC group) and inhibited by BBR treatment (Fig. 3F). In contrast to NE, α1-AT protein expression in the HFHC group was significantly lower compared with that in the SC group (P<0.05), and α1-AT levels were significantly upregulated in the BBR group compared with the HFHC group (P<0.05) (Fig. 3G). This NE/α1-AT balance is consistent with the reduced inflammatory foci and ALT levels observed in the ApoE−/− mice following BBR treatment.
BBR hepatoprotection is associated with the CXCR4/CXCL12 signaling pathway
Localization of inflammatory cells to the liver is an essential step in the progression of NASH (21). The active form of NE is externalized during neutrophil activation at inflammatory sites, thus helping to promote inflammatory and immune responses (20,22). The present study investigated whether NE elevation affected the expression of inflammatory chemokines and cytokines. The HFHC diet stimulated the expression of TNF-α, MCP-1, IL-6, CXCR7 and CXCL11 in the mouse liver compared with mice fed a standard diet (data not shown). However, although BBR treatment reduced the expression of these cytokines and chemokines, no statistically significant differences were observed between the HFHC and BBR group (data not shown).
Previous studies have suggested that the CXCR4/CXCL12 signaling pathways serves a key role in maintaining neutrophil homeostasis (23). Liver mRNA levels of CXCR4 and CXCL12 were significantly lower in the BBR group compared with the HFHC group (P<0.05; Fig. 4A and B). Immunochemistry staining yielded similar results for the protein levels of CXCR4 and CXCL12 (Fig. 4C). Gene expression analysis found no significant differences in the expression of mitogen-activated protein kinase 14, extracellular signal-regulated kinase 1/2 or and B-cell lymphoma 2-associated agonist of cell death between the HFHC and BBR groups (data not shown). However, RAS, PLC-β, PI3K, AKT, NF-κB, IL-1 and IL-8 expression was significantly attenuated by BBR treatment (P<0.05; Fig. 4D-J).
Figure 4.
BBR reducing the expression of inflammatory cytokine, chemokines and associated genes. Hepatic expression of (A) CXCR4 and (B) CXCL12 mRNA. (C) Representative slides illustrating CXCR4 and CXCL12-stained liver sections from the three experimental groups. Hepatic expression of (D) RAS, (E) PLC-β, (F) PI3K, (G) AKT, (H) NF-κβ, (I) IL-1 and (J) IL-8 mRNA was determined by quantitative polymerase chain reaction analysis and normalized to GAPDH. *P<0.05 vs. the HFHC group; ∆P<0.05, ∆∆P<0.01 vs. the BBR group. CXCR4, C-X-C chemokine receptor type 4; CXCL12, C-X-C motif chemokine 12; BBR, berberine; HFHC, high-fat high-cholesterol; SC, standard chow; PLC-β, phospholipase C-β; PI3K, phosphatidylinositol-3 kinase; AKT, protein kinase B; NF-κβ, nuclear factor-κβ; IL, interleukin.
Discussion
ApoE−/− mice are well-established and typically used for studies of NAFLD (15). In the present study, ApoE−/− mice fed a HFHC diet presented with hepatic steatosis, ballooning and increased hepatic inflammation, similar to the clinical findings of human NASH. The principal findings of the present study demonstrate that BBR improves the histological and biochemical effects of NASH in mice.
BBR is known to improve lipid metabolism disorders via multipathway mechanisms (24). In the present study, BBR treatment reduced weight gain, hypertriglyceridemia and hypercholesterolemia in ApoE−/− mice fed a HFHC diet; however, the decrease was not statistically significant, which suggests that lipid metabolism signaling pathways are not the primary target of BBR. Histological examination demonstrated that the hepatoprotective effect of BBR in the ApoE−/− mouse NASH model was associated with anti-inflammatory activities.
