Abstract
Aims
To present a brief review on discussions from “Do we understand any more about lower urinary tract interstitial cells?” session at the 2013 International Consultation on Incontinence-Research Society (ICI-RS) meeting in Bristol, UK.
Methods
Discussion focused on bladder interstitial cell (IC) subtypes, their localization and characterization, and communication between themselves, the urothelium, and detrusor smooth muscle. The role of ICs in bladder pathologies and new methods for studying ICs were also addressed.
Results
ICs have been studied extensively in the lower urinary tract and have been characterized based on comparisons with ICs of Cajal in the gastro-intestinal tract. In fetal bladders it is believed that ICs drive intrinsic contractions to expel urine through the urachus. These contractions diminish postpartum as bladder innervation develops. Voiding in human neonates occurs when filling triggers a spinal cord reflex that contracts the detrusor; in rodents, maternal stimulation of the perineum triggers voiding. Following spinal cord injury, intrinsic contractions, and spinal micturition reflexes develop, similar to those seen during neonatal development. These enhanced contractions may stimulate nociceptive and mechanosensitive afferents contributing to neurogenic detrusor overactivity and incontinence. The IC-mediated activity is believed to be initiated in the lamina propria by responding to urothelial factors. These IC may act syncytially through gap junction coupling and modulate detrusor activity through unknown mechanisms.
Conclusion
There has been a great deal of information discovered regarding bladder ICs, however, many of their (patho)physiological functions and mechanisms are still unclear and necessitates further research.
Keywords: detrusor overactivity, interstitial cells, lamina propria, LUTS
INTRODUCTION
It has been demonstrated that newborn rats,1 pigs,2 and human infants3 have bladder ICs with similar morphological and immunological properties to their adult counterparts. Bladder ICs have been defined according to ultrastructural features4–6 and are distinct from cells with similar morphologies including myofibroblasts and telocytes. These ICs are important for the development and function of the urinary bladder as long term treatment with the c-kit inhibitor, imatinib, results in impaired bladder development, and contractility.1 It has been suggested that intrinsic activity in neonatal and pathological bladders is associated with an increase in IC number and coupling.7 This is supported by findings that patients with overactive bladders have both increased numbers of ICs and enhanced sensitivity to imatinib.8 Accordingly, ICs have an important role in bladder development, function and pathology, and merit further research. The information presented is a brief summary of current knowledge on bladder ICs as discussed at the ICI-RS 2013 meeting. For more comprehensive reviews refer to.8,9
IC SUBTYPES, LOCALIZATION, AND CHARACTERIZATION
Bladder ICs were initially identified by their morphological similarity to ICs of Cajal (i.e., spindle shaped with extensive processes) and selective responses to nitric oxide donors.10,11 The similarities has raised the possibility of bladder ICs also being pacemakers like ICs of Cajal to regulate the contractile activity of smooth muscle. Although, ICs of Cajal and bladder ICs appear to share morphological and receptor expression characteristics, they appear to differ functionally.
There have been progressively more cell surface markers identified on bladder IC, including c-kit tyrosine receptor kinase,12 platelet-derived growth factor α13 and β14 receptors (PDGFRα and β), and CD3415 all of which are markers for hematopoietic stem cells. This, along with differential localization of ICs in the bladder wall, has brought about the concept of distinct IC subtypes mediating various processes including tissue regeneration and remodeling (myofibroblasts and telocytes), signal transduction, or pacemaker activity (ICC-like cells, Table I). The use of novel markers is crucial to identifying new IC subtypes. PDGFRα is co-expressed with c-kit and vimentin-positive cells in rat bladders9; and nucleoside triphosphate diphosphohydrolyase2 (NTPDase2) and anoctamine- 1 (Ca2+ activated chloride channel, Ano1) co-label CD34 positive cells in mouse bladders.14 Pacemaker-like activity has been associated with c-kit16 or PDGFRα17 positive ICs. The relationships of other IC markers to specific functions have yet to be determined.
TABLE I.
