Abstract
What is a good life and how it can be achieved is one of the fundamental issues. When considering a good life, there is a division between hedonic (pleasure attainment) and eudaimonic well-being (meaning pursuing and self-realization). However, an integrated approach that can compare the brain functional and structural differences of these two forms of well-being is lacking. Here, we investigated how the individual tendency to eudaimonic well-being relative to hedonic well-being, measured using eudaimonic and hedonic balance (EHB) index, is reflected in the functional and structural features of a key network of well-being—the default mode network (DMN). We found that EHB was positively correlated with functional connectivity of bilateral ventral medial prefrontal cortex within anterior DMN and bilateral precuneus within posterior DMN. Brain morphometric analysis showed that EHB was also positively correlated with gray matter volume in left precuneus. These results demonstrated that the relative dominance of one form of well-being to the other is reflected in the morphometric characteristics and intrinsic functions of DMN.
Keywords: eudaimonic and hedonic balance, eudaimonic well-being, hedonic well-being, default mode network, functional connectivity, voxel-based morphometry
Introduction
What is a good life and how it can be achieved is one of the fundamental issues, which has a great impact on our practices to make human society better (Ryan and Deci, 2001; Huta and Waterman, 2013). Dating back to ancient Greece, significant strides have been made in distinguishing between hedonic and eudaimonic well-being when considering the nature of a good life (Ryan and Deci, 2001). The hedonic approach focuses on happiness and defines well-being as pleasure attainment. In contrast, the eudaimonic approach defines well-being in terms of meaning, purpose, self-potential and self-realization (Waterman, 1993; Ryan and Deci, 2001). As each form of well-being provides different answers to what a good life is, revealing the brain mechanisms of hedonic and eudaimonic well-being holds promise for enriching our comprehension of well-being.
Psychologically, hedonic well-being relates more strongly to excitement-seeking, extraversion and present-orientated, whereas eudaimonic well-being relates more strongly to self-connection, introversion and temporal integration (Huta and Ryan, 2010; Huta, 2012; Baumeister et al., 2013). For example, compared to individuals with hedonic well-being dominance, individuals with eudaimonic well-being dominance devoted more time to self-reflection to identify one’s true self (Huta, 2012) and thought more frequently about their past and future (Baumeister et al., 2013). These characteristics of hedonic and eudaimonic well-being are in line with functions of the default mode network (DMN), i.e. introspectively oriented mental activity at rest (e.g. self-reflection, theory of mind, mind wandering, episodic memory and future episodic thought) (Raichle et al., 2001; Buckner et al., 2008).
Recent neuroimaging studies on well-being have revealed neural correlates of hedonic or eudaimonic well-being within and outside of DMN (Heller et al., 2013; Lewis et al., 2014; Luo et al., 2014; Kong et al., 2015, 2016; Sato et al., 2015). For example, our previous study using resting-state functional magnetic resonance imaging (R-fMRI) approach found that the hedonic well-being was associated with decreased resting-state functional connectivity (RSFC) within core areas of DMN (Luo et al., 2016). Self-reported meaning in life was shown positively correlated with connectivity of medial temporal lobe, a subnetwork of the DMN (Waytz et al., 2015). As core regions of DMN, such as medial prefrontal cortex (MPFC), not only involved in pleasure attachment, but also implicated in self-representation, introspective self-referential cognition and consciousness, DMN may play a fundamental role in connecting hedonic and eudaimonic well-being (Kringelbach and Berridge, 2009).
To date, few study have examined hedonic and eudaimonic well-being in a single study. The only pioneering study using resting-state electroecncephalography found that greater left than right superior frontal activation was positively associated with both forms of well-being, and left frontal activation predicted eudaimonic but not hedonic well-being when positive affect is statistically controlled (Urry et al., 2004). Thus, an integrated approach that can compare the functional and structural differences of these two forms of well-being with high spatial resolution is lacking.
Here, we filled this gap by using structural and functional MRI techniques and an eudaimonic and hedonic balance (EHB) index. Conceptually, EHB quantifies the balance between eudaimonic and hedonic well-being, or in other words, which form of well-being is more dominant. This could be an interesting trait that shows meaningful individual differences. Operationally, it is defined as the difference between Z-standardized scores of Psychological Well-being and the Positive Affect subscale of the Positive and Negative Affect Schedule (PANAS). This definition is based on the approach used in Cox et al. (2012), which measures the balance between affective empathy and cognitive empathy. EHB index has high ecological validity and reflects the fact that individuals live with both hedonic and eudaimonic well-being in daily life, though some with greater tendency to pursue pleasure and others with greater tendency to self-realization. Using EHB index is necessary also because it allows us to investigate eudaimonic and hedonic well-being integrally, even when two measures were highly correlated.
Previous studies reported that eudaimonic well-being, relative to hedonic well-being, is correlated more strongly with self, and past or future thinking (Huta and Ryan, 2010; Huta, 2012; Baumeister et al., 2013). Thus, we predicted that EHB score will be positively correlated with functional connectivity of core DMN areas implicated in these functions, such as MPFC and precuneus (Raichle et al., 2001; Buckner et al., 2008; Andrews-Hanna et al., 2010). That is, a eudaimonic dominance would exhibited enhanced functional connectivity within DMN and a hedonic dominance would exhibited decreased DMN functional connectivity. Furthermore, as eudaimonic or hedonic dominance is a relatively stable trait, EHB may also be associated with structural features within the DMN. Thus, we predicted that EHB would be related to DMN morphometry, such as gray matter volume.
