SUMMARY
Circadian clocks regulate various aspects of photoreceptor physiology, but their contribution to photoreceptor development and function is unclear. Cone photoreceptors are critical for color vision. Here, we define the molecular function of circadian activity within cone photoreceptors and reveal a role for the clock genes Bmal1 and Per2 in regulating cone spectral identity. ChIP analysis revealed that BMAL1 binds to the promoter region of the thyroid hormone-activating enzyme type 2 iodothyronine deiodinase (Dio2) and thus regulates the expression of Dio2. Thyroid hormone treatment resulted in a partial rescue of the phenotype caused by the loss of Bmal1, thus revealing a functional relationship between Bmal1 and Dio2 in establishing cone photoreceptor identity. Furthermore, Bmal1 and Dio2 are required to maintain cone photoreceptor functional integrity. Overall, our results suggest a mechanism by which circadian proteins can locally regulate the availability of thyroid hormone and influence tissue development and function.
In Brief
Short and medium wavelength opsins in cone photoreceptors are required for color vision. Sawant et al. show that core circadian clock genes are required for cone opsin expression, gradient maintenance, and cone function and that these effects are mediated by regulation of thyroid hormone signaling.
INTRODUCTION
Vertebrate retinal photoreceptors are specialized light-sensing neurons comprised of rods and cones. Rods are mainly active under dim illumination (photopic) conditions, while cones mediate high acuity vision under bright daylight (scotopic) conditions and are essential for color vision. Functional differences between rods and cones are due to the presence of different light-sensing opsins expressed in respective photoreceptors. Unlike rods, which express just one type of opsin (rhodopsin), cone opsins are widely divergent and classified based on their spectral sensitivity. Humans and closely related primates with trichromatic color vision have three types of cone opsins - short (S), medium (M) and long (L) wavelength-sensitive, while most mammals with dichromatic color vision have only S and M (Applebury et al., 2000; Haverkamp et al., 2005; Nathans, 1999). Despite cone diversity in number and type between species, both gene regulatory networks and developmental patterning in cones exhibit similarities (Viets et al., 2016). In mice, retinal cones are arranged in a dorso-ventral gradient, with M cones predominantly in the dorsal region and S cones enriched in the ventral region (Applebury et al., 2000).
Thyroid hormone (TH) signaling plays an essential role in formation of the dorso-ventral cone gradient. Animals lacking the thyroid hormone receptor TRβ2 lack M opsin expression and show S opsin expression throughout the retina (Ng et al., 2001). Similarly, loss of RXRγ, which heterodimerizes with TRβ2, results in S opsin expression in the dorsal retina, but M opsin expression remains unaffected (Roberts et al., 2005). TH is predominantly secreted into the circulation as an inactive prohormone thyroxin (T4). In the target tissue, most cytoplasmic T4 is converted into active triiodothyronine (T3) by the activity of enzyme type 2 iodothyronine deiodinase (DIO2), while the related protein DIO3 inactivates T4 and T3 by converting them into reverse T3 and T2, respectively (Gereben et al., 2008). T3 generated by DIO2 contributes to the nuclear pool of T3 and serves as ligand for the thyroid hormone receptors such as TRβ2 (Wu and Koenig, 2000). A number of studies have documented circadian and seasonal dependent oscillations in circulating levels of T3 and intracellular levels of Dio2 and Dio3 (Barrett et al., 2007; Bedolla and Torre, 2011; Ono et al., 2008; Peliciari-Garcia et al., 2016; Watanabe et al., 2007). These observations raise the possibility that T3 availability within the tissues could be under a circadian regulation, however molecular mechanisms underlying this regulation are unknown.
Circadian rhythms are generated through the activity of a set of proteins that form an autoregulatory transcriptional and translational feedback loop. The positive transcriptional arm of the loop is mediated by activity of the heterodimeric transcription factors BMAL1 and CLOCK that bind to E-Box motifs of the Period (Per) and Cryptochrome (Cry) genes and other clock-controlled genes to activate transcription. PER and CRY proteins also form heterodimers and repress their own transcription by blocking BMAL1/CLOCK mediated activation. BMAL1 and CLOCK also activate transcription of the nuclear receptor genes Rev-Erbα and Rorα. REVERBα inhibits Bmal1 transcription, whereas RORα enhances it (McMahon et al., 2014; Tosini et al., 2008). Transcripts and proteins encoded by clock genes have been detected in the cells of the inner nuclear layer (INL), ganglion cell layer (GCL), retinal pigmented epithelium (RPE), in cones and at very low levels in rods (Hiragaki et al., 2014; Liu et al., 2012; Mustafi et al., 2013; Ruan et al., 2006). Nonetheless, their role in photoreceptor development and function has not been determined.
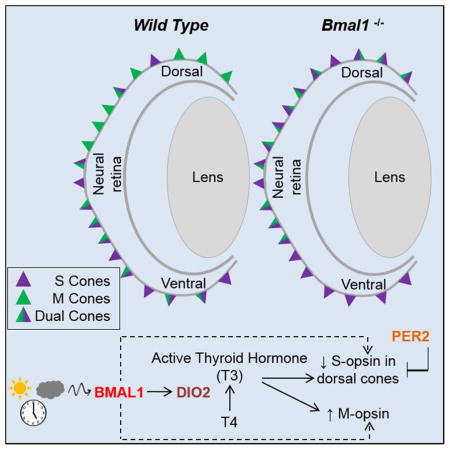
Here we find that Bmal1 loss from mouse cones results in ectopic expression of S opsin in the dorsal retina, while conversely, Per2 loss results in decreased S opsin expression in the dorsal retina. BMAL1 mediates its action in part by regulating Dio2 as we report that Dio2 loss-of-function mutants and Dio2/Bmal1 double heterozygotes exhibit similar S opsin phenotypes. In addition, we use ChIP sequencing to show that Dio2 is a BMAL1 transcriptional target and thus retinal Dio2 expression could be under circadian control. Morever, we show that T3 can partially rescue the phenotypes caused by Bmal1 loss. Finally, our studies indicate that Bmal1 and Dio2 are required for cone receptor maintenance, as loss of either promotes progressive defects in photoreceptor function. These studies suggest a function of the circadian clock in cone photoreceptor activities. Our findings have implication for cone degenerative diseases, as a cone clock or its output signals could exert its effects on retinal health by regulating gene expression, signaling and metabolism.
RESULTS
Bmal1 is required for maintenance of S opsin gradient
The dorso-ventral gradient of cone opsins is known to be regulated by TH and its receptors (Glaschke et al., 2011; Ng et al., 2001; Pessoa et al., 2008; Roberts et al., 2006). We have previously shown that light can regulate TH levels in the murine eye and can affect rod photoreceptor development (Sawant et al., 2015). Light is a strong entrainment signal for the circadian clock and TH is an important regulator of metabolic processes. Hence we hypothesized that the effects of light may be due to desynchronization between the retinal circadian clock and TH signaling during photoreceptor development. To determine if disruption of the circadian clock could affect photoreceptor development, we used the photoreceptor specific Crx-Cre transgene to delete Bmal1 from all photoreceptors. Bmal1 deletion was confirmed by qPCR and western blot analysis on the entire retina (Figure S1). Though the transcript was significantly reduced at P17, we only detected a significant reduction in BMAL1 protein at P24 (Figure S1 AC). Despite the loss of Bmal1 from all photoreceptors, rod development or function was not affected as assessed by dark adapted ERG a-wave amplitude (Figure S1 D) and rhodopsin western blots (Figure S1 F). However, we did observe an ERG response that was indicative of cone photoreceptors being functionally compromised. (Figure S1 E). This led us to further investigate if the cone photoreceptors were affected by the loss of Bmal1.
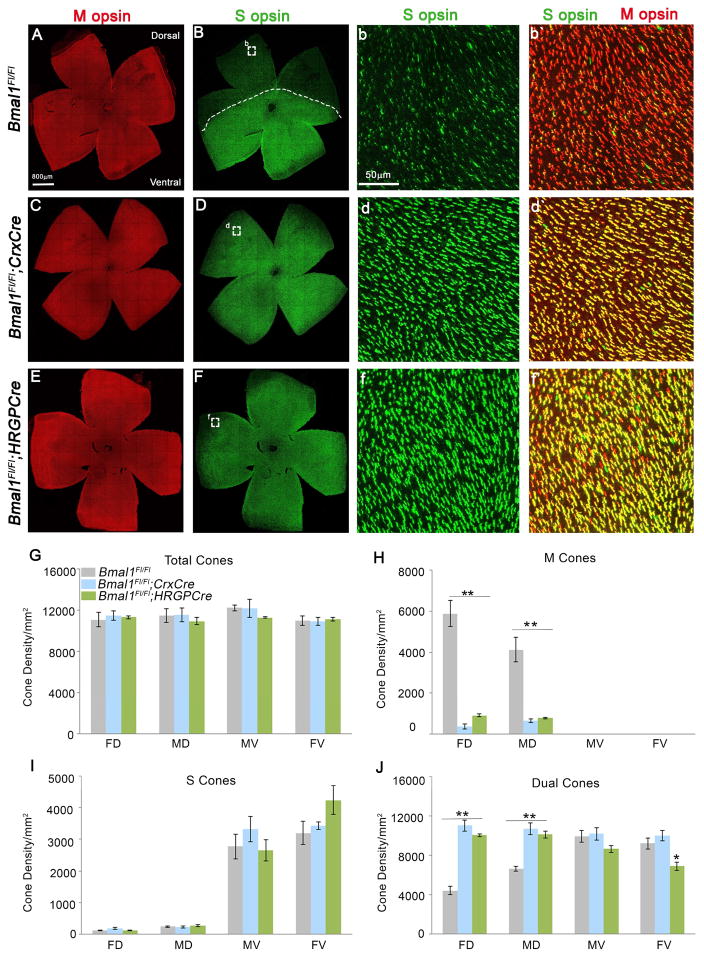
We coimmunolabeled the retinae with antibodies to S and M opsin to visualize the cones. In the litter mate control retina, the S opsin expressing cones were concentrated in the ventral retina with very few S opsin positive cones in the dorsal retina (Figure 1B, b and b′). By contrast, in Bmal1 conditional mutants (CKO) there is a drastic disruption in the regular patterning and distribution of S opsin expressing cones with all the cones in the dorsal retina now expressing S opsin. (Figure 1D, d and d′). In the Crx-Cre transgene, the Cre expression occurs as early as embryonic day 12 (E12), a developmental time when progenitors are committed to becoming photoreceptors. Early loss of Bmal1 could result in a cone/rod fate switch but the total number of cones were unaffected suggesting that Bmal1 alters opsin expression but not cone/rod fate (Figure 1G). To conclusively determine the effects of Bmal1 deletion on cone photoreceptors, we used the cone specific HRGP-Cre transgene to delete Bmal1 (Le et al., 2004, 2006). Similar to the phenotype detected in the Crx-Cre; Bmal1Fl/Fl retina, cone specific deletion of Bmal1 resulted in S opsin expression in the dorsal retina (Figure 1F, f and f′). We quantified the density of cones expressing either only S opsin (Figure 1I), only M opsin (Figure 1H), both S and M opsin (Figure 1J) and the total number of cones (Figure 1G) in the various regions of the retina as shown in the schematic (Figure S2).
