Abstract
[Ru(terpy)(bdq)NO]3+ (TERPY) is a nitric oxide (NO) donor that promotes relaxation of the mesenteric artery and aorta in rats. We sought to investigate whether it acts as both an NO donor and endothelial NO synthase (eNOS) activator, as shown previously for nitroglycerin. Human umbilical vein endothelial cells (HUVECs) and human embryonic kidney 293 cells transfected with empty vector (HEK) or eNOS cDNA (HEK-eNOS) were treated with TERPY (1 μM) for different lengths of time. eNOS expression, dimerization, and Ser1177 phosphorylation, caveolin-1 (Cav-1) oligomerization, Cav-1 Tyr14 phosphorylation were evaluated by Western blotting. Studies also assessed the production of reactive oxygen/nitrogen species (ROS/RNS) in HUVECs and HEK-eNOS cells. In HEK cells devoid of eNOS, TERPY released NO without additional stimulus indicating that is an NO donor. Moreover, in HEK-eNOS cells, TERPY-induced NO production that was blocked by L-NAME. In addition, TERPY increased ROS and ONOO– production which were blocked by more than 80% by BH4 (essential eNOS co-factor) and eNOS siRNA. These results suggest that TERPY-induced ROS and ONOO− production were originated from eNOS. HUVECs stimulated with TERPY showed increased eNOS Ser1177 and Cav-1 Tyr14 phosphorylation, and decreased eNOS dimerization, Cav-1 oligomerization, and Cav-1/eNOS interaction after 20 min. It suggests that TERPY induces eNOS hyperactivation and uncoupling by disrupting Cav-1/eNOS interaction and depleting BH4. Endothelium-dependent vasodilation in response to NO donor TERPY is associated with eNOS activation and uncoupling, and thereby appears to be mediated, at least in part, via eNOS-dependent ROS/RNS production.
Keywords: NO donor, TERPY, eNOS uncoupling, caveolin-1, eNOS hyperactivation
Graphical abstract
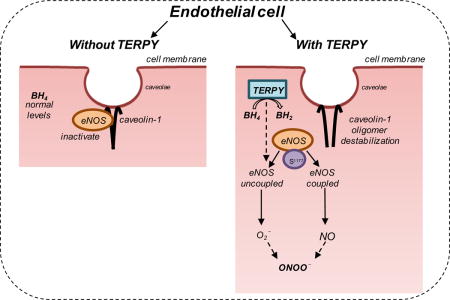
1. Introduction
Endothelial nitric oxide synthase (eNOS), comprised of reductase and oxygenase domains connected by calmodulin (CaM), functions as a dimer [1]. Nicotinamide adenine-dinucleotide phosphate (NADPH), flavin adenine dinucleotide (FAD), and flavin mononucleotide (FMN) are co-substrates of eNOS, whereas (6R-)5,6,7,8-tetrahydro-L-biopterin (BH4) and L-arginine are cofactors. When CaM affinity for eNOS increases, it promotes the alignment of two monomeric subunits, enabling the reductase domain of one eNOS molecule to transfer NADPH-derived electrons via FAD and FMN to the heme moiety in the oxygenase domain of a second juxtaposed eNOS molecule [2]. In the oxygenase domain, BH4 facilitates electron transfer upon L-arginine oxidation forming NO and L-citrulline [3].
In blood vessels, NO is primarily produced by eNOS and it is an important modulator of vascular tone [4]. However, NO is a highly reactive free radical [5] and its half-life in biological tissues is estimated to be less than 6 seconds [6]. Therefore, NO donors have become an attractive pharmacological tool to study cellular mechanisms of NO action and commonly used therapeutically, especially in patients with cardiovascular disease characterized by a reduction in NO bioavailability.
[Ru(terpy)(bdq)NO]3+ (TERPY) is a ruthenium complex NO donor that promotes a hypotensive effect with greater magnitude in hypertensive rats when compared with their normotensive controls [7]; [8]. The TERPY-induced hypotensive effect is slow, long lasting, and it does not lead to reflex tachycardia [7]. Furthermore, TERPY promotes relaxation in aorta [7]; [9] and in resistance vessels [10] from hypertensive and normotensive rats.
It was demonstrated that TERPY oxidizes BH4 to dihydrobiopterin (BH2) [11]. When BH4 bioavailability is limiting, electron transfer from eNOS flavins become uncoupled from L-arginine oxidation and hydrogen peroxide or superoxide anion are generated by uncoupled eNOS [12];[13];[14]; [15]. Moreover, in aortas of normotensive rats, the presence of endothelium or NOS activity attenuated the vasodilator effect of TERPY [11]. However, in aorta from spontaneously hypertensive rats (SHR), the effect of TERPY was entirely distinct from that observed in Wistar rats in that it was improved by endothelium or NOS activity [9].
In this work, we have demonstrated that TERPY increases the NO production by endothelial cells, in addition to donating NO. As the pharmacological interaction between TERPY and NOS is not completely understood, this study aimed to evaluate the effect of TERPY on NOS activity in endothelial cells.
2. Methods
2.1 Cell culture
Human umbilical vein endothelial cells (HUVECs)
HUVECs were purchased from Vec Technologies (Rensselaer, NY). They were cultured in growth medium (EGM-2 plus rhEGF 0.5 mL, ascorbic acid 0.5 mL, hydrocortisone 0.2 mL, heparin 0.5 mL; VEGF 0.5 mL; GA-1000 0.5 mL, R3-IGF-1 0.5 mL, rhFGF-B 2.0 mL) from Lonza (Walkersville, MD) supplemented with 10% of fetal bovine serum (FBS) from Gemini Bio-Products (West Sacramento, CA) and used at passages 4–6.
Wild-type (WT) human embryonic kidney (HEK) 293 and HEK cells stably overexpressing eNOS cDNA (HEK-eNOS)
HEK cells were purchased from the American Type Culture Collection (Rockville, MD). eNOS cDNA (in pcDNA3.1) was transduced in HEK 293 cells using Lipofectamine 2000 from Invitrogen (Carlsbad, CA) according to the manufacturer’s instructions. They were cultured in Dulbecco’s modified eagle medium (DMEM 1x) from Corning Cellgro (Manassas, VA) supplemented with 10% FBS and 1% penicillin/streptomycin from Invitrogen (Carlsbad, CA).
2.2 Drugs and Reagents
The NO donor compound TERPY (MW: 951.00) was synthesized in the Analytical Chemistry Laboratory at the Department of Physics and Chemistry of the Faculty of Pharmaceutical Sciences of Ribeirão Preto, as described by de Lima et al. [16]. The structure of TERPY was demonstrated by Bonaventura et al. [11] and Munhoz et al. [7]. eNOS si-RNA was purchased from QIAGEN (Valencia, CA). Nω-nitro-L-arginine methyl ester hydrochloride (L-NAME, non-selective NOS inhibitor), dihydroethidium (DHE, fluorescence probe for measurement of ROS), catalase-polyethylene glycol (PEG-catalase, C4963), calcium ionophore (A23187), 4-amino-5-(4-chlorophenyl)-7-(t-butyl) pyrazolo [3, 4-d] pyrimidine (PP2, Src kinase inhibitor), catalase (C1345), superoxide dismutase (SOD, S8160), superoxide dismutase-polyethylene glycol (PEG-SOD, S9549) and uric acid (U0881) were obtained from Sigma-Aldrich (St. Louis, MO). Coumarin-7-boronic acid (7-CBA, fluorescent probe for detection of peroxynitrite, ONOO–) and tetrahydro-L-biopterin hydrochloride (BH4) were obtained from Cayman Chemical (Ann Arbor, MI). 4-Amino-5-methylamino-2′,7′-difluorofluorescein diacetate (DAF-FM, fluorescence probe for detection of NO) was obtained from Invitrogen (Carlsbad, CA). CuFL, a fluorescein-based Cu(II) NO sensor, was obtained from Strem Chemicals (Newburyport, MA). Rabbit anti-phospho-eNOS (Ser1177) was obtained from Cell Signaling Technology (Danvers, MA). Mouse anti-eNOS (610297), rabbit anti-Cav-1 polyclonal (610060), mouse anti-Cav-1 monoclonal (610406), mouse anti-phospho-Cav-1 (pTyr14, 611339) and mouse anti-actin (612657) were obtained from BD Biosciences (San Jose, CA). Mouse anti-GAPDH (sc-25778) was from Santa Cruz Biotechnology (Dallas, TX). Rabbit anti-phospho-eNOS (Tyr657) was obtained from ECM Biosciences (Versailles, KY).