Materials and Methods
Participants
Participants were 154 healthy undergraduate or postgraduate students, who volunteered as part of an ongoing study investigating the association between brain imaging, temporal cognition and well-being (Luo et al., 2014, 2016). Fourteen participants were excluded due to excessive head motion (see Functional MRI data preprocessing and analyses section for details), and another two were excluded due to missing self-report measures. Final sample includes 138 participants (males/females, 52/86; mean age = 21.10 ± 1.69). All participants were right-handed and had normal or corrected-to-normal vision, with no history of neurological conditions or psychiatric episodes. In accordance with the Declaration of Helsinki, written informed consent was obtained from all participants. The study protocol was approved by the Southwest University Brain Imaging Center Institutional Review Board.
Eudaimonic and hedonic balance
We used EHB to quantify the tendency to eudaimonic or hedonic well-being. The EHB index, a relative measure, was defined as the difference between eudaimonic and hedonic well-being. A positive EHB score indicates a dominance of eudaimonic well-being, while a negative EHB score indicates a dominance of hedonic well-being. The trait level of hedonic well-being was measured using the Positive Affect subscales of the PANAS (Watson et al., 1988; Heller et al., 2013), a measure of hedonic well-being with high reliability (Cronbach's = 0.87).
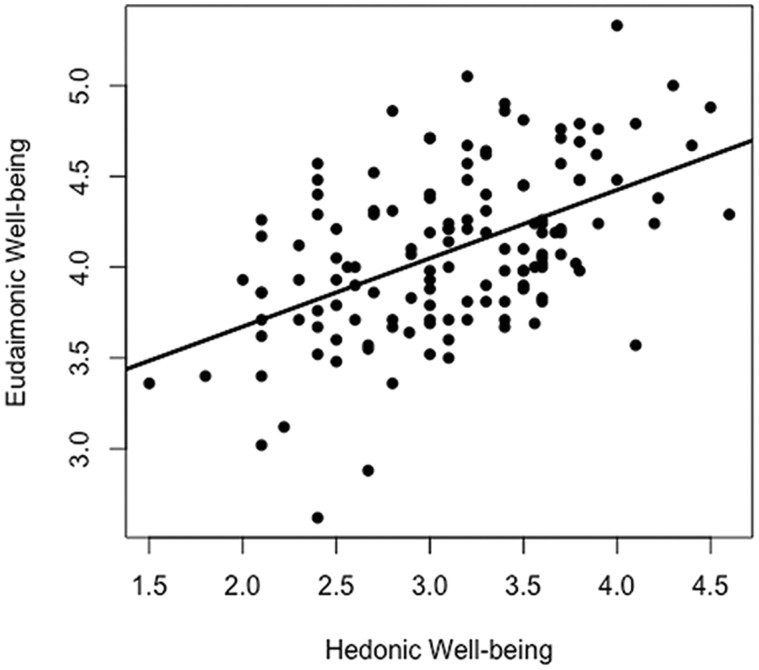
The trait level of eudaimonic well-being was assessed using the Chinese version of the 42-item Psychological Well-being (Ryff, 1989), a reliable measure of eudaimonic well-being (Heller et al., 2013; Lewis et al., 2014; Kong et al., 2015). In our sample, a good reliability was also observed (Cronbach’s α = 0.92). The eudaimonic well-being can be further divided into two components: self- and other-focused eudaimonic well-being. The self-focused eudaimonic well-being index is composed of environmental mastery, personal growth, purpose in life and self-acceptance (Cronbach’s α = 0.90). Other-focused eudaimonic well-being index is composed of positive relations with others (Cronbach’s α = 0.79) (Barrett-Cheetham et al., 2016). The correlation between hedonic and eudaimonic well-being was shown in Figure 1.
Fig. 1.
Correlations between hedonic and eudaimonic well-being.
Imaging data acquisition
Imaging data were acquired on a 3.0-T scanner (Magnetom Trio, Siemens, Erlangen, Germany). Eight minutes of functional images were acquired using a single-shot, gradient-recalled echo planar imaging sequence (TR = 2000 ms, TE = 30 ms, flip angle = 90°, 32 axial slices, FOV = 192 × 192 cm, acquisition matrix = 64 × 64, slice thickness = 3 mm, without gap, voxel size = 3 × 3 × 4 mm), when participants were instructed to rest with their eyes closed, not to think of anything in particular and not to fall asleep. To minimize head motion, participants’ head were restricted with foam cushions. High-resolution T1-weighted anatomical images were also acquired in sagittal orientation using a 3D magnetization prepared rapid gradient-echo sequence (176 slices, TR = 1900 ms, TE = 2.53 ms, flip angle = 9°, resolution = 256 × 256 and voxel size = 1 × 1 × 1 mm).
Functional MRI data preprocessing and analyses
Preprocessing
Functional images were preprocessed using data processing assistant and R-fMRI (version 2.2, http://www.restfmri.net/forum/DPARSF) (Chao-Gan and Yu-Feng, 2010). The preprocessing steps included slice timing, head motion correction, spatial normalization and smoothing (4 mm full-width at half maximum Gaussian kernel).
