Abstract
The ability to adaptively regulate emotion is essential for mental and physical well-being. How should we organize the myriad ways people attempt to regulate their emotions? We explore the utility of a framework that distinguishes among four fundamental classes of emotion regulation strategies. The framework describes each strategy class in terms their behavioral characteristics, underlying psychological processes and supporting neural systems. A key feature of this multi-level framework is its conceptualization of the psychological processes in terms of two orthogonal dimensions that describe (i) the nature of the emotion regulation goal (ranging from to implicit to explicit) and (ii) the nature of the emotion change process (ranging from more automatic to more controlled). After describing the core elements of the framework, we use it to review human and animal research on the neural bases of emotion regulation and to suggest key directions for future research on emotion regulation.
Keywords: emotion regulation, prefrontal cortex, amygdala, implicit, explicit
Introduction
If life is full of emotional peaks and valleys, then our ability to regulate emotion helps us to navigate this landscape effectively. Indeed, emotion regulation can be used to lighten the burden of sadness, resist temptations or overcome fears. Given its importance in maintaining mental and physical well-being (Gross and John, 2003; Diener, 2009), emotion regulation has increasingly been the focus of behavioral and neuroscience research. Indeed, between 2002 and 2012, there was a 40-fold increase in the number of papers including the phrase ‘emotion regulation’ (Gross, 2013). This growth has been spurred by studies in both humans and animals examining diverse forms of behavior that have emotion regulatory consequences.
How should we organize the myriad ways we can regulate emotions, their underlying neural bases and the ever-expanding literature examining them? While emotion regulation strategies can be distinguished in many ways (Ochsner and Gross, 2005; Berkman and Lieberman, 2009; Hartley and Phelps, 2009; Gyurak et al., 2011; Ochsner et al., 2012; Gross, 2015), we focus on a high-level classification scheme that trades on the distinction between explicit and implicit forms of regulation. In recognition of the potential value of an explicit/implicit distinction, some reviews already have begun to explore its application to an emotion regulation context (e.g. Berkman and Lieberman, 2009; Gyurak et al., 2011; Etkin et al., 2015). One issue, however, is that these reviews offer differing descriptions of ‘implicit’ regulation, sometimes focusing on the lack of a conscious and explicit goal to regulate and other times arguing that it occurs automatically and/or without need for cognitive control. As such, it remains unclear how best to unify the diverse literatures on emotion regulation and to draw conclusions about the common or distinct mechanisms underlying different kinds of explicit or implicit strategies. Our aim is to further this discussion by providing a comprehensive, multi-level framework that accounts for the range of behaviors currently referred to as emotion regulation and explains what makes different types of explicit and implicit emotion regulation strategies similar to or different from one another—as well as other forms of emotion regulation that do not easily fit into either category.
A key feature of our framework is its conceptualization of the psychological operations underlying emotion regulation strategies in terms of two orthogonal dimensions: (i) the nature of the emotion regulation goal (ranging from implicit/nonconscious to explicit/conscious) and (ii) the nature of the emotion change process (ranging from more automatic to more controlled). As we explicate below, this framework has several advantages. First, identifying two dimensions, rather than one, allows us to distinguish between neural systems supporting the regulation goal vs the change process. Second, the framework offers clear criteria for defining implicit and explicit regulation based on specific features of goal and process, giving rise to four distinct classes of emotion regulation, which allows it to additionally account for strategies that share features of both implicit and explicit regulation. Third, our formulation is guided by the success of similar frameworks in other areas of research, such as the study of memory, where the explicit/implicit distinction fostered research by specifying how qualitatively different behavioral expressions of memory were supported by distinct—but related—sets of underlying psychological processes and neural systems (Schacter and Tulving, 1994).
As such, the framework describes each class of regulation strategies at three levels: in terms their behavioral characteristics, underlying psychological processes, and supporting neural systems. This approach leverages both behavioral and brain data to constrain psychological theorizing about the goal vs process distinction, which in turn builds a framework robust enough to account for multiple kinds of data. That said, the multi-level framework we advocate for here, as is the case for all such frameworks, rests on the notion that converging evidence from different DVs (dependent variables), which may be at different levels of analysis (e.g. behavioral vs brain measures) is needed to constrain the inferences we draw about the process level. The process level is, after all, unobservable, and behavioral and brain measures are all we have to triangulate on its nature. As such, we believe both kinds of measures are needed to understand the nature of the psychological level of analysis. However, given that—at present—the brain data are ambiguous with respect to whether specific prefrontal systems [e.g. dlPFC vs ventral medial prefrontal cortex (vmPFC)] implement explicit vs implicit regulatory goals or controlled vs automatic change processes, as of now the best data for classifying strategies are behavioral. Therefore, while the article highlights the neural systems that have been associated with each class of emotion regulation, future studies are needed to tease apart specific neural functions.
With these considerations in mind, this article is divided into six parts. The first lays out the definitional logic of our proposed framework with a particular emphasis on distinguishing different combinations of regulation goals and emotion change processes that give rise to four different classes of emotion regulation strategies. The second through fifth parts review behavioral and neural evidence relevant to each strategy class. The final part summarizes the benefits of the framework and considers potential basic and translational applications.
A multi-level framework for explicit and implicit emotion regulation
To understand how emotions are regulated, it is important to first consider how they are generated. We adopt an inclusive definition of emotions as valenced multi-system—behavioral, cognitive, physiological and experiential—reactions occasioned by appraisals of a situation’s relevance to current or chronic goals, needs or values (Cacioppo et al., 2000; Scherer et al., 2001). Emotion generation is subserved by multiple neural systems, each supporting distinct types of emotion-relevant computations, different combinations of which will be engaged depending upon the nature of the stimulus and one’s response, including the amygdala, important for detecting, encoding and triggering responses to goal-relevant stimuli in general and potential threats in particular (Phelps, 2006; Davis and Whalen, 2001; Cunningham and Brosch, 2012; Janak and Tye, 2015); the ventral striatum, involved in learning the reinforcement/reward value of stimuli (O'doherty, 2004; Delgado, 2007) and the insula, which may support awareness of body states in the context of emotion (Critchley et al., 2004; Craig, 2009; Zaki et al., 2012).
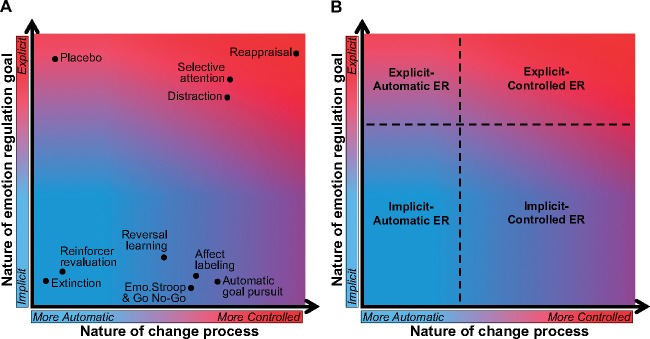
In our framework, explicit and implicit regulation are differentiated along two orthogonal psychological dimensions (Figure 1A): (i) the nature of the emotion regulation goal, ranging from implicit/nonconscious to explicit/conscious and (ii) the nature of the emotion change process, ranging from more automatic to more controlled. This yields four classes of emotion regulation strategies (Figure 1B), named according to their characteristics on the goal and process dimensions (e.g. ‘explicit controlled’). Two classes capture the majority of commonly studied forms of regulation: Explicit-controlled emotion regulation, which involves an explicit regulation goal and controlled change processes, and implicit-automatic emotion regulation, which involves an implicit regulation goal and more automatic change processes. The other two classes are hybrids that arise from less commonly studied—but nonetheless potentially important—combinations of regulatory goals and processes (i.e. explicit automatic and implicit controlled). In the next sections, we describe the goal and process dimensions in greater detail to explain how different combinations of these dimensions give rise to these four classes of emotion regulation.
Fig. 1.
Description of the psychological dimensions involved in different kinds of emotion regulation. Location on the y-axis depicts the nature of the emotion regulation goal, and location on the x-axis depicts the nature of the change process. (A) Dots represent the typically studied instantiations of emotion regulation strategies. (B) Dashed lines indicate the rough boundaries of four classes of emotion regulation.
Dimension 1: implicit vs explicit goals
The goal dimension (the y-axis of Figure 1A) describes the nature of the emotion regulation goal, which can vary from implicit to explicit. We define goals as mental representations of potential internal states and/or states of the world, which can be either explicit and consciously held or implicit and nonconscious (Hassin et al., 2009). Explicit emotion regulation goals involve a conscious desire to change one’s emotions (e.g. the goal to feel happier), whereas implicit goals do not involve the conscious desire to change emotional responding. Although it may seem counterintuitive to describe some emotion regulation goals as implicit, our conceptualization is guided by recent advances in the study of self-regulation (Berkman and Lieberman, 2009; Payer et al., 2012) as well as automatic goal pursuit, which demonstrates that goals can be activated and shape behavior outside of conscious awareness (Custers and Aarts, 2010).
