ABSTRACT
Polyamines (PAs) are polycationic compounds found in all living organisms and play crucial roles in growth and survival. PAs interact with and modulate the functions of anionic macromolecules such as DNA, RNA and proteins. LHR1/PUT3 is a polyamine influx transporter localized in the plasma membrane in Arabidopsis. In our recent paper in The Plant Journal,1 we demonstrated that LHR1/PUT3 has a pivotal role in stabilizing the mRNAs of several important heat stress responsive genes under high temperature. In this short communication, we discuss about a putative pathway for modulating the PUT3 transport activity and the significance of evolutionary variations in PUT3 in Arabidopsis.
KEYWORDS: Gene regulation, heat stress, LHR1, mRNA stability, polyamine transporter, PUT3
LHR1/PUT3 is localized in the plasma membrane and mediates cellular uptake of polyamines (PAs) in Arabidopsis. Unlike the signaling via activating MAPK pathways or producing ROS in response to heat stress,2 fast elevation of the cellular concentrations of PAs through LHR1/PUT3 thus stabilizing the existing heat stress-induced mRNAs could be a quick response to high temperature. Although how PAs stabilize mRNAs is still a question to be addressed, our findings suggest that PAs function selectively in mRNAs under heat stress. Exogenously applied PAs seemed only reduce degradation of the transcripts with relatively short half-life, such as HSFA2, HSP101 and HSP70 transcripts, whereas HSP18.2 and HSP17.4Cl transcripts with relatively long half-life did not show an increase of mRNA stability by PA treatment. RNA-seq analysis also indicated that, after 2 hours heat treatment, the expression levels of most of the HSP100 and HSP70 genes are much lower in lhr1/put3 mutant, while the transcripts of most HSP40 and HSP20 genes showed comparable or even higher expression levels in the lhr1/put3 mutant than that in the wildtype. Analysis of the induction and decline of HSP gene transcripts by using the publicly available data from AtGenExpress(http://jsp.weigelworld.org/expviz/expviz.jsp) revealed that some of the HSP100 and HSP70 genes transcripts indeed have shorter half-life than that of the HSP20 genes, as exemplified in Fig. 1. The heat-induced mRNAs of At1g74310 (HSP101) and At2g32120 (HSP70T-2) declined to nearly basal level after 1 hour recovery at room temperature. Nevertheless, At3g46230 (HSP17.4) and At1g59860 (encoding a HSP20-like protein) transcripts declined much slower during recovery at room temperature. These results suggest that cellular PAs may selectively stabilize heat-induced mRNAs with relatively short half-life.
Figure 1.
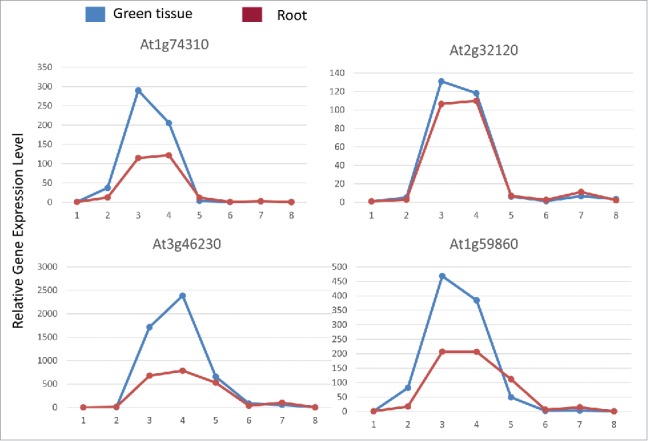
Relative expression levels of some heat-inducible HSP genes in root and green tissue of Arabidopsis after heat stress treatments and recovery at room temperature. Blue line represents gene expression level in green tissue and red line represents gene expression level in root. Shown are normalized values relative to the values of 0.25 hour heat treatment. 1. 38°C treatment of 0.25 hour; 2. 38°C treatment of 0.5 hour; 3. 38°C treatment of 1 hour; 4. 38°C treatment of 3 hours; 5. 3-hour 38°C treatment followed by 1 hour recovery at 25°C; 6. 3-hour 38°C treatment followed by 3 hour recovery at 25°C; 7. 3-hour 38°C treatment followed by 9 hour recovery at 25°C; 8. 3-hour 38°C treatment followed by 21 hour recovery at 25°C.