As head motion is a major concern for R-fMRI data analyses, we controlled head motion at different levels. First, we performed outlier analyses based on mean framewise displacement (FD), a summary measure of overall head motion (Power et al., 2012). Eight participants were excluded due to head motion outside of 1.5 s.d. of the group mean (0.122 ± 0.061 mm, threshold = 0.214 mm); second, we checked the number of volumes with an FD < 0.2 mm for each participant. Six participants were excluded due to having < 150 volumes with FD < 0.20 mm (Power et al., 2012); finally, we included mean FD in the group analysis as a nuisance regressor to account for the residual effect of motion.
DMN identification
After preprocessing, we concatenated the image data of 138 participants and performed group spatial independent component analysis (ICA) on the concatenated data to separate fMRI signal into spatially independent networks using Group ICA of fMRI Toolbox (GIFT) (icatb.sourceforge.net) (Calhoun et al., 2001). The optimal number of components was set to 32, according to the minimum description length criteria for source estimation (Li et al., 2007). To perform group ICA, dimensionality of the data was first reduced using principle component analysis. Then, the reduced data were concatenated over the time domain using the infomax algorithm. To ensure the reliability of the derived components, the infomax algorithm was repeated 20 times by running the ICASSO toolbox, using both ‘randinit’ and ‘bootstrap’ methods (Himberg et al., 2004). Individual maps and time courses for each participant were then back-reconstructed and calibrated using Z values to normalize the signal. As Z values represented the contribution of the voxels to the independent component, it is commonly thought that Z values can be indirectly used to measure the functional connectivity within the network (Beckmann et al., 2005; Liao et al., 2010).
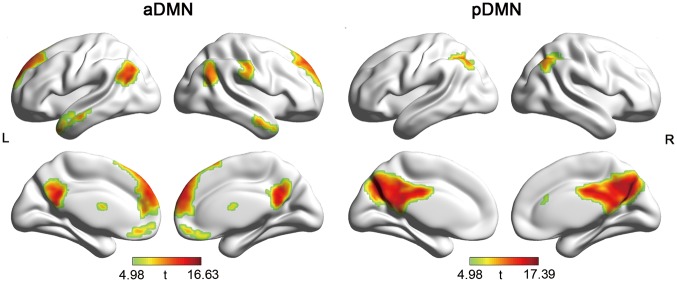
Components corresponding to the DMN were identified in two steps: first, we performed a spatial template matching procedure using a DMN template delineated by Smith et al. (2009). Two components having the highest spatial overlap with the template were identified (r = 0.32 and 0.17). Then, these two components were further confirmed by visual inspection and guided by previously reported DMN (Buckner et al., 2008; Andrews-Hanna et al., 2010). One component mainly includes MPFC, anterior and posterior cingulate cortex (ACC/PCC) (anterior DMN: aDMN), and the other includes precuneus and PCC (posterior DMN: pDMN) (Figure 2). The stability of these two components were both above 0.97 assessed using ICASSO.
Fig. 2.
Spatial pattern of aDMN and pDMN. L, left; R, right.
Association between DMN intrinsic functional connectivity and the balance of well-beings
Statistical analyses were performed using SPM8 (http://www.fil.ion.ucl.ac.uk/spm/software/spm8/). To associate functional connectivity of DMN with EHB, multiple regression analyses were performed. First, for each DMN component, individual maps of all participants were entered into random effect one-sample t-tests (family-wise error, P < 0.05, k > 20) and create a sample-specific component map (Figure 2). Each component map was used as a mask in the group analysis to restrict the results within DMN areas. Second, we carried out multiple regression analysis with EHB as the covariate of interests, and gender, age and mean FD as nuisance covariates. Multiple comparisons were corrected using 10 000 Monte Carlo simulation (Ledberg et al., 1998), implemented in AlphaSim program within AFNI (http://afni.nimh.nih.gov/pub/dist/doc/program_help/AlphaSim.html) (P < 0.05, minimum cluster size are 30 voxels/810 mm3 and 27 voxels/729 mm3 for aDMN and pDMN, respectively).
Secondary analyses
In addition to EHB, we performed secondary analyses to examine the unique effect associated with hedonic after controlling for eudaimonic well-being and vice versa. Although two forms of well-beings are highly correlated, no multicollinearity among the regressors were found when both forms of well-beings were entered into the same general linear model (variance inflation factor was 1.52, below the cutoff criterion of 5) (Studenmund, 2000). Age, gender and head motion were included as nuisance covariates. Multiple comparison correction procedure was identic to the EHB analyses.
Specificity analyses
To confirm the specificity of our results, we performed additional two sets of analyses. First, we examined whether DMN connectivity was associated with measures other than EHB, such as anxious arousal. Anxious arousal is measured using the Anxious Arousal subscale of Mood and Anxiety symptom Questionnaire (Clark and Watson, 1991). This subscale is composed of 17 items and measures the level of somatic tension and hyperarousal (Cronbach’s α = 0.77). We chose this measure because it has no direct relationship with hedonic or eudaimonic well-being. Second, we examined whether EHB was associated with functional connectivity of networks other than DMN. We chose two visual networks (medial and lateral visual networks) for this purpose (see Supplementary Figure S1 for spatial map of medial and lateral visual networks). The regression analyses and multiple comparison correction were identical to the main EHB and DMN analyses.
Structural MRI data preprocessing and analyses: voxel-based morphometry
To examine the anatomical basis underlying the association between EHB and DMN functional connectivity, we performed voxel-based morphometry (VBM) analysis using FSL-VBM (Douaud et al., 2007, http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FSLVBM), an optimized VBM protocol (Good et al., 2001; Smith et al., 2004). Preprocessing included brain extraction, gray matter segmentations and non-linear registration to Montreal Neurological Institute (MNI) 152 standard space. Then, the gray matter segmented images were used to create a study-specific template. After that, all native gray matter images were non-linearly registered to this template and ‘modulated’ to correct for local expansion (or contraction) due to the non-linear component of the spatial transformation. The modulated gray matter images were then smoothed with an isotropic Gaussian kernel with a sigma of 3 mm.