Figure 1. Bmal1 is required for maintenance of S opsin gradient.
(A–F) P24 retinal flat mount preparations coimmunolabeled with S opsin (green) and M opsin (red) from control (A and B) and Bmal1 conditional mutants (C–F). (B) Dotted line separating low S opsin expressing dorsal (superior) region from S opsin enriched ventral (inferior) region in control retina. (D and F) Loss of dorso-ventral S opsin gradient in Bmal1 conditional mutants. (b–f′) Representative high magnification images of the region within the square from control (b and b′) and Bmal1 conditional mutants (d, d′, f and f′).
(G–J) Quantification of total cones (G), genuine M opsin cones (H), genuine S opsin cones (I), and dual S+M opsins cones (J) in 20x fields across far dorsal (FD), mid dorsal (MD), mid ventral (MV) and far ventral (FV) regions of the retina indicating that deletion of Bmal1 results in ectopic S opsin expression in the dorsal retina. Error bars are ± SEM and n=5–11. Results were analyzed using one-way ANOVA followed by Tukey post test. * indicates P<0.05 and ** indicates P<0.01.
We observed a significant reduction (>85%) in the number of M opsin expressing cones (Figure 1H) and a concurrent increase in the density of dual-cones (Figure 1J). Overall there is a decrease in M opsin expressing cones throughout the retina and this decrease was more evident in the far ventral region of the retina, in the HRGP-Cre; Bmal1Fl/Fl animals (Figure S3 D). Immunofluorescence data was validated using western blot analysis (Figure S3 C) and real time qPCR (Figure S3 A–B). In agreement with our observation, both the M opsin transcript and protein was significantly lower in the CKO retina. Thus, these results demonstrate that core circadian clock gene Bmal1 is required for the maintenance of S opsin gradient and regulates the expression of M opsin in the mouse retina. Interestingly, we observe a similar phenotype in the retinas from genetically deleted Clock animals (Figure S4). BMAL1/CLOCK heterodimer regulates the transcription of target genes which include other clock genes like Period and Cryptochrome. Thus, the loss of either Bmal1 or Clock resulting in the same phenotype strengthens our hypothesis that core clock genes are important for cone photoreceptor development.
Per2 is required for overall S opsin levels but not for maintaining the S opsin gradient
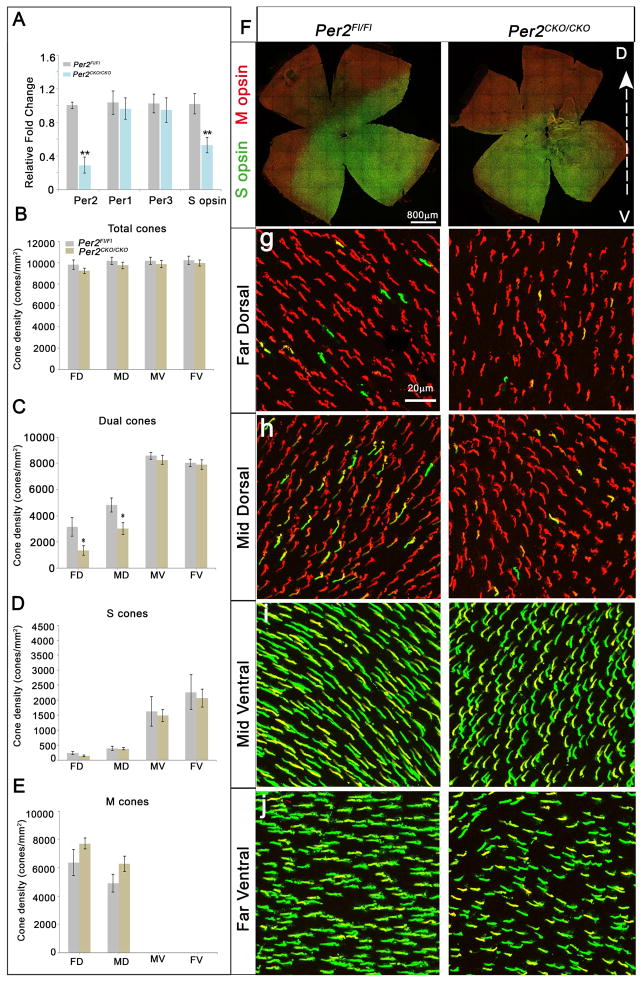
Bmal1 and Period are known to have opposite effects on cellular processes and opposite phase for their rhythmic expression pattern (Bouchard-Cannon et al., 2013; Jensen et al., 2012). It has been demonstrated that in mice lacking both Per1 and Per2, S opsin levels are reduced (Ait-Hmyed et al., 2013) and this phenotype is exactly opposite to our observations with Bmal1 deletion. Based on previous analysis (Hiragaki et al., 2014; Liu et al., 2012; Ruan et al., 2006), Per1 appears to be largely restricted to the INL while Per2 seems to be more cone specific, suggesting a distinct function for the different Per genes. To determine if photoreceptor specific loss of Per2 would have the opposite effects to the loss of Bmal1, we used a floxed allele for Per2 (Figure S5 A) and deleted Per2 using the Crx-Cre transgene (Figure S5 C). We confirmed loss of Per2 by qRT-PCR analysis. Loss of Per2 resulted in the reduction of S opsin transcript but Per1 and Per3 were unchanged (Figure 2A). Although the overall dorso-ventral gradient of cone opsins was not altered in Per2 CKO retinas (Figure 2F), there was a significant decrease in the number of S opsin positive cones in the dorsal region compared to the control group (Figure 2C, g–h). Thus our data suggest that Per2 is expressed in the cones (Figure S5 A) and loss of Per2 results in reduced S opsin levels but S opsin gradient was unaffected. It will be interesting to determine if the loss of Cryptochromes, would result in similar phenotypes as loss of Period. Nevertheless, our findings suggest that Bmal1 and Per2 have an opposing effect on S opsin expression.
Figure 2. Per2 is required for overall S opsin levels but not for maintaining S opsin gradient.
(A) Relative expression of Per2, Per1, Per3 and S opsin transcripts in P24 control and Crx-Cre; Per2Fl/Fl retinas using qRT-PCR. Fold change is relative to expression in the control group. Error bars are ± SEM and n=4. ** indicates P<0.01.
(F–j) P42 retinal flat mount preparations coimmunolabeled with S opsin (green) and M opsin (red) for control (F-left panel) and Per2 conditional mutants (F-right panel). The S opsin gradient is not perturbed in the Crx-Cre; Per2Fl/Fl animals, but decrease in S opsin expression was observed at high magnification, (g–j right panels) compared to the control (g–j left panels). D-dorsal and V-ventral.
(B–E) Quantification of total cones (B), dual S+M opsins cones (C), genuine S opsin cones (D), and genuine M opsin cones (E) in 20x fields across far dorsal (FD), mid dorsal (MD), mid ventral (MV) and far ventral (FV) regions of the retina. Error bars are ± SEM and n=5–6. Results were analyzed using Student’s t-test. * indicates P<0.05.