We have identified several different instantiations of implicit goals. The first is a goal that is activated non-consciously (e.g. through priming), but in other situations, could be explicitly held. The second is a chronically active goal that is important for survival, such as the goal to identify, respond to and accurately represent the value of goal-relevant stimuli (Schultz et al., 1997; Ledoux, 2012). The third is an incidental goal. In this case, there is no explicit goal to regulate emotion and the performance of a task—whose overt goal is not emotion-related—has the incidental effect of altering emotional responses (Lieberman et al., 2007). An incidental goal is logically distinct from the first two types of implicit goals, which involve an active goal to change emotion. Importantly, in many instances where emotion change occurs and an individual has no conscious goal to regulate, it may be difficult to determine whether it was guided by one type of implicit goal or another. Therefore, we group these three types of goals under the umbrella heading of implicit because they support emotion change in the absence of a conscious goal. Future research is needed to differentiate the underlying mechanisms and behavioral effects.
Both explicit and implicit emotion regulation goals can be generated internally or externally. Internally generated goals come from an individual’s thoughts or mental representations, such as the explicit decision to reappraise a stimulus or the implicit and chronic, continuously operating goal to identify and respond to salient stimuli (Ledoux, 2012) and to accurately represent their value (Schultz et al., 1997). Externally generated goals come from outside the individual, including another person or a stimulus, such as an explicit placebo expectation derived from experimenter instructions or a goal that is primed nonconsciously by cues present in a specific situation (Shidlovski and Hassin, 2011).
Dimension 2: controlled vs automatic change processes
The process dimension (the x-axis of Figure 1A) describes the nature of the emotion change process, which can range from more automatic to more controlled. The more automatic the change process, the more non-conscious is its operation, it engages few to no top-down control processes, and it tends to incrementally introduce changes in affective responding that may accrue over time. As such, automatic emotion change processes often involve affective learning, where an organism experiences shifts in the context or contingent outcomes associated with a stimulus and learns to update its prior affective value and/or adopt a new affective response to the stimulus. An example is extinction (Figure 1A), where an organism reduces or extinguishes affective responses to a stimulus because it learns that it is no longer predictive of a previously associated outcome (e.g. a shock).
As we move right along the process continuum, strategies become less automatic and more controlled. Strategies in the middle of the continuum involve some top-down control processes, such as those supporting selection of goal-appropriate, and inhibition of goal-inappropriate, responses. An example is selective attention (Figure 1A), in which an individual focuses their attention on the neutral aspects of an affective stimulus, instead of the affective features.
On the far right side of the process continuum are strategies that engage controlled processes to a greater degree, which could include processes supporting selection and inhibition as well as maintenance and manipulation of information in working memory. A strategy could be considered, ‘more controlled’ for multiple reasons, including engaging controlled processes that are of multiple different kinds, that are individually more complex, that operate for longer durations or some combination of these features. An example controlled strategy is reappraisal (Figure 1A), in which an individual effortfully attempts to describe and characterize an emotional stimulus in terms that change their initial emotional response.
Differentiating classes of regulatory strategies
Although the nature of both the goals and change processes vary along a continuum, four different classes of regulation arise from different combinations of goals and processes. Critically, explicit and implicit are clearly defined as terms describing the goals to regulate. The crossing of type of goal and type of process generates four distinct areas within Figure 1B. Explicit-controlled emotion regulation involves explicit goals that initiate regulation supported by controlled processes, whereas implicit-automatic emotion regulation involves opposite goal and process features—incidental or implicit goals that initiate regulation supported by automatic processes. The first hybrid class, implicit-controlled emotion regulation, involves implicit goals and depends on change processes that are more controlled. The second hybrid class, explicit-automatic emotion regulation, involves explicit goals and change processes that are more automatic.
In addition to distinguishing these four classes of emotion regulation, the framework provides a means to gain a more complete understanding of the psychological processes that underlie specific emotion regulation strategies. Variability in regulatory behavior—in the way in which any individual strategy can be implemented—can be conceptualized in terms of shifts along the goal and process axes. Consider reappraisal, which in its most-studied instantiation is putatively an explicit-controlled emotion regulation strategy, represented within the upper right area of the goal/process space (Figure 1A), corresponding to an explicit goal to change emotions using cognitive control processes. While not typically studied, there may be instances where one rethinks the meaning of a situation without having the conscious intention to change emotion or where the reliance of reappraisal on controlled processes diminishes—for example, after some degree of reappraisal training and/or practice (Denny and Ochsner, 2014; Denny et al., 2015). Dieters who repeatedly attempt to control desire for food may provide an everyday instance of this shift: At first, the goal to reappraise is conscious, and the act of reappraising food is conscious and effortful, but over time, both could become more automatic. While to date no work has directly addressed this issue (for food or any other stimuli), the framework highlights the boundaries of our current knowledge that could be extended in future studies that test reappraisal under experimental conditions that limit the availability of cognitive resources, such as under cognitive load. Whatever the outcome of such studies, the key idea is that typically studied forms of reappraisal might exemplify explicit-controlled regulation, but other variants of reappraisal, including forms that become more implicit with practice, could all be located within different regions of the goal/process space. Figure 2 illustrates the theoretical locations that reappraisal and other emotion regulation strategies could occupy depending on the way they are implemented. Finally, it is important to note that changes in goal and process will be associated with shifts in the underlying neural systems, and here again the framework highlights the boundaries of our current knowledge. As previewed above—and as detailed below—more research is needed to clarify the association of specific control systems with regulatory goals vs processes because the lion’s share of extant work examines just two of the four possible ways that explicit vs implicit goals and controlled vs automatic processes can combine to support emotion regulation strategies.
Fig. 2.
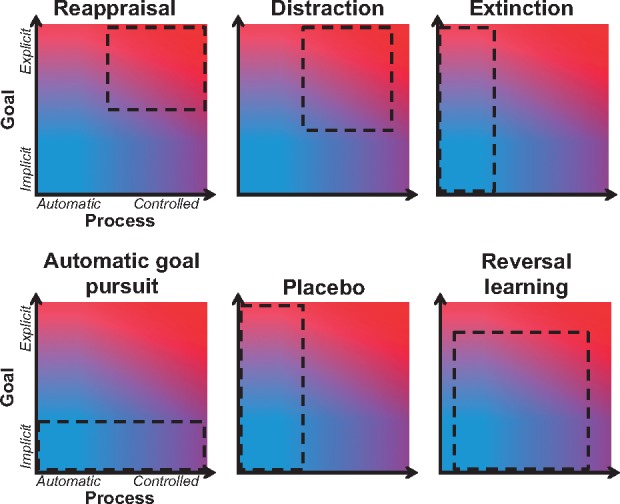
Theoretical locations of different emotion regulation strategies within the goal and process space. Dashed lines indicate the locations that could be occupied by different instantiations of an emotion regulation strategy.
With these definitions in place, we can now review extant behavioral and brain data to support and elaborate the idea that these four classes of emotion regulation can be differentiated by the two-dimensional space of Figure 1. In each section below, we describe behavioral examples (which we refer to as strategies whose names are italicized in the text and are represented in Figure 1A) and what we know about the neural systems generally implicated in each class of regulation (see Figure 3 for overview). While we limit our review to the self-regulation of emotion, we acknowledge that social/interpersonal forms of emotion regulation represent an important new area of research that may benefit from the types of distinctions made here (Zaki and Williams, 2013; Coan and Maresh, 2014; Reeck et al., 2016).
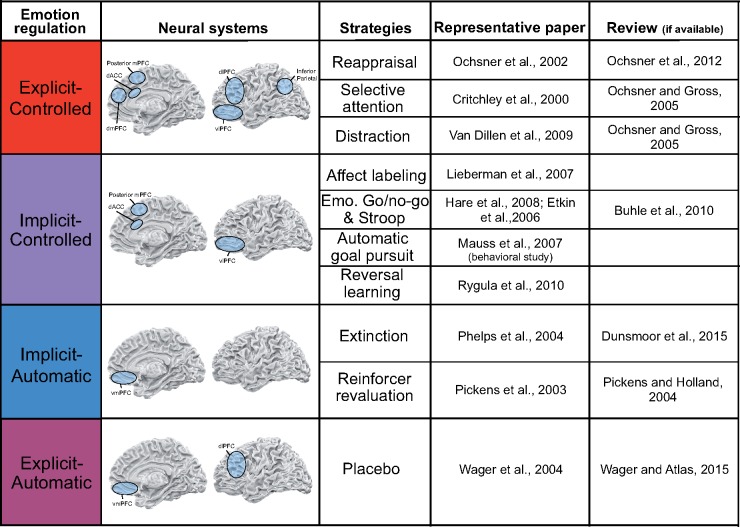
Fig. 3.
Four classes of emotion regulation, their neural systems, and behavioral strategies. The neural systems involved in each class of emotion regulation are illustrated. The specific regions and combinations of regions involved may vary, in particular for explicit-controlled and implicit-automatic regulation. The regions depicted were chosen because they represent the current literature.
Explicit-controlled emotion regulation
We know the most about explicit-controlled forms of emotion regulation, which have dominated human emotion regulation research for the last 15 years. Per the definitions offered above, explicit-controlled emotion regulation strategies are consciously initiated by individuals who are aware that they are regulating and are supported by controlled processes.
Three explicit-controlled emotion regulation strategies have received the most attention: selective attention, distraction and reappraisal. Selective attention (Figure 1A) involves moving the focus of attention toward, or away from, affective stimuli or certain features of them, whereas distraction is the process of keeping our minds occupied with irrelevant cognitions so that the amount of attention devoted to a stimulus is diminished (Ochsner and Gross, 2005). Reappraisal (Figures 1A and 2) involves intentionally changing how we think about and describe a stimulus’s meaning so as to alter our emotional response to it (Gross, 1998).
