ABSTRACT
Japanese encephalitis (JE) is the leading cause of viral neurological disease and disability in Asia. Some 50–80% of children with clinical JE die or have long-term neurologic sequelae. Since there is no cure, human vaccination is the only effective long-term control measure, and the World Health Organization recommends that at-risk populations receive a safe and effective vaccine. Four different types of JE vaccines are currently available: inactivated mouse brain-derived vaccines, inactivated Vero cell vaccines, live attenuated SA 14–14–2 vaccines and a live recombinant (chimeric) vaccine. With the rapidly increasing demand for and availability and use of JE vaccines, countries face an important decision in the selection of a JE vaccine. This article provides a comprehensive review of the available safety literature for the live attenuated SA 14–14–2 JE vaccine (LAJEV), the most widely used new generation JE vaccine. With well-established effectiveness data, a single dose of LAJEV protects against clinical JE disease for at least 5 years, providing a long duration of protection compared with inactivated mouse brain-derived vaccines. Since 1988, about 700 million doses of the LAJEV have been distributed globally. Our review found that LAJEV is well tolerated across a wide age range and can safely be given to children as young as 8 months of age. While serious adverse events attributable to LAJEV have been reported, independent experts have not found sufficient evidence for causality based on the available data.
KEYWORDS: adverse events, Japanese encephalitis, live attenuated SA 14–14–2 vaccine, serious adverse events, vaccine safety
Background
Japanese encephalitis (JE)—a mosquito-borne, neglected tropical disease—is the leading cause of viral neurological disease and disability in Asia.1,2 Approximately 3 billion people living in Asia, including 700 million children, are at risk for JE, with an estimated 67,900 JE cases occurring annually in JE-endemic countries.2 The burden due to death and disability is often underestimated, while determinants for developing overt neurologic disease following infection are not fully understood.3 JE primarily affects children and confers lifelong immunity to those infected.4 There is no cure for JE; about 20–30% of JE cases are fatal and of those who survive, 30–50% have long-term neurologic sequelae.5
Human vaccination is the only effective long-term control measure against JE, and the World Health Organization (WHO) recommends that at-risk populations receive a safe and effective vaccine through the national immunization program.1 The 15 JE vaccines currently in use fall into 4 classes: inactivated, mouse brain-derived vaccines; inactivated, Vero cell culture-derived vaccines; live, attenuated SA-14–14–2 vaccines; and a live, recombinant (chimeric) vaccine.6 Thus far, the inactivated, mouse brain-derived JE vaccine has successfully reduced JE disease in several countries; however, barriers to wider use have included a complex multi-dose schedule, a difficult production process and uncertain supply, high vaccine price, and safety concerns.7,8 Due to these concerns, in 2005, the Japanese government withdrew its recommendation for routine immunization with the inactivated, mouse brain-derived JE vaccine.9-11 In reviewing this decision, the WHO Global Advisory Committee on Vaccine Safety (GACVS) determined there was no causal link suggesting an increased risk for acute disseminated encephalomyelitis associated with the inactivated mouse brain-derived JE vaccine.9-11 However, the WHO recommended that the inactivated mouse brain-derived JE vaccine be gradually replaced by new-generation JE vaccines.1,9,10
The newer JE vaccines, available in several endemic countries, fall into 3 classes. First, there are inactivated Vero cell culture-derived JE vaccines developed from various JE strains (e.g., Beijing-1, Beijing P-3, Kolar strain [(JEV 821564 XY]).6 Each vaccine strain follows different schedules for doses and boosters; for example, the Vero cell culture-derived JE vaccine derived from an attenuated SA 14–14–2 strain generally requires a primary immunization of 2 intramuscular doses administered 4 weeks apart for children 2 months of age or older in endemic regions.6,12 A second class includes a live, attenuated SA 14–14–2 JE vaccine (LAJEV), derived from the SA 14–14–2 viral strain of a genetically stable, neuro-attenuated JE virus, which requires a single dose for children 9 months of age or older and confers protection against clinical JE disease for at least 5 y.13 A third class consists of a novel live, attenuated, recombinant (chimeric) JE vaccine (JE-CV) comprising the structural genes of SA 14–14–2 virus and nonstructural genes of yellow fever 17D virus, which has been licensed for children 9 months of age or older as a 2-dose vaccine given subcutaneously, and confers 5 y of protection against clinical JE disease.14 The WHO recommends a single-dose of JE-CV over a 2-dose schedule.6 Furthermore, the WHO has underscored the continued importance of rigorously monitoring all JE vaccine failures.
Of these 3 classes, LAJEV has become the most widely used vaccine in endemic countries in Asia.6,8,15,16 The vaccine was first licensed in China in 1988 after initial trials showed no significant local reactions, systemic reactions, nor serious adverse events (SAEs) in follow-up periods ranging from 14 to 21 d after vaccination.17,18 Since licensure, approximately 700 million doses of LAJEV have been produced in China (personal communication between S. Halstead and Dr. Yu, July, 2016). It is the first Chinese vaccine to be used internationally on a large scale. In 2013, LAJEV became WHO-prequalified at the Chengdu Institute of Biological Products, Chengdu, China. In 2014, Global Alliance for Vaccines and Immunization (GAVI) invited eligible countries to apply for financial support to conduct national JE vaccine catch-up campaigns and to introduce JE vaccine into their routine national immunization programs.19,20
As countries face the decision to introduce or update JE vaccines in their national immunization schedules, a comprehensive review of the available safety literature for LAJEV is warranted, especially as results from more methodologically rigorous safety studies have been recently published. In this paper, we reviewed published data on LAJEV since 1988 and synthesized the available evidence to determine vaccine safety according to the following topics: a) local reactions; b) systemic reactions; and c) serious adverse events (SAEs).
Materials and methods
Search strategy
We searched for all articles in the English language in PUBMED, EMBASE, and Web of Science using the following indexed and free-text search terms: “Japanese encephalitis,” “live attenuated,” “SA 14–14–2,” “vaccine,” and “safety” in multiple combinations. Snowball searching through the references of retrieved articles supplemented our search results. We also reviewed PMS reports, 2 from China (2005–2012 and 2009–2012)21 and one from South Korea (2002–08).21-23 In addition, we contacted Chengdu Institute of Biological Products for other vaccine safety data that may not be publicly accessible.
