ABSTRACT
Soil salinity-alkalinity is one of abiotic stresses that lead to plant growth inhibition and yield loss. It has recently been indicated that plant growth promoting rhizobacteria (PGPR) can enhance the capacity of plants to counteract negative effects caused by adverse environments. However, whether PGPR confers increased saline-alkaline resistance of plants and the underlying mechanisms remain unclear. We thus investigated the effects of Bacillus licheniformis (strain SA03) on Chrysanthemum plants grown under saline-alkaline conditions. Soil inoculation with SA03 significantly mitigated saline-alkaline stress in plants with augmented photosynthesis, biomass and survival rates. Moreover, the inoculated plants accumulated more Fe and less Na+ content than the non-inoculated plants under the stress. However, the inoculation with SA03 failed to trigger a series of saline-alkaline stress responses in abscisic acid (ABA)- and nitric oxide (NO)-deficient plants. Furthermore, NO acted as a secondary messenger of ABA to regulate the stress responses and tolerance in Chrysanthemum plants. Therefore, these findings indicated that B. licheniformis SA03 could be employed to improve saline-alkaline tolerance of plants by mediating cellular ABA levels.
KEYWORDS: Abscisic acid, iron acquisition, plant growth promoting rhizobacteria, soil alkalinity
Soil salinity and alkalinity often exist meanwhile because of the complexity of soil property.1 It is estimated that over 800 million hectares of soils are seriously affected by excessive salinity-alkalinity worldwide, including 434 and 397 million hectares of saline and alkaline soils.2 Soil alkalization mainly results from the accumulation of NaHCO3 and Na2CO3.3 Salinity-alkalinity has increasingly become a major limiting factor for plant growth and yield in semi-arid and arid areas.4-6 Plants have to experience high pH, ionic toxicity, and osmotic stress simultaneously under saline-alkaline stress.4-6 Until now, great progress has been gained in understanding the mechanisms of plant adaption to salt stress.7-9 However, lack of researches exists on the mechanisms of plants to tolerate saline-alkaline stress.
Many works have recently been made to improve abiotic stress tolerance mainly via transgene technology, whereas transgenic plants must face rigorous food and environmental safety trials.9-11 In addition, application of several important molecules such as polyamines and ABA can also increase the resistance of plants to various stresses.12-14 However, these experiments are often confined to small field regions because of its high cost. Hence, it is an urgent need to develop an efficient strategy to improve the capability of plants to resist saline-alkaline stress. During long-term evolution, plants can recruit a wide range of beneficial soil microbes to colonize in the rhizosphere, and this mutualistic interaction can help plants survival under unfavorable environments. Beneficial soil microbes can regulate plant growth, and improve abiotic and biotic stress tolerance in plants.15-17 Use of soil bacteria to induce plant stress resistance may offer some striking advantages: (1) low costing of isolation, propagation and inoculation of soil bacteria; (2) several bacterial strains can benefit both monocots and dicot plants; (3) the capability of one stress-resistant plant species can be easily transferred to the others by soil inoculation. Thus, it will be a great potential to utilize soil microbes for improving saline-alkaline tolerance of plants.
Our recent study revealed that the capability of plants to tolerate saline-alkaline stress was significantly enhanced by beneficial soil bacteria, implying that this bacteria strain (Bacillus licheniformis SA03) might regulate certain signal transduction pathways for plant's adaptation to adverse conditions.18 However, the processes of plant-microbe interaction are exceedingly complicate. To better understand the mechanisms of the microbe-regulated stress tolerance, transcriptomic analyses were used to identify DEGs between the control and inoculated plants. A majority of some genes associated with abiotic stresses such as water deprivation, salt stress and wounding was observed in the upregulated DEGs. It is well known that ABA plays a cardinal role in modulating abiotic stress responses and tolerance in various plant species.19 Thus, we speculated that B. licheniformis SA03 conferred increased saline-alkaline stress resistance partially by mediating ABA-dependent signaling pathways.