Our understanding of explicit-controlled emotion regulation comes largely from studies of reappraisal. Reappraisal can be used to change the intensity, quality or duration of various kinds of emotional responses in accordance with regulation goals (Ochsner et al., 2012; Gross, 2015). Most work has examined the use of reappraisal to decrease responses to aversive visual stimuli (e.g. photographic images). Although there are many ways to reappraise (e.g. McRae et al., 2012; Ochsner et al., 2012), a canonical version of such a task presents aversive pictures and asks participants to reappraise them by reinterpreting depicted scenes in a less negative way.
To date, our knowledge of the neural mechanisms underlying explicit-controlled emotion regulation comes almost entirely from studies in humans rather than in animals, in large part because complex, consciously directed cognitive processes are difficult to study in animal models. Of human studies, the majority have focused on reappraisal, and of those, nearly all involve imaging. Selective attention studies have used a disparate array of methods that often lack behavioral measures verifying how well attention has been controlled (Ochsner and Gross, 2005; Buhle et al., 2010), thereby limiting the conclusions that can be drawn. The few studies examining distraction have used more consistent and reliable methods, and their results are highlighted here.
Our understanding of the neural systems for explicit-controlled emotion regulation builds on prior cognitive neuroscience research suggesting that cognitive control is implemented through the influence of domain general PFC-based control systems on posterior and subcortical systems that represent specific kinds of sensory or mnemonic information (Knight et al., 1999; Miller and Cohen, 2001). This influence is possible due to the intrinsic and extrinsic anatomical connections of PFC/parietal control regions (Miller and Cohen, 2001; Miller and D'esposito, 2005), which allow them to affect processing in other parts of the brain.
Four such control systems (Figures 3 and 4) have been most strongly implicated in explicit-controlled emotion regulation: dorsolateral prefrontal cortex (dlPFC), ventrolateral prefrontal cortex (vlPFC), dorsal anterior cingulate cortex (dACC) and dorsal medial prefrontal cortex (dmPFC) (Diekhof et al., 2011; Ochsner et al., 2012; Buhle et al., 2014). DLPFC is active during both distraction (Van Dillen et al., 2009; Kanske et al., 2010) and reappraisal (Buhle et al., 2014), which both involve holding in mind regulatory goals and manipulating strategy-relevant content while controlling the focus of attention on strategy relevant information, all of which are thought to depend on a dlPFC-parietal control network (Miller and Cohen, 2001; Cocchi et al., 2013). Reappraisal involves the vlPFC, likely because it requires the selection of goal-appropriate interpretations and inhibition of inappropriate ones, both functions of vlPFC (Aron et al., 2004; Cohen and Lieberman, 2010). While distraction sometimes recruits vlPFC, direct comparisons suggest that reappraisal recruits it to a greater extent (McRae et al., 2010), presumably because selecting appropriate reinterpretations is essential to reappraisal. Distraction (Kanske et al., 2010; McRae et al., 2010) and reappraisal (Buhle et al., 2014) engage the dACC and adjacent posterior dmPFC, which monitor conflicts between intended and actual behavioral outcomes and signal when appropriate adjustments in control are needed (Botvinick et al., 2001; Badre and Wagner, 2004; Botvinick et al., 2004; Carter and Van Veen, 2007). Rostral portions of dmPFC, thought to play a key role in mentalizing (Zaki and Ochsner, 2012), have been implicated in some studies of reappraisal, likely to support monitoring and reflection on one’s own emotional states and reflection on and reinterpretation of the mental states of stimulus persons (Ochsner et al., 2012; Buhle et al., 2014).
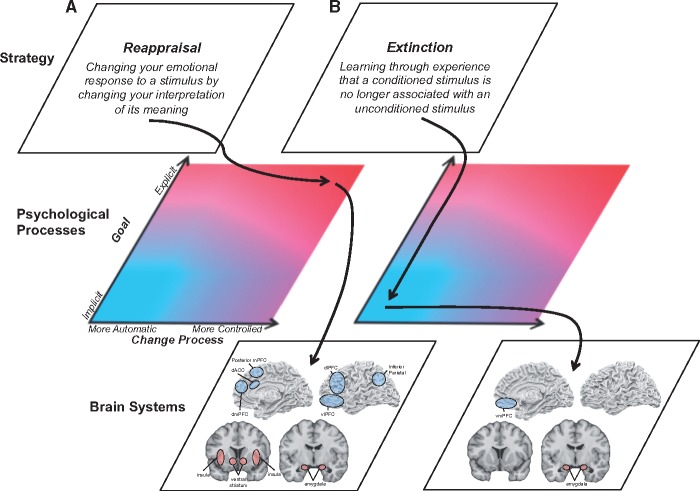
Fig. 4.
Description of emotion regulation strategies at multiple-levels. Reappraisal (A) and extinction (B) are described at the levels of behavior, psychological processes and brain systems. Brain regions colored blue are thought to be involved in regulation, whereas brain regions colored red are thought to be modulated by regulation.
Four additional kinds of data suggest that the regions supporting explicit-controlled regulation serve domain general cognitive control functions. First, structural data show that gray matter volume in the right vlPFC predicts successful behavioral performance on both a reappraisal task and a stop-signal task, which is a common measure of response inhibition (Tabibnia et al., 2011). Second, PFC damage impairs virtually all ‘cold’ forms of cognitive control (Miller and Cohen, 2001) and the same may be true for reappraisal: a left frontal stroke patient was unable to spontaneously generate reappraisals (Salas et al., 2013) and group of patients with focal frontal unilateral lesions were slower to generate reappraisals (Salas et al., 2014). Third, working memory capacity predicts behavioral indicators of reappraisal success (Schmeichel et al., 2008). Fourth, better performance on a battery of memory and cognitive control tasks predicts greater decreases in amygdala activity during reappraisal (Winecoff et al., 2011).
Explicit-controlled strategies modulate activity in various systems for triggering affective responses, including the amygdala, ventral striatum and insula. During the down-regulation of negative emotion, amygdala activity is consistently reduced by distraction (Van Dillen et al., 2009; Kanske et al., 2010; McRae et al., 2010) and reappraisal (Buhle et al., 2014). Insula activity is reduced by distraction from pain (Wager et al., 2013) and sometimes by distraction from negative emotion (Van Dillen et al., 2009). And ventral striatum activity has been reduced by distraction (Delgado et al., 2008; Martin and Delgado, 2011) and reappraisal (Kober et al., 2010).
Implicit-automatic emotion regulation
Implicit-automatic emotion regulation describes strategies initiated by implicit goals to change affect that are implemented by more automatic processes. Two main implicit-automatic emotion regulation strategies have been studied to date: extinction and reinforcer revaluation (Figures 1A and 2). Both involve learning through experience that the affective value of a stimulus has changed because the outcome (e.g. pain or reward) that was associated with it has been removed or altered. In extinction, a stimulus that previously predicted an aversive outcome (e.g. a painful shock) now no longer does, and in reinforcer revaluation, a stimulus that previously was associated with some outcome (e.g. a large reward) now predicts a different outcome (e.g. a smaller reward). We consider these phenomena to be implicit-automatic emotion regulation because (i) updating the affective value of a stimulus causes corresponding changes in behavioral indicators of emotional response; (ii) there is no explicit goal to regulate emotion and (iii) emotional responses are changed by processes that operate largely, if not entirely, automatically (Ledoux, 2015).
Extinction and reinforcer revaluation have received considerable attention in both human and animal studies (Pickens and Holland, 2004; Delgado et al., 2006; Murray et al., 2007; Quirk and Mueller, 2007), which suggest that they are supported by the medial orbitofrontal cortex and vmPFC (Figures 3 and 4). For simplicity and to be consistent with the majority of human neuroimaging research, we use the term vmPFC here.
The vmPFC is thought of as a hub that integrates inputs from diverse brain systems to compute and update representations of the affective value of stimuli to support contextually appropriate responses to them (Roy et al., 2012). The vmPFC combines information about one’s current context, goals, motivational states and learning history to provide an integrated representation of the expected (subjective, affective) value(s) of actions, stimuli and outcomes (Schoenbaum et al., 2011; Roy et al., 2012; Ochsner and Gross, 2014; Rudebeck and Murray 2014). The vmPFC is uniquely suited to integrate this information, as it is reciprocally interconnected with regions that (i) identify what stimuli are present—including lateral temporal regions representing perceptual features of stimuli (Öngür and Price, 2000), (ii) indicate the initial valuation of these stimuli and their features—including the amygdala (Amaral and Price, 1984) and ventral striatum (Öngür and Price, 2000; Haber and Knutson, 2010), which provide information about the relevance of stimuli to affective goals and (iii) provide important contextual information that may constrain the range of appropriate responses—including medial temporal regions that may have encoded specific prior experiences with stimuli (Price and Drevets, 2009), subcortical and cortical (e.g. insula) regions representing current motivational (e.g. hunger) and other body states (e.g. pain) (Cavada et al., 2000; Price, 2007), and lateral prefrontal and cingulate regions representing current task goals (Cavada et al., 2000; Price, 2007).
