Abstract
In this article, we argued that the term stress has served as a valuable heuristic, helping researchers to integrate traditions that illuminate different stages of the process linking stressful life events to disease. We provided a short history of three traditions in the study of stress: the epidemiological, psychological, and biological. The epidemiological tradition focuses on defining which circumstances and experiences are deemed stressful on the basis of consensual agreement that they constitute threats to social or physical well-being. The psychological tradition focuses on individuals’ perceptions of the stress presented by life events on the basis of their appraisals of the threats posed and the availability of effective coping resources. The biological tradition focuses on brain-based perturbations of physiological systems that are otherwise essential for normal homeostatic regulation and metabolic control. The foci of these three traditions have informed elements of a stage model of disease, wherein events appraised as stressful are viewed as triggering affective states that in turn engender behavioral and biological responses having possible downstream implications for disease.
Keywords: stress, stress and disease, stress mechanisms
In an article in this issue of Perspectives in Psychological Science, Kagan (2016, this issue) voices a concern that has been discussed for more than half a century, namely, that the term stress has been so promiscuously invoked and applied in such a biased fashion as to render it of little utility (see, for example, Appley & Trumbull, 1967). In turn, because stress is used inconsistently across disciplines with different methodological traditions and different levels of analysis, it is often difficult to understand how research from these fields fits together. In this commentary, we provide a brief history of how the term stress has been used in research on humans. We propose that stress be viewed broadly as a set of constructs representing stages in a process by which environmental demands that tax or exceed the adaptive capacity of an organism occasion psychological, behavioral, and biological responses that may place persons at risk for disease.
Traditions of Studying Stress
There are several traditions of studying stress, most notably the epidemiological, psychological, and biological (Cohen, Kessler, & Underwood Gordon, 1995). Fifty years ago, each of these traditions was pursued by different networks of researchers, but the last 20 years have seen increasing integration of these approaches.
The epidemiologic tradition
In the epidemiologic tradition, investigators assess the objective levels of stress posed by individual life events. Implicit in this approach is that a specific life event generates an equivalent amount of stress for all individuals. Estimates of objective levels are based on normative (average) ratings of the stress associated with each event by individuals in the population being studied or by trained judges. However, definitions of stress used to assign objective (normative) levels have varied.
In an early approach, Holmes and Rahe (1967) proposed that the more change inherent in adapting to a life event, the greater the stress associated with that event. In turn, this approach suggests that stress is cumulative, with each additional event adding to the overall burden of adaptation required of the individual. This was the theory behind the development of early stressful life event scales. For example, in the Social Readjustment Rating Scale (SRRS; Holmes & Masuda, 1974), the amount of change required by each of a list of 43 “major” life events was determined by normative ratings obtained from a panel of judges drawn from the population. The change scores (known as life change units, or LCU) for all events that an individual reported as occurring in a specified period (usually a year) were summed to generate a measure of total change required to adapt to experienced events. Examples of events on the SRRS include marriage (LCU = 50), divorce (LCU = 73), being fired at work (LCU = 47), retirement (LCU = 45), pregnancy (LCU = 40), and taking a vacation (LCU = 13).
Although the adaptation (change) model has been endorsed by some (see Turner & Wheaton, 1995), it has not held up empirically (e.g., it implied that positive events would have similar effects as negative ones) and largely has been replaced by a definition of stressors as events that are consensually seen as undesirable or threatening (e.g., Brown & Harris, 1989: Paykel, Prusoff, & Uhlenhuth, 1971; Ross & Mirowsky, 1979; Vinokur & Selzer, 1975). Here self-reported stressful life event scales are made up of events consensually seen as negative (threatening), such as job loss, death of a close other, and legal problems. Typically, these are not weighted (weighted indices do not generally increase the correlation with disease; Turner & Wheaton, 1995) but are scored by counting the total number of negative events that are endorsed.
Another example of a method for assessing stressful life events on their objective level of threat is the Life Events and Difficulties Schedule (LEDS; Brown & Harris, 1989). Unlike simple checklists of common life stressors, the LEDS is a structured interview used to probe for details of reported events and their surrounding context. Each event (informed by the circumstances surrounding the event) then is rated for threat by a group of trained raters who use prior ratings of similar events by other LEDS raters as anchors for rating new events. The raters then enter their own ratings in a master database, resulting in a growing dictionary of ratings of the “objective” (consensual) long-term threat associated with many different life events (Wethington, Brown, & Kessler, 1995). In the LEDS, stress is not assumed to increase with each additional event. Instead, any single event meeting the LEDS-defined threat-severity threshold suffices to mark the presence of sufficient stress to put a person at risk for disease. This convention did not stem from theoretical considerations but rather from empirical evidence that a single severe event is enough to predict depressive episodes (Brown & Harris, 1989). It is notable that those with a single severe event have also been found to have increased risk for a range of other psychiatric and physical disorders (reviewed in Brown & Harris, 1989).