Study selection
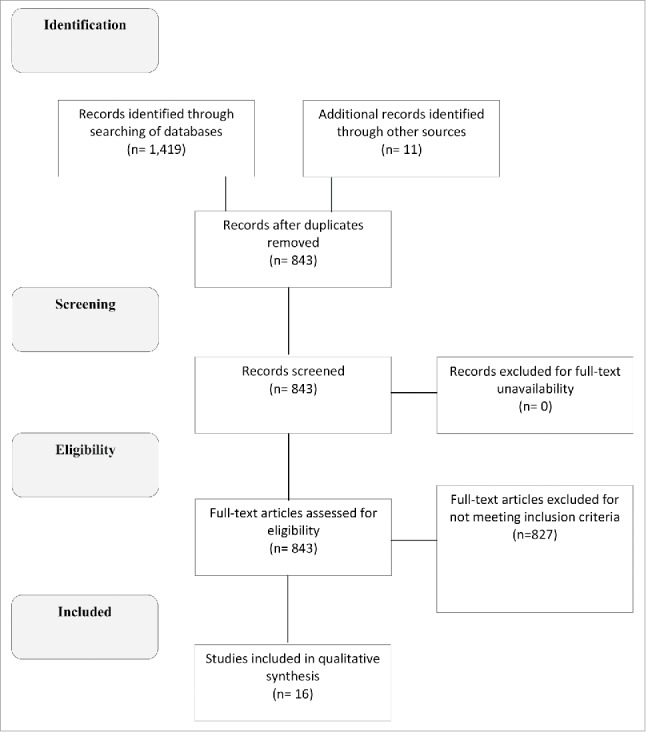
Titles and abstracts identified through the search strategy were screened for inclusion if: 1) children received primary immunization with the licensed, LAJEV produced by Chengdu Institute of Biological Products containing titers of at least 5.4 log PFU per 0.5 ml dose; and 2) children were monitored for local (e.g., erythema, swelling, induration, pain) and/or systemic (e.g., fever, lethargy, insomnia, irritability, seizure, anorexia, nausea, vomiting, diarrhea, cough, and rash) reactions following vaccination. Studies were excluded if they: 1) examined other versions of LAJEV produced by other manufacturers; 2) were conducted before 1988 (when a standardized vaccine titer was not mandated post-licensure); 3) included less than 30 study participants; 4) were primarily immunogenicity studies and did not include safety data; and/or 5) lacked or had limited details on methodology. Disagreements about the inclusion of an article were collectively discussed by the authors before a decision was reached. Appendix Figure A1 provides additional information on the study selection process.
Data extraction
Articles were screened and data abstracted by the first 2 co-authors, and discrepancies were reviewed to reach agreement. The following data were extracted from included articles: author, publication year, country, study design, number and age of subjects, episodes of AEs and/or SAEs experienced post-vaccination, duration of follow-up and any other vaccines delivered concomitantly or before the administration of LAJEV.
Findings
Overview of included studies
An overview of the 16 studies that met the inclusion criteria are summarized in Table 1. The studies, which were conducted between 1995 and 2015 in various Asian countries, consist of 7 prospective cohort studies,8,24-29 3 PMS studies,21-23 2 open-label, nonrandomized single arm trials,30,31 one prospective randomized trial with a control group,32 2 observer-blind, randomized controlled trials (RCT) comparing LAJEV to JE-CV,33,34 and one 4-arm, double-blind RCT assessing lot-to-lot consistency of LAJEV.35
Table 1.
Overview of clinical studies providing safety data on the live attenuated SA 14–14–2 JE vaccine in children.
| Year | Authors | Study country | Study design | Number of vaccinated children | Age of children | Number of vaccine doses | Concomitant vaccines | Duration of follow-up per child | Children with serious adverse events related to vaccine |
|---|---|---|---|---|---|---|---|---|---|
| 1997 | Liu32 | China | Prospective randomized trial with control group, block randomization, of primary and booster immunization in Chengdu, Sichuan province; 1995 | 13,275 | 1, 2, 6 years | 1 or 2 depending on age | None | 30 d(subsample of 266 subjects were examined at days 1,2,3 and 7) | None |
| 1999 | Sohn24 | South Korea | Prospective cohort study of primary immunization in 3 private chronic care facilities in Seoul (2) and Kyungki province (1); 1998 | 68 | 1–3 years | 1 | None | 30 days | None |
| 2008 | Glovax22 | South Korea | Post-marketing surveillance study of primary immunization in 8 research centers across South Korea; 2002–2008 | 606 | 10 months – 13 years | 1 | None | 30 days | None |
| 2008 | Gatchalian8 | Philippines | Prospective cohort study of primary immunization in a single center in Manila; 2005–2006 | 571 | 8–10 months | 1 | Measles | 28 days | None |
| 2011 | Chotpitayasunondh25 | Thailand | Prospective open-label single center trial of primary immunization in a single center in Bangkok; 2006–2007 | 150 | 9–15 months | 1 | None | 28 days | None |
| 2012 | Ranganath26 | India | Prospective cohort study of immunization campaign in Kolar, Karnataka; 2007 | 1,640 | 10–15 years | 1 | None | 28 d | None |
| 2013 | Choi27 | South Korea | Prospective cohort study of booster immunization in 3 tertiary level hospitals in South Korea; 2009–2010 | 68 | 5–7 years | 1 booster(already received 1 or 2 doses previously at ages 1–2 years) | None | 28 days | None |
| 2014 | Wijesinghe31 | Sri Lanka | Prospective randomized open-label trial of primary immunization in a single center in Colombo; 2007–2008 | 278 | 9 months | 1 | Measles | 1 y | None |
| 2014 | Liu23 | China | Post-marketing surveillance study of 2- or 3-dose series in Guangdong, China; 2005–2012 | 23 million doses | 8 months, 18–24 months, 6 years | 3 dose series before 2009; 2 dose series post-2009 (ages 8 months 18–24 months) | Largely administered alone; some cases concurrently administered with measles only, measles-rubella, or diphtheria-tetanus-pertussis (acellular) vaccines | 3 month reporting period via passive surveillance | 36, including 2 deaths(difficult to assess causality) |
| 2014 | Wang21 | China | Post-marketing surveillance study of primary immunization throughout 26 provinces in China; 2009–2012 | 62.39 million doses released (number of doses administered is unknown) | 1–3 years | N/A | None | 1 y | 70, including 4 deaths(difficult to assess causality) |
| 2014 | Zaman35 | Bangladesh | Lot-to-lot consistency randomized controlled trial of primary immunization in Matlab and Mirpur; 2012 | 818 | 10–12 months | 1 | None | 28 days | None |
| 2014 | Feroldi33 | Thailand | Observer-blind randomized controlled trial of primary immunization with LAJEV or JE-CV in 3 sites in Thailand; 2010 | 300 | 9–18 months | 1 | None | 6 months | None |
| 2014 | Kim34 | South Korea | Observer-blind randomized controlled trial of primary immunization with LAJEV or JE-CV in 10 sites in South Korea; 2011–2013 | 274 | 12–24 months | 1 | None | 6 months | 2 |
| 2015 | Kwon28 | South Korea | Prospective cohort study of 2-dose primary immunization in 5 centers across South Korea; 2007–2009 | 90 | 1–3 years | 2 (1 y interval) | None | 42 d after each vaccination | None |
| 2016 | Sanchayan29 | Sri Lanka | Prospective cohort study of primary immunization in Jaffna district; 2012–2015 | 3,041 | 9 months | 1 | None | 45 days | 15 |
| 2016 | Wijesinghe30 | Sri Lanka | Open-label nonrandomized single arm study of primary immunization in children previously vaccinated with inactivated mouse brain- derived JE vaccine in Colombo district; 2007 | 305 | 2–5 years | 1 | None; Children who previously received 2 or 3 doses of inactivated mouse brain-derived JE vaccine, were again vaccinated with LAJEV | 10 months | None |
LAJEV = live, attenuated SA 14–14–2 JE vaccine; JE-CV = live, attenuated, recombinant (chimeric) JE vaccine.
Study subjects and vaccination
Study participants were healthy male or female children ranging from 8 months to 15 y of age. LAJEV was administered subcutaneously as a single 0.5 ml dose. In most of the studies,22,24-26,29,30,35,36 children were administered only one dose of LAJEV with the exception of 5 studies.8,27,28,31,32 In 2 RCTs, children were randomized 1:1 to receive either LAJEV or JE-CV.33,34 One study that administered a single dose of LAJEV required children to have previously received the inactivated mouse brain-derived vaccine.30 All studies, except one29 which did not clearly specify, excluded children from vaccination if they had visible signs of sickness (e.g., a fever, cough or cold); however, the stringency of the exclusion criteria applied varied by study.
Assessment of safety
Local and/or systemic reactions were monitored across all trials at various time intervals. Most studies monitored children for local reactions or AEs for at least 30 minutes after each vaccination.8,22,24,25,28,30,31,33-36 After vaccination, subjects were generally followed for SAEs at least 28 d and some up to one year following the last study vaccination. In addition to clinic and home visits, parents were encouraged to record and/or report any local and systemic symptoms following vaccination in some studies.8,24,28,29,31,35,36
In contrast, the 2 PMS studies in China21,23 tracked AEs following immunization (AEFI) using a national passive surveillance system, the Chinese Center for Disease Control (CDC) database, which includes AEFI report card data submitted by health staff, manufacturers and patients.37 A PMS study in South Korea actively assessed the occurrence of AEs and SAEs following LAJEV vaccination by administering a series of 6 surveys annually over a 6 y period across 19 hospitals and clinics.22
Local reactions
Local reactions such as erythema (0.2%–1.1%), swelling (0.4%–1.54%), pain (0.9–17%) and signs and/or symptoms at the injection site (3%) were reported across studies that administered only one LAJEV dose,22,25,26,35 except in a prospective cohort study among South Korean children,24 in which neither local nor systemic reactions were reported. In a study in Sri Lanka, a local reaction at the injection site or induration was reported in 12 out of 2878 infants over a 14 day follow-up period.29 In another study from Sri Lanka, all solicited local reactions (including erythema, induration, and pain) were non-severe in children 2 y of age (74 children with local reactions out of 151 children) and 5 y of age (106 out of 154) who were previously vaccinated with the inactivated, mouse brain-derived JE vaccine before being vaccinated with a single dose of LAJEV.30
In 2 RCTs conducted in Thailand and South Korea where LAJEV and JE-CV were compared, solicited reactions at the injection site were common.33,34 In Thailand, tenderness, erythema and swelling were the most common local reactions reported in both LAJEV (37.5%, 23.0%, 7.9%) and JE-CV (30.1%, 17.8, 6.2%) vaccination groups.33 Similarly, in South Korea, pain and erythema followed by swelling were the most common local reactions reported in LAJEV (27.7%, 24.1%, 7.3%) and JE-CV (25.5%, 16.8%, 4.4%) vaccination groups.34 In both studies, one child vaccinated with LAJEV experienced severe injection site pain or tenderness, which spontaneously resolved.33,34
Solicited local reactions following immunization were also observed in 2 South Korean studies which administered a 2 dose primary LAJEV series28 and a booster dose of LAJEV.27 Children that received the 2 dose primary LAJEV series reported solicited local reactions such as erythema (24.6% vs 13.3%), pain/tenderness (20.3% vs 8.9%), and swelling (11.6% vs 7.8%) more frequently after the second dose versus the first dose.28 Interestingly, this study reported a higher frequency of local reactions following the second dose of LAJEV compared with the first, but also noted that the geometric mean titer increased by 6.5-fold in the second dose compared with the first dose.28 Solicited local reactions, such as, erythema (4.8%), pain (4.8%) and swelling (3.2%) were also observed at the 4 week follow-up, among children that received a booster dose.27 In a large prospective study in China, local reactions such as injection site tenderness (0.4%), hives (0.4%), and rash (2.2%) were reported over 7 d in a closely observed sub-sample of 266 children who received either their first or second LAJEV dose.32
Two studies in which both LAJEV and measles vaccine (MV) were administered found most solicited local reactions among children to be mild and transient.8,31 A study from the Philippines, which randomized infants to one of 3 study groups (group 1: receive LAJEV at 8 months and MV at 9 months; group 2: receive both LAJEV and MV at 9 months; or group 3: MV at 9 months and LAJEV at 10 months), found that 12.1–27.0% of infants across the groups experienced a solicited local reaction (e.g., erythema, pain, swelling, or induration) within 7 d of vaccination.8 However, none of these local reactions were classified as severe, and only 2.1–8.0% reactions were considered moderately severe. A study from Sri Lanka which co-administered LAJEV and MV to children, similarly reported solicited local reactions (e.g., erythema, induration or pain) among 12.2% of children within 0–3 d and 0.7% of children between days 4–7; however, no reactions were classified as severe.31 Neither study reported safety issues associated with the co-administration of the 2 vaccines.8,31
The occurrence of local reactions following LAJEV immunization was also documented in the 3 PMS studies we reviewed. A PMS study in Guangdong, China examining 23 million LAJEV doses between 2005 to 2012 recorded a total of 1,426 AEFIs, of which 1,390 were classified as non-serious.23 Though 3% of these cases were classified as location reactions (e.g., erythema, swelling, and local or sterile abscess), most of the remaining cases were attributed to systemic reactions including fever or allergic reactions. A 2009–2012 national PMS study in China found erythema and swelling (13.73%), rash (7.17%), and induration (5.76%) were the most commonly reported local reactions among 6,024 AEFIs cases; in this study “the reported rate of AEFI with 6,024 cases was 96.55 per million doses using lot release data as denominator” (62.39 million LAJEV doses).21 In comparison, only 3 cases out of 606 vaccinated children in a PMS study in South Korea reported local reactions, which included erythema or swelling.22
Systemic reactions
A majority of studies we reviewed using a single dose administration of LAJEV reported mild and time-limited systemic reactions following immunization regardless of follow-up time.25,26,35 For example, in a prospective cohort study in Bangkok, Thailand during the first week following immunization, cough (16%), rhinorrhea (10.7%) and fever >38.0°C (9.3%) were commonly reported systemic reactions.25 Similarly, in a lot-to-lot consistency study in Bangladesh, solicited systemic reactions, such as fever (3.2%), but also diarrhea (2.2%), drowsiness (0.7%) or vomiting (1.8%) were reported among vaccinated children within 7 d of the vaccination.35 In a study in Sri Lanka, fever (> 37.8°C) was noted among 133 infants immunized with a single dose of LAJEV, of whom 39% and 44% reported recovering within one day and 2 days, respectively.29 Fifty infants sought care and 6 infants were hospitalized, though, the specific circumstances of the hospitalization were not described. In addition, irritability (41%) was the most commonly reported systemic reaction, which mothers noted was likely related to pain and fever from the vaccination.29
Over a longer follow-up period of 30 days, fever (4.9%–11.3%), cough (3.4%–12.6%) and irritability (1%–3.8%) remained the most commonly reported systemic reactions across 3 single dose studies from South Korea, India and Bangladesh, and a Chinese PMS study that documented systemic reactions following either one or 2 LAJEV doses.24,26,32,35 Less reported systemic reactions included headache (2.6%),26 nausea/vomiting (1%–1.1%),24,26 as well as self-limited skin rashes (1%) and loss of appetite (1%).24
Fever (3.2%) was the most commonly solicited systemic reaction reported among South Korean children that received a booster dose, followed by unsolicited reports of systemic reactions including upper respiratory tract infection (9.67%) and pneumonia (1.61%) over the 4 week follow-up period.27 Similarly, in a 2 dose LAJEV safety evaluation in South Korea, fever was common after the first dose (4.4%) and second dose (7.2%), but no fever ≥ 40°C was reported.28 All other events reported after each dose were considered mild and self-limiting.28 Among children who were previously vaccinated with inactivated, mouse brain-derived JE vaccine and then vaccinated with a single dose of LAJEV in Sri Lanka, solicited systematic reactions were largely non-severe.30 Among 151 2 and 154 5 y old children, anorexia (n = 28; n = 16), fever (n = 12; n = 16), crying (n = 15; n = 8), and insomnia (n = 13; n = 5) were reported. Only 2 severe systemic reactions (irritability and diarrhea) were reported overall.
In 2 studies which administered both LAJEV and MV, solicited systemic reactions were also reported.8,31 Specifically, in a study in the Philippines, of those vaccinated first with LAJEV, 34% of infants experienced a mild systemic reaction (including anorexia, crying, diarrhea, drowsiness, insomnia, irritability, vomiting, and fever).8 Moderate (22%) and severe (2%) reactions were reported less frequently. Within 7 d of receiving LAJEV dose alone, mild (37.5–38.6°C) and moderate (38.7–39.9°C) fevers were noted in 6.0% and in 18.0% of infants, respectively. Similarly, in a study in Sri Lanka in which LAJEV and MV were co-administered, fevers were noted in 7.6% of infants within 3 d of receiving the vaccines, and 5.0% within 4 to 7 d of the vaccines.31 However, no cases of severe fever were reported in either study.
Fever was also a commonly reported systemic reaction in PMS studies in China.21,23 Fever was the most reported AEFI in Guandong in 2005–201223 and nationally in 2009–2012.21 In a Guandong PMS study,the reported rates were provided by temperatures 37.1–37.5°C (1.20 per million), 37.6–38.5°C (6.74 per million), and ≥ 38.6°C (22.37 per million).23 In a national PMS study, a single reported rate of fever per 76.76 per million dose distributed was provided.21 By comparison, a PMS study in South Korea found that upper respiratory infections (8.91%) and cough (6.60%) were the most commonly reported systemic reactions among vaccinated children.22 Systemic allergic reactions including generalized rash, urticaria, and angioedema were also observed in the 2005–2012 PMS study in Guandong, China.23
Solicited systemic reactions were observed in 2 RCTs conducted in Thailand and South Korea.33,34 In Thailand, irritability and loss of appetite were the most commonly reported in both LAJEV (38.2%, 35.5%), and JE-CV (28.1%; 21.9%) groups.33 Fever was more commonly reported in LAJEV (21.7%) than JE-CV group (16.4%). In South Korea, similar trends were observed in solicited systemic reactions in LAJEV and JE-CV groups: irritability (26.3% vs. 22.6%), loss of appetite (29.2% vs. 27.7%), and fever (25% vs. 24.6%).34
Serious adverse events
Many studies that documented SAEs determined that all reported SAEs were unrelated to LAJEV administration.8,25,26,28,30,31,33,35 One study reported no “significant” AEs,24 but others did not fully describe or address the issue of causality,22,27 or were unable to make strong assessment due to study design23 or the use of a passive surveillance system.22 However, SAEs associated with LAJEV vaccination were noted in 2 studies.29,34
A RCT study conducted in 1995 in China observed SAEs such as encephalitis, meningitis or hospitalizations due to all causes, but noted that they were no more commonly observed among vaccinated children compared with non-vaccinated children.32 Diarrhea (0.8%) was reported in a closely observed subsample.32 Acute diarrhea or severe pneumonia was also documented among 10 of 818 vaccinated children in a lot-to-lot consistency study in Bangladesh, but none of the cases were determined to be vaccine-related.35
In a 2005–2012 PMS study in Guandong, China, out of a total of 1,426 AEs (61.24 per million doses) reported, there were 36 SAEs (2.5%), including 2 deaths, 11 hospitalizations, 19 life-threatening events, and 4 cases of severe disability.23 The 2 deaths following vaccination were reported as due to viral hypothalamic encephalitis and viral hemorrhagic encephalitis. Thirty-one neurological events were reported, including febrile convulsion (14 cases), asphyxia (n = 4), seizure (n = 3), viral encephalitis (n = 3), encephalopathy (n = 2), acute disseminated encephalomyelitis (n = 2) among others (n = 3). Most of the febrile convulsion cases were reported on the day of vaccination, which the study noted was “closely associated” with high fever; the study also noted that “in most cases, high fever was correlated with infection.” Due to the design of this PMS study, a strong assessment of causality was not possible. Another Chinese 2009–2012 national PMS study recorded 70 SAEs over 366 d following vaccination which included central nervous system damage (n = 34), serious immunological reactions (n = 13), blood system diseases (n = 12), peripheral nervous system disease (n = 2), serious infection (n = 3), and other diseases (n = 6).21 In 14 SAE cases, LAJEV was co-administered with another vaccine. Of the 70 SAEs, 4 resulted in deaths, which were reported in children 8 months to 1 y of age. These deaths were due to (1) viral encephalitis and serious pneumonia, (2) serious pneumonia, respiration-circulation failure, (3) serious infection, and (4) malnutrition and severe dehydration. In the death due to malnutrition and severe dehydration, the child was co-vaccinated with other vaccines (meningococcal A conjugate vaccine, hepatitis B vaccine, diphtheria-tetanus-acellular pertussis vaccine, and oral polio vaccine). Due to the use of a passive surveillance system in this PMS study, causality was assessed largely by reviewing clinical symptoms and signs and laboratory examinations.
