Abstract
The kidney is a primary organ for filtration of the blood and elimination of drugs and xenobiotics. These active reabsorptive and secretory processes can result in acute kidney injury as a result of these concentrative properties. Classic measures of acute kidney injury are hampered by their ability to accurately assess function before irreversible damage has occurred. This review will discuss efforts to refine the clinical utility of standard biomarkers as well as the development of novel biomarkers of nephrotoxicity.
Keywords: Acute Kidney Injury, Biomarkers, Nephrotoxicity
The kidneys receive approximately 25% of total cardiac blood flow and are responsible for the maintaining of circulatory fluid homeostasis while serving as a primary organ of xenobiotic elimination and detoxification. The functional filtration unit of the kidney is the renal nephron with approximately 1 million nephrons per kidney. There are three key functional components of the renal nephron: the passive filtration of the blood by the glomeruli, and both the active reabsorption and secretion of solutes via tubular epithelia. These tubular epithelia, particularly the proximal tubule, are enriched with integral membrane proteins responsible for facilitated and active transport processes which have the potential to concentrate compounds within a cell to significantly higher levels than what is observed in circulation [1]. Intracellular accumulation has been considered to be the primary driver behind xenobiotic-induced nephropathy leading to observable acute kidney injury (AKI), chronic kidney disease (CKD) and, with time, end-stage renal disease (ESRD) requiring the use of renal replacement therapy (RRT) as a method of disease intervention.
A current major challenge is the ability to accurately predict toxin-induced kidney injury, whether it be for existing prescription medications, clinical trials for new pharmaceuticals, or for risk assessment due to environmental exposures of xenobiotics. A particular clinical concern is for early detection of AKI. Classic criteria for the diagnosis of AKI include observing a decrease in the clearance of creatinine (detected via a rise in serum creatinine (sCr)), and/or oliguria, determined by measuring timed total urine output, and monitoring an increase in the circulation levels of blood urea nitrogen (BUN). While sCr, urine output, and BUN levels are considered a staple in the classification of kidney injury, by the time one typically observes changes in these measures, significant and potentially irreversible damage may have already occurred. In response to this concern, there has been a quest to identify new, more sensitive, biomarkers of AKI [2]. The accuracy of predicting and identifying an early toxic event has been further improved with the discovery of novel urinary biomarkers, including measurements of miRNA and secreted proteins. The iterative improvement of nephrotoxic biomarkers will not only allow for an earlier detection of xenobiotic-induced kidney injury but also potentially reduce the likelihood of patient mortality through earlier intervention.
AKI definitions have been continually redefined over the past 10 years with an overall goal to increase the sensitivity and specificity of detection. In 2002, the Acute Dialysis Quality Initiative (ADQI) group defined the foundation of diagnosing AKI using the RIFLE (Risk, Injury, Failure, Loss, ESRD) classification, whose primary specific diagnostic biomarker of renal function was the use of sCr and BUN combined with urine output. Using a combination of these markers, severity of AKI was established by substantial increases in sCr and/or loss of urine output indicating progressive stages of sensitivity (Risk, Injury and Failure) and two clinical outcomes of specificity (Loss and End-stage renal disease) [3, 4]. Further advancements to the definition of AKI were later made in 2005, by the AKIN (The Acute Kidney Injury Network) group, evaluating both the hydration of the patient at the time of diagnostic biomarker measurement as well as a refinement on the use of sCr determining GFR changes [5]. Finally in 2012, KDIGO (Kidney Disease: Improving Global Outcomes) refined the definition of AKI by implementing both differences between RIFLE and AKIN into simplified stages of AKI defined as an increase in sCr ≥0.3 mg/dL (≥26.5 μmol/L) within 48 h; or an increase in sCr to ≥1.5 times baseline, which is known or presumed to have occurred within the prior 7 days or a urine volume of <0.5 mL/kg/h for 6 h [6]. In 2014, Zeng et al. evaluated AKI incidence between the varying refinements of definitions of AKI using a retrospective cohort study. Results showed AKI incidence was highest according to the KDIGO definition (18.3%) followed by the AKIN (16.6%), and RIFLE (16.1%), definitions. Paralleled by additional studies, Zeng et al. observed AKI incidence associated with an increased rate of mortality and increased hospital costs [7].
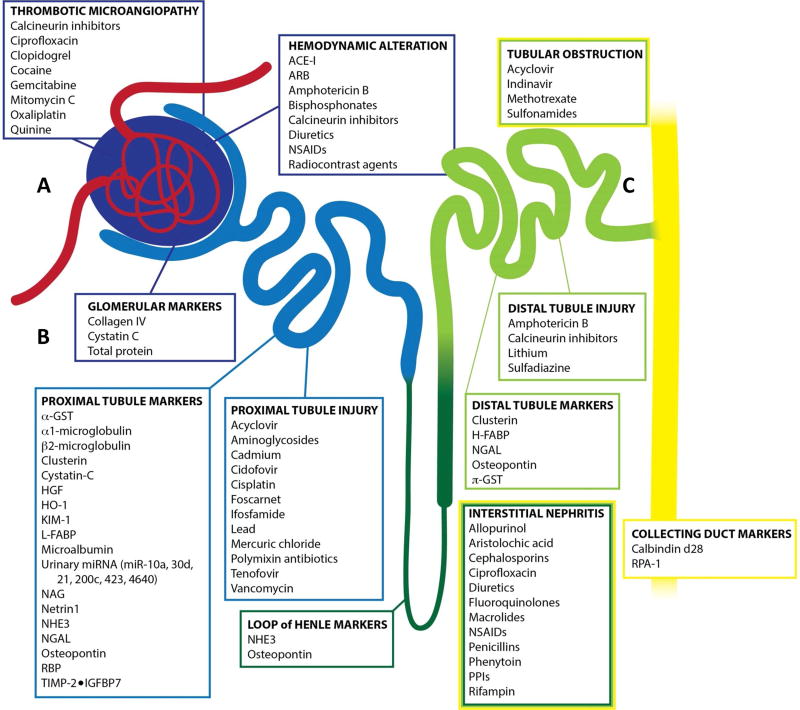
In addition to defining AKI clinically, pathophysiology of drug/xenobiotic-induced AKI can be defined by measurable damage to site-specific segments of the nephron which include: glomerular capillaries, mesangium, podocytes, parietal epithelial cells, proximal tubule, distal tubule, collecting ducts, and interstitium [8–12] [Figure 1]. There are a multitude of compounds that possess nephrotoxic properties which include: chemotherapeutics (cisplatin), antiretroviral therapeutics (tenofovir), analgesics (NSAIDs), contrast agents for imaging, heavy metal pollutant (cadmium), and some classes of antibiotics (aminoglycoside/polymyxin) [13–17].
Figure 1. Diagram of renal nephron indicating toxicant, area of injury, and origin of biomarker.
A) Passive filtration is the first process achieved by the glomerulus where toxicant-induced AKI can result in thrombotic microangiopathy and/or hemodynamic alterations. B) In juxtaposition to the glomerulus, the proximal tubule is a primary location of toxicant-induced AKI resulting in proximal tubule injury and loss of integrity leading to downstream accumulation of biomarkers in the urine. C) The remaining tubule structures (Loop of Henle, Distal Tubule, Collecting Duct) are additional structures of the nephron that can become compromised upon toxicant-induced AKI resulting in interstitial nephritis and biomarker accumulation within the urine. (adapted from Casaret and Doull’s Toxicology: The Basic Science of Poisons)
Despite increased sensitivity to detecting clinical AKI, sCr and BUN response may be significantly delayed following kidney injury requiring the use of emerging urinary biomarkers to detect both early events of injury as well as segment-specific nephrotoxicity [18]. In 2009, the Predictive Safety Testing Consortium (PSTC) released a biomarker qualification data submission indicating emerging urinary biomarkers, in addition to sCr and BUN, which include: urinary kidney injury molecule-1 (KIM-1), urinary beta2-microglobulin(B2M), urinary total protein, urinary albumin, urinary clusterin, urinary cystatin c (Cys C), and urinary trefoil factor 3 (TFF-3) in good laboratory practice rat toxicology studies to monitor xenobiotic-induced kidney injury [19]. In a later report in 2014, the PSTC, with letters of support from the European Medicines Agency and Food and Drug Administration, encouraged further evaluation of emerging biomarkers including osteopontin (OPN) and neutrophil gelatinase-associated lipocalin (NGAL) [20].
This review will describe currently available biomarkers that are frequently used in preclinical/clinical investigations as well as emerging biomarkers of nephrotoxicity. It is important to note that, relative to the proximal tubule, segments of the renal nephron (glomerulus, distal tubule, collecting duct, etc.) are targets of xenobiotic-induced nephropathy as well, yet their biomarker sensitivity and specificity profiles are limited and require ongoing investigations to identify suitable markers.
Frequently investigated biomarkers of nephrotoxicity
Kidney Injury Molecule-1 (KIM-1)
First discovered in rats as an upregulated protein in response to ischemia, KIM-1 is a type 1 transmembrane protein, containing immunoglobulin and mucin domains. In response to acute kidney injury, the N-terminal immunoglobulin and mucin ectodomain are cleaved from the apical surface of the proximal tubule and shed in the urine [21]. KIM-1 is an excellent biomarker of AKI, as demonstrated in preclinical and clinical investigations, including a recent study by Pavkovic et al. evaluating KIM-1 as a urinary biomarker for drug-induced AKI in humans [22]. Compared to healthy volunteers, Pavkovic et al. showed significant levels of KIM-1 (relative to urinary creatinine) in the urine of patients in two cohort studies who were or were not clinically diagnosed with AKI following either an overdose of acetaminophen or intraoperative cisplatin therapy, with resultant chronic interstitial nephritis [23]. Furthermore, as demonstrated in a rat model, Luo et al. observed concentration-dependent gentamicin-induced renal injury leading to a significant induction of KIM-1 in the urine across multiple days. Additional findings showed significant differences of clinical biomarkers (BUN/sCr) only being observed at later points of collection (day 7) and at the highest concentration treated; when compared to KIM-1, these findings demonstrate the lack of early sensitivity and delayed response to detect acute kidney injury [24].
Interleukin-18 (IL-18)
From the IL-1 cytokine family, interferon-γ-inducing factor (now recognized as interleukin-18, IL-18) is a proinflammatory cytokine that is activated and released upon cleavage via caspase 1. Primarily expressed in proximal tubule cells and intercalated renal cells (late distal convoluted tubule, connecting tubule, and the collecting duct), IL-18 has been shown to accumulate in the urine in response to acute kidney injury. In 2013, Zubowska et al. confirmed preclinical findings on the induction of IL-18 following nephropathy by observing a significant increase of IL-18 in the urine of children who were being treated with nephrotoxic chemotherapies [25]. Additionally, a meta-analysis by Lin et al. analyzed 11 different studies with approximately 2800 people in total and observed a higher chance of early detection of AKI using IL-18 when compared to sCr [26]. Furthermore, in a simulated clinical trial of AKI using data obtained from prospective cohort study of patients with at least 1 risk factor for AKI, Parikh et al. observed a potential increase in proportion of patients experiencing AKI following inclusion of urinary IL-18 monitoring [27].
Neutrophil Gelatinase-associated Lipocalin (NGAL)
NGAL is a small protease-resistant secreted protein filtered by the glomerulus and extensively reabsorbed by the proximal tubule. The utility of NGAL as a predictive biomarker for AKI was first suggested from a study by Mishra et al. where increases in both transcripts and protein were observed in mice in response to renal ischemia [28]. Tubular injury, reflecting a decrease in NGAL reabsorption and accumulation in the urine, has been demonstrated in a number of studies including cisplatin-induced nephrotoxicity in mice. Mishra et al. observed cisplatin-induced tubule cell necrosis and apoptosis in addition to rapid induction of NGAL in both tubule epithelial cells as well as an accumulation and detection of NGAL in the urine just 3 hours after dosing [29]. Luo et al. later confirmed the significant induction of NGAL as a nephrotoxicity biomarker in both a dose- and time-dependency with gentamicin treated rats. However, clinical examples reveal an uncertainty when using NGAL as a urinary biomarker of cyclosporine A (CsA) treatment for children with nephrotic syndrome [24]. Gacka et al. concluded that NGAL cannot be used alone as a marker of cyclosporine A-nephrotoxicity but has potential benefits when monitoring nephrotoxicity in a chronic setting [30]. In contrast to CsA studies, a multicenter prospective cohort study by Nickolas et al. showed that urinary NGAL was significantly better at diagnosing intrinsic AKI compared to other biomarkers (sCr, KIM-1, IL-18, FABP, CysC) and more effective in predicting increasing RIFLE classes[31].
Liver-type Fatty Acid-binding Protein (L-FABP)
Primarily located in the both the convoluted and straight segments of the proximal tubules, the liver-type fatty acid-binding protein (L-FABP) binds long-chain fatty acids (LCFA) and transports them for β-oxidation (in mitochondria/peroxisomes). Using established human FABP transgenic mice, Matsui et al. observed a significant induction of L-FABP in the proximal tubules following exposure to aristolochic acid, a carcinogenic plant alkaloid with known nephrotoxicity [32]. Significant induction of FABP was observed for both mRNA and protein, with concomitantly increased urinary levels when compared to control mice. Additional findings by Jabłonowska et al. showed statistically higher concentrations of urinary L-FABP (normalized to creatinine) in HIV-infected individuals receiving antiretroviral therapy compared to healthy controls [33].
Novel Biomarkers of AKI
Urine Tissue Inhibitor of Metalloproteinase 2 and Insulin-like Growth Factor Binding Protein 7 [TIMP-2 ● IGFBP7]
Expressed in renal tubular cells, TIMP-2 and IGFBP7 are G1 cell-cycle arrest proteins induced as a consequence of AKI. TIMP-2 is an inhibitor of matrix metalloproteinase activity while IGFBP7 is a regulator of IGF signaling. In two separate multicenter observational studies (Discovery and Validation) of critically ill patients at risk for AKI, Kashani et al. observed significantly elevated urinary IGFBP7 and TIMP-2 when stratifying AKI risk compared to other urinary biomarkers of AKI (NGAL, CysC, KIM-1, IL-18, pi-GST, FABP) [34]. Additional clinical cohort studies by Bihorac et al. and Hoste et al. defined cutoff values of urinary [TIMP-2 ● IGFBP7] improving the identification of patients at high risk of AKI [35, 36].
Myo-inositol oxygenase (MIOX)
Expressed extensively in the proximal tubule, myo-inositol oxygenase (MIOX) catabolizes glucose intermediates (myo-inositol) which enter the pentose phosphate pathway. Additionally, MIOX plays a critical role in antioxidant and oxidant responses regulated by Nrf2 [37]. In vitro studies show MIOX overexpression leads to enhanced generation of ROS in the presence of cisplatin [38]. Dutta et al. observed, using both MIOX null and overexpressing mice, that MIOX null mice are resistant to cisplatin-induced nephrotoxicity whereas MIOX overexpressing mice displayed enhanced injury [39]. In work by Gaut et al. investigators developed an immunoassay to detect plasma MIOX as a biomarker of AKI in both animal and human species. Plasma levels of MIOX were significantly higher and detected 54 hours earlier than creatinine in mice experiencing AKI as well as critically ill patients diagnosed with AKI compared to patients without AKI [40].
Heme oxygenase-1 (HO-1)
Responsible for the degradation of heme via conversion to biliverdin, HO-1 is induced in the proximal tubule and exerts cytoprotective effects in response to renal injury. In similarity to MIOX, HO-1 cytoprotective antioxidant activity is also regulated by Nrf2 [41]. Earlier reports show HO-1 to be nephro-protective as evidenced by induction in response to cisplatin-induced AKI using a rat model [42]. Using a mouse model to assess four different models of AKI (ischemia/reperfusion, glycerol-induced rhabdomyolysis, cisplatin nephrotoxicity, and bilateral ureteral obstruction), Zager et al. observed significant increases of HO-1 gene expression and therefore increased concentrations in both the plasma and urine 4 hours later. These findings were then supported with a clinical investigation revealing patients with AKI to have significantly higher levels of both plasma and urine HO-1 compared to patients without AKI, patients with CKD, or patients with ESRD [43]. More recently, using a high throughput approach for predictive nephrotoxicity assessment, Adler et al. screened a panel of over 40 nephrotoxicants using primary human proximal tubular epithelial cells and showed HO-1 to significantly upregulate after exposure at the mRNA and protein level [44].
Urinary miRNA
MicroRNAs are endogenous short (~22 nucleotides) RNA molecules that regulate expression primarily via inhibition of mRNA translation, with lesser effects on transcript stability. Because extracellular miRNA are stable at room temperature and changes in pH coupled with the ability to amplify these targets with PCR, miRNAs have the potential to serve as excellent urinary biomarkers. For example, Wang et al. observed a significant induction of urinary miR-10a and miR-30d in mice experiencing renal ischemia reperfusion or streptozotocin-induced renal injury. The investigators further evidenced the mouse model findings with a cohort of healthy individuals and patients experiencing focal segmental glomerulosclerosis (FSGS); showing FSGS patients to have significantly elevated levels of urinary miR-10a and miR-30d [45]. Additionally, Ramachandran et al. identified 4 miRNAs (miR-21, 200c, 423, 4640) significantly different between cohort groups of patients with AKI and healthy volunteers[46]. Furthermore, Pavkovic et al. observed statistically significant elevated concentrations in urinary miRNA (miR-21, 423, and 200c) and KIM-1 in patients who were diagnosed with AKI following either an overdose of acetaminophen or intraoperative cisplatin therapy [23].
Concluding remarks and future directions
To achieve early diagnosis and enhanced detection of toxin-induced AKI, continual refinement of established biomarkers coupled with identification and implementation of novel biomarkers is necessary. The work of Adler et al. identified HO-1 as a better in vitro biomarker of AKI in primary proximal tubule epithelial cells in both traditional 2D cultures as well as within a novel 3D system that recapitulates the proximal tubule [44]. The clinical utility of HO-1 as a biomarker of nephrotoxicity is limited however, as it is ubiquitously expressed. But, the use of 3D microphysiological models of the proximal tubule will facilitate identification of novel tubule-specific biomarkers of nephrotoxicity [47]. While technological advancements are improving to increase both sensitivity and specificity of biomarkers, there remains a critical need for the implementation of diverse approaches that span in vitro, in vivo, and clinical investigations. In summary, early detection of AKI should be the primary goal of clinicians and drug developers, as AKI progression to CKD is extremely costly, potentially fatal to the patient, and can halt new drug development in clinical trials unless proper diagnostic tools are available.
Highlights.
Standard measures of nephrotoxicity are serum creatinine, blood urea nitrogen and urine output.
sCr, BUN & urine output temporal changes are inadequate for assessment of acute kidney injury.
Refinement and development of novel biomarkers is required to improve early detection of AKI.
Acknowledgments
We would like to express our thanks to Dr. Catherine M. Lockhart for expert graphical illustration support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
References of special interest (18, 27, 34 & 44)
References of outstanding interest (6, 19 & 23)
- 1.Yin J, Wang J. Renal drug transporters and their significance in drug-drug interactions. Acta Pharm Sin B. 2016;6(5):363–373. doi: 10.1016/j.apsb.2016.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferguson MA, Vaidya VS, Bonventre JV. Biomarkers of nephrotoxic acute kidney injury. Toxicology. 2008;245(3):182–193. doi: 10.1016/j.tox.2007.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bellomo R, Kellum JA, Mehta R, Palevsky PM, Ronco C. Acute dialysis quality initiative ii: The vicenza conference. Current opinion in critical care. 2002;8(6):505–508. doi: 10.1097/00075198-200212000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P Acute Dialysis Quality Initiative w. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: The second international consensus conference of the acute dialysis quality initiative (adqi) group. Critical care. 2004;8(4):R204–212. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Levin A Acute Kidney Injury N. Acute kidney injury network: Report of an initiative to improve outcomes in acute kidney injury. Critical care. 2007;11(2):R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levin A, Stevens PE. Summary of kdigo 2012 ckd guideline: Behind the scenes, need for guidance, and a framework for moving forward. Kidney Int. 2014;85(1):49–61. doi: 10.1038/ki.2013.444. [DOI] [PubMed] [Google Scholar]