The epidemiological approach also has included studies of exposure to single, consensually determined threatening events, such as unemployment (Jin, Shah, & Svovoda, 1997), divorce (Kitson, & Morgan, 1990), bereavement (Bowling, 1987), economic strain (Lallukka, Lahelma, & Rankonan, 2013), and caregiving for the chronically ill (Kiecolt-Glaser, Marucha, Mercado, Malarkey, & Glaser, 1995; Schulz, Visintainer, & Williamson, 1990). Reliance on these single-event indicators reflects an underlying assumption that certain types of events are sufficient to generate substantial levels of threat. These predominantly include threats relating to central social roles (e.g., worker, spouse, or parent; Lepore, 1995) and to the integrity of interpersonal relationships (Bolger, DeLongis, Kessler, & Schilling, 1989; Cohen et al., 1998; Rook, 1984). They also tend to be chronic, with the event or its implications lasting months or even years (Cohen et al., 1998).
Overall, while the definition of what constitutes a stressful life event has varied over time, the epidemiological perspective has defined stress primarily by reference to independent ratings that reflect how others, in aggregate, judge the negative impact of particular events. Such measures have been successful in predicting morbidity (e.g., depression, respiratory infections, and coronary heart disease), disease progression (e.g., HIV-AIDS, wound healing, and autoimmune diseases) and mortality (reviewed by Cohen, Janicki-Deverts, & Miller, 2007).
The psychological tradition
According to the epidemiological approach, individuals’ experience of stress is inferred from their exposure to a life event independently judged as threatening or requiring adaptive adjustment. In contrast, the psychological perspective stems from the observation that experiencing the same event can be stressful for some individuals but not others. Hence, according to the psychological perspective, a stressful experience cannot be inferred by uniform reference to any particular event. Rather, such an inference necessarily depends on how such an event is construed by the individual. This approach is represented by Lazarus and Folkman’s (Lazarus, 1966; Lazarus & Folkman, 1984) seminal work on stress appraisal, which proposes that people appraise both the degree of potential threat posed by events and the availability of resources needed to cope with them. Threat appraisals of events are influenced by the imminence of harm and the intensity, duration, and potential controllability of the event, as well as by individuals’ beliefs about themselves and the environment, their values and commitments, and related personality dispositions. Coping appraisals may focus on actions designed to directly alter the perceived threatening event or on the viability of thoughts or actions that are intended to change emotional and behavioral responses to the event. A threat appraisal without the belief that effective coping responses are available is experienced as stress, which engenders emotional responses including worry, fear, and anxiety.
An example of the use of the appraisal approach in a specific domain is the Karasek Job Control Questionnaire (JCQ). Perceived job stress is defined as present when individuals rate their own jobs as simultaneously high in work demand and low in personal control over the work setting (e.g., Karasek, Baker, Marxer, Ahlbom, & Theorell, 1981). Others have assessed individuals’ appraisals of the threat or negative impact associated with engaging in specific social roles, such as work, marriage, or parenthood (see review by Lepore, 1995). This perspective has also been applied to stressful life event scales, such that subjective (self-generated) ratings of the negative impact of endorsed events are summed instead of the objective, consensual ratings used in earlier instruments (e.g., Sarason, Johnson, & Siegel, 1978).
A different approach to tapping individuals’ perceptions of stress has focused on global (event-independent) appraisals. For example, the Perceived Stress Scale (Cohen, Kamarck, & Mermelstein, 1983) focuses on experiences during the last month, assessing the degree to which people feel that the demands in their lives exceed their abilities to cope effectively. Similarly, an adaptation of the JCQ (perceptions of high demand and low control) has been used to assess non-work-related stress in daily life (e.g., Kamarck, Muldoon, Shiffman, & Sutton-Tyrrell, 2007). Over the years, investigators have assessed other stressor-related appraisals in addition to the dimension of threat, including appraisals of injustice, time pressure, harm or loss, and distress intolerance (Monroe & Kelley, 1995).
Overall, researchers holding the psychological perspective generally have defined stress as an experience that occurs when individuals simultaneously appraise events as threatening or otherwise harmful and their coping resources as inadequate. Like objective measures of events, measures of perceived stress also have been useful in predicting subsequent risk for morbidity and mortality (e.g., Keller et al., 2012; Nielson, Kristensen, Schnohr, & Grønbæk, 2008; Wisnivesky, Lorenzo, Feldman, Leventhal, & Halm, 2010).
The biological tradition
In the biological approach, the impact of defined stressors is indexed via perturbations of physiological systems that are otherwise essential for homeostatic regulation and metabolic control. An assumption in the biological tradition is that these physiological perturbations or reactions provide support over the short term for adaptive behavioral action or coping. Over the long term, however, such physiological reactions may prove maladaptive and relate to risk for disease.