In fact, plants inevitably escape two major problems including Fe uptake and Na+ toxicity under saline-alkaline stress.4-6,20 Soil inoculation with B. licheniformis SA03 improved saline-alkaline tolerance, which likely contributed to controlling cellular Fe and Na+ homeostasis effectively.18 As expectedly, the SA03-inoculated plants had more shoot and root Fe contents compared with the controls under the stress, indicating that the inoculated plants owned a strong Fe acquisition machine. Saline-tolerant plants often maintain low shoot Na+ content and a concurrent high K+ content.19,21 Our recent study showed that the SA03-inoculated plants exhibited extremely lower Na+/K+ ratio than the controls under saline-alkaline stress.18 Importantly, cellular ABA levels are positively related to the accumulation of Na+ and K+ in plants. Increased ABA levels significantly inhibits net K+ efflux and concurrently induces net Na+ efflux and H+ influx in plants by modulating several Na+/H+ and K+ antiporters/channels, implying that a fine regulation of cellular ABA levels is an adaptive strategy for plants to cope with negative effects imposed by salt stress.19 Intriguingly, exogenous ABA increases the reutilization and transport of Fe from roots to shoots in Fe-deficient Arabidopsis plants.22 Additionally, ABA is involved in the regulation of the activity of plasma membrane H+-ATPase, thereby mediating the release of protons to plant rhizospere.23 A result of our study has shown that the inoculation of plants with B. licheniformis SA03 can markedly increase the activity of plasma membrane H+-ATPase under saline-alkaline stress.18 Higher activity of H+-ATPase may enhance rhizospheric acidification for counteracting high soil pH and increasing Fe availability, indicating that ABA-induced a great increase of H+-ATPase activity helps plants adapt to saline-alkaline conditions.
Due to unknown genome sequence information about Chrysanthemum morifolium vs Chuju, it was difficult to develop several ABA-deficient or -insensitive mutants. Pharmacological analyses were thus used to examine the effects of ABA on the resistance of plants to saline-alkaline stress. This result indicated that the SA03-inoculated plants treated with FLU, an inhibitor of ABA biosynthesis, exhibited similar phenotypes with the controls under saline-alkaline stress.18 Moreover, the content of ABA was significantly higher in the inoculated plants than the controls under saline-alkaline stress. However, treatments with FLU abolished beneficial effects of SA03 on the growth of host plants under the stress, as evidenced by changes of physiological indexes. Similarly, FLU exposure remarkably increased alkaline-mediated damages to plant cells, implying that cellular ABA levels were associated with the ability of plants to resist alkaline stress.14
The inoculation with SA03 evidently affected the accumulation of NO in Chrysanthemum plants under saline-alkaline stress, which was consistent with the changing tendency of cellular ABA.18 NO has well been demonstrated to regulate abiotic stress responses in plants, which tightly collaborates with ABA and other hormones.24-26 However, the mechanisms by which NO, in coordination with ABA, modulate the impact of abiotic stress remains elusive. So, does ABA act upstream of NO to regulate a series of saline-alkaline response in SA03-inoculated plants? Previously, NO functions as a second messenger of ABA to modulate salt stress responses in plants by enhancing the activities of antioxidant enzymes and Na+/H+ antiporters.27 However, NO negatively regulate the perception of ABA, and function as a vital factor to finely mediate the intensity or magnitude of ABA-stimulated responses.28 It remained unknown how the functional interactions between NO and ABA contributed to SA03-regulated stress responses in plants. For this purpose, cellular NO levels were examined in the inoculated plants exposed to FLU, showing that the biosynthesis of NO was seriously repressed in the FLU-treated plants under saline-alkaline stress.18 Moreover, treatments with c-PTIO, a scavenger of NO, markedly downregulated the transcription of Fe acquisition- and Na+ transported-related genes in the inoculated plants.18 There was indistinct difference in phenotypic and physiological traits between the FLU- and c-PTIO-treated inoculated plants. More importantly, c-PTIO exposure did not affect cellular ABA levels in the inoculated plants under saline-alkaline stress.18 Thus, these results indicated that SA03 conferred saline-alkaline resistance in host plants, involving the activation of ABA-mediated NO signaling pathways.
