ABSTRACT
Plasma membrane microdomains are plasma membrane sub-compartments enriched in sphingolipids and sterols, and composed by a specific set of proteins. They are involved in recognizing signal molecules, transducing these signals, and controlling endocytosis and exocytosis processes. In a recent study, applying biochemical and microscopic methods, we characterized the soybean GmFWL1 protein, a major regulator of soybean nodulation, as a new membrane microdomain-associated protein. Interestingly, upon rhizobia inoculation of the soybean root system, GmFWL1 and one of its interacting partners, GmFLOT2/4, both translocate to the root hair cell tip, the primary site of interaction and infection between soybean and Rhizobium. The role of GmFWL1 as a plasma membrane microdomain-associated protein is also supported by immunoprecipitation assays performed on soybean nodules, which revealed 178 GmFWL1 protein partners including a large number of microdomain-associated proteins such as GmFLOT2/4. In this addendum, we provide additional information about the identity of the soybean proteins repetitively identified as GmFWL1 protein partners. Their function is discussed especially in regard to plant-microbe interactions and microbial symbiosis. This addendum will provide new insights in the role of plasma membrane microdomains in regulating legume nodulation.
KEYWORDS: Flotillins, FWL1, legume nodulation, plasma membrane microdomains
Plasma membrane microdomains
The plasma membrane plays an essential role in the perception and transduction of environmental signals and in cell-to-cell communication. In the plasma membrane, microdomains are sub-compartments, which display unique lipid and protein compositions. Their biologic function is distinct from the surrounding plasma membrane. For instance, plasma membrane microdomains are proposed to regulate, among others, signal transduction, exocytosis and endocytosis, and actin cytoskeleton organization.1
Role of microdomain-associated proteins during legume nodulation
We recently characterized GmFWL1 (Glyma.09G187000), a soybean protein regulating nodulation,2 as a microdomain-associated proteins.3 Notably, we identified 178 soybean proteins interacting with GmFWL1 from isolated nodules. Among them, 10 proteins were repetitively characterized as GmFWL1 partners across the three independent biologic replicates (Table 1). Mining the soybean transcriptome atlas,4 the genes encoding these GmFWL1 partners were highly and preferentially expressed in underground tissue, including three genes highly preferentially expressed in soybean nodules (Glyma.05G029800, Glyma.06G065600, and Glyma.13G065000). These three genes encode SPFH/Band 7/PHB domain–containing membrane-associated proteins, well-characterized plasma membrane microdomain-associated proteins. Interestingly, the Arabidopsis thaliana rthologs of Glyma.13G065000 and Glyma.05G029800, HYPERSENSITIVE INDUCED REACTION 1 (AtHIR1, AT1G69840) and HYPERSENSITIVE INDUCED REACTION 4 (AtHIR4, At5g62740), respectively, were previously characterized for their role in plant immunity.5,6 Notably, supporting its role as part of the plant defense system, AtHIR1 encodes a plasma membrane-associated protein capable to oligomerize upon pathogen perception.5
Table 1.
Transcriptional activity of 10 soybean genes encoding GmFWL1 protein partners (Libault et al, 2010). Color code shows the expression level from low (green color) to high (red color). SAM: shoot apical meristem, HAI: hour after innoculation; UN/IN: B. japonicum un/inoculated; RH: root hair cell.
 |
Another GmFWL1 protein partner is the soybean GmFLOT2/4 (Glyma.06G065600) which is encoded by a gene orthologous to the Medicago truncatula FLOT2 and FLOT4 genes.3 These two genes encode regulators of the early events of the Medicago nodulation process.7 In Medicago, the “flotillin” family is composed by 11 members (e-value<2.3e-169 for 10 out the 11 member; Medtr3g065540 e-value is 1.8e-36 due to its shorter coding sequence). Among them, pairs of tandem duplicated flotillin genes were repetitively noticed (Fig. 1). Mining the soybean genome, we identified only two flotillin genes, Glyma.06G065600 (e-value = 2.5e-256) and Glyma.04G064400 (e-value = 4.1e-21), which encode 482 and 97 amino acid proteins, respectively. Due to its short coding sequence and relatively low expression in soybean tissues, Glyma.04G064400 could be a potential pseudogene. In Arabidopsis, AtFLOT1 (At5g25250) has been characterized for its role in the endocytosis process independently of the clathrin mediation.8 Similar function in plants is also supported by studies conducted on mammalian systems where flotillin proteins are playing an active role during endocytosis.9 Hypothesizing that soybean flotillin might also control endocytosis, it is attempting to suggest that FWL1 and FLOT2/4 may form a protein complex to mediate the formation then elongation of the infection thread, a tubular structure allowing the infection of the plant by the symbiotic bacteria, and, ultimately, the endocytosis of the bacteroids into the infected plant nodule cells.