As described by Kagan, the biological tradition was influenced heavily by Selye’s (1956) early work, in which stress was equated with chronic activation of one of the body’s principal neuroendocrine axes, the hypothalamic–pituitary–adrenal (HPA) system. This tradition also has deep roots in experimental psychophysiology and psychosomatic medicine, where responses of the autonomic nervous system have long figured prominently as markers of stress (Cannon, 1932; Mason, 1971; Weiner, 1992). Measured stress indicators thus commonly include the HPA-derived hormone, cortisol, and the sympathoadrenal-medullary (SAM) mediators, epinephrine and norepinephrine, as well as autonomically regulated, peripheral physiological indices like heart rate and blood pressure. Patterns of response across these parameters of physiology often vary with differences in the stimuli that evoke them, but in general, their stimulus-dependent activation, when excessive, persistent, or repeated often, has been viewed as a biological instantiation of stress (Cohen et al., 1995; Smyth, Zawadzki, & Gerin, 2013). As alluded to by Kagan, one problematic issue inherent to the biological tradition is that there are no agreed-upon thresholds that define stress for any of these parameters.
Biological research on stress in humans historically has emphasized laboratory studies in which participants are exposed to experimental challenges (stressors). These studies are used to identify and elucidate biological mechanisms that may mediate effects of environmental stressors on more distal outcomes. In such studies, the participants are exposed to tasks and stimuli of brief duration that share certain features with objectively defined stressors, such as aversiveness, conflict, uncontrollability, social threat, or demands for time-pressured coping. So the point is not that these tasks and stimuli are “stress” but that they model features of epidemiologically studied stressors in a controlled setting, allowing for instrumented investigation of evoked physiological reactions. By extension, the types of autonomic and neuroendocrine responses typically assessed in such studies, when repeatedly elicited in the context of naturally occurring stressful life events, are thought to promote systemic biological and cellular changes that are conducive to disease, such as altered metabolic, immune, respiratory, and cardiovascular functioning. Accordingly, the impact of stressor-evoked physiological reactions is thought to constitute a primary pathway connecting stressful events and appraisals to physical health outcomes.
A second line of biological research on stress focuses on stable individual differences in the magnitude of stressor-evoked physiological reactions, particularly cardiovascular, HPA, and cellular immune reactions to acute psychological stressors (e.g., Krantz & Manuck, 1984; Manuck, 1994; Manuck, Cohen, Rabin, Muldoon, & Bachen, 1991; Marsland, Bachen, Cohen, Rabin, & Manuck, 2002). A reason for this focus is that individuals with a tendency to exhibit heightened blood pressure reactivity when measured in laboratory settings, for instance, also are more likely to exhibit preclinical vascular disease and suffer from premature cardiovascular mortality (Carroll et al., 2012; Chida & Steptoe, 2010; Gianaros et al., 2002; Jennings et al., 2004). Similarly, individual differences in cellular immune reactivity relate to indicators of weakened immunity (e.g., blunted antibody responses to Hepatitis B antigen following vaccination; Marsland, Cohen, Rabin, & Manuck, 2001), and analogous individual differences in HPA activation moderate infectious disease susceptibility in response to naturally occurring stressful life events (Cohen et al., 2002).
A broader view of the biology of stress in terms of dysregulated systems (e.g., overactivation, underactivation, delayed recovery, counterregulatory rebound) has begun to supplant earlier emphases equating stress with only overactivation of the SAM and HPA (McEwen, 1998). For example, research on the HPA response suggests that while cortisol does increase under acute stress, the HPA response to chronic stressors can be more complex, variously including a diminished cortisol release, glucocorticoid insensitivity, or alterations in the cortisol diurnal rhythm (Miller, Chen, & Zhou, 2007; Miller, Cohen, & Ritchey, 2002). These patterns of response may affect immune and cardiovascular parameters that contribute to disease pathogenesis (e.g., Cohen et al., 2012; Kiecolt-Glaser et al., 2005; Matthews, Schwartz, Cohen, & Seeman, 2006).
Finally, a recent direction in research on human stress biology has been to characterize the brain systems that appraise psychological and social stressors, as well as generate downstream physiological reactions that might relate to disease risk (Muscatell & Eisenberger, 2012). Specifically, investigators in this area have used functional magnetic resonance imaging (fMRI) and other methods to measure neural activity while people complete aversive tasks or process threatening stimuli that are modeled from laboratory-based studies of stress physiology. During imaging, this neural activity is measured along with peripheral physiological reactions (e.g., blood pressure) that have been implicated in disease risk (Gianaros & Wager, 2015). Evidence from these brain-imaging studies indicates that psychological and social stressors engage a network of cortical and limbic regions, particularly regions of the medial and lateral prefrontal cortex, cingulate, insula, and amygdala. Evoked patterns of activity across these and other networked brain regions are presumed to correspond to the neural processes supporting stressor appraisals and possibly emotional experience, responding, and regulation (Gianaros & Wager, 2015). Moreover, changes in neural activity in these same regions are associated with peripheral measures of stress physiology, including disease-relevant parameters of autonomic, neuroendocrine, cardiovascular, and immune physiology (Muscatell & Eisenberger, 2012).