Meta-analyses of human imaging studies have demonstrated that vmPFC is the primary (but not only) region whose activity tracks the current subjective value of food, money or various goods (Chib et al., 2009), its activity increases for highly valued stimuli or when extinction requires that these values are updated (Wik et al., 1997; Gottfried and Dolan, 2004; Phelps et al., 2004) and decreases for devalued stimuli (Gottfried et al., 2003; Kerfoot et al., 2007). Importantly, the vmPFC tracks the subjective value for stimuli as a function of one’s chronic goals—for example, in successful dieters, vmPFC activity correlates with evaluations of how tasty and healthy foods are vs only tastiness in non-dieters (Hare et al., 2009; Hare et al., 2011). Here we should point out that valuation processes, like the ones just reviewed, may not be completely implicit. That is, individuals may have some awareness that how they value a stimulus is changing/has changed. We consider valuation processes to be implicit-automatic when there is no explicit goal to regulate emotion and emotional responses are changed by processes that operate largely automatically.
Structural studies also support the role of the vmPFC in representing subjective value/preferences based on current information about contingencies rather than stored preferences (Schoenbaum et al., 2011). For example, in humans, vmPFC lesions impair the initial learning (Camille et al., 2011b) and expression of simple stimulus preferences (Fellows and Farah, 2007), render preference hierarchies unstable (Camille et al., 2011a) and disrupt evaluation of social stimuli such as facial expressions (Hornak et al., 1996; Heberlein et al., 2008) and stereotyped groups (Milne and Grafman, 2001). In both animal and human research, vmPFC lesions disrupt retention of the newly learned values acquired in extinction (Morgan et al., 1993; Quirk et al., 2000), and in humans, vmPFC thickness predicts retention of extinguished responses (Milad et al., 2005). Additionally, individuals with vmPFC lesions exhibit behaviors suggesting an impaired ability to integrate contextual information (e.g. social norms) to guide their behavior, including general disruptions of social behavior and emotional experience (Hornak et al., 2003), teasing strangers in inappropriate ways (Beer et al., 2003) and failing to recognize when others commit a faux pas (Stone et al., 1998). Most critically, individuals with vmPFC lesions show heightened amygdala responses to aversive images, providing evidence that the vmPFC is involved in regulating the amygdala (Motzkin et al., 2015). These studies highlight the myriad functions that have been attributed to the vmPFC, and its specific roles are still under debate (Delgado et al., 2016).
Implicit-controlled emotion regulation
Implicit-controlled emotion regulation is characterized by an implicit emotion regulatory goal and the engagement of controlled processes. Research on its neural bases has not been systematic, coming primarily from human imaging work suggesting that it engages combinations of the neural systems observed in explicit-controlled and implicit-automatic regulation (Figure 3). Most such studies have used selective attention tasks relying on brain regions involved in cognitive control, including lateral PFC regions critical for holding in mind rules or selecting/inhibiting responses, and posterior mPFC/dACC regions important for performance monitoring and evaluating the need for cognitive control (Miller and Cohen, 2001; Wager and Smith, 2003; Shenhav et al., 2016).
Implicit-controlled strategies occupy a large swath of the two-dimensional space because their underlying psychological processes arise from several different combinations of goal and process. We have identified two main types of implicit-controlled strategies and discuss what is known about their underlying neural systems below.
The first type includes strategies where the regulatory goal is incidental and regulation is simply a by-product of using top-down control to perform another task. Here, two types of experimental tasks have been commonly used. The first involves selectively attending to perceptual features of stimuli, such as the emotional Stroop (Figure 1A) (Pessoa, 2005; Etkin et al., 2006; Buhle et al., 2010) or emotional go-nogo (Figure 1A) (Hare et al., 2005; Eigsti et al., 2006; Hare et al., 2008; Berkman et al., 2009; Casey et al., 2011). In these tasks, affective stimuli are encountered while pursuing an explicit task goal (e.g. ‘ignore the words and name the emotion expression’ or ‘press a key when you see fearful faces’) that does not involve the conscious intention to regulate emotion but nonetheless engages controlled processes. Performing these tasks tends to engage lateral prefrontal regions (e.g. dlPFC and vlPFC) and mPFC/dACC and modulate activity in regions implicated in encoding appetitive or aversive stimulus features (Ochsner and Gross, 2005; Buhle et al., 2010). The second example involves selectively attending/responding to semantic features of stimuli. This type of implicit-controlled regulation is exemplified by affect labeling tasks (Figure 1A) where participants have the explicit goal of deciding which of two semantic labels (e.g. ‘fear’) is the best match for a target stimulus (a fear face) but have no conscious intention to change their affective responses. The act of selecting the semantic label engages regions implicated in controlled processes (e.g. right vlPFC) and reduces behavioral (e.g. self-report) and neural (e.g. amygdala activity) markers of affective responding (Lieberman et al., 2007; Cohen and Lieberman, 2010; Lieberman et al., 2011; Payer et al., 2012; Torrisi et al., 2013; Burklund et al., 2014).
The second type of implicit-controlled strategy includes those that involve the use of controlled processes initiated by implicit goals that are externally-generated (e.g. by priming) or internally-generated (e.g. by a chronically active goal). Again, two kinds of research are relevant here. First, in studies of automatic goal pursuit (Figures 1A and 2) an externally given goal is activated outside of awareness and guides subsequent behavior (Bargh et al., 2001). Although there is a rich behavioral literature on automatic goal pursuit in non-affective contexts (Custers and Aarts, 2010; Hassin, 2013), only recently has it been studied as a form of emotion regulation. For example, reductions in anger (Mauss et al., 2007) and physiological reactivity (Williams et al., 2009) may follow from presentation of regulation-related words embedded in a scrambled sentences paradigm presented as unrelated to a main task. Interestingly, priming emotion regulation in this way may be most effective for individuals who do not habitually use explicit-controlled reappraisal (Mauss et al., 2007), suggesting that explicitly and implicitly triggering a given strategy may involve different mechanisms. Although no imaging studies have examined primed emotion regulation, one study demonstrated that top-down control processes and lateral PFC can be engaged by non-consciously primed conflict (Lau and Passingham, 2007).
The second example comes from studies where an internally generated implicit goal, such as the chronically active goal to maintain accurate representations of affective value, can drive engagement of controlled processes to update affective responses. Perhaps the most well-studied example is reversal learning (Figures 1A and 2), where an organism learns that one stimulus of a pair is initially associated with reward, but then this association flips and the organism must update the affective values associated with both stimuli. Some studies suggest that reversal learning involves the kind of vmPFC-dependent value updating found in implicit-automatic regulation (Hornak et al., 2004; Murray et al., 2007; Schiller et al., 2008; Fellows, 2011). However, studies using repeated/serial reversals, which foster the development and application of rules, find that the vlPFC is involved in both human fMRI (Cools et al., 2002; Remijnse et al., 2005; Mitchell et al., 2008; Hampshire et al., 2012) and animal lesion studies (Rygula et al., 2010; Rudebeck et al., 2013). The animal studies are particularly striking because they suggest that previous findings of vmPFC lesions impairing reversal are attributable to damaging fibers of passage connecting the amygdala and vlPFC. Excitotoxic lesions targeting only cortical tissue and sparing these fibers do not impair reversal (Rudebeck et al., 2013). As such, at present, we consider reversal learning to be best characterized as a form of implicit-controlled regulation, because in some cases, like repeated reversals, more than one kind of process—including controlled processes—may support it.
Explicit-automatic emotion regulation
Explicit-automatic emotion regulation involves an explicit goal to change emotion, with regulatory change depending on automatic processes (cf. Bargh, 1989). This type of regulation has received the least empirical attention in neuroscience research, but there is at least one well-studied behavioral phenomenon that has the characteristics of explicit-automatic regulation: placebo effects (Figure 1A). Placebo effects involve the expectation or belief that a sham treatment is effective in changing the way one will respond to a stimulus without relying on top-down control processes to cause placebo-related changes in response to a stimulus (Wager and Atlas, 2015).
Most neuroscience research has probed effects of placebo on pain in humans. Behaviorally, numerous studies have shown that consciously holding the expectation that a placebo treatment (e.g. a cream) is effective reduces the experience of pain (e.g. unpleasant heat). In the brain (Figure 3), placebo treatment is generally accompanied by increased recruitment of several regions, including vmPFC, dlPFC, lateral OFC, ventral striatum and periaqueductal gray, with activity in these regions thought to support the maintenance of information about the context and the creation of placebo-related expectations/appraisals (Wager and Atlas, 2015). Accompanying these activity increases are reductions in activity in pain-sensitive brain regions including the medial thalamus, anterior insula, and dACC, periaqueductal gray and secondary somatosensory cortex-dorsal posterior insula (Wager and Atlas, 2015). Placebo beliefs also can regulate other types of affective responses, including the experience of disgust and associated insula activity (Schienle et al., 2013).