2.3 Western Blotting
After serum deprivation in culture medium for 3–5 h, treatment was performed with different reagents in confluent HUVECs, HEK-WT, and HEK-eNOS cells. The samples were lysed on ice in RIPA buffer (50 mM Tris-HCl, 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS, pH 7.4 ± 0.2) containing 1% of protease and phosphatase inhibitors cocktail (Sigma-Aldrich, St. Louis, MO). The lysates were centrifuged (20 minutes, 12,000 rpm, 4°C) and protein concentration was quantified by Lowry method [17]. Lysates were boiled in 4× Laemmli sample buffer (Bio-Rad Laboratories, Hercules, CA) and 10% mercaptoethanol and after that they were subjected to SDS polyacrylamide gel electrophoresis (SDS-PAGE) and blotted onto nitrocellulose membranes. The membranes were blocked with 5% blotting-grade nonfat dry milk (Bio-Rad, Hercules, CA) in Tris-buffered saline (TBS plus 0.05% Tween-20, 60 min) and incubated with primary antibodies overnight at 4°C, followed by subsequent incubation with secondary antibodies (mouse or rabbit) for 1 hour at room temperature. Proteins were visualized with enhanced chemiluminescence substrate (Super Signal West Pico or Femto, Thermo Scientific, Waltham, MA).
Low-Temperature SDS-PAGE analyses of eNOS dimer/monomer and Cav-1 oligomer/monomer
Cultured cells were lysed on ice in buffer (RIPA) and lysates were under non-denaturing conditions. Samples were prepared with 5x SDS loading buffer (250 mM Tris, 10% SDS, 20% glycerol, 0.02% bromophenol blue) plus 10% dithiothreitol (DTT 1M) and were loaded on 8–12% polyacrylamide gels. During the electrophoresis process and transfer of proteins to nitrocellulose membrane, buffers were placed in an ice-water bath and the whole apparatus was kept at 4°C. The monomer and dimer forms of eNOS and oligomer and monomer forms of Cav-1 were incubated with specific polyclonal antibodies and detected by chemiluminescence substrate (Pierce).
2.4 Co-immunoprecipitation (co-IP)
Co-immunoprecipitation (co-IP) was performed as previously described [18]; [19], with the following modifications. We performed the treatment with TERPY in confluent HUVECs plated on 100 mm dishes. Cells were lysed in 2% octyl-D-glucoside (ODG) buffer (50 mM Tris-HCl, 150 mM NaCl, 1 mM EDTA, 1 mM NaF, 1% protease inhibitor cocktail, 1 mM sodium orthovanadate) and centrifuged (20 minutes, 13,000 rpm, 4°C). Supernatants were collected and rotated overnight at 4°C with specific antibodies (anti–rabbit immunoglobulin IgG, rabbit anti-eNOS polyclonal or mouse anti-Cav-1 monoclonal). After that, the lysates were rotated for 2 hours at 4°C with protein A/G PLUS-agarose beads. Samples containing beads were washed eight times in Tris-buffer (50 mM Tris-HCl, 150 mM NaCl, 1 mM EDTA, 1 mM NaF, 0,5% NP-40). The proteins were eluted with Laemmli sample buffer and then the samples were boiled for 10 minutes at 100°C and eNOS or Cav-1 were detected by Western blot analysis.
2.5 siRNA-mediated eNOS depletion
HUVECs seeded in 60 mm wells were grown to ~40% confluence and transfected with siRNA specific for eNOS or scrambled siRNA at the final concentration of 100 nM. After 72 hours, cells were stimulated and lysed at 4° for Western blot analysis.
2.6 Analysis of eNOS activity by DAF-FM or NO sensor CuFL fluorescence probes
Cells were grown in 96-well black assay plates (Corning Incorporated, Corning, NY), washed twice with HBSS, and incubated with media containing 2.5 μM DAF-FM or 1 μM CuFL plus 1 mM L-arginine for 60 minutes. Before stimulation, the cells were pretreated with 100 μM BH4 (30 minutes), 100 μM L-NAME (30 minutes) or 1 mM L-NAME (30 minutes). Then, treatment of the same cells was performed with different reagents (vehicle, 1 μM TERPY or 5 μM A23187) for times indicated. Fluorescence produced by benzotriazole or CuFL was read using the bottom-read mode of a SpectraMax M5 Microplate Reader (Molecular Advices, Sunnyvale, CA) and fluorescence of DAF-FM was measured using Excitation: 488 nm/Emission: 530 nm.
2.7 Measurement of superoxide anion using DHE probe
Cells were grown in 96-well black assay plates (Corning Incorporated, Corning, NY), washed twice with HBSS, and incubated with media containing 2.5 μM DHE plus 1 mM L-arginine for 60 minutes. Before stimulation, the cells were pretreated with 100 μM BH4 (30 minutes), 100 μM L-NAME (30 minutes), 1 mM L-NAME (30 minutes), 300 U/mL SOD (60 minutes), 300 U/mL PEG-SOD (60 minutes) or 300 μM uric acid (120 minutes). Then, treatment of the same cells was performed with different reagents (vehicle or 1 μM TERPY) for times indicated. Fluorescence produced by DHE was measured using wavelength 370 nm/420 nm (Excitation/Emission) in SpectraMax M5 Microplate Reader (Molecular Advices, Sunnyvale, CA).
2.8 Measurement of ONOO– production by coumarin-7-boronate acid (7-CBA)
Cells were grown in 6-well assay plates (Corning Incorporated, Corning, NY), washed twice with HBSS, and incubated with media containing 20 μM 7-CBA plus 1 mM L-arginine for 60 minutes. Before stimulation, some samples were pretreated with 100 μM BH4, 300 U/mL Catalase (30 minutes), 300 U/mL PEG-cat (30 minutes), 300 U/mL SOD (60 minutes), 300 U/mL PEG-SOD (60 minutes) or 300 μM uric acid (120 minutes). Then, treatment with different reagents (vehicle or 1μM TERPY) was performed for the times indicated. The supernatant was collected, placed in 96-well assay plates, and fluorescence analysis of the oxidation product 7-OH-coumarin (COH) [20] was performed on a SpectraMax M5 Microplate Reader (Molecular Advices, Sunnyvale, CA). Fluorescence was measured using 350 nm (excitation) and 450 nm (emission). The results were normalized to total cellular protein.
2.9 Densitometry and statistics
Densitometry of protein bands was performed with ImageJ software (http://rsbweb.nih.gov/ij/). Comparison of two groups was conducted using Student’s t test, and three or more groups were compared using one-way ANOVA with Tukey’s post hoc testing. Experimental data are presented as mean ± standard error of the mean (SEM). All statistical tests were performed two-sided and non-blinded using GraphPad Prism software for Mac (GraphPad Software, La Jolla, CA). P values <0.05 were considered statistically significant.
3. Results
NO production induced by TERPY is dependent, in part, on eNOS
It is known that eNOS phosphorylation on Ser1177 promotes NO production. Thus, we examined NO production stimulated by TERPY in HUVECs, WT-HEK and HEK-eNOS cells. These results are shown in two different ways: using DAF-FM diacetate probe in HUVECs and via Cu(II) fluorescein-based NO sensor (CuFL) in WT-HEK and HEK-eNOS cells. TERPY induced an increase in DAF-FM fluorescence in HUVECs which was blocked by approximately 50% in cells pretreated with L-NAME (100 μM or 1 mM) (Figure 1A). Also, we stimulated HUVECs with A23187 (5 μM) as a positive control (Figure 1B) and NO production was blocked by L-NAME (100 μM). Using the CuFL fluorescent copper probe, TERPY increased NO level in WT-HEK cells (Figure 1C) which may be promoted by its direct effect as a NO donor. In non-treated HEK-eNOS cells, we observed time-dependent basal NO production which was significantly increased after addition of A23187 (5 μM) or TERPY (1 μM) and partially blocked by L-NAME (1 mM) (Figure 1D). These data indicate that NO production in HUVECs and HEK-eNOS stimulated by TERPY is partially dependent on eNOS.
Figure 1. NO production induced by TERPY is dependent in part on eNOS.