Although findings from these human imaging studies have begun to clarify the role of the brain in linking psychological and social stressors to physiological reactions that may contribute to disease risk, several core questions remain. One such question is whether appraisal and effector mechanisms for physiological stress responding are overlapping and inseparable processes at the level of the brain. Alternative possibilities are that appraisal and effector response processes are initiated in parallel or are sequenced over time in a chain across networked brain regions. Finally, researchers have not been able to resolve whether the stressor-evoked neural activity changes that covary simultaneously with peripheral physiological responses correspond only to the efferent commands for these responses or also to the afferent representation of peripheral physiological changes. The afferent representation of these physiological changes through feedback mechanisms to the brain may themselves serve to influence emotional experiences and the appraisal of events that are encoded as stressors (Gianaros & Wager, 2015).
A Stage Model From Life Events to Disease
One approach to integrating the different traditions of studying stress is to view the various definitions as stages of a process linking environmental events to disease (cf. Cohen et al., 1995; see Fig. 1). Environmental (stressful life) events can result in brain-based stress appraisals (threatening or not). If an event is appraised as a threat, it will trigger affective responses (e.g., worry, fear, or anxiety) and alter the functions of the HPA, SAM, and other regulatory and neuroendocrine systems (e.g., parasympathetic nervous system activity and gonadal steroids), having downstream implications for disease onset and severity.
Fig. 1.
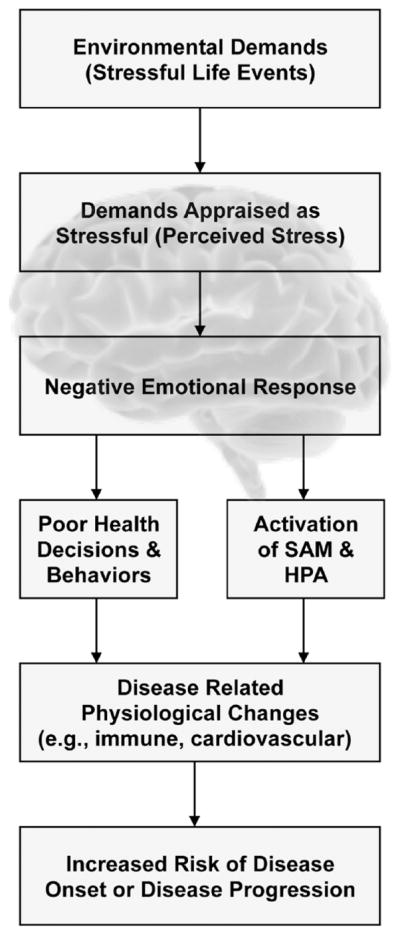
A heuristic model of the stress process designed to illustrate the potential integration of the environmental, psychological, and biological definitions of stress. Although the figure presents an ordered series of stages, we recognize that other mechanisms may be at play, and all the stages may not be required. Moreover, although the model is unidirectional, we recognize that there are potential feedback loops as well. HPA = hypothalamic–pituitary–adrenal system, SAM = sympatho-adrenal-medullary mediators.
Brain-based stress appraisals also may affect health-relevant behaviors via affective responses and higher-level cognitions. For instance, behaviors used to cope with perceived negative events (e.g., decreased exercise and sleep) or with emotional responses to negative events (e.g., increased smoking and alcohol consumption) provide important pathways by which stressful events may influence disease risk. Additionally, stress heightens forms of impulsive decision making such as delay discounting—a relative preference for immediate rewards over larger rewards delayed in time—that in turn may promote a variety of health-impairing behaviors including cigarette smoking and substance abuse (Bickel & Marsch, 2001; Fields, Lange, Ramos, Thamotharan, & Rassu, 2014; Sweitzer, Donny, Dierker, Flory, & Manuck, 2008). Finally, appraisals of stress and their affective sequelae can influence interpretations of physiological sensations, such as defining sensations as symptoms and symptom clusters as diseases that in turn influence health-care seeking, and adherence to medical regimens (Cohen & Williamson, 1991; Erickson, Creswell, Verstynen, & Gianaros, 2014; Pennebaker, 1983; Smyth et al., 2013).
The stage model implies that each sequential component of the stress process is more proximal to and hence more predictive of illness outcomes than the preceding. For example, a disease-relevant biological-stress-response measure should be a better predictor than measures of stressful life events or perceived stress. We want to emphasize that this is a heuristic model designed to illustrate the potential integration of the environmental, psychological, and biological approaches. Although the model presents an ordered series of stages, we recognize that other mechanisms may be at play, and all the stages may not be required. For example, environmental demands can put persons at risk for disorder even when appraisal does not result in perceptions of stress and negative emotional responses; the process of coping itself (even when it is successful and environmental demands are appraised as benign) may directly result in physiological and behavioral changes that place a person at risk for disease (Cohen, Evans, Krantz, & Stokols, 1986). Finally, although the figure presents the model as unidirectional, we recognize that there are feedback loops (e.g., depressed affect may result in negatively biased appraisal of the threat posed or of coping resources).