Previous studies have identified that fat and cholesterol-induced inflammatory molecules are associated with liver injury (25). Neutrophils are typically the first immune cells to initiate the inflammatory signaling pathway cascade in response to obesity and are able to exacerbate chronic inflammation by recruiting other immune cells (26). A previous study by our group demonstrated that NASH mice with neutrophil depletion had lower serum ALT levels, liver inflammation and mRNA levels of proinflammatory genes (6). It is now generally accepted that, as an important enzyme produced by neutrophils, NE is able to promote inflammatory responses in several disease models (26). Our group previously demonstrated that the NE inhibitor sivelestat was able to recapitulate the effects of neutrophil depletion in ApoE−/− mice given a HFHC diet (6). In the present study, a significant increase was observed in NE expression and activation in the serum and liver tissues of mice receiving a HFHC diet. These data suggest that inflammation may be associated with NE-dependent liver injury in mice with HFHC diet-induced NASH, and that treatment with BBR reverses this effect. An imbalance between NE and its natural inhibitor α1-AT contributes to the development of obesity and subsequent inflammation (8). The present study demonstrated that HFHC diet-induced NASH was associated with a significant increase in the NE/α1-AT ratio in a mouse model. BBR treatment increased α1-AT expression in the serum and liver tissues of the NASH mice, suggesting that the protective effect of BBR against NASH development is associated with alleviation of the positive feed-forward loop between the imbalance of NE and α1-AT.
The results of the present study suggest that there is crosstalk between the NE and CXCR4/CXCL12 signaling pathway in NASH mice. It has previously been suggested that neutrophil homeostasis is tightly regulated by the CXCR4/CXCL12 signaling pathway (23). It has also been reported that CXCR4 was markedly upregulated in patients with NASH (27), and that the CXCL12/CXCR4 signaling pathway contributes to the enhanced recruitment of cluster of differentiation 4+ T-cells in NASH (21). In addition, a previous study demonstrated that NE directly interacts with CXCL12 in vitro (28). In the present study, it was observed that NE and CXCR4/CXCL12 expression were simultaneously blocked by BBR treatment. Furthermore, a significant induction of PI3K/AKT/NF-κB gene expression in HFHC-fed mice was observed in the present study, and this was significantly reduced by BBR treatment. This is consistent with previous observations that activation of the PI3K/AKT/NF-κB signaling pathways occurs in patients and mice with NAFLD (29,30), which may contribute to the increased expression of inflammatory, chemotaxis and cell proliferation genes (31). Notably, a previous study reported that NE expression resulted in overexpression of IL-8 (28). This is in accordance with the findings of the present study, which demonstrated an upregulation of IL-8 and IL-1 in the HFHC-fed mice, and that this upregulation was significantly attenuated by BBR treatment.
In conclusion, the results of the present study suggest that BBR has a hepatoprotective effect and highlighted a potential molecular mechanism underlying this effect. In the murine model of HFHC-diet induced NASH used in the present study, NE may trigger a proinflammatory cascade via the CXCR4/CXCL12 signaling pathway, which further increases hepatocyte inflammation and damage. BBR was identified to suppress NE overexpression by restoring the balance between NE and α1-AT levels, and inhibiting the inflammatory CXCR4/CXCL12 signaling pathway. These results indicate that BBR is a candidate agent for the treatment of NASH.
Acknowledgements
The present study was supported by the Natural Science Foundation of Zhejiang, China (grant nos. LY14H070004, LY15H070004, LY17H030009 and LQ17H070002) and the Traditional Chinese Medical Science and Technology Project of Zhejiang Province (grant nos. 2015ZA067 and 2015ZA141).