The group analyses were restricted within three spherical region of interests (ROIs) (radius = 8 mm), defined based on the peak voxel coordinates in the RSFC analysis. Non-parametric voxel-wise statistical testing was performed using FSL randomize with 5000 permutations. Regressors include EHB, gender and age. Cluster significance was determined using the threshold-free cluster enhancement techniques (Smith and Nichols, 2009). The number of ROIs (n = 3) was also Bonferroni corrected (P < 0.05/3 = 0.017). Lastly, the VBM values of the ‘survived’ ROIs were correlated with its RSFC values to test for the structural and functional association.
Results
Behavioral measures of well-being
The sample demographics were showed in Table 1. There is no significant correlation between EHB and participant measures, including age, sex and head motion (P > 0.10). Hedonic (3.12 ± 0.61) and eudaimonic well-being (4.09 ± 0.46) scores were moderately correlated (r = 0.51, P < 0.001) (Figure 1). EHB scores range from −2.76 to 2.22. The percent of participants showing eudaimonic and hedonic dominance were close (50.7 vs 49.3%).
Table 1.
Demographics
| Mean ± s.d. | Range | r/t (P value) | |
|---|---|---|---|
| Age (year) | 21.10 ± 1.69 | 18–25 | −0.14 (0.11) |
| Female sex, % | 62.3% | −0.54 (0.59) | |
| Head motion (mm) | 0.108 ± 0.038 | 0.04–0.22 | 0.07 (0.39) |
| Eudaimonic well-being | 4.09 ± 0.46 | 2.62–5.33 | 0.50 (<0.001) |
| Hedonic well-being | 3.12 ± 0.61 | 1.5–4.6 | 0.50 (<0.001) |
| EHB | 0.00 ± 1.00 | −2.76–2.22 |
Note: n = 138. For continuous variables, Pearson’s correlation between demographic variable and EHB was computed. For categorical variable (i.e. sex), independent samples t-Test were performed to test for the group differences in EHB.
DMN intrinsic functional connectivity and the balance of well-beings
Spatial pattern of DMNs obtained from the group ICA analyses are shown in Figure 2 on surface map using BrainNet Viewer (Xia et al., 2013). Visual inspection indicated that the aDMN was mainly composed of the MPFC, ACC, a small portion of the PCC/precuneus, and bilateral inferior parietal gyrus. The pDMN mainly included the PCC and precuneus.
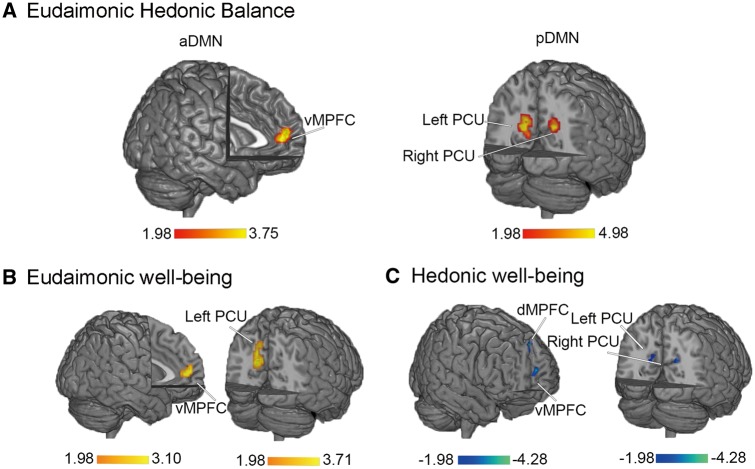
Multiple regression analysis indicated that the Z values of the bilateral vMPFC (portion of the aDMN) were positively correlated with EHB, and the Z values of the bilateral precuneus (portion of the pDMN) were also positively correlated with EHB (Figure 3A, Table 2). That is, individuals with greater eudaimonic dominance had greater DMN connectivity and individuals with greater hedonic dominance had smaller DMN connectivity.
Fig. 3.
aDMN and pDMN regions where functional connectivity is correlated with EHB (A), eudaimonic (B) or hedonic well-being (C). vMPFC, ventral MPFC; dMPFC, dorsal MPFC; PCU, precuneus.
Table 2.
DMN regions where within network functional connectivity is significantly correlated with EHB, hedonic well-being, or eudaimonic well-being
| Anatomical region | Side | BAs | MNI |
Voxel | Peak | |||
|---|---|---|---|---|---|---|---|---|
| x | y | z | Size | t-value | ||||
| EHB | aDMN | |||||||
| Ventral MPFC | B | 10 | −6 | 54 | 12 | 40 | 3.75 | |
| pDMN | ||||||||
| Precuneus | L | 31 | −15 | −63 | 21 | 67 | 4.08 | |
| Precuneus | R | 31 | 21 | −54 | 27 | 36 | 4.51 | |
| Hedonic well-being | aDMN | |||||||
| Ventral MPFC | B | 10 | −6 | 54 | 12 | 32 | −4.08 | |
| Dorsal MPFC | B | 9 | 6 | 48 | 42 | 47 | −4.28 | |
| pDMN | ||||||||
| Precuneus | L | 31 | −15 | −66 | 21 | 48 | −3.35 | |
| Precuneus | R | 31 | 18 | −54 | 27 | 54 | −4.28 | |
| Eudaimonic well-being | aDMN | |||||||
| Ventral MPFC | B | 10 | −6 | 51 | 9 | 34 | 3.10 | |
| pDMN | ||||||||
| Precuneus | L | 31 | −15 | −63 | 21 | 70 | 3.72 | |
Note: Side refers to the hemisphere (B, bilateral; R, right; L, left). Brodmann areas (BAs), coordinates of peak t-value in MNI space, volume in voxels and peak t-values are specified for each region showing significant correlations.