Overall, this perspective allows evidence from any of the stages of stress to be integrated with downstream counterparts, for instance, as in testing hypotheses positing mediating mechanisms. The ultimate aim is to elucidate all points in pathways leading from stressful events and their appraisals to specific clinical outcomes, although impediments of cost and practicality and the inherent limitations of human research hinder full realization of this goal. Nonetheless, increasing progress has been made in establishing predicted links across two or more stages so that gaps in the understanding of stress-illness relationships are being filled (cf. Miller, Chen, & Cole, 2009). Particularly informative is the emergence of experimental models of disease susceptibility and studies of disease-relevant biological processes mediated by stress-evoked autonomic and neuroendocrine reactions. For example, a series of studies has shown that chronic stress (assessed as either life events or globally perceived stress) heightens susceptibility to the common cold among individuals inoculated with an upper respiratory virus (e.g., Cohen, Tyrrell, & Smith, 1993). Recent experimental findings suggest this effect is mediated by downregulation of the glucocorticoid receptor, which diminishes cortisol-dependent modulation of inflammatory responses to infection and, in turn, promotes worsened cold symptomatology (Cohen et al., 2012). In another example, aggregated measures of momentary task demands in daily life (assessed by ecological momentary assessment) were found to predict progression of carotid artery atherosclerosis over a 6-year follow-up among individuals who were not on drugs to lower blood pressure. Moreover, this association was partially mediated by higher ambulatory blood pressures among those reporting high subjective demand (Kamarck, Shiffman, Sutton-Tyrrell, Muldoon, & Tepper, 2012). Together, studies of this kind show researchers’ progress in accounting for how stress promotes disease or worsens symptomatology in affected individuals, although such studies typically stop short of establishing causal mechanisms. This research is often only possible with suitable animal models, which in the ideal are coextensive with human research (e.g., Capitanio & Cole, 2015; Cohen et al., 1997; Kaplan et al., 1987; Manuck, Marsland, Kaplan, & Williams, 1995).
In sum, we believe that the term stress has served as a valuable heuristic that has helped to highlight the similarities between research findings on different stages of the process linking environmental events to disease. Admittedly, the term stress is used too broadly and sometimes in confusing ways. However, the diversity of perspectives that the term has spawned has provided a basis for understanding the processes through which environmental adversities influence disease processes. We hope that future studies will include multiple stages of the model (from environment through brain to disease) in order to further validate this approach as a conceptual tool for understanding the stress process.
Acknowledgments
The authors are grateful to Denise Janicki-Deverts and Tom Kamarck for their comments on an earlier version of this article.
Footnotes
Author Contributions
All the authors contributed to writing of the manuscript.
Declaration of Conflicting Interests
The authors declared that they had no conflicts of interest with respect to their authorship or the publication of this article.
References
- Appley MH, Trumbull R, editors. Psychological stress: Issues in research. New York, NY: Appleton-Century-Crofts; 1967. [Google Scholar]
- Bickel WK, Marsch L. Toward a behavioral economic understanding of drug dependence: Delay discounting processes. Addiction. 2001;96:73–86. doi: 10.1046/j.1360-0443.2001.961736.x. [DOI] [PubMed] [Google Scholar]
- Bolger N, DeLongis A, Kessler RC, Schilling EA. Effects of daily stress on negative mood. Journal of Personality and Social Psychology. 1989;57:808–818. doi: 10.1037//0022-3514.57.5.808. [DOI] [PubMed] [Google Scholar]
- Bowling A. Mortality after bereavement: A review of the literature on survival periods and factors affecting survival. Social Science & Medicine. 1987;24:117–124. doi: 10.1002/gps.930090603. [DOI] [PubMed] [Google Scholar]
- Brown GW, Harris TO. Life events and illness. New York, NY: Guilford Press; 1989. [Google Scholar]
- Cannon WB. The wisdom of the body. New York, NY: Norton; 1932. [Google Scholar]