General Classification of Interstitial Cell-Like Cells in the Urinary Bladder, Including Potential Functions and Cell–Cell Interactions
| Cell types | Functions | Interactions |
|---|---|---|
| Myofibroblasts | Muscle progenitor | Smooth muscle, urothelium, nerve terminals |
| Interstitial cells of Cajal-like | Pacemaker cell | Smooth muscle, urothelium, nerve terminals |
| Telocytes | Inter-cellular communication | Urothelium, nerve terminals, capillaries, inflammatory cells |
Gene (splice variants) and protein (isoforms) signatures are needed to characterize IC populations. ICs in lamina propria and detrusor have different ionic currents18,19 suggesting these cells may have specialized functionality related to their location. However, there are also species and tissue-specific differences, for example, three Ano1 exons are expressed in human male bladders.20 This is important as pharmacological agents might discriminate between receptor/channel isoforms and have implications for the development of animal models. Of interest, however, is that c-kit mutant mice and rats have unaffected bladder IC networks.21 Ano1 knockout mice have a very severe gut phenotype, which lacks peristalsis22; but quantitative analysis of the gut IC network structure properties revealed no changes between wild type and knockout mice.23 There are no reports on bladder ICs in this animal model. It is unknown, which protein isoform, network characteristics in pathophysiology, and compensatory mechanisms might be behind the lack of visible changes. IC markers have also been identified in human bladder biopsies24,25 and alterations in ICs have been linked to pathological conditions.26 However, there are little to no functional studies using human tissue samples and this is a deficit that needs to be addressed.
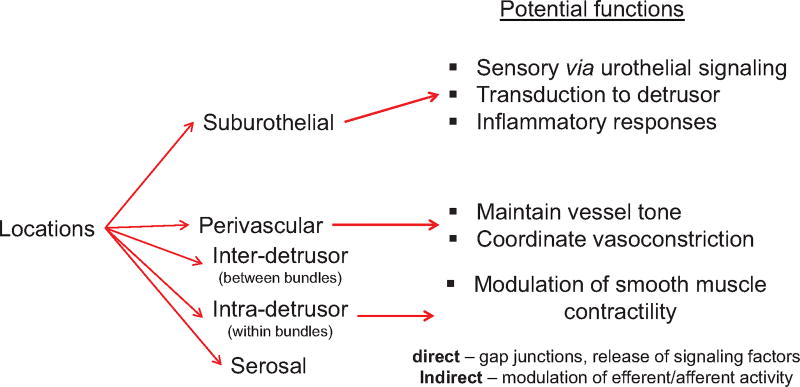
The location of ICs could also be significant in respect to their function. Bladder ICs are located throughout the lamina propria, between detrusor muscle bundles (intra-muscular), within muscle bundles (inter-muscular)27 and around blood vessels (perivascular)6 (Fig. 1). These ICs may be involved in regulating the activity of smooth muscle or nerve terminals in their respective locations.18,28,29 Isolated lamina propria ICs differ from those of the muscle layer when challenged with various receptor agonists, for example, lamina propria ICs readily respond to ATP but not ACh.18 This difference has led to the concept of lamina propria ICs being potential transducers for urothelial signaling factors (e.g., ATP) to the detrusor. Similarly muscle ICs may more readily respond to neurotransmitters that mediate detrusor contractility (e.g., ACh) due to their proximity to efferent terminals.29
Fig. 1.
Localization of interstitial cells in the urinary bladder and their hypothesized functional significance.
IC CELL–CELL INTERACTIONS
ICs in the bladder lamina propria (i.e., suburothelium) and the detrusor layer are characterized by connexin (Cx) labeling, in particular of Cx43,14,30 and in lamina propria ICs are associated with gap junctions between adjacent cells. ICs also demonstrate large, spontaneous transient inward currents mediated in part through Ca2+ -activated Cl− currents,18 possibly Ano1,14 and activated by exogenous agents such as ATP and other purinergic agonists, or reduction of extra-cellular pH.31 Thus, it is likely that electrical signals can flow between cells and an IC network can act as a functional syncytium. Moreover, the ability to propagate electrical signals may be enhanced in conditions associated with detrusor overactivity as the number of ICs is greatly increased.31,32 An intriguing observation is that physical abutment of adjacent ICs, without the formation of gap junctions, augments the electrical activity of individual cells33––this enhancement was attenuated by imatinib.31 The exact mechanism for the enhancement is unclear but demonstrates the importance of cell–cell contact in signal amplification through the IC networks.
