Sun-loving plants react to changes in light quality caused by neighbouring plants via “Shade Avoidance” responses, including vertical elongation, upward orientation of leaves and reduced branching/tillering. Such responses are favoured by natural selection because they increase the fitness of individuals, but can be disadvantageous for crops. We took a step towards the development of varieties of wheat with reduced shade avoidance by inducing mutations followed by phenotypic screening. The most promising mutant line did not differ in height from non-mutated cultivars under normal conditions or neutral shade, but elongated much less in strong far-red light.
Keywords: Cereals, forward screening, induced mutations, mutant screening, plasticity, red:far-red
Abstract
Several researchers have hypothesized that shade avoidance behaviour is favoured by natural selection because it increases the fitness of individuals. Shade avoidance can be disadvantageous for crops, however, because it reduces allocation of resources to reproductive yield, increases the risk of lodging and reduces weed suppression. One approach to develop varieties with reduced shade avoidance and enhanced agronomic performance is by inducing mutations followed by phenotypic screening. We treated spring wheat seeds with ethyl methanesulfonate and screened the seedlings repeatedly under green filters for plants showing reduced elongation of the first leaf sheath and second leaf lamina. The shade avoidance responses of five promising mutant lines were further compared to non-mutated plants in a climate chamber experiment with added far-red light. Two of the selected lines displayed significantly reduced elongation under all light treatments while two lines showed reduced elongation only in added far-red light. The most promising mutant line did not differ in height from the non-mutated cultivar in neutral light, but elongated 20.6% less in strong far-red light. This traditional forward approach of screening mutagenized spring wheat produced plants with reduced shade avoidance responses. These mutants may generate new molecular handles to modify the reaction of plants to changes in light spectral distribution in traditional and novel cultivation systems.
Introduction
Plants possess sensory mechanisms to detect changes in ambient light caused by adjacent vegetation. Leaf chlorophylls and carotenoids absorb mostly red (R) and blue light. Thus, light that is transmitted through vegetation is depleted in red and strongly enriched in far-red (FR; Smith 2000; Franklin and Whitelam 2005). Even before direct shading takes place, FR light reflected from neighbouring plants lowers the R:FR light ratio, acting as an early signal of neighbour proximity (Casal et al. 1986; Ballaré et al. 1990). Later, the depletion of red and blue light signals a transition from neighbour detection to real competition (de Wit et al. 2016). The natural R:FR ratio varies from 1 to 1.2 in sunlight above a canopy, gradually decreasing to <0.2 under a dense canopy (Gundel et al. 2014). Changes in the R:FR light ratio are detected by signal-transducing photoreceptors, phytochromes, and result in morphological responses termed the ‘shade avoidance syndrome’. These responses include a strong elongation of stem-like organs, upward orientation of leaves (hyponasty), reduced branching or tillering and early flowering (Smith and Whitelam 1997; Ballaré and Casal 2000; Franklin 2008).
While shade avoidance increases the fitness of an individual in a crowded plant population (Schmitt et al. 1995), helping it to reach the canopy and avoid being shaded by neighbours, it is likely to reduce population yield of crops, as the elongation of the plants is achieved at the expense of leaf area, tillering and root growth (Casal et al. 1986; Kasperbauer and Karlen 1994; Ruberti et al. 2012; but see Ballaré et al. 1991). Reduced allocation of resources to roots has a negative impact on the anchoring capacity of the crop plants and increases the risk of lodging (Sparkes and King 2008) and water depletion. In line with this, several studies have suggested that reduced shade avoidance responses could increase cereal crop yields (Smith 1992; Holt 1995; Sawers et al. 2005; Carriedo et al. 2016).
In addition to these direct effects, we have argued that the shade avoidance response is detrimental to a crop’s ability to suppress weeds at high density. Cereals have greater potential to suppress weeds than previously thought and this potential can be realized by increasing the density and spatial uniformity of the crop (Weiner et al. 2001). While high plant density offers increased weed suppression, the earlier competition triggers earlier shade avoidance responses. The upward orientation of the plant leaves and reduced tillering under shade avoidance allow light to penetrate deeper into the canopy, thereby reducing the shading of weeds by the crop population. Consequently, we hypothesize that reduced shade avoidance by the crop will increase weed suppression at high density (Weiner et al. 2010), because shade avoidance is an individual, defensive strategy to avoid being shaded, while weed suppression at high density is an offensive, group strategy to shade weeds when they are still small.
Thus, reduced shade avoidance offers a promising target for breeding, since wild type shade avoidance is an evolutionary mechanism favoured by natural selection because it benefits the individual, but at a cost for the population (Weiner et al. 2010; Denison 2012). The aim of this study was to explore the possibility of developing spring wheat plants with reduced shade avoidance responses. As knowledge of the genetic control of shade avoidance in wheat remains limited, we used a traditional forward genetic screening of EMS mutagenized material. Such an approach could generate new phenotypes with altered light signalling downstream of the primary light receptors and provide new molecular handles to alter plant reactions to changes in the light spectrum.
Methods
Plant material and growth conditions
The glasshouse experiments were conducted in 2009–12 (Table 1) at Frederiksberg (55°41′N, 12°32′E) and the climate chamber work was located at Taastrup, University of Copenhagen, Denmark. The initial seed material consisted of selfed and bulk harvested M2 seeds from six spring wheat cultivars (Amaretto, Vinjett, Triso, CPBT W93, Dragon and Dacke), treated with three concentrations of ethyl methanesulfonate (0.038 M, 0.075 M and 0.15 M; M0880-100G, Sigma, Copenhagen, Denmark). Seeds were sown in trays (TEKU JP3050/230H and VEFI PK60) or in pots (Pöppelmann Plastik Skandinavien Aps, Odense, Denmark) in sphagnum substrate (Pindstrup Substrate 1&7; Pindstrup Mosebrug A/S, Ryomgaard, Denmark) and watered with 2 g of Topsin WG (Tiofanatmetyl 700 g kg−1, Nordisk Alkali AB, Malmoe, Sweden) dissolved in 10 L of water, to deter fungal infections. Seedlings were sub-irrigated (greenhouse) or drip-irrigated (growth chambers) with a full nutrient solution (‘Gødningsblanding’, Plant Facilties, Department of Plant and Environmental Sciences, University Copenhagen) at pH 5.5. The glasshouses provided supplementary light from 600 W SON-T Green Power lamps (E40, Philips, Eindhoven, Netherlands) 16 h a day, unless otherwise stated. The day/night temperatures were 15/12 °C and ventilation started when the temperature increased 3 °C above the set point.
Table 1.
Summary of the selection steps to obtain mutants with reduced shade avoidance.
| Experiments, R:FR | Time | Seeds | Mutants | Methods | Selected |
|---|---|---|---|---|---|
| Greenhouse screening 1, R:FR 0.65 | 2009 | M2 | 1000000 | Bulk trays, phenotype screening, green filters, reduced red and blue light | 13850 |
| Greenhouse screening 2, R:FR 0.65 | 2009 | M3 | 7200 | Mutant lines, phenotype screening green filters, reduced red and blue light | 248 |
| Greenhouse selection 1, R:FR 1.1 and 0.16 | 2010 | M4 | 163 lines | Measured traits, green and neutral filters, reduced red and blue light | 36 |
| Greenhouse selection 2, R:FR 1.1 and 0.16 | 2011 | M5 | 36 lines | Measured traits, green and neutral filters, reduced red and blue light | 5 |
| Climate chamber, R:FR 2.5, 0.9 and 0.4 | 2012 | M5 | 5 lines | Measured traits, far-red LED modules, added far-red light | 2 |
Many filters have been used in earlier shade avoidance experiments (Hiraut-Bron et al. 2001; Causin and Wulff 2003; Pierik et al. 2005; Weijschedé et al. 2006). In this study, LEE Pale green 138 filters were used for the initial visual screening in early spring, and LEE Fern green 122 filters and Lutrasil Pro 23 (neutral shade) were used in the subsequent experiments, carried out in the summer (LEE filters, Andover, UK and Lutrasil Pro 23 g m−2, Freudenberg Vliesstoffe KG, Germany). The Pale green 138 filter had a triple transmission peak in the range 495–555 nm, the Fern green 122 filter a double transmission peat in the range 510–535 nm, while the neutral shade reduced all wavelengths approximately equally in the range 400–750 nm.
Screening 1—visual selection of divergent phenotypes under green shading
Approximately 1 million mutated M2 seeds were sown in trays under green filters (LEE 138) that reduced the R:FR ratio (655–665:725–735 nm) from 1.1 to 0.65, and photosynthetic active radiation (PAR) by 40 %. Four weeks after sowing, at a 3–4 leaf stage, plants were screened for individuals that did not display characteristic traits for shade avoidance, that is rapid elongation of leaf sheaths and laminae, reduced chlorophyll concentration, leaf hyponasty and reduced tillering. The selected plants had shorter leaf sheaths and/or laminae and darker green leaves than other plants, and some had started tillering. These selected mutants were transplanted into 10 cm pots and moved outdoors in April. At maturity, the self-pollinated plants were harvested and threshed, and grains produced by each plant were kept separately.