- Capitanio JP, Cole SW. Social instability and immunity in rhesus monkeys: The role of the sympathetic nervous system. Philosophical Transactions of the Royal Society B: Biological Sciences. 2015;370 doi: 10.1098/rstb.2014.0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll D, Ginty AT, Der G, Hunt K, Benzevall M, Phillips AC. Increased blood pressure reactions to acute mental stress are associated with 16-year cardiovascular disease mortality. Psychophysiology. 2012;49:1444–1448. doi: 10.1111/j.1469-8986.2012.01463.x. [DOI] [PubMed] [Google Scholar]
- Chida Y, Steptoe A. Greater cardiovascular responses to laboratory mental stress are associated with poor subsequent cardiovascular risk status: A meta-analysis of prospective evidence. Hypertension. 2010;55:1026–1032. doi: 10.1161/HYPERTENSIONAHA.109.146621. [DOI] [PubMed] [Google Scholar]
- Cohen S, Evans GW, Krantz DS, Stokols D. Behavior, health, and environmental stress. New York, NY: Plenum Press; 1986. [Google Scholar]
- Cohen S, Frank E, Doyle WJ, Skoner DP, Rabin BS, Gwaltney JM., Jr Types of stressors that increase susceptibility to the common cold in adults. Health Psychology. 1998;17:214–223. doi: 10.1037/0278-6133.17.3.214. [DOI] [PubMed] [Google Scholar]
- Cohen S, Hamrick N, Rodriguez MS, Feldman PJ, Rabin BS, Manuck SB. Reactivity and vulnerability to stress-associated risk for upper respiratory illness. Psychosomatic Medicine. 2002;64:302–310. doi: 10.1097/00006842-200203000-00014. [DOI] [PubMed] [Google Scholar]
- Cohen S, Janicki-Deverts D, Doyle WJ, Miller GE, Frank E, Rabin BS, Turner RB. Chronic stress, glucocorticoid receptor resistance, inflammation, and disease risk. PNAS: Proceedings of the National Academy of Sciences of the United States of America. 2012;109:5995–5999. doi: 10.1073/pnas.1118355109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Janicki-Deverts D, Miller GE. Psychological stress and disease. Journal of the American Medical Association. 2007;298:1685–1687. doi: 10.1001/jama.298.14.1685. [DOI] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. Journal of Health and Social Behavior. 1983;24:385–396. Retrieved from http://www.jstor.org/stable/2136404. [PubMed] [Google Scholar]
- Cohen S, Kessler RC, Underwood Gordon L. Strategies for measuring stress in studies of psychiatric and physical disorders. In: Cohen S, Kessler RC, Underwood Gordon L, editors. Measuring stress: A guide for health and social scientists. New York, NY: Oxford University Press; 1995. pp. 3–26. [Google Scholar]
- Cohen S, Line S, Manuck SB, Rabin BS, Heise E, Kaplan JR. Chronic social stress, social status, and susceptibility to upper respiratory infections in nonhuman primates. Psychosomatic Medicine. 1997;59:213–221. doi: 10.1097/00006842-199705000-00001. [DOI] [PubMed] [Google Scholar]
- Cohen S, Tyrrell DAJ, Smith AP. Life events, perceived stress, negative affect, and susceptibility to the common cold. Journal of Personality and Social Psychology. 1993;64:131–140. doi: 10.1037/0022-3514.64.1.131. [DOI] [PubMed] [Google Scholar]
- Cohen S, Williamson G. Stress and infectious disease in humans. Psychological Bulletin. 1991;109:5–24. doi: 10.1037/0033-2909.109.1.5. [DOI] [PubMed] [Google Scholar]
- Erickson KI, Creswell JD, Verstynen TD, Gianaros PJ. Health neuroscience: Defining a new field. Current Directions in Psychological Science. 2014;23:446–453. doi: 10.1177/0963721414549350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields SA, Lange K, Ramos A, Thamotharan S, Rassu F. The relationship between stress and delay discounting: A meta-analytic review. Behavioral Pharmacology. 2014;25:434–444. doi: 10.1097/FBP.0000000000000044. [DOI] [PubMed] [Google Scholar]
- Gianaros PJ, Bleil ME, Muldoon MF, Jennings JR, Sutton-Tyrrell K, McCaffery JM, Manuck SB. Is cardiovascular reactivity associated with atherosclerosis among hypertensives? Hypertension. 2002;40:742–747. doi: 10.1161/01.HYP.0000035707.57492.EB. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros PJ, Wager TD. Brain-body pathways linking psychological stress and physical health. Current Directions in Psychological Science. 2015;24:313–321. doi: 10.1177/0963721415581476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes TH, Masuda M. Life changes and illness susceptibility. In: Dohrenwend BS, Dohrenwend BP, editors. Stressful life events: Their nature and effects. New York, NY: Wiley; 1974. pp. 45–72. [Google Scholar]
- Holmes TH, Rahe RH. The Social Readjustment Rating Scale. Journal of Psychosomatic Research. 1967;11:213–218. doi: 10.1016/0022-3999(67)90010-4. [DOI] [PubMed] [Google Scholar]
- Jennings JR, Kamarck TW, Everson-Rose SA, Kaplan GA, Manuck SB, Salonen JT. Exaggerated blood pressure responses during mental stress are prospectively related to enhanced carotid atherosclerosis in middle-aged Finnish men. Circulation. 2004;110:2198–2203. doi: 10.1161/01.CIR.0000143840.77061.E9. [DOI] [PubMed] [Google Scholar]