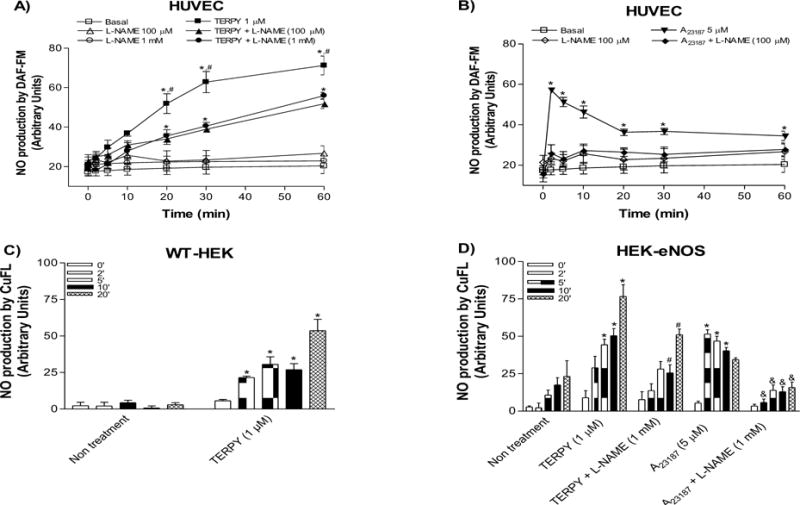
(A) Nitric oxide (NO) was measured by DAF-FM probe in human umbilical vein endothelial cells (HUVECs) stimulated by TERPY 1 μM (A) and A23187 5 μM (B) as a positive control. TERPY-induced NO production was inhibited in part by L-NAME (100 μM or 1 mM) (A). CuFL NO sensor was used in wild-type human embryonic kidney 293 cells (WT-HEK) and in HEK cells stably transduced with eNOS cDNA (HEK-eNOS) to detect NO level directly liberated by TERPY (C,D). HEK-eNOS, in absence or presence of L-NAME (1 mM), were stimulated with TERPY (1 μM) or A23187 (5 μM) (D). *p<0.05 indicates statistical difference versus basal condition (non- treatment or basal, n=3–5). #p<0.05 indicates statistical difference between TERPY versus TERPY + L-NAME (n=3–5). &p<0.05 indicates statistical difference between A23187 versus A23187 + L-NAME (n=3–5). “n” indicates independent experiments.
TERPY-induced eNOS dysfunction: eNOS uncoupling, ROS and ONOO– production
HUVECs were exposed to TERPY (1 μM) for 1 to 60 minutes. As shown in Figures 2A and 2B, the ratio of monomeric to dimeric eNOS was significantly higher after 5 minutes of treatment with TERPY. Accumulation of eNOS in its monomeric form was observed for up to 60 minutes, indicative of eNOS uncoupling. After TERPY treatment, no difference was observed in total eNOS expression (Figure 2C). Next, to evaluate whether TERPY-induced eNOS uncoupling is associated with ROS and ONOO– production, we incubated HUVECs with DHE and 7-CBA respectively, in the absence (control) or presence of TERPY. TERPY (1 μM) induced an increase ROS (Figures 3A, 3B and 3F) and ONOO– (Figure 3D) production. The source of ROS and ONOO– was confirmed to be eNOS, since L-NAME (100 μM or 1 mM) blocked the increase in DHE fluorescence (Figures 3A and 3F) induced by TERPY. Moreover, in absence of eNOS (HUVECs treated with eNOS siRNA), the fluorescence intensity of 7-CBA following stimulation with TERPY was abolished (Figure 3C), as well as no fluorescent products in the presence of DHE were observed in WT-HEK after TERPY’s stimulation (Figure 3E). Moreover, the both superoxide scavengers SOD and PEG-SOD were able to decrease the fluorescence intensity of DHE (Figure 3B) or 7-CBA (Figure 3D), showing that TERPY-induced eNOS uncoupling were followed by ROS and ONOO– production. However, uric acid did not change the fluorescence intensity of DHE (Figure 3B) or 7-CBA (Figure 3D) stimulated by TERPY.
Figure 2. TERPY-induced changes in eNOS dimerization in HUVECs.
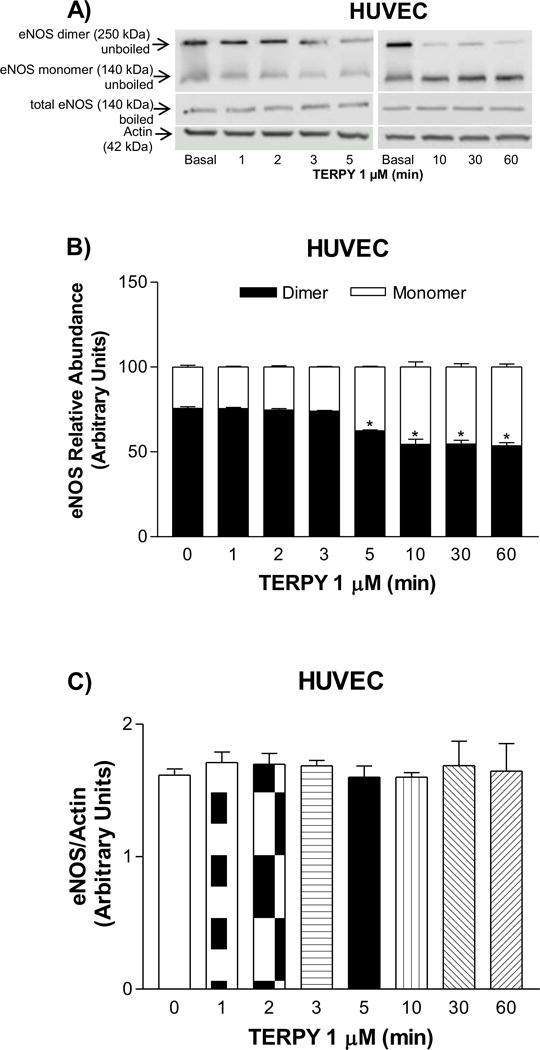
(A) Representative Western blot of eNOS dimer/monomer distribution in unboiled samples (top blot), total eNOS expression observed in boiled samples (middle blot), and actin (bottom blot; loading control) in human umbilical vein endothelial cells (HUVECs). (B) Basal and time-dependent changes in eNOS dimer and monomer induced by TERPY (1 μM) as compared to (C) total eNOS expression in boiled samples. *Indicates p<0.05 versus basal condition (n=3–5). “n” indicates independent experiments.
Figure 3. TERPY-induced eNOS dysfunction in HUVECs and HEK-eNOS.
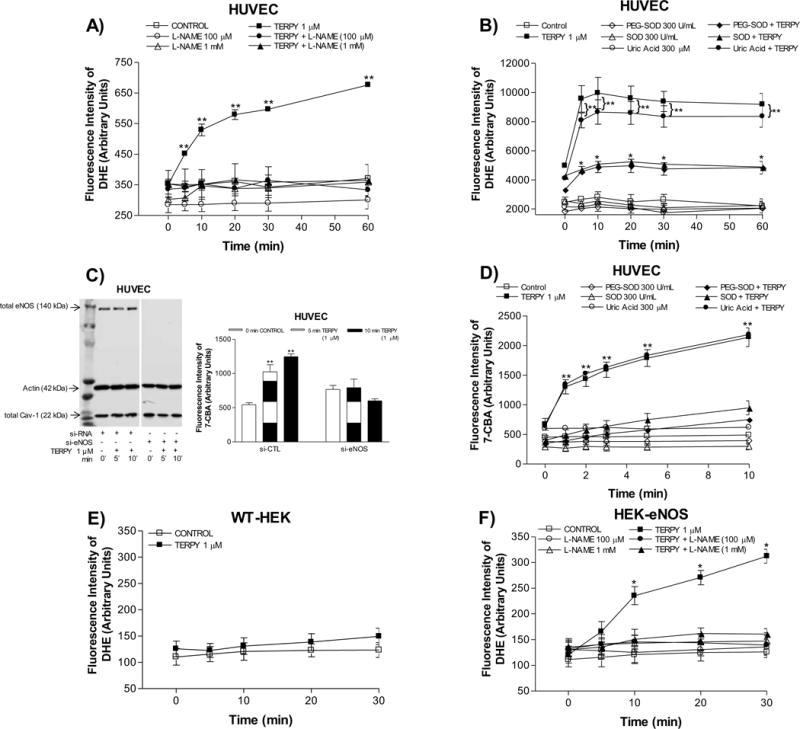
Reactive oxygen species were measured using dihydroethidium (DHE) in human umbilical vein endothelial cells (HUVECs) (A,B), wild-type human embryonic kidney 293 cells (HEK-WT) (E) and HEK cells stably transduced with eNOS cDNA (HEK-eNOS) (F) in absence (Control) or presence of TERPY (1 μM) for times indicated. (C) and (D) Peroxynitrite was measured using coumarin-7-boronic acid (7-CBA) in HUVECs. (C) Representative Western blot of total eNOS, actin, and total Cav-1 in control si-RNA (right lane) and eNOS si-RNA treated cells (left lane). (A) and (F) L-NAME (100 μM or 1 mM) inhibited the TERPY-stimulated fluorescence intensity of DHE. (B) and (D) SOD (300 U/mL) and PEG-SOD (300 U/mL) decreased the fluorescence intensity of DHE and 7-CBA stimulated by TERPY, but not uric acid (300 μM). *p<0.05 indicates difference between TERPY, TERPY + SOD or TERPY + PEG-SOD versus basal condition (control, n=3–5) and **p<0.01 indicates difference between TERPY or TERPY + uric acid versus basal condition (control, n=3–5). “n” indicates independent experiments.