Although placebo treatments recruit multiple PFC systems, current behavioral data tell us more about the underlying processes and indicate the way in which they affect change does not require cognitive control or working memory. Evidence supporting this view comes from a study showing that the magnitude of placebo-induced analgesia was not impacted by concurrent completion of a working memory task (Buhle et al., 2012). This suggests that placebo effects can occur without substantial conscious effort, perhaps because—in contrast to explicit-controlled strategies—placebo effects do not require ongoing conscious manipulation of appraisals of the painful stimulus. This difference between reappraisal and placebo effects was hit home in a recent imaging study showing that placebo acts directly on activity in pain-sensitive regions, whereas reappraisal changes connectivity between pain-sensitive regions and regions related to reward (Woo et al., 2015). Taken together, these studies highlight the importance of understanding brain activity in the context of behavior. While dlPFC is engaged during placebo, its role is likely limited to holding the expectation in mind, rather than the conscious adjustment of appraisals.
Implications and future directions
This multi-level framework organizes several distinct literatures and methodologies from both animal and human research to describe the relationships among and differences between broad classes of emotion regulatory phenomena. In so doing, it provides a common, unified language for talking about the wide range of regulatory phenomena and helps clarify the boundaries between what we already understand and what future work should explore. Having summarized evidence supporting this framework, in this final section, we consider how it clarifies knowledge boundaries, charts a path for future research and may have translational applications.
Clarifying knowledge boundaries and charting future directions
Our premise was that the behavior-brain link is best understood in terms of the psychological mechanisms that connect them (Figure 4). As such, we proposed a multi-level framework that turns on a key distinction at the psychological level between explicit and implicit forms of regulation in terms of their reliance on explicit vs implicit regulatory goals, with further specification needed to indicate whether these goals change emotion via processes that operate in a controlled vs automatic fashion. This framework is complementary to the existing process model of emotion regulation (Gross, 1998; Ochsner et al., 2012) and distinct from other proposed frameworks (Berkman and Lieberman, 2009; Gyurak et al., 2011) in that it uses two orthogonal psychological dimensions to define four related, but distinct classes of emotion regulation strategies. Two classes—explicit-controlled and implicit-automatic—define distinct forms of commonly studied emotion regulation in terms of underlying goals and processes, rather than just one or the other, as has been the case in prior formulations of the explicit vs implicit distinction. The framework also provides a means of conceptualizing two more classes of regulation—implicit-controlled and explicit-automatic—that have been characterized previously only as ‘implicit’ or ‘explicit’ without clarifying how they differ from other forms of regulation that the framework slots into the explicit-controlled and implicit-automatic categories.
Another benefit of the framework is that it provides a means to conceptualize the various ways in which a given emotion regulation strategy can be implemented (Figure 2). Importantly, it also makes predictions about how the neural systems will change based on movement on the goal or process axes. Thinking about regulatory strategies in this way highlights the need for future work to systematically vary the degree of awareness and intentionality for regulatory goals and identify factors determining if and when the implementation of a strategy can rely less on controlled processes.
The framework also makes clear what we know about the brain systems involved in explicit-controlled and implicit-automatic regulation (Figure 3) and what requires future study. For example, extant work shows that explicit-controlled and implicit-automatic regulation differentially depend on lateral PFC versus vmPFC (Figure 4). However, because the explicit-controlled/implicit-automatic distinction confounds the goal and process dimensions, we do not know whether one or both dimensions underlie the difference in PFC recruitment. Future work is needed to determine whether some brain regions—or networks of regions that interact in different ways, depending on the means of regulation employed—are more associated with explicit vs implicit goals and/or automatic versus controlled regulatory processes. Studies of hybrid regulation could address these questions because they involve different combinations of regulatory goals and processes. At present, however, there are too few studies of each type of hybrid strategy with methods that differ in multiple ways to draw firm conclusions.
For simplicity, we limited the framework to the goal and process dimensions. However, other dimensions may be useful to consider, including the temporal dimension of emotion regulation. Future work could address at least three key questions. First, how does each class of regulation affect the different emotion generation stages described in the process model? Second, do the classes differ in how quickly they move from activation of a regulatory goal to changing an emotional response? Explicit-controlled regulation, for example, is typically studied with fMRI tasks that have immediate effects on responses to unique stimuli, whereas implicit-automatic regulation has been studied primarily in animals with learning tasks that involve incremental changes in responding to repeated stimuli. More human studies are needed on implicit-automatic, implicit-controlled and explicit-automatic strategies, and we should examine the role of learning/practice in all classes. This leads to a third question—do the classes of regulation differ in how durable are their effects on emotional responding? The effects of explicit-controlled regulation may last from minutes to days, depending on the degree of regulatory practice (Shurick et al., 2012; Denny and Ochsner, 2014; Silvers et al., 2014; Denny et al., 2015). Regarding implicit-automatic strategies, we know that the effect of extinction is also durable and lasts days to weeks (e.g. Quirk, 2002), but extinction memories are sensitive to context and are more fragile than the original conditioned association (Dunsmoor et al., 2015). Future work could ask whether explicit-controlled strategies become more automatic and implicit with practice, which in turn could lead to a shift in the frontal regions recruited from dorsal and lateral to ventral and medial.
Translational applications
In describing the behaviors, processes and neural systems supporting different forms of regulation, the framework provides a foundation for understanding sources of normal and abnormal variability in emotion regulation ability. Immaturity, due to a still developing brain, or deficits, due to clinical disorders or aging, of brain systems that support specific kinds of emotion regulation will render some forms of regulation less effective for certain individuals. The framework suggests which kinds of regulation are likely to be the most problematic or beneficial for a given group. For example, relative to young adults, reappraisal ability may be diminished in children and adolescents (Silvers et al., 2012; Silvers et al., 2014), older adults (Urry et al., 2006; Winecoff et al., 2011; Tucker et al., 2012) and individuals with depression (Johnstone et al., 2007) or borderline personality disorder (Koenigsberg et al., 2009), in part, because of lateral PFC dysfunction. Future work could test the framework’s prediction that implicit-automatic strategies, which depend more on vmPFC, may be more effective for such groups.
Different situational and contextual factors also influence regulation success (Doré et al., 2016), and future work could probe how the four classes of regulatory strategies are affected by them. For instance, recent work suggests that stress may impair explicit-controlled regulation (Raio et al., 2013), perhaps by disrupting prefrontal function (Arnsten, 2009; van Ast et al., 2014). We predict that implicit-automatic strategies would not be as impacted by stress because stress potentiates non-PFC dependent, habitual responses (Arnsten, 2009). Similarly, certain forms of regulation may be better suited to particular affective stimuli or experiences. For instance, aversive image intensity affects explicit-controlled strategy choice (Sheppes et al., 2011).
Conclusion
Our knowledge of emotion regulation strategies has grown over the last 15 years, and we have identified a diverse set of behavioral phenomena that modulate emotional responding. Understanding the neural systems involved in these various strategies requires a novel framework that can account for the wide range of relevant phenomena and provide an organizing structure that is informed by knowledge about behavior, psychological processes and the brain. We proposed a framework that identifies four different classes of emotion regulation strategies—explicit-controlled, implicit-automatic, implicit-controlled and explicit-automatic—which are differentiated based on two orthogonal psychological dimensions that describe the nature of the emotion regulation goal and the nature of the emotion change process. While this framework helps organize our understanding of extant research, it also makes clear how many important questions remain unanswered, and charts directions for future work to address them.
Funding
This work was supported by the National Institute on Aging (AG043463 to K.N.O.); the National Institute of Child Health and Human Development (HD069178 to K.N.O.); the National Institute of Mental Health (MH090964 to K.N.O.) and the National Institute on Alcohol Abuse and Alcoholism (AA023704 postdoctoral fellowship to L.M.B.).
Conflict of interest. None declared.
