ABSTRACT
Single-bud cuttings of Vitis vinifera L exposed to forced growing conditions were used to investigate the involvement of phytohormones, abscisic acid (ABA), auxin (Aux) and cytokinin (CK) in the release of buds from the ED and in bud-sprouting. This artificial system imitates and hastens the natural sprouting that occurs in spring. Temporal expression analysis of genes related to phytohormones synthesis, showed an early drop in the expression of ABA biosynthesis gene that preceded an increase in Aux and CK biosynthesis genes. Bud-break is headed by the activation of all structures of the latent bud, especially the differentiation of the inflorescence and the development of the early stages of floral organs. Therefore, resumption of cell division and increases in respiration are essential for the activation of the bud. Temporal expression analysis of the cell cycle and respiration genes indicate that an increase in cell division go before the increase in respiration. These results, together with results indicating that the cell cycle genes are upregulated by Aux and CK, suggest that the events before the bud-break, start with a reduction in ABA content, followed by an increase in the content of Aux and CK, which activates the machinery of the cell cycle, which eventually would cause an increase in respiration.
KEYWORDS: Cell cycle genes, endodormancy release, grapevine buds, phytohormones, respiratory genes
A general characteristic of deciduous fruit tress grown in temperate zones is that their reproductive cycle extends over two consecutive growing seasons separated by a dormancy period between autumn and spring. In grapevines, during the spring and summer of the first season, the meristem of the latent bud (LB) initiate the alternate production of leaf primordia (LP) and uncommitted primordia (UCM) or anlagen.1 The anlagen have the potential to differentiate into tendrils or inflorescence primordia (IP).2,3 By the end of the summer, the short day (SD)-photoperiod triggers the entrance of the LB into endodormancy (ED).4,5 To release from the ED, the bud requires to accumulate a certain amount of chill during the winter (Chilling requirements), and when the environmental conditions are permissive, bud growth resumes and the meristems produces further LP and IP that develops to generate flower primordia (FP) and bud-break is induced.6,7 Artificial sprouting of single-bud cuttings under forced conditions can mimic and hasten the natural sprouting occurring in spring. Recently, using this artificial bud sprouting system we found that the expression of ABA biosynthesis gene VvNCED1 strongly decreased during the first week of incubation, while cytokinin (CK) biosynthesis genes ISOPENTENYL TRANSFERASE (VvIPTs), LONELY GUY (VvLOG1) and auxin (Aux) biosynthesis gene (VvYUC3) increased their expression after 2 and 3 weeks of incubation, respectively.8 These results indicate that phytohormones play a key role in the activation of grapevine buds before bud-break.
Phytohormones regulate the expression of cell cycle genes in grape somatic embryos
A close functional link between phytohormones and regulatory proteins of the cell-cycle has been reported in the literature.9 Because grape somatic embryos (GSE) contain meristematic tissue, and are highly responsive to phytohormones, they were used to study the effect of CK and Aux on the expression of cell cycle genes. Results showed that Aux upregulated the expression of the cyclin dependent kinase genes VvCDKB1.2 and VvCDKB2 and cyclins VvCYCA1, VvCYCA2.2, VvCYCA3, VvCYCB, VvCYCD3.2a and VvCYCd3.2b. While CK has no effect on VvCDKs but upregulated VvCYCA2.2, VvCYCA3 and VvCYCD3.2a (Fig. 1).
Figure 1.
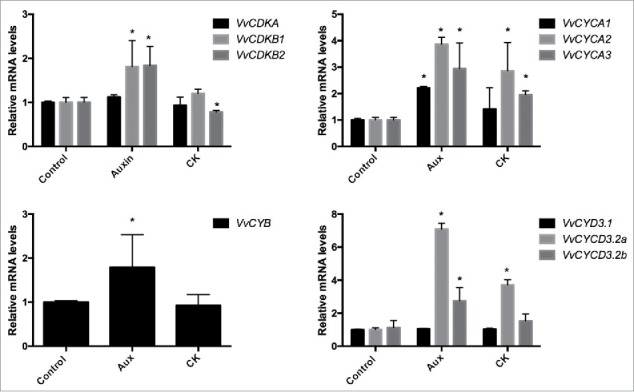
Effects of Aux and CK on the expression of cell cycle genes in grape somatic embryos. Grape somatic embryos (GSE) mostly at the globular or heart-shape stage were transferred from subculture of semi-solid growth regulator free X6 medium to liquid X6 medium and incubated with 10 µM indoleacetic acid (IAA), 2 µM benzilaminopurine (6-BPA) and water as control, respectively. Samples were shaken at 115 rpm for 72 h. Afterwards, the liquid medium was discarded and the GSE were frozen until used. Gene expression analysis was performed by RT-qPCR normalized to VvUBIQUITIN. Values are referred to control samples and correspond to the average of 3 biologic replicates, bars represent s.d. and asterisks indicate significant differences with respect to control samples (Dunnett´s multiple comparison test p < 0.05).
Temporal expression of cell cycle genes
Temporal expression analysis of cell cycle genes showed that cyclin dependent-kinase VvCDKB1 and VvCDKB2 increased their expression level after 2 weