ABSTRACT
The phytohormones auxin and cytokinin are key regulators of plant development, and both regulate almost all aspects of plant growth and development. Communication between auxin-cytokinin signaling pathways has been the subject of intense research. However, few studies have focused specifically on the development of the early gynoecium. We have recently discovered that cytokinin signaling plays a role in the regulation of auxin biosynthesis and transport in the ovary region of the gynoecium, and that the transcription factor SPATULA (SPT) is necessary. Here, we provide evidence that indicates that cytokinin and auxin have a synergistic relationship at the medial domain during gynoecium development, and that SPT is important for this interaction.
KEYWORDS: Cytokinin, Auxin, SPATULA, gynoecium, hormonal communication
The flower is the reproductive unit in angiosperms, and its origin contributed to angiosperm evolution and diversification.1,2 The female reproductive part is called the gynoecium, a highly complex organ with great diversity of forms.3,4 The Arabidopsis gynoecium is a complex structure, which consists of two congenitally fused carpels that arise from the gynoecial primordia at the center of the flower. A key event during gynoecium development is the establishment of the Carpel Margin Meristem (CMM). The CMM is an important meristematic tissue that gives rise to different tissues that are very important for sexual reproduction: the placenta, ovules, septum, transmitting tract, style, and stigma. All these tissues and structures are in the medial domain, and the two carpel walls (or ovary walls) form the lateral domains.5,6
The phytohormones auxin and cytokinin are key regulators of plant growth and development. The interactions between auxin and cytokinin play a crucial role in several and significant developmental processes such as maintenance of stem-cells, and vascular and root development.7-10 However, it is only recently that we have begun to understand the molecular mechanisms of the interaction between the auxin and cytokinin pathways, which can be antagonistic or synergistic (compared to the yin-yang concept), where their combined activity has a greater effect than just the sum of their separate effects.11-13 Recently, we have proposed a model, in which cytokinin signaling is important during the early steps of CMM and septum development in the gynoecium.14 Furthermore, in the medial domain, active cytokinin signaling results in the activation of the auxin biosynthesis gene TRYPTOPHAN AMINOTRANSFERASE OF ARABIDOPSIS 1 (TAA1) and the auxin efflux transporter gene PIN-FORMED 3 (PIN3). In this domain of the ovary of the young gynoecium (CMM and septa primordia), there is no expression of the auxin response DR5 reporter, suggesting that the produced auxin in the medial domain is redistributed towards the repla and valves (lateral domain) in a PIN-dependent mode.14 Most likely, afterwards the auxin is transported to the apical part of the gynoecium, creating an auxin flux resulting upwards growth of the gynoecial tube.15
Moreover, we identified SPATULA (SPT),16 a member of the basic Helix-Loop-Helix (bHLH) transcription factor family, as a positive regulator of cytokinin signaling in the medial region of the ovary.14 Our results demonstrate that SPT positively controls the cytokinin signaling output, in part through type-B ARABIDOPSIS RESPONSE REGULATOR (ARR) gene activation, at least by direct regulation of ARR1.14 On the other hand, it has been shown that SPT modulates auxin signaling during gynoecium and style-stigma development.17,18 These observations raised the question of whether the SPT gene could also be involved in the modulation of auxin signaling in the ovary region of the gynoecium. To answer this question, we crossed the auxin response reporter line DR5rev::GFP19 to the spt-2 mutant and the 35S::SPT line to investigate the spatial distribution of auxin signaling in the CMM and septum in these genotypes.
In the wild-type style-stigma region, the DR5 signal is mainly seen as a ring ‘around’ the style at stage 9, as it has been previously demonstrated15,17,20,21 (Fig. 1A, B). However, in the ovary region, DR5 signal is mainly confined to presumptive provasculature cells and presumptive ovule primordia (Fig. 1C),14,22 and no GFP signal was detected in the septum or transmitting tract at stage 12 (Fig. 1), as reported before.14 While DR5 signal is absent in these medial tissues, TCS signal is clearly present there.14,20,22
Figure 1.
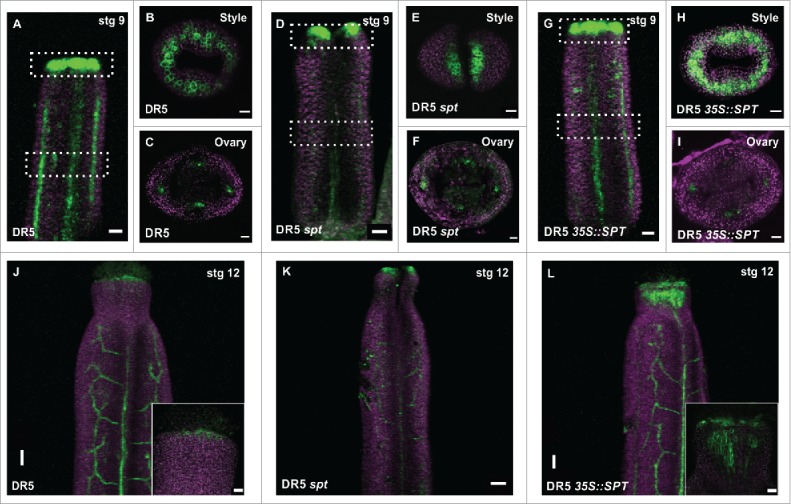
SPT affects the auxin response in the gynoecium. (A-L) Confocal laser scanning microscope (CLSM) imaging of the fluorescence signal of the auxin transcriptional response reporter DR5rev::GFP in stage 9 gynoecia of wild type (A-C), spt-2 (D-F), and 35S::SPT (G-I). White pointed boxes in (A, D, G) indicate the regions of observation, from the top (B, E, H) or in transverse sections (C, F, I). (J-K) DR5 signal expression in stage 12 gynoecia of wild type (J), spt-2 (K), and 35S::SPT (L). GFP signal in green; Propidium iodide (PI) counter stain in purple. Scale bars: 50 µm (J-L), 20 µm (A, D, G; inset in J, L), 10 µm (B, C, E, F, H, I).
On the other hand, in the spt mutant the DR5 signal failed to form this ring-shape expression pattern in the style region but was observed in two separate regions, probably due to the lack of fused tissue (Fig. 1D, E), which is consistent with previous analysis.17,23 Interestingly, in the ovary region, DR5 signal was still detected in the presumptive provasculature cells in the spt mutant (Fig. 1). The DR5 signal, however, was not clearly defined and in some occasions, we observed a moderate expansion of its expression (Fig. 1). In contrast, ectopic SPT expression caused a mild reduction in the DR5 fluorescence signal in the provasculature cells of the ovary (Fig. 1I). Although, in the style-stigma region of 35S::SPT gynoecia, the DR5 signal is increased (Fig. 1H, L). In summary, this data suggests that SPT affects the auxin-signaling response during gynoecium development.
Auxin and cytokinin interact in complex ways, either antagonistically or synergistically, depending on the developmental context.7,10,11,13,14,21,24 To test whether auxin application can change the cytokinin signaling response, and whether the change requires a functional SPT, we treated inflorescence apices of the cytokinin response reporter TCS::GFP7 and of spt-2 TCS::GFP with the auxin Indole 3-Acetic Acid (IAA). In young gynoecia stages (stage 7–9), TCS signal is absent in the spt-2 mutant background, because SPT is necessary for cytokinin signaling in the medial domain in the ovary at those stages.14 However, from gynoecium stage 10 onwards, a SPT-independent TCS signal can be observed (inset in Fig. 2E).14 In the wild type TCS::GFP line, auxin application led to an increase of the TCS signal in the presumptive provasculature cells and septa primordia (as an example, a stage 10 gynoecium is presented in Fig. 2B). In young spt gynoecia, the TCS signal remained absent even when gynoecia are treated with IAA. In untreated stage 10 spt gynoecia, TCS signal was detectable in the septum, which coincides with the transmitting tract formation (Fig. 2C). However, at this stage, the spt TCS::GFP auxin-treated gynoecia showed a lack of induction of the TCS signal (Fig. 2D, E). The results of these experiments indicate that auxin potentiates the cytokinin response, in a SPT-dependent manner. Moreover, and strikingly, the TCS signal not only could not be induced by auxin in the spt mutant background, but even the low signal observed in stage 10 gynoecia completely disappeared. This suggests that SPT is needed to maintain TCS signaling in stage 10–12 gynoecia in the presence of a high auxin concentration. Recently, it has been reported that a high auxin concentration disrupts the protein-protein interaction between the transcription factors ETTIN (ETT/ARF3) and INDEHISCENT (IND), both important for gynoecium development.25 So maybe something similar happens with the complex that permits cytokinin signaling (TCS signal) at stage 10–12 gynoecia; the complex, without SPT, would not be stable in the presence of a high auxin concentration.
Figure 2.
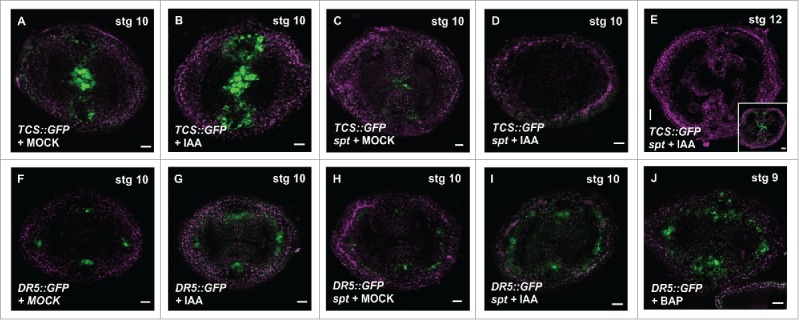
Synergistic relationship between auxin and cytokinin signaling in the gynoecium. (A-E) CLSM imaging of the fluorescence signal of the cytokinin transcriptional response reporter TCS::GFP in transverse sections of the ovary, without or with auxin (100 μM IAA for 48 hours) treatment. TCS signal in wild type gynoecia treated for 48 hours with Mock (A) or IAA (B), spt-2 treated for 48 hours with Mock (C) or IAA (D-E). (E inset) TCS signal in a stage 12 spt-2 gynoecium of a non-treated plant. (F-I) CLSM imaging of the fluorescence signal of the auxin transcriptional response reporter DR5rev::GFP in transverse sections of the ovary, without or with auxin (100 μM IAA for 48 hours) treatment. DR5 signal in wild type stage 10 gynoecia treated for 48 hours with Mock (F) or IAA (G), spt-2 treated for 48 hours with Mock (H) or IAA (I). (J) DR5 signal in a wild type stage 9 gynoecium treated for 48 hours with cytokinin (100 μM BAP; 6-Benzylaminopurine). GFP signal in green; PI counter stain in purple. Scale bars: 20 µm (E and inset), 10 µm (A-D, F-J).
Next, we evaluated the effects of auxin application on the DR5 reporter line in the gynoecium. The IAA treatment of wild type plants led to an increase in DR5 signal, mainly in the medial and lateral provasculature cells, as expected (Fig. 2G). As mentioned above, the DR5 signal in the spt mutant background is a little bit less defined, but not too much affected (Fig. 2H). When these spt DR5rev::GFP plants were treated with IAA, the DR5 signal increased (Fig. 2I), similar as what happened in a wild type background. The results suggest that the auxin signaling response to exogenous auxin in the ovary is not dependent on SPT. This is also in line with previous reports that auxin can complement the apical fusion defects in the style-region of the spt gynoecium.26
Several studies indicated the existence of a synergistic effect between auxin and cytokinin signaling in several significant developmental processes.11,13 We recently found that cytokinin signaling promotes auxin biosynthesis and transport in a SPT-dependent manner.14 In this study, we demonstrate that auxin increases cytokinin signaling in the ovary, also in a SPT-dependent manner. However, it is still unclear how auxin signaling interacts with SPT, which we are investigating at the moment. Furthermore, vice versa, we have observed that increased cytokinin potentiates auxin signaling activity in the ovary (i.e., cytokinin applications lead to increased activity of the DR5 reporter (Fig. 2J)), forming a robust circuit (Fig. 3).
Figure 3.
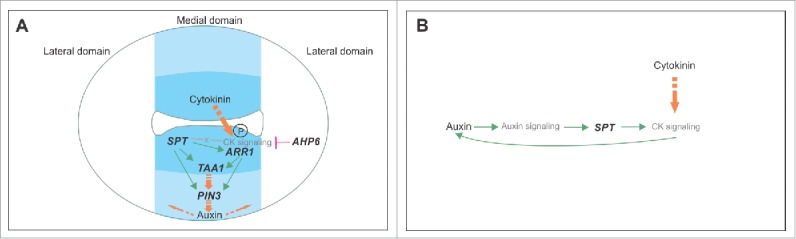
Models of the regulatory network in early gynoecium development integrating SPT, cytokinin signaling, and auxin signaling, and their synergistic interaction. (A) Previously published model of the regulatory network active in the ovary region in the young gynoecium.14 The transcription factor SPT enables cytokinin signaling at the medial region in part by transcriptionally activating the type-B ARR1 transcription factor. The protein becomes active upon phosphorylation by a phosphorelay cascade initiated when cytokinin is present. Subsequently, both SPT and ARR1 transcriptionally activate the auxin biosynthesis gene TAA1 and the auxin transporter PIN3, probably resulting in an auxin flux. SPT most likely also affects other components of the cytokinin signaling pathway. In the lateral domain, the cytokinin signaling repressor AHP6 restricts cytokinin signaling to the medial domain. (B) Model of the synergistic relationship between auxin and cytokinin signaling in the ovary region of the gynoecium. Auxin positively affects cytokinin signaling in a SPT-dependent manner, and cytokinin positively affects auxin production, thereby positively affecting auxin signaling.
In summary, all these results indicate a synergistic relationship between the two hormones in the ovary region of the young gynoecium, and support the notion that SPT plays an important role in this synergistic relationship.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
JIRO was supported by the Mexican National Council of Science and Technology (CONACyT) with a PhD fellowship (210085). Work in the SDF laboratory was financed by the CONACyT grants CB-2012-177739, FC-2015-2/1061, and INFR-2015-253504, and NMM by the CONACyT grants CB-2011-165986 and CB-2015-255069. SDF acknowledges the support of the European Union H2020-MSCA-RISE-2015 project ExpoSEED (grant no. 691109).
References
- 1.Zahn LM, Leebens-Mack J, DePamphilis CW, Ma H, Theissen G. To B or Not to B a flower: the role of DEFICIENS and GLOBOSA orthologs in the evolution of the angiosperms. J Hered. 2005;96:225–40. doi: 10.1093/jhered/esi033. [DOI] [PubMed] [Google Scholar]
- 2.Smyth DR. Morphogenesis of flowers–our evolving view. Plant Cell. 2005;17:330–41. doi: 10.1105/tpc.104.030353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Endress PK, Igersheim A. Gynoecium diversity and systematics of the Laurales. Bot J Linn Soc. 1997;125:93–168. doi: 10.1111/j.1095-8339.1997.tb02250.x. [DOI] [Google Scholar]
- 4.Staedler YM, Weston PH, Endress PK. Comparative gynoecium structure and development in Calycanthaceae (Laurales). Int J Plant Sci. 2009;170:21–41. doi: 10.1086/593045. [DOI] [Google Scholar]
- 5.Bowman JL, Baum SF, Eshed Y, Putterill J, Alvarez J. Molecular genetics of gynoecium development in Arabidopsis. Curr Top Dev Biol. 1999;45:155–205. doi: 10.1016/S0070-2153(08)60316-6. [DOI] [PubMed] [Google Scholar]
- 6.Reyes-Olalde JI, Zuñiga-Mayo VM, Chavez Montes RA, Marsch-Martinez N, de Folter S. Inside the gynoecium: At the carpel margin. Trends Plant Sci. 2013;18:644–55. doi: 10.1016/j.tplants.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 7.Muller B, Sheen J. Cytokinin and auxin interaction in root stem-cell specification during early embryogenesis. Nature. 2008;453:1094–7. doi: 10.1038/nature06943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao Z, Andersen SU, Ljung K, Dolezal K, Miotk A, Schultheiss SJ, Lohmann JU. Hormonal control of the shoot stem-cell niche. Nature. 2010;465:1089–92. doi: 10.1038/nature09126. [DOI] [PubMed] [Google Scholar]
- 9.Bishopp A, Help H, El-Showk S, Weijers D, Scheres B, Friml J, Benková E, Mähönen AP, Helariutta Y. A mutually inhibitory interaction between auxin and cytokinin specifies vascular pattern in roots. Curr Biol. 2011;21:917–26. doi: 10.1016/j.cub.2011.04.017. [DOI] [PubMed] [Google Scholar]
- 10.De Rybel B, Adibi M, Breda AS, Wendrich JR, Smit ME, Novak O, Yamaguchi N, Yoshida S, Van Isterdael G, Palovaara J, et al.. Plant development. Integration of growth and patterning during vascular tissue formation in Arabidopsis. Science. 2014;345:1255215. doi: 10.1126/science.1255215. [DOI] [PubMed] [Google Scholar]
- 11.El-Showk S, Ruonala R, Helariutta Y. Crossing paths: Cytokinin signalling and crosstalk. Development. 2013;140:1373–83. doi: 10.1242/dev.086371. [DOI] [PubMed] [Google Scholar]
- 12.Wolters H, Jurgens G. Survival of the flexible: Hormonal growth control and adaptation in plant development. Nat Rev Genet. 2009;10:305–17. doi: 10.1038/nrg2558. [DOI] [PubMed] [Google Scholar]