Treatment with BH4 prevented eNOS uncoupling induced by TERPY
Oxidation, and thus functional depletion of BH4, is associated with eNOS dysfunction and uncoupling [13–15]. To determine whether eNOS uncoupling occurs directly or indirectly in response to treatment with TERPY, cells were treated with 100 μM BH4 for 30 minutes prior to stimulation with TERPY. In non-boiled gels, eNOS dimerization (>250 kDa) was reduced and eNOS monomers (140 kDa) accumulated (Figure 4A and 4B) consistent with TERPY-induced eNOS uncoupling in HUVECs. However, TERPY-induced eNOS uncoupling was prevented in presence of BH4 (Figures 4A and 4B). In absence of TERPY, BH4 had no effect indicating basal levels were not limiting and eNOS was fully coupled in untreated HUVECs (Figure 4A and 4B). In boiled samples, neither TERPY nor BH4 had an effect on total eNOS protein (Figure 4C). To assess eNOS function, we measured the production of ROS (Figure 4D) and ONOO– (Figure 4E) in HUVECs stimulated by TERPY with and without pretreatment with BH4. As shown in Figure 4D, TERPY induced a significant increase in DHE fluorescence which was abolished in cells pretreated with BH4 suggesting TERPY increases ROS production via uncoupled eNOS. In addition, we observed that TERPY promoted a significant increase in ONOO– production which was blocked by BH4 (Figure 4E). Zielonka et al. [21] showed through the HPLC analysis the stoichiometry of the reaction, indicating that one molecule of CBA reacts with a molecule of ONOO–, producing COH with the overall yield of ~81%. Thus, ONOO– reacts with the coumarin boronate at least a million times faster than hydrogen peroxide (H2O2). Since 7-CBA can also detect hydroperoxides, we used catalase (300 U/mL) and PEG-Cat (300 U/mL) to remove H2O2 and we observed no additional effect on blocked of ONOO– level stimulated by TERPY (Figure 4E).
Figure 4. Treatment with BH4 prevented eNOS uncoupling induced by TERPY.
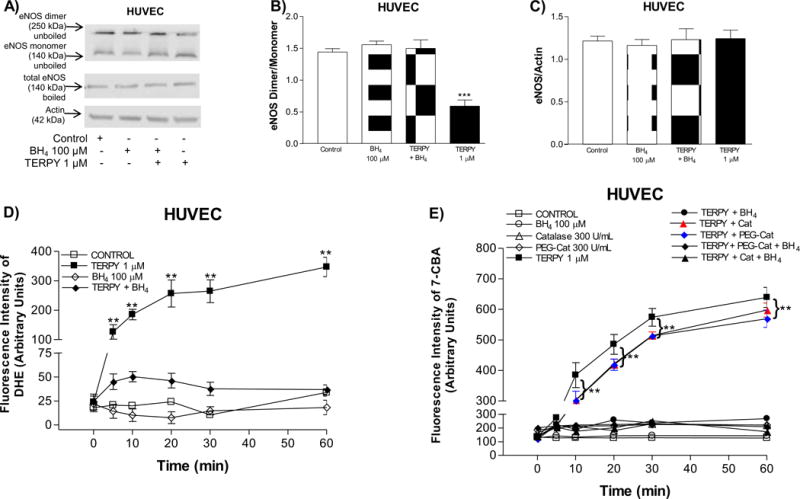
(A) Representative Western blot of eNOS dimer/monomer distribution in unboiled samples (top blot), total eNOS expression (middle blot) and actin (bottom blot) in boiled samples of human umbilical vein endothelial cells (HUVECs) in absence (Control) or presence of BH4 (100 μM). TERPY (1 μM) was added for the times indicated before lysis. (B) and (C) summarized data. (D) Reactive oxygen species measurement by dihydroethidium (DHE). (E) Peroxynitrite measurement by coumarin-7-boronic acid (7-CBA). Catalase (Cat, 300 U/mL) and PEG-Catalase (PEG-Cat, 300 U/mL) were used to remove hydrogen peroxide. **Indicates p<0.01 between TERPY, TERPY + PEG-Cat or TERPY + Cat versus basal conditions (n=3–5). ***Indicates p<0.001 between TERPY versus basal conditions (n=3–5). “n” indicates independent experiments.
TERPY-induced Cav-1 oligomer destabilization
Next, we evaluated whether TERPY had an effect on Cav-1 oligomer stability, which we had shown previously to be rapidly modulated upon NO-mediated s-nitrosylation of critical Cys residues and subsequent phosphorylation of Cav-1 Tyr14 [22]. As shown in Figures 5A and 5B, treatment of HUVECs with TERPY for 20 minutes reduced Cav-1 oligomer expression which was associated with an increase in Cav-1-Tyr14 phosphorylation (Figures 5C and 5D), indicative of cav-1 oligomers destabilization. On the other hand, there was no change in total Cav-1 expression (denatured samples heated to 100°C) (Figures 5G and 5H). Moreover, we showed that phosphorylation of Cav-1 (Tyr14) was following by phosphorylation of Src (Tyr418) and eNOS (Ser1177). Src inhibitor PP2 blocked Src and Cav-1 phosphorylation.
Figure 5. TERPY-induced destabilization of Cav-1 oligomers.
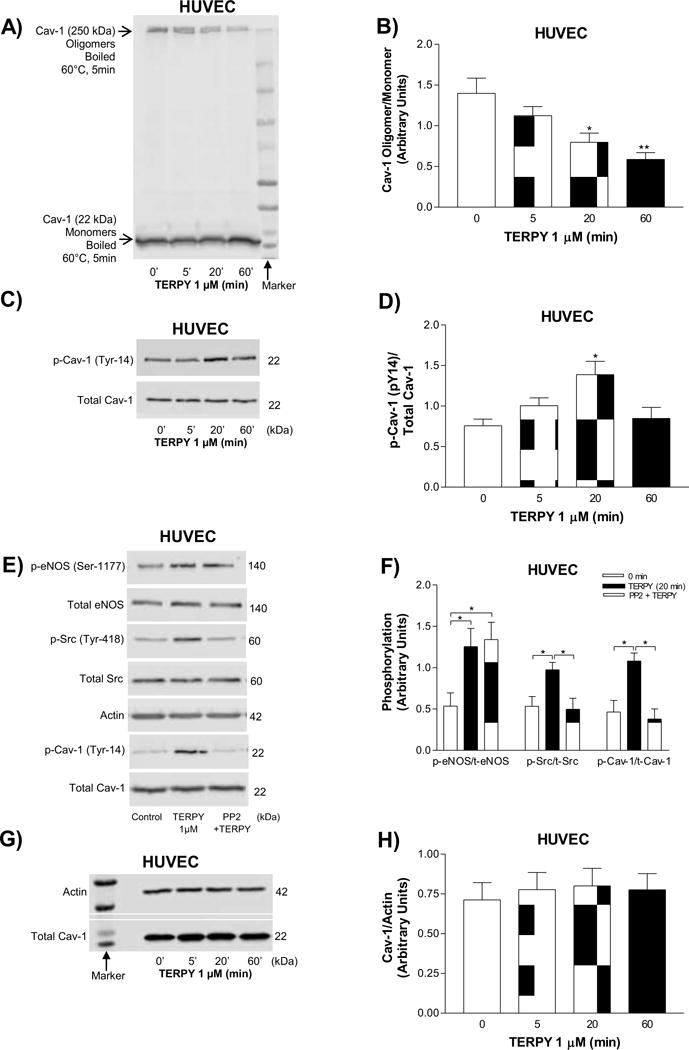
(A) Representative Western blot of Cav-1 oligomer/monomer distribution and (B) summarized data. (C) Representative Western blot of Cav-1 Tyr-14 phosphorylation and (D) summarized data. (E) Representative Western blot of phosphorylation of eNOS-Ser1177, Src-Tyr418, and Cav-1-Tyr14 in absence or presence of PP2 (Src kinase inhibitor) for times indicated and (F) summarized data. (G) Representative Western blot of total Cav-1 expression and (H) summarized data. All experiments were performed in human umbilical vein endothelial cells (HUVECs) exposed to the TERPY (1 μM) for times indicated. *p<0.05 and **p<0.01 indicate statistical difference versus basal condition (n=3–5). “n” indicates independent experiments.
TERPY reduced eNOS/Cav-1 interaction and promoted eNOS-Ser1177 hyperphosphorylation
Cav-1 oligomer destabilization induced by TERPY may modulate the interaction between eNOS and Cav-1 and thereby affect eNOS activity. To assess this, we immunoprecipitated Cav-1 and blotted for Cav-1 and eNOS in HUVECs treated with TERPY. As shown in Figures 6A and 6B, TERPY, in a time-dependent manner, reduced eNOS/Cav-1 interaction. Next, HUVECs and HEK-eNOS were treated with TERPY for 5 to 60 minutes and eNOS phosphorylation on active site Ser1177 was assessed with a peak effect at about 20 minutes in both HUVECs (Figures 6C and 6D) and HEK-eNOS cells (Figures 6E and 6F). TERPY reduced the association between eNOS and Cav-1 and this effect was associated with an increase in eNOS phosphorylation. Moreover, is known that eNOS phosphorylation at Tyr657 residue regulates negatively eNOS activity. However, we did not observe changing at eNOS Tyr657 phosphorylation (Figures 6C and 6D).