Screening 2—visual selection for lines with homozygous mutations under green shading
In October 2009, the M3 seeds obtained from the previous screening were sown in rows of 10 seeds per parent plant on trays placed under green filters (LEE 138). The plants were screened as before, 4 weeks after sowing, at the 3–4 leaf stage, selecting for mutations for reduced elongation responses under green shading. The selected lines were transplanted into 10 cm pots, left to self-pollinate and later harvested and threshed.
Selection 1—selection for extreme elongation behaviour in low R:FR light
In June 2010, the shade avoidance responses of 163 selected mutant lines were compared to the four non-mutated cultivars they originated from. Two sets of 6 seeds per mutant line and 30 seeds per non-mutated cultivar were sown in 10 cm pots that were placed on two 16 m2 tables in the glasshouse. One table was covered with a LEE Fern green 122 filter that reduced the R:FR ratio from 1.13 to 0.16 and PAR by 60 %. The other table was covered with several layers of neutral Lutrasil Pro 23 fabric to reduce PAR by 60 % without changing the R:FR ratio. No supplemental light was used during the experiment. Both tables were divided into six blocks. Within each block, one plant of every mutant line and two plants of the non-mutated cultivars were placed in a random order on trays, 10 cm apart. Border plants were positioned around the table edges to reduce edge effects.
In two pilot experiments conducted in 2009 and 2010, the length of the first leaf sheath (soil surface to first leaf ligule) and the second leaf lamina (stalk to the tip of the leaf) resulted in the most reliable measures of early elongation responses. In this screening experiment, these measurements were taken once a week for 3 weeks, starting 3 weeks after sowing. It is known that reduced R:FR ratios suppress tillering in grasses (Casal et al. 1986; Evers et al. 2006). As tillering may enhance weed suppression at early stages, the number of tillers was recorded at the last measurement. Three weeks after the first sowing the experiment was repeated in all details.
The mutant lines were ranked according to their elongation response (difference in the length of the first leaf sheath and of the second leaf lamina between neutral and green shading), using random effects estimates (Robinson 2001). Based on the ranking of the lines and a phenotypic evaluation of the mature mutant plants, vigorous and seed-setting lines with a high variance in elongation responses were selected for the next experiment. To reduce the number of dwarf mutations and the number of lines, so they could be handled in pot experiments, lines were considered only if they had a final mean plant height of at least 60 % of the mean of the non-mutated cultivars.
Selection 2—selection for mutant lines with reduced elongation in low R:FR light
In June 2011, the selected 36 mutant lines were grown once more under green (LEE122) and neutral shade (Lutrasil Pro 23), and their elongation responses compared to the non-mutated cultivars. According to the availability of seeds, two sets of 5–30 seeds per each mutant line and 30 seeds per non-mutated cultivar were sown in 10 cm pots and placed in a fully randomized order on two glasshouse tables. No supplemental light was used in the experiment. Measurements were conducted exactly as the year before and the experiment was repeated in all respects 3 weeks after the sowing of the first experiment. The mutant lines were again ranked according to the difference in the length of the first leaf sheath and second leaf lamina under neutral versus green shading, using random effects estimates (Robinson 2001). Based on the ranking of the lines and a phenotypic evaluation of the mature mutant plants, four vigorous, seed-setting lines with very low elongation responses and one interesting strong tillering phenotype were selected for further testing.
Selection for mutant lines with reduced elongation in added FR light
In November 2011, the shade avoidance responses of the five most promising mutants were measured in a reduced R:FR light environment. Instead of reducing the R light with green filters, the plants were exposed to two levels of added FR light. The experiment was conducted in three climate chambers with a 3.3 m2 growing area (Conviron Walk-In, PGV 56, Winnipeg, Canada) equipped with eight metal-halide lamps (Osram Powerstar 400W/D Pro HQI-BT, Osram, Munich, Germany). In the first chamber, a R:FR light ratio of 0.4 was supplied by installing 12 FR LED production modules (Philips GreenPower LED150, 39 µmol m−2 s−1 for 33W, Philips, Eindhoven, the Netherlands) under HQI-BT lamps (as used in Pierik et al. 2005), in the second chamber a R:FR ratio of 0.9 was supplied using four modules. In a third chamber, the R:FR light ratio was 2.5 without added FR light. Wooden dummies were used to compensate for the different numbers of LED modules in the three chambers and to give even shade and PAR levels that were as close as possible viz: 348 ± 20.3, 327 ± 15.7 and 344 ± 20.1 µmol m−2 s−1 (average of 20 positions ± SD) in the three climate chambers, respectively. Measurements were taken with an AvaSpec-2048 spectrometer (Avantes, Apeldoorn, the Netherlands).
Ten 13 cm pots with three seeds per pot for each of the five mutant lines and their two parent cultivars were prepared for each chamber. After germination, the plants were thinned to one seedling per pot. The pots of each line were evenly distributed in the chambers on four trolleys and the order of the trolleys was changed three times a week. The plants received drip irrigation with full nutrient solution and were subjected to 16/8 h of light/dark period and 20/16 °C day/night temperatures.
Measurements of all leaf sheath and lamina lengths were taken once a week for 5 weeks after germination, and tillers were counted after the last measurement. The level of spike development was recorded three times a week for 10 weeks and disk samples for chlorophyll measurements were taken 11 weeks after sowing. After 14 weeks, the plants were harvested. From each chamber five plants per line were randomly taken for leaf area determination by scanning the leaves of five tillers per plant (LI-COR 3100 Area meter, LI-COR, Lincoln, NE). All plant material was dried in 80 °C for 24 h, after which leaf, stem and spike biomass were weighed.
Chlorophyll extraction
Four disks of 5 mm or two disks of 7 mm in diameter (depending on the width of the leaf) were punched from the centre of the third leaf of each plant. The samples were weighed, and placed in vials containing 3 mL dimethylformamide (DMF, Sigma–Aldrich, Brøndby, Denmark). The vials were placed in a dark fridge, in 4 °C for 24 h to extract chlorophyll. The absorbance of the chlorophyll solution was then measured with a spectrophotometer (Unicam UV1, Unicam, Cambridge, UK) at 647.0 and 664.5 nm, and chlorophyll a and b concentrations were calculated according to Inskeep and Bloom (1985).
Statistical analysis
Ranking of mutant lines with reference to treatment response was done by means of mixed linear models. Specifically, elongation of leaf sheaths and leaf laminae were modelled including main effects of R:FR ratios, parent/mutant status, cultivar and time of recording as well as random effects of line and line within treatment. From these models, random effects estimates of line within treatment were obtained by restricted maximum likelihood (REML) estimation. The change in random effects estimates within line due to treatment was used to rank the lines (Robinson 2001).
For the chosen mutant lines and their parental lines, leaf sheath and leaf lamina sizes above zero at different stages and over time were analysed with mixed linear models. This model included a combined effect of treatment and line adjusted for the combined effect of stage and time. A random effect of plant was included in the model to accommodate repeated measurements of the same plants. Post hoc comparisons of parents and mutants were based on REML estimation and adjusted for multiple testing by means of the single-step procedure (Hothorn et al. 2008). Reported P-values correspond to Z-tests and were evaluated at a 5 % significance level. All analyses were performed in R version 2.15 (www.r-project.org).
Results
Screening 1 and 2
The initial screening of approximately 1 million mutated seedlings under green filters resulted in the selection of 13850 individuals with reduced apical elongation, darker and broader leaves, shorter than average leaf sheaths or early tillering. Of the selected plants, 7200 individuals produced seeds which were sown in rows under a green filter to obtain mutant offspring with short stature phenotypes deviating from the parent cultivars. From these rows 248 lines were chosen and evaluated at maturity for vigour and seed set, resulting in 163 mutant lines used in subsequent experiments (Table 1).
Selection 1 and 2 with reduced red light
In the first selection experiment, 163 mutant lines and 4 non-mutated original cultivars were placed under green and neutral filters to select for mutant lines with different elongation responses to shading. In this experiment, most of the plants responded to green shading with strong elongation: the length of the first leaf sheath was on average 7.5 mm (P < 0.001) longer and the second leaf lamina 9.33 mm (P < 0.001) longer in plants grown under green than under neutral shade. The plants under neutral shade had more tillers (P < 0.001) than under green shade (Table 2). No significant difference was detected in the average elongation or tillering responses between the cultivars and the between the 163 mutants due to strong variation in responses of the mutants to reduced red light. After ranking the mutant lines according to their elongation response (difference between the random effects estimates of the first leaf sheath and second leaf lamina length between green and neutral shading) and evaluating the ranked lines at maturity for vigour and seed set, and discarding individuals shorter than 60% of the final height of the original cultivars, 36 mutant lines with extremely strong and extremely weak shade avoidance responses were selected for the second experiment.
Table 2.