- 13.Schaller GE, Bishopp A, Kieber JJ. The Yin-Yang of Hormones: Cytokinin and Auxin interactions in plant development. Plant Cell. 2015;27:44–63. doi: 10.1105/tpc.114.133595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reyes-Olalde JI, Zuniga-Mayo VM, Serwatowska J, Chavez Montes RA, Lozano-Sotomayor P, Herrera-Ubaldo H, Gonzalez-Aguilera KL, Ballester P, Ripoll JJ, Ezquer I, et al.. The bHLH transcription factor SPATULA enables cytokinin signaling, and both activate auxin biosynthesis and transport genes at the medial domain of the gynoecium. PLoS Genet. 2017;13:e1006726. doi: 10.1371/journal.pgen.1006726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Larsson E, Roberts CJ, Claes AR, Franks RG, Sundberg E. Polar auxin transport is essential for medial versus lateral tissue specification and vascular-mediated valve outgrowth in Arabidopsis gynoecia. Plant Physiol. 2014;166:1998–2012. doi: 10.1104/pp.114.245951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heisler MG, Atkinson A, Bylstra YH, Walsh R, Smyth DR. SPATULA, a gene that controls development of carpel margin tissues in Arabidopsis, encodes a bHLH protein. Development. 2001;128:1089–98. [DOI] [PubMed] [Google Scholar]
- 17.Moubayidin L, Ostergaard L. Dynamic control of auxin distribution imposes a bilateral-to-radial symmetry switch during gynoecium development. Curr Biol. 2014;24:2743–8. doi: 10.1016/j.cub.2014.09.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schuster C, Gaillochet C, Lohmann JU. Arabidopsis HECATE genes function in phytohormone control during gynoecium development. Development. 2015;142:3343–50. doi: 10.1242/dev.120444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Friml J, Vieten A, Sauer M, Weijers D, Schwarz H, Hamann T, Offringa R, Jürgens G. Efflux-dependent auxin gradients establish the apical-basal axis of Arabidopsis. Nature. 2003;426:147–53. doi: 10.1038/nature02085. [DOI] [PubMed] [Google Scholar]
- 20.Marsch-Martinez N, Ramos-Cruz D, Irepan Reyes-Olalde J, Lozano-Sotomayor P, Zuñiga-Mayo VM, de Folter S. The role of cytokinin during Arabidopsis gynoecia and fruit morphogenesis and patterning. Plant J. 2012;72:222–34. doi: 10.1111/j.1365-313X.2012.05062.x. [DOI] [PubMed] [Google Scholar]
- 21.Marsch-Martinez N, de Folter S. Hormonal control of the development of the gynoecium. Curr Opin Plant Biol. 2016;29:104–14. doi: 10.1016/j.pbi.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 22.Reyes-Olalde JI, Marsch-Martinez N, de Folter S. Imaging early stages of the female reproductive structure of Arabidopsis by confocal laser scanning microscopy. Dev Dyn. 2015;244:1286–90. doi: 10.1002/dvdy.24301. [DOI] [PubMed] [Google Scholar]
- 23.Girin T, Paicu T, Stephenson P, Fuentes S, Korner E, O'Brien M, Sorefan K, Wood TA, Balanzá V, Ferrándiz C, et al.. INDEHISCENT and SPATULA interact to specify carpel and valve margin tissue and thus promote seed dispersal in Arabidopsis. Plant Cell. 2011;23:3641–53. doi: 10.1105/tpc.111.090944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marsch-Martinez N, Reyes-Olalde JI, Ramos-Cruz D, Lozano-Sotomayor P, Zuniga-Mayo VM, de Folter S. Hormones talking: Does hormonal cross-talk shape the Arabidopsis gynoecium? Plant Signal Behav. 2012;7:1698–701. doi: 10.4161/psb.22422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simonini S, Deb J, Moubayidin L, Stephenson P, Valluru M, Freire-Rios A, Sorefan K, Weijers D, Friml J, Østergaard L. A noncanonical auxin-sensing mechanism is required for organ morphogenesis in Arabidopsis. Genes Dev. 2016;30:2286–96. doi: 10.1101/gad.285361.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Staldal V, Sohlberg JJ, Eklund DM, Ljung K, Sundberg E. Auxin can act independently of CRC, LUG, SEU, SPT and STY1 in style development but not apical-basal patterning of the Arabidopsis gynoecium. New Phytol. 2008;180:798–808. doi: 10.1111/j.1469-8137.2008.02625.x. [DOI] [PubMed] [Google Scholar]