- 7.Zeng X, McMahon GM, Brunelli SM, Bates DW, Waikar SS. Incidence, outcomes, and comparisons across definitions of aki in hospitalized individuals. Clin J Am Soc Nephrol. 2014;9(1):12–20. doi: 10.2215/CJN.02730313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fabrizi F, Aghemo A, Fogazzi GB, Moroni G, Passerini P, D'Ambrosio R, Messa P. Acute tubular necrosis following interferon-based therapy for hepatitis c: Case study with literature review. Kidney Blood Press Res. 2013;38(1):52–60. doi: 10.1159/000355753. [DOI] [PubMed] [Google Scholar]
- 9.Hirschberg R. Renal complications from bisphosphonate treatment. Curr Opin Support Palliat Care. 2012;6(3):342–347. doi: 10.1097/SPC.0b013e328356062e. [DOI] [PubMed] [Google Scholar]
- 10.Weisbord SD, Ramkumar M, LaPidus SA, McBride DJ, Palevsky PM. Functional renal artery obstruction following percutaneous kidney biopsy. Nephrol Dial Transplant. 2005;20(6):1274–1275. doi: 10.1093/ndt/gfh828. [DOI] [PubMed] [Google Scholar]
- 11.Khan M, Ortega LM, Bagwan N, Nayer A. Crystal-induced acute kidney injury due to ciprofloxacin. J Nephropathol. 2015;4(1):29–31. doi: 10.12860/jnp.2015.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Farry JK, Flombaum CD, Latcha S. Long term renal toxicity of ifosfamide in adult patients-5 year data. Eur J Cancer. 2012;48(9):1326–1331. doi: 10.1016/j.ejca.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 13.Beebe A, Seaworth B, Patil N. Rifampicin-induced nephrotoxicity in a tuberculosis patient. Journal of Clinical Tuberculosis and Other Mycobacterial Diseases. 2015;1(13–15) doi: 10.1016/j.jctube.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Waheed S, Attia D, Estrella MM, Zafar Y, Atta MG, Lucas GM, Fine DM. Proximal tubular dysfunction and kidney injury associated with tenofovir in hiv patients: A case series. Clin Kidney J. 2015;8(4):420–425. doi: 10.1093/ckj/sfv041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rigatto MH, Behle TF, Falci DR, Freitas T, Lopes NT, Nunes M, Costa LW, Zavascki AP. Risk factors for acute kidney injury (aki) in patients treated with polymyxin b and influence of aki on mortality: A multicentre prospective cohort study. J Antimicrob Chemother. 2015;70(5):1552–1557. doi: 10.1093/jac/dku561. [DOI] [PubMed] [Google Scholar]
- 16.Bird ST, Etminan M, Brophy JM, Hartzema AG, Delaney JA. Risk of acute kidney injury associated with the use of fluoroquinolones. CMAJ. 2013;185(10):E475–482. doi: 10.1503/cmaj.121730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gupta A, Biyani M, Khaira A. Vancomycin nephrotoxicity: Myths and facts. Neth J Med. 2011;69(9):379–383. [PubMed] [Google Scholar]
- 18.Bonventre JV, Vaidya VS, Schmouder R, Feig P, Dieterle F. Next-generation biomarkers for detecting kidney toxicity. Nat Biotechnol. 2010;28(5):436–440. doi: 10.1038/nbt0510-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dieterle F, Sistare F, Goodsaid F, Papaluca M, Ozer JS, Webb CP, Baer W, Senagore A, Schipper MJ, Vonderscher J, Sultana S, et al. Renal biomarker qualification submission: A dialog between the fda-emea and predictive safety testing consortium. Nat Biotechnol. 2010;28(5):455–462. doi: 10.1038/nbt.1625. [DOI] [PubMed] [Google Scholar]
- 20.Phillips JA, Holder DJ, Ennulat D, Gautier JC, Sauer JM, Yang Y, McDuffie E, Sonee M, Gu YZ, Troth SP, Lynch K, et al. Rat urinary osteopontin and neutrophil gelatinase-associated lipocalin improve certainty of detecting drug-induced kidney injury. Toxicol Sci. 2016;151(2):214–223. doi: 10.1093/toxsci/kfw038. [DOI] [PubMed] [Google Scholar]