Figure 6. TERPY decreases eNOS/Cav-1 interaction and promotes eNOS hyperphosphorylation at Serine1177.
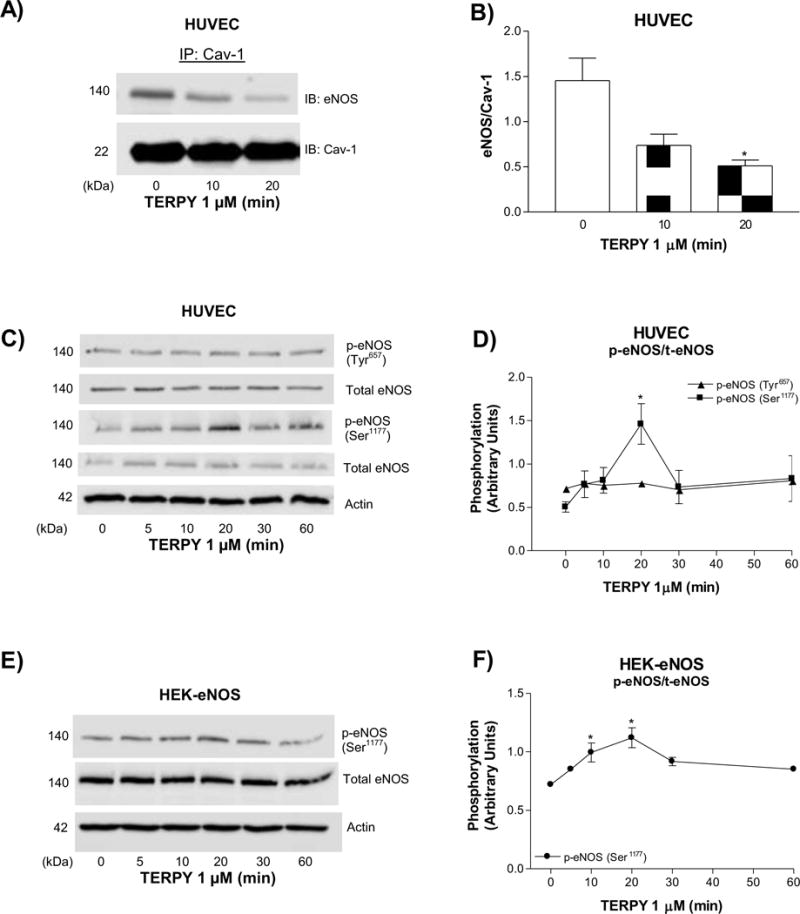
(A) Interaction of Cav-1 and eNOS determined by co-immunoprecipitation (Co-IP) in human umbilical vein endothelial cells (HUVECs) exposed to TERPY (1 μM). (C) Phosphorylation of eNOS Ser1177 and eNOS Tyr657 in HUVECs stimulated with TERPY (1 μM) for times indicated and (D) summarized data. (E) Phosphorylation of eNOS Ser1177 in human embryonic kidney 293 cells transduced with eNOS cDNA (HEK-eNOS) and (F) summarized data. *p<0.05 indicates statistical difference versus basal condition (n=3–5). “n” indicates independent experiments.
4. Discussion
Besides the well-known effect of TERPY as a NO donor [7];[8];[9];[10]; [11], our work elucidates a secondary effect of TERPY as an eNOS activator and uncoupling factor. This effect of TERPY was associated with NO, ONOO–, and ROS production, BH4 depletion, Cav-1-Tyr14 phosphorylation and oligomer destabilization, and a decrease in Cav-1/eNOS interaction. In its phosphorylated form, Cav-1 might impair the localization of Cav-1 in the plasma membrane and thereby, render it less able to bind and inactivate eNOS. Alternatively, uncoupled eNOS may not be able to bind or be inhibited by Cav-1, leading to sustained eNOS phosphorylation and sustained NO production. In fact, in cells treated with TERPY, eNOS Ser1177 phosphorylation persisted for 20 minutes and it was associated with NO production. Thus, TERPY is not simply an NO donor, rather, it also promotes sustained NO, ONOO–, and ROS production, consistent with eNOS activation and uncoupling.
Kojima et al., [23] developed di-amino-fluorescein (DAF), 4-amino-5-methylamino-2′,7′-difluorofluorescein (DAF-FM) and the cell-permeable DAF-FM diacetate to detect NO in many different cells types and using several methodologies. Other molecules as the NO2 and N2O3 are intermediate that activates DAF-FM through the formation of one electron oxidation product of DAF, which then combines with NO to form DAF-T [24]. Despite some disadvantages in using DAF-FM diacetate to detect NO, different studies have been using this probe to measure NO [25]; [26]. Unlike existing fluorescent sensors, the construct - a Cu(II) complex of a fluorescein modified with an appended metal-chelating ligand (FL) - can directly and immediately pick up NO formed or released rather than a derivative reactive nitrogen species. The NO-induced fluorescence involves reduction of the complex to Cu(I) with release of the nitrosated ligand, which occurs irreversibly. NO production was detected through nanomole concentrations of NO in both constitutive and inducible NO synthases (cNOS and iNOS, respectively) in mammalian cultured cells in a concentration- and time-dependent manner [27]; [28]. In our study, we used two different NO sensors (DAF-FM diacetate and CuFL) to confirm that TERPY is a NO donor and to show that NO production induced by TERPY is dependent, in part, on eNOS (Figure 1A and D).
Sullivan and Pollock [29] suggested that eNOS can exist in two forms: coupled and uncoupled, with the uncoupled enzyme residing mainly in the cytosol and the coupled dimeric form associated with the membrane. Schmidt et al., [30] confirmed this hypothesis by showing that HUVECs exhibit basal BH4 levels of 0.3 pmol/mg, and as a consequence, eNOS may not be fully coupled. When we analyzed the eNOS dimer/monomer ratio in HUVECs under basal conditions, we observed that eNOS exists primarily as dimers (Figure 2B) and that addition of BH4 did not have a statistically significant effect on dimerization (Figure 4B). When we treated HUVECs with TERPY for 1 to 60 minutes, we observed a remarkable increase in eNOS monomerization (<50% decrease in dimer/monomer ratio) after 5 minutes of treatment indicating eNOS was becoming uncoupled, with a fraction of the total eNOS pool remaining coupled (Figure 2A–B). eNOS uncoupling induced by TERPY was reversed by pretreatment of cells with BH4, and thus dependent on BH4 depletion (Figure 4).
Ravi et al. [31] demonstrated in bovine aortic endothelial cells (BAECs) that exogenous NO can promote S-nitrosylation and act as an inhibitor of eNOS activity associated with decrease in eNOS dimer levels. However, eNOS could be maintained in a completely dimeric state after increasing the concentration of thioredoxin and thioredoxin reductase system or in the presence of the reducing agent DTT (5 mM). Also, Chen et al. [32] demonstrated that two cysteine residues, Cys689 and Cys908, can be S-glutathionylated modifying their own conformation that would disrupt FAD–FMN alignment, interrupting electron transfer between the flavins resulting in S-glutathionylation dependent-eNOS-uncoupling, changing NO to superoxide anion generation. The eNOS uncoupling induced by S-glutathionylation in BAECs treated with 1,3-bis(2-chloroethyl)-1-nitrosourea (BCNU) was blocked by DTT.
In our study, the eNOS monomerization was not due to S-nitrosylation or S-glutathionylation, since we used DTT (10%) in all samples to perform western blot in non-denaturing conditions. Furthermore, S-glutathionylation is increased in hypertensive vessels, resulting in impaired endothelium-dependent vasodilation [32]. However, we showed previously that vasodilator effect of TERPY is improved by endothelium only in hypertensive rats [9].
TERPY-induced eNOS monomerization can be explained by the fact that TERPY releases an active metabolic ([Ru(H2O)(bdq)(terpy)2+) that is able to oxidize BH4 to BH2 [11]. Several studies have shown that reduced levels of BH4 increase eNOS oxidase activity [13];[14]; [15], and in fact we demonstrated superoxide anion production and ONOO− formation in HUVECs stimulated with TERPY (Figure 3) suggesting that eNOS oxidase activity and dysfunction may be induced by TERPY in healthy endothelial cells. When we treated the HUVECs with BH4, the eNOS uncoupling was prevented (Figure 4A and 4B).