- Jin RL, Shah CP, Svovoda TJ. The impact of unemployment on health: A review of evidence. Journal of Public Health Policy. 1997;18:275–401. [Google Scholar]
- Kagan J. An overly permissive extension. Perspective on Psychological Science. 2016;11:442–450. doi: 10.1177/1745691616635593. [DOI] [PubMed] [Google Scholar]
- Kamarck TW, Muldoon MF, Shiffman S, Sutton-Tyrrell K. Experiences of demand and control during daily life are predictors of carotid atherosclerotic progression among healthy men. Health Psychology. 2007;26:324–332. doi: 10.1037/0278-6133.26.3.324. [DOI] [PubMed] [Google Scholar]
- Kamarck TW, Shiffman S, Sutton-Tyrrell K, Muldoon MF, Tepper P. Daily psychological demands are associated with 6 year progression of carotid artery atherosclerosis. Psychosomatic Medicine. 2012;74:432–4399. doi: 10.1097/PSY.0b013e3182572599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan JR, Manuck SB, Adams MR, Weingand KW, Clarkson TB. Inhibition of coronary atherosclerosis by propranolol in behaviorally predisposed monkeys fed an atherogenic diet. Circulation. 1987;76:1364–1372. doi: 10.1161/01.cir.76.6.1364. [DOI] [PubMed] [Google Scholar]
- Karasek RA, Baker D, Marxer F, Ahlbom A, Theorell T. Job decision latitude, job demands, and cardiovascular disease: A prospective study of Swedish men. American Journal of Public Health. 1981;71:694–705. doi: 10.2105/AJPH.71.7.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller A, Litzelman K, Wisk LE, Maddox T, Cheng ER, Creswell PD, Witt WP. Does the perception that stress affects health matter? The association with health and mortality. Health Psychology. 2012;31:677–684. doi: 10.1037/a0026743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Loving TJ, Stowell JR, Malarkey WB, Lemeshow S, Dickinson SL, Glaser R. Hostile marital interactions, proinflammatory cytokine production, and wound healing. Archives of General Psychiatry. 2005;62:1377–1384. doi: 10.1001/archpsyc.62.12.1377. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Marucha PT, Mercado AM, Malarkey WB, Glaser R. Slowing of wound healing by psychological stress. The Lancet. 1995;346:1194–1196. doi: 10.1016/s0140-6736(95)92899-5. [DOI] [PubMed] [Google Scholar]
- Kitson GC, Morgan LA. The multiple consequences of divorce: A decade review. Journal of Marriage and Family. 1990;52:913–924. doi: 10.2307/353310. [DOI] [Google Scholar]
- Krantz DS, Manuck SB. Acute psychophysiologic reactivity and risk of cardiovascular disease: A review and methodological critique. Psychological Bulletin. 1984;96:435–465. doi: 10.1037/0033-2909.96.3.435. [DOI] [PubMed] [Google Scholar]
- Lallukka T, Lahelma E, Rankonen O. Changes in economic difficulties and subsequent sickness absence: A prospective register-linkage study. BMJ Open. 2013;3:e002212. doi: 10.1136/bmjopen-2012-002212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarus RS. Psychological stress and the coping process. New York, NY: McGraw-Hill; 1966. [Google Scholar]
- Lazarus RS, Folkman S. Stress, appraisal, and coping. New York, NY: Springer; 1984. [Google Scholar]
- Lepore SJ. Measurement of chronic stressors. In: Cohen S, Kessler RC, Underwood Gordon L, editors. Measuring stress: A guide for health and social scientists. New York, NY: Oxford University Press; 1995. pp. 102–120. [Google Scholar]
- Manuck SB. Cardiovascular reactivity in cardiovascular disease: “Once more unto the breach. International Journal of Behavioral Medicine. 1994;1:4–31. doi: 10.1207/s15327558ijbm0101_2. [DOI] [PubMed] [Google Scholar]
- Manuck SB, Cohen S, Rabin BS, Muldoon MF, Bachen EA. Individual differences in cellular immune response to stress. Psychological Science. 1991;2:111–115. doi: 10.1111/j.1467-9280.1991.tb00110.x. [DOI] [Google Scholar]
- Manuck SB, Marsland AS, Kaplan JR, Williams JK. The pathogenicity of behavior and its neuroendocrine mediation: An example from coronary artery disease. Psychosomatic Medicine. 1995;57:275–283. doi: 10.1097/00006842-199505000-00009. [DOI] [PubMed] [Google Scholar]
- Marsland AL, Bachen EA, Cohen S, Rabin B, Manuck S. Stress, immune reactivity, and susceptibility to infectious disease. Physiology & Behavior. 2002;77:711–716. doi: 10.1016/S0031-9384(02)00923-X. [DOI] [PubMed] [Google Scholar]
- Marsland AL, Cohen S, Rabin BS, Manuck SB. Associations between stress, trait negative affect, and acute immune reactivity and antibody response to Hepatitis B vaccination. Health Psychology. 2001;20:4–11. doi: 10.1037/0278-6133.20.1.4. [DOI] [PubMed] [Google Scholar]
- Mason JW. A re-evaluation of the concept of non-specificity in stress theory. Journal of Psychiatric Research. 1971;8:323–333. doi: 10.1016/0022-3956(71)90028-8. [DOI] [PubMed] [Google Scholar]