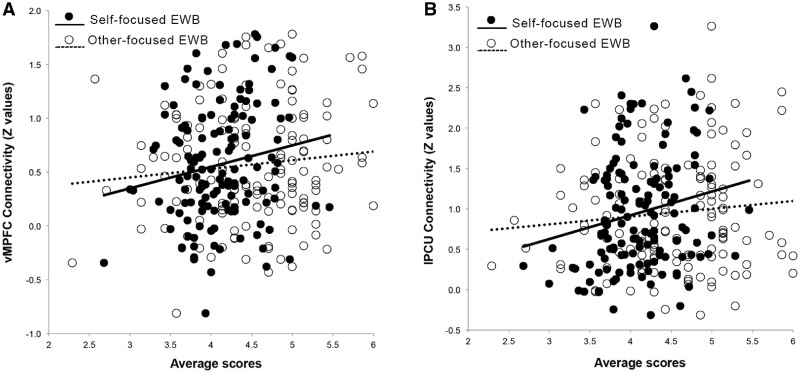
As a large body of literature showed that DMN hyperconnectivity was associated with rumination and depression (Greicius et al., 2007; Sheline et al., 2010; Berman et al., 2011; Whitfield-Gabrieli and Ford, 2012), we further disentangle the functional significance of DMN hyperconnectivity by correlating the DMN connectivity strength with two subcomponents of eudaimonic well-being. Specifically, we extracted the connectivity strength from regions showing significant associations with eudaimonic well-being (after controlling for hedonic well-being) and conducted Person’s correlation between their connectivity and the two subscales of eudaimonic well-being. We found that the Z values of the vMPFC were positively correlated with the self-focused eudaimonic well-being (r = 0.17, P = 0.046) but not other-focused eudaimonic well-being (r = 0.11, P > 0.05). The same pattern was observed for the left precuneus: correlation was significant for self-focused eudaimonic well-being (r = 0.19, P = 0.023) but not for other-focused eudaimonic well-being (r = 0.01, P > 0.05) (Figure 4).
Fig. 4.
Correlations between self- and other-focused eudaimonic well-being (EWB) and DMN functional connectivity: vMPFC (A) and precunues (B).
Consistent with our primary analyses, secondary analyses showed that eudaimonic well-being was positively correlated with intrinsic functional connectivity of vMPFC and precuneus after controlling for hedonic well-being (Figure 3B, Table 2). In contrast, hedonic well-being was negatively correlated with functional connectivity of dMPFC, vMPFC and precuneus after controlling for eudaimonic well-being (Figure 3C, Table 2). Our specificity analyses indicated that DMN functional connectivity was not significantly associated with anxious arousal; Furthermore, the functional connectivity of visual networks were not correlated with EHB. These results provide additional evidence to support that the correlations between EHB and DMN functional connectivity are specific.
VBM and the balance of well-being
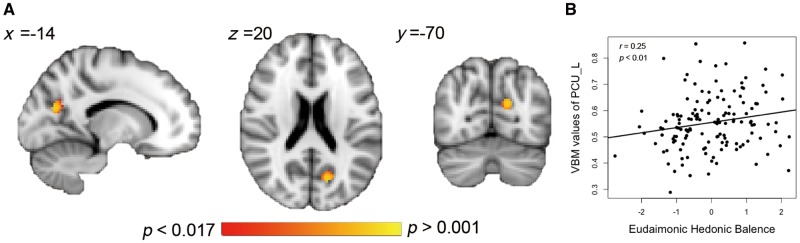
ROI-based analyses revealed a significant positive correlation between EHB and gray matter volume of the left precuneus (MNI coordinate: −14, −70, 20; cluster size = 100, P = 0.003, Bonferroni corrected) (Figure 5). No significant correlations were found between bilateral vMPFC or right precuneus and EHB. The gray matter volume value of left precuneus was marginally correlated with its RSFC values (r = 0.15, P = 0.075).
Fig. 5.
VBM analyses. (A) DMN subregions where gray matter volume is significantly correlated with EHB. (B) Correlations between EHB and gray matter volume of the left precuneus (PCU_L).
Discussion
In the present work, we used structural and R-fMRI to investigate how predominance of a certain form of well-being is reflected in the functional and structural characteristics of DMN. We demonstrated that EHB were positively correlated with the intrinsic functional connectivity of bilateral vMPFC within aDMN and bilateral precuneus within pDMN. Furthermore, our brain morphometry analysis highlighted the association between gray matter volume in the left precuneus and EHB. These results provided novel evidence to support the role of DMN in one’s inclination toward hedonic or eudaimonic well-being.