References
- Amaral D.G., Price J.L. (1984). Amygdalo-cortical projections in the monkey (Macaca fascicularis). Journal of Comparative Neurology, 230, 465–96. [DOI] [PubMed] [Google Scholar]
- Arnsten A.F. (2009). Stress signalling pathways that impair prefrontal cortex structure and function. Nature Reviews Neuroscience, 10, 410–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron A.R., Robbins T.W., Poldrack R.A. (2004). Inhibition and the right inferior frontal cortex. Trends in Cognitive Sciences, 8, 170–7. [DOI] [PubMed] [Google Scholar]
- Badre D., Wagner A.D. (2004). Selection, integration, and conflict monitoring: assessing the nature and generality of prefrontal cognitive control mechanisms. Neuron, 41, 473–87. [DOI] [PubMed] [Google Scholar]
- Bargh J.A. (1989). Conditional automaticity: varieties of automatic influence in social perception and cognition. Unintended Thought, 3, 51–69. [Google Scholar]
- Bargh J.A., Gollwitzer P.M., Lee-Chai A., Barndollar K., Trotschel R. (2001). The automated will: nonconscious activation and pursuit of behavioral goals. Journal of Personality and Social Psychology, 81, 1014–27. [PMC free article] [PubMed] [Google Scholar]
- Beer J.S., Heerey E.A., Keltner D., Scabini D., Knight R.T. (2003). The regulatory function of self-conscious emotion: insights from patients with orbitofrontal damage. Journal of Personality and Social Psychology, 85, 594.. [DOI] [PubMed] [Google Scholar]
- Berkman E.T., Burklund L., Lieberman M.D. (2009). Inhibitory spillover: Intentional motor inhibition produces incidental limbic inhibition via right inferior frontal cortex. Neuroimage, 47, 705–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkman E.T., Lieberman M.D. (2009). Using neuroscience to broaden emotion regulation: theoretical and methodological considerations. Social and Personality Psychology Compass, 3, 475–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinick M.M., Braver T.S., Barch D.M., Carter C.S., Cohen J.D. (2001). Conflict monitoring and cognitive control. Psychological Review, 108, 624.. [DOI] [PubMed] [Google Scholar]
- Botvinick M.M., Cohen J.D., Carter C.S. (2004). Conflict monitoring and anterior cingulate cortex: an update. Trends in Cognitive Sciences, 8, 539–46. [DOI] [PubMed] [Google Scholar]
- Buhle J., Wager T., Smith E. (2010). Using the Stroop task to study emotion regulation In: Hassin R., Ochsner K. N., Trope Y., editors. Self Control in Society, Mind, and Brain. New York: Oxford University Press. [Google Scholar]
- Buhle J.T., Silvers J.A., Wager T.D., et al. (2014). Cognitive reappraisal of emotion: a meta-analysis of human neuroimaging studies. Cerebral Cortex, 24, 2981–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhle J.T., Stevens B.L., Friedman J.J., Wager T.D. (2012). Distraction and placebo two separate routes to pain control. Psychological Science, 23, 246–53. [DOI] [PubMed] [Google Scholar]
- Burklund L.J., Creswell J.D., Irwin M.R., Lieberman M.D. (2014). The common and distinct neural bases of affect labeling and reappraisal in healthy adults. Front Psychol, 5, 221.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacioppo J.T., Berntson G.G., Larsen J.T., Poehlmann K.M., Ito T.A. (2000). The psychophysiology of emotion. Handbook of Emotions, 2, 173–91. [Google Scholar]
- Camille N., Griffiths C.A., Vo K., Fellows L.K., Kable J.W. (2011a). Ventromedial frontal lobe damage disrupts value maximization in humans. The Journal of Neuroscience, 31, 7527–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camille N., Tsuchida A., Fellows L.K. (2011b). Double dissociation of stimulus-value and action-value learning in humans with orbitofrontal or anterior cingulate cortex damage. The Journal of Neuroscience, 31, 15048–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter C.S., Van Veen V. (2007). Anterior cingulate cortex and conflict detection: an update of theory and data. Cognitive, Affective, & Behavioral Neuroscience, 7, 367–79. [DOI] [PubMed] [Google Scholar]
- Casey B.J., Somerville L.H., Gotlib I.H., et al. (2011). Behavioral and neural correlates of delay of gratification 40 years later. Proc Natl Acad Sci U S A, 108, 14998–5003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavada C., Tejedor J., Cruz-Rizzolo R.J., Reinoso-Suárez F. (2000). The anatomical connections of the macaque monkey orbitofrontal cortex. A review. Cerebral Cortex, 10, 220–42. [DOI] [PubMed] [Google Scholar]
- Chib V.S., Rangel A., Shimojo S., O'doherty J.P. (2009). Evidence for a common representation of decision values for dissimilar goods in human ventromedial prefrontal cortex. The Journal of Neuroscience, 29, 12315–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coan J.A., Maresh E.L. (2014). Social baseline theory and the social regulation of emotion In: Gross J. J., Thompson R. H., editors. Handbook of Emotion Regulation .2nd edn, New York, NY: Guilford Press. [Google Scholar]
- Cocchi L., Zalesky A., Fornito A., Mattingley J.B. (2013). Dynamic cooperation and competition between brain systems during cognitive control. Trends in Cognitive Sciences, 17, 493–501. [DOI] [PubMed] [Google Scholar]
- Cohen J.R., Lieberman M.D. (2010). The common neural basis of exerting self-control in multiple domains. Self Control in Society, Mind, and Brain 141–62. [Google Scholar]
- Cools R., Clark L., Owen A.M., Robbins T.W. (2002). Defining the neural mechanisms of probabilistic reversal learning using event-related functional magnetic resonance imaging. The Journal of Neuroscience, 22, 4563–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig A. (2009). How do you feel—now? The anterior insula and human awareness. Nature Reviews Neuroscience, 10, 59–70. [DOI] [PubMed] [Google Scholar]
- Critchley H., Daly E., Phillips M., et al. (2000). Explicit and implicit neural mechanisms for processing of social information from facial expressions: a functional magnetic resonance imaging study. Human Brain Mapping, 9, 93–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley H.D., Wiens S., Rotshtein P., Öhman A., Dolan R.J. (2004). Neural systems supporting interoceptive awareness. Nature Neuroscience, 7, 189–95. [DOI] [PubMed] [Google Scholar]
- Cunningham W.A., Brosch T. (2012). Motivational salience: amygdala tuning from traits, needs, values, and goals. Current Directions in Psychological Science, 21, 54–9. [Google Scholar]
- Custers R., Aarts H. (2010). The unconscious will: how the pursuit of goals operates outside of conscious awareness. Science, 329, 47–50. [DOI] [PubMed] [Google Scholar]
- Davis M., Whalen P.J. (2001). The amygdala: vigilance and emotion. Molecular Psychiatry, 6, 13–34. [DOI] [PubMed] [Google Scholar]
- Delgado M.R. (2007). Reward-related responses in the human striatum. Annals of the New York Academy of Sciences, 1104, 70–88. [DOI] [PubMed] [Google Scholar]
- Delgado M.R., Beer J.S., Fellows L.K., et al. (2016). Viewpoints: dialogues on the functional role of the ventromedial prefrontal cortex. Nature Neuroscience, 19, 1545–52. [DOI] [PubMed] [Google Scholar]
- Delgado M.R., Gillis M.M., Phelps E.A. (2008). Regulating the expectation of reward via cognitive strategies. Nature Neuroscience, 11, 880–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado M.R., Olsson A., Phelps E.A. (2006). Extending animal models of fear conditioning to humans. Biological Psychology, 73, 39–48. [DOI] [PubMed] [Google Scholar]
- Denny B.T., Inhoff M.C., Zerubavel N., Davachi L., Ochsner K.N. (2015). Getting over it long-lasting effects of emotion regulation on amygdala response. Psychological Science 0956797615578863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denny B.T., Ochsner K.N. (2014). Behavioral effects of longitudinal training in cognitive reappraisal. Emotion, 14, 425.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekhof E.K., Geier K., Falkai P., Gruber O. (2011). Fear is only as deep as the mind allows: a coordinate-based meta-analysis of neuroimaging studies on the regulation of negative affect. Neuroimage, 58, 275–85. [DOI] [PubMed] [Google Scholar]
- Diener E. (2009). Subjective Well-Being The Science of Well-Being. Amsterdam: Springer. [Google Scholar]
- Doré B.P., Silvers J.A., Ochsner K.N. (2016). Toward a personalized science of emotion regulation. Social and Personality Psychology Compass, 10, 171–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunsmoor J.E., Niv Y., Daw N., Phelps E.A. (2015). Rethinking extinction. Neuron, 88, 47–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eigsti I.M., Zayas V., Mischel W., et al. (2006). Predicting cognitive control from preschool to late adolescence and young adulthood. Psychol Sci, 17, 478–84. [DOI] [PubMed] [Google Scholar]
- Etkin A., Büchel C., Gross J.J. (2015). The neural bases of emotion regulation. Nature Reviews Neuroscience, 16, 693–700. [DOI] [PubMed] [Google Scholar]
- Etkin A., Egner T., Peraza D.M., Kandel E.R., Hirsch J. (2006). Resolving emotional conflict: a role for the rostral anterior cingulate cortex in modulating activity in the amygdala. Neuron, 51, 871–82. [DOI] [PubMed] [Google Scholar]
- Fellows L.K. (2011). Orbitofrontal contributions to value-based decision making: evidence from humans with frontal lobe damage. Annals of the New York Academy of Sciences, 1239, 51–8. [DOI] [PubMed] [Google Scholar]
- Fellows L.K., Farah M.J. (2007). The role of ventromedial prefrontal cortex in decision making: judgment under uncertainty or judgment per se? Cerebral Cortex, 17, 2669–74. [DOI] [PubMed] [Google Scholar]
- Gottfried J.A., Dolan R.J. (2004). Human orbitofrontal cortex mediates extinction learning while accessing conditioned representations of value. Nature Neuroscience, 7, 1144–52. [DOI] [PubMed] [Google Scholar]
- Gottfried J.A., O'doherty J., Dolan R.J. (2003). Encoding predictive reward value in human amygdala and orbitofrontal cortex. Science, 301, 1104–7. [DOI] [PubMed] [Google Scholar]