Based on our recent findings, a proposed model that illustrates SA03-regulated saline-alkaline stress responses was shown (Fig. 1). Soil salinity-alkalinity results in low Fe availability and high Na+ toxicity for plants. The inoculation of plants with SA03 remarkably ameliorated adverse effects imposed by saline-alkaline stress. Combined transcriptomic, biochemical, and pharmacological analyses indicated that the inoculation with SA03 significantly improved the tolerance of plants to saline-alkaline stress through stimulation of ABA-induced NO synthesis. Although induction of ABA accumulation in host plants by SA03 was involved in activating a series of adaptive mechanisms, some unclear questions remained: How is cellular ABA levels in plants mediated by this bacterial strain SA03? Does ABA function as a secondary messenger of certain molecules released by SA03 to regulate saline-alkaline stress responses? To clarify the mechanisms by which this bacterial strain SA03 regulated saline-alkaline stress responses of host plants, further investigation is required.
Figure 1.
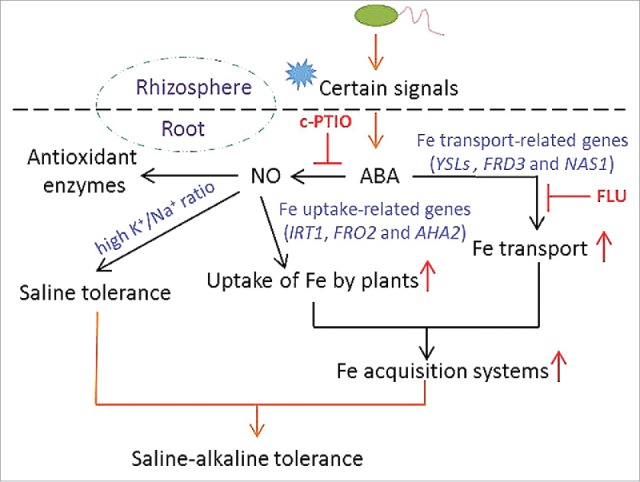
A schematic representation of B. licheniformis SA03-induced saline-alkaline tolerance in Chrysanthemum plants. The inoculation with SA03 mitigates adverse impacts caused by saline-alkaline stress in plants by modulating cellular ABA levels. Soil inoculation triggered a series of adaptive mechanisms such as increased antioxidant enzymatic activities, increased Fe acquisition, and decreased Na+ accumulation in plants, which were primarily associated with NO-mediated signaling pathways. Red upright arrows indicate increase in effects.
Disclosure of potential conflicts of interest
No potential conflicts of interest are disclosed.
Funding
We are grateful for financial support from the National Natural Science Foundation of China (31600210, 21607002), the Research Foundation of the Ministry of Agriculture (BOFC2015KB02), the National Sparking Plan Project (2015GA710013, 2015GA710014), the Key Research Project of the Anhui Science and Technology Committee (1301032151, 15CZZ03102), the Natural Science Foundation of Anhui Province (1508085QD74, 1608085MC59), and the Research Foundation of Anhui Science and Technology University (ZRC2014403).