In a RCT in Thailand comparing LAJEV and JE-CV, SAEs were reported in both groups; however, none were determined to be associated with either vaccine.33 Among children previously vaccinated with inactivated, mouse brain-derived JE vaccine, 26 SAEs were observed in 22 two year old children and 9 SAEs in 8 five year old children; however, none were determined to be related to LAJEV vaccination.30 SAEs were also observed in a study from Sri Lanka when LAJEV was co-administered with MV.31 Overall, 16.2% (n = 45) of infants experienced a non-vaccine-related SAE, most commonly resulting from gastroenteritis (4%), viral infections (2.9%) and lower respiratory tract infections (2.5%). No deaths or life threatening illnesses occurred and illnesses were resolved without any serious sequelae. Study investigators determined no SAEs were related to either vaccine.
SAEs associated with LAJEV vaccination were noted in 2 studies. A study in Sri Lanka assessing the safety of LAJEV noted that 15 out of 2,878 infants followed for 14 d experienced SAEs which were “consistent with causal association” to LAJEV.29 These infants had fever (n = 2), febrile convulsions (n = 6), papular urticaria (n = 1), acute gastroenteritis (n = 2), and diarrhea (n = 4). The study reported “no deaths” or “life threatening AEFIs.” In a RCT in South Korea comparing LAJEV and JE-CV, SAEs were reported in both the LAJEV group (13.1%) and the JE-CV group (12.4%).34 The SAEs included 2 cases of fever which required hospitalization and were determined to be associated with LAJEV vaccination; the 2 cases were treated and the children continued in the study. The study authors did not specifically comment on whether these cases fully resolved.
Discussion
Our review indicates that LAJEV is a safe vaccine. While SAEs attributable to LAJEV administration have been reported, independent experts have not found sufficient evidence for causality based on the available data. In some studies, AEs and SAEs were reported without an assessment of causality, and thus, it is important to note that AEs and SAEs can be due to chance. Our review found that LAJEV is well tolerated across a wide age range (8 months to 15 years) and may be safely administered to children as young as 8 months of age. A recent study reported that a single dose of LAJEV also can be safely administered to adults, generating immunity among both seronegative and naturally seropositive adults.38 Furthermore, data on the co-administration of LAJEV with MV shows an acceptable short-term safety profile,15 which may encourage endemic JE countries to revise their national immunization programs to reflect a co-administration of the 2 vaccines as in Sri Lanka.39 An immunogenicity study examining the co-administration of LAJEV and MV just completed in China,40 and an immunogenicity and safety study is underway in the Philippines to evaluate the co-administration of LAJEV and the measles-mumps-rubella (MMR) vaccine which pending the results, may turn into an official recommendation for interested countries.41
Limitations to our review included the small number of eligible studies for analysis as well as several differences between studies that prevented clear comparisons. The differences occurred in the areas of monitoring and measurement of local and systemic reactions following immunization, data collection methods, frequency of study visits, the types of outcomes documented, and reporting mechanisms (active vs. passive). Those studies with active safety monitoring following immunization with LAJEV while smaller, allowed more rigorous evaluation of AEs; those studies with passive safety reporting and surveillance also helped establish LAJEV's safety. Both types of studies were used to inform decisions around LAJEV recommendations and regulatory action.
Additional limitations also encompassed differences in the level of investigator and clinical trial site experience. We suspect some studies may have under-reported AEs and SAEs, which may have resulted from less recognition and/or reporting of AEs and SAEs by study participants over prolonged time periods, especially when there was no active surveillance. The PMS studies in China also recognized the limitation of underreporting due incomplete or erroneous documentation of cases. In spite of these limitations, the safety profile of LAJEV has been consistent and acceptable across the studies, and the risk-benefit ratio remains favorable.