Our primary and secondary analyses highlighted the role of vMPFC and precuneous hyperconnectivity in eudaimonic well-being dominance and eudaimonic well-being alone. These results seem to contradict with previous work on hedonic well-being and the depression literature (Greicius et al., 2007; Sheline et al., 2010; Berman et al., 2011; Whitfield-Gabrieli and Ford, 2012) but are consistent with a recent eudaimonic well-being study (Waytz et al., 2015), which showed that meaning in life was positively correlated with a subnetwork of the DMN. When decomposing eudaimonic well-being into self-focus and other-focus well-being, we found that DMN hyperconnectivity was only correlated with self-focus but not other-focus well-being.
Self-focused attention can be adaptive (i.e. self-reflection) or maladaptive (i.e. self-rumination). Self-reflection is a form of positive self-focus that is motivated by curiosity or epistemic interest in the self and confers benefits to mental health; whereas, self-rumination is a form of negative, chronic self-focus is motivated by perceived threat, losses to the self and is associated with depression and neuroticism (Trapnell and Campbell, 1999; Takano and Tanno, 2009). As eudaimonic well-being is associated with higher level of adaptation and mental health, and more self-connected (Huta, 2012; Baumeister et al., 2013), it is possible that people with higher levels of eudaimonic well-being frequently engaged in self-reflection, rather than self-rumination. Thus, our results of EHB and eudaimonic well-being support the idea that the functional role of DMN is not limited to maladaptation and lower level of well-being but also to adaptation and higher level of well-being.
We found that eudaimonic dominance (a high positive EHB score) is not only associated with higher intrinsic functional connectivity but also with larger gray matter volume in precuneus. The convergence between structural and functional results in precuneus indicates that the observed association between intrinsic functional connectivity, and EHB could be partially explained by the gray matter volume. This convergence also highlights the critical role of precuneus in the stable trait of balance between eudaimonic and hedonic well-being. Precunues, the posterior core of DMN, subserves visuospatial imagery, episodic memory retrieval and episodic future thought (Cavanna and Trimble, 2006; Schacter et al., 2007; Fransson and Marrelec, 2008; Uddin et al., 2009). Functionally, the overall connectivity of pDMN was shown positively correlated with meaning in life, a core feature in eudaimonic well-being (Waytz et al., 2015). Structurally, gray matter volume of precuneus was shown positively correlated with the emotional intensity and purpose in life (Sato et al., 2015). In contrast, decreased functional connectivity of medial parietal cortex was associated with the inclination to hedonic well-being (Luo et al., 2016).
As thinking about past and future vs the present enhances the meaning of life or eudaimonic well-being (Routledge et al., 2011; Waytz et al., 2015; Sedikides et al., 2016), increased intrinsic functional connectivity of pDMN may indicate that people with eudaimonic dominance are more likely to beyond here and now and draw meaning from past or future events (Baumeister et al., 2013), which in turn enhances the feeling of purpose and meaningfulness (Waytz et al., 2015).
Our EHB-related results replicated and extended previous work examining either hedonic or eudaimonic well-being. Our work suggests that EHB is a sensitive index that can capture the neural characteristics of relative dominance of hedonic and eudaimonic well-being. We recommend that this relative measure should be considered in future neuroimaging, clinical and psychopathological studies, given its capability of integrating two distinctive but related forms of well-being and its high ecological validity.
The present work has several limitations. First, we focused our investigation within DMN due to its fundamental role in EHB. It will be of merit for future work to extend to other networks using advanced analytics such as network analysis. Second, our study demonstrated the correlational relationship between EHB and DMN. Future studies using neural modulation technique to establish the causal relationship are needed. Third, we only compared the neural basis of people with eudaimonic and hedonic dominance. Logically, four categories are possible according to different levels of two forms of well-being: people with high eudaimonic and high hedonic well-being; people with low eudaimonic and high hedonic well-being; people with high eudaimonic and low hedonic well-being; people with low eudaimonic and low hedonic well-being. It will be interesting for future studies to characterize brain functional and structural features of these four well-being subgroups. Fourth, this study performed on a relatively homogenous sample, with the narrow range of age (18–25), the similar education level (undergraduate or graduate) and the same culture (Chinese). Future studies with more diverse sample, such as cross-culture studies, are warranted to generalize the results, given the impact of sociodemographic factors on well-being.
Conclusion
Using both functional and structural MRI techniques, we investigated the neural correlates underlying EHB. Our results highlighted vMPFC and precuneus as neural basis of well-being balance and suggested that enhanced functional connectivity of vMPFC and precuneus was positively correlated with EHB. Furthermore, the left precuneus morphometry was also positively correlated with EHB. These findings provided insights into the associations between EHB and the intrinsic functional and structural architecture of DMN in the brain. This study may provide an avenue for future causal studies with diverse sample.
Funding
This work was supported by the National Natural Science Foundation of China (31600913 to Y.L.); the Youth foundation for Humanities and Social Science Research of Ministry of Education (15XJC190001 to Y.L.); the China Postdoctoral Science Foundation (2016M590918 and 2017T100724 to Y.L.); the Shaanxi Postdoctoral Science Foundation (2016BSHTDZZ10 to Y.L.); the Young Talent fund of University Association for Science and Technology in Shaanxi, China (20160210 to Y.L.); the Ministry of Education of China (MOE) Key Project of Philosophy and Social Science (11JZD044 to X.Y.); the major project of philosophy and social science of Chongqing (2010CQZDW07 to X.H.), and the Research Team’s Construction Project from the Faculty of Psychology in Southwest University (2012) (TR201201-1 to X.H.).
