Abstract
The lower urinary tract’s main functions are storage and elimination. The micturition reflex pathway is modulated by the spinobulbospinal reflex pathway as well as higher brain centers involved in the voluntary micturition control. Micturition is sensitive to numerous injuries, resulting in various types of dysfunction. Animal studies indicate that lower urinary tract dysfunction partly depends on plasticity of the neural pathways. Reflex plasticity is associated with changes in ion channels, receptors, and numerous mediators. Animal models may aid in understanding the mechanisms leading to pathologic conditions and the plasticity in reflex pathways to the lower urinary tract after neurogenic lesions.
Keywords: Lower urinary tract, Afferents, Animal model, Central nervous system
Key points
-
•
Due to the complexity of the neural mechanisms regulating the lower urinary tract, micturition is sensitive to a wide variety of injuries and diseases, resulting in neurogenic lower urinary tract dysfunction.
-
•
In animal models of cerebral infarction (CI) produced by occlusion of middle cerebral artery, the balance between excitatory glutamatergic neurons and inhibitory glycinergic or GABAergic in the brain might be disrupted, leading to neurogenic lower urinary tract dysfunction.
-
•
In animal models of parkinson disease (PD) produced by disruption of nigrostriatal dopaminergic pathways, bladder overactivity is primarily induced by disruption of D1-like dopamine receptor-mediated inhibition of the micturition reflex.
-
•
In animal models of multiple sclerosis (MS) induced by experimental encephalomyelitis, neurogenic lower urinary tract dysfunction associated with detrusor overactivity is developed as seen in patients with MS.
-
•
In animal models of spinal cord injury (SCI), hyperexcitability of C-fiber bladder afferents is a major pathophysiological basis of neurogenic lower urinary tract dysfunction, and various neural plasticities in peripheral and central nervous systems are identified.
Introduction
The functions of the lower urinary tract to store and periodically eliminate urine depend on neural reflexes located in the brain, spinal cord, and peripheral ganglia.1 Coordination between the bladder and urethra maintain storage phase and work reciprocally. Thus, urine storage and elimination depend greatly on the central nervous system. This dependence on the central nervous system distinguishes the lower urinary tract from many other visceral structures, such as the gastrointestinal tract and cardiovascular system that maintain a certain level of function because of the local pace-making mechanism inside the organ, even after elimination of extrinsic neural input. In addition, voiding is under voluntary control and depends on learned behavior that develops during maturation of the central nervous system, whereas many other visceral organs are regulated involuntary.2, 3
Due to the complexity of the neural control of lower urinary tract, micturition is sensitive to numerous injuries, medical diseases, and drugs that affect the nervous system. Neurologic mechanisms are an important consideration in the diagnosis and treatment of voiding disorders. Thus, this article focuses on neurophysiologic mechanisms in the control of lower urinary tract function and their alterations that contribute to the pathologic conditions involved in the central nervous system, such as cerebral infarction (CI), Parkinson disease (PD), multiple sclerosis (MS), spinal cord injury (SCI), and spina bifida.
Neurophysiology of the lower urinary tract
Bladder and Urethra
The lower urinary tract is composed of the bladder and the urethra, the 2 functional units for storage (the bladder body, or reservoir) and elimination (the bladder neck and urethra, or outlet) of urine. The bladder and urethra function reciprocally. As the bladder fills during the urine storage phase, the detrusor remains quiescent, with a little change in intravesical pressure, adapting to the increasing volume by increasing the length of its muscle cells. Furthermore, neural pathways that stimulate the bladder for micturition are quiescent during this phase, and inhibitory pathways are active.4, 5
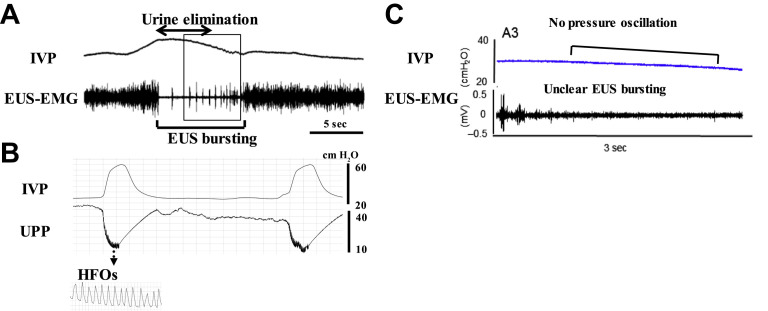
In normal rats, external urethral sphincter (EUS)–electromyogram (EMG) recordings, which are widely used for evaluating the urethral function, exhibit tonic activity before onset of voiding and bursting activity during voiding (Fig. 1 ). This EUS bursting during voiding is characterized by clusters of high-frequency spikes separated by low tonic activity, and produces rhythmic contractions and a relaxation of EUS that present a pumping action of EUS.6, 7, 8, 9, 10, 11, 12 The EUS bursting activity and pressure oscillations in cystometrograms are abolished by bungarotoxin, a neuromuscular blocker or pudendal nerve transection in rats,10, 13, 14 suggesting that the EUS pumping activity plays an important role in efficient bladder emptying. This bursting activity is thought to generate high-frequency oscillations (HFOs) measured by urethral perfusion pressure, which are mainly shown in some species, such as rat and dog.2 However, in normal mice, only a minority exhibits bursting-like EUS activity, and it has little impact on voiding efficiency,11 indicating that there are species differences regarding the neural control of bladder and sphincter function during voiding. However, the precise mechanisms of voluntary and reflex controls of bladder and urethra in humans still need to be clarified, although in brain imaging studies using functional MRI, various brain regions are identified to be involved in the storage and voiding phases of micturition.15
Fig. 1.
Representative recordings of simultaneous measurement of intravesical pressure (IVP) and EUS-EMG activity or urethral perfusion pressure (UPP) in a rat (A and B) or a mouse (C). The EUS-EMG exhibits tonic activity before the onset of voiding and bursting activity during voiding (A). The bursting produces rhythmic contractions and relaxation of the EUS and is thought to generate a urethral pumping action during voiding, which is seen as high-frequency oscillations (HFOs) on UPP (B). In contrast, most mice exhibited reduced EUS activity without bursting and no obvious pressure oscillation on the cystometrogram during voiding.
([A, C] Adapted from Kadekawa K, Yoshimura N, Majima T, et al. Characterization of bladder and external urethral activity in mice with or without SCI–a comparison study with rats. Am J Physiol Regul Integr Comp Physiol 2016;310:R752–8; and [B] From Miyazato M, Sasatomi K, Hiragata S, et al. Suppression of detrusor-sphincter dysynergia by GABA-receptor activation in the lumbosacral spinal cord in spinal cord-injured rats. Am J Physiol Regul Integr Comp Physiol 2008;295:R336–42.)
The lower urinary tract is peripherally innervated by parasympathetic, sympathetic, and somatic peripheral nerves that are components of intricate efferent and afferent circuitry derived from the brain and the spinal cord. The neural circuits act as an integrated complex of reflexes that regulates micturition, allowing the lower urinary tract to be in either a storage or elimination mode.
Efferent Pathways
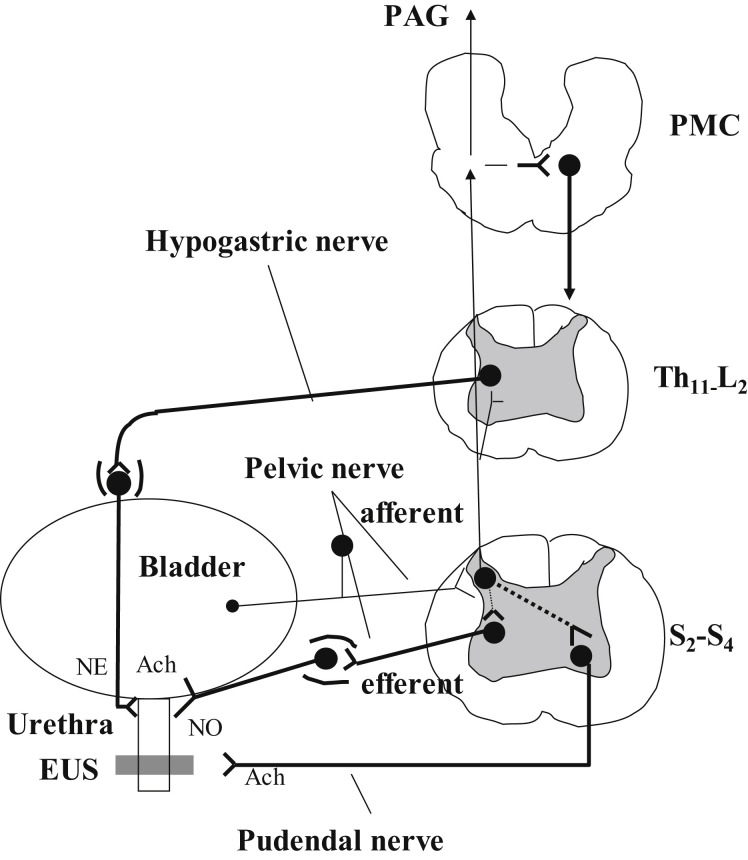
The smooth muscles of the bladder (the detrusor) are innervated primarily by parasympathetic nerves, whereas those of the bladder neck and urethra (the internal sphincter) are innervated by sympathetic nerves. The striated muscles of the EUS receive their primary innervation from somatic nerves (Figs. 2 and 3 ).
Fig. 2.
Efferent pathways: Major preganglionic and postganglionic neural pathways from the spinal cord to the lower urinary tract. The sympathetic hypogastric nerve, emerging from the inferior mesenteric ganglion, stimulates urethral smooth muscle. The parasympathetic pelvic nerve, emerging from the pelvic ganglion, stimulates bladder detrusor muscle and inhibits urethral smooth muscle. The somatic pudendal nerve stimulates striated muscle of the EUS. Afferent pathways: Ascending afferent inputs from the spinal cord passes through neurons in the periaqueductal gray (PAG) to upper brain regions and the pontine micturition (PMC). ACh, acetylcholine; NE, norepinephrine; NO, nitric oxide; S2-S4, sacral segments of the spinal cord; T11-L2, thoracolumbar segments of the spinal cord.