References
- 1.Farrell GC, van Rooyen D, Gan L, Chitturi S. NASH is an inflammatory disorder: Pathogenic, prognostic and therapeutic implications. Gut Liver. 2012;6:149–171. doi: 10.5009/gnl.2012.6.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tiniakos DG, Vos MB, Brunt EM. Nonalcoholic fatty liver disease: Pathology and pathogenesis. Annu Rev Pathol. 2010;5:145–171. doi: 10.1146/annurev-pathol-121808-102132. [DOI] [PubMed] [Google Scholar]
- 3.Gadd VL, Skoien R, Powell EE, Fagan KJ, Winterford C, Horsfall L, Irvine K, Clouston AD. The portal inflammatory infiltrate and ductular reaction in human nonalcoholic fatty liver disease. Hepatology. 2014;59:1393–1405. doi: 10.1002/hep.26937. [DOI] [PubMed] [Google Scholar]
- 4.Mócsai A. Diverse novel functions of neutrophils in immunity, inflammation, and beyond. J Exp Med. 2013;210:1283–1299. doi: 10.1084/jem.20122220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Uchida Y, Freitas MC, Zhao D, Busuttil RW, Kupiec-Weglinski JW. The protective function of neutrophil elastase inhibitor in liver ischemia/reperfusion injury. Transplantation. 2010;89:1050–1056. doi: 10.1097/TP.0b013e3181d45a98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zang S, Wang L, Ma X, Zhu G, Zhuang Z, Xun Y, Zhao F, Yang W, Liu J, Luo Y, et al. Neutrophils play a crucial role in the early stage of nonalcoholic steatohepatitis via neutrophil elastase in mice. Cell Biochem Biophys. 2015;73:479–487. doi: 10.1007/s12013-015-0682-9. [DOI] [PubMed] [Google Scholar]
- 7.Zang S, Ma X, Zhuang Z, Liu J, Bian D, Xun Y, Zhang Q, Zhao F, Yang W, Liu J, et al. Increased ratio of neutrophil elastase to α1-antitrypsin is closely associated with liver inflammation in patients with nonalcoholic steatohepatitis. Clin Exp Pharmacol Physiol. 2016;43:13–21. doi: 10.1111/1440-1681.12499. [DOI] [PubMed] [Google Scholar]
- 8.Mansuy-Aubert V, Zhou QL, Xie X, Gong Z, Huang JY, Khan AR, Aubert G, Candelaria K, Thomas S, Shin DJ, et al. Imbalance between neutrophil elastase and its inhibitor α1-antitrypsin in obesity alters insulin sensitivity, inflammation, and energy expenditure. Cell Metab. 2013;17:534–548. doi: 10.1016/j.cmet.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kulkarni SK, Dhir A. Berberine: A plant alkaloid with therapeutic potential for central nervous system disorders. Phytother Res. 2010;24:317–324. doi: 10.1002/ptr.2968. [DOI] [PubMed] [Google Scholar]
- 10.Battu SK, Repka MA, Maddineni S, Chittiboyina AG, Avery MA, Majumdar S. Physicochemical characterization of berberine chloride: A perspective in the development of a solution dosage form for oral delivery. AAPS Pharm Sci Tech. 2010;11:1466–1475. doi: 10.1208/s12249-010-9520-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li J, Pan Y, Kan M, Xiao X, Wang Y, Guan F, Zhang X, Chen L. Hepatoprotective effects of berberine on liver fibrosis via activation of AMP-activated protein kinase. Life Sci. 2014;98:24–30. doi: 10.1016/j.lfs.2013.12.211. [DOI] [PubMed] [Google Scholar]
- 12.Sun X, Zhang X, Hu H, Lu Y, Chen J, Yasuda K, Wang H. Berberine inhibits hepatic stellate cell proliferation and prevents experimental liver fibrosis. Biol Pharm Bull. 2009;32:1533–1537. doi: 10.1248/bpb.32.1533. [DOI] [PubMed] [Google Scholar]
- 13.Ye D, Li FY, Lam KS, Li H, Jia W, Wang Y, Man K, Lo CM, Li X, Xu A. Toll-like receptor-4 mediates obesity-induced non-alcoholic steatohepatitis through activation of X-box binding protein-1 in mice. Gut. 2012;61:1058–1067. doi: 10.1136/gutjnl-2011-300269. [DOI] [PubMed] [Google Scholar]
- 14.Van Herck H, Baumans V, Brandt CJ, Boere HA, Hesp AP, van Lith HA, Schurink M, Beynen AC. Blood sampling from the retro-orbital plexus, the saphenous vein and the tail vein in rats: Comparative effects on selected behavioural and blood variables. Lab Anim. 2001;35:131–139. doi: 10.1258/0023677011911499. [DOI] [PubMed] [Google Scholar]
- 15.Jeon S, Park YJ, Kwon YH. Genistein alleviates the development of nonalcoholic steatohepatitis in ApoE(−/−) mice fed a high-fat diet. Mol Nutr Food Res. 2014;58:830–841. doi: 10.1002/mnfr.201300112. [DOI] [PubMed] [Google Scholar]