Figure 1.
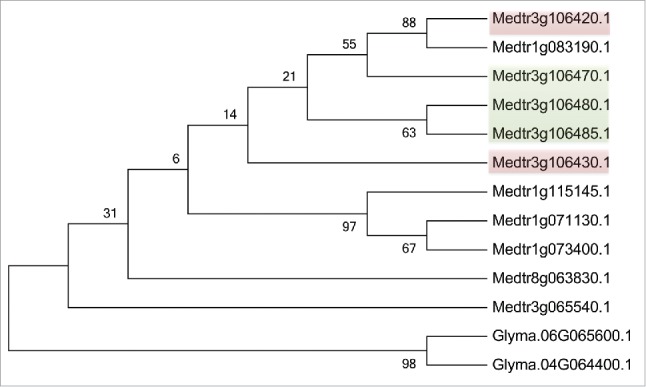
Phylogenetic analysis of flotillin family members in Medicago truncatula and Glycine max. Deduced amino acid sequences were aligned using ClustalW2 and the maximum likelihood phylogenetic tree was generated based on the JTT matrix-based model. The bootstrap consensus tree inferred from 100 replicates. Color boxes highlight tandemly duplicated genes.
Beside FWL1 and flotillin proteins, remorins, which are also well-characterized plasma membrane microdomain-associated proteins, are also controlling the nodulation process. In Medicago, protein complementation assays revealed the interaction between SYMREM1 and Nod factor receptor like kinases (NFP, LYK3 and DMI2), suggesting a potential role of SYMREM1 in Nod factor signal perception.10 Considering that in Medicago, MtFLOT4 and MtLYK3 co-localized in the root hair cell tip in response to rhizobia infection, and that the soybean GmFLOT2/4 and GmFWL1 display a similar translocation at the tip of the root hair upon rhizobial inoculation, we hypothesize that FWL1, flotillin, and remorin work together with Nod factor receptors during the early stages of rhizobial infection.
Microdomain-associated protein FWL1 interaction partners
The H+-ATPase protein encoded by Glyma.09 g056300 interacts with GmFWL1. Glyma.09 g056300 is preferentially expressed in underground tissue (i.e., the root system and root hair cells) (Table 1). Its homolog in Arabidopsis, AtAHA1, regulates the guard cell turgor pressure by interacting other plasma membrane-associated protein.11 The regulation of stomata aperture is the consequence of environmental changes, such as drought stress, and light perception.12,13 In addition, in response to pathogens, AtAHA1 also regulates the jasmonic acid pathway to mediate microbial infection through the stomata.14 Supporting the role of H+-ATPase during nodulation, the Medicago H+-ATPase MtAHA5 has been characterized as a regulator of the very early stage of the nodulation process: at the time of the interaction between Medicago plants and their symbiotic rhizobia.15
Plasma membrane intrinsic proteins (PIP) are also important in controlling the nodulation process based on the interaction of the proteins encoded by the Glyma.8G015300, Glyma.02G073600 and Glyma.14G061500 genes, and GmFWL1 (Table 1). These proteins belong to the PIP1 and PIP2 subgroups. The PIP family proteins are involved in plant response to environmental stresses, such as water deficiency, salt, heavy metal and cold stresses.16 For instance, GmPIP1;6 confers salt tolerance to soybean plants.17 In addition, PIPs are also important in controlling plant response to biotic stresses, such as plant-microbe symbiosis and plant immune response to pathogens.18-20 Furthermore, in Arabidopsis, Li et al. (2011) reported that the PIP2;1 aquaporin co-localizes and co-migrates with FLOT1, supporting the location of PIP2 into plasma membrane microdomains.21
Three vacuolar ATPase proteins were also repetitively identified as GmFWL1 partners in nodules (Table 1). These three proteins belong to different components of the ATPase complex: Glyma.08G218500, Glyma.08G224400, and Glyma.05G213800 encode a D, an A, and an E isoform 3 subunits, respectively. The vacuolar ATPases are proton pumps that responsible for acidification of intracellular compartments, including endosomes, lysosomes, phagosomes.22 In Arabidopsis, the vacuolar H+-ATPases, A subunit, A1 are required for secretory and endocytic trafficking.23
Conclusions and perspectives
These ten soybean proteins repetitively interacting with GmFWL1 in nodules belong to four protein families. Among them, only flotillins have been well characterized for their role during legume nodulation,7 while H+-ATPase, PIP and vacuolar ATPase are described as regulators of plant responses to biotic and abiotic stresses. Notably, most of them are channel proteins located in the plasma membrane allowing the regulation of plant cell homeostasis. In addition, it is important to note that flotillins, PIPs and vacuolar ATPases regulate the endocytosis process or membrane internalization, processes that relevant to the infection of plant cell by nitrogen-fixing symbiotic bacteria. Such observation suggests that plasma membrane microdomians might play a critical role in the recognition of the symbiotic bacteria, then in the initiation and progression of the infection thread. Ultimately, microdomains could also regulate the final endocytosis-like process occurring in the nodule and resulting in the formation of the symbiosomes.