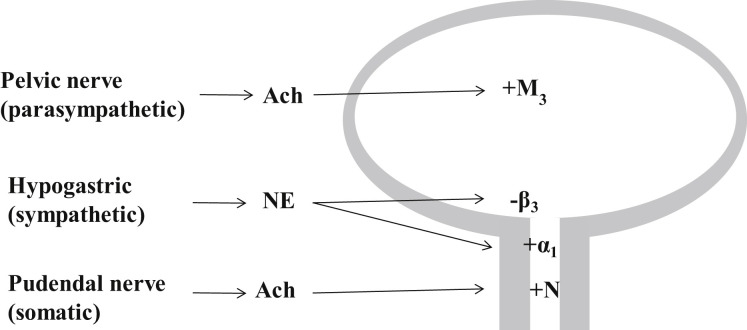
Fig. 3.
Innervation of the lower urinary tract: The parasympathetic pelvic nerve stimulates the bladder detrusor muscle, mediated by muscarinic receptors (M3) being activated by ACh. The sympathetic hypogastric nerve stimulates urethral smooth muscle and inhibits bladder detrusor, mediated by α1-adrenergic and β3-adrenergic receptors, respectively. The somatic pudendal nerve stimulates striated muscle of the EUS, mediated by ACh activating nicotinic (N) receptors. ACh, acetylcholine; NE, norepinephrine. Plus and minus signs indicate neural stimulation and inhibition, respectively.
Parasympathetic nerves
The efferent parasympathetic pathway provides the major excitatory innervation of the detrusor.5 Preganglionic axons emerge, as does the pelvic nerve, from the sacral parasympathetic nucleus in the intermediolateral column of sacral spinal segments S2 to S4 in humans and synapse in the pelvic ganglia, as well as in small ganglia on the bladder wall, releasing acetylcholine (ACh). Excitation of postsynaptic neurons by ACh is mediated by nicotinic receptors. Postganglionic axons continue for a short distance in the pelvic nerve and terminate in the detrusor layer where they release ACh to induce contractions of the smooth muscle fibers of the detrusor. This stimulatory effect of ACh at the postganglionic axon terminal is mediated by muscarinic receptors in detrusor cells. Two muscarinic subtypes, M2 and M3, are known to be present in the bladder; although M2 is most abundant in detrusor cells, the M3 subtype is the major receptor mediating stimulation of detrusor contractions.5, 16, 17 Parasympathetic postganglionic nerves also release nonadrenergic, noncholinergic transmitters (adenosine triphosphate [ATP]), which act on P2X1 purinoceptors.18
In addition to the parasympathetic stimulation of bladder smooth muscle, some postsynaptic parasympathetic neurons exert a relaxation effect on urethral smooth muscle, most likely via transmission of nitric oxide (NO).5, 17, 19, 20 Thus, as the bladder contracts during the elimination phase, the internal urethral sphincter relaxes to facilitate the bladder emptying.
Sympathetic nerves
Sympathetic nerves stimulate smooth muscle contraction in the urethra and bladder neck and cause relaxation of the detrusor. Preganglionic sympathetic neurons are located in the intermediolateral column of thoracolumbar cord segments T11 to L2 in humans.5, 17 Most of the preganglionic fibers synapse with postganglionic neurons in the inferior mesenteric ganglia. The preganglionic neurotransmitter is ACh, which acts via nicotinic receptors in the postganglionic neurons. Postganglionic axons travel in the hypogastric nerve and release norepinephrine (NE) at their terminals. The major terminals are in the urethra and bladder neck, as well as in the bladder body. NE stimulates contraction of urethral and bladder neck smooth muscle via α1-adrenoceptors and causes relaxation of detrusor via β2-adrenoceptors and β3-adrenoceptors, the latter being predominant.21
Somatic nerves
Somatic nerves provide excitatory innervation to the striated muscles of the EUS and pelvic floor. The efferent motoneurons are located in Onuf nucleus, along the lateral border of the ventral horn in sacral spinal cord segments S2 to S4 in humans.5, 22 The motoneuron axons are carried in the pudendal nerve and release ACh at their terminals. The ACh acts on nicotinic receptors in the striated muscle, inducing muscle contraction to maintain closure of the EUS.5, 22, 23
Afferent Pathways
The pelvic, hypogastric, and pudendal nerves carry sensory information in afferent fibers from the lower urinary tract to the lumbosacral spinal cord.22, 24, 25 The most important afferents for initiating micturition are those passing in the pelvic nerve to sacral spinal cord. These afferents are small myelinated Aδ and unmyelinated C fibers, which convey information from receptor in the bladder wall to second-order neurons in the spinal cord.26 Aδ bladder afferents in the cat respond in a graded manner to passive distension, as well as to active contraction of the bladder. In contrast, unmyelinated C-fiber bladder afferents in the cat are insensitive to mechanical stimuli and commonly do not respond to even high levels of intravesical pressure.27, 28 In the cat, silent C-fiber afferents have specialized function, such as the signaling of inflammatory or noxious events in the lower urinary tract. In the rat, Aδ and C-fiber bladder afferents cannot be distinguished because both types of afferents consist of mechanosensitive and chemosensitive populations.27 The properties of Aδ and C-fiber bladder afferent nerves in humans are unknown.
Bladder Urothelium
The urothelium, which has been traditionally viewed as a passive barrier, also has specialized sensory and signaling properties that allow it to respond to chemical and mechanical stimuli and to engage in chemical communication with neighboring nerves or myofibroblasts in the underlying lamina, which comprises the bladder mucosa with the urothelial layer.18, 29, 30 These urothelial properties include (1) expression of receptors for ACh, NE, tachykinins, and agonists for transient receptor potential (TRP) channels, such as TRPV1, TRPV4, and TRPV8; (2) close physical association with afferent nerves; and (3) ability to release chemical mediators, such as ATP, ACh, nerve growth factor (NGF), and NO, which also can influence reflex bladder contractions.18, 29 The somata of the pelvic and pudendal afferent nerves are located in dorsal root ganglia at sacral segments S2 to S4; the somata of the hypogastric nerve are located in dorsal root ganglia at thoracolumbar segments T11–L2.
Neural Circuits Controlling Micturition
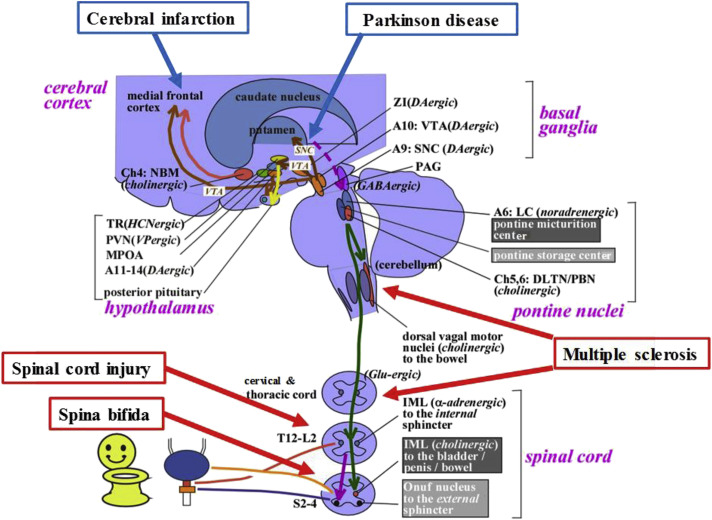
Coordination between the bladder and urinary sphincter is mediated by a complex neural control system that is located in the brain, spinal cord, and peripheral ganglia (Fig. 4 ). Storage function is primarily maintained by the spinal-cord reflex, which enhances the activity of the sympathetic and somatic nerves innervating the EUS. This function is also facilitated by the pontine storage center, which lies ventrolateral to the pontine micturition center (PMC), the hypothalamus, the cerebellum, the basal ganglia, and the frontal cortex. In those without neurologic deficits, the micturition reflex depends on the spinobulbospinal reflex, which involves the periaqueductal gray (PAG) in the midbrain and in the PMC.31 The PAG is thought to mediate switching from storage to voiding, and the PMC, which is located in or adjacent to the locus coeruleus, has spinal descending fibers containing glutamate as a major excitatory neurotransmitter, which project to the sacral cord intermediate column. Fibers that contain γ-aminobutyric acid (GABA) and glycine as inhibitory neurotransmitters also project from the PMC to the Onuf nucleus. These fibers are able to suppress urethral sphincter activity during voiding. In addition, the mechanism of switching from storage to voiding in the PAG is thought to be regulated by higher brain structures, such as the hypothalamus and prefrontal cortex. Overall, owing to the complexities of the neural mechanisms regulating the LUT, a wide variety of neurologic diseases, such as CI, PD, MS, SCI, and spina bifida, affect micturition and are able to induce lower urinary tract (LUT) dysfunction (see Fig. 4).
Fig. 4.