Dio2 is expressed in the cone photoreceptors
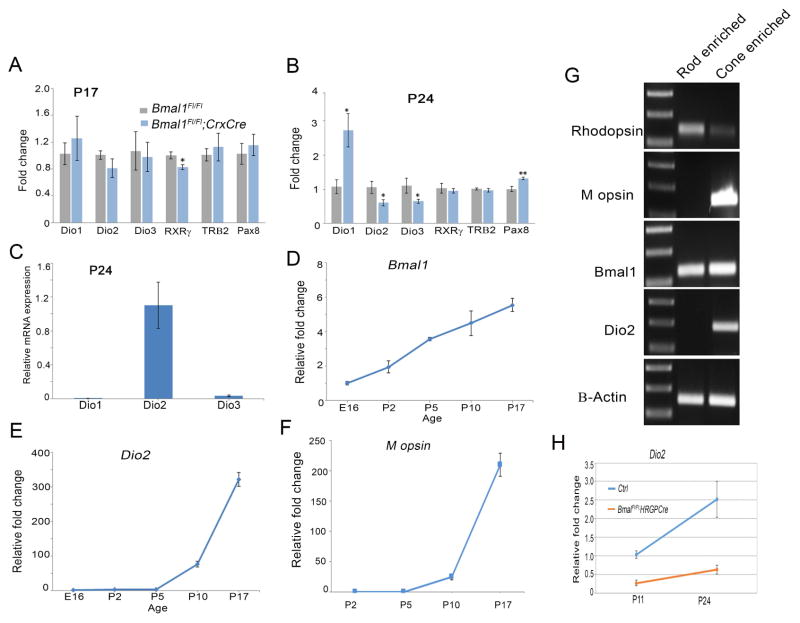
A number of genes involved in thyroid hormone signaling pathway such as Trβ2, Pax8 and Dio3 are known to play a vital role in establishing the cone opsin gradient (Applebury et al., 2007; Glaschke et al., 2010; Ng et al., 2001; Ng et al., 2010; Pessoa et al., 2008). To determine if any of these genes were transcriptionally altered by loss of Bmal1, we analyzed their transcript levels using qRT-PCR at P17 and P24, the ages when we can detect lower levels of Bmal1 transcript in the Bmal1 CKO retina (Figure S1). We only tested for Dio1, Dio2, Dio3, RXRγ, Trβ2 and Pax8 transcripts as these could play a role in cone opsin gradient formation. Except for RXRγ which was significantly reduced in the Bmal1 CKO retina, none of the other transcripts were affected at P17. RXRγ is a cone marker and is expressed in the developing cones (Roberts et al., 2005). Thus the observed reduction in the RXRγ transcript, could be due to a delay in cone photoreceptor development in the Bmal1 CKO. Accordingly at P24 RXRγ levels were not different between the control and CKO retina (Figure 3B). Interestingly all the deiodinases were altered by the loss of Bmal1. However, neither Dio1 or Dio3 is known to be expressed at any significant levels in the post natal retina compared to Dio2, thus making it less likely that these deiodinases have any role to play in regulating S opsin expression in the post natal retina. (Figure 3C) (Ng et al., 2017). Moreover, loss of Dio3 results in apoptosis of cone photoreceptors, whereas in the Bmal1 CKO retina, cone numbers are not significantly altered (Figure 1G). On the other hand, in the postnatal retina, the relative expression profile for the Dio2 transcript is very similar to that of M opsin expression (Figure 3E–F) and coincides with the peak period of photoreceptor outer segment extension (LaVail, 1973). Similarly, Bmal1 transcript levels also increase postnatally but the increase is not as exponential as seen for the Dio2 or M opsin transcripts. The transcript analysis was performed using the entire retina and these transcriptional changes reflect global changes within the retina. To determine if Dio2 was specifically expressed in the cone photoreceptors, we performed qPCR analysis on isolated cones and rods at P24. Despite the presence of some contaminating rods in our cone enriched population, Dio2 transcripts could only be detected in the cone enriched population (Figure 3G). Importantly, there is a significant increase in Dio2 transcript between P11 and P24 in the sorted cones (Figure 3H). Similar to the results acquired from the RNA analysis of the whole retina, Dio2 transcript levels are significantly lower in the isolated cones from the Bmal CKO (Figure 3H) at P11 and P24. Taken together these results indicate that Dio2 is expressed in the cone photoreceptors and Dio2 levels are reduced in Bmal1 CKO retinas.
Figure 3. Dio2 levels are altered in Bmal1 conditional mutants and Dio2 is expressed in the cone photoreceptors.
(A–B) Relative mRNA expression of Dio1, Dio2, Dio3, Rxrγ, Trβ2 and Pax8 in control and Bmal1 conditional mutant retinas using qRT-PCR. Fold change is relative to expression in the control group.
(C) Relative retinal mRNA expression of Dio1 and Dio3 compared to that of Dio2 at P24 indicating that retinal Dio2 expression is much higher than that of Dio1 and Dio3.
(D–F) Relative mRNA expression of retinal Bmal1, Dio2 and M opsin over the developmental ages indicated.
(G) PCR products of rhodopsin, M opsin, Bmal1, Dio2 and β-actin from FAC sorted cone and rod enriched populations.
(H) Relative Dio2 mRNA expression in cone-enriched population from control (tdTomato; Bmal1 Fl/+; Hrgp-Cre) and Bmal1 conditional mutant (tdTomato; Bmal1 Fl/Fl; Hrgp-Cre) animals at P11 and P24. Dio2 expression in the control group was significantly higher at P24 compared to P11.
Error bars are ± SEM and n=3–4. Results were analyzed using Student’s t-test.* indicates P<0.05 and ** indicates P<0.01.
Loss of Dio2 results in ectopic S opsin expression in the dorsal retina
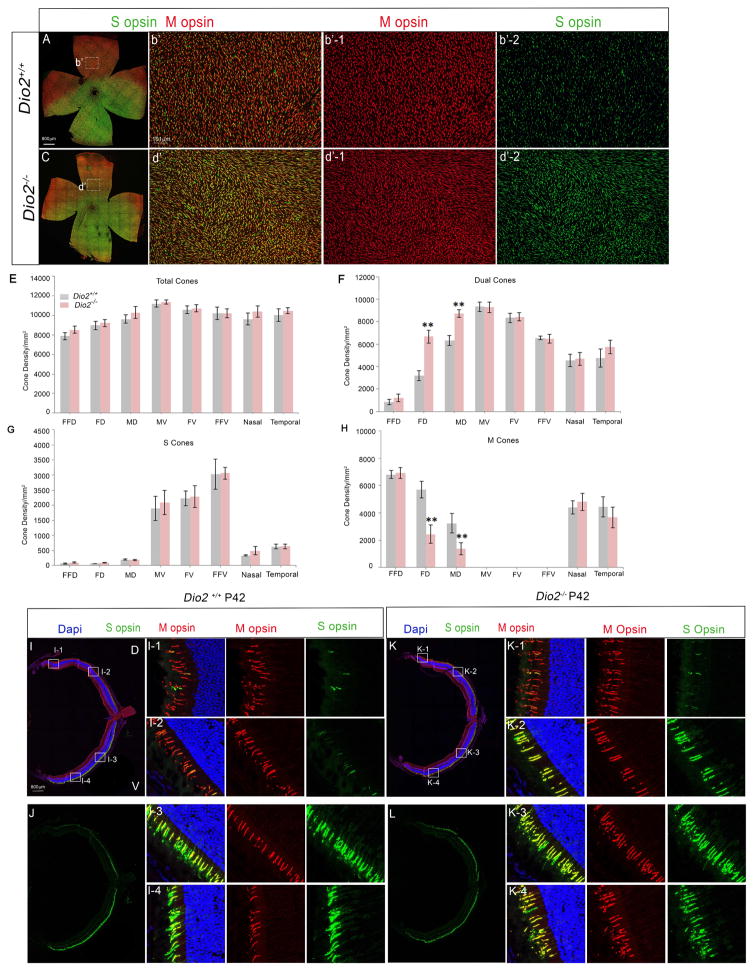
Next, we wanted to test if the ectopic S opsin expression in the Bmal1 CKO retina is due to alterations in Dio2 expression. We analyzed the retinas from animals that lack Dio2 due to the insertion of a null mutation in the Dio2 gene. We observed an increase in the number of S opsin expressing cones in the dorsal retina and disruption of dorso-ventral S opsin gradient in Dio2−/−animals (Figure 4C, d′, d′-2, K-2 and L) compared to the controls (Figure 4A, b′, b′-2, I-2 and J). Similar to Bmal1 CKO retina, Dio2 deletion did not affect total number of cones, but density of dual cones is increased with a concurrent decrease in genuine M cone density (Figure 4E–H). The phenotype is more pronounced in the far dorsal and mid dorsal region, but the cones in the extreme periphery region (FFD-far far dorsal) are unaffected (Figure 4E–H). However, this phenotype is persistent up to 6 weeks (Figure 4K-2). The phenotype of Dio2−/− animals though mild is similar to that of the Bmal1 CKO animals suggesting that both Bmal1 and Dio2 are required for maintaining the S opsin gradient in the mouse retina.
Figure 4. Ablation of Dio2 ectopic S opsin expressing cones in the dorsal retina.
(A–d) P24 retinal flat mount preparations coimmunolabeled with antibodies to S opsin (green) and M opsin (red) from control (A) and Dio2 mutant (C). Representative high magnification images of the dorsal retina from control (b′) and Dio2 mutant (d′) showing increased density of dual cones in Dio2−/− retina.
(E–H) Quantification of total cones (E), dual S+M opsins cones (F), genuine S opsin cones (G), and genuine M opsin cones (G) in 20x fields across far far dorsal (FFD), far dorsal (FD), mid dorsal (MD), mid ventral (MV), far ventral (FV) and far far ventral (FFV) regions of the retina. Error bars are ± SEM and n=6. Results were analyzed using Student’s t-test. * indicates P<0.05 and ** indicates P<0.01.
(I–L) P42 retinal sections coimmunolabeled with S opsin (green) and M opsin (red) for control (I and J) and Dio2 mutant (K and L) indicating that in the Dio2−/− mutant the phenotype persists in the adults. (l1–4, K1–4) High magnification images of the boxed regions.
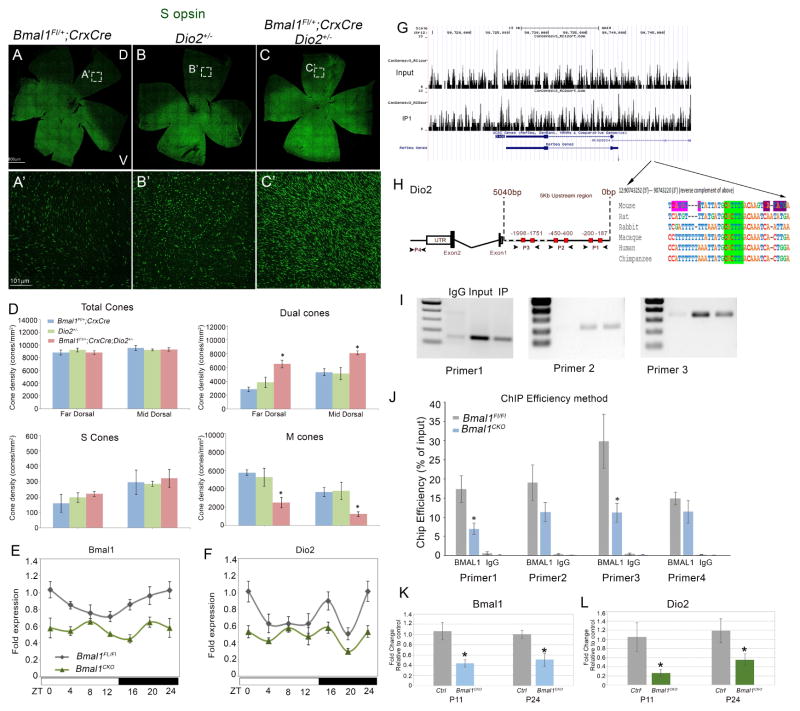
BMAL1 regulates Dio2 expression
Bmal1 and Dio2 mutants have similar phenotype suggesting that the two signaling pathways could cooperate in regulating S opsin expression. BMAL1 is a transcription factor, therefore, one possibility is that BMAL1 directly regulates Dio2 expression. If the two pathways are interacting, then partially compromising the function of both pathways should result in an additive effect. To test this hypothesis, we quantitated the S cone density in the Bmal1 CKO; Dio2+/− double heterozygous retinas and compared it to single heterozygotes of Bmal1 CKO and Dio2. The S opsin gradient was disrupted in the double heterozygous mutant animals and the number of S opsin positive cones were significantly higher (Figure 5C, C′, D) compared to single heterozygous animals (Figure 5A′, B′, D). We detected the maximal changes in the dorsal retina and hence quantitated the cone densities in the dorsal region. These findings suggest that there is a genetic interaction between Bmal1 and Dio2 and these pathways are important regulators of S opsin patterning in the dorsal retina.