- Matthews KA, Schwartz J, Cohen S, Seeman T. Diurnal cortisol decline is related to coronary calcification: CARDIA study. Psychosomatic Medicine. 2006;68:657–661. doi: 10.1097/01.psy.0000244071.42939.0e. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Protective and damaging effects of stress mediators. New England Journal of Medicine. 1998;338:171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- Miller GE, Chen E, Cole SW. Health psychology: Developing biologically plausible models linking the social world and physical health. Annual Review of Psychology. 2009;60:501–524. doi: 10.1146/annurev.psych.60.110707.163551. [DOI] [PubMed] [Google Scholar]
- Miller GE, Chen E, Zhou ES. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychological Bulletin. 2007;133:25–45. doi: 10.1037/0033-2909.133.1.25. [DOI] [PubMed] [Google Scholar]
- Miller GE, Cohen S, Ritchey AK. Chronic psychological stress and the regulation of pro-inflammatory cytokines: A glucocorticoid resistance model. Health Psychology. 2002;21:531–541. doi: 10.1037/0278-6133.21.6.531. [DOI] [PubMed] [Google Scholar]
- Monroe SM, Kelley JM. Measurement of stress appraisal. In: Cohen S, Kessler RC, Underwood Gordon L, editors. Measuring stress: A guide for health and social scientists. New York, NY: Oxford University Press; 1995. pp. 122–147. [Google Scholar]
- Muscatell KA, Eisenberger NI. A social neuroscience perspective on stress and health. Social & Personality Psychology Compass. 2012;6:890–904. doi: 10.1111/j.1751-9004.2012.00467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielson NR, Kristensen TS, Schnohr P, Grønbæk M. Perceived stress and cause-specific mortality among men and women: Results from a prospective cohort study. American Journal of Epidemiology. 2008;166:481–491. doi: 10.1093/aje/kwn157. [DOI] [PubMed] [Google Scholar]
- Paykel ES, Prusoff BA, Uhlenhuth EH. Scaling of life events. Archives of General Psychiatry. 1971;25:340–347. doi: 10.1001/archpsyc.1971.01750160052010. [DOI] [PubMed] [Google Scholar]
- Pennebaker J. The psychology of physical symptoms. New York, NY: Springer-Verlag; 1983. [Google Scholar]
- Rook KS. The negative side of social interaction: Impact on psychological well-being. Journal of Personality and Social Psychology. 1984;46:1097–1108. doi: 10.1037/0022-3514.46.5.1097. [DOI] [PubMed] [Google Scholar]
- Ross CE, Mirowsky J. A comparison of life event weighting schemes: Change, undesirability, and effect proportional indices. Journal of Health and Social Behavior. 1979;20:166–177. Retrieved from http://www.jstor.org/stable/2136437. [PubMed] [Google Scholar]
- Sarason IG, Johnson JH, Siegel JM. Assessing the impact of life changes: Development of the Life Experiences Survey. Journal of Consulting and Clinical Psychology. 1978;46:932–946. doi: 10.1037/0022-006X.46.5.932. [DOI] [PubMed] [Google Scholar]
- Schulz R, Visintainer P, Williamson GM. Psychiatric and physical morbidity effects of caregiving. The Journal of Gerontology. 1990;45:181–191. doi: 10.1093/geronj/45.5.P181. [DOI] [PubMed] [Google Scholar]
- Selye H. The stress of life. New York, NY: McGraw-Hill; 1956. [Google Scholar]
- Smyth J, Zawadzki M, Gerin W. Stress and disease: A structural and functional analysis. Social & Personality Psychology Compass. 2013;7:217–227. doi:10.111/spc3. 12020. [Google Scholar]
- Sweitzer MM, Donny EC, Dierker LC, Flory JD, Manuck SB. Delay discounting and smoking: Association with the Fagerstrom Test for Nicotine Dependence but not cigarettes smoked per day. Nicotine & Tobacco Research. 2008;10:1571–1575. doi: 10.1080/14622200802323274. [DOI] [PubMed] [Google Scholar]
- Turner RJ, Wheaton B. Checklist measurement of stressful life events. In: Cohen S, Kessler RC, Underwood Gordon L, editors. Measuring stress: A guide for health and social scientists. New York, NY: Oxford University Press; 1995. pp. 29–58. [Google Scholar]
- Vinokur A, Selzer ML. Desirable vs. undesirable life events: Their relationship to stress and mental disease. Journal of Personality and Social Psychology. 1975;32:329–337. doi: 10.1037/0022-3514.32.2.329. [DOI] [PubMed] [Google Scholar]
- Weiner H. Perturbing the organism: The biology of stressful experience. Chicago, IL: University of Chicago Press; 1992. [Google Scholar]
- Wethington E, Brown GW, Kessler RC. Interview measurements of stressful life events. In: Cohen S, Kessler RC, Gordon LU, editors. Measuring stress: A guide for health and social scientists. New York, NY: Oxford University Press; 1995. pp. 59–79. [Google Scholar]
- Wisnivesky JP, Lorenzo J, Feldman JM, Leventhal H, Halm EA. The relationship between perceived stress and morbidity among adult inner-city asthmatics. Journal of Asthma. 2010;47:100–104. doi: 10.3109/02770900903426989. doi:0.3109/02770900903426989. [DOI] [PubMed] [Google Scholar]