Neural circuitry relevant to micturition. The lower urinary tract consists of 2 major components: the bladder, and the urethra. The bladder is mainly innervated by the parasympathetic pelvic nerve. The urethra is innervated by the sympathetic hypogastric nerve and somatic pudendal nerve, respectively. Urinary storage depends on the reflex arc of the sacral spinal cord. The storage reflex is thought to be tonically facilitated by the brain, particularly the pontine storage center. The storage function is thought to be further facilitated by the hypothalamus, cerebellum, basal ganglia, and frontal cortex. Central cholinergic fibers from the nucleus basalis Meynert (NBM; also called the Ch4 cell group) seem to facilitate urinary storage. Micturition depends on the reflex arc of the brainstem and spinal cord, which involves the midbrain PAG and the PMC (located in or adjacent to the locus coeruleus [LC]). The voiding function is thought to be initiated by the hypothalamus and prefrontal cortex, which overlap the storage-facilitating area. CI induces the neural damage in the cerebrum. PD is primarily induced by degeneration of dopaminergic (DAgic) neurons in the substantia nigra pars compacta (SNC). MS is induced by focal demyelization of the central nervous system at various levels. SCI is induced by complete or incomplete neural damages of the spinal cord at different levels. Spina bifida is caused by a failure of the caudal neural tube to fuse normally in early development, thus often inducing the damage of the lumbosacral spinal cord and resulting in myelomeningocele, in which the spinal cord and neural elements are exposed. A, adrenergic or noradrenergic; DLTN, dorsolateral tegmental nucleus; GABA, γ-aminobutyric acid; HCN, hypocretinergic; IML, intermediolateral cell column; L, lumbar; MPOA, medial preoptic area; PBN, parabrachial nucleus; PVN, paraventricular nucleus; S, sacral; SNC, substantia nigra pars compacta; T, thoracic; TR, tuberous region; VTA, ventral tegmental area; ZI, zona incerta.
Disease-induced changes in micturition-animal models
Terminology
This section summarizes the findings of basic science research in neurogenic lower urinary tract dysfunction using animal models of human diseases, such as CI, PD, MS, spinal cord injury, and spina bifida. At present, the terminology of lower urinary tract dysfunction in basic animal research is not yet standardized. Also, it is not known which urodynamic findings in animals directly correspond to human conditions of neurogenic lower urinary tract dysfunction. Therefore, this article avoids the use of the term overactive bladder, which is a symptom-based disease definition, when describing the urodynamic findings of animal models. Also, based on the international continence society standardization, detrusor overactivity (DO) in humans is defined as the unexpected bladder muscle (the detrusor) contraction during bladder filling. Thus, the term DO is used only when animals exhibit nonvoiding bladder contractions during the storage phase in cystometric analyses. In other cases in which frequent voiding with reduced voided volume in voiding behavior studies or reduced intercontraction intervals with decreased bladder capacity during cystometry are observed, the condition is called bladder overactivity to avoid the confusion with DO. In addition, the term detrusor sphincter dyssynergia (DSD) is used when the tonic activity of EUS-EMG is increased or the urethral pressure becomes positive values above 0 cmH2O in urethral perfusion pressure measurements during voiding bladder contractions.
Cerebral Infarction
Cerebrovascular accident is a serious neurologic event and it can cause temporary or permanent neurogenic lower urinary tract dysfunction to patients.32 In CI survivors, there is also a high prevalence of urinary incontinence varying from 12% to 79%, depending on time after CI.33, 34 In CI, the neurologic dysfunction is caused by a focal brain damage due to ischemia and/or hemorrhage. When the brain damage is located in a small area in the right frontal region of cerebrum, which is involved in the control of micturition, it may predominantly result in bladder overactivity and urgency urinary incontinence.1, 35, 36
A rat model of CI produced by occlusion of the middle cerebral artery with a flamed 4-0 monofilament nylon inserted into the internal carotid artery has been shown to exhibit bladder overactivity as evidenced by reduced bladder capacity during awake cystometry.37 In another rat model of bladder overactivity without brain damage, midbrain ischemia is induced by anastomosis between the right external jugular vein and the right common carotid artery with partial obstruction of the left common carotid artery.38 The mechanism underlying lower urinary tract dysfunction after CI remains unclear. However, using animal models of CI, it has been reported that N-methyl-D-aspartate (NMDA) glutamatergic excitatory mechanisms play an important role in bladder overactivity induced by CI. Pretreatment with MK-801, an NMDA receptor agonist, can prevent bladder overactivity in CI rats.39 Rats with CI also exhibit the upregulation of D2-like dopamine receptor-mediated excitatory mechanisms and alteration in dopaminergic-glutamatergic interaction in the brain.40, 41, 42 Disruption of GABAergic43 and glycinergic44 inhibitory mechanisms in the brain is also involved to enhance the micturition reflex. Thus, the balance between excitatory glutamatergic neurons and inhibitory glycinergic or GABAergic might be impaired after CI, which results in bladder overactivity.
In addition to bladder dysfunction, CI reportedly induces urethral dysfunction. In a recent study in CI rats, leak point pressure was 29% lower compared with normal rats,45 and duloxetine, an NE and serotonin reuptake inhibitor, failed to enhance the sneeze-induced urethral continence reflex,45 suggesting that CI impairs the urethral continence mechanism to induce stress urinary incontinence. Robinson and Bloom46 postulated that ischemic lesions may interrupt the biogenic amine-containing axons ascending from the brainstem to the cerebral cortex, leading to decreased availability of biogenic amines in limbic structures of the frontal and temporal lobes, as well as in the basal ganglia. The monoamine theory postulates that depression is associated with low levels of monoamines, particularly NE, serotonin, and dopamine.47 In addition, monoaminergic descending pathways projecting through the dorsolateral spinal column are instrumental in the regulation of pain.48 Thus, ascending and descending pathways through the raphe nuclei and the locus coeruleus, which are the major sources of spinal serotonergic and noradrenergic pathways, respectively,49 in the brain stem may be disrupted by the stroke lesion, which may result in impaired urethral continence function.
Parkinson Disease
PD is a degenerative disorder of central nervous system characterized by muscle rigidity, tremor, and a slow physical movement. These symptoms result from decreased stimulation of the motor cortex by the basal ganglia, usually caused by the insufficient formation and action of dopamine, which is produced in the pathways from the substantia nigra to the striatum in the midbrain. Patients with PD also often have lower urinary tract symptoms, such as nocturia, increased urinary frequency, and urinary incontinence, which overlap with those of overactive bladder symptoms. The reported incidence of such symptoms ranges from 27% to 63.9% across different studies.50 The most common finding in the urodynamic study is DO, shown by uninhibited contractions during bladder filling, which result from impaired activity of the central nervous system, especially in the nigrostriatal dopaminergic pathways.51, 52 A previous functional brain imaging study has shown some overlaps (PAG, thalamus, putamen, and insula), as well as differences (pons or anterior cingulate gyrus only in healthy volunteers) in brain activation sites during filling between healthy volunteers and subjects with PD who had DO.53
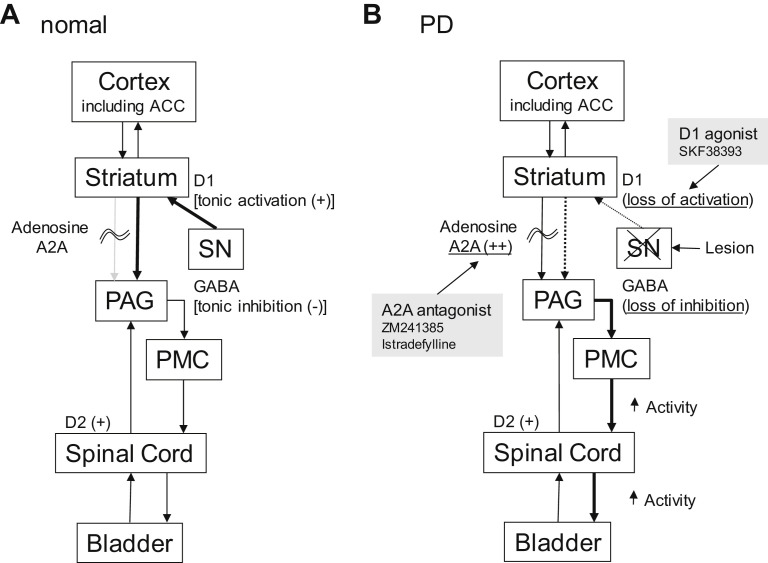
The underlying mechanisms inducing bladder dysfunction in PD have been investigated in animal studies. Fig. 5 shows a hypothetical diagram drawn from animal models of bladder dysfunction in PD. In monkeys, disruption of nigrostriatal dopaminergic pathways induced by the neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) produces PD-like motor symptoms accompanied by bladder overactivity shown by frequent urination with reduced voided volume.54, 55, 56 A rat model of PD induced by a unilateral 6-hydroxydopamine injection into the substantia nigra exhibits a similar type of bladder overactivity.57, 58 In these animal models, bladder overactivity was suppressed by enhancement of D1-like receptors with SKF 38393 or pergolide,55, 56, 57 suggesting that bladder overactivity in PD is primarily induced by disruption of D1-like dopamine receptor-mediated inhibition of the micturition reflex.
Fig. 5.
A hypothetical diagram demonstrates working model of bladder dysfunction in PD. Micturition reflex is controlled by spinobulbospinal pathways through PAG in midbrain and PMC in brainstem. This neural circuit is under control of higher centers, including anterior cingulate cortex (ACC) and other cortex regions. (A) Under normal conditions tonic inhibition from ACC suppress micturition reflex. Tonic firing (+) of dopaminergic neurons in SN activates dopamine D1 receptors expressed on GABAergic inhibitory neurons in the striatum to induce tonic GABAergic inhibition (−) of the micturition reflex at the level of PAG. At the same time, D1 receptor stimulation suppresses the activity of adenosinergic neurons, which exert an excitatory effect on micturition via adenosine A2A receptors (+). (B) In PD, dopaminergic neurons in the substantia nigra pars compacta (SN) are lost (lesion), leading to the loss of dopamine D1 receptors activation (D1 [loss of activation]), which results in reduced activation inhibitory GABAergic neurons in the striatum (GABA [loss of inhibition]). At the same time, reduced D1 receptor stimulation enhances the adenosinergic mechanism to stimulate adenosine A2A receptors (adenosine A2A [++]), leading to facilitation of the spinobulbospinal pathway controlling the micturition reflex pathway. Administration of dopamine D1 receptor agonist (SKF 38393) can restore the GABAergic nerve activity and suppress A2A receptor-mediated activation to reduce bladder overactivity in PD. Also, administration of adenosine A2A antagonists (ZM241385 or istradefylline) can suppress A2A receptor-mediated activation of the micturition reflex to reduce bladder overactivity in PD. Dopamine D2 receptors (D2 [+]) expressed in the spinal cord enhances the micturition reflex.