Greater connectivity in an interstitial network may thus appear as larger, better-coordinated spontaneous electrical, or contractile activity across the bladder wall. Electrical signals and Ca2+ waves propagate from the suburothelium to the detrusor and this activity is augmented in detrusor overactivity.31 Moreover, localized micromotions of the bladder wall are more sustained and of higher frequency in human subjects experiencing increased sensations on bladder filling.34 Thus, in overactive bladders, ICs in the bladder,35 and urethra36 may act as pacemakers similar to ICs of Cajal in the gastrointestinal tract.37
ICS IN BLADDER PATHOLOGY
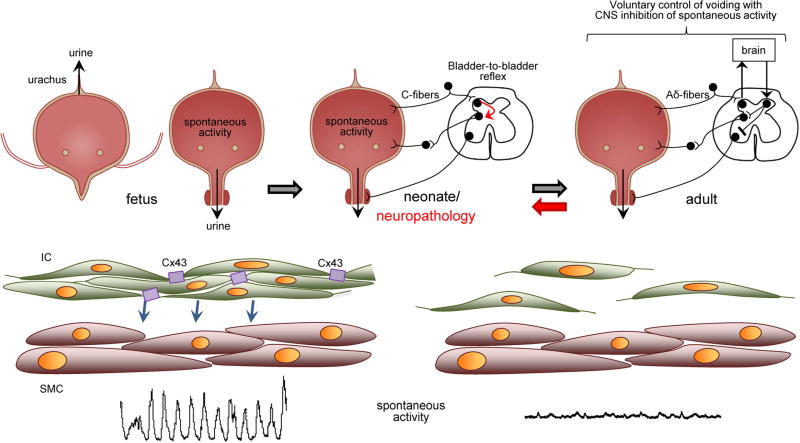
The role of ICs in normal bladder pathology is still unclear. However, in pathologies such as spinal cord injury, neurogenic bladder dysfunction, and partial bladder outlet obstruction (PBOO) there is prominent spontaneous detrusor contractions. Changes to bladder ICs in these conditions include; increased IC numbers,7 morphologies and distributions,38 and gap junction connectivity throughout the bladder wall,7 as mentioned in the previous section. The enhanced coupling between ICs is believed to facilitate the coordinated stimulation of the detrusor to enhance intrinsic contractile activity and can lead to detrusor overactivity. The coupling of ICs may occur as a compensatory mechanism in response to a disruption of bladder innervation, similar to the situation in neonatal stages. Studies with neonatal rodent bladders have demonstrated large amplitude intrinsic detrusor contractions and voiding facilitated by a bladder–spinal cord reflex pathway.39 It is hypothesized that ICs respond to bladder filling by enhancing the intrinsic detrusor contractions to trigger the spinal voiding reflex (Fig. 2). In situations such as spinal cord injury where neural input is disrupted, remodeling, and coupling of ICs could be a mechanism to aid voiding.
Fig. 2.
Proposed mechanism for interaction of IC-mediated spontaneous contractions and spinal reflex pathways. Urine is expelled from the fetal bladder via the urachus in utero. In neonates, IC forms a syncytium through gap junctions that allow for coordinated activation of detrusor smooth muscle. These large magnitude contractions can stimulate mechanosensitive afferents that activate a bladder-to-bladder spinal pathways that triggers micturition. The spinal reflex pathway is lost in adulthood and voiding becomes regulated entirely by the CNS. Along with neural remodeling in the spinal cord, there is decreased connectivity between bladder IC and spontaneous activity. In neuropathologies, a spinal reflex loop similar to that in neonates can form, along with increased bladder IC connectivity. This may be a initially compensatory mechanism to allow voiding in the absence of CNS input. However, in conditions such as spinal cord injury this can lead to detrusor sphincter dyssynergia due to uncontrolled neural remodeling.