- Gross J.J. (1998). The emerging field of emotion regulation: an integrative review. Review of General Psychology, 2, 271–99. [Google Scholar]
- Gross J.J. (2013). Emotion regulation: taking stock and moving forward. Emotion, 13, 359.. [DOI] [PubMed] [Google Scholar]
- Gross J.J. (2015). Emotion regulation: current status and future prospects. Psychological Inquiry, 26, 1–26. [Google Scholar]
- Gross J.J., John O.P. (2003). Individual differences in two emotion regulation processes: implications for affect, relationships, and well-being. Journal of Personality and Social Psychology, 85, 348–62. [DOI] [PubMed] [Google Scholar]
- Gyurak A., Gross J.J., Etkin A. (2011). Explicit and implicit emotion regulation: a dual-process framework. Cognition and Emotion, 25, 400–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber S.N., Knutson B. (2010). The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology, 35, 4–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampshire A., Chaudhry A.M., Owen A.M., Roberts A.C. (2012). Dissociable roles for lateral orbitofrontal cortex and lateral prefrontal cortex during preference driven reversal learning. Neuroimage, 59, 4102–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare T.A., Camerer C.F., Rangel A. (2009). Self-control in decision-making involves modulation of the vmPFC valuation system. Science, 324, 646–8. [DOI] [PubMed] [Google Scholar]
- Hare T.A., Malmaud J., Rangel A. (2011). Focusing attention on the health aspects of foods changes value signals in vmPFC and improves dietary choice. The Journal of Neuroscience, 31, 11077–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare T.A., Tottenham N., Davidson M.C., Glover G.H., Casey B.J. (2005). Contributions of amygdala and striatal activity in emotion regulation. Biological Psychiatry, 57, 624–32. [DOI] [PubMed] [Google Scholar]
- Hare T.A., Tottenham N., Galvan A., Voss H.U., Glover G.H., Casey B.J. (2008). Biological substrates of emotional reactivity and regulation in adolescence during an emotional go-nogo task. Biol Psychiatry, 63, 927–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley C.A., Phelps E.A. (2009). Changing fear: the neurocircuitry of emotion regulation. Neuropsychopharmacology, 35, 136–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassin R.R. (2013). Yes it can on the functional abilities of the human unconscious. Perspectives on Psychological Science, 8, 195–207. [DOI] [PubMed] [Google Scholar]
- Hassin R.R., Aarts H., Eitam B., Custers R., Kleiman T. (2009). Non-conscious goal pursuit and the effortful control of behavior. Oxford Handbook of Human Action 549–66. [Google Scholar]
- Heberlein A.S., Padon A.A., Gillihan S.J., Farah M.J., Fellows L.K. (2008). Ventromedial frontal lobe plays a critical role in facial emotion recognition. J Cogn Neurosci, 20, 721–33. [DOI] [PubMed] [Google Scholar]
- Hornak J., Bramham J., Rolls E.T., et al. (2003). Changes in emotion after circumscribed surgical lesions of the orbitofrontal and cingulate cortices. Brain, 126, 1691–712. [DOI] [PubMed] [Google Scholar]
- Hornak J., O'doherty J., Bramham J., et al. (2004). Reward-related reversal learning after surgical excisions in orbito-frontal or dorsolateral prefrontal cortex in humans. Journal of Cognitive Neuroscience, 16, 463–78. [DOI] [PubMed] [Google Scholar]
- Hornak J., Rolls E.T., Wade D. (1996). Face and voice expression identification in patients with emotional and behavioural changes following ventral frontal lobe damage. Neuropsychologia, 34, 247–61. [DOI] [PubMed] [Google Scholar]
- Janak P.H., Tye K.M. (2015). From circuits to behaviour in the amygdala. Nature, 517, 284–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone T., Van Reekum C.M., Urry H.L., Kalin N.H., Davidson R.J. (2007). Failure to regulate: counterproductive recruitment of top-down prefrontal-subcortical circuitry in major depression. The Journal of Neuroscience, 27, 8877–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanske P., Heissler J., Schönfelder S., Bongers A., Wessa M. (2010). How to regulate emotion? neural networks for reappraisal and distraction. Cerebral Cortex, 21, 1379–88. [DOI] [PubMed] [Google Scholar]
- Kerfoot E.C., Agarwal I., Lee H.J., Holland P.C. (2007). Control of appetitive and aversive taste-reactivity responses by an auditory conditioned stimulus in a devaluation task: a FOS and behavioral analysis. Learning & Memory, 14, 581–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight R.T., Richard Staines W., Swick D., Chao L.L. (1999). Prefrontal cortex regulates inhibition and excitation in distributed neural networks. Acta Psychologica, 101, 159–78. [DOI] [PubMed] [Google Scholar]
- Kober H., Mende-Siedlecki P., Kross E.F., et al. (2010). Prefrontal-striatal pathway underlies cognitive regulation of craving. Proceedings of the National Academy of Sciences, U.S.A, 107, 14811–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigsberg H.W., Fan J., Ochsner K.N., et al. (2009). Neural correlates of the use of psychological distancing to regulate responses to negative social cues: a study of patients with borderline personality disorder. Biological Psychiatry, 66, 854–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau H.C., Passingham R.E. (2007). Unconscious activation of the cognitive control system in the human prefrontal cortex. The Journal of Neuroscience, 27, 5805–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledoux J. (2015). Anxious: Using the Brain to Understand and Treat Fear and Anxiety, New York: Penguin. [Google Scholar]
- Ledoux J.E. (2012). Evolution of human emotion: a view through fear. Progress in Brain Research, 195, 431.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman M.D., Eisenberger N.I., Crockett M.J., Tom S.M., Pfeifer J.H., Way B.M. (2007). Putting feelings into words: Affect labeling disrupts amygdala activity in response to affective stimuli. Psychological Science, 18, 421–8. [DOI] [PubMed] [Google Scholar]
- Lieberman M.D., Inagaki T.K., Tabibnia G., Crockett M.J. (2011). Subjective responses to emotional stimuli during labeling, reappraisal, and distraction. Emotion, 11, 468.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin L.N., Delgado M.R. (2011). The influence of emotion regulation on decision-making under risk. Journal of Cognitive Neuroscience, 23, 2569–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauss I.B., Cook C.L., Gross J.J. (2007). Automatic emotion regulation during anger provocation. Journal of Experimental Social Psychology, 43, 698–711. [Google Scholar]
- Mcrae K., Ciesielski B., Gross J.J. (2012). Unpacking cognitive reappraisal: goals, tactics, and outcomes. Emotion, 12, 250.. [DOI] [PubMed] [Google Scholar]
- Mcrae K., Hughes B., Chopra S., Gabrieli J.D., Gross J.J., Ochsner K.N. (2010). The neural bases of distraction and reappraisal. Journal of Cognitive Neuroscience, 22, 248–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad M.R., Quinn B.T., Pitman R.K., Orr S.P., Fischl B., Rauch S.L. (2005). Thickness of ventromedial prefrontal cortex in humans is correlated with extinction memory. Proceedings of the National Academy of Sciences of the United States of America, 102, 10706–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller B.T., D'esposito M. (2005). Searching for “the top” in top-down control. Neuron, 48, 535–8. [DOI] [PubMed] [Google Scholar]
- Miller E.K., Cohen J.D. (2001). An integrative theory of prefrontal cortex function. Annual Review of Neuroscience, 24, 167–202. [DOI] [PubMed] [Google Scholar]
- Milne E., Grafman J. (2001). Ventromedial prefrontal cortex lesions in humans eliminate implicit gender stereotyping . The Journal of Neuroscience, 21, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell D., Rhodes R., Pine D., Blair R. (2008). The contribution of ventrolateral and dorsolateral prefrontal cortex to response reversal. Behavioural Brain Research, 187, 80–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan M.A., Romanski L.M., Ledoux J.E. (1993). Extinction of emotional learning: contribution of medial prefrontal cortex. Neuroscience Letters, 163, 109–13. [DOI] [PubMed] [Google Scholar]
- Motzkin J.C., Philippi C.L., Wolf R.C., Baskaya M.K., Koenigs M. (2015). Ventromedial prefrontal cortex is critical for the regulation of amygdala activity in humans. Biological Psychiatry, 77, 276–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray E.A., O'doherty J.P., Schoenbaum G. (2007). What we know and do not know about the functions of the orbitofrontal cortex after 20 years of cross-species studies. The Journal of Neuroscience, 27, 8166–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'doherty J.P. (2004). Reward representations and reward-related learning in the human brain: Insights from neuroimaging. Current Opinion in Neurobiology, 14, 769–76. [DOI] [PubMed] [Google Scholar]
- Ochsner K.N., Gross J.J. (2005). The cognitive control of emotion. Trends in Cognitive Sciences, 9, 242–9. [DOI] [PubMed] [Google Scholar]
- Ochsner K.N., Gross J.J. (2014). The neural bases of emotion and emotion regulation: a valuation perspective In: Gross J. J., Thompson R. H., editors. Handbook of Emotion Regulation, 2nd edn,New York, NY: Guilford Press. [Google Scholar]
- Ochsner K.N., Silvers J.A., Buhle J.T. (2012). Functional imaging studies of emotion regulation: a synthetic review and evolving model of the cognitive control of emotion. Annals of the New York Academy of Sciences, 1251, E1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Öngür D., Price J. (2000). The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cerebral Cortex, 10, 206–19. [DOI] [PubMed] [Google Scholar]
- Payer D.E., Baicy K., Lieberman M.D., London E.D. (2012). Overlapping neural substrates between intentional and incidental down-regulation of negative emotions. Emotion, 12, 229.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L. (2005). To what extent are emotional visual stimuli processed without attention and awareness? Current Opinion in Neurobiology, 15, 188–96. [DOI] [PubMed] [Google Scholar]