(Modified from Kitta T, Chancellor MB, de Groat WC, et al. Role of the anterior cingulate cortex in the control of micturition reflex in a rat model of Parkinson's disease. J Urol 2016;195:1613–20.)
Levodopa for treatment of PD patients often worsens DO due to activation of dopamine D2-receptors.59 In addition, in a rat model of PD, bladder overactivity was suppressed by an adenosine A2A receptor antagonist, ZM 241385, suggesting that enhanced activity of the adenosine A2A system in the brain contribute to bladder overactivity associated with PD.60 The adenosine A2A receptor-expressing neural pathways are very likely located downstream of D1 receptor expressing pathways in the control of micturition because inhibition of bladder activity by D1 receptor activation can induce the partial suppression of adenosine A2A receptor-mediated excitatory mechanisms in the rat model of PD.60 Kitta and colleagues61 also reported that intravenous ZM 241385 dose-dependently increased the amplitude of evoked potentials in the anterior cingulate cortex in a rat PD model but not in sham operated rats, suggesting that anterior cingulate cortex neurons have an inhibitory role in bladder control and an executive function, including decision-making in the micturition reflex. Clinical studies of adenosine A2A receptor antagonists also provided promising results of motor dysfunction in PD subjects.62, 63 Istradefylline, an adenosine A2A receptor antagonist, has been already approved and launched for patients with PD, which also may be a promising candidate for treatment of lower urinary tract dysfunction in patients with PD.64 To support this assumption, a recent open-labeled clinical study reported that treatment with istradefylline, a selective adenosine A2A receptor antagonist, for 12 weeks significantly improved lower urinary tract symptoms in 13 male PD patients64 although a larger-sized, placebo-controlled randomized study is needed to confirm the results.
In addition, a previous study65 shows that the injection of human amniotic-fluid-derived stem cells and bone-marrow-derived mesenchymal stem cells into the medial forebrain bundle improves bladder dysfunction in rat models of PD and stem-cell injection did improve cystometric parameters 14 days after implantation. This effect is not sustained and the number of stem cells gradually decreases with time. After injection, human stem cells were found to express superoxide dismutase-2, and modulated the expression of interleukin-6 and glial cell–derived neurotrophic factor by host cells. Thus, the injected stem cells seem to act on the juxtacrine or paracrine system, although the precise mechanism is yet to be determined.
Multiple Sclerosis
MS is a chronic disease with focal demyelization of the central nervous system at various levels, causing a wide spectrum of neurologic manifestation. The onset of MS ranges from 20 to 40 years, with more than 80% patients suffering from lower urinary tract symptoms.66 The storage symptoms, such as frequency, urgency, and urge incontinence, are more common but voiding symptoms, such as weak stream, straining, and large residual urine, are also commonly seen in MS patients.67, 68, 69 The exact causes of the development of MS are still unknown; however, experimental autoimmune encephalomyelitis is among the most commonly used and characterized animal model of MS using mice or rats.70, 71, 72 Coronavirus-induced encephalomyelitis in mice was also reported to develop neurogenic lower urinary tract dysfunction that is compatible with DO, often seen in patients with MS.73, 74 Thus, these findings are in line with the current view that bladder dysfunction in MS is associated with spinal cord demyelination and, subsequently, disruption of pathways between the lumbosacral spinal cord and the PMC.
In addition to the neurogenic dysfunction in the central nervous system, there is also evidence showing the damage in peripheral nerves and urothelial function of the bladder. For example, Lamarre and colleagues74 demonstrated a deficit in the nerve-evoked cholinergic component of bladder contraction. Negoro and colleagues75 also reported that pannexin1, a member of the gap junction protein expressed in the bladder urothelium, provides a positive feedback loop for inflammatory responses in bladder dysfunction in an MS mouse model with experimental autoimmune encephalomyelitis.
Spinal Cord Injury
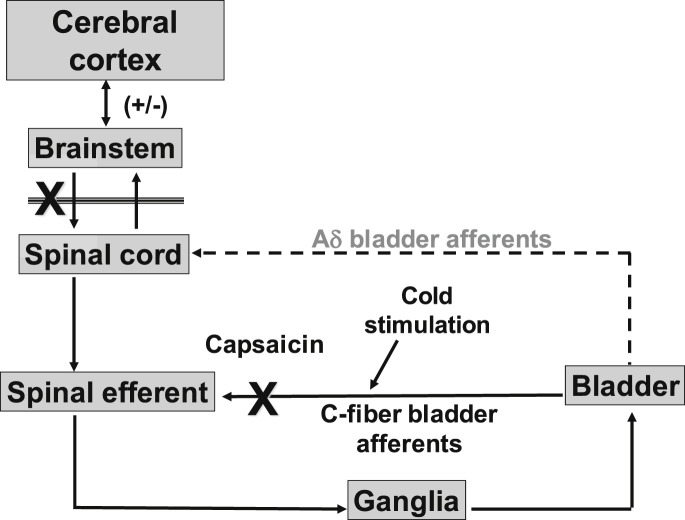
SCI initially induces areflexic bladder and urinary retention, followed by the emergence of automatic micturition and, eventually, DO mediated by spinal reflex mechanisms. This reflex mimics a neonatal exteroceptive micturition reflex that is activated by the mother licking the perineal region for the expelling of urine.76, 77 Maximal voiding pressure is increased, voiding efficiency is reduced, and bladder undergoes marked hypertrophy.6, 8, 78 Bladder-sphincter coordination is impaired, leading to DSD.79, 80, 81, 82, 83 Thus, the organization of the micturition reflex shows marked changes after SCI (Fig. 6 ). Any injury to the spinal cord, such as blunt, degenerative, developmental, vascular, infectious, traumatic, and idiopathic injury, can cause voiding dysfunction. In cats with chronic thoracic spinal cord transection, micturition is induced by C-fiber afferent pathways. It has been demonstrated that in chronic spinalized cats, desensitization of TRPV1-expressing C-fiber afferent pathways by subcutaneously administered capsaicin, a C-fiber neurotoxin that binds to TRPV1 receptors, completely blocked DO shown by nonvoiding bladder contractions during the storage phase, whereas capsaicin had no inhibitory effects on reflex bladder contractions in spinal intact cats.80, 84 Thus, it is plausible that C-fiber bladder afferents that usually do not respond to bladder distension27, 28 become mechanosensitive and initiate automatic micturition after SCI.
Fig. 6.
A hypothetical diagram demonstrates micturition reflex pathways after SCI. SCI rostral to the lumbosacral spinal cord level eliminates voluntary and supraspinal control of voiding. Over a period of several weeks following SCI, a spinal reflex mechanism emerges, which is triggered by unmyelinated C-fiber bladder afferents. Aδ-fiber bladder afferent inputs are ineffective. Stimulation of C-fiber bladder afferents by instillation of ice water into the bladder (cold stimulation) activates voiding responses. Pretreatment with capsaicin that desensitizes TRPV1-expressing C-fiber afferent pathways reduces detrusor overactivity in chronic SCI rats but does not block the micturition reflex in normal rats.
In a rat model of SCI induced by complete transection of the thoracic spinal cord, increased excitability of C-fiber bladder afferents after SCI also induces DO, as evidenced by nonvoiding bladder contractions before micturition, because desensitizing C-fiber afferents by systemic capsaicin administration completely suppressed these nonvoiding bladder contractions without affecting the voiding reflex.6, 85 Desensitization of C-fiber afferent pathways by capsaicin pretreatment also reduces DSD in chronic SCI rats.86 Furthermore, it has also been shown in the cat that C-fiber bladder afferents are responsible for cold-induced bladder reflexes via TRPM8 receptors.29, 87 Chronic SCI in humans also causes the emergence of an unusual bladder reflex that is elicited by infusion of cold water into the bladder, which is blocked by intravesical capsaicin treatment.88, 89, 90 Thus, it is likely that cold-sensitive and capsaicin-sensitive C-fiber bladder afferents contribute to the emergence of DO and DSD after SCI.
The mechanisms inducing hyperexcitability of C-fiber afferent pathways after SCI has also been investigated in SCI animal models. In rats, it has been shown that bladder afferent neurons undergo both morphologic91 and physiologic changes, including a shift from high-threshold tetrodotoxin (TTX)-resistant Na+ channel type to the low-threshold, TTX-sensitive Na+ channel type,83, 92 as well as downregulation of low-threshold A-type K+ channels that is associated with decreased expression of Kv1.4 α-subunit following SCI.93 In addition, other factors are also involved in the genesis of DO after SCI. For example, activation of neurokinin-1 receptors, as well as increased expression of vasoactive intestinal polypeptide, and pituitary adenylate cyclase-activating polypeptide in the lumbosacral spinal cord plays a role in the emergence of DO after SCI.86, 94 TRP receptors in the suburothelial nerve fibers, such as TRPV1 or TRPA1, are also involved in C-fiber bladder hyperexcitability that contributes to neurogenic DO in SCI.95, 96
It has also been speculated that SCI-induced DO is mediated by the action of neurotrophic factors, such as NGF or brain-derived neurotrophic factor (BDNF). Animal studies demonstrated that chronic administration of NGF into the spinal cord or into the bladder wall induced DO and increased excitability of bladder afferent neurons.97, 98, 99 Immunoneutralization of NGF in the spinal cord reduced NGF levels in the L6 to S1 dorsal root ganglia, which contain bladder afferent neurons, and also suppressed DO and DSD.86, 100 Thus, a combination of peripheral and central NGF action is likely to be involved in the emergence of DO or DSD. In addition, although the role of BDNF has not been well-defined, a previous study by Frias and colleagues101 demonstrated that BDNF may be an important regulator of neurogenic DO appearance and maintenance because the early-phase administration of BDNF inhibited neurogenic DO, whereas, at later stages, BDNF sequestration reduced neurogenic DO in a rat model of SCI.