Selection experiments in 2010 and 2011, in which mutated spring wheat plants and their parental cultivars were placed under green and neutral filters to study the elongation responses of their first leaf sheath and second leaf lamina and their tillering behaviour to vegetative shading.
| Difference | Estimate | SE | z value | Pr (>|z|) | Confidence interval | ||
|---|---|---|---|---|---|---|---|
| lower | upper | ||||||
| Selection 1 (2010) | |||||||
| Length of 1st leaf sheath (mm) | Neutral–green shade | −7.50 | 0.42 | −17.82 | <0.001 | −8.33 | −6.68 |
| Cultivar–mutant | 14.17 | 7.28 | 1.95 | 0.264 | −0.10 | 28.45 | |
| Length of 2nd leaf lamina (mm) | Neutral–green shade | −9.33 | 1.60 | −5.83 | <0.001 | −12.47 | −6.19 |
| Cultivar–mutant | 74.41 | 31.79 | 2.34 | 0.138 | 12.09 | 136.73 | |
| Number of tillers | Neutral–green shade | 0.50 | 0.03 | 17.38 | <0.001 | 0.44 | 0.56 |
| Cultivar–mutant | 0.17 | 0.07 | 2.36 | 0.087 | 0.03 | 0.31 | |
| Selection 2 (2011) | |||||||
| Length of 1st leaf sheath (mm) | Neutral–green shade | −4.07 | 0.51 | −7.91 | <0.001 | −5.08 | −3.06 |
| Cultivar–mutant | 3.32 | 4.19 | 0.79 | 0.983 | −4.89 | 11.53 | |
| Length of 2nd leaf lamina (mm) | Neutral–green shade | −9.54 | 2.30 | −4.15 | <0.001 | −14.04 | −5.03 |
| Cultivar–mutant | 21.55 | 21.98 | 0.98 | 0.939 | −21.54 | 64.63 | |
| Number of tillers | Neutral–green shade | 0.52 | 0.04 | 13.06 | <0.001 | 0.44 | 0.60 |
| Cultivar–mutant | 0.00 | 0.16 | −0.03 | 1.000 | −0.31 | 0.30 | |
In the second selection experiment, the elongation responses of these 36 mutant lines and the 4 non-mutated cultivars were ranked to select for the mutant lines with the lowest shade avoidance responses to reduced red light. In this experiment, most of the plants responded to the low R:FR ratio under the green filter with strong elongation of the first leaf sheath (4.07 mm, P < 0.001) and the second leaf lamina (9.54 mm, P = 0.001), and with reduced tillering (P < 0.001, Table 2). After ranking the mutant lines according to their elongation responses (Table 3) four promising Amaretto mutant lines (A9, A20, A48 and A151) were selected because they displayed less elongation of the second leaf lamina. In two of these mutants, the first leaf sheaths also elongated less under green shading than non-mutated Amaretto whilst having only a slightly reduced mature plant height. An interesting mutant of Triso (T104) was also chosen for further testing due to its strongly increased tillering under green shade, even though it elongated more than the non-mutated Triso (Table 3).
Table 3.
Mutant lines from the selection experiment in 2011, ranked according to the difference in elongation of the plants’ first leaf sheath (a) and the second leaf lamina length (b) under green and neutral shade using random effects estimates.
| (a) First leaf sheath | (b) Second leaf lamina | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Cultivar | Line | Type | Difference green–neutral | Plant height (mm) | Cultivar | Line | Type | Difference green–neutral | Plant height (mm) |
| Amaretto | 151 | −1.27 | 678 | Amaretto | 151 | −1.18 | 678 | ||
| 48 | −0.46 | 668 | 48 | −0.95 | 668 | ||||
| 164 | Parent | −0.05 | 730 | 133 | −0.91 | 698 | |||
| 133 | −0.02 | 698 | 150 | −0.47 | 710 | ||||
| 20 | −0.01 | 732 | 9 | −0.34 | 670 | ||||
| 9 | 0.14 | 670 | 20 | −0.27 | 732 | ||||
| 128 | 0.42 | 641 | 128 | 0.00 | 641 | ||||
| 50 | 0.44 | 610 | 6 | 0.57 | 764 | ||||
| 150 | 0.46 | 710 | 50 | 0.70 | 610 | ||||
| 39 | 0.58 | 440 | 5 | 0.85 | 500 | ||||
| 130 | 0.68 | 723 | 164 | Parent | 0.89 | 730 | |||
| 6 | 0.84 | 764 | 39 | 1.00 | 440 | ||||
| 5 | 1.35 | 500 | 130 | 1.43 | 723 | ||||
| Vinjett | 140 | −1.79 | 599 | Vinjett | 157 | −1.61 | 589 | ||
| 157 | −0.85 | 589 | 153 | −1.09 | 618 | ||||
| 142 | −0.38 | 631 | 139 | −0.57 | 619 | ||||
| 153 | 0.04 | 618 | 140 | −0.25 | 599 | ||||
| 165 | Parent | 0.24 | 713 | 142 | −0.17 | 631 | |||
| 159 | 0.27 | 688 | 165 | Parent | 0.35 | 713 | |||
| 65 | 0.73 | 578 | 159 | 0.50 | 688 | ||||
| 139 | 1.42 | 619 | 65 | 1.34 | 578 | ||||
| 55 | 1.47 | 569 | 59 | 1.36 | 323 | ||||
| 59 | 2.58 | 323 | 55 | 1.81 | 569 | ||||
| Triso | 160 | −1.05 | 500 | Triso | 162 | −1.81 | 396 | ||
| 166 | Parent | −0.89 | 688 | 105 | −1.08 | 619 | |||
| 162 | −0.57 | 396 | 103 | −0.96 | 485 | ||||
| 107 | −0.49 | 663 | 95 | −0.62 | 606 | ||||
| 103 | −0.42 | 485 | 166 | Parent | −0.38 | 688 | |||
| 145 | −0.41 | 460 | 160 | −0.14 | 500 | ||||
| 95 | −0.32 | 606 | 107 | −0.07 | 663 | ||||
| 93 | −0.27 | 532 | 93 | 0.02 | 532 | ||||
| 105 | −0.12 | 619 | 97 | 0.43 | 557 | ||||
| 97 | 0.10 | 557 | 163 | 0.79 | 539 | ||||
| 101 | 0.82 | 677 | 145 | 1.14 | 460 | ||||
| 104 | 1.14 | 636 | 101 | 1.61 | 677 | ||||
| 163 | 1.52 | 539 | 104 | 1.73 | 636 | ||||
| CPBT-W93 | 115 | −2.42 | 548 | CPBT-W93 | 167 | Parent | −1.48 | 613 | |
| 167 | Parent | −1.59 | 613 | 115 | −1.43 | 548 | |||
| 117 | −1.30 | 474 | 117 | −0.85 | 474 | ||||
| 119 | −0.57 | 636 | 119 | 0.12 | 636 | ||||
Climate chamber experiment with added far-red light
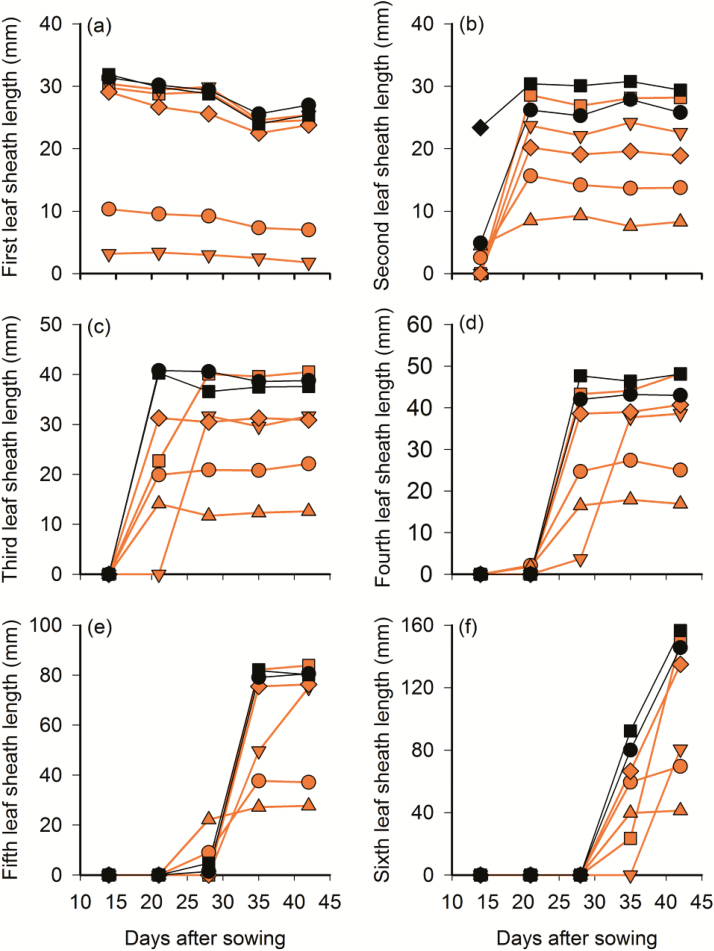
When the five mutants showing reduced shade avoidance behaviour and the non-mutated cultivars were placed in climate chambers with supplemental FR light, four of the five mutants showed reduced leaf sheath elongation in the lowest R:FR ratio compared to the non-mutated cultivars (Fig. 1). Differences in leaf sheath length between mutant and wild type was most pronounced for mutant lines A9 and T104 (20.98 and 31.30 mm) but statistically significant also in lines A48 and A151 (10.84 and 6.61 mm, Table 4).