In China, where JE vaccines have been included in the national childhood immunization programs since 2008, PMS data generated by the Chinese national surveillance system from 2009–2012 determined that LAJEV had a reasonable safety profile.21 A 2005–2012 PMS study in Guangdong, China reinforced this finding; AEs were most commonly mild, occurring within 3 to 5 d following LAJEV, and neurologic events were observed rarely.23 It is important to note that the rates of AEs reported with LAJEV are comparable to those reported with other JE vaccines.42,43 A recent review quantifying the benefits of LAJEV vaccination concluded that since the inclusion of LAJEV in the national Chinese immunization program that provides all immunizations at no cost, the nationwide JE incidence has declined considerably and yielded significant economic and social benefits.44 In 2001, South Korea was the first country outside of China to license LAJEV and since then, its national regulatory agency has conducted PMS studies22 and concluded that LAJEV has an acceptable safety profile.23
Through JE vaccination campaigns, India has successfully immunized over 62 million (78.8%) children between one to 15 y of age with LAJEV without evidence of vaccine-related serious illnesses or deaths.45 After a 2006 campaign reached greater than 9.3 million children in India, a PMS study documented no SAEs following immunization, but was unable to reconcile the reports of 65 hospitalizations, including 22 deaths.46 These reports prompted an evaluation by an independent expert committee, which concluded that no direct causality had been established between these events and the administration of LAJEV. After reviewing this report, the WHO GACVS recommended that future immunization campaigns be accompanied by strengthened monitoring and investigation of AEFIs. When the GACVS reconvened in 2007 and 2008 to review the available safety data, including new reports from South Korea and the Philippines, it concluded that the short-term safety profile of LAJEV is satisfactory and that LAJEV could safely be co-administered with MV to children 9 months of age.8,22
India and Nepal have seen a significant reduction in JE cases and deaths post-introduction of LAJEV.47,48 Both countries have seen an improvement in acute encephalitis syndrome and JE lab surveillance systems as well as increased awareness about JE disease prevention through vaccination. In Sri Lanka, due to increasing reports of AEs associated with the inactivated mouse brain-derived JE vaccine, and the desire to keep costs down while expanding the target population to include vulnerable adults in high-risk areas in addition to children, the Sri Lankan Ministry of Health transitioned from the inactivated mouse brain-derived JE vaccine to LAJEV after conducting a safety study.31 Other countries in the region have also implemented strategies to scale up LAJEV immunization efforts. In 2015, Lao People's Democratic Republic became the first GAVI-eligible country to conduct catch-up campaigns for children 9 months to 15 y of age with Cambodia and Nepal following suit in 2016.49 Cambodia has also launched a national JE vaccination campaign following which the vaccine will be included in the national routine immunization program.50 Having conducted surveillance showing active circulation of JE virus and learning from the experiences of regional partners, Bangladesh and Indonesia are planning or expanding their national JE immunization programs, potentially first through vaccination campaigns and then routine immunization programs.
A JE control target has been supported by the 2005 World Health Assembly resolution on disability, and is consistent with the goals and objectives of WHO, United Nations Children's Fund (UNICEF), GAVI, and World Bank as well as several countries that face a high risk of JE infection. Through safe and effective JE immunization, JE control is feasible. Safety data and introduction experiences summarized in this review strengthen the evidence base for LAJEV safety, and may inform the decision-making of countries considering introducing a vaccine for JE control.
Appendix
Figure A1.
Source: Authors' review of published articles from peer-reviewed and gray literature.
Disclosure of potential conflicts of interest
The authors report no conflict of interest.
References
- [1].World Health Organization. Japanese Encephalitis Vaccines: WHO position paper - February 2015 Weekly epidemiological record: Relevé épidémiologique hebdomadaire. World Health Organization; 2015; 69-88. Weekly epidemiological record Report No.: 9, 90. http://www.who.int/wer/2015/wer9009.pdf
- [2].Campbell GL, Hills SL, Fischer M, Jacobson JA, Hoke CH, Hombach JM, Marfin AA, Solomon T, Tsai TF, Tsu VD. Estimated global incidence of Japanese encephalitis: a systematic review. Bulletin World Health Organization. 2011;89(10):766-74. doi: 10.2471/BLT.10.085233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Halstead SB, Thomas SJ. Japanese encephalitis: new options for active immunization. Clin Infect Dis. 2010;50(8):1155-64. doi: 10.1086/651271. PMID:20218889 [DOI] [PubMed] [Google Scholar]
- [4].Solomon T. Flavivirus encephalitis. N Eng J Med. 2004;351(4):370-8. doi: 10.1056/NEJMra030476 [DOI] [PubMed] [Google Scholar]
- [5].Fischer M, Hills S, Staples E, Johnson B, Yaich M, Solomon T. Japanese encephalitis prevention and control: advances, challenges, and new initiatives. 2008. [accessed 2016March15]. http://www.asmscience.org/content/book/10.1128/9781555815592.ch06
- [6].SAGE Working Group on Japanese encephalitis vaccine Background Paper on Japanese Encephalitis Vaccines. Geneva, Switzerland: World Health Organization; 2014:1-74. http://www.who.int/immunization/sage/meetings/2014/october/1_JE_Vaccine_Background_Paper.pdf [Google Scholar]
- [7].Zanin MP, Webster DE, Martin JL, Wesselingh SL. Japanese encephalitis vaccines: moving away from the mouse brain. Expert Rev Vaccines. 2003;2(3):407-16. doi: 10.1586/14760584.2.3.407. PMID:12903806 [DOI] [PubMed] [Google Scholar]