Furthermore, SCI can induce peripheral changes in urothelial and detrusor muscle function in the bladder to stimulate bladder afferents and induce bladder overactivity. For example, the urothelial release of ATP that can activate bladder afferents through P2X2/3 ATP receptors is shown to be increased in a rat model of SCI.102, 103 Also, previous studies have reported that urothelially activated intrinsic detrusor contractions that are sensitive to L-type Ca2+ channels blockers, such as nifedipine, are enhanced in the SCI rat bladder104 and that the SCI-induced intrinsic detrusor activity is linked to the increased firing of single unit bladder afferent fibers in SCI mice.105
In the central nervous system, glutamate is a major excitatory amino acid, whereas glycine and GABA are major inhibitory neurotransmitters and act to inhibit the micturition reflex at supraspinal and/or spinal sites.106 Intravenous application of an α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) glutamatergic receptor antagonist is reported to eliminate DSD.107 Glycine and GABA have additive or synergistic inhibitory effects on bladder activity.108 Hypofunction of glycinergic or GABAergic mechanisms in the lumbosacral spinal cord induces lower urinary tract dysfunction, such as DO or DSD, after SCI in rats.12, 109, 110 Intrathecal, intravenous, or dietary glycine inhibits both bladder and urethral activity in normal and SCI rats.109, 111 Therefore, glycine might be useful agent for the treatment of DO. Also, intrathecal muscimol and baclofen (GABAA and GABAB agonists, respectively) in SCI rats inhibit nonvoiding bladder contractions by suppressing C-fiber bladder afferents,110 and they improve DSD as well.12 Baclofen is approved for treatment of DO in SCI patients,112 but this agent has not been widely used because the therapeutic window of this drug is modest with the limited dose range due to side effects. Gene delivery of glutamic acid decarboxylase (GAD), the GABA synthesis enzyme, by using nonreplicating herpes simplex virus vectors inhibits DO or DSD without affecting voiding contraction in SCI rats.113, 114 Therefore, GAD gene therapy can restore urine storage function without affecting voiding function; therefore, it would be more beneficial than drug therapy for the treatment of urinary problems in SCI patients. In addition, a recent study using rats with partial SCI induced by bilateral dorsal lesions of the thoracic spinal cord has demonstrated that the treatment with a P2X7 ATP receptor antagonist at the injured spinal cord region reduced DO induced after SCI and that P2X7 receptors were expressed in CD11b-positive microglia cells.115 Because the ATP release from the spinal cord during bladder distension is reportedly increased in SCI rats compared with spinal intact rats,116 it is conceivable that the ATP-mediated excitatory mechanism in the spinal cord via P2X7 receptors expressed in microglia cells is enhanced to induce neurogenic DO after SCI. Overall, various levels of changes in both central and peripheral nervous systems, as well as in the lower urinary tract seem to contribute to neurogenic lower urinary tract dysfunction such as DO after SCI.
Although DO during urine storage is similarly observed in both rats and mice after SCI,105, 117 the behavior of EUS during voiding is quite different in these 2 species.11 In SCI rats, EUS bursting occurs during voiding bladder contractions, which coincides with small-amplitude intravesical pressure oscillations during cystometry. However, SCI mice do not exhibit clear EUS bursting or intravesical pressure oscillation but rather exhibit intermittent voiding with slow large-amplitude reductions in intravesical pressure, which occur during periods of reduced EUS activity.11 α-Bungarotoxin improved voiding by reducing urethral outlet resistance in SCI rats14; however, in normal rats, the toxin reduced voiding, probably by suppressing high-frequency phasic sphincter activity necessary for efficient urine elimination in normal animals. Thus, the reflex EUS pumping activity recovers and enables achieving efficient voiding, even after SCI in rats, whereas it is not the case in SCI mice.11 These results suggest that the difference in voiding efficiency after SCI in these 2 species might be due to the difference in urethral activity during the voiding phase, which should be taken into account in the basic research of SCI-induced neurogenic lower urinary tract dysfunction such as DSD. In addition, bilateral transection of hypogastric nerve, which provides the major sympathetic input to the urinary bladder and proximal urethra, improved voiding dysfunction in SCI rats118 although the role of hypogastric nerves in SCI mice has not been investigated.
Spina Bifida
The birth prevalence of spina bifida is at approximately 30 per 100,000 in the United States. Spina bifida is caused by a failure of the caudal neural tube to fuse normally in early development, thus often inducing the damage of the lumbosacral spinal cord. Myelomeningocele, in which the spinal cord and neural elements are exposed, is the most common and clinically severe of the open spina bifida defects. Thus, it causes variable impact on the somatic, parasympathetic, and sympathetic innervation of the lower urinary tract to affect the ability to store and empty urine.119
Although the basic research on neurogenic lower urinary tract dysfunction induced by spinal bifida is limited, fetal rats with retinoic acid-induced myelomeningocele120 have been used as an animal model of spina bifida to investigate bladder dysfunction. It has been reported that fetuses with myelomeningocele from pregnant Sprague-Dawley rats that were gavage fed with retinoic acid showed a reduction in KCl or bethanechol-induced muscle strip contractility and decreased nerve density in the bladder, whereas bladder smooth muscle of fetal myelomeningocele rats was morphologically normal.121, 122 A recent study has also shown that the number of interstitial cells of Cajal in the bladder is decreased in fetal myelomeningocele rats.123 Thus, this animal model of spinal bifida and myelomeningocele seems to be useful to study the denervation process in the bladder and the possible damage of coordinating activity of bladder smooth muscles, although it is not likely to be suitable for the postnatal investigation of spina bifida-induced lower urinary tract dysfunction.
Summary
The lower urinary tract has 2 main functions, storage and elimination of urine. These highly, coordinated functions are regulated by a complex neural control system located in the brain and spinal cord (ie, central nervous system). Due to the complexity of these systems, micturition is sensitive to various diseases, such as CI, PD, MS, SCI, and spinal bifida. Studies in animals for these diseases are helpful to investigate the mechanism involved in the genesis and the plasticity in reflex pathways to the lower urinary tract after lesions, although care should be taken in extrapolating observations made in animals when applying them to human disease conditions.
Footnotes
Disclosure: N. Yoshimura has acted as a consultant for Astellas Pharma and Kyorin Pharmaceutical and has received research grants from Astellas Pharma and GlaxoSmithKline. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the article apart from those disclosed.
The authors’ research has been supported in part by grants from the National Institutes of Health (Grant Number P01DK093424 and R01DK088836), the Department of Defense (W81XWH-11-1-0763), and the Paralyzed Veterans of America (2793).
References
- 1.Fowler C.J., Griffiths D., de Groat W.C. The neural control of micturition. Nat Rev Neurosci. 2008;9:453–466. doi: 10.1038/nrn2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Groat W.C., Griffiths D., Yoshimura N. Neural control of the lower urinary tract. Compr Physiol. 2015;5:327–396. doi: 10.1002/cphy.c130056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Groat W.C., Yoshimura N. Anatomy and physiology of the lower urinary tract. Handbook Clin Neurol. 2015;130:61–108. doi: 10.1016/B978-0-444-63247-0.00005-5. [DOI] [PubMed] [Google Scholar]
- 4.Park J.M., Bloom D.A., McGuire E.J. The guarding reflex revisited. Br J Urol. 1997;80:940–945. doi: 10.1046/j.1464-410x.1997.00488.x. [DOI] [PubMed] [Google Scholar]
- 5.Yoshimura N., de Groat W.C. Neural control of the lower urinary tract. Int J Urol. 1997;4:111–125. doi: 10.1111/j.1442-2042.1997.tb00156.x. [DOI] [PubMed] [Google Scholar]
- 6.Cheng C.L., de Groat W.C. The role of capsaicin-sensitive afferent fibers in the lower urinary tract dysfunction induced by chronic spinal cord injury in rats. Exp Neurol. 2004;187:445–454. doi: 10.1016/j.expneurol.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 7.Chen S.C., Fan W.J., Lai C.H. Jason Chen JJ, Peng CW. Effect of a 5-HT(1A) receptor agonist (8-OH-DPAT) on the external urethral sphincter activity in the rat. J Formos Med Assoc. 2012;111:67–76. doi: 10.1016/j.jfma.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 8.Kruse M.N., Belton A.L., de Groat W.C. Changes in bladder and external urethral sphincter function after spinal cord injury in the rat. Am J Physiol. 1993;264:R1157–R1163. doi: 10.1152/ajpregu.1993.264.6.R1157. [DOI] [PubMed] [Google Scholar]