Figure 1.
Leaf sheath growth patterns of five spring wheat mutant lines and their non-mutated parent cultivars in added FR light (R:FR 0.4). Mutants depicted in orange and non-mutated cultivars in black lines and symbols. Red circles: A9, red squares: A20, inverted triangles: A48, triangles: T104, diamonds: A151, black circles: Amaretto, black squares: Triso.
Table 4.
Leaf sheath and lamina lengths of five selected spring wheat mutant lines compared with non-mutated cultivars of their origin when grown with enhanced FR light, n = 10.
| R:FR | Estimate (mm) | SE | Pr (>|z|) | Confidence interval | ||
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| Leaf sheaths | ||||||
| A9—Amaretto | 2.5 | −16.69 | 1.570 | <0.001 | −19.77 | −13.62 |
| 0.9 | −17.24 | 1.573 | <0.001 | −20.32 | −14.15 | |
| 0.4 | −20.98 | 1.579 | <0.001 | −24.07 | −17.88 | |
| A20—Amaretto | 2.5 | 1.67 | 1.583 | 0.987 | −1.43 | 4.77 |
| 0.9 | 1.42 | 1.554 | 0.997 | −1.63 | 4.46 | |
| 0.4 | −4.03 | 1.521 | 0.105 | 7.01 | −1.05 | |
| A48—Amaretto | 2.5 | −1.31 | 1.647 | 0.999 | −4.54 | 1.92 |
| 0.9 | −6.15 | 1.608 | 0.002 | −9.30 | −2.99 | |
| 0.4 | −10.84 | 1.581 | <0.001 | −13.94 | −7.74 | |
| T104—Triso | 2.5 | −20.94 | 1.601 | <0.001 | −24.08 | −17.81 |
| 0.9 | −27.94 | 1.577 | <0.001 | −31.03 | −24.85 | |
| 0.4 | −31.30 | 1.561 | <0.001 | −34.36 | −28.24 | |
| A151—Amaretto | 2.5 | −3.77 | 1.577 | 0.203 | −6.86 | −0.68 |
| 0.9 | −4.22 | 1.539 | 0.082 | −7.23 | −1.20 | |
| 0.4 | −6.61 | 1.514 | <0.001 | −9.58 | −3.64 | |
| Leaf laminas | ||||||
| A9—Amaretto | 2.5 | −44.23 | 5.500 | <0.001 | −55.01 | −33.45 |
| 0.9 | −33.05 | 5.549 | <0.001 | −43.93 | −22.18 | |
| 0.4 | −38.65 | 5.531 | <0.001 | −49.49 | −27.81 | |
| A20—Amaretto | 2.5 | −17.86 | 5.531 | 0.016 | −28.70 | −7.02 |
| 0.9 | −11.07 | 5.503 | 0.391 | −21.85 | −0.28 | |
| 0.4 | −10.15 | 5.471 | 0.507 | −20.87 | 0.58 | |
| A48—Amaretto | 2.5 | −60.13 | 5.643 | <0.001 | −71.19 | −49.07 |
| 0.9 | −48.13 | 5.618 | <0.001 | −59.14 | −37.12 | |
| 0.4 | −58.55 | 5.547 | <0.001 | −69.43 | −47.68 | |
| T104—Triso | 2.5 | −85.27 | 5.475 | <0.001 | −96.00 | −74.54 |
| 0.9 | −92.43 | 5.431 | <0.001 | −103.07 | −81.79 | |
| 0.4 | −76.86 | 5.428 | <0.001 | −87.50 | −66.22 | |
| A151—Amaretto | 2.5 | −13.72 | 5.539 | 0.146 | −24.58 | −2.86 |
| 0.9 | −7.24 | 5.492 | 0.884 | −18.01 | 3.52 | |
| 0.4 | −13.17 | 5.448 | 0.169 | −23.84 | −2.49 | |
The mutants A48 and A151 showed no significant difference in leaf sheath growth from the non-mutated lines in R:FR 2.5, but displayed signs of reduced elongation at R:FR 0.9 and responded with significantly reduced elongation to low (0.4) R:FR light (68.9 % and 20.6 % total reduction in elongation compared to parental lines, Table 4, Fig. 2). Although these mutants reached a slightly shorter final height in near neutral light (R:FR 0.9) than did non-mutated cultivars (A48: 11.4 % and A151: 6.8 %, Table 6) their reduced elongation was clearly a gradual response to increased shade (Table 4). The leaf lamina responses of these two mutants differed. While the leaf laminae of A48 were shorter than those of non-mutated plants in all light treatments (P < 0.001, Table 4) those of A151 did not differ from the non-mutated plants in any of the treatments. The lack of a gradual response to shading in the length of the second leaf lamina in added FR light, while evident in the green filter experiment (Table 3), may be due to the stronger reduction of the R:FR ratio under the green filters (R:FR 0.16) than in the climate chambers (R:FR 0.4)
Figure 2.
Selected mutant lines A9, A20, A48, T104 and A151 and two non-mutated cultivars Amaretto and Triso, grown in R:FR 2.5, 0.9 and 0.4. Photos were taken 8 weeks after sowing. Plants grown in R:FR 0.4 show a faster development, earlier spike emergence and anthesis than plants in R:FR 2.5.
Table 6.
Growth parameters of mutants and non-mutated cultivars grown in three R:FR light regimes in climate chambers. Measures taken 14 weeks after sowing, n = 10.
| R:FR | Mutant, cultivar | Plant height (cm) | Total dry matter (g) | Stem weight (g) | Leaf weight (g) | Leaf area (cm2) | Leaf:stem ratio | Number of spikes | Chlorophyll a:b ratio | Total chlorophyll (µg m−2) |
|---|---|---|---|---|---|---|---|---|---|---|
| A9 | 73.0 ± 9.9 | 42.9 ± 17.1 | 20.0 ± 6.2 | 10.5 ± 1.6 | 1882.6 ± 300.7 | 0.58 ± 0.20 | 15.4 ± 5.1 | 4.7 ± 0.82 | 0.48 ± 0.24 | |
| A20 | 85.3 ± 4.5 | 52.2 ± 10.3 | 23.8 ± 4.6 | 8.7 ± 1.4 | 1273.5 ± 197.2 | 0.37 ± 0.06 | 16.5 ± 1.6 | 3.6 ± 0.31 | 0.93 ± 0.12 | |
| A48 | 71.9 ± 5.5 | 28.1 ± 6.8 | 15.5 ± 4.5 | 7.6 ± 2.0 | 1211.6 ± 88.1 | 0.53 ± 0.17 | 12.5 ± 2.7 | 3.7 ± 0.39 | 0.74 ± 0.12 | |
| 2.5 | T104 | 73.4 ± 15.3 | 32.1 ± 12.6 | 19.0 ± 7.8 | 7.9 ± 2.3 | 1755.6 ± 577.5 | 0.47 ± 0.18 | 19.9 ± 10.3 | 4.2 ± 0.16 | 0.48 ± 0.08 |
| A151 | 80.8 ± 7.1 | 54.4 ± 13.5 | 23.9 ± 6.5 | 8.4 ± 1.9 | 1053.7 ± 256.5 | 0.37 ± 0.09 | 16.2 ± 3.9 | 3.8 ± 0.26 | 0.81 ± 0.12 | |
| Amaretto | 92.9 ± 6.8 | 67.4 ± 3.4 | 24.5 ± 1.7 | 7.2 ± 1.3 | 1096.9 ± 101.0 | 0.29 ± 0.04 | 16.0 ± 1.8 | 3.1 ± 0.17 | 1.05 ± 0.07 | |