SOD (cell-impermeable) and PEG-SOD (cell-permeable) are superoxide scavengers, while uric acid is a natural scavenger of ONOO−. For the constant formation of ONOO− is necessary the presence of superoxide and NO. When we treated HUVECs with SOD or PEG-SOD, the fluorescence intensity of DHE and 7-CBA stimulated by TERPY was reduced as expected (Figure 3). However, uric acid did not change the fluorescence intensity of DHE or 7-CBA induced by TERPY. We believed that the concentration of uric acid (300 μM) or the treatment-time (2 hours) were not enough to decrease, at least, the fluorescence intensity of 7-CBA. Uric acid (in the concentration higher than 300 μM and in treatment-time higher than 6 hours) decreased expression and activity of eNOS, reduced NO bioavailability, enhanced ROS generation and induced the apoptosis in HUVECs [33];[34];[35]; [36]. Park et al. [37] showed that attenuated NO production induced by uric acid could be related with decreasing the interaction between eNOS and calmodulin. On the other hand, Papežíková et al. [38] showed that the treatment of HUVECs with uric acid (300 μM) for 2 hours did not change NO bioavailability or eNOS Ser1177 phosphorylation, but a higher concentration (600 μM) was able to reduce NO production and decrease eNOS Ser1177 phosphorylation. In this way, we used uric acid 300 μM (physiological concentration) to avoid the unwanted effects on eNOS activity.
It was previously demonstrated that BH2 competes with BH4 for binding to eNOS, however, BH2 does not provide electrons for reductive oxygen activation [39]; [40]. Moreover, Karuppiah et al. [41] using a combination of gene silencing and pharmacological approaches, demonstrated that reduced levels of pterin bioavailability serves to suppress eNOS uncoupling. Thus, the competition between BH2 and BH4 for specific sites on eNOS could suppress eNOS uncoupling. We observed that part of the total eNOS pool remained coupled, or in dimeric form, as shown in Figure 2A–B. In this configuration, dimeric eNOS is still able to be activated and to synthesize NO. The activity of eNOS is regulated by its subcellular localization, phosphorylation on multiple residues, by post-translational lipid modifications, and through its interaction with different proteins [42], including Cav-1 which was shown previously to maintain eNOS in an inactive state [43].
Caveolin-1 degradation induced by oxidative stress [44]; [45] and NO-dependent destabilization of Cav-1 oligomers [22];[46]; [47] was previously observed. TERPY induced the destabilization of Cav-1 oligomers in association with an increase in Cav-1-Tyr14 phosphorylation, as shown in Figure 5. Chen et al. [48] showed that the NO molecule derived from NO donor DEA NONOate induces Src activation and thereby allowing Cav-1 phosphorylation on Tyr14 as we observed in our results in Figure 5E–F. The Src-dependent Cav-1-Tyr14 phosphorylation induced by the NO donor TERPY was inhibited by Src inhibitor PP2. Unlike observed by Chen et al. [48], we showed that TERPY decreased eNOS/Cav-1 interaction that could be associated with TERPY-induced Cav-1 oligomer destabilization. Cav-1 phosphorylation and monomerization [22,41] might impair its ability to remain in the plasma membrane and thereby, deter association of eNOS with Cav-1 as demonstrated in Figure 6A–B. In fact, various studies have shown that loss of caveolin-1 [22];[49]; [51] or disruption of eNOS/Cav-1 binding [43];[52]; [53] promotes the hyper-activation of eNOS. We showed that TERPY promoted sustained phosphorylation of the Ser1177 residue in eNOS (Figure 6C–F) and furthermore, the increase in DAF-FM fluorescence in HUVECs and HEK-eNOS stimulated by TERPY was decreased in presence of L-NAME (Figure 1A and 1C). These results suggest that eNOS is activated by TERPY. Although the phosphorylation of eNOS at tyrosines is less investigated, mutation analyses revealed that Tyr657 phosphorylation attenuates eNOS enzyme activity, presumably in order to limit the detrimental consequences of maintained high NO output in situations of redox stress [54];[55]; [56]. In this way, we analyzed TERPY-induced eNOS-phosphorylation at Tyr657. Our result showed that eNOS Tyr657 phosphorylation is not modulate by TERPY.
Mao et al. [57] showed that nitroglycerin, another NO donor, results in Cav-1 modification and depletion leading to eNOS hyperactivation and uncoupling. In the present study, our data support the hypothesis that TERPY promotes the oxidization of BH4 to BH2 resulting in eNOS uncoupling prior to the destabilization of Cav-1 oligomers, but ultimately also promote eNOS hyperactivation and NO production by endothelial cells.
5. Conclusion
Taken together, these results suggest that in addition to being an NO donor, TERPY also increases ROS and RNS production by affecting eNOS activity. We demonstrate that TERPY promotes eNOS uncoupling and Cav-1 oligomer destabilization and consequently, promotes sustained eNOS Ser1177 phosphorylation in endothelial cells. Thus, chemical and biological molecules that are being developed to increase NO bioavailability may do so, at least in part, by increasing eNOS activity. Future studies should consider the possibility that these agents may also promote eNOS uncoupling, ROS and RNS production, and ultimately endothelial dysfunction.
Supplementary Material
Supplemental Figure S1: Representative Western blot of total eNOS, actin, and total Cav-1 in control si-RNA (right lane) and eNOS si-RNA treated cells (left lane) treated with TERPY (1 μM) or not. The left lane shows that efficiency of the eNOS si-RNA is not 100%.
Highlights.
-
-
TERPY promotes eNOS uncoupling
-
-
TERPY increases oxidant production
-
-
TERPY promotes Cav-1 and Src phosphorylation
-
-
TERPY decreases Cav-1/NOS interaction leading to eNOS hyperphosphorylation
-
-
TERPY is a NO-donor that can modulate eNOS activity
Acknowledgments
We would like to thank Professor Dr. Randall O. Dull for allowing the use of his research lab.
Funding Source
This work was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico [Grant numbers 400164/2014-0; 232217/2014-9 (LMB, CA)] and National Institute of Health [Grant numbers HL60678 and HL125356 (RDM)].
List of nonstandard abbreviations
- TERPY
[Ru(terpy)(bdq)NO]3+
- eNOS
endothelial nitric oxide synthase
- SHR
spontaneously hypertensive rats
- Cav-1
caveolin-1
- NO
nitric oxide
- CaM
calmodulin
- FAD
flavin adenine dinucleotide
- FMN
flavin mononucleotide
- BH4
(6R-)5,6,7,8-tetrahydro-L-biopterin
- BH2
dihydrobiopterin
- HUVECs
human umbilical vein endothelial cells
- WT-HEK
wild-type human embryonic kidney 293 cell line
- HEK-eNOS
HEK cells stably expressing eNOS
- co-IP
co-immunoprecipitation
- L-NAME
Nω-nitro-L-arginine methyl ester hydrochloride
- DHE
dihydroethidium
- 7-CBA
coumarin-7-boronic acid
- DAF-FM
4-amino-5-methylamino-2′,7′-difluorofluorescein diacetate
- CuFL
Cu(II) fluorescein-based NO sensor
- A23187
calcium ionophore
- H2O2
hydrogen peroxide
- PP2
4-amino-5-(4-chlorophenyl)-7-(t-butyl) pyrazolo [3, 4-d] pyrimidine
- COH
7-OH-coumarin
- DTT
dithiothreitol
- PEG-catalase
catalase polyethylene glycol
- ONOO−
peroxynitrite
- SOD
superoxide dismutase
- PEG-SOD
superoxide dismutase-polyethylene glycol
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
Conceived and designed the experiments: SRP ZC SDSO RDM.
Performed the experiments: SRP ZC SDSO.
Analyzed the data: SRP ZC SDSO RDM.
Provided the drugs and contributed to the writing of the manuscript: SRP LMB RS da S MGB CA RDM.
Final approval of the version to be submitted: SRP ZC SDSO RS da S LMB MGB CA RDM
Conflict of interest
The authors have no conflicts of interest to declare.