Abbreviations
- ABA
abscisic acid
- c-PTIO
2-(4-carboxyphenyl)-4,4,5,5–tetramethylimidazoline -1-oxyl-3-oxide
- DEGs
differentially expressed genes
- FLU
fluridone
- NO
nitric oxide
References
- 1.Zhang Y, Zhang H, Zou ZR, Liu Y, Hu XH. Deciphering the protective role of spermidine against saline-alkaline stress at physiological and proteomic levels in tomato. Phytochemistry. 2015;110:13–21. doi: 10.1016/j.phytochem.2014.12.021. [DOI] [PubMed] [Google Scholar]
- 2.Martinez-Beltran J, Manzur CL. Overview of salinity problems in the world and FAO strategies to address the problem. In Proceedings of the International Salinity Forum, April 25-27. Riverside, California. 2005:311–13 [Google Scholar]
- 3.Shi D, Sheng Y. Effect of various salt-alkaline mixed stress conditions on sunflower seedlings and analysis of their stress factors. Environ Exp Bot. 2005;54:8–21. doi: 10.1016/j.envexpbot.2004.05.003 [DOI] [Google Scholar]
- 4.Li Q, Yang A, Zhang WH. Efficient acquisition of iron confers greater tolerance to saline-alkaline stress in rice (Oryza sativa L.). J Exp Bot. 2016;67:6431–44. doi: 10.1093/jxb/erw407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang CW, Xu HH, Wang LL, Liu J, Shi D, Wang DL. Comparative effects of salt-stress and alkali-stress on the growth, photosynthesis, solute accumulation, and ion balance of barley plants. Photosynthetica. 2009;47:79–86. doi: 10.1007/s11099-009-0013-8 [DOI] [Google Scholar]
- 6.Zhou C, Guo JS, Zhu L, Xiao X, Xie Y, Zhu J, Ma ZY, Wang JF. Paenibacillus polymyxa BFKC01 enhances plant iron absorption via improved root systems and activated iron acquisition mechanisms. Plant Physiol Biochem. 2016;105:162–73. doi: 10.1016/j.plaphy.2016.04.025. [DOI] [PubMed] [Google Scholar]
- 7.Sun XC, Xu L, Wang Y, Luo XB, Zhu XW, Kinuthia KB, Nie SS, Feng HY, Li C, Liu LW. Transcriptome-based gene expression profiling identifies differentially expressed genes critical for salt stress response in radish (Raphanus sativus L). Plant Cell Rep. 2016;35:329–46. doi: 10.1007/s00299-015-1887-5. [DOI] [PubMed] [Google Scholar]
- 8.Takahashi R, Nishio T, Ichizen N, Takano T. Salt-tolerant reed plants contain lower Na+ and higher K+ than salt-sensitive reed plants. Acta Physiol Plant. 2007;29:431–38. doi: 10.1007/s11738-007-0052-3 [DOI] [Google Scholar]
- 9.Tang RJ, Yang Y, Yang L, Liu H, Wang CT, Yu MM, Gao XS, Zhang HX. Poplar calcineurin B-like proteins PtCBL10A and PtCBL10B regulate shoot salt tolerance through interaction with PtSOS2 in the vacuolar membrane. Plant Cell Environ. 2014;37:573–88. doi: 10.1111/pce.12178. [DOI] [PubMed] [Google Scholar]
- 10.Zhu L, Guo J, Zhu J, Zhou C. Enhanced expression of EsWAX1 improves drought tolerance with increased accumulation of cuticular wax and ascorbic acid in transgenic Arabidopsis. Plant Physiol Biochem. 2014;75:24–35. doi: 10.1016/j.plaphy.2013.11.028. [DOI] [PubMed] [Google Scholar]
- 11.Eisenstein M. Discovery in a dry spell. Nature. 2013;501:S7–S9. doi: 10.1038/501S7a. [DOI] [PubMed] [Google Scholar]
- 12.Savvides A, Ali S, Tester M, Fotopoulos V. Chemical priming of plants against multiple abiotic stresses: mission possible? Trends Plant Sci. 2016;21:329–40. doi: 10.1016/j.tplants.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 13.Zhu XF, Wang B, Song WF, Zheng SJ, Shen RF. Putrescine alleviates iron deficiency via NO-dependent reutilization of root cell-wall Fe in Arabidopsis. Plant Physiol. 2016;170:558–67. doi: 10.1104/pp.15.01617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wei LX, Lv BS, Wang MM, Ma HY, Yang HY, Liu XL, Jiang CJ, Liang ZW. Priming effect of abscisic acid on alkaline stress tolerance in rice (Oryza sativa L.) seedlings. Plant Physiol Biochem. 2015;90:50–7. doi: 10.1016/j.plaphy.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 15.Zhou C, Zhu L, Ma ZY, Wang JF. Bacillus amyloliquefaciens SAY09 increases cadmium resistance in plants by activation of auxin-mediated signaling pathways. Genes. 2017;8:173. doi: 10.3390/genes8070173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang JF, Zhou C, Xiao X, Xie Y, Zhu L, Ma ZY. Enhanced iron and selenium uptake in plants by volatile emissions of Bacillus amyloliquefaciens (BF06). Appl Sci. 2017;71:85. doi: 10.3390/app7010085 [DOI] [Google Scholar]