ICs may also be involved with sensory processing, a concept derived from their close approximation to afferent nerves.40 Additionally ICs respond to a number of signaling factors including ATP,18 ACh,28 prostaglandins,41 and NO,11 but their functional roles are still unclear. Certain pathologies have been linked to increased release of urothelial signaling factors such as ATP (e.g., interstitial cystitis/painful bladder syndrome, spinal cord injury),42,43 which could potentiate IC activity. Indeed, it has been demonstrated that there is increased P2X344 and possibly muscarinic M3-receptors in bladder ICs following PBOO.45 Thus it could be hypothesized that the combined effect of increased receptor expression and signaling factor release could further potentiate IC activity and exacerbate detrusor overactivity and/or alter sensory processing.
NEW METHODS FOR STUDYING IC
Novel approaches, including subtype characterization, development of animal models, pharmacological, and cellinteraction studies are used to shed light on the functional role of ICs and in turn their clinical relevance. Although these studies have yielded important information on the characteristics of these cells, there are still a number of unresolved questions regarding functionality, particularly their interactions with other tissues.
Studying cell-to-cell communications, while difficult in intact tissues, can be done using cultured cells. Optical mapping of confluent strips of ICs from normal rodent bladders placed between strips of urothelial and smooth muscle cells may be used to determine their role in urothelial to smooth muscle communication. When the urothelial cells were stretched by osmotic distension, signals propagated from the urothelial to smooth muscle. When the ICs were removed, the smooth muscle did not respond to stretch.46 In addition, ICs from spinal cord transected rodents exhibited rhythmic Ca2+ transients, which could directly communicate with smooth muscle causing it to contract.42 Such in-line culture based systems allow mapping of information flow between cell types in a controlled manner. In addition, different IC subtypes could be isolated using cell sorting methods targeting different surface markers. Cell sorting has been utilized to separate ICs from human mucosal samples for culturing purposes47 and this technique could be further developed to isolate based on other IC surface markers.
Crucial for understanding the role of ICs in bladder sensation is the determination of whether they directly interact with afferent nerves in the bladder wall. This can be studied using pseudorabies viruses that express the Ca2+ sensor, GCaMP.48 These viruses can be injected into the L6–S2 (pelvic) and T13–L2 (hypo-gastric) ganglia to label their afferent projections to the bladder wall. Studies using this approach have demonstrated that afferent nerves communicate with urothelial cells but not ICs (preliminary data presented by the Kanai lab at the ICI-RS 2013 meeting).
SUMMARY AND RESEARCH QUESTIONS
ICs potentially have a key role in driving the symptomology of various bladder disorders, but the exact nature of IC function under normal physiology and pathophysiology has yet to be elucidated. Further experimental evidence is required for associating specific IC markers/subtypes or ICs from different parts of the bladder wall with specialized functionality, and this would have to be followed by investigations in bladder pathology models. Additionally, the characteristics of isolated cells may not necessarily reflect their role in vivo therefore methodologies to examine select IC subtypes in situ should also be considered.
Their needs to be further characterization of newly identified IC markers in different animal models (e.g., mice, rats, guinea pigs, pigs, etc.) and comparisonsmade to human tissues. This needs to be extended to pathological models including; spinal cord injury, partial bladder outlet obstruction, diabetes, and neurodegenerative diseases. IC-specific studies could be performed in transgenic models such as Ano1 knockout mice or c-kit mutant mice to determine if ICs are indeed responsible for driving detrusor overactivity following a pathological insult. In this way, a more thorough cellular characterization could be performed including histology, electrophysiology, and organ bath studies that can be correlated with cystometric parameters.
Some of the research questions derived from the discussion at the ICI-RS 2013 meeting that warrant addressing are the following:
What is the role of urethral ICs in lower urinary tract dysfunction?
Can bladder IC surface markers be used to differentiate their subtypes and functionality?
Do IC networks propagate regenerative electrical signals in the lamina propria and detrusor layers?
What are the end target tissues for IC networks: urothelium, afferent or efferent nerves, blood vessels or detrusor smooth muscle?
Are novel markers such as Ano1 potential therapeutic targets for the treatment of bladder pathologies?
Footnotes
Conflict of interest: none.