Figure 5. Bmal1 regulates Dio2 expression.
(A–C) retinal flat mount images and (A′–C′) high magnification images from dorsal region of the retina indicating increase in S opsin (green) positive cones in double heterozygous retina (C′) compared to single heterozygous of Bmal1 (A′) and Dio2 (B′).
(D) Quantification of total cones, dual S+M opsins cones, genuine S opsin cones, and genuine M opsin cones in 20x fields across far dorsal (FD) and mid dorsal (MD) regions of the retina. Error bars are ± SEM and n=4–5. Results were analyzed using Student’s t-test. * indicates P<0.05.
(E–F) Circadian oscillations in retinal Bmal1 (E) and Dio2 (F) transcripts from P21 animals of the indicated genotypes. Solid white and black bars on the X-axis indicate light and dark phase, respectively. Bmal1 and Dio2 levels were significantly lower in the Bmal1 CKO group compared to the control group (F1, 47=18.83; P<0.001 and F1, 47=13.50; P<0.001, respectively). Error bars are ± SEM and n=4. Results were analyzed using two-way ANOVA followed by Fisher LSD post-hoc test with genotype and ZT as two independent factors.
(H) Sequence analysis of mouse Dio2 upstream region that contain known E-boxes (red boxes) that bind to BMAL1. P1, P2 and P3 indicate the primer sets designed to amplify the DNA sequence (I) from the Input, BMAl1 IP (Immunoprecipitated) and IgG IP samples. (J) ChIP-qPCR indicating decrease in ChIP efficiency in P24 Bmal1 CKO compared to control. IgG was used a a non specific antibody control for the ChIP experiments. Error bars are ± SEM and n=3. Results were analyzed using Student’s t-test. * indicates P<0.05.
(G) Coverage plots of genomic sequence surrounding Dio2 gene in input and BMAL1 IP retinal samples (P3). Arrows indicate the region in the coverage plot with multiple peaks is a region within the Dio2 promoter [Chr12: 90743252 (5′) --- 90743220 (3′)] that is highly conserved among different species and contains a conserved E box (green highlight). –indicates spaces in alignment. In mouse, the conserved E-box is accompanied by another BMAL1 E-box (CATGTG) (pink) separated by 7 bases and a known CLOCK binding E-box (CATATG) (violet) separated by 6 bases.
(K–L) Relative expression of Bmal1 (K) and Dio2 (L) transcripts in cone-enriched population from control (tdTomato; Bmal1 Fl/+; Hrgp-Cre) and Bmal1 conditional mutant (tdTomato; Bmal1 Fl/Fl; Hrgp-Cre) animals at P11 and P24. Error bars are ± SEM and n=3. Results were analyzed using Student’s t-test. * indicates P<0.05.
Next we wanted to test if we could detect any oscillation in the Bmal1 and Dio2 transcripts within the retina over a circadian time period. We observed rhythmicity in Bmal1 expression, with peak expression at ZT0 (beginning of the light phase) and lowest expression at ZT12 (beginning of dark phase) (Figure 5E). In the Bmal1 CKO, as expected, the overall levels of Bmal1 transcript were significantly lower (P<0.001) but Bmal1 expression levels were not significantly different among different ZTs (Figure 5E). This is surprising given that only 50% of the transcript is reduced by the loss of Bmal1 from the photoreceptors (Figure S1). This data suggests that the circadian oscillations of Bmal1 in the entire retina could be compromised due to deletion of Bmal1 from the photoreceptors. Dio2 transcript also exhibited cyclic oscillations fairly similar to that of Bmal1 except for ZT 20 (Figure 5F). The peak expression for retinal Dio2 could be detected at ZT 0 and was significantly higher compared to its lowest expression observed just before and during the dark phase (Figure 5F). Overall oscillations of the Dio2 transcript was eliminated in Bmal1 CKO retinas as none of the time points were significantly different. To confirm that Bmal1 deletion would result in loss of Dio2, we performed qPCR analysis on sorted cones for Bmal1 and Dio2 transcripts. There is more than 50% reduction in the Bmal1 and Dio2 transcripts at P11 and at P24 (Figure 5K and L). The residual expression for Bmal1 and Dio2 is due to the activity of the transgene as Cre driven conversion of a reporter transgene was used to isolate the cones (see experimental procedures for details). This data suggest that BMAL1 could be directly regulating Dio2 expression within the cones.
We then wanted to determine if BMAL1 controls Dio2 expression. First we performed in-silico analysis of the Dio2 promoter to establish if any of the known canonical (5′-CANNTG-3′) BMAL1 binding E-box elements were present. We identified 6 E-box elements (Jensen et al., 2012; Rey et al., 2011) within 5 kb of the annotated transcription start site of the Dio2 gene (Figure 5H). Of these 6, there is one highly conserved E-box in the region that is accompanied by another BMAL1 E-box (CATGTG) separated by 7bp (Figure 5H right panel). Interestingly, there is also a known CLOCK binding E-box (CATATG) site right next to the BMAL1 E-box (Yoshitane et al., 2014) separated by 6 bases. This in-silico analysis strongly suggested that Dio2 might be a target of BMAL1. To validate our in-silico analysis, we performed chromatin immunoprecipitation (ChIP) assay on the retinal tissues using a BMAL1 antibody and an IgG control antibody. We could specifically amplify the amplicons from the BMAL1 ChIPed DNA samples but not the IgG samples, with primers that span across the 6 E-boxes (Figure 5I). We also performed qPCR analysis on the input and ChIPed DNA with the same primer sets and one primer (P4) that was unrelated to the BMAL1 E-boxes. This analysis indicated a decrease in ChIP efficiency (Figure 5J) for these sites in Bmal1 CKO, further suggesting that BMAL1 can bind to the Dio2 promoter. It should be noted that the ChIP efficiency for the primers is variable and this is due to the entire retina being used for the ChIP-Seq. Again, in the ChIPed DNA samples obtained with IgG, we did not detect Dio2 (Figure 5J). We also validated the ChIP efficiency using primers designed against known BMAL1 E-boxes around upstream region of Per2 gene (Figure S6 A). ChIP-Seq coverage plots for the Dio2 gene and 5′ upstream region further confirmed our observations that Dio2 is a target of BMAL1 (Figure 5G). Thus our data demonstrate that Dio2 expression significantly varies over the circadian day and regulation of Dio2 by BMAL1 is important for spatial distribution of the S opsin expressing cones.
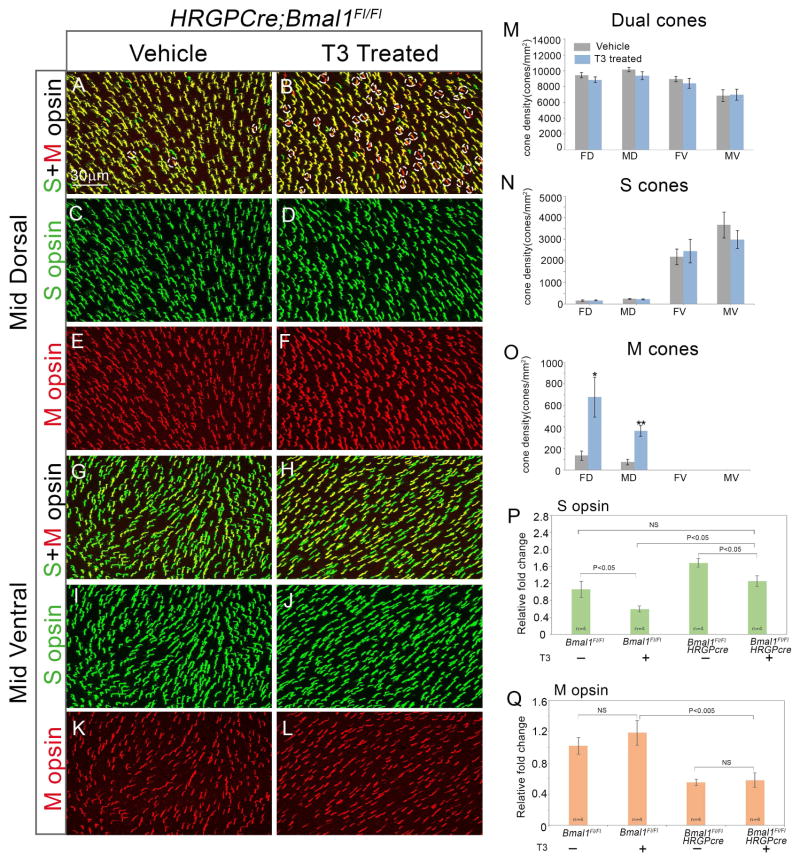
Partial recovery of opsin expression after T3 administration
The phenotype in Bmal1 CKO animals is very similar to that observed in adult rodents with induced hypothyroidism, where terminally differentiated M opsin cones in the dorsal retina convert to S cones (Glaschke et al., 2011; Pessoa et al., 2008; Roberts et al., 2006). Our data raises the possibility that in the Bmal1 CKO retina, due to the loss of Dio2, a local reduction in T3 levels could result in an hypothyroid-like cone opsin phenotype. If our hypothesis is correct then we would predict that we should rescue the phenotype in the Bmal1 CKO by providing exogenous T3 to the animals. To achieve this, we provided T3 via drinking water to Bmal1Fl/Fl and Hrgp-Cre; Bmal1Fl/Fl littermates from P20–P50 and analyzed their retina for S and M opsin expression. As predicted, T3 supplementation resulted in a decrease in S opsin expression in the dual cones and they begin to appear as genuine M opsin expressing cones (Figure 6A–B, dotted ovals). The far and mid dorsal region were most affected by T3 supplementation (Figure 6O). We validated the immunofluorescence data with transcript analysis. T3 treatment results in an overall reduction in S opsin transcript (Figure 6P). It is interesting to note that at P50, S opsin transcript levels were similar in the vehicle control WT group and T3 treated CKO group (Figure 6P). Though T3 treatment did not result in complete reversal of S opsin gradient as assessed by immunofluorescence staining, we could detect a noticeable rescue in the S opsin transcript levels. It would be interesting to maintain the animals on T3 for longer periods to determine if longer treatment can result in complete reversal of S opsin gradient. However, high levels of T3 can also affect cone survival and overall affect the health of the animals (Ma et al., 2014). Nonetheless, our data demonstrates that T3 supplementation can partially reverse the cone opsin phenotype resulting from Bmal1 deletion.