References
- 1.Andrew PJ, Mayer B. Enzymatic function of nitric oxide synthases. Cardiovasc Res. 1999;43:521–531. doi: 10.1016/s0008-6363(99)00115-7. [DOI] [PubMed] [Google Scholar]
- 2.Förstermann U, Sessa WC. Nitric oxide synthases: regulation and function. Eur Heart J. 2012;33:829–837. doi: 10.1093/eurheartj/ehr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mayer B, Hemmens B. Biosynthesis and action of nitric oxide in mammalian cells. Trends Biochem Sci. 1997;22:477–481. doi: 10.1016/s0968-0004(97)01147-x. [DOI] [PubMed] [Google Scholar]
- 4.Valance P, Chan N. Endothelial function and nitric oxide: clinical relevance. Heart. 2001;85:342–350. doi: 10.1136/heart.85.3.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lorente L, Aller MA, Arias JL, Arias J. Nitric oxide (NO) is a highly reactive free radical with a multitude of organ specific regulatory functions. Ann Surg. 1996;224:688–689. doi: 10.1097/00000658-199611000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kelm M, Feelisch M, Deussen A, Schrader J, Strauer BE. The role of nitric oxide in the control of coronary vascular tone in relation to partial oxygen pressure, perfusion pressure and flow. J Cardiovasc Pharmacol. 1991;17:S95–S99. [Google Scholar]
- 7.Munhoz FC, Potje SR, Pereira AC, Daruge MG, da Silva RS, Bendhack LM, Antoniali C. Hypotensive and vasorelaxing effects of the new NO-donor [Ru(terpy)(bdq)NO(+)](3+) in spontaneously hypertensive rats. Nitric Oxide. 2012;26:111–117. doi: 10.1016/j.niox.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 8.Rodrigues GJ, Pereira AC, Vercesi JA, de Lima RG, da Silva RS, Bendhack LM. Long-lasting hypotensive effect in renal hypertensive rats induced by nitric oxide released from a ruthenium complex. J Cardiovasc Pharmacol. 2012;60:193–198. doi: 10.1097/FJC.0b013e31825bacc4. [DOI] [PubMed] [Google Scholar]
- 9.Potje SR, Munhoz FC, Perassa LA, Graton ME, Pereira AA, Nakamune AC, da Silva RS, Bendhack LM, Sumida DH, Antoniali C. Mechanisms underlying the hypotensive and vasodilator effects of Ru(terpy)(bdq)NO]3+, a nitric oxide donor, differ between normotensive and spontaneously hypertensive rats. Eur J Pharmacol. 2014;15:222–229. doi: 10.1016/j.ejphar.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 10.Araújo AV, Pereira AC, Grando MD, da Silva RS, Bendhack LM. The new NO donor Terpy induces similar relaxation in mesenteric resistance arteries of renal hypertensive and normotensive rats. Nitric Oxide. 2013;30:47–53. doi: 10.1016/j.niox.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 11.Bonaventura D, Lunardi CN, Rodrigues GJ, Neto MA, Vercesi JA, de Lima RG, da Silva RS, Bendhack LM. Endothelium negatively modulates the vascular relaxation induced by nitric oxide donor, due to uncoupling NO synthase. J Inorg Biochem. 2009;103:1366–1374. doi: 10.1016/j.jinorgbio.2009.07.015. [DOI] [PubMed] [Google Scholar]
- 12.Vásquez-Vivar J, Kalyanaraman B, Martásek P, Hogg N, Masters BS, Karoui H, Tordo P, Pritchard KA., Jr Superoxide generation by endothelial nitric oxide synthase: the influence of cofactors. Proc Natl Acad Sci USA. 1998;95:9220–9225. doi: 10.1073/pnas.95.16.9220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wever RM, van Dam T, van Rijn HJ, de Groot F, Rabelink TJ. Tetrahydrobiopterin regulates superoxide and nitric oxide generation by recombinant endothelial nitric oxide synthase. Biochem Biophys Res Commun. 1997;237:340–344. doi: 10.1006/bbrc.1997.7069. [DOI] [PubMed] [Google Scholar]
- 14.Landmesser U, Dikalov S, Price SR, McCann L, Fukai T, Holland SM, Mitch WE, Harrison DG. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J Clin Invest. 2003;111:1201–1209. doi: 10.1172/JCI14172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laursen JB, Somers M, Kurz S, McCann L, Warnholtz A, Freeman BA, Tarpey M, Fukai T, Harrison DG. Endothelial regulation of vasomotion in apoE-deficient mice: implications for interactions between peroxynitrite and tetrahydrobiopterin. Circulation. 2001;103:1282–1288. doi: 10.1161/01.cir.103.9.1282. [DOI] [PubMed] [Google Scholar]
- 16.de Lima RG, Sauaia MG, Bonaventura D, Tedesco AC, Bendhack LM, da Silva RS. Influence of ancillary ligand L in the nitric oxide photo release by the [Ru(L)(terpy)NO]3+ complex and its vasodilator activity based on visible light irradiation. Inorg Chim Acta. 2006;359:2543–2549. [Google Scholar]
- 17.Lowry OH, Rosebrough NJ, Farr AL, Randal RJ. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 18.Busconi L, Michel T. Endothelial nitric oxide synthase. N-terminal myristoylation determines subcellular localization. J Biol Chem. 1993;268:8410–8413. [PubMed] [Google Scholar]
- 19.Feron O, Michel JB, Sase K, Michel T. Dynamic regulation of endothelial nitric oxide synthase: complementary roles of dual acylation and caveolin interactions. Biochemistry. 1998;37:193–200. doi: 10.1021/bi972307p. [DOI] [PubMed] [Google Scholar]
- 20.Zielonka J, Sikora A, Hardy M, Joseph J, Dranka BP, Kalyanaraman B. Boronate probes as diagnostic tools for real time monitoring of peroxynitrite and hydroperoxides. Chem Res Toxicol. 2012;25:1793–1799. doi: 10.1021/tx300164j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zielonka J, Sikora A, Joseph J, Kalyanaraman B. Peroxynitrite is the major species formed from different flux ratios of co-generated nitric oxide and superoxide, Direct reaction with boronate-based fluorescent probe. J Biol Chem. 2010;285:14210–14216. doi: 10.1074/jbc.M110.110080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bakhshi FR, Mao M, Shajahan AN, Piegeler T, Chen Z, Chernaya O, Sharma T, Elliot WM, Szulcek R, Bogaard HJ, Comhair S, Erzurum S, van Nieuw Amerongen GP, Bonini MG, Minshall RD. Nitrosation-dependent caveolin-1 phosphorylation, ubiquitination, and degradation and its association with idiopathic pulmonary arterial hypertension. Pulm Circ. 2013;3:816–830. doi: 10.1086/674753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kojima H, Nakatsubo N, Kikuchi K, Kawahara S, Kirino Y, Nagoshi H, Hirata Y, Nagano T. Detection and imaging of nitric oxide with novel fluorescent indicators: diaminofluoresceins. Anal Chem. 1998;70:2446–2453. doi: 10.1021/ac9801723. [DOI] [PubMed] [Google Scholar]
- 24.Wardman P. Fluorescent and luminescent probes for measurement of oxidative and nitrosative species in cells and tissues: progress, pitfalls, and prospects. Free Radic Biol Med. 2007;43:995–1022. doi: 10.1016/j.freeradbiomed.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 25.Zhou X, He P. Improved measurements of intracellular nitric oxide in intact microvessels using 4,5-diaminofluorescein diacetate. Am J Physiol Heart Circ Physiol. 2011;301:H108–H114. doi: 10.1152/ajpheart.00195.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xian JA, Guo H, Li B, Miao YT, Ye JM, Zhang SP, Pan XB, Ye CX, Wang AL, Hao XM. Measurement of intracellular nitric oxide (NO) production in shrimp haemocytes by flow cytometry. Fish Shellfish Immunol. 2013;35:2032–2039. doi: 10.1016/j.fsi.2013.10.014. [DOI] [PubMed] [Google Scholar]
- 27.Lim MH. Preparation of a copper-based fluorescent probe for nitric oxide and its use in mammalian cultured cells. Nat Protoc. 2007;2:408–415. doi: 10.1038/nprot.2007.43. [DOI] [PubMed] [Google Scholar]
- 28.Lim MH, Xu D, Lippard S. Visualization of nitric oxide in living cells by a copper-based fluorescent probe. Nat Chem Biol. 2006;2:375–380. doi: 10.1038/nchembio794. [DOI] [PubMed] [Google Scholar]
- 29.Sullivan JC, Pollock JS. Coupled and uncoupled NOS: separate but equal? Uncoupled NOS in endothelial cells is a critical pathway for intracellular signaling. Circ Res. 2006;98:717–719. doi: 10.1161/01.RES.0000217594.97174.c2. [DOI] [PubMed] [Google Scholar]