- 9.Liu G., Lin Y.H., Yamada Y. External urethral sphincter activity in diabetic rats. Neurourol Urodyn. 2008;27:429–434. doi: 10.1002/nau.20543. [DOI] [PubMed] [Google Scholar]
- 10.Maggi C.A., Giuliani S., Santicioli P. Analysis of factors involved in determining urinary bladder voiding cycle in urethan-anesthetized rats. Am J Physiol. 1986;251:R250–R257. doi: 10.1152/ajpregu.1986.251.2.R250. [DOI] [PubMed] [Google Scholar]
- 11.Kadekawa K., Yoshimura N., Majima T. Characterization of bladder and external urethral activity in mice with or without spinal cord injury–a comparison study with rats. Am J Physiol Regul Integr Comp Physiol. 2016;310:R752–R758. doi: 10.1152/ajpregu.00450.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miyazato M., Sasatomi K., Hiragata S. Suppression of detrusor-sphincter dysynergia by GABA-receptor activation in the lumbosacral spinal cord in spinal cord-injured rats. Am J Physiol Regul Integr Comp Physiol. 2008;295:R336–R342. doi: 10.1152/ajpregu.90315.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peng C.W., Chen J.J., Cheng C.L. Role of pudendal afferents in voiding efficiency in the rat. Am J Physiol Regul Integr Comp Physiol. 2008;294:R660–R672. doi: 10.1152/ajpregu.00270.2007. [DOI] [PubMed] [Google Scholar]
- 14.Yoshiyama M., deGroat W.C., Fraser M.O. Influences of external urethral sphincter relaxation induced by alpha-bungarotoxin, a neuromuscular junction blocking agent, on voiding dysfunction in the rat with spinal cord injury. Urology. 2000;55:956–960. doi: 10.1016/s0090-4295(00)00474-x. [DOI] [PubMed] [Google Scholar]
- 15.Griffiths D. Functional imaging of structures involved in neural control of the lower urinary tract. Handbook Clin Neurol. 2015;130:121–133. doi: 10.1016/B978-0-444-63247-0.00007-9. [DOI] [PubMed] [Google Scholar]
- 16.Chapple C.R., Yamanishi T., Chess-Williams R. Muscarinic receptor subtypes and management of the overactive bladder. Urology. 2002;60:82–88. doi: 10.1016/s0090-4295(02)01803-4. [discussion: 88–9] [DOI] [PubMed] [Google Scholar]
- 17.de Groat W.C., Yoshimura N. Pharmacology of the lower urinary tract. Annu Rev Pharmacol Toxicol. 2001;41:691–721. doi: 10.1146/annurev.pharmtox.41.1.691. [DOI] [PubMed] [Google Scholar]
- 18.Birder L., de Groat W., Mills I. Neural control of the lower urinary tract: peripheral and spinal mechanisms. Neurourol Urodyn. 2010;29:128–139. doi: 10.1002/nau.20837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bennett B.C., Kruse M.N., Roppolo J.R. Neural control of urethral outlet activity in vivo: role of nitric oxide. J Urol. 1995;153:2004–2009. [PubMed] [Google Scholar]
- 20.Yono M., Yamamoto Y., Yoshida M. Effects of doxazosin on blood flow and mRNA expression of nitric oxide synthase in the spontaneously hypertensive rat genitourinary tract. Life Sci. 2007;81:218–222. doi: 10.1016/j.lfs.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nomiya M., Yamaguchi O. A quantitative analysis of mRNA expression of alpha 1 and beta-adrenoceptor subtypes and their functional roles in human normal and obstructed bladders. J Urol. 2003;170:649–653. doi: 10.1097/01.ju.0000067621.62736.7c. [DOI] [PubMed] [Google Scholar]
- 22.Thor K.B., Morgan C., Nadelhaft I. Organization of afferent and efferent pathways in the pudendal nerve of the female cat. J Comp Neurol. 1989;288:263–279. doi: 10.1002/cne.902880206. [DOI] [PubMed] [Google Scholar]
- 23.Blaivas J.G. The neurophysiology of micturition: a clinical study of 550 patients. J Urol. 1982;127:958–963. doi: 10.1016/s0022-5347(17)54147-6. [DOI] [PubMed] [Google Scholar]
- 24.Morgan C., deGroat W.C., Nadelhaft I. The spinal distribution of sympathetic preganglionic and visceral primary afferent neurons that send axons into the hypogastric nerves of the cat. J Comp Neurol. 1986;243:23–40. doi: 10.1002/cne.902430104. [DOI] [PubMed] [Google Scholar]
- 25.Andersson K.E., Wein A.J. Pharmacology of the lower urinary tract: basis for current and future treatments of urinary incontinence. Pharmacol Rev. 2004;56:581–631. doi: 10.1124/pr.56.4.4. [DOI] [PubMed] [Google Scholar]
- 26.Mallory B., Steers W.D., De Groat W.C. Electrophysiological study of micturition reflexes in rats. Am J Physiol. 1989;257:R410–R421. doi: 10.1152/ajpregu.1989.257.2.R410. [DOI] [PubMed] [Google Scholar]
- 27.de Groat W.C., Yoshimura N. Afferent nerve regulation of bladder function in health and disease. Handb Exp Pharmacol. 2009;194:91–138. doi: 10.1007/978-3-540-79090-7_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Habler H.J., Janig W., Koltzenburg M. Activation of unmyelinated afferent fibres by mechanical stimuli and inflammation of the urinary bladder in the cat. J Physiol. 1990;425:545–562. doi: 10.1113/jphysiol.1990.sp018117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Birder L.A. Urothelial signaling. Auton Neurosci. 2010;153:33–40. doi: 10.1016/j.autneu.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Andersson K.E., McCloskey K.D. Lamina propria: the functional center of the bladder? Neurourol Urodyn. 2014;33:9–16. doi: 10.1002/nau.22465. [DOI] [PubMed] [Google Scholar]
- 31.Kakizaki H., Kita M., Wada N. Models for sensory neurons of dorsal root ganglia and stress urinary incontinence. Neurourol Urodyn. 2011;30:653–657. doi: 10.1002/nau.21138. [DOI] [PubMed] [Google Scholar]
- 32.Sakakibara R. Lower urinary tract dysfunction in patients with brain lesions. Handbook Clin Neurol. 2015;130:269–287. doi: 10.1016/B978-0-444-63247-0.00015-8. [DOI] [PubMed] [Google Scholar]
- 33.Brittain K.R., Peet S.M., Castleden C.M. Stroke and incontinence. Stroke. 1998;29:524–528. doi: 10.1161/01.str.29.2.524. [DOI] [PubMed] [Google Scholar]
- 34.Kolominsky-Rabas P.L., Hilz M.J., Neundoerfer B. Impact of urinary incontinence after stroke: results from a prospective population-based stroke register. Neurourol Urodyn. 2003;22:322–327. doi: 10.1002/nau.10114. [DOI] [PubMed] [Google Scholar]
- 35.Griffiths D. Clinical studies of cerebral and urinary tract function in elderly people with urinary incontinence. Behav Brain Res. 1998;92:151–155. doi: 10.1016/s0166-4328(97)00187-3. [DOI] [PubMed] [Google Scholar]
- 36.Griffiths D.J. Cerebral control of bladder function. Curr Urol Rep. 2004;5:348–352. doi: 10.1007/s11934-004-0081-z. [DOI] [PubMed] [Google Scholar]
- 37.Yokoyama O., Komatsu K., Ishiura Y. Change in bladder contractility associated with bladder overactivity in rats with cerebral infarction. J Urol. 1998;159:577–580. doi: 10.1016/s0022-5347(01)63987-9. [DOI] [PubMed] [Google Scholar]
- 38.Yotsuyanagi S., Narimoto K., Namiki M. Mild brain ischemia produces bladder hyperactivity without brain damage in rats. Urol Int. 2006;77:57–63. doi: 10.1159/000092936. [DOI] [PubMed] [Google Scholar]
- 39.Yokoyama O., Yoshiyama M., Namiki M. Glutamatergic and dopaminergic contributions to rat bladder hyperactivity after cerebral artery occlusion. Am J Physiol. 1999;276:R935–R942. doi: 10.1152/ajpregu.1999.276.4.R935. [DOI] [PubMed] [Google Scholar]
- 40.Yokoyama O., Yoshiyama M., Namiki M. Interaction between D2 dopaminergic and glutamatergic excitatory influences on lower urinary tract function in normal and cerebral-infarcted rats. Exp Neurol. 2001;169:148–155. doi: 10.1006/exnr.2001.7639. [DOI] [PubMed] [Google Scholar]
- 41.Yokoyama O., Yoshiyama M., Namiki M. Role of the forebrain in bladder overactivity following cerebral infarction in the rat. Exp Neurol. 2000;163:469–476. doi: 10.1006/exnr.2000.7391. [DOI] [PubMed] [Google Scholar]
- 42.Yokoyama O., Yoshiyama M., Namiki M. Changes in dopaminergic and glutamatergic excitatory mechanisms of micturition reflex after middle cerebral artery occlusion in conscious rats. Exp Neurol. 2002;173:129–135. doi: 10.1006/exnr.2001.7833. [DOI] [PubMed] [Google Scholar]
- 43.Kanie S., Yokoyama O., Komatsu K. GABAergic contribution to rat bladder hyperactivity after middle cerebral artery occlusion. Am J Physiol Regul Integr Comp Physiol. 2000;279:R1230–R1238. doi: 10.1152/ajpregu.2000.279.4.R1230. [DOI] [PubMed] [Google Scholar]
- 44.Sugaya K., Nishijima S., Miyazato M. Central nervous control of micturition and urine storage. J Smooth Muscle Res. 2005;41:117–132. doi: 10.1540/jsmr.41.117. [DOI] [PubMed] [Google Scholar]
- 45.Miyazato M., Kitta T., Kaiho Y. Effects of duloxetine on urethral continence reflex and bladder activity in rats with cerebral infarction. J Urol. 2015;194:842–847. doi: 10.1016/j.juro.2015.03.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Robinson R.G., Bloom F.E. Pharmacological treatment following experimental cerebral infarction: implications for understanding psychological symptoms of human stroke. Biol Psychiatry. 1977;12:669–680. [PubMed] [Google Scholar]
- 47.Krishnan V., Nestler E.J. The molecular neurobiology of depression. Nature. 2008;455:894–902. doi: 10.1038/nature07455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Loubinoux I., Kronenberg G., Endres M. Post-stroke depression: mechanisms, translation and therapy. J Cell Mol Med. 2012;16:1961–1969. doi: 10.1111/j.1582-4934.2012.01555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Holstege J.C., Kuypers H.G. Brainstem projections to spinal motoneurons: an update. Neuroscience. 1987;23:809–821. doi: 10.1016/0306-4522(87)90160-6. [DOI] [PubMed] [Google Scholar]