- 21.Ichimura T, Bonventre JV, Bailly V, Wei H, Hession CA, Cate RL, Sanicola M. Kidney injury molecule-1 (kim-1), a putative epithelial cell adhesion molecule containing a novel immunoglobulin domain, is up-regulated in renal cells after injury. J Biol Chem. 1998;273(7):4135–4142. doi: 10.1074/jbc.273.7.4135. [DOI] [PubMed] [Google Scholar]
- 22.Sabbisetti VS, Ito K, Wang C, Yang L, Mefferd SC, Bonventre JV. Novel assays for detection of urinary kim-1 in mouse models of kidney injury. Toxicol Sci. 2013;131(1):13–25. doi: 10.1093/toxsci/kfs268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pavkovic M, Robinson-Cohen C, Chua AS, Nicoara O, Cardenas-Gonzalez M, Bijol V, Ramachandran K, Hampson L, Pirmohamed M, Antoine DJ, Frendl G, et al. Detection of drug-induced acute kidney injury in humans using urinary kim-1, mir-21, -200c, and -423. Toxicol Sci. 2016;152(1):205–213. doi: 10.1093/toxsci/kfw077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luo QH, Chen ML, Sun FJ, Chen ZL, Li MY, Zeng W, Gong L, Cheng AC, Peng X, Fang J, Tang L, et al. Kim-1 and ngal as biomarkers of nephrotoxicity induced by gentamicin in rats. Mol Cell Biochem. 2014;397(1–2):53–60. doi: 10.1007/s11010-014-2171-7. [DOI] [PubMed] [Google Scholar]
- 25.Zubowska M, Wyka K, Fendler W, Mlynarski W, Zalewska-Szewczyk B. Interleukin 18 as a marker of chronic nephropathy in children after anticancer treatment. Dis Markers. 2013;35(6):811–818. doi: 10.1155/2013/369784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin X, Yuan J, Zhao Y, Zha Y. Urine interleukin-18 in prediction of acute kidney injury: A systemic review and meta-analysis. J Nephrol. 2015;28(1):7–16. doi: 10.1007/s40620-014-0113-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parikh CR, Moledina DG, Coca SG, Thiessen-Philbrook HR, Garg AX. Application of new acute kidney injury biomarkers in human randomized controlled trials. Kidney Int. 2016;89(6):1372–1379. doi: 10.1016/j.kint.2016.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mishra J. Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. Journal of the American Society of Nephrology. 2003;14(10):2534–2543. doi: 10.1097/01.asn.0000088027.54400.c6. [DOI] [PubMed] [Google Scholar]
- 29.Mishra J, Mori K, Ma Q, Kelly C, Barasch J, Devarajan P. Neutrophil gelatinase-associated lipocalin: A novel early urinary biomarker for cisplatin nephrotoxicity. Am J Nephrol. 2004;24(3):307–315. doi: 10.1159/000078452. [DOI] [PubMed] [Google Scholar]
- 30.Gacka E, Zyczkowski M, Bogacki R, Paradysz A, Hyla-Klekot L. The usefulness of determining neutrophil gelatinase-associated lipocalin concentration excreted in the urine in the evaluation of cyclosporine a nephrotoxicity in children with nephrotic syndrome. Dis Markers. 2016;2016 doi: 10.1155/2016/6872149. (6872149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nickolas TL, Schmidt-Ott KM, Canetta P, Forster C, Singer E, Sise M, Elger A, Maarouf O, Sola-Del Valle DA, O'Rourke M, Sherman E, et al. Diagnostic and prognostic stratification in the emergency department using urinary biomarkers of nephron damage: A multicenter prospective cohort study. J Am Coll Cardiol. 2012;59(3):246–255. doi: 10.1016/j.jacc.2011.10.854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matsui K, Kamijo-Ikemorif A, Sugaya T, Yasuda T, Kimura K. Renal liver-type fatty acid binding protein (l-fabp) attenuates acute kidney injury in aristolochic acid nephrotoxicity. Am J Pathol. 2011;178(3):1021–1032. doi: 10.1016/j.ajpath.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jablonowska E, Wojcik K, Piekarska A. Urine liver-type fatty acid-binding protein and kidney injury molecule-1 in hiv-infected patients receiving combined antiretroviral treatment based on tenofovir. AIDS Res Hum Retroviruses. 2014;30(4):363–369. doi: 10.1089/aid.2013.0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kashani K, Cheungpasitporn W, Ronco C. Biomarkers of acute kidney injury: The pathway from discovery to clinical adoption. Clin Chem Lab Med. 2017 doi: 10.1515/cclm-2016-0973. [DOI] [PubMed] [Google Scholar]