Supplementary Material
Acknowledgements
The authors thank all the participants who took part in our project. They also thank the anonymous referee for valuable comments and suggestions.
Supplementary data
Supplementary data are available at SCAN online.
Conflict of interest. None declared.
References
- Andrews-Hanna J.R., Reidler J.S., Sepulcre J., Poulin R., Buckner R.L. (2010). Functional-anatomic fractionation of the brain’s default network. Neuron, 65, 550–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett-Cheetham E., Williams L.A., Bednall T.C. (2016). A differentiated approach to the link between positive emotion, motivation, and eudaimonic well-being. The Journal of Positive Psychology, 11, 595–608. [Google Scholar]
- Baumeister R.F., Vohs K.D., Aaker J.L., Garbinsky E.N. (2013). Some key differences between a happy life and a meaningful life. The Journal of Positive Psychology, 8, 505–16. [Google Scholar]
- Beckmann C.F., DeLuca M., Devlin J.T., Smith S.M. (2005). Investigations into resting-state connectivity using independent component analysis. Philosophical Transactions of the Royal Society B: Biological Sciences, 360, 1001–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman M.G., Peltier S., Nee D.E., Kross E., Deldin P.J., Jonides J. (2011). Depression, rumination and the default network. Social Cognitive and Affective Neuroscience, 6, 548–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner R.L., Andrews-Hanna J.R., Schacter D.L. (2008). The brain’s default network - anatomy, function, and relevance to disease. Year in Cognitive Neuroscience, 1124, 1–38. [DOI] [PubMed] [Google Scholar]
- Calhoun V.D., Adali T., Pearlson G.D., Pekar J.J. (2001). A method for making group inferences from functional MRI data using independent component analysis. Human Brain Mapping, 14, 140–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanna A.E., Trimble M.R. (2006). The precuneus: a review of its functional anatomy and behavioural correlates. Brain, 129, 564–83. [DOI] [PubMed] [Google Scholar]
- Chao-Gan Y., Yu-Feng Z. (2010). DPARSF: a MATLAB toolbox for “pipeline” data analysis of resting-state fMRI. Frontiers in Systems Neuroscience, 4, 13.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark L.A., Watson D. (1991). Tripartite model of anxiety and depression: psychometric evidence and taxonomic implications. Journal of Abnormal Psychology, 100, 316–36. [DOI] [PubMed] [Google Scholar]
- Cox, C.L., Uddin, L.Q., Di Martino, A., Castellanos, F.X., Milham, M.P., Kelly, C. (2012). The balance between feeling and knowing: affective and cognitive empathy are reflected in the brain's intrinsic functional dynamics. Social Cognitive and Affective Neuroscience, 7, 727–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douaud G., Smith S., Jenkinson M., et al. (2007). Anatomically related grey and white matter abnormalities in adolescent-onset schizophrenia. Brain, 130, 2375–86. [DOI] [PubMed] [Google Scholar]
- Fransson P., Marrelec G. (2008). The precuneus/posterior cingulate cortex plays a pivotal role in the default mode network: evidence from a partial correlation network analysis. NeuroImage, 42, 1178–84. [DOI] [PubMed] [Google Scholar]
- Good C.D., Johnsrude I.S., Ashburner J., Henson R.N., Friston K.J., Frackowiak R.S. (2001). A voxel-based morphometric study of ageing in 465 normal adult human brains. NeuroImage, 14, 21–36. [DOI] [PubMed] [Google Scholar]
- Greicius M.D., Flores B.H., Menon V., et al. (2007). Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biological Psychiatry, 62, 429–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller A.S., van Reekum C.M., Schaefer S.M., et al. (2013). Sustained striatal activity predicts eudaimonic well-being and cortisol output. Psychological Science, 24, 2191–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himberg J., Hyvarinen A., Esposito F. (2004). Validating the independent components of neuroimaging time series via clustering and visualization. NeuroImage, 22, 1214–22. [DOI] [PubMed] [Google Scholar]
- Huta V. (2012). Linking peoples’ pursuit of eudaimonia and hedonia with characteristics of their parents: parenting styles, verbally endorsed values, and role modeling. Journal of Happiness Studies, 13, 47–61. [Google Scholar]
- Huta V., Ryan R.M. (2010). Pursuing pleasure or virtue: the differential and overlapping well-being benefits of hedonic and eudaimonic motives. Journal of Happiness Studies, 11, 735–62. [Google Scholar]
- Huta V., Waterman A.S. (2014). Eudaimonia and its distinction from hedonia: developing a classification and terminology for understanding conceptual and operational definitions. Journal of Happiness Studies, 15, 1425–56. [Google Scholar]
- Kong F., Liu L., Wang X., Hu S., Song Y., Liu J. (2015). Different neural pathways linking personality traits and eudaimonic well-being: a resting-state functional magnetic resonance imaging study. Cognitive, Affective, & Behavioral Neuroscience, 15, 299–309. [DOI] [PubMed] [Google Scholar]
- Kong F., Xue S., Wang X. (2016). Amplitude of low frequency fluctuations during resting state predicts social well-being. Biological Psychology, 118, 161–8. [DOI] [PubMed] [Google Scholar]
- Kringelbach M.L., Berridge K.C. (2009). Towards a functional neuroanatomy of pleasure and happiness. Trends in Cognitive Sciences, 13, 479–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledberg A., Akerman S., Poland P.F. (1998). Estimation of the probabilities of 3D clusters in functional brain images. NeuroImage, 8, 113–28. [DOI] [PubMed] [Google Scholar]