In yeast, 4 plasma membrane transporters, DUR3, SAM3, GAP1 and AGP2, and 2 protein kinases, PTK1 and PTK2, were found to modulate polyamine uptake.3 PTK2 was identified to phosphorylate thus regulate the activity of the polyamine uptake transporter DUR3. Homology search has identified a group of CIPKs (CBL-Interacting Protein Kinases) including SOS2 (CIPK24) in Arabidopsis sharing significant similarity with the yeast PTK2. SOS2, together with SOS1 and SOS3, constitute a so-called SOS signaling pathway that plays a crucial role in plant salt resistance.4 Interestingly, both sos1 and sos2 mutant seedlings exhibited paraquat resistant phenotype as lhr1/put3 mutant did (Fig. 2). In addition, RCD1, a regulator of oxidative stress response, was found to interact with the C-terminal tail of the plasma membrane Na+/H+ antiporter SOS1, and rcd1 mutant also showed paraquat resistance.5,6 Based on these findings, we hypothesize that SOS2 may functionally resemble the yeast PTK2 and phosphorylate LHR1/PUT3 to activate its transport activity, while SOS1 may serve as a harbor to recruit SOS2 to the plasma membrane and RCD1 perhaps functions as a regulator for the complex formation. Our ongoing research is attempting to verify this hypothesis by using genetic and biochemical studies.
Figure 2.

The lhr1/put3, sos1 and sos2 mutant showed paraquat resistant phenotype. Five-day-old seedlings grown in ½ MS agar medium were transferred to the same medium supplemented with 0.1 μM paraquat. Picture was taken at the 10th day after the transfer. Primary root growth shows the sensitivity of the genotypes to paraquat. WT, Col-0 wild type; Col-gl1, Col-0 with the gl1 mutation. The lhr1 mutant is in the background of Col-0, while sos1 and sos2 mutants are in the background of Col-gl1.
The LHR1/PUT3 gene exhibits allelic variations in natural populations in Arabidopsis, and 5 out of 22 tested ecotypes have a non-functional PUT3 allele (put3-).7 It was found that all these 5 ecotypes originated from northern latitudes or at high elevations, where stress-inducible high temperatures are rare. Thus, loss of function of PUT3 gene in these ecotypes may be associated with disuse of this gene for high temperature response during evolution. We also found that 4 out of these 5 ecotypes with put3- alleles showed extremely late flowering under long-day photoperiod and requires lengthy vernalization treatment of flowering. This observation further supports that put3- ecotypes may be originated from cold zones and have evolved mechanisms to cope with long-time low temperatures for flowering time control. It would be interesting to address whether loss of function of PUT3 gene is only because it is evolutionarily disposable since high temperature stress has not been a stress factor for these ecotypes and/or nonfunctional PUT3 is also associated with vernalization and flowering.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgment
We thank Dr. Yun Shen for his technical support.
Funding
This work was supported by the National Natural Science Foundation of China (grant #31470365).
References
- 1.Shen Y, Ruan Q, Chai H, Yuan Y, Yang W, Chen J, Xin Z, Shi H. The Arabidopsis polyamine transporter LHR1/PUT3 modulates heat responsive gene expression by enhancing mRNA stability. Plant J. 2016;88:1006–21. doi: 10.1111/tpj.13310. [DOI] [PubMed] [Google Scholar]
- 2.Zhu J-K. Abiotic stress signaling and responses in plants. Cell 2016;167:313–24. doi: 10.1016/j.cell.2016.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Uemura T, Kashiwagi K, Igarashi K. Polyamine uptake by DUR3 and SAM3 in Saccharomyces cerevisiae. J Biol Chem. 2007;282:7733–41. doi: 10.1074/jbc.M611105200. [DOI] [PubMed] [Google Scholar]
- 4.Zhu J-K. Cell signaling under salt, water and cold stresses. Curr Opin Plant Biol. 2001;4:401–6; PMID:11597497; https://doi.org/ 10.1016/S1369-5266(00)00192-8. [DOI] [PubMed] [Google Scholar]
- 5.Katiyar-Agarwal S, Zhu J, Kim K, Agarwal M, Fu X, Huang A, Zhu J-K. The plasma membrane Na+/H+ antiporter SOS1 interacts with RCD1 and functions in oxidative stress tolerance in Arabidopsis. Proc Natl Acad Sci U S A. 2006;103:18816–21. doi: 10.1073/pnas.0604711103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chung J-S, Zhu J-K, Bressan RA, Hasegawa PM, Shi H. Reactive oxygen species mediate Na+-induced SOS1 mRNA stability in Arabidopsis. Plant J. 2008;53:554–65. doi: 10.1111/j.1365-313X.2007.03364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fujita M, Fujita Y, Iuchi S, Yamada K, Kobayashi Y, Urano K, Kobayashi M, Yamaguchi-Shinozaki K, Shinozaki K. Natural variation in a polyamine transporter determines paraquat tolerance in Arabidopsis. Proc Natl Acad Sci U S A. 2012;109:6343–7. doi: 10.1073/pnas.1121406109. [DOI] [PMC free article] [PubMed] [Google Scholar]