- 17.Zhou C, Ma ZY, Zhu L, Xiao X, Xie Y, Zhu J, Wang JF. Rhizobacterial strain Bacillus megaterium BOFC15 induces cellular polyamine changes that improve plant growth and drought resistance. Int J Mol Sci. 2016;17:976. doi: 10.3390/ijms17060976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou C, Zhu L, Xie Y, Li FY, Xiao X, Ma ZY, Wang JF. Bacillus licheniformis SA03 confers increased saline-alkaline tolerance in Chrysanthemum plants by induction of abscisic acid accumulation. Front Plant Sci. 2017;8:1143. doi: 10.3389/fpls.2017.01143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang J, Yu H, Zhang Y, Wang Y, Li M, Zhang J, Duan L, Zhang M, Li Z. Increased abscisic acid levels in transgenic maize overexpressing AtLOS5 mediated root ion fluxes and leaf water status under salt stress. J Exp Bot. 2016;67:1339–55. doi: 10.1093/jxb/erv528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abadía J, Vazquez S, Rellan-Alvarez R, El-Jendoubi H, Abadía A, Alvarez-Fernandez A, Lopez-Millan AF. Towards a knowledge-based correction of iron chlorosis. Plant Physiol Biochem. 2011;49:471–82. doi: 10.1016/j.plaphy.2011.01.026. [DOI] [PubMed] [Google Scholar]
- 21.Anschütz U, Becker D, Shabala S. Going beyond nutrition: regulation of potassium homoeostasis as a common denominator of plant adaptive responses to environment. J Plant Physiol. 2014;171:670–87. doi: 10.1016/j.jplph.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 22.Lei GJ, Zhu XF, Wang ZW, Dong F, Dong NY, Zheng SJ. Abscisic acid alleviates iron deficiency by promoting root iron reutilization and transport from root to shoot in Arabidopsis. Plant Cell Environ. 2014;37:852–63. doi: 10.1111/pce.12203. [DOI] [PubMed] [Google Scholar]
- 23.Xu W, Jia L, Shi W, Liang J, Zhou F, Li Q, Zhang J. Abscisic acid accumulation modulates auxin transport in the root tip to enhance proton secretion for maintaining root growth under moderate water stress. New Phytol. 2013;197:139–50. doi: 10.1111/nph.12004. [DOI] [PubMed] [Google Scholar]
- 24.León J, Castillo MC, Coego A, Lozano-Juste J, Mir R. Diverse functional interactions between nitric oxide and abscisic acid in plant development and responses to stress. J Exp Bot. 2014;65:907–21. doi: 10.1093/jxb/ert454. [DOI] [PubMed] [Google Scholar]
- 25.Tanou G, Molassiotis A, Diamantidis G. Hydrogen peroxide- and nitric oxide-induced systemic antioxidant prime-like activity under NaCl-stress and stress-free conditions in citrus plants. J Plant Physiol. 2009;166:1904–13. doi: 10.1016/j.jplph.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 26.Chen WW, Yang JL, Qin C, Jin CW, Mo JH, Ye T, Zheng SJ. Nitric oxide acts downstream of auxin to trigger root ferric-chelate reductase activity in response to iron deficiency in Arabidopsis. Plant Physiol. 2010;154:810–9. doi: 10.1104/pp.110.161109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Y, Tan J, Guo Z, Lu S, He S, Shu W, Zhou B. Increased abscisic acid levels in transgenic tobacco over-expressing 9 cis-epoxycarotenoid dioxygenase influence H2O2 and NO production and antioxidant defences. Plant Cell Environ. 2009;32:509–19. doi: 10.1111/j.1365-3040.2009.01945.x. [DOI] [PubMed] [Google Scholar]
- 28.Lozano-Juste J, León J. Nitric oxide modulates sensitivity to ABA. Plant Signal Behav. 2010;5:314–6. doi: 10.1104/pp.109.148023. [DOI] [PMC free article] [PubMed] [Google Scholar]