Figure 6. Partial rescue of Bmal1 conditional mutants after T3 administration.
(A–F) High magnification images from dorsal region of the retina indicating a concurrent decrease in S opsin expression and increase in M opsin expression resulting in the appearance of more M opsin cones (dotted ovals) in T3 treated Bmal1 conditional mutants (B) compared to the untreated vehicle group (A). (G–L) High magnification images from ventral region of the retina. (M–O) Quantification of dual S+M opsins cones (M), genuine S opsin cones (N), and genuine M opsin cones (O) in 20x fields across far dorsal (FD), mid dorsal (MD), mid ventral (MV) and far ventral (FV) regions of the retina. Error bars are ± SEM and n=5. Results were analyzed using Student’s t-test. * indicates P<0.05 and ** indicates P<0.01.
(P–Q) Relative retinal expression of S opsin (P) and M opsin (Q) transcript in P50 Bmal1Fl/Fl and Hrgp-Cre; Bmal1Fl/Fl animals from T3 supplemented and vehicle control groups using qRT-PCR. Fold change is relative to the Bmal1Fl/Fl vehicle control group. Error bars are ± SEM and n=4. Results were analyzed using two-way ANOVA followed by Tukey post-hoc test with genotype and treatment (T3 V/S Vehicle) as two independent factors.
Bmal1 and Dio2 are required for adult cone function and maintenance
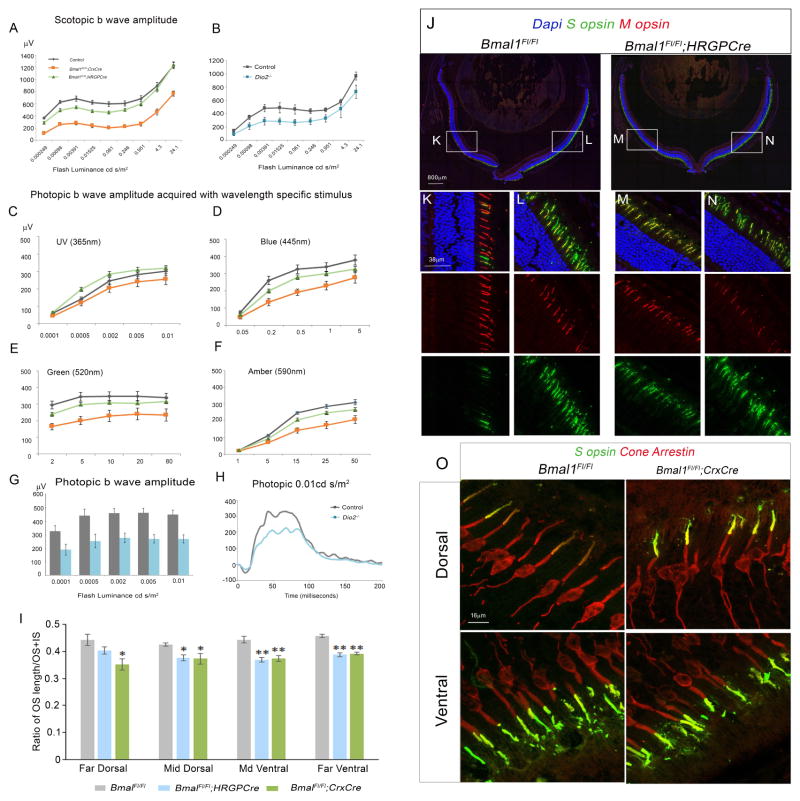
Next, we wanted to test if the dorsal increase in the S opsin cones results in functional changes and if Bmal1 CKO cones exhibit stronger responses towards short wavelength of light. We performed wavelength specific ERG testing in mice at 6 weeks of age to assess if the observed changes in opsin expression result in impaired cone function. In HRGP-Cre; Bmal1Fl/Fl mice, UV light flashes (365nm) that excites S opsin cones elicited a statistically significant increased response at various flash stimuli compared to the control group (Figure 7C). Other wavelengths of light which include blue (445nm), green (520nm) and amber (590nm) light sources showed significantly lower b-wave amplitudes compared to the control group (Figure 7D–F). Cone b-wave ERG responses in Crx-Cre; Bmal1Fl/Fl mice were also significantly decreased for all wavelengths tested compared to the control group. Although cone b-wave response for UV light was significantly lower in Crx-Cre; Bmal1Fl/Fl mice (Figure 7A), the degree of reduction is less severe than the degree of reduction for other wavelengths (Figure 7C–F) (P=0.024 V/S P<0.001). These results indicate that cone specific deletion of Bmal1 results in an increase in S opsin cone responses and a decrease in M opsin cone responses.
Figure 7. Bmal1 and Dio2 are required for adult cone function and maintenance.
(A–B) Dark-adapted b-wave responses were decreased in 6 weeks old Bmal1 CKO (A) and Dio2−/−mutants (B) compared to the reponses recorded from the respective littermate controls.
(C–F) Light adapted ERG b-wave responses in 6 weeks old Bmal1 CKO and littermate control mice using 365 nm (C), 445 nm (D), 520 nm (E) and 590 nm (F) of light sources. (C) b-wave responses at 365 nm were significantly higher in the HRGP-Cre; Bmal1Fl/Fl group (F2, 65=18.74; P<0.001). (D–F) b-wave responses at 445 nm (F2, 65=35.25; P<0.001), 520 nm (F2, 65=41.08; P<0.001) and 590 nm (F2, 65=40.36; P<0.001) were significantly lower in both Bmal1 conditional mutant groups compared to the control group.
(G–H) Light-adapted b-wave responses were significantly decreased in Dio2 mutants at 6 weeks of age. Error bars are ± SEM and n=4–8. Results were analyzed using two-way ANOVA followed by Tukey post-hoc test with genotype and flash intensity as two independent factors.
(J–N) Dorsal and ventral retinal sections from 3 month old control animal (K and L,) and Bmal1 CKO (M and N,) labeled with S opsin (green) and M opsin (red). (O) Dorsal and ventral retinal sections from 3 month old control animal and Bmal1 CKO labeled with S opsin (green) and Cone arrestin (red). (I) Quantification of cone outer segment length (OS). The cones from Bmal1 CKO retina have shorter OS compared to littermate controls suggesting a role for Bmal1 in maintenance of the photoreceptors. Results were analyzed using one-way ANOVA followed by Tukey post-hoc test.* indicates P<0.05 and ** indicates P<0.01.
We performed standard ERGs on Dio2−/− animals at 6 weeks of age. Dio2−/− mice exhibited approximately 40% reduction in light-adapted cone b-wave response to increasing intensity of achromatic flashes that excite both S and M opsins as compared to control mice (Figure 7G–H). As expected, dark-adapted a-wave was not drastically affected in Dio2−/− mice compared to their respective littermate controls (Figure S6 B) since we could not find detectable levels of Dio2 in the rods (Figure 3G). We also observed a significant reduction in dark-adapted b-wave responses in Bmal1 CKOs (Figure 7A) as well as in the Dio2−/− mice (Figure 7B). As this response is known to reflect the activity of the bipolar cells, this reduction could be due to the loss of Bmal1 in bipolar cells. This certainly could be the case as we can detect very weak levels of Cre protein in cells of INL besides the photoreceptor (data not shown) and the Crx-Cre CKO animals exhibit much stronger phenotype compared to the HRGP-Cre CKO animals. However, the reduced dark adapted b-wave amplitude in the HRGP-Cre CKO animals also suggest that the Scotopic b-wave response is in part due to compromised cone function. In summary, these results indicate that Bmal1 and Dio2 are required for cone photoreceptor function.
Our ChIP-Seq analysis with the BMAL1 antibody identified 752 target genes and none of these appeared in the Bmal1 CKO group (Figure S7 and Data File S1, S2). Many of these targets were involved in pathways that regulate cellular homeostasis (Data File S3, S4). This prompted us to investigate if the loss of Bmal1 would lead to long term changes in the cones. We assessed the retina from 3 month-old animals for cone outer segment (OS) defects. The majority of the cones in Bmal1 CKO animals appear to have shorter OS compared to the controls (Figure 7K–N). To further evaluate this phenotype, we measured cone OS length compared to the combined OS and IS (inner segment) length, using cone arrestin and S opsin to mark the cones (Zhu et al., 2002). In 3 month-old animals, loss of Bmal1 resulted in a significant decrease in cone OS length across the entire retina (Figure 7I,O). In the HRGP-Cre CKO animals, the OS length of the cones in the far dorsal region was slightly shorter in comparison to the cones from the control animals, but not stastically significant. This could be due to the late onset of Hrgp-Cre and we speculate that this phenotype will probably deteriorate over time. We also observed a small (~21%) but statistically significant reduction in cone arrestin transcript levels in Bmal1 CKO retinas at 5 months of age (Figure S6 C). This data suggest that Bmal1 is also required for cone function and maintenance of the cone outer segment.