- [8].Gatchalian S, Yao Y, Zhou B, Zhang L, Yoksan S, Kelly K, Neuzil KM, Yaïch M, Jacobson J. Comparison of the immunogenicity and safety of measles vaccine administered alone or with live, attenuated Japanese encephalitis SA 14-14-2 vaccine in Philippine infants. Vaccine. 2008;26(18):2234-41. doi: 10.1016/j.vaccine.2008.02.042. PMID:18394765 [DOI] [PubMed] [Google Scholar]
- [9].World Health Organization Japanese encephalitis vaccines. Wkly Epidemiol Rec. 2006;81(34/35):331-40. PMID:16933380 [PubMed] [Google Scholar]
- [10].World Health Organization Global Advisory Committee on Vaccine Safety, 9–10 June 2005. Weekly Epidemiol Rec. 2005;80(28):242-7 [PubMed] [Google Scholar]
- [11].Konishi E, Kitai Y, Tabei Y, Nishimura K, Harada S. Natural Japanese encephalitis virus infection among humans in west and east Japan shows the need to continue a vaccination program. Vaccine. 2010;28(14):2664-70. doi: 10.1016/j.vaccine.2010.01.008. PMID:20080072 [DOI] [PubMed] [Google Scholar]
- [12].World Health Organization Summary of Key Points - WHO Position Paper on Vaccines against Japanese Encephalitis (JE) February 2015. 2015. [accessed 2016March28]. http://www.who.int/immunization/policy/position_papers/pp_je_feb2015_presentation.pdf?ua=1
- [13].Yu Y. Phenotypic and genotypic characteristics of Japanese encephalitis attenuated live vaccine virus SA14-14-2 and their stabilities. Vaccine. 2010;28(21):3635-41. doi: 10.1016/j.vaccine.2010.02.105. PMID:20226891 [DOI] [PubMed] [Google Scholar]
- [14].Desai K, Coudeville L, Bailleux F. Modelling the long-term persistence of neutralizing antibody in adults after one dose of live attenuated Japanese encephalitis chimeric virus vaccine. Vaccine. 2012;30(15):2510-5. doi: 10.1016/j.vaccine.2012.02.005. PMID:22342547 [DOI] [PubMed] [Google Scholar]
- [15].World Health Organization WHO | Japanese encephalitis. WHO. 2015. December [accessed 2016March15]. http://www.who.int/mediacentre/factsheets/fs386/en/
- [16].Tandan JB, Ohrr H, Sohn YM, Yoksan S, Ji M, Nam CM, Halstead SB. Single dose of SA 14-14-2 vaccine provides long-term protection against Japanese encephalitis: a case-control study in Nepalese children 5 years after immunization. drjbtandan@yahoo.com. Vaccine. 2007;25(27):5041-5. doi: 10.1016/j.vaccine.2007.04.052. PMID:17521781 [DOI] [PubMed] [Google Scholar]
- [17].Ma WX, Yu YX, Wang SG, Zhou BL, Ma YH, Feng SY. A large-scale study on the safety and serological efficacy of live attenuated Japanese encephalitis vaccine. Chinese J Biol. 1993;6(4):1-5 [Google Scholar]
- [18].Xin YY, Ming ZG, Peng GY, Jian A, Min LH. Safety of a live-attenuated Japanese encephalitis virus vaccine (SA14-14-2) for children. Am J Tropical Med Hygiene. 1988;39(2):214-7. doi: 10.4269/ajtmh.1988.39.214 [DOI] [PubMed] [Google Scholar]
- [19].World Health Organization Newly accessible Japanese encephalitis vaccine will make saving children easier in developing countries. Media Centre | WHO. 2013. [accessed 2016June11]. http://www.who.int/mediacentre/news/releases/2013/japanese_encephalitis_20131009/en/ [Google Scholar]
- [20].Gavi, the Vaccine Alliance Japanese encephalitis vaccines: Frequently asked questions February 2014 page. 2014. http://www.gavi.org/Library/Documents/GAVI-documents/Guidelines-and-forms/Japanese-Encephalitis-vaccines-FAQ/ [Google Scholar]
- [21].Wang Y, Dong D, Cheng G, Zuo S, Liu D, Du X. Post-marketing surveillance of live-attenuated Japanese encephalitis vaccine safety in China. Vaccine. 2014;32(44):5875-9. doi: 10.1016/j.vaccine.2014.08.001. PMID:25173477 [DOI] [PubMed] [Google Scholar]
- [22].Glovax Summary of Live attenuated SA14-14-2 Japanese Encephalitis vaccine post-marketing surveillance study in Korea (2002–2008). Seoul: Glovax Clinical Research and Regulation Regulatory Affairs; 2008 [Google Scholar]
- [23].Liu Y, Lin H, Zhu Q, Wu C, Zhao Z, Zheng H. Safety of Japanese encephalitis live attenuated vaccination in post-marketing surveillance in Guangdong, China, 2005–2012. Vaccine. 2014;32(15):1768-73. doi: 10.1016/j.vaccine.2013.11.107. PMID:24503272 [DOI] [PubMed] [Google Scholar]
- [24].Sohn YM, Park MS, Rho HO, Chandler LJ, Shope RE, Tsai TF. Primary and booster immune responses to SA14-14-2 Japanese encephalitis vaccine in Korean infants. Vaccine. 1999;17(18):2259-64. doi: 10.1016/S0264-410X(99)00006-7. PMID:10403593 [DOI] [PubMed] [Google Scholar]
- [25].Chotpitayasunondh T, Sohn YM, Yoksan S, Min J, Ohrr H. Immunizing children aged 9 to 15 months with live attenuated SA14-14-2 Japanese encephalitis vaccine in Thailand. J Medical Association of Thailand = Chotmaihet Thangphaet. 2011;94:S195-203 [PubMed] [Google Scholar]
- [26].Ranganath BG, Hiremath SG. Adverse events following immunisation with SA 14-14-2 Japanese encephalitis vaccine in children of Kolar in Karnataka. J Indian Medical Association. 2012;110(1):10-12 [PubMed] [Google Scholar]
- [27].Choi UY, Lee SY, Kim KH, Kim DS, Choi KM, Cha SH, Kang JH. Is a booster dose necessary in children after immunization with live attenuated Japanese encephalitis vaccine? J Trop Pediatr 2013;59(5), 423–5; doi: 10.1093/tropej/fmt043 [DOI] [PubMed] [Google Scholar]
- [28].Kwon HJ, Lee SY, Kim KH, Kim DS, Cha SH, Jo DS, Kang JH. The immunogenicity and safety of the Live-attenuated SA 14-14-2 Japanese Encephalitis vaccine given with a two-dose primary schedule in children. J Korean Med Sci. 2015;30(5):612-6. doi: 10.3346/jkms.2015.30.5.612. PMID:25931793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Sanchayan K, Fernandopulle R, Amarasinghe A, Thiyahiny SN, Ranganathan SS. Safety of live attenuated Japanese encephalitis vaccine given at the age of 9 months in National Immunisation Programme of Sri Lanka. Ceylon Medical J. 2016;61(3):99-105. doi: 10.4038/cmj.v61i3.8344 [DOI] [PubMed] [Google Scholar]