- 50.Ogawa T., Sakakibara R., Kuno S. Prevalence and treatment of LUTS in patients with PD or multiple system atrophy. Nat Rev Urol. 2017;14(2):79–89. doi: 10.1038/nrurol.2016.254. [DOI] [PubMed] [Google Scholar]
- 51.Araki I., Kitahara M., Oida T. Voiding dysfunction and Parkinson's disease: urodynamic abnormalities and urinary symptoms. J Urol. 2000;164:1640–1643. [PubMed] [Google Scholar]
- 52.Araki I., Kuno S. Assessment of voiding dysfunction in Parkinson's disease by the international prostate symptom score. J Neurol Neurosurg Psychiatry. 2000;68:429–433. doi: 10.1136/jnnp.68.4.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kitta T., Kakizaki H., Furuno T. Brain activation during detrusor overactivity in patients with Parkinson's disease: a positron emission tomography study. J Urol. 2006;175:994–998. doi: 10.1016/S0022-5347(05)00324-1. [DOI] [PubMed] [Google Scholar]
- 54.Albanese A., Jenner P., Marsden C.D. Bladder hyperreflexia induced in marmosets by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Neurosci Lett. 1988;87:46–50. doi: 10.1016/0304-3940(88)90143-7. [DOI] [PubMed] [Google Scholar]
- 55.Yoshimura N., Erdman S.L., Snider M.W. Effects of spinal cord injury on neurofilament immunoreactivity and capsaicin sensitivity in rat dorsal root ganglion neurons innervating the urinary bladder. Neuroscience. 1998;83:633–643. doi: 10.1016/s0306-4522(97)00376-x. [DOI] [PubMed] [Google Scholar]
- 56.Yoshimura N., Mizuta E., Kuno S. The dopamine D1 receptor agonist SKF 38393 suppresses detrusor hyperreflexia in the monkey with parkinsonism induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) Neuropharmacology. 1993;32:315–321. doi: 10.1016/0028-3908(93)90151-r. [DOI] [PubMed] [Google Scholar]
- 57.Yoshimura N., Seki S., Erickson K.A. Histological and electrical properties of rat dorsal root ganglion neurons innervating the lower urinary tract. J Neurosci. 2003;23:4355–4361. doi: 10.1523/JNEUROSCI.23-10-04355.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kitta T., Matsumoto M., Tanaka H. GABAergic mechanism mediated via D receptors in the rat periaqueductal gray participates in the micturition reflex: an in vivo microdialysis study. Eur J Neurosci. 2008;27:3216–3225. doi: 10.1111/j.1460-9568.2008.06276.x. [DOI] [PubMed] [Google Scholar]
- 59.Brusa L., Petta F., Pisani A. Central acute D2 stimulation worsens bladder function in patients with mild Parkinson's disease. J Urol. 2006;175:202–206. doi: 10.1016/S0022-5347(05)00058-3. [discussion: 206–7] [DOI] [PubMed] [Google Scholar]
- 60.Kitta T., Chancellor M.B., de Groat W.C. Suppression of bladder overactivity by adenosine A2A receptor antagonist in a rat model of Parkinson disease. J Urol. 2012;187:1890–1897. doi: 10.1016/j.juro.2011.12.062. [DOI] [PubMed] [Google Scholar]
- 61.Kitta T., Chancellor M.B., de Groat W.C. Role of the anterior cingulate cortex in the control of micturition reflex in a rat model of Parkinson's disease. J Urol. 2016;195:1613–1620. doi: 10.1016/j.juro.2015.11.039. [DOI] [PubMed] [Google Scholar]
- 62.Pinna A., Wardas J., Simola N. New therapies for the treatment of Parkinson's disease: adenosine A2A receptor antagonists. Life Sci. 2005;77:3259–3267. doi: 10.1016/j.lfs.2005.04.029. [DOI] [PubMed] [Google Scholar]
- 63.Pinna A., Volpini R., Cristalli G. New adenosine A2A receptor antagonists: actions on Parkinson's disease models. Eur J Pharmacol. 2005;512:157–164. doi: 10.1016/j.ejphar.2005.01.057. [DOI] [PubMed] [Google Scholar]
- 64.Kitta T., Yabe I., Takahashi I. Clinical efficacy of istradefylline on lower urinary tract symptoms in Parkinson's disease. Int J Urol. 2016;23(10):893–894. doi: 10.1111/iju.13160. [DOI] [PubMed] [Google Scholar]
- 65.Soler R., Fullhase C., Hanson A. Stem cell therapy ameliorates bladder dysfunction in an animal model of Parkinson disease. J Urol. 2012;187:1491–1497. doi: 10.1016/j.juro.2011.11.079. [DOI] [PubMed] [Google Scholar]
- 66.Awad S.A., Gajewski J.B., Sogbein S.K. Relationship between neurological and urological status in patients with multiple sclerosis. J Urol. 1984;132:499–502. doi: 10.1016/s0022-5347(17)49710-2. [DOI] [PubMed] [Google Scholar]
- 67.de Seze M., Ruffion A., Denys P. Genulf. The neurogenic bladder in multiple sclerosis: review of the literature and proposal of management guidelines. Mult Scler. 2007;13:915–928. doi: 10.1177/1352458506075651. [DOI] [PubMed] [Google Scholar]
- 68.Betts C.D., D'Mellow M.T., Fowler C.J. Urinary symptoms and the neurological features of bladder dysfunction in multiple sclerosis. J Neurol Neurosurg Psychiatry. 1993;56:245–250. doi: 10.1136/jnnp.56.3.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McCombe P.A., Gordon T.P., Jackson M.W. Bladder dysfunction in multiple sclerosis. Expert Rev Neurother. 2009;9:331–340. doi: 10.1586/14737175.9.3.331. [DOI] [PubMed] [Google Scholar]
- 70.Mannie M., Swanborg R.H., Stepaniak J.A. Experimental autoimmune encephalomyelitis in the rat. Curr Protoc Immunol. 2009;Chapter: 15 doi: 10.1002/0471142735.im1502s85. Unit: 15.2. [DOI] [PubMed] [Google Scholar]
- 71.McCarthy D.P., Richards M.H., Miller S.D. Mouse models of multiple sclerosis: experimental autoimmune encephalomyelitis and Theiler's virus-induced demyelinating disease. Methods Mol Biol. 2012;900:381–401. doi: 10.1007/978-1-60761-720-4_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mizusawa H., Igawa Y., Nishizawa O. A rat model for investigation of bladder dysfunction associated with demyelinating disease resembling multiple sclerosis. Neurourol Urodyn. 2000;19:689–699. doi: 10.1002/1520-6777(2000)19:6<689::aid-nau7>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 73.McMillan M.T., Pan X.Q., Smith A.L. Coronavirus-induced demyelination of neural pathways triggers neurogenic bladder overactivity in a mouse model of multiple sclerosis. Am J Physiol Renal Physiol. 2014;307:F612–F622. doi: 10.1152/ajprenal.00151.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lamarre N.S., Braverman A.S., Malykhina A.P. Alterations in nerve-evoked bladder contractions in a coronavirus-induced mouse model of multiple sclerosis. PLoS One. 2014;9:e109314. doi: 10.1371/journal.pone.0109314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Negoro H., Lutz S.E., Liou L.S. Pannexin 1 involvement in bladder dysfunction in a multiple sclerosis model. Sci Rep. 2013;3:2152. doi: 10.1038/srep02152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.de Groat W.C. Plasticity of bladder reflex pathways during postnatal development. Physiol Behav. 2002;77:689–692. doi: 10.1016/s0031-9384(02)00919-8. [DOI] [PubMed] [Google Scholar]
- 77.Sugaya K., De Groat W.C. Micturition reflexes in the in vitro neonatal rat brain stem-spinal cord-bladder preparation. Am J Physiol. 1994;266:R658–R667. doi: 10.1152/ajpregu.1994.266.3.R658. [DOI] [PubMed] [Google Scholar]
- 78.Kruse M.N., Bennett B., De Groat W.C. Effect of urinary diversion on the recovery of micturition reflexes after spinal cord injury in the rat. J Urol. 1994;151:1088–1091. doi: 10.1016/s0022-5347(17)35189-3. [DOI] [PubMed] [Google Scholar]
- 79.de Groat W.C., Yoshimura N. Mechanisms underlying the recovery of lower urinary tract function following spinal cord injury. Prog Brain Res. 2006;152:59–84. doi: 10.1016/S0079-6123(05)52005-3. [DOI] [PubMed] [Google Scholar]
- 80.de Groat W.C., Kawatani M., Hisamitsu T. Mechanisms underlying the recovery of urinary bladder function following spinal cord injury. J Auton Nerv Syst. 1990;30(Suppl):S71–S77. doi: 10.1016/0165-1838(90)90105-r. [DOI] [PubMed] [Google Scholar]
- 81.de Groat W.C., Yoshimura N. Changes in afferent activity after spinal cord injury. Neurourol Urodyn. 2010;29:63–76. doi: 10.1002/nau.20761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.de Groat W.C., Yoshimura N. Plasticity in reflex pathways to the lower urinary tract following spinal cord injury. Exp Neurol. 2012;235:123–132. doi: 10.1016/j.expneurol.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yoshimura N. Bladder afferent pathway and spinal cord injury: possible mechanisms inducing hyperreflexia of the urinary bladder. Prog Neurobiol. 1999;57:583–606. doi: 10.1016/s0301-0082(98)00070-7. [DOI] [PubMed] [Google Scholar]
- 84.Cheng C.L., Liu J.C., Chang S.Y. Effect of capsaicin on the micturition reflex in normal and chronic spinal cord-injured cats. Am J Physiol. 1999;277:R786–R794. doi: 10.1152/ajpregu.1999.277.3.R786. [DOI] [PubMed] [Google Scholar]
- 85.Cheng C.L., Ma C.P., de Groat W.C. Effect of capsaicin on micturition and associated reflexes in chronic spinal rats. Brain Res. 1995;678:40–48. doi: 10.1016/0006-8993(95)00212-9. [DOI] [PubMed] [Google Scholar]
- 86.Seki S., Sasaki K., Igawa Y. Suppression of detrusor-sphincter dyssynergia by immunoneutralization of nerve growth factor in lumbosacral spinal cord in spinal cord injured rats. J Urol. 2004;171:478–482. doi: 10.1097/01.ju.0000088340.26588.74. [DOI] [PubMed] [Google Scholar]