| Triso | 93.5 ± 6.9 | 65.9 ± 13.7 | 23.8 ± 5.2 | 6.4 ± 1.0 | 1007.2 ± 167.6 | 0.28 ± 0.06 | 16.6 ± 2.3 | 3.1 ± 0.37 | 1.07 ± 0.12 | |
| A9 | 84.7 ± 8.7 | 58.8 ± 14.1 | 23.9 ± 4.5 | 10.3 ± 1.6 | 1658.1 ± 221.4 | 0.44 ± 0.07 | 17.1 ± 1.9 | 3.7 ± 0.46 | 0.68 ± 0.19 | |
| A20 | 86.1 ± 5.6 | 53.9 ± 12.6 | 22.1 ± 2.9 | 7.4 ± 0.7 | 930.9 ± 107.3 | 0.34 ± 0.03 | 16.7 ± 2.2 | 3.0 ± 0.20 | 0.98 ± 0.09 | |
| A48 | 83.2 ± 3.5 | 36.3 ± 6.9 | 17.9 ± 3.6 | 6.7 ± 0.7 | 910.5 ± 27.5 | 0.38 ± 0.05 | 10.5 ± 1.4 | 3.0 ± 0.21 | 0.86 ± 0.05 | |
| 0.9 | T104 | 89.9 ± 5.3 | 44.1 ± 8.6 | 23.6. ± 5.1 | 7.4 ± 1.3 | 1399.4 ± 229.0 | 0.31 ± 0.02 | 22.3 ± 4.8 | 4.0 ± 0.20 | 0.48 ± 0.05 |
| A151 | 87.5 ± 5.7 | 52.2 ± 3.6 | 19.1 ± 2.4 | 6.3 ± 1.0 | 892.4 ± 152.2 | 0.33 ± 0.03 | 13.7 ± 1.9 | 3.2 ± 0.22 | 0.88 ± 0.08 | |
| Amaretto | 93.9 ± 7.1 | 59.7 ± 10.2 | 18.0 ± 5.3 | 5.5 ± 1.3 | 825.1 ± 219.1 | 0.33 ± 0.14 | 15.1 ± 3.3 | 2.9 ± 0.29 | 1.02 ± 0.12 | |
| Triso | 97.3 ± 5.5 | 64.4 ± 6.6 | 21.0 ± 2.3 | 5.5 ± 0.6 | 820.3 ± 105.0 | 0.26 ± 0.03 | 16.3 ± 3.6 | 3.1 ± 0.15 | 1.00 ± 0.04 | |
| A9 | 88.4 ± 6.6 | 51.9 ± 23.6 | 22.0 ± 7.2 | 8.1 ± 1.3 | 1219.0 ± 94.3 | 0.39 ± 0.09 | 13.9 ± 2.3 | 3.6 ± 0.44 | 0.57 ± 0.18 | |
| A20 | 92.7 ± 2.9 | 62.1 ± 11.9 | 27.1 ± 5.1 | 8.3 ± 1.1 | 1067.5 ± 194.3 | 0.31 ± 0.05 | 15.7 ± 2.8 | 3.0 ± 0.25 | 0.93 ± 0.14 | |
| A48 | 86.5 ± 3.4 | 37.1 ± 6.6 | 18.9 ± 3.4 | 6.2 ± 0.9 | 797.3 ± 46.8 | 0.33 ± 0.03 | 9.6 ± 0.5 | 2.7 ± 0.16 | 0.87 ± 0.10 | |
| 0.4 | T104 | 89.7 ± 6.3 | 39.8 ± 10.3 | 20.4 ± 4.9 | 5.4 ± 1.1 | 880.4 ± 119.1 | 0.27 ± 0.03 | 18.0 ± 3.9 | 3.6 ± 0.27 | 0.51 ± 0.08 |
| A151 | 88.8 ± 4.8 | 58.7 ± 14.9 | 20.4 ± 5.6 | 6.6 ± 1.1 | 740.5 ± 264.2 | 0.34 ± 0.09 | 14.2 ± 3.0 | 3.0 ± 0.11 | 0.87 ± 0.08 | |
| Amaretto | 100.0 ± 4.1 | 75.2. ± 6.2 | 26.1 ± 1.4 | 6.5 ± 0.6 | 1008.3 ± 217.1 | 0.25 ± 0.03 | 15.2 ± 2.4 | 2.9 ± 0.24 | 0.91 ± 0.15 | |
| Triso | 99.8 ± 4.5 | 71.7 ± 9.8 | 24.2 ± 3.0 | 5.3 ± 0.8 | 740.5 ± 120.5 | 0.22 ± 0.02 | 14.9 ± 1.9 | 3.0 ± 0.37 | 0.92 ± 0.19 |
In near neutral light (R:FR 0.9), A48 had, on average, a 10.4 % larger leaf area than non-mutated Amaretto, in spite of having a fewer tillers (10.5 vs. 15.1). It also had thick, dark green leaves and the ears emerged on average 11 days later than those of the non-mutated Amaretto (Table 5). The mutant A151 on the other hand strongly resembled the phenotype of the non-mutated Amaretto (Fig. 2), having similar leaf area (8.2% larger), leaf to stem ratio (0.3) and number of tillers (13.7 vs. 15.1) as the non-mutated Amaretto (Table 6, Fig. 2).
Table 5.
Onset of spike emergence for spring wheat grown in climate chambers in three R:FR light regimes, recorded for 70 days after sowing, n = 10.
| Spike emergence | |||
|---|---|---|---|
| Mutant, cultivar | R:FR 2.5 | R:FR 0.9 | R:FR 0.4 |
| Days ± SE | Days ± SE | Days ± SE | |
| A9 | 67 ± 2.50 | 60 ± 4.06 | 59 ± 4.65 |
| A20 | 61 ± 1.93 | 54 ± 0.84 | 53 ± 1.58 |
| A48 | 69 ± 1.15 | 62 ± 2.21 | 61 ± 0.63 |
| T104 | >70 | 68 ± 0.00 | 66 ± 2.79 |
| A151 | 63 ± 2.59 | 55 ± 1.69 | 52 ± 2.04 |
| Amaretto | 58 ± 4.44 | 51 ± 2.80 | 51 ± 2.42 |
| Triso | 57 ± 1.91 | 52 ± 1.85 | 50 ± 0.97 |
The mutant lines A9 and T104 had significantly shorter leaf sheaths and laminae than the non-mutated cultivars in all three light treatments (P < 0.001, Table 4). These two lines resembled a wheat phenotype with shorter internode segments along its culm that has been characterized as Rht8 semi-dwarf mutant with reduced cell elongation and reduced sensitivity to brassinosteroids (Gasperini et al. 2012). Differences in leaf sheath and lamina length of lines A9 and T104 compared to non-mutated plants were not explicitly triggered by the reduced R:FR ratio. Both lines also had a much larger leaf area than the non-mutated cultivars in near neutral light (A9: 101 % and T104: 70.6 %), but their leaf area was strongly reduced by the FR light (Table 6). Both mutant lines produced thin and pale leaves, with a lower concentration of chlorophyll than the non-mutated cultivars (33.3 % and 52 %, respectively at R:FR 0.9, Table 6, Fig. 2). Line T104 in particular had narrow leaves and a profusely tillering phenotype with many spikes (22 vs. 16 in non-mutated cultivars). Finally, mutant A20 showed no significant difference in elongation behaviour to the non-mutated Amaretto (Table 4). In near-neutral light, the plants were on average only 8.3 % smaller at harvest, had 9.7 % lower total dry biomass and 3.9 % lower chlorophyll concentration in their leaves than the non-mutated Amaretto plants (Table 6, Fig. 2).
In general, added FR light in the climate chambers significantly increased the final height of the plants (10.67 cm, P < 0.001) but reduced leaf area (−402.97 cm2, P < 0.001) and leaf weight (−1.46 g, P < 0.001) and thus also the leaf to stem ratio (−0.11, P < 0.001, Table 7). Plants in added FR light also showed a significant reduction in the chlorophyll a:b ratio (−0.63, P < 0.001, Table 7), indicating the enlargement of the chlorophyll antenna size (Webb and Melis 1995; Neidhardt et al. 1998).
Table 7.