References
- 1.Gevaert T, Hutchings G, Everaerts W, et al. Administration of imatinib mesylate in rats impairs the neonatal development of intramuscular interstitial cells in bladder and results in altered contractile properties. Neurourol Urodyn. 2013 doi: 10.1002/nau.22415. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 2.Vahabi B, Sellers DJ, Bijos DA, et al. Phasic contractions in urinary bladder from juvenile versus adult pigs. PLoS ONE. 2013;8:e58611, 1–10. doi: 10.1371/journal.pone.0058611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Piaseczna-Piotrowska AM, Dzieniecka M, Kulig A, et al. Different distribution of c-kit positive interstitial cells of Cajal-like in children’s urinary bladders. Folia Histochem Cytobiol. 2011;49:431–5. doi: 10.5603/fhc.2011.0061. [DOI] [PubMed] [Google Scholar]
- 4.Popescu LM, Gherghiceanu M, Cretoiu D, et al. The connective connection: Interstitial cells of Cajal (ICC) and ICC-like cells establish synapses with immunoreactive cells. Electron microscope study in situ. J Cell Mol Med. 2005;9:714–30. doi: 10.1111/j.1582-4934.2005.tb00502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Drake MJ, Fry CH, Eyden B. Structural characterization of myofibroblasts in the bladder. BJU Int. 2006;97:29–32. doi: 10.1111/j.1464-410X.2006.05818.x. [DOI] [PubMed] [Google Scholar]
- 6.Cunningham RM, Larkin P, McCloskey KD. Ultrastructural properties of interstitial cells of Cajal in the Guinea pig bladder. J Urol. 2011;185:1123–31. doi: 10.1016/j.juro.2010.10.037. [DOI] [PubMed] [Google Scholar]
- 7.Ikeda Y, Fry C, Hayashi F, et al. Role of gap junctions in spontaneous activity of the rat bladder. Am J Physiol Renal Physiol. 2007;293:F1018–25. doi: 10.1152/ajprenal.00183.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McCloskey KD. Interstitial cells of Cajal in the urinary tract. Handb Exp Pharmacol. 2011;202:233–254. doi: 10.1007/978-3-642-16499-6_11. [DOI] [PubMed] [Google Scholar]
- 9.McCloskey KD. Bladder interstitial cells: An updated review of current knowledge. Acta Physiol (Oxf) 2013;207:7–15. doi: 10.1111/apha.12009. [DOI] [PubMed] [Google Scholar]
- 10.Smet PJ, Jonavicius J, Marshall VR, et al. Distribution of nitric oxide synthaseimmunoreactive nerves and identification of the cellular targets of nitric oxide in guinea-pig and human urinary bladder by cGMP immunohistochemistry. Neuroscience. 1996;71:337–48. doi: 10.1016/0306-4522(95)00453-x. [DOI] [PubMed] [Google Scholar]
- 11.Gillespie JI, Markerink-van Ittersum M, de Vente J. cGMP-generating cells in the bladder wall: Identification of distinct networks of interstitial cells. BJU Int. 2004;94:1114–24. doi: 10.1111/j.1464-410X.2004.05186.x. [DOI] [PubMed] [Google Scholar]
- 12.McCloskey KD, Gurney AM. Kit positive cells in the guinea pig bladder. J Urol. 2002;168:832–6. [PubMed] [Google Scholar]
- 13.Koh BH, Roy R, Hollywood MA, et al. Platelet-derived growth factor receptoralpha cells in mouse urinary bladder: A new class of interstitial cells. J Cell Mol Med. 2012;16:691–700. doi: 10.1111/j.1582-4934.2011.01506.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu W, Zeidel ML, Hill WG. Cellular expression profile for interstitial cells of cajal in bladder––A cell often misidentified as myocyte or myofibroblast. PLoS ONE. 2012;7:e48897. doi: 10.1371/journal.pone.0048897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rasmussen H, Hansen A, Smedts F, et al. CD34-positive interstitial cells of the human detrusor. Apmis. 2007;115:1260–6. doi: 10.1111/j.1600-0643.2007.00759.x. [DOI] [PubMed] [Google Scholar]
- 16.Biers SM, Reynard JM, Doore T, et al. The functional effects of a c-kit tyrosine inhibitor on guinea-pig and human detrusor. BJU Int. 2006;97:612–6. doi: 10.1111/j.1464-410X.2005.05988.x. [DOI] [PubMed] [Google Scholar]