- [30].Wijesinghe PR, Abeysinghe MRN, Yoksan S, Yao Y, Zhou B, Zhang L, Fleming JA, Marfin AA, Victor JC. Immunogenicity of live attenuated Japanese encephalitis SA 14-14-2 vaccine among Sri Lankan children with previous receipt of inactivated JE vaccine. Vaccine. 2016;34(48):5923-8. doi: 10.1016/j.vaccine.2016.10.028. PMID:27773472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Wijesinghe PR, Abeysinghe MN, Yoksan S, Yao Y, Zhou B, Zhang L, Yaich M, Neuzil KM, Victor JC. Safety and immunogenicity of live-attenuated Japanese encephalitis SA 14-14-2 vaccine co-administered with measles vaccine in 9-month-old infants in Sri Lanka. Vaccine. 2014;32(37):4751-7. doi: 10.1016/j.vaccine.2014.06.036. PMID:24951871 [DOI] [PubMed] [Google Scholar]
- [32].Liu Z-L, Hennessy S, Strom BL, Tsai TF, Wan C-M, Tang S-C, Xiang C-F, Bilker WB, Pan X-P, Yao Y-J, et al.. Short-term safety of live attenuated Japanese encephalitis vaccine (SA14-14-2): results of a randomized trial with 26,239 subjects. J Infect Dis. 1997;176(5):1366-9. doi: 10.1086/517323. PMID:9359740 [DOI] [PubMed] [Google Scholar]
- [33].Feroldi E, Pancharoen C, Kosalaraksa P, Chokephaibulkit K, Boaz M, Meric C, Hutagalung Y, Bouckenooghe A. Primary immunization of infants and Toddlers in Thailand with Japanese Encephalitis Chimeric virus vaccine in comparison with SA14-14-2 a randomized study of immunogenicity and safety. Pediatric Infect Dis J. 2014;33(6):643-9. doi: 10.1097/INF.0000000000000276 [DOI] [PubMed] [Google Scholar]
- [34].Kim DS, Houillon G, Jang GC, Cha S-H, Choi S-H, Lee J, Kim HM, Kim JH, Kang JH, Kim J-H, et al.. A randomized study of the immunogenicity and safety of Japanese encephalitis chimeric virus vaccine (JE-CV) in comparison with SA14-14-2 vaccine in children in the Republic of Korea. Hum Vaccines Immunotherapeutics. 2014;10(9):2656-63. doi: 10.4161/hv.29743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Zaman K, Naser AM, Power M, Yaich M, Zhang L, Ginsburg AS, Luby SP, Rahman M, Hills S, Bhardwaj M, et al.. Lot-to-lot consistency of live attenuated SA 14-14-2 Japanese encephalitis vaccine manufactured in a good manufacturing practice facility and non-inferiority with respect to an earlier product. Vaccine. 2014;32(46):6061-6. doi: 10.1016/j.vaccine.2014.09.012. PMID:25239483 [DOI] [PubMed] [Google Scholar]
- [36].Haldar D, Das S, Bisoi S, Basu M, Roy SK, Sinha A. Post-marketing Surveillance on Safety of Japanese Encephalitis Vaccine SA 14-14-2 in Burdwan District of West Bengal, India. 2014. [accessed 2016March28]. http://www.journalofcomprehensivehealth.co.in/archive/jan2014/PDF/Original%20Article1.pdf
- [37].China Food and Drug Administration National Guideline for Surveillance of adverse events following immunization. [accessed 2017January12]. http://www.sda.gov.cn/WS01/CL0056/57717.html
- [38].Khan S, Kakati S, Dutta P, Chowdhury P, Borah J, Topno R, Jadhav S, Mohapatra P, Mahanta J, Gupte M. Immunogenicity & safety of a single dose of live-attenuated Japanese encephalitis vaccine SA 14-14-2 in adults. Indian J Medical Res. 2016;144(6):886. doi: 10.4103/ijmr.IJMR_712_15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Ministry of Health & Indigenous Medicine Change of the National Immunization Schedule: MMR, LJE vaccination. 2015. [accessed 2016September13]. http://www.epid.gov.lk/web/images/pdf/Circulars/MMR_LJEV/mmr_ljv_vaccination.pdf
- [40].ClinicalTrials.gov Immunogenicity of Co-administration of Measles and Japanese Encephalitis Vaccines. 2016. September 11 [accessed 2016September13]. https://clinicaltrials.gov/ct2/show/NCT02643433
- [41].ClinicalTrials.gov Immunogenicity and safety of Japanese encephalitis vaccine when given with measles-mumps-rubella vaccine. ClinicalTrials.gov. 2016. September 11 [accessed 2016September13]. https://clinicaltrials.gov/show/NCT02880865
- [42].Li X, Ma S-J, Liu X, Jiang L-N, Zhou J-H, Xiong Y-Q, Ding H, Chen Q. Immunogenicity and safety of currently available Japanese encephalitis vaccines: a systematic review. Hum Vaccines Immunotherapeutics. 2014;10(12):3579-93. doi: 10.4161/21645515.2014.980197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Wang S-Y, Cheng X-H, Li J-X, Li X-Y, Zhu F-C, Liu P. Comparing the immunogenicity and safety of 3 Japanese encephalitis vaccines in Asia-Pacific area: A systematic review and meta-analysis. Hum Vaccines Immunotherapeutics. 2015;11(6):1418-25. doi: 10.1080/21645515.2015.1011996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Gao X, Li X, Li M, Fu S, Wang H, Lu Z, Cao Y, He Y, Zhu W, Zhang T, et al.. Vaccine Strategies for the Control and Prevention of Japanese Encephalitis in Mainland China, 1951–2011. PLoS Neglected Tropical Dis. 2014. [accessed 2016June11];8(8):e3015 http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4133196/. doi: 10.1371/journal.pntd.0003015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Immunization Division, Department of Family Welfare, Ministry of Health and Family Welfare, Government of India Operational Guide Japanese Encephalitis Vaccination in India. 2010. [accessed 2016March15]. http://www.iapcoi.com/hp/Dec%2025th/Guidelines-Japanese-Encephalitis,%20MoHFW,%20September%202010%5B2%5D.pdf
- [46].SAS Post Marketing Study of primary immunization in Burdwan, West Bengal and Bellary, Karnataka (2006–2007). New Delhi: SAS; 2008 [Google Scholar]
- [47].Upreti SR, Janusz KB, Schluter WW, Bichha RP, Shakya G, Biggerstaff BJ, Shrestha MM, Sedai TR, Fischer M, Gibbons RV, et al.. Estimation of the Impact of a Japanese Encephalitis Immunization Program with Live, Attenuated SA 14-14-2 Vaccine in Nepal. Am J Tropical Med Hygiene. 2013;88(3):464-8. doi: 10.4269/ajtmh.12-0196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].VK R. AES/JE Surveillance - Directorate of National Vector Borne Disease Control Programme, Government of India Ministry of Health & Family Welfare. Presented at Bi-Regional Meeting on the Control of Japanese Encephalitis; 8 June 2009; Bangkok, Thailand. [Google Scholar]
- [49].Gavi, the Vaccine Alliance Japanese encephalitis vaccine support. [accessed 2016June11]. http://www.gavi.org/support/nvs/japanese-encephalitis/
- [50].UNICEF - Media Centre Nation-wide Japanese Encephalitis vaccination campaign launched in Cambodia and to be introduced into routine immunization schedule. UNICEF. 2016. [accessed 2017January11]. https://www.unicef.org/cambodia/12681_25295.html