DISCUSSION
Cells in the rodent retina exhibit circadian activity in processes such as melatonin synthesis (Tosini and Menaker, 1998), disk shedding (LaVail, 1980), and ion channel function (Huang et al., 2015). Nonetheless, whether a circadian clock regulates photoreceptor activity in the mammalian retina has remained unclear, due in part to difficulty in analyzing low levels of expression of various clock genes in those tissues. Here we show that the circadian clock genes Bmal1 and Per2 are required to maintain the spectral identity of the cone photoreceptors and Bmal1 mediates its effect by regulating thyroid hormone signaling within the cone photoreceptors.
Circadian rhythmicity in visual responses
ERG responses from Bmal1 CKO animals have been reported (Storch et al., 2007), but assessment of cone subtypes and distribution in mutant mice has not been analyzed. Importantly, Storch et al, reported that light-adapted b-wave ERG responses in wild-type mice exhibit circadian rhythmicity, a response abolished in animals lacking retinal Bmal1. Based on their observation, the authors concluded that Bmal1 is required for generation of circadian rhythms in the inner retinal neurons. Here, we observed similar reductions in light-adapted b-wave responses in photoreceptor-specific and cone-specific Bmal1 knockout mice. Although we did not test mutant mice at different circadian times, our data suggests that differences in light-driven responses observed by Storch et al., could be due to the presence of a functional circadian clock within the cones. Similar circadian changes in cone function have been reported for mice deficient in Rev-Erbα−/−, which show an increase in dark-adapted b-wave amplitude, a modest increase in light-adapted b-wave amplitude, and unaltered dark-adapted a-wave amplitude (Ait-Hmyed Hakkari et al., 2016). Our data provides further evidence for the existence of a cone specific clock that is required for both developmental and mature functional responses. However, further studies are needed to determine if the cone clock drives rhythmic expression of other retinal factors.
Thyroid hormone and other transcription factors controlling developmental cone opsin gradient
Multiple transcription factors and external signals such as TH control temporal and spatial expression of cone opsins. In mice, peak levels of TH (Alonso et al., 2007; Sawant et al., 2015), TH transporters (Henning and Szafranski, 2016) and Dio2 (Campos-Barros et al., 2000) are detected in the retina around P12–P18, during the period of photoreceptor development. Based on the expression pattern for S and M opsin, it has been proposed that initially all cones express S opsin by default and then active suppression of the S opsin occurs in the dorsal retina, followed by M opsin expression (Mears et al., 2001; Ng et al., 2001). S opsin suppression in the dorsal retina requires protein-protein interaction between thyroid hormone-bound TRβ2 and RXRγ (Roberts et al., 2005). Bmal1 deletion by Crx-Cre did not disrupt the S opsin gradient at P6 (Figure S6 D), despite the fact that Crx-Cre is expressed as early as E12. This observation suggests that either Bmal1 is not expressed in cone photoreceptors before P6, or Bmal1 is essential only after P6. We have not confirmed if Bmal1 is expressed in the cones before P6, but Bmal1 deletion using the late onset HRGP-Cre results in a phenotype that is similar to that observed in the Crx-Cre CKO and strongly suggests that Bmal1 is probably expressed after P6. Despite the important role played by TRβ2 in regulating M opsin expression, there is a considerable delay between detection of high levels of TRβ2 expression and M opsin expression (Applebury et al., 2007; Ng et al., 2009), suggesting that additional factors cooperate with TRβ2 and RXRγ in this activity. One such candidate RORα, when genetically deleted results in decreased expression of S opsin, M opsin and cone arrestin, indicating that RORα is required for overall cone development (Fujieda et al., 2009). In the Bmal1 CKO, RORα levels remain unchanged both at P17 or at P24 (data not shown). Other transcription factors such as GTF2IRD1 and chicken ovalbumin upstream promoter-transcription factor (COUP-TF) nuclear receptors are implicated in S opsin suppression (Masuda et al., 2014; Satoh et al., 2009), and Coup-tfI (Nr2f1) was identified as a BMAL1 target in our ChIP-Seq analysis (data not shown). Our results raise the possibility that BMAL1 is a master regulator that is required for synchronized and coordinated expression of multiple target genes that can regulate S opsin suppression within the dorsal retina, although further investigations are needed to support this hypothesis.
The role of the circadian clock and thyroid hormone in cone maintenance
It is well documented that TH is required for cone survival and elevated TH levels in adult mice cause cone death (Ma et al., 2014). Similar results have been observed using a zebrafish model in which morpholino-mediated transient knockdown of all three deiodinases promoted photoreceptor loss (Houbrechts et al., 2016). Reduction in levels of thyroid hormone also has a protective effect on cone survival in animal models of retinal degeneration (Ma et al., 2014). Our data suggests that Bmal1 and Dio2 are required for cone maintenance in adults. Accordingly, we can detect impairment in cone function at P90 in the photoreceptor specific and cone specific Bmal1 CKO and in Dio2 mutants. Compromised cone function could be the result of the shorter cone outer segments in the Bmal1 CKO animals compared to their WT cohorts of the same age. These results suggest a role for TH signaling in adult tissue maintenance. Though TH signaling plays an important role in developing tissues and is a key mediator of growth, role of TH signaling in adult tissue maintenance is still unexplored (For a review, see (Mendoza and Hollenberg, 2017)). It is also important to note that both Bmal1 and Dio2 transcripts are rhythmically expressed in the retina, although the functional significance of this pattern needs to be determined. Our findings in Dio2−/− mice as well as the genetic interaction between Bmal1 and Dio2 reported here, suggest that cone maintenance is dependent on BMAL1-mediated Dio2 signaling. Moreover, by providing T3 to the Bmal1 CKO animals we can bypass the requirement for Dio2 and partially revert some of the dual cones to M only cones. Based on this analysis we also cannot exclude the possibility that besides Dio2 and thyroid hormone there are additional signaling pathways that are regulated by Bmal1 and are important in the patterning of S and M opsin expressing cones in the mouse retina. Nevertheless the partial rescue results suggest that it is possible to revert opsin expression in adult cones and that opsin expression could be manipulated for therapeutic purposes.
In summary, we show that loss of the circadian clock gene Bmal1 disrupts the dorso-ventral gradient of S and M opsins and increases S opsin expression in the entire retina, whereas loss of Per2, reduces S opsin expression. We also show that the levels of the transcript encoding the thyroid-activating enzyme Dio2 oscillate within the retina during the circadian day and the circadian clock protein BMAL1 binds to the Dio2 promoter. Our findings suggest a functional role for the circadian clock in the mammalian cone photoreceptor development and provides evidence for a continual role for thyroid hormone signaling in cone photoreceptor maintenance. Our findings maybe of relevance in other tissues, where the circadian clock could alter tissue function by directly regulating Dio2 expression and thus TH signaling.
EXPERIMENTAL PROCEDURES
Mouse Strains
Transgenic mice carrying the Dio2 mutation (Stock No: 018985), (Schneider et al., 2001), Bmal1flox/flox (Stock No: 018985), (Storch et al., 2007), tdTomato (Stock No: 007914) were purchased from The Jackson Laboratory, Bar Harbor, ME. Other mice used in this study are HRGP-Cre (Le et al., 2004, 2006), and Crx-Cre (Nishida et al., 2003). Details about mice breeding are included in supplemental experimental procedures. Animal use protocol was approved by the Institutional Animal Care and Use Committees at Cleveland Clinic, and all procedures adhered to the ARVO guidelines for the Use of Animals in Vision Research.
Quantitative Real-Time PCR
Relative fold changes in mRNA expression were determined using the comparative Ct method (2−ΔΔCt method) and details of RNA isolation, cDNA synthesis and qRT-PCR are mentioned in supplemental experimental procedures.
Immunohistochemistry
Dissected retinas were fixed in 4% PFA for 90 min at room temperature (RT), washed in PBS several times, permeabilized for 30 min using 1% Triton X-100 and blocked for 1 hr at RT with PBS containing 3% BSA and 0.03% Triton X-100. Retinas for flatmount preparation were incubated with primary antibodies [(Goat anti S Opsin, sc-14363, Santa Cruz Biotechnology, Dallas, TX, USA) and (rabbit anti M opsin, AB5405, Millipore, Billerica, MA, USA)] (1:100) for 4 days at 4°C. For cryosectioing, dorso-ventral orientation of mouse eye was determined using guidelines described previously (Wagner et al., 2000). Details about antibodies and immunohistochemistry procedure is described in supplemental experimental procedures.
Cell Isolation by FACS
Retinal dissociation was done using enzyme liberase (Roche Diagnostics, Indianapolis, IN, USA). Cell isolations using fluroscence-activated cell sorting (FACS) were performed on a FACSAriaII (BD Biosciences, San Jose, CA, USA) at the Cleveland Clinic flow cytometry core facility. More information about FACS protocol is described in supplemental experimental procedures.
T3 Supplementation
Oral T3 supplementation (3 μg/mL) via drinking water was done as described previously (Hamidi et al., 2010). T3 supplementation was started either on the day of weaning (P20) (for Figure 6A–O) or on P10 (for Figure 6P–Q) and terminated on P50. Animals were sacrificed on P50. Due to increased lethality associated with T3 supplementation before P10, all rescue experiments were done after P10.
Chromatin Immunoprecipitation (ChIP) Assay
P24 mouse retinas (14–28) and P3 retinas (28–32) were used for the chromatin immunoprecipitation (ChIP) assay using a rabbit anti-BMAL1 antibody (2 μg/sample, ab3350, Abcam, Cambridge, UK) and a non-immune rabbit IgG (2 μg/sample, 12-370, Abcam, Millipore, Billerica, MA, USA). ChIP protocol used in this study was adapted from a protocol recommended for mouse retinal tissues previously (Peng and Chen, 2013). Ultrasonicator (Model S2, Covaris, Inc. Woburn, MA, USA) was used for fragmentation of chromatin DNA from mouse retinas. ChIP DNA was used for PCR analysis. See supplemental information (Table S1) for a list of primers used. Sequencing on ChIP samples was performed on either single read HiSeq 2500 or HiSeq 4000 (Illumina, San Diego, CA, USA). Sequences from ChIP samples and input controls were aligned to the GRCm38 mouse reference genome (mm10) with bowtie2 (Langmead and Salzberg, 2012). Peak calling was done using HOMER peak caller. See supplemental experimental procedures for more details about ChIP-Seq and data analysis.