- 17.Lee H, Koh BH, Peri LE, et al. Functional expression of SK channels in murine detrusor PDGFR+ cells. J Physiol. 2013;591:503–13. doi: 10.1113/jphysiol.2012.241505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu C, Sui GP, Fry CH. Purinergic regulation of guinea pig suburothelial myofibroblasts. J Physiol. 2004;559:231–43. doi: 10.1113/jphysiol.2004.067934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCloskey KD. Characterization of outward currents in interstitial cells from the guinea pig bladder. J Urol. 2005;173:296–301. doi: 10.1097/01.ju.0000141581.00922.f4. [DOI] [PubMed] [Google Scholar]
- 20.Ferrera L, Caputo A, Ubby I, et al. Regulation of TMEM16A chloride channel properties by alternative splicing. J Biol Chem. 2009;284:33360–8. doi: 10.1074/jbc.M109.046607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Okada S, Kojima Y, Kubota Y, et al. Attenuation of bladder overactivity in KIT mutant rats. BJU Int. 2011;108:E97–103. doi: 10.1111/j.1464-410X.2010.09870.x. [DOI] [PubMed] [Google Scholar]
- 22.Hwang SJ, Blair PJ, Britton FC, et al. Expression of anoctamin 1/TMEM16A by interstitial cells of Cajal is fundamental for slow wave activity in gastrointestinal muscles. J Physiol. 2009;587:4887–904. doi: 10.1113/jphysiol.2009.176198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gao J, Du P, O’Grady G, et al. Numerical metrics for automated quantification of interstitial cell of Cajal network structural properties. J R Soc Interface/R Soc. 2013;10:20130421. doi: 10.1098/rsif.2013.0421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnston L, Woolsey S, Cunningham RM, et al. Morphological expression of KIT positive interstitial cells of Cajal in human bladder. J Urol. 2010;184:370–7. doi: 10.1016/j.juro.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Monaghan KP, Johnston L, McCloskey KD. Identification of PDGFRalpha positive populations of interstitial cells in human and guinea pig bladders. J Urol. 2012;188:639–47. doi: 10.1016/j.juro.2012.03.117. [DOI] [PubMed] [Google Scholar]
- 26.Piaseczna-Piotrowska A, Dzieniecka M, Samolewicz E, et al. Distribution of interstitial cells of Cajal in the neurogenic urinary bladder of children with myelomeningocele. Adv Med Sci. 2013:1–6. doi: 10.2478/ams-2013-0002. [DOI] [PubMed] [Google Scholar]
- 27.Davidson RA, McCloskey KD. Morphology and localization of interstitial cells in the guinea pig bladder: Structural relationships with smooth muscle and neurons. J Urol. 2005;173:1385–90. doi: 10.1097/01.ju.0000146272.80848.37. [DOI] [PubMed] [Google Scholar]
- 28.Johnston L, Carson C, Lyons AD, et al. Cholinergic-induced Ca2+ signaling in interstitial cells of Cajal from the guinea pig bladder. Am J Physiol Renal Physiol. 2008;294:F645–55. doi: 10.1152/ajprenal.00526.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gillespie JI, Markerink-van Ittersum M, De Vente J. Interstitial cells and cholinergic signalling in the outer muscle layers of the guinea-pig bladder. BJU Int. 2006;97:379–85. doi: 10.1111/j.1464-410X.2006.05989.x. [DOI] [PubMed] [Google Scholar]
- 30.Sui GP, Rothery S, Dupont E, et al. Gap junctions and connexin expression in human suburothelial interstitial cells. BJU Int. 2002;90:118–29. doi: 10.1046/j.1464-410x.2002.02834.x. [DOI] [PubMed] [Google Scholar]
- 31.Fry CH, Young JS, Jabr RI, et al. Modulation of spontaneous activity in the overactive bladder: The role of P2Y agonists. Am J Physiol Renal Physiol. 2012;302:F1447–54. doi: 10.1152/ajprenal.00436.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roosen A, Datta SN, Chowdhury RA, et al. Suburothelial myofibroblasts in the human overactive bladder and the effect of botulinum neurotoxin type A treatment. Eur Urol. 2009;55:1440–8. doi: 10.1016/j.eururo.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 33.Sui GP, Wu C, Roosen A, et al. Modulation of bladder myofibroblast activity: Implications for bladder function. AmJ Physiol Renal Physiol. 2008;295:F688–97. doi: 10.1152/ajprenal.00133.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Drake MJ, Harvey IJ, Gillespie JI, et al. Localized contractions in the normal human bladder and in urinary urgency. BJU Int. 2005;95:1002–5. doi: 10.1111/j.1464-410X.2005.05455.x. [DOI] [PubMed] [Google Scholar]