- 35.Bihorac A, Chawla LS, Shaw AD, Al-Khafaji A, Davison DL, Demuth GE, Fitzgerald R, Gong MN, Graham DD, Gunnerson K, Heung M, et al. Validation of cell-cycle arrest biomarkers for acute kidney injury using clinical adjudication. Am J Respir Crit Care Med. 2014;189(8):932–939. doi: 10.1164/rccm.201401-0077OC. [DOI] [PubMed] [Google Scholar]
- 36.Hoste EA, McCullough PA, Kashani K, Chawla LS, Joannidis M, Shaw AD, Feldkamp T, Uettwiller-Geiger DL, McCarthy P, Shi J, Walker MG, et al. Derivation and validation of cutoffs for clinical use of cell cycle arrest biomarkers. Nephrol Dial Transplant. 2014;29(11):2054–2061. doi: 10.1093/ndt/gfu292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nayak B, Kondeti VK, Xie P, Lin S, Viswakarma N, Raparia K, Kanwar YS. Transcriptional and post-translational modulation of myo-inositol oxygenase by high glucose and related pathobiological stresses. J Biol Chem. 2011;286(31):27594–27611. doi: 10.1074/jbc.M110.217141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun L, Dutta RK, Xie P, Kanwar YS. Myo-inositol oxygenase overexpression accentuates generation of reactive oxygen species and exacerbates cellular injury following high glucose ambience: A new mechanism relevant to the pathogenesis of diabetic nephropathy. J Biol Chem. 2016;291(11):5688–5707. doi: 10.1074/jbc.M115.669952. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 39.Dutta RK, Kondeti VK, Sharma I, Chandel NS, Quaggin SE, Kanwar YS. Beneficial effects of myo-inositol oxygenase deficiency in cisplatin-induced aki. J Am Soc Nephrol. 2016 doi: 10.1681/ASN.2016070744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gaut JP, Crimmins DL, Ohlendorf MF, Lockwood CM, Griest TA, Brada NA, Hoshi M, Sato B, Hotchkiss RS, Jain S, Ladenson JH. Development of an immunoassay for the kidney-specific protein myo-inositol oxygenase, a potential biomarker of acute kidney injury. Clin Chem. 2014;60(5):747–757. doi: 10.1373/clinchem.2013.212993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Loboda A, Damulewicz M, Pyza E, Jozkowicz A, Dulak J. Role of nrf2/ho-1 system in development, oxidative stress response and diseases: An evolutionarily conserved mechanism. Cell Mol Life Sci. 2016;73(17):3221–3247. doi: 10.1007/s00018-016-2223-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Agarwal A, Balla J, Alam J, Croatt AJ, Nath KA. Induction of heme oxygenase in toxic renal injury: A protective role in cisplatin nephrotoxicity in the rat. Kidney International. 1995;48(4):1298–1307. doi: 10.1038/ki.1995.414. [DOI] [PubMed] [Google Scholar]
- 43.Zager RA, Johnson AC, Becker K. Plasma and urinary heme oxygenase-1 in aki. J Am Soc Nephrol. 2012;23(6):1048–1057. doi: 10.1681/ASN.2011121147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Adler M, Ramm S, Hafner M, Muhlich JL, Gottwald EM, Weber E, Jaklic A, Ajay AK, Svoboda D, Auerbach S, Kelly EJ, et al. A quantitative approach to screen for nephrotoxic compounds in vitro. J Am Soc Nephrol. 2016;27(4):1015–1028. doi: 10.1681/ASN.2015010060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang N, Zhou Y, Jiang L, Li D, Yang J, Zhang CY, Zen K. Urinary microrna-10a and microrna-30d serve as novel, sensitive and specific biomarkers for kidney injury. PLoS One. 2012;7(12):e51140. doi: 10.1371/journal.pone.0051140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ramachandran K, Saikumar J, Bijol V, Koyner JL, Qian J, Betensky RA, Waikar SS, Vaidya VS. Human mirnome profiling identifies micrornas differentially present in the urine after kidney injury. Clin Chem. 2013;59(12):1742–1752. doi: 10.1373/clinchem.2013.210245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weber EJ, Chapron A, Chapron BD, Voellinger JL, Lidberg KA, Yeung CK, Wang Z, Yamaura Y, Hailey DW, Neumann T, Shen DD, et al. Development of a microphysiological model of human kidney proximal tubule function. Kidney Int. 2016;90(3):627–637. doi: 10.1016/j.kint.2016.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]