Differences in the growth and yield parameters of five selected spring wheat mutant lines and the non-mutated cultivars of their origin in response to different R:FR light regimes and in response to mutation, n=10
| Measured traits | Compared treatments | Estimate | Std. Error | Pr(>|z|) | Confidence | interval |
|---|---|---|---|---|---|---|
| lower | upper | |||||
| Plant height (cm) | R:FR (0.9–2.5) | 7.37 | 1.707 | <0.001 | 4.03 | 10.72 |
| R:FR (0.4–2.5) | 10.67 | 1.707 | <0.001 | 7.33 | 14.02 | |
| cultivar-mutant | 12.11 | 2.268 | <0.001 | 7.67 | 16.56 | |
| Leaf area (cm2) | R:FR (0.9–2.5) | -263.20 | 85.540 | 0.008 | -430.85 | -95.55 |
| R:FR (0.4–2.5) | -402.97 | 85.380 | <0.001 | -570.31 | -235.62 | |
| cultivar-mutant | -263.18 | 216.620 | 0.590 | -687.77 | 161.40 | |
| Leaf weight (g) | R:FR (0.9–2.5) | -1.09 | 0.360 | 0.009 | -1.79 | -0.38 |
| R:FR (0.4–2.5) | -1.46 | 0.360 | <0.001 | -2.16 | -0.75 | |
| cultivar-mutant | -1.64 | 0.911 | 0.232 | -3.43 | 0.14 | |
| Stem weight(g) | R:FR (0.9–2.5) | -0.73 | 1.369 | 0.959 | -3.41 | 1.96 |
| R:FR (0.4–2.5) | 1.20 | 1.369 | 0.802 | -1.48 | 3.89 | |
| cultivar-mutant | 1.78 | 1.848 | 0.748 | -1.84 | 5.40 | |
| Leaf to stem ratio | R:FR (0.9–2.5) | -0.07 | 0.026 | 0.022 | -0.12 | -0.02 |
| R:FR (0.4–2.5) | -0.11 | 0.026 | <0.001 | -0.16 | -0.06 | |
| cultivar-mutant | -0.11 | 0.044 | 0.035 | -0.20 | -0.03 | |
| Total dry matter(g) | R:FR (0.9–2.5) | 3.53 | 1.731 | 0.144 | 0.14 | 6.93 |
| R:FR (0.4–2.5) | 7.60 | 1.731 | <0.001 | 4.20 | 10.99 | |
| cultivar-mutant | 22.42 | 7.520 | 0.024 | 5.80 | 35.28 | |
| Total chlorophyll (µg/cm-2) | R:FR (0.9–2.5) | 0.05 | 0.034 | 0.416 | -0.02 | 0.12 |
| R:FR (0.4–2.5) | 0.01 | 0.034 | 0.999 | -0.06 | 0.07 | |
| cultivar-mutant | 0.26 | 0.147 | 0.260 | 0.03 | 0.54 | |
| chlorophyll a to b ratio | R:FR 0.9 | -0.48 | 0.114 | <0.001 | -0.71 | -0.26 |
| R:FR 0.4 | -0.63 | 0.114 | <0.001 | -0.85 | -0.41 | |
| cultivar-mutant | -0.50 | 0.308 | 0.329 | -1.10 | 0.11 | |
| Number of spikes | R:FR 0.9 | -0.01 | 0.042 | 0.994 | -0.10 | 0.07 |
| R:FR 0.4 | -0.11 | 0.042 | 0.048 | -0.19 | -0.02 | |
| cultivar-mutant | 0.03 | 0.139 | 0.999 | -0.24 | 0.30 |
In summary, two of the final mutant lines showed significantly reduced shade avoidance responses in low R:FR light responding to light composition with respect to leaf sheath elongation. Leaf lamina elongation showed reduced responses only in the stronger shade treatment (green filter). Of these two lines, A151 showed a 20.6 % reduced leaf sheath elongation compared to the non-mutated Amaretto plants and is the most promising candidate for further studies. While A48 showed an even stronger reduction in elongation (68.9%), it differed from the non-mutated cultivar in several aspects (extremely low early vigour, delayed development, thicker and shorter leaves and reduced number of tillers). A151, on the other hand, strongly resembled the original cultivar throughout its development, reaching almost the same final height but without a rapid elongation response to shading (Fig. 2).
Discussion
Shade avoidance in crops
It could be expected that half a century of breeding for short cultivars with a high reproductive output would already have selected against shade avoidance responses in modern wheat germplasm, since there is a trade-off between elongation responses and yield. However, a study of 20th century Argentinian wheat cultivars showed that selection for higher yield has not reduced shade avoidance responses. On the contrary, the magnitude of the responses is greater in modern than in older cultivars (Ugarte et al. 2010). Our results, particularly those from the climate chambers, support these findings.
The original cultivars in our experiments responded with strong elongation to both reduced R and to increased FR light. Thus, it seems that in spite of the introduction of dwarfing genes to more than 70 % of current commercial wheat varieties (Gale et al. 1985; Evans 1998) these plants still maintain their ability for rapid elongation in shade. This may not have been of great practical significance because of suppression of shade-induced elongation through the use of stem-shortening growth regulators and routine application of herbicides. This may have lessened the urgency for the development of cultivars with weak shade avoidance responses when grown at high density. Increases in the yields of many cereals achieved over the last 60 years through breeding have been in large part based on selecting short cultivars with higher harvest index and tolerance of high sowing densities (Fischer and Edmeades 2010). Furthermore, under a given set of growth conditions, the yield of a cultivar is not only affected by management and plant density but also by competition for resources from weeds, which are more efficiently suppressed by higher crop densities (Weiner et al. 2001). The use of agrochemicals such as stem-shortening growth regulators is being questioned in many countries. Consequently, the development of cultivars with low shade avoidance responses is becoming more desirable and is likely to make a contribution to raising grain yield in future years.
More than 20 semi-dwarfing loci (Rht) and 25 alleles are known to be associated with the semi-dwarf growth habit of wheat (Konzak 1987). Though the reduced height of the plants has allowed the partitioning of more assimilates to the developing grains (Evans 1993), dwarfing alone may not eliminate seed yield loss due to density-triggered, light-induced growth elongation. Thus, the objective of this study was not to reduce the size of the cultivars per se, but to diminish the plants’ plasticity in response to light quality, especially at early growth stages, thus reducing the yield reduction caused by shade-induced elongation. In other words, we wish to preserve the height and form of plants grown at low densities when they are grown at the high densities typical of modern cereal farming.
In recent decades, much progress has been made in understanding the complex interactions of light receptors, transcription factors and plant hormones involved in shade avoidance through studying mutants of Arabidopsis thaliana. In addition to phytochromes, blue light sensing cryptochromes and phototropins and plant hormones, notably gibberellic acid, ethylene, brassinosteroids and auxin also play a role in regulating the growth and development of shaded plants (McCormac et al. 1992; Chen et al. 2004; Pierik et al. 2004; Vandenbussche et al. 2005; Franklin 2008; Xu et al. 2015; de Wit et al. 2016). However, extending the findings of a dicot model species such as Arabidopsis to monocot crop plants presents major challenges. Extensive genome rearrangements, duplication events and lost genes between monocot and dicot lineages render the identification of orthologous relationships difficult (Bennetzen 2007). While many eco-physiological experiments on the effects of shade avoidance have been conducted in crop species (Ballaré et al. 1987, 1991; Ghersa et al. 1994), relatively little is known about the genetic light signal transduction networks in monocots (Mathews and Sharrock 1996; Sawers et al. 2005), particularly in allohexaploid wheat.
Light treatments for shade avoidance experiments
The spectral light environment changes gradually during crop growth. Sunlight contains blue, red and far-red light at high intensities and the R:FR ratio is just above one (Gundel et al. 2014). When plants start to sense near neighbours, they are responding to a R:FR ratio that has decreased to <1 due to reflection of FR from nearby plants (de Wit et al. 2016). In dense shade, the R:FR can be as low as 0.2 or less (Gundel et al. 2014). As competition for light increases, the canopy closes and PAR decreases, blue light will decrease to levels in which signalling through the blue light-absorbing cryptochrome occurs (de Wit et al. 2016). Thus, during crop establishment, the entire plant is exposed to R:FR >1 but when the canopy closes, the ratio gradually decreases due to first reflection from neighbours, and later, to transmission of light through leaves. However, cereal crops are grown in open environments so the top of the canopy will always be exposed to a high R:FR ratio. This complicates matters when it comes to simulating shade in experimental setups.
In our first two screenings we used green filters resulting in modest decrease in the R:FR ratio (to 0.65). This eliminated the most severely dwarfed mutants and reduced the number to a manageable set of 36 for more detailed selection experiments. To further separate mutants with different shade avoidance responses, morphological studies were performed, and the green filter was changed to decrease the R:FR ratio to 0.16. A neutral grey filter with the same reduction of PAR was used as control.
In both the screening and selection experiments, the green filter decreased blue irradiance in the top of the canopy and this could also have enhanced the response to low R:FR (de Wit et al. 2016). However, with a very large number of mutants to deal with at the initial stages this compromise was considered acceptable. In the last experiment where only five mutants and two parent cultivars were involved, it became possible to avoid any blue light effect by enhancing FR intensities directly in climate chambers using metal halide lamps with a higher R:FR ratio than natural sunlight. This ensured a maximum photo-conversion of the phytochrome system from the physiologically inactive Pr form that absorbs red light to the physiologically active Pfr form that absorbs FR and prevents FR induced elongation growth (Xu et al. 2015). The use of LED modules to create a low R:FR ratio enabled us to create light environments with similar PAR and maintain high irradiance of blue wavelengths on the top of the canopy, while creating three levels of R:FR.
In the field, an entire plant will experience reduction of R:FR and blue light from the top of the canopy only if it is growing under a tall canopy. When working with crops that are grown in full sun, it would be difficult if not impossible to artificially create a light environment where both the R:FR ratio and blue light are high at the top of the canopy. Therefore, as in all controlled experiments using filters or FR light sources, our results may be slightly stronger than those we would expect in the field. We know only one previous study of how a gradient of R:FR down through a canopy affects shoot elongation (Weijschedé et al. 2006)
Reducing shade avoidance responses
Two successful strategies used to investigate shade avoidance have been the overexpression of phytochrome photoreceptors (Keller et al. 1989; Clough et al. 1995; Jordan et al. 1995) and the study of phytochrome deficient plants (Whitelam and Smith 1991; Childs et al. 1997; Sawers et al. 2002; Sheehan et al. 2007). Due to the intricate light and hormonal signalling networks, these approaches carry a high probability of undesirable pleiotropic effects. Furthermore, morphological responses to heterologous expression of PHY phytochrome genes can vary strongly between (Sawers et al. 2005) and within species (Kay et al. 1989; Nagatani et al. 1991; Kebrom and Brutnell 2007), making it difficult to apply conclusions drawn from one species to another.