Funding
This work was supported by the National Science Foundation under Grant IOS-1339194.
References
- 1.Falk J, Thoumine O, Dequidt C, Choquet D, Faivre-Sarrailh C. NrCAM coupling to the cytoskeleton depends on multiple protein domains and partitioning into lipid rafts. Mol Biol Cell. 2004;15:4695–709. doi: 10.1091/mbc.E04-03-0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Libault M, Zhang XC, Govindarajulu M, Qiu J, Ong YT, Brechenmacher L, Berg RH, Hurley-Sommer A, Taylor CG, Stacey G, et al.. A member of the highly conserved FWL (tomato FW2.2-like) gene family is essential for soybean nodule organogenesis. Plant J. 2010;62:852–64. doi: 10.1111/j.1365-313X.2010.04201.x. [DOI] [PubMed] [Google Scholar]
- 3.Qiao Z, Brechenmacher L, Smith B, Strout GW, Mangin W, Taylor C, Russell SD, Stacey G, Libault M.. The GmFWL1 (FW2-2-like) nodulation gene encodes a plasma membrane microdomain associated protein. Plant Cell Environ. 2017;40:1442–1455. doi: 10.1111/pce.12941. [DOI] [PubMed] [Google Scholar]
- 4.Libault M, Farmer A, Joshi T, Takahashi K, Langley RJ, Franklin LD, He J, Xu D, May G, Stacey G. An integrated transcriptome atlas of the crop model Glycine max, and its use in comparative analyses in plants. Plant J. 2010;63:86–99. [DOI] [PubMed] [Google Scholar]
- 5.Qi Y, Tsuda K, Nguyen le V, Wang X, Lin J, Murphy AS, Glazebrook J, Thordal-Christensen H, Katagiri F. Physical association of Arabidopsis hypersensitive induced reaction proteins (HIRs) with the immune receptor RPS2. J Biol Chem. 2011;286:31297–307. doi: 10.1074/jbc.M110.211615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Faulkner C. Receptor-mediated signaling at plasmodesmata. Front Plant Sci. 2013;4:521.doi: 10.3389/fpls.2013.00521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haney CH, Long SR. Plant flotillins are required for infection by nitrogen-fixing bacteria. Proc Natl Acad Sci U S A. 2010;107:478–83. doi: 10.1073/pnas.0910081107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li R, Liu P, Wan Y, Chen T, Wang Q, Mettbach U, Baluska F, Samaj J, Fang X, Lucas WJ, et al.. A membrane microdomain-associated protein, Arabidopsis Flot1, is involved in a clathrin-independent endocytic pathway and is required for seedling development. Plant Cell. 2012;24:2105–22. doi: 10.1105/tpc.112.095695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Otto GP & Nichols BJ. The roles of flotillin microdomains–endocytosis and beyond. J Cell Sci. 2011;124:3933–40.doi: 10.1242/jcs.092015. [DOI] [PubMed] [Google Scholar]
- 10.Lefebvre B, Timmers T, Mbengue M, Moreau S, Hervé C, Tóth K, Bittencourt-Silvestre J, Klaus D, Deslandes L, Godiard L, et al.. A remorin protein interacts with symbiotic receptors and regulates bacterial infection. Proc Natl Acad Sci U S A. 2010;107:2343–8. doi: 10.1073/pnas.0913320107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hashimoto-Sugimoto M, Higaki T, Yaeno T, Nagami A, Irie M, Fujimi M, Miyamoto M, Akita K, Negi J, Shirasu K, et al.. A Munc13-like protein in Arabidopsis mediates H+-ATPase translocation that is essential for stomatal responses. Nat Commun. 2013;4:2215. doi: 10.1038/ncomms3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vasseur F, Pantin F, Vile D. Changes in light intensity reveal a major role for carbon balance in Arabidopsis responses to high temperature. Plant Cell Environ. 2011;34:1563–76. doi: 10.1111/j.1365-3040.2011.02353.x. [DOI] [PubMed] [Google Scholar]