- 35.Wang Y, Fang Q, Lu Y, et al. Effects of mechanical stretch on interstitial cells of Cajal in guinea pig bladder. J Surg Res. 2010;164:e213–9. doi: 10.1016/j.jss.2010.04.040. [DOI] [PubMed] [Google Scholar]
- 36.Hashitani H. Interaction between interstitial cells and smooth muscles in the lower urinary tract and penis. J Physiol. 2006;576:707–14. doi: 10.1113/jphysiol.2006.116632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hirst GD, Edwards FR. Generation of slow waves in the antral region of guinea-pig stomach––A stochastic process. J Physiol. 2001;535:165–80. doi: 10.1111/j.1469-7793.2001.00165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Johnston L, Cunningham RM, Young JS, et al. Altered distribution of interstitial cells and innervation in the rat urinary bladder following spinal cord injury. J Cell Mol Med. 2012;16:1533–43. doi: 10.1111/j.1582-4934.2011.01410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kruse MN, de Groat WC. Consequences of spinal cord injury during the neonatal period on micturition reflexes in the rat. Exp Neurol. 1994;125:87–92. doi: 10.1006/exnr.1994.1010. [DOI] [PubMed] [Google Scholar]
- 40.Wiseman OJ, Fowler CJ, Landon DN. The role of the human bladder lamina propria myofibroblast. BJU Int. 2003;91:89–93. doi: 10.1046/j.1464-410x.2003.03802.x. [DOI] [PubMed] [Google Scholar]
- 41.Rahnama’i MS, van Koeveringe GA, Essers PB, et al. Prostaglandin receptor EP1 and EP2 site in guinea pig bladder urothelium and lamina propria. J Urol. 2010;183:1241–7. doi: 10.1016/j.juro.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 42.Sun Y, Chai TC. Effects of dimethyl sulphoxide and heparin on stretchactivated ATP release by bladder urothelial cells from patients with interstitial cystitis. BJU Int. 2002;90:381–5. doi: 10.1046/j.1464-410x.2002.02912.x. [DOI] [PubMed] [Google Scholar]
- 43.Khera M, Somogyi GT, Kiss S, et al. Botulinum toxin A inhibits ATP release from bladder urothelium after chronic spinal cord injury. Neurochem Int. 2004;45:987–93. doi: 10.1016/j.neuint.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 44.Li Y, Xue L, Miao Q, et al. Expression and electrophysiological characteristics of P2×3 receptors in interstitial cells of Cajal in rats with partial bladder outlet obstruction. BJU Int. 2013;111:843–51. doi: 10.1111/j.1464-410X.2012.11408.x. [DOI] [PubMed] [Google Scholar]
- 45.Grol S, van Koeveringe GA, de Vente J, et al. Regional differences in sensory innervation and suburothelial interstitial cells in the bladder neck and urethra. BJU Int. 2008;102:870–7. doi: 10.1111/j.1464-410X.2008.07752.x. [DOI] [PubMed] [Google Scholar]
- 46.Kanai A, Zabbarova I, Ikeda Y, et al. Sophisticated models and methods for studying neurogenic bladder dysfunction. Neurourol Urodyn. 2011;30:658–67. doi: 10.1002/nau.21120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Woodman JR, Mansfield KJ, Lazzaro VA, et al. Immunocytochemical characterisation of cultures of human bladder mucosal cells. BMC Urol. 2011;11:5. doi: 10.1186/1471-2490-11-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Granstedt AE, Kuhn B, Wang SS, et al. Calcium imaging of neuronal circuits in vivo using a circuit-tracing pseudorabies virus. Cold Spring Harbor Protoc. 2010;2010 doi: 10.1101/pdb.prot5410. pdb prot5410. [DOI] [PMC free article] [PubMed] [Google Scholar]