- Phelps E.A. (2006). Emotion and cognition: insights from studies of the human amygdala. Annual Review of Psychology, 57, 27–53. [DOI] [PubMed] [Google Scholar]
- Phelps E.A., Delgado M.R., Nearing K.I., Ledoux J.E. (2004). Extinction learning in humans: role of the amygdala and vmPFC. Neuron, 43, 897–905. [DOI] [PubMed] [Google Scholar]
- Pickens C.L., Holland P.C. (2004). Conditioning and cognition. Neuroscience & Biobehavioral Reviews, 28, 651–61. [DOI] [PubMed] [Google Scholar]
- Pickens C.L., Saddoris M.P., Setlow B., Gallagher M., Holland P.C., Schoenbaum G. (2003). Different roles for orbitofrontal cortex and basolateral amygdala in a reinforcer devaluation task. The Journal of Neuroscience, 23, 11078–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price J.L. (2007). Definition of the orbital cortex in relation to specific connections with limbic and visceral structures and other cortical regions. Annals of the New York Academy of Sciences, 1121, 54–71. [DOI] [PubMed] [Google Scholar]
- Price J.L., Drevets W.C. (2009). Neurocircuitry of mood disorders. Neuropsychopharmacology, 35, 192–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk G.J. (2002). Memory for extinction of conditioned fear is long-lasting and persists following spontaneous recovery. Learning & Memory, 9, 402–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk G.J., Mueller D. (2007). Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology, 33, 56–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk G.J., Russo G.K., Barron J.L., Lebron K. (2000). The role of ventromedial prefrontal cortex in the recovery of extinguished fear. The Journal of Neuroscience, 20, 6225–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raio C., Orederu T., Palazzolo L., Shurick A., Phelps E. (2013). Cognitive emotion regulation fails the stress test. Proceedings of the National Academy of Sciences, 110, 15139–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeck C., Ames D.R., Ochsner K.N. (2016). The social regulation of emotion: an integrative, cross-disciplinary model. Trends in Cognitive Sciences, 20, 47–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remijnse P.L., Nielen M.M., Uylings H.B., Veltman D.J. (2005). Neural correlates of a reversal learning task with an affectively neutral baseline: an event-related fMRI study. Neuroimage, 26, 609–18. [DOI] [PubMed] [Google Scholar]
- Roy M., Shohamy D., Wager T.D. (2012). Ventromedial prefrontal-subcortical systems and the generation of affective meaning. Trends in Cognitive Sciences, 16, 147–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudebeck P.H., Murray E.A. (2014). The orbitofrontal oracle: cortical mechanisms for the prediction and evaluation of specific behavioral outcomes. Neuron, 84, 1143–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudebeck P.H., Saunders R.C., Prescott A.T., Chau L.S., Murray E.A. (2013). Prefrontal mechanisms of behavioral flexibility, emotion regulation and value updating. Nature Neuroscience, 16, 1140–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rygula R., Walker S.C., Clarke H.F., Robbins T.W., Roberts A.C. (2010). Differential contributions of the primate ventrolateral prefrontal and orbitofrontal cortex to serial reversal learning. The Journal of Neuroscience, 30, 14552–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas C.E., Gross J.J., Rafal R.D., Viñas-Guasch N., Turnbull O.H. (2013). Concrete behaviour and reappraisal deficits after a left frontal stroke: a case study. Neuropsychological Rehabilitation, 23, 467–500. [DOI] [PubMed] [Google Scholar]
- Salas C.E., Gross J.J., Turnbull O.H. (2014). Reappraisal generation after acquired brain damage: the role of laterality and cognitive control. Front Psychol, 5, 242, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter D.L., Tulving E. (1994). Memory Systems 1994, Cambridge, Mass: MIT Press. [Google Scholar]
- Scherer K.R., Schorr A.E., Johnstone T.E. (2001). Appraisal Processes in Emotion: Theory, Methods, Research, Oxford: Oxford University Press. [Google Scholar]
- Schienle A., Übel S., Schöngßüner F., Ille R., Scharmüller W. (2013). Disgust regulation via placebo: an fMRI study. Social Cognitive and Affective Neuroscience, 9, 985–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller D., Levy I., Niv Y., Ledoux J.E., Phelps E.A. (2008). From fear to safety and back: reversal of fear in the human brain. The Journal of Neuroscience, 28, 11517–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmeichel B.J., Volokhov R.N., Demaree H.A. (2008). Working memory capacity and the self-regulation of emotional expression and experience. Journal of Personality and Social Psychology, 95, 1526.. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G., Takahashi Y., Liu T.L., Mcdannald M.A. (2011). Does the orbitofrontal cortex signal value? Annals of the New York Academy of Sciences, 1239, 87–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W., Dayan P., Montague P.R. (1997). A neural substrate of prediction and reward. Science, 275, 1593–9. [DOI] [PubMed] [Google Scholar]
- Shenhav A., Cohen J.D., Botvinick M.M. (2016). Dorsal anterior cingulate cortex and the value of control. Nature Neuroscience, 19, 1286–91. [DOI] [PubMed] [Google Scholar]
- Sheppes G., Scheibe S., Suri G., Gross J.J. (2011). Emotion-regulation choice. Psychological Science, 22, 1391–6. [DOI] [PubMed] [Google Scholar]
- Shidlovski D., Hassin R.R. (2011). When pooping babies become more appealing the effects of nonconscious goal pursuit on experienced emotions. Psychological Science, 22, 1381–5. [DOI] [PubMed] [Google Scholar]
- Shurick A.A., Hamilton J.R., Harris L.T., Roy A.K., Gross J.J., Phelps E.A. (2012). Durable effects of cognitive restructuring on conditioned fear. Emotion, 12, 1393.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvers J.A., Mcrae K., Gabrieli J.D., Gross J.J., Remy K.A., Ochsner K.N. (2012). Age-related differences in emotional reactivity, regulation, and rejection sensitivity in adolescence. Emotion, 12, 1235.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvers J.A., Shu J., Hubbard A.D., Weber J., Ochsner K.N. (2014). Concurrent and lasting effects of emotion regulation on amygdala response in adolescence and young adulthood. Developmental Science, 18, 771–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone V.E., Baron-Cohen S., Knight R.T. (1998). Frontal lobe contributions to theory of mind. Journal of Cognitive Neuroscience, 10, 640–56. [DOI] [PubMed] [Google Scholar]
- Tabibnia G., Monterosso J.R., Baicy K., et al. (2011). Different forms of self-control share a neurocognitive substrate. The Journal of Neuroscience, 31, 4805–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrisi S.J., Lieberman M.D., Bookheimer S.Y., Altshuler L.L. (2013). Advancing understanding of affect labeling with dynamic causal modeling. Neuroimage, 82: 481–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker A.M., Feuerstein R., Mende-Siedlecki P., Ochsner K.N., Stern Y. (2012). Double dissociation: circadian off-peak times increase emotional reactivity; aging impairs emotion regulation via reappraisal. Emotion, 12, 869.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urry H.L., Van Reekum C.M., Johnstone T., et al. (2006). Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. The Journal of Neuroscience, 26, 4415–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Ast V., Spicer J., Smith E., et al. (2014). Brain mechanisms of social threat effects on working memory. Cerebral Cortex, 26(2): 544–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dillen L.F., Heslenfeld D.J., Koole S.L. (2009). Tuning down the emotional brain: an fMRI study of the effects of cognitive load on the processing of affective images. Neuroimage, 45, 1212–9. [DOI] [PubMed] [Google Scholar]
- Wager T.D., Atlas L.Y. (2015). The neuroscience of placebo effects: connecting context, learning and health. Nature Reviews Neuroscience, 16, 403–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager T.D., Atlas L.Y., Lindquist M.A., Roy M., Woo C.W., Kross E. (2013). An fMRI-based neurologic signature of physical pain. The New England Journal of Medicine, 368, 1388–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager T.D., Rilling J.K., Smith E.E., et al. (2004). Placebo-induced changes in fMRI in the anticipation and experience of pain. Science, 303, 1162–7. [DOI] [PubMed] [Google Scholar]
- Wager T.D., Smith E.E. (2003). Neuroimaging studies of working memory. Cognitive, Affective, & Behavioral Neuroscience, 3, 255–74. [DOI] [PubMed] [Google Scholar]
- Wik G., Elbert T., Fredrikson M., Hoke M., Ross B. (1997). Magnetic brain imaging of extinction processes in human classical conditioning. Neuroreport, 8, 1789–92. [DOI] [PubMed] [Google Scholar]
- Williams L.E., Bargh J.A., Nocera C.C., Gray J.R. (2009). The unconscious regulation of emotion: nonconscious reappraisal goals modulate emotional reactivity. Emotion, 9, 847–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winecoff A., Labar K.S., Madden D.J., Cabeza R., Huettel S.A. (2011). Cognitive and neural contributors to emotion regulation in aging. Social Cognitive and Affective Neuroscience, 6, 165–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo C.W., Roy M., Buhle J.T., Wager T.D. (2015). Distinct brain systems mediate the effects of nociceptive input and self-regulation on pain. PLoS Biol, 13, e1002036.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki J., Davis J.I., Ochsner K.N. (2012). Overlapping activity in anterior insula during interoception and emotional experience. Neuroimage, 62, 493–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki J., Ochsner K.N. (2012). The neuroscience of empathy: progress, pitfalls and promise. Nature Neuroscience, 15, 675–80. [DOI] [PubMed] [Google Scholar]
- Zaki J., Williams W.C. (2013). Interpersonal emotion regulation. Emotion, 13, 803.. [DOI] [PubMed] [Google Scholar]