There have been to date few studies on phytochromes and the transgenic overexpression of phytochrome photoreceptors in wheat (Carr-Smith et al. 1994). It has been shown that the overexpression of the oat PHYA gene in wheat inhibits coleoptile elongation in continuous FR light, increases the synthesis of anthocyanin and promotes leaf unrolling (Shlumukov et al. 2001; Sineshchekov et al. 2001). There is no information available concerning how these transgenic plants develop and what growth characteristics they possess, however.
Reduced leaf sheath and lamina lengths in low R:FR light have also been used to measure effects in tobacco and tomato overexpressing oat PHYA (Boylan and Quail 1989; Robson et al. 1996) and in rice mutants overexpressing the Arabidopsis PHYA (Garg et al. 2006). A drawback in many of these transgenic shade avoidance phenotypes has been the severe dwarfism caused by such major modifications of primary light sensors. For these reasons, we discarded strong dwarfing phenotypes in the early steps of the screening process and only selected mutants with normal height. This gave us four out of five selected mutant lines that showed reduced elongation of the first leaf sheath or second leaf lamina in added FR light, compared with non-mutated plants. Several studies have shown that elongation responses to low R:FR ratios are predominantly mediated by phytochrome A and B, as mutants deficient in phyA or phyB display dramatically elongated phenotypes with early flowering (Nagatani et al. 1991; Devlin et al. 1992, 1997; Halliday et al. 1994; Yanovsky et al. 1995). However, little is yet known about effectors of light signalling downstream to the primary light receptors in monocots, especially in wheat.
Future molecular studies of the mutants obtained from our experiments may reveal novel molecular handles, which can be used to modify responses to changed light distributions. This approach presents fewer pleiotropic complications than does modification of primary light receptors. Testing the performance of these lines in field experiments, especially in high-density cultivation systems, will show if the reduced elongation in response to neighbours will result in the desired increases in grain yield.
Conclusions
Shade avoidance can be disadvantageous for crop plants, because it reduces allocation of resources to reproductive yield, increases the risk of lodging, and reduces weed suppression at high crop density. We succeeded in producing lines of spring wheat with reduced shade avoidance using a forward approach with induced mutations and phenotypic screening. These mutants may be useful in developing new wheat varieties with reduced shade avoidance responses, and in generating molecular handles to modify the reaction of plants to changed light quality.
Sources of Funding
This research was supported by the Program of Excellence of the University of Copenhagen.
Contributions by the Authors
S.B.A. and W.W. designed the study. W.W., S.B.A. and E.R. performed the experiments. C.B.P. and W.W. did the statistical analyses. J.W. obtained the funding. All authors contributed to writing the article.
Conflicts of Interest
None declared.
Acknowledgements
The authors wish to thank Sally Nordlund Andersen, Benoit Foucault, Christine Heimes, Allan Højgaard Jensen, Lars Pødenphant Kjær, Uffe Lauridsen, Pawel Lewinski, Antoine Monier, Mai-Britt Sauer, Leila Therese Traore, Justin Wynns, Krystian Zandecki and Agata Zywert for help with sowing, screening, taking measurements and threshing seeds. Special thanks for Sylwia Stojak for taking the final measures of the climate chamber experiment. The authors are also very grateful for the technical help of Mads Nielsen and Allan Esben Hansen and comments of anonymous reviewers on an earlier version of this article.
Literature Cited
- Ballaré CL, Casal JJ. 2000. Light signals perceived by crop and weed plants. Field Crops Research 67:149–160. [Google Scholar]
- Ballaré CL, Sánchez RA, Scopel AL, Casal JJ, Ghersa CM. 1987. Early detection of neighbour plants by phytochrome perception of spectral changes in reflected sunlight. Plant, Cell and Environment 10:551–557. [Google Scholar]
- Ballaré CL, Scopel AL, Sánchez RA. 1990. Far-red radiation reflected from adjacent leaves: an early signal of competition in plant canopies. Science 247:329–332. [DOI] [PubMed] [Google Scholar]
- Ballaré CL, Scopel AL, Sánchez RA. 1991. On the opportunity cost of the photosynthate invested in stem elongation reactions mediated by phytochrome. Oecologia 86:561–567. [DOI] [PubMed] [Google Scholar]
- Bennetzen JL. 2007. Patterns in grass genome evolution. Current Opinion in Plant Biology 10:176–181. [DOI] [PubMed] [Google Scholar]
- Boylan MT, Quail PH. 1989. Oat phytochrome is biologically active in transgenic tomatoes. The Plant Cell 1:765–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr-Smith HD, Johnson CB, Plumpton C, Butcher GW, Thomas B. 1994. The kinetics of type I phytochrome in green, light-grown wheat (Triticum aestivum L.). Planta 194:136–142. [DOI] [PubMed] [Google Scholar]
- Carriedo LG, Maloof JN, Brady SM. 2016. Molecular control of crop shade avoidance. Current Opinion in Plant Biology 30:151–158. [DOI] [PubMed] [Google Scholar]
- Casal JJ, Sánchez RA, Deregibus VA. 1986. The effect of plant density on tillering: the involvement of R/FR ratio and the proportion of radiation intercepted per plant. Environmental and Experimental Botany 26:365–371. [Google Scholar]
- Causin H, Wulff RD. 2003. Changes in the responses to light quality during ontogeny in Chenopodium album. Canadian Journal of Botany 81:152–163. [Google Scholar]
- Chen M, Chory J, Fankhauser C. 2004. Light signal transduction in higher plants. Annual Review of Genetics 38:87–117. [DOI] [PubMed] [Google Scholar]
- Childs KL, Miller FR, Cordonnier-Pratt MM, Pratt LH, Morgan PW, Mullet JE. 1997. The sorghum photoperiod sensitivity gene, ma3, encodes a phytochrome b. Plant Physiology 113:611–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough RC, Casal JJ, Jordan ET, Christou P, Vierstra RD. 1995. Expression of functional oat phytochrome a in transgenic rice. Plant Physiology 109:1039–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit M, Keuskamp DH, Bongers FJ, Hornitschek P, Gommers CM, Reinen E, Martínez-Cerón C, Fankhauser C, Pierik R. 2016. Integration of phytochrome and cryptochrome signals determines plant growth during competition for light. Current Biology 26:3320–3326. [DOI] [PubMed] [Google Scholar]
- Denison RF. 2012. Darwinian agriculture. How understanding evolution can improve agriculture. Princeton, NJ: Princeton University Press. [Google Scholar]
- Devlin PF, Rood SB, Somers DE, Quail PH, Whitelam GC. 1992. Photophysiology of the elongated internode (ein) mutant of Brassica rapa: ein mutant lacks a detectable phytochrome b-like polypeptide. Plant Physiology 100:1442–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devlin PF, Somers DE, Quail PH, Whitelam GC. 1997. The Brassica rapa elongated internode (ein) gene encodes phytochrome b. Plant Molecular Biology 34:537–547. [DOI] [PubMed] [Google Scholar]
- Evers JB, Vos J, Andrieu B, Struik PC. 2006. Cessation of tillering in spring wheat in relation to light interception and red: far-red ratio. Annals of Botany 97:649–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans LT. 1993. Crop evolution, adaptation and yield. Cambridge, UK: Cambridge University Press. [Google Scholar]
- Evans LT. 1998. Feeding the ten billion: plant and population growth. Cambridge, UK: Cambridge University Press. [Google Scholar]
- Fischer RA, Edmeades GO. 2010. Breeding and cereal yield progress. Crop Science 50:S85–S98. [Google Scholar]
- Franklin KA. 2008. Shade avoidance. The New Phytologist 179:930–944. [DOI] [PubMed] [Google Scholar]
- Franklin KA, Whitelam GC. 2005. Phytochromes and shade-avoidance responses in plants. Annals of Botany 96:169–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale MD, Youssefian S. 1985. Dwarfing genes in wheat. In: Russell GE, eds. Progress in plant breeding. London, UK: Butterworth-Heinemann, 1–35. [Google Scholar]
- Garg AK, Sawers RJ, Wang H, Kim JK, Walker JM, Brutnell TP, Parthasarathy MV, Vierstra RD, Wu RJ. 2006. Light-regulated overexpression of an Arabidopsis phytochrome a gene in rice alters plant architecture and increases grain yield. Planta 223:627–636. [DOI] [PubMed] [Google Scholar]
- Gasperini D, Greenland A, Hedden P, Dreos R, Harwood W, Griffiths S. 2012. Genetic and physiological analysis of rht8 in bread wheat: an alternative source of semi-dwarfism with a reduced sensitivity to brassinosteroids. Journal of Experimental Botany 63:4419–4436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghersa CM, Martinez-Ghersa MA, Casal JJ, Kaufman M, Roush ML, Deregibus VA. 1994. Effect of light on winter wheat (Triticum aestivum) and Italian ryegrass (Lolium multiflorum). Weed Technology 8:37–45. [Google Scholar]