- 13.Higaki T, Hashimoto-Sugimoto M, Akita K, Iba K, & Hasezawa S. Dynamics and environmental responses of PATROL1 in Arabidopsis subsidiary cells. Plant and Cell Physiology. 2013;55(4):773–80. doi: 10.1093/pcp/pct151. [DOI] [PubMed] [Google Scholar]
- 14.Zhou Z, Wu Y2, Yang Y2, Du M1, Zhang X1, Guo Y2, Li C1, Zhou JM. An Arabidopsis plasma membrane proton ATPase modulates JA signaling and is exploited by the Pseudomonas syringae effector protein AvrB for stomatal invasion. Plant Cell. 2015;27:2032–41. doi: 10.1105/tpc.15.00466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nguyen TT, Volkening JD, Rose CM, Venkateshwaran M, Westphall MS, Coon JJ, Ané JM, Sussman MR. Potential regulatory phosphorylation sites in a Medicago truncatula plasma membrane proton pump implicated during early symbiotic signaling in roots. FEBS Lett. 2015;589:2186–93. doi: 10.1016/j.febslet.2015.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith-Espinoza C, Richter A, Salamini F, Bartels D. Dissecting the response to dehydration and salt (NaCl) in the resurrection plant Craterostigma plantagineum. Plant Cell Environm. 2003;26:1307–15.doi: 10.1046/j.0016-8025.2003.01055.x. [DOI] [Google Scholar]
- 17.Zhou L, Wang C, Liu R, Han Q, Vandeleur RK, Du J, Tyerman S, Shou H. Constitutive overexpression of soybean plasma membrane intrinsic protein GmPIP1; 6 confers salt tolerance. BMC Plant Biol. 2014;14:181. doi: 10.1186/1471-2229-14-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zou J, Rodriguez-Zas S, Aldea M, Li M, Zhu J, Gonzalez DO, Vodkin LO, DeLucia E, Clough SJ. Expression profiling soybean response to Pseudomonas syringae reveals new defense-related genes and rapid HR-specific downregulation of photosynthesis. Molec Plant-Microbe Interact. 2005;18:1161–74. doi: 10.1094/MPMI-18-1161. [DOI] [PubMed] [Google Scholar]
- 19.Bárzana G, Aroca R, Bienert GP, Chaumont F, Ruiz-Lozano JM. New insights into the regulation of aquaporins by the arbuscular mycorrhizal symbiosis in maize plants under drought stress and possible implications for plant performance. Mol Plant-Microbe Interact. 2014;27:349–63. doi: 10.1094/MPMI-09-13-0268-R. [DOI] [PubMed] [Google Scholar]
- 20.Aroca R, Porcel R, & Ruiz-Lozano JM. How does arbuscular mycorrhizal symbiosis regulate root hydraulic properties and plasma membrane aquaporins in Phaseolus vulgaris under drought, cold or salinity stresses? New Phytol. 2007;173:808–16. doi: 10.1111/j.1469-8137.2006.01961.x. [DOI] [PubMed] [Google Scholar]
- 21.Li X, Wang X, Yang Y, Li R, He Q, Fang X, Luu DT, Maurel C, Lin J. Single-molecule analysis of PIP2; 1 dynamics and partitioning reveals multiple modes of Arabidopsis plasma membrane aquaporin regulation. Plant Cell. 2011;23:3780–97. doi: 10.1105/tpc.111.091454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maxson ME & Grinstein S. The vacuolar-type H+-ATPase at a glance–more than a proton pump. 2014;127:4987–4993. doi: 10.1242/jcs.158550. [DOI] [PubMed] [Google Scholar]
- 23.Dettmer J, Hong-Hermesdorf A, Stierhof Y-D, Schumacher K. Vacuolar H+-ATPase activity is required for endocytic and secretory trafficking in Arabidopsis. Plant Cell. 2006;18:715–30. doi: 10.1105/tpc.105.037978. [DOI] [PMC free article] [PubMed] [Google Scholar]