- 87.Fall M., Lindstrom S., Mazieres L. A bladder-to-bladder cooling reflex in the cat. J Physiol. 1990;427:281–300. doi: 10.1113/jphysiol.1990.sp018172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Geirsson G., Fall M., Lindstrom S. The ice-water test–a simple and valuable supplement to routine cystometry. Br J Urol. 1993;71:681–685. doi: 10.1111/j.1464-410x.1993.tb16065.x. [DOI] [PubMed] [Google Scholar]
- 89.Geirsson G., Lindstrom S., Fall M. Positive bladder cooling test in neurologically normal young children. J Urol. 1994;151:446–448. doi: 10.1016/s0022-5347(17)34984-4. [DOI] [PubMed] [Google Scholar]
- 90.Geirsson G., Fall M., Sullivan L. Clinical and urodynamic effects of intravesical capsaicin treatment in patients with chronic traumatic spinal detrusor hyperreflexia. J Urol. 1995;154:1825–1829. [PubMed] [Google Scholar]
- 91.Kruse M.N., Bray L.A., de Groat W.C. Influence of spinal cord injury on the morphology of bladder afferent and efferent neurons. J Auton nervous Syst. 1995;54:215–224. doi: 10.1016/0165-1838(95)00011-l. [DOI] [PubMed] [Google Scholar]
- 92.Yoshimura N., de Groat W.C. Plasticity of Na+ channels in afferent neurones innervating rat urinary bladder following spinal cord injury. J Physiol. 1997;503(Pt 2):269–276. doi: 10.1111/j.1469-7793.1997.269bh.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Takahashi R., Yoshizawa T., Yunoki T. Hyperexcitability of bladder afferent neurons associated with reduction of Kv1.4 alpha-subunit in rats with spinal cord injury. J Urol. 2013;190:2296–2304. doi: 10.1016/j.juro.2013.07.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zvarova K., Dunleavy J.D., Vizzard M.A. Changes in pituitary adenylate cyclase activating polypeptide expression in urinary bladder pathways after spinal cord injury. Exp Neurol. 2005;192:46–59. doi: 10.1016/j.expneurol.2004.10.017. [DOI] [PubMed] [Google Scholar]
- 95.Santos-Silva A., Charrua A., Cruz C.D. Rat detrusor overactivity induced by chronic spinalization can be abolished by a transient receptor potential vanilloid 1 (TRPV1) antagonist. Auton Neurosci. 2012;166:35–38. doi: 10.1016/j.autneu.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 96.Andrade E.L., Meotti F.C., Calixto J.B. TRPA1 antagonists as potential analgesic drugs. Pharmacol Ther. 2012;133:189–204. doi: 10.1016/j.pharmthera.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 97.Lamb K., Gebhart G.F., Bielefeldt K. Increased nerve growth factor expression triggers bladder overactivity. J Pain. 2004;5:150–156. doi: 10.1016/j.jpain.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 98.Yoshimura N., Bennett N.E., Hayashi Y. Bladder overactivity and hyperexcitability of bladder afferent neurons after intrathecal delivery of nerve growth factor in rats. J Neurosci. 2006;26:10847–10855. doi: 10.1523/JNEUROSCI.3023-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zvara P., Vizzard M.A. Exogenous overexpression of nerve growth factor in the urinary bladder produces bladder overactivity and altered micturition circuitry in the lumbosacral spinal cord. BMC Physiol. 2007;7:9. doi: 10.1186/1472-6793-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Seki S., Sasaki K., Fraser M.O. Immunoneutralization of nerve growth factor in lumbosacral spinal cord reduces bladder hyperreflexia in spinal cord injured rats. J Urol. 2002;168:2269–2274. doi: 10.1016/S0022-5347(05)64369-8. [DOI] [PubMed] [Google Scholar]
- 101.Frias B., Santos J., Morgado M. The role of brain-derived neurotrophic factor (BDNF) in the development of neurogenic detrusor overactivity (NDO) J Neurosci. 2015;35:2146–2160. doi: 10.1523/JNEUROSCI.0373-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Smith C.P., Gangitano D.A., Munoz A. Botulinum toxin type A normalizes alterations in urothelial ATP and NO release induced by chronic spinal cord injury. Neurochem Int. 2008;52:1068–1075. doi: 10.1016/j.neuint.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Munoz A., Somogyi G.T., Boone T.B. Modulation of bladder afferent signals in normal and spinal cord-injured rats by purinergic P2X3 and P2X2/3 receptors. BJU Int. 2012;110:E409–E414. doi: 10.1111/j.1464-410X.2012.11189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ikeda Y., Kanai A. Urotheliogenic modulation of intrinsic activity in spinal cord-transected rat bladders: role of mucosal muscarinic receptors. Am J Physiol Ren Physiol. 2008;295:F454–F461. doi: 10.1152/ajprenal.90315.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.McCarthy C.J., Zabbarova I.V., Brumovsky P.R. Spontaneous contractions evoke afferent nerve firing in mouse bladders with detrusor overactivity. J Urol. 2009;181:1459–1466. doi: 10.1016/j.juro.2008.10.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Shapiro S. Neurotransmission by neurons that use serotonin, noradrenaline, glutamate, glycine, and gamma-aminobutyric acid in the normal and injured spinal cord. Neurosurgery. 1997;40:168–176. doi: 10.1097/00006123-199701000-00037. [discussion: 177] [DOI] [PubMed] [Google Scholar]
- 107.Yoshiyama M., Nezu F.M., Yokoyama O. Influence of glutamate receptor antagonists on micturition in rats with spinal cord injury. Exp Neurol. 1999;159:250–257. doi: 10.1006/exnr.1999.7124. [DOI] [PubMed] [Google Scholar]
- 108.Miyazato M., Sugaya K., Nishijima S. Rectal distention inhibits bladder activity via glycinergic and GABAergic mechanisms in rats. J Urol. 2004;171:1353–1356. doi: 10.1097/01.ju.0000099840.09816.22. [DOI] [PubMed] [Google Scholar]
- 109.Miyazato M., Sugaya K., Nishijima S. Inhibitory effect of intrathecal glycine on the micturition reflex in normal and spinal cord injury rats. Exp Neurol. 2003;183:232–240. doi: 10.1016/s0014-4886(03)00175-4. [DOI] [PubMed] [Google Scholar]
- 110.Miyazato M., Sasatomi K., Hiragata S. GABA receptor activation in the lumbosacral spinal cord decreases detrusor overactivity in spinal cord injured rats. J Urol. 2008;179:1178–1183. doi: 10.1016/j.juro.2007.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Miyazato M., Sugaya K., Nishijima S. Dietary glycine inhibits bladder activity in normal rats and rats with spinal cord injury. J Urol. 2005;173:314–317. doi: 10.1097/01.ju.0000141579.91638.a3. [DOI] [PubMed] [Google Scholar]
- 112.Steers W.D., Meythaler J.M., Haworth C. Effects of acute bolus and chronic continuous intrathecal baclofen on genitourinary dysfunction due to spinal cord pathology. J Urol. 1992;148:1849–1855. doi: 10.1016/s0022-5347(17)37048-9. [DOI] [PubMed] [Google Scholar]
- 113.Miyazato M., Sugaya K., Goins W.F. Herpes simplex virus vector-mediated gene delivery of glutamic acid decarboxylase reduces detrusor overactivity in spinal cord-injured rats. Gene Ther. 2009;16:660–668. doi: 10.1038/gt.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Miyazato M., Sugaya K., Saito S. Suppression of detrusor-sphincter dyssynergia by herpes simplex virus vector mediated gene delivery of glutamic acid decarboxylase in spinal cord injured rats. J Urol. 2010;184:1204–1210. doi: 10.1016/j.juro.2010.04.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Munoz A., Yazdi I.K., Tang X. Localized inhibition of P2X7R at the spinal cord injury site improves neurogenic bladder dysfunction by decreasing urothelial P2X3R expression in rats. Life Sci. 2016;171:60–67. doi: 10.1016/j.lfs.2016.12.017. [DOI] [PubMed] [Google Scholar]
- 116.Salas N.A., Somogyi G.T., Gangitano D.A. Receptor activated bladder and spinal ATP release in neurally intact and chronic spinal cord injured rats. Neurochem Int. 2007;50:345–350. doi: 10.1016/j.neuint.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wada N., Shimizu T., Takai S. Post-injury bladder management strategy influences lower urinary tract dysfunction in the mouse model of spinal cord injury. Neurourol Urodyn. 2016 doi: 10.1002/nau.23120. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yoshiyama M., de Groat W.C. Effect of bilateral hypogastric nerve transection on voiding dysfunction in rats with spinal cord injury. Exp Neurol. 2002;175:191–197. doi: 10.1006/exnr.2002.7887. [DOI] [PubMed] [Google Scholar]
- 119.Snow-Lisy D.C., Yerkes E.B., Cheng E.Y. Update on Urological Management of Spina Bifida from Prenatal Diagnosis to Adulthood. J Urol. 2015;194:288–296. doi: 10.1016/j.juro.2015.03.107. [DOI] [PubMed] [Google Scholar]
- 120.Danzer E., Schwarz U., Wehrli S. Retinoic acid induced myelomeningocele in fetal rats: characterization by histopathological analysis and magnetic resonance imaging. Exp Neurol. 2005;194:467–475. doi: 10.1016/j.expneurol.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 121.Danzer E., Kiddoo D.A., Redden R.A. Structural and functional characterization of bladder smooth muscle in fetal rats with retinoic acid-induced myelomeningocele. Am J Physiol Ren Physiol. 2007;292:F197–F206. doi: 10.1152/ajprenal.00001.2006. [DOI] [PubMed] [Google Scholar]
- 122.Shen J., Zhou G., Chen H. Morphology of nervous lesion in the spinal cord and bladder of fetal rats with myelomeningocele at different gestational age. J Pediatr Surg. 2013;48:2446–2452. doi: 10.1016/j.jpedsurg.2013.08.021. [DOI] [PubMed] [Google Scholar]
- 123.Tekin A., Karakus O.Z., Hakguder G. Distribution of interstitial cells of Cajal in the bladders of fetal rats with retinoic acid induced myelomeningocele. Turk J Urol. 2016;42:285–289. doi: 10.5152/tud.2016.98474. [DOI] [PMC free article] [PubMed] [Google Scholar]