Electroretinogram Recordings
P24 or adult 6 week old mice were dark-adapted overnight and then anesthetized using ketamine (40mg/kg) and xylazine (8mg/kg). Pupils were dilated using 1% tropicamide (Bausch and Lomb, Tampa, FL, USA), 2.5% phenylephrine hydrochloride (Akorn Inc., Lake Forest, IL, USA), and 1% cyclopentolate (Bausch and Lomb). Eyes were anesthetized with 0.5% proparacaine hydrochloride (Akorn, Inc.). Mice were placed on a temperature regulated heating pad and ERGs were recorded using an Espion E3 ColorDome Full field Ganzfeld (Diagnosys, LLC, Lowell, MA, USA) as described previously (Ng et al., 2010). Details about rod (scotopic) and wavelength specific photopic responses are mentioned in supplemental experimental procedures.
Statistical Analysis
Statistical significance was determined using SigmaPlot 10.0 software (Systat Software, San Jose, CA, USA). Results comparing two groups were analyzed using Student’s t test. Results in which more than two groups are involved were analyzed using either one-way or two- way ANOVA followed by Tukey post-hoc test or in some case Fisher LSD post-hoc test.
Supplementary Material
Highlights.
Circadian clock genes Bmal1 and Per2 are required for spatial patterning of cone opsins
BMAL1 controls expression of the thyroid hormone activating gene Dio2
Regulation of Dio2 by BMAL1 is required for cone spectral identity
Bmal1 and Dio2 are required for visual function and cone integrity
Acknowledgments
This research was funded by R01 EY027077-01 (S.R.), RPB1503 (S.R.), E.Matilda Ziegler Foundation - MZF1408 (S.R.), Knights Templar Eye Foundation award (S.R.), BrightFocus Foundation award (V.L.B.), California Table Grape Commission research grant (V.L.B), Genentech grant (V.L.B.), VA Merit Award i01-BX002754 (I.S.S.), a National Eye Institute P30-EY025585 core grant and Cleveland Clinic Foundation startup funds (S.R.). We are grateful to Rebecca Fuller, Leigh Perkins and Joey Tate for their assistance in the laboratory, Biological Resources Unit and Veterinary Services at Cleveland Clinic for their assistance with mouse colony management, Genomics Core at the Case Western Reserve University, University of Chicago and Cleveland Clinic for their assistance with ChIP-Seq.
Footnotes
AUTHOR CONTRIBUTIONS
O.B.S., and S.R. designed the research. Experiments and investigations were conducted by O.B.S., A.M.H., O.F.Z., R.C., V.L.B., I.S.S., and S.R. Data analysis was done by O.B.S., R.C., I.S.S., and S.R. Manuscript was written by O.B.S. and S.R. Final version of the manuscript was reviewed and approved by all the authors.
Supplemental Information includes seven figures, a primer list table, experimental procedures and data files related to ChIP-Seq data analysis.
Data File S1, related to Figure 7 and S7. Target genes
Data File S2, Related to Figure 7 and S7. Trusted Targets
Data File S3, Related to Figure 7 and S7. Biological Process
Data File S4, Related to Figure 7 and S7. Pathway
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ait-Hmyed Hakkari O, Acar N, Savier E, Spinnhirny P, Bennis M, Felder-Schmittbuhl MP, Mendoza J, Hicks D. Rev-Erbalpha modulates retinal visual processing and behavioral responses to light. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2016;30:3690–3701. doi: 10.1096/fj.201600414R. [DOI] [PubMed] [Google Scholar]
- Ait-Hmyed O, Felder-Schmittbuhl MP, Garcia-Garrido M, Beck S, Seide C, Sothilingam V, Tanimoto N, Seeliger M, Bennis M, Hicks D. Mice lacking Period 1 and Period 2 circadian clock genes exhibit blue cone photoreceptor defects. Eur J Neurosci. 2013;37:1048–1060. doi: 10.1111/ejn.12103. [DOI] [PubMed] [Google Scholar]
- Alonso M, Goodwin C, Liao X, Page D, Refetoff S, Weiss RE. Effects of maternal levels of thyroid hormone (TH) on the hypothalamus-pituitary-thyroid set point: studies in TH receptor beta knockout mice. Endocrinology. 2007;148:5305–5312. doi: 10.1210/en.2007-0677. [DOI] [PubMed] [Google Scholar]
- Applebury ML, Antoch MP, Baxter LC, Chun LL, Falk JD, Farhangfar F, Kage K, Krzystolik MG, Lyass LA, Robbins JT. The murine cone photoreceptor: a single cone type expresses both S and M opsins with retinal spatial patterning. Neuron. 2000;27:513–523. doi: 10.1016/s0896-6273(00)00062-3. [DOI] [PubMed] [Google Scholar]
- Applebury ML, Farhangfar F, Glosmann M, Hashimoto K, Kage K, Robbins JT, Shibusawa N, Wondisford FE, Zhang H. Transient expression of thyroid hormone nuclear receptor TRbeta2 sets S opsin patterning during cone photoreceptor genesis. Developmental dynamics: an official publication of the American Association of Anatomists. 2007;236:1203–1212. doi: 10.1002/dvdy.21155. [DOI] [PubMed] [Google Scholar]
- Barrett P, Ebling FJ, Schuhler S, Wilson D, Ross AW, Warner A, Jethwa P, Boelen A, Visser TJ, Ozanne DM, et al. Hypothalamic thyroid hormone catabolism acts as a gatekeeper for the seasonal control of body weight and reproduction. Endocrinology. 2007;148:3608–3617. doi: 10.1210/en.2007-0316. [DOI] [PubMed] [Google Scholar]
- Bedolla DE, Torre V. A component of retinal light adaptation mediated by the thyroid hormone cascade. PLoS One. 2011;6:e26334. doi: 10.1371/journal.pone.0026334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard-Cannon P, Mendoza-Viveros L, Yuen A, Kaern M, Cheng HY. The circadian molecular clock regulates adult hippocampal neurogenesis by controlling the timing of cell-cycle entry and exit. Cell reports. 2013;5:961–973. doi: 10.1016/j.celrep.2013.10.037. [DOI] [PubMed] [Google Scholar]
- Campos-Barros A, Amma LL, Faris JS, Shailam R, Kelley MW, Forrest D. Type 2 iodothyronine deiodinase expression in the cochlea before the onset of hearing. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:1287–1292. doi: 10.1073/pnas.97.3.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujieda H, Bremner R, Mears AJ, Sasaki H. Retinoic acid receptor-related orphan receptor alpha regulates a subset of cone genes during mouse retinal development. Journal of neurochemistry. 2009;108:91–101. doi: 10.1111/j.1471-4159.2008.05739.x. [DOI] [PubMed] [Google Scholar]
- Gereben B, Zavacki AM, Ribich S, Kim BW, Huang SA, Simonides WS, Zeold A, Bianco AC. Cellular and molecular basis of deiodinase-regulated thyroid hormone signaling. Endocrine reviews. 2008;29:898–938. doi: 10.1210/er.2008-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaschke A, Glosmann M, Peichl L. Developmental changes of cone opsin expression but not retinal morphology in the hypothyroid Pax8 knockout mouse. Investigative ophthalmology & visual science. 2010;51:1719–1727. doi: 10.1167/iovs.09-3592. [DOI] [PubMed] [Google Scholar]
- Glaschke A, Weiland J, Del Turco D, Steiner M, Peichl L, Glosmann M. Thyroid hormone controls cone opsin expression in the retina of adult rodents. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2011;31:4844–4851. doi: 10.1523/JNEUROSCI.6181-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamidi S, Aliesky H, Chen CR, Rapoport B, McLachlan SM. Variable suppression of serum thyroxine in female mice of different inbred strains by triiodothyronine administered in drinking water. Thyroid: official journal of the American Thyroid Association. 2010;20:1157–1162. doi: 10.1089/thy.2010.0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haverkamp S, Wassle H, Duebel J, Kuner T, Augustine GJ, Feng G, Euler T. The primordial, blue-cone color system of the mouse retina. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2005;25:5438–5445. doi: 10.1523/JNEUROSCI.1117-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henning Y, Szafranski K. Age-Dependent Changes of Monocarboxylate Transporter 8 Availability in the Postnatal Murine Retina. Frontiers in cellular neuroscience. 2016;10:205. doi: 10.3389/fncel.2016.00205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiragaki S, Baba K, Coulson E, Kunst S, Spessert R, Tosini G. Melatonin signaling modulates clock genes expression in the mouse retina. PLoS One. 2014;9:e106819. doi: 10.1371/journal.pone.0106819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houbrechts AM, Vergauwen L, Bagci E, Van Houcke J, Heijlen M, Kulemeka B, Hyde DR, Knapen D, Darras VM. Deiodinase knockdown affects zebrafish eye development at the level of gene expression, morphology and function. Molecular and cellular endocrinology. 2016;424:81–93. doi: 10.1016/j.mce.2016.01.018. [DOI] [PubMed] [Google Scholar]
- Huang CC, Shi L, Lin CH, Kim AJ, Ko ML, Ko GY. A new role for AMP-activated protein kinase in the circadian regulation of L-type voltage-gated calcium channels in late-stage embryonic retinal photoreceptors. Journal of neurochemistry. 2015;135:727–741. doi: 10.1111/jnc.13349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen LD, Cao Z, Nakamura M, Yang Y, Brautigam L, Andersson P, Zhang Y, Wahlberg E, Lanne T, Hosaka K, et al. Opposing effects of circadian clock genes bmal1 and period2 in regulation of VEGF-dependent angiogenesis in developing zebrafish. Cell reports. 2012;2:231–241. doi: 10.1016/j.celrep.2012.07.005. [DOI] [PubMed] [Google Scholar]
- Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat Methods. 2012;9:357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaVail MM. Kinetics of rod outer segment renewal in the developing mouse retina. The Journal of cell biology. 1973;58:650–661. doi: 10.1083/jcb.58.3.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaVail MM. Circadian nature of rod outer segment disc shedding in the rat. Investigative ophthalmology & visual science. 1980;19:407–411. [PubMed] [Google Scholar]