- Gundel PE, Pierik R, Mommer L, Ballaré CL. 2014. Competing neighbors: light perception and root function. Oecologia 176:1–10. [DOI] [PubMed] [Google Scholar]
- Halliday KJ, Koornneef M, Whitelam GC. 1994. Phytochrome b and at least one other phytochrome mediate the accelerated flowering response of Arabidopsis thaliana l. To low red/far-red ratio. Plant Physiology 104:1311–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraut-Bron V, Robin C, Varlet-Grancher C, Guckert A. 2001. Phytochrome mediated effects on leaves of white clover: consequences for light. Annals of Botany 88:737–743. [Google Scholar]
- Holt JS. 1995. Plant responses to light: a potential tool for weed management. Weed Science 43:474–482. [Google Scholar]
- Hothorn T, Bretz F, Westfall P. 2008. Simultaneous inference in general parametric models. Biometrical Journal. Biometrische Zeitschrift 50:346–363. [DOI] [PubMed] [Google Scholar]
- Inskeep WP, Bloom PR. 1985. Extinction coefficients of chlorophyll a and b in n,n-dimethylformamide and 80% acetone. Plant Physiology 77:483–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan ET, Hatfield PM, Hondred D, Talon M, Zeevaart JA, Vierstra RD. 1995. Phytochrome a overexpression in transgenic tobacco. Correlation of dwarf phenotype with high concentrations of phytochrome in vascular tissue and attenuated gibberellin levels. Plant Physiology 107:797–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasperbauer MJ, Karlen DL. 1994. Plant spacing and reflected far-red light effects on phytochrome-regulated photosynthase allocation in corn seedlings. Crop Science 34:1564–1569. [Google Scholar]
- Kay SA, Keith B, Shinozaki K, Chye ML, Chua NH. 1989. The rice phytochrome gene: structure, autoregulated expression, and binding of gt-1 to a conserved site in the 5′ upstream region. The Plant Cell 1:351–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kebrom TH, Brutnell TP. 2007. The molecular analysis of the shade avoidance syndrome in the grasses has begun. Journal of Experimental Botany 58:3079–3089. [DOI] [PubMed] [Google Scholar]
- Keller JM, Shanklin J, Vierstra RD, Hershey HP. 1989. Expression of a functional monocotyledonous phytochrome in transgenic tobacco. The embo Journal 8:1005–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konzak CF. 1987. Mutations and mutation breeding. In: Heyne EC, ed. Wheat and wheat improvement. Madison, WI: American Society of Agronomy, 428–443. [Google Scholar]
- Mathews S, Sharrock RA. 1996. The phytochrome gene family in grasses (poaceae): a phylogeny and evidence that grasses have a subset of the loci found in dicot angiosperms. Molecular Biology and Evolution 13:1141–1150. [DOI] [PubMed] [Google Scholar]
- McCormac AC, Whitelam GC, Boylan MT, Quail PH, Smith H. 1992. Contrasting responses of etiolated and light-adapted seedlings to red:far-red ratio: a comparison of wild-type, mutant and transgenic plants has revealed differential functions of members of the phytochrome family. Journal of Plant Physiology 140:707–714. [Google Scholar]
- Nagatani A, Chory J, Furuya M. 1991. Phytochrome B is not detectable in the hy3 mutant of Arabidopsis, which is deficient in responding to end-of-day far-red light treatments. Plant and Cell Physiology 32:1119–1122. [Google Scholar]
- Neidhardt J, Benemann JR, Zhang L, Melis A. 1998. Photosystem II repair and chloroplast recovery from irradiance stress: relationship between chronic photoinhibition, light-harvesting chlorophyll antenna size and photosynthetic productivity in Dunaliella salina (green algae). Photosynthesis Research 56:175–184. [Google Scholar]
- Pierik R, Cuppens ML, Voesenek LA, Visser EJ. 2004. Interactions between ethylene and gibberellins in phytochrome-mediated shade avoidance responses in tobacco. Plant Physiology 136:2928–2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierik R, Millenaar FF, Peeters AJ, Voesenek LA. 2005. New perspectives in flooding research: the use of shade avoidance and Arabidopsis thaliana. Annals of Botany 96:533–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson GK. 2001. That BLUP is a good thing: the estimation of random effects. Statistical Science 6:15–32. [Google Scholar]
- Robson PR, McCormac AC, Irvine AS, Smith H. 1996. Genetic engineering of harvest index in tobacco through overexpression of a phytochrome gene. Nature Biotechnology 14:995–998. [DOI] [PubMed] [Google Scholar]
- Ruberti I, Sessa G, Ciolfi A, Possenti M, Carabelli M, Morelli G. 2012. Plant adaptation to dynamically changing environment: the shade avoidance response. Biotechnology Advances 30:1047–1058. [DOI] [PubMed] [Google Scholar]
- Sawers RJ, Linley PJ, Farmer PR, Hanley NP, Costich DE, Terry MJ, Brutnell TP. 2002. Elongated mesocotyl1, a phytochrome-deficient mutant of maize. Plant Physiology 130:155–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawers RJ, Sheehan MJ, Brutnell TP. 2005. Cereal phytochromes: targets of selection, targets for manipulation? Trends in Plant Science 10:138–143. [DOI] [PubMed] [Google Scholar]
- Schmitt J, McCormack AC, Smith H. 1995. A test of the adaptive plasticity hypothesis using transgenic and mutant plants disabled in phytochrome-mediated elongation responses to neighbors. American Naturalist 146:937–953. [Google Scholar]
- Sheehan MJ, Kennedy LM, Costich DE, Brutnell TP. 2007. Subfunctionalization of phyb1 and phyb2 in the control of seedling and mature plant traits in maize. The Plant Journal 49:338–353. [DOI] [PubMed] [Google Scholar]
- Shlumukov LR, Barro F, Barcelo P, Lazzeri P, Smith H. 2001. Establishment of far-red high irradiance responses in wheat through transgenic expression of an oat phytochrome A gene. Plant, Cell and Environment 24:703–712. [Google Scholar]
- Sineshchekov V, Koppel L, Shlumukov L, Barro F, Barcelo P, Lazzeri P, Smith H. 2001. Fluorescence and photochemical properties of phytochromes in wild-type wheat and a transgenic line overexpressing an oat phytochrome A (PHYA) gene: functional implications. Plant, Cell and Environment 24:1289–1297. [Google Scholar]
- Smith H. 1992. The ecological functions of the phytochromes family. Clues to a transgenic programme of crop improvement. Photochemistry and Photobiology 56:815–822. [Google Scholar]
- Smith H. 2000. Phytochromes and light signal perception by plants—an emerging synthesis. Nature 407:585–591. [DOI] [PubMed] [Google Scholar]
- Smith H, Whitelam GC. 1997. The shade avoidance syndrome: multiple responses mediated by multiple phytochromes. Plant, Cell and Environment 20:840–844. [Google Scholar]
- Sparkes DL, King M. 2008. Disentangling the effects of PAR and R:FR on lodging-associated characters of wheat (Triticum aestivum). Annals of Applied Biology 152:1–9. [Google Scholar]
- Ugarte CC, Trupkin SA, Ghiglione H, Slafer G, Casal JJ. 2010. Low red/far-red ratios delay spike and stem growth in wheat. Journal of Experimental Botany 61:3151–3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbussche F, Pierik R, Millenaar FF, Voesenek LA, Van Der Straeten D. 2005. Reaching out of the shade. Current Opinion in Plant Biology 8:462–468. [DOI] [PubMed] [Google Scholar]
- Webb MR, Melis A. 1995. Chloroplast response in Dunaliella salina to irradiance stress (effect on thylakoid membrane protein assembly and function). Plant Physiology 107:885–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weijschedé J, Martínková J, de Kroon H, Huber H. 2006. Shade avoidance in Trifolium repens: costs and benefits of plasticity in petiole length and leaf size. The New Phytologist 172:655–666. [DOI] [PubMed] [Google Scholar]
- Weiner J, Andersen SB, Wille WK, Griepentrog HW, Olsen JM. 2010. Evolutionary agroecology: the potential for cooperative, high density, weed-suppressing cereals. Evolutionary Applications 3:473–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner J, Griepentrog H-W, Kristensen L. 2001. Suppression of weeds by spring wheat Triticum aestivum increases with crop density and spatial uniformity. Journal of Applied Ecology 38:784–790. [Google Scholar]
- Whitelam GC, Smith H. 1991. Retention of phytochrome-mediated shade avoidance responses in phytochrome-deficient mutants of Arabidopsis, cucumber and tomato. Journal of Plant Physiology 139:119–125. [Google Scholar]
- Xu X, Paik I, Zhu L, Huq E. 2015. Illuminating progress in phytochrome-mediated light signaling pathways. Trends in Plant Science 20:641–650. [DOI] [PubMed] [Google Scholar]
- Yanovsky MJ, Casal JJ, Whitelam GC. 1995. Phytochrome A, phytochromes B and HY4 are involved in hypocotyl growth responses to natural radiation in Arabidopsis: weak de-etiolation of the phyA mutant under dense canopies. Plant Cell and Environment 18:788–794. [Google Scholar]