- Lewis G.J., Kanai R., Rees G., Bates T.C. (2014). Neural correlates of the ‘good life’: eudaimonic well-being is associated with insular cortex volume. Social Cognitive and Affective Neuroscience, 9, 615–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y.O., Adali T., Calhoun V.D. (2007). Estimating the number of independent components for functional magnetic resonance imaging data. Human Brain Mapping, 28, 1251–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao W., Chen H., Feng Y., et al. (2010). Selective aberrant functional connectivity of resting state networks in social anxiety disorder. NeuroImage, 52, 1549–58. [DOI] [PubMed] [Google Scholar]
- Luo Y., Huang X., Yang Z., Li B., Liu J., Wei D. (2014). Regional homogeneity of intrinsic brain activity in happy and unhappy individuals. PLoS One, 9, e85181.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y., Kong F., Qi S., You X., Huang X. (2016). Resting-state functional connectivity of the default mode network associated with happiness. Social Cognitive and Affective Neuroscience, 11, 516–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power J.D., Barnes K.A., Snyder A.Z., Schlaggar B.L., Petersen S.E. (2012). Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage, 59, 2142–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle M.E., MacLeod A.M., Snyder A.Z., Powers W.J., Gusnard D.A., Shulman G.L. (2001). A default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America, 98, 676–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Routledge C., Arndt J., Wildschut T., et al. (2011). The past makes the present meaningful: nostalgia as an existential resource. Journal of Personality and Social Psychology, 101, 638–52. [DOI] [PubMed] [Google Scholar]
- Ryan R.M., Deci E.L. (2001). On happiness and human potentials: a review of research on hedonic and eudaimonic well-being. Annual Review of Psychology, 52, 141–66. [DOI] [PubMed] [Google Scholar]
- Ryff C.D. (1989). Happiness is everything, or is it? Explorations on the meaning of Psychological Well-being. Journal of Personality and Social Psychology, 57, 1069–81. [Google Scholar]
- Sato W., Kochiyama T., Uono S., et al. (2015). The structural neural substrate of subjective happiness. Scientific Reports, 5, 16891.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter D.L., Addis D.R., Buckner R.L. (2007). Remembering the past to imagine the future: the prospective brain. Nature Reviews Neuroscience, 8, 657–61. [DOI] [PubMed] [Google Scholar]
- Sedikides C., Wildschut T., Cheung W.-Y., et al. (2016). Nostalgia fosters self-continuity: uncovering the mechanism (social connectedness) and consequence (eudaimonic well-being). Emotion, 16, 524–39. [DOI] [PubMed] [Google Scholar]
- Sheline Y.I., Price J.L., Yan Z.Z., Mintun M.A. (2010). Resting-state functional MRI in depression unmasks increased connectivity between networks via the dorsal nexus. Proceedings of the National Academy of Sciences of the United States of America, 107, 11020–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.M., Fox P.T., Miller K.L., et al. (2009). Correspondence of the brain’s functional architecture during activation and rest. Proceedings of the National Academy of Sciences of the United States of America, 106, 13040–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.M., Jenkinson M., Woolrich M.W., et al. (2004). Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage, 23(Suppl 1), S208–19. [DOI] [PubMed] [Google Scholar]
- Smith, S.M., Nichols, T.E. (2009). Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. NeuroImage, 44, 83–98. [DOI] [PubMed] [Google Scholar]
- Studenmund A. (2000). Using Econometrics: A Practical Guide, 4th edn.Boston, MA: Addison Wesley. [Google Scholar]
- Takano K., Tanno Y. (2009). Self-rumination, self-reflection, and depression: self-rumination counteracts the adaptive effect of self-reflection. Behaviour Research and Therapy, 47, 260–4. [DOI] [PubMed] [Google Scholar]
- Trapnell P.D., Campbell J.D. (1999). Private self-consciousness and the five-factor model of personality: distinguishing rumination from reflection. Journal of Personality and Social Psychology: Personality Processes and Individual Differences, 76, 284–304. [DOI] [PubMed] [Google Scholar]
- Uddin L.Q., Kelly A.M., Biswal B.B., Castellanos F.X., Milham M.P. (2009). Functional connectivity of default mode network components: correlation, anticorrelation, and causality. Human Brain Mapping, 30, 625–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urry H.L., Nitschke J.B., Dolski I., et al. (2004). Making a life worth living - neural correlates of well-being. Psychological Science, 15, 367–72. [DOI] [PubMed] [Google Scholar]
- Waterman A.S. (1993). Two conceptions of happiness: contrasts of personal expressiveness (eudaimonia) and hedonic enjoyment. Journal of Personality and Social Psychology, 64, 678. [Google Scholar]
- Watson D., Clark L.A., Tellegen A. (1988). Development and validation of brief measures of positive and negative affect - the PANAS scales. Journal of Personality and Social Psychology, 54, 1063–70. [DOI] [PubMed] [Google Scholar]
- Waytz A., Hershfield H.E., Tamir D.I. (2015). Mental simulation and meaning in life. Journal of Personality and Social Psychology, 108, 336–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S., Ford J.M. (2012). Default mode network activity and connectivity in psychopathology. Annual Review of Clinical Psychology, 8, 49–76. [DOI] [PubMed] [Google Scholar]
- Xia M., Wang J., He Y. (2013). BrainNet Viewer: a network visualization tool for human brain connectomics. PLoS One, 8, e68910.. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.