- Le YZ, Ash JD, Al-Ubaidi MR, Chen Y, Ma JX, Anderson RE. Targeted expression of Cre recombinase to cone photoreceptors in transgenic mice. Molecular vision. 2004;10:1011–1018. [PubMed] [Google Scholar]
- Le YZ, Ash JD, Al-Ubaidi MR, Chen Y, Ma JX, Anderson RE. Conditional gene knockout system in cone photoreceptors. Advances in experimental medicine and biology. 2006;572:173–178. doi: 10.1007/0-387-32442-9_26. [DOI] [PubMed] [Google Scholar]
- Liu X, Zhang Z, Ribelayga CP. Heterogeneous expression of the core circadian clock proteins among neuronal cell types in mouse retina. PLoS One. 2012;7:e50602. doi: 10.1371/journal.pone.0050602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma H, Thapa A, Morris L, Redmond TM, Baehr W, Ding XQ. Suppressing thyroid hormone signaling preserves cone photoreceptors in mouse models of retinal degeneration. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:3602–3607. doi: 10.1073/pnas.1317041111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda T, Zhang X, Berlinicke C, Wan J, Yerrabelli A, Conner EA, Kjellstrom S, Bush R, Thorgeirsson SS, Swaroop A, et al. The transcription factor GTF2IRD1 regulates the topology and function of photoreceptors by modulating photoreceptor gene expression across the retina. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2014;34:15356–15368. doi: 10.1523/JNEUROSCI.2089-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon DG, Iuvone PM, Tosini G. Circadian organization of the mammalian retina: from gene regulation to physiology and diseases. Progress in retinal and eye research. 2014;39:58–76. doi: 10.1016/j.preteyeres.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mears AJ, Kondo M, Swain PK, Takada Y, Bush RA, Saunders TL, Sieving PA, Swaroop A. Nrl is required for rod photoreceptor development. Nature genetics. 2001;29:447–452. doi: 10.1038/ng774. [DOI] [PubMed] [Google Scholar]
- Mendoza A, Hollenberg AN. New insights into thyroid hormone action. Pharmacology & therapeutics. 2017;173:135–145. doi: 10.1016/j.pharmthera.2017.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustafi D, Kevany BM, Genoud C, Bai X, Palczewski K. Photoreceptor phagocytosis is mediated by phosphoinositide signaling. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2013;27:4585–4595. doi: 10.1096/fj.13-237537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathans J. The evolution and physiology of human color vision: insights from molecular genetic studies of visual pigments. Neuron. 1999;24:299–312. doi: 10.1016/s0896-6273(00)80845-4. [DOI] [PubMed] [Google Scholar]
- Ng L, Hurley JB, Dierks B, Srinivas M, Salto C, Vennstrom B, Reh TA, Forrest D. A thyroid hormone receptor that is required for the development of green cone photoreceptors. Nature genetics. 2001;27:94–98. doi: 10.1038/83829. [DOI] [PubMed] [Google Scholar]
- Ng L, Liu H, St Germain DL, Hernandez A, Forrest D. Deletion of the Thyroid Hormone-Activating Type 2 Deiodinase Rescues Cone Photoreceptor Degeneration but Not Deafness in Mice Lacking Type 3 Deiodinase. Endocrinology. 2017;158:1999–2010. doi: 10.1210/en.2017-00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng L, Lyubarsky A, Nikonov SS, Ma M, Srinivas M, Kefas B, St Germain DL, Hernandez A, Pugh EN, Jr, Forrest D. Type 3 deiodinase, a thyroid-hormone-inactivating enzyme, controls survival and maturation of cone photoreceptors. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2010;30:3347–3357. doi: 10.1523/JNEUROSCI.5267-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng L, Ma M, Curran T, Forrest D. Developmental expression of thyroid hormone receptor beta2 protein in cone photoreceptors in the mouse. Neuroreport. 2009;20:627–631. doi: 10.1097/WNR.0b013e32832a2c63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida A, Furukawa A, Koike C, Tano Y, Aizawa S, Matsuo I, Furukawa T. Otx2 homeobox gene controls retinal photoreceptor cell fate and pineal gland development. Nat Neurosci. 2003;6:1255–1263. doi: 10.1038/nn1155. [DOI] [PubMed] [Google Scholar]
- Ono H, Hoshino Y, Yasuo S, Watanabe M, Nakane Y, Murai A, Ebihara S, Korf HW, Yoshimura T. Involvement of thyrotropin in photoperiodic signal transduction in mice. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:18238–18242. doi: 10.1073/pnas.0808952105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peliciari-Garcia RA, Previde RM, Nunes MT, Young ME. Interrelationship between 3,5,3 -triiodothyronine and the circadian clock in the rodent heart. Chronobiology international. 2016;33:1444–1454. doi: 10.1080/07420528.2016.1229673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng GH, Chen S. Double chromatin immunoprecipitation: analysis of target co-occupancy of retinal transcription factors. Methods Mol Biol. 2013;935:311–328. doi: 10.1007/978-1-62703-080-9_22. [DOI] [PubMed] [Google Scholar]
- Pessoa CN, Santiago LA, Santiago DA, Machado DS, Rocha FA, Ventura DF, Hokoc JN, Pazos-Moura CC, Wondisford FE, Gardino PF, et al. Thyroid hormone action is required for normal cone opsin expression during mouse retinal development. Investigative ophthalmology & visual science. 2008;49:2039–2045. doi: 10.1167/iovs.07-0908. [DOI] [PubMed] [Google Scholar]
- Rey G, Cesbron F, Rougemont J, Reinke H, Brunner M, Naef F. Genome-wide and phase-specific DNA-binding rhythms of BMAL1 control circadian output functions in mouse liver. PLoS biology. 2011;9:e1000595. doi: 10.1371/journal.pbio.1000595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts MR, Hendrickson A, McGuire CR, Reh TA. Retinoid X receptor (gamma) is necessary to establish the S-opsin gradient in cone photoreceptors of the developing mouse retina. Investigative ophthalmology & visual science. 2005;46:2897–2904. doi: 10.1167/iovs.05-0093. [DOI] [PubMed] [Google Scholar]
- Roberts MR, Srinivas M, Forrest D, Morreale de Escobar G, Reh TA. Making the gradient: thyroid hormone regulates cone opsin expression in the developing mouse retina. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:6218–6223. doi: 10.1073/pnas.0509981103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan GX, Zhang DQ, Zhou T, Yamazaki S, McMahon DG. Circadian organization of the mammalian retina. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:9703–9708. doi: 10.1073/pnas.0601940103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh S, Tang K, Iida A, Inoue M, Kodama T, Tsai SY, Tsai MJ, Furuta Y, Watanabe S. The spatial patterning of mouse cone opsin expression is regulated by bone morphogenetic protein signaling through downstream effector COUP-TF nuclear receptors. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2009;29:12401–12411. doi: 10.1523/JNEUROSCI.0951-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawant O, Horton AM, Shukla M, Rayborn ME, Peachey NS, Hollyfield JG, Rao S. Light-Regulated Thyroid Hormone Signaling Is Required for Rod Photoreceptor Development in the Mouse Retina. Investigative ophthalmology & visual science. 2015;56:8248–8257. doi: 10.1167/iovs.15-17743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider MJ, Fiering SN, Pallud SE, Parlow AF, St Germain DL, Galton VA. Targeted disruption of the type 2 selenodeiodinase gene (DIO2) results in a phenotype of pituitary resistance to T4. Molecular endocrinology. 2001;15:2137–2148. doi: 10.1210/mend.15.12.0740. [DOI] [PubMed] [Google Scholar]
- Storch KF, Paz C, Signorovitch J, Raviola E, Pawlyk B, Li T, Weitz CJ. Intrinsic circadian clock of the mammalian retina: importance for retinal processing of visual information. Cell. 2007;130:730–741. doi: 10.1016/j.cell.2007.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosini G, Menaker M. The clock in the mouse retina: melatonin synthesis and photoreceptor degeneration. Brain research. 1998;789:221–228. doi: 10.1016/s0006-8993(97)01446-7. [DOI] [PubMed] [Google Scholar]
- Tosini G, Pozdeyev N, Sakamoto K, Iuvone PM. The circadian clock system in the mammalian retina. BioEssays: news and reviews in molecular, cellular and developmental biology. 2008;30:624–633. doi: 10.1002/bies.20777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viets K, Eldred K, Johnston RJ., Jr Mechanisms of Photoreceptor Patterning in Vertebrates and Invertebrates. Trends Genet. 2016;32:638–659. doi: 10.1016/j.tig.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner E, McCaffery P, Drager UC. Retinoic acid in the formation of the dorsoventral retina and its central projections. Developmental biology. 2000;222:460–470. doi: 10.1006/dbio.2000.9719. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Yamamura T, Watanabe M, Yasuo S, Nakao N, Dawson A, Ebihara S, Yoshimura T. Hypothalamic expression of thyroid hormone-activating and -inactivating enzyme genes in relation to photorefractoriness in birds and mammals. American journal of physiology Regulatory, integrative and comparative physiology. 2007;292:R568–572. doi: 10.1152/ajpregu.00521.2006. [DOI] [PubMed] [Google Scholar]
- Wu Y, Koenig RJ. Gene regulation by thyroid hormone. Trends in endocrinology and metabolism: TEM. 2000;11:207–211. doi: 10.1016/s1043-2760(00)00263-0. [DOI] [PubMed] [Google Scholar]
- Yoshitane H, Ozaki H, Terajima H, Du NH, Suzuki Y, Fujimori T, Kosaka N, Shimba S, Sugano S, Takagi T, et al. CLOCK-controlled polyphonic regulation of circadian rhythms through canonical and noncanonical E-boxes. Mol Cell Biol. 2014;34:1776–1787. doi: 10.1128/MCB.01465-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Li A, Brown B, Weiss ER, Osawa S, Craft CM. Mouse cone arrestin expression pattern: light induced translocation in cone photoreceptors. Molecular vision. 2002;8:462–471. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.