- 30.Schmidt K, Kolesnik B, Gorren AC, Werner ER, Mayer B. Cell type-specific recycling of tetrahydrobiopterin by dihydrofolate reductase explains differential effects of 7,8-dihydrobiopterin on endothelial nitric oxide synthase uncoupling. Biochem Pharmacol. 2014;90:246–253. doi: 10.1016/j.bcp.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ravi K, Brennan LA, Levic S, Ross PA, Black SM. S-nitrosylation of endothelial nitric oxide synthase is associated with monomerization and decreased enzyme activity. Proc Natl Acad Sci USA. 2003;101:2619–2624. doi: 10.1073/pnas.0300464101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen CA, Wang TY, Varadharaj S, Reyes LA, Hemann C, Talukder MA, Chen YR, Druhan LJ, Zweier JL. S-glutathionylation uncouples eNOS and regulates its cellular and vascular function. Nature. 2010;468:1115–1118. doi: 10.1038/nature09599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Papezikova I, Pekarova M, Lojek A, Kubala L. The effect of uric acid on homocysteine-induced endothelial dysfunction in bovine aortic endothelial cells. Neuro Endocrinol Lett. 2009;30:112–115. [PubMed] [Google Scholar]
- 34.Li P, Zhang L, Zhang M, Zhou C, Lin N. Uric acid enhances PKC-dependent eNOS phosphorylation and mediates cellular ER stress: a mechanism for uric acid-induced endothelial dysfunction. Int J Mol Med. 2016;37:989–997. doi: 10.3892/ijmm.2016.2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cai W, Duan X, Liu Y, Yu J, Tang Y, Liu Z, Jiang S, Zhang C, Liu J, Xu J. Uric acid induces endothelial dysfunction by activating the HMGB1/RAGE signaling pathway. Biomed Res Int. 2017;2017:4391920. doi: 10.1155/2017/4391920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang Z, Hong Q, Zhang X, Xiao W, Wang L, Cui S, Feng Z, Lv Y, Cai G, Chen X, Wu D. Aldose reductase mediates endothelial cell dysfunction induced by high uric acid concentrations. Cell Commun Signal. 2017;15:3. doi: 10.1186/s12964-016-0158-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Park JH, Jin YM, Hwang S, Cho DH, Kang DH, Jo I. Uric acid attenuates nitric oxide production by decreasing the interaction between endothelial nitric oxide synthase and calmodulin in human umbilical vein endothelial cells: a mechanism for uric acid-induced cardiovascular disease development. Nitric Oxide. 2013;1:36–42. doi: 10.1016/j.niox.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 38.Papežíková I, Pekarová M, Kolářová H, Klinke A, Lau D, Baldus S, Lojek A, Kubala L. Uric acid modulates vascular endothelial function through the down regulation of nitric oxide production. Free Radic Res. 2013;47:82–88. doi: 10.3109/10715762.2012.747677. [DOI] [PubMed] [Google Scholar]
- 39.Presta A, Siddhanta U, Wu C, Sennequier N, Huang L, Abu-Soud HM, Erzurum S, Stuehr DJ. Comparative functioning of dihydro- and tetrahydropterins in supporting electron transfer, catalysis, and subunit dimerization in inducible nitric oxide synthase. Biochemistry. 1998;37:298–310. doi: 10.1021/bi971944c. [DOI] [PubMed] [Google Scholar]
- 40.Crabtree MJ, Smith CL, Lam G, Goligorsky MS, Gross SS. Ratio of 5,6,7,8-tetrahydrobiopterin to 7,8-dihydrobiopterin in endothelial cells determines glucose-elicited changes in NO vs. superoxide production by eNOS. Am J Physiol Heart Circ Physiol. 2008;294:H1530–H1540. doi: 10.1152/ajpheart.00823.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Karuppiah K, Druhan LJ, Chen CA, Smith T, Zweier JL, Sessa WC, Cardounel AJ. Suppression of eNOS-derived superoxide by caveolin-1: a biopterin-dependent mechanism. Am J Physiol Heart Circ Physiol. 2011;301:H903–H911. doi: 10.1152/ajpheart.00936.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fulton D, Gratton JP, Sessa WC. Post-translational control of endothelial nitric oxide synthase: why isn’t calcium/calmodulin enough? J Pharmacol Exp Ther. 2001;299:818–824. [PubMed] [Google Scholar]
- 43.Ju H, Zou R, Venema VJ, Venema RC. Direct interaction of endothelial nitric-oxide synthase and caveolin-1 inhibits synthase activity. J Biol Chem. 1997;272:18522–18525. doi: 10.1074/jbc.272.30.18522. [DOI] [PubMed] [Google Scholar]
- 44.Luanpitpong S, Talbott SJ, Rojanasakul Y, Nimmannit U, Pongrakhananon V, Wang L, Chanvorachote P. Regulation of lung cancer cell migration and invasion by reactive oxygen species and caveolin-1. J Biol Chem. 2010;285:38832–38840. doi: 10.1074/jbc.M110.124958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mougeolle A, Poussard S, Decossas M, Lamaze C, Lambert O, Dargelos E. Oxidative stress induces caveolin-1 degradation and impairs caveolae functions in skeletal muscle cells. Plos One. 2015;10:e0122654. doi: 10.1371/journal.pone.0122654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li H, Brodsky S, Basco M, Romanov V, De Angelis DA, Goligorsky MS. Nitric oxide attenuates signal transduction. Circ Res. 2001;88:229–236. doi: 10.1161/01.res.88.2.229. [DOI] [PubMed] [Google Scholar]
- 47.Zimnicka AM, Husain YS, Shajahan AN, Sverdlov M, Chaga O, Chen Z, Toth PT, Klomp J, Karginov AV, Tiruppathi C, Malik AB, Minshall RD. Src-dependent phosphorylation of caveolin-1 Tyr-14 promotes swelling and release of caveolae. Mol Biol Cell. 2016;27:2090–2106. doi: 10.1091/mbc.E15-11-0756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen Z, Bakhshi FR, Shajahan AN, Sharma T, Mao M, Trane A, Bernatchez P, Amerongen GP, Bonini MG, Skidgel RA, Malik AB, Minshall RD. Nitric oxide–dependent Src activation and resultant caveolin-1 phosphorylation promote eNOS/caveolin-1 binding and eNOS inhibition. Mol Biol Cell. 2012;23:1388–1398. doi: 10.1091/mbc.E11-09-0811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schubert W, Frank PG, Woodman SE, Hyogo H, Cohen DE, Chow CW, Lisanti MP. Microvascular hyperpermeability in caveolin-1 (−/−) knock-out mice. Treatment with a specific nitric-oxide synthase inhibitor, L-NAME, restores normal microvascular permeability in Cav-1 null mice. J Biol Chem. 2002;277:40091–40098. doi: 10.1074/jbc.M205948200. [DOI] [PubMed] [Google Scholar]
- 50.Zhao YY, Zhao YD, Mirza MK, Huang JH, Potula HH, Vogel SM, Brovkovych V, Yuan JX, Wharton J, Malik AB. Persistent eNOS activation secondary to caveolin-1 deficiency induces pulmonary hypertension in mice and humans through PKG nitration. J Clin Invest. 2009;119:2009–2018. doi: 10.1172/JCI33338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Siddiqui MR, Komarova YA, Vogel SM, Gao X, Bonini MG, Rajasingh J, Zhao YY, Brovkovych V, Malik AB. Caveolin-1-eNOS signaling promotes p190RhoGAP-A nitration and endothelial permeability. J Cell Biol. 2011;193:841–850. doi: 10.1083/jcb.201012129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Feron O, Saldana F, Michel JB, Michel T. The Endothelial Nitric-oxide Synthase-Caveolin Regulatory Cycle. J Biol Chem. 1998;273:3125–3128. doi: 10.1074/jbc.273.6.3125. [DOI] [PubMed] [Google Scholar]
- 53.Rizzo V, McIntosh DP, Oh P, Schnitzer JE. In situ flow activates endothelial nitric oxide synthase in luminal caveolae of endothelium with rapid caveolin dissociation and calmodulin association. J Biol Chem. 1998;273:34724–34729. doi: 10.1074/jbc.273.52.34724. [DOI] [PubMed] [Google Scholar]
- 54.García-Cardeña G, Fan R, Stern DF, Liu J, Sessa WC. Endothelial nitric oxide synthase is regulated by tyrosine phosphorylation and interacts with caveolin-1. J Biol Chem. 1996;271:27237–27240. doi: 10.1074/jbc.271.44.27237. [DOI] [PubMed] [Google Scholar]
- 55.Loot AE, Schreiber JG, Fisslthaler B, Fleming I. Angiotensin II impairs endothelial function via tyrosine phosphorylation of the endothelial nitric oxide synthase. J Exp Med. 2009;206:2889–2896. doi: 10.1084/jem.20090449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Heiss EH, Dirsch VM. Regulation of eNOS Enzyme Activity by Posttranslational Modification. Curr Pharm Des. 2014;220:3503–3513. doi: 10.2174/13816128113196660745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mao M, Varadarajan S, Fukai T, Bakhshi FR, Chernaya O, Dudley SC, Jr, Minshall RD, Bonini MG. Nitroglycerin tolerance in caveolin-1 deficient mice. PLoS One. 2014;9:e104101. doi: 10.1371/journal.pone.0104101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure S1: Representative Western blot of total eNOS, actin, and total Cav-1 in control si-RNA (right lane) and eNOS si-RNA treated cells (left lane) treated with TERPY (1 μM) or not. The left lane shows that efficiency of the eNOS si-RNA is not 100%.