- 16.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 17.Wienken CJ, Baaske P, Duhr S, Braun D. Thermophoretic melting curves quantify the conformation and stability of RNA and DNA. Nucleic Acids Res. 2011;39:e52. doi: 10.1093/nar/gkr035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 19.Elgazar-Carmon V, Rudich A, Hadad N, Levy R. Neutrophils transiently infiltrate intra-abdominal fat early in the course of high-fat feeding. J Lipid Res. 2008;49:1894–1903. doi: 10.1194/jlr.M800132-JLR200. [DOI] [PubMed] [Google Scholar]
- 20.Pham CT. Neutrophil serine proteases: Specific regulators of inflammation. Nat Rev Immunol. 2006;6:541–550. doi: 10.1038/nri1841. [DOI] [PubMed] [Google Scholar]
- 21.Boujedidi H, Robert O, Bignon A, Cassard-Doulcier AM, Renoud ML, Gary-Gouy H, Hemon P, Tharinger H, Prévot S, Bachelerie F, et al. CXCR4 dysfunction in non-alcoholic steatohepatitis in mice and patients. Clin Sci (Lond) 2015;128:257–267. doi: 10.1042/CS20130833. [DOI] [PubMed] [Google Scholar]
- 22.Snelgrove RJ, Jackson PL, Hardison MT, Noerager BD, Kinloch A, Gaggar A, Shastry S, Rowe SM, Shim YM, Hussell T, Blalock JE. A critical role for LTA4H in limiting chronic pulmonary neutrophilic inflammation. Science. 2010;330:90–94. doi: 10.1126/science.1190594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eash KJ, Means JM, White DW, Link DC. CXCR4 is a key regulator of neutrophil release from the bone marrow under basal and stress granulopoiesis conditions. Blood. 2009;113:4711–4719. doi: 10.1182/blood-2008-09-177287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Q, Xiao X, Feng K, Wang T, Li W, Yuan T, Sun X, Sun Q, Xiang H, Wang H. Berberine moderates glucose and lipid metabolism through multipathway mechanism. Evid Based Complement Alternat Med. 2011;2011:pii:924851. doi: 10.1155/2011/924851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bartelt A, Orlando P, Mele C, Ligresti A, Toedter K, Scheja L, Heeren J, Di Marzo V. Altered endocannabinoid signalling after a high-fat diet in Apoe(−/−) mice: Relevance to adipose tissue inflammation, hepatic steatosis and insulin resistance. Diabetologia. 2011;54:2900–2910. doi: 10.1007/s00125-011-2274-6. [DOI] [PubMed] [Google Scholar]
- 26.Talukdar S, Oh DY, Bandyopadhyay G, Li D, Xu J, McNelis J, Lu M, Li P, Yan Q, Zhu Y, et al. Neutrophils mediate insulin resistance in mice fed a high-fat diet through secreted elastase. Nat Med. 2012;18:1407–1412. doi: 10.1038/nm.2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu H, Li J, Tillman B, Morgan TR, French BA, French SW. TLR3/4 signaling is mediated via the NFκB-CXCR4/7 pathway in human alcoholic hepatitis and non-alcoholic steatohepatitis which formed Mallory-Denk bodies. Exp Mol Pathol. 2014;97:234–240. doi: 10.1016/j.yexmp.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Korkmaz B, Horwitz MS, Jenne DE, Gauthier F. Neutrophil elastase, proteinase 3, and cathepsin G as therapeutic targets in human diseases. Pharmacol Rev. 2010;62:726–759. doi: 10.1124/pr.110.002733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sharma M, Mitnala S, Vishnubhotla RK, Mukherjee R, Reddy DN, Rao PN. The riddle of nonalcoholic fatty liver disease: Progression from nonalcoholic fatty liver to nonalcoholic steatohepatitis. J Clin Exp Hepatol. 2015;5:147–158. doi: 10.1016/j.jceh.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pisonero-Vaquero S, Martínez-Ferreras Á, García-Mediavilla MV, Martínez-Flórez S, Fernández A, Benet M, Olcoz JL, Jover R, González-Gallego J, Sánchez-Campos S. Quercetin ameliorates dysregulation of lipid metabolism genes via the PI3K/AKT pathway in a diet-induced mouse model of nonalcoholic fatty liver disease. Mol Nutr Food Res. 2015;59:879–893. doi: 10.1002/mnfr.201400913. [DOI] [PubMed] [Google Scholar]
- 31.Teicher BA, Fricker SP. CXCL12 (SDF-1)/CXCR4 pathway in cancer. Clin Cancer Res. 2010;16:2927–2931. doi: 10.1158/1078-0432.CCR-09-2329. [DOI] [PubMed] [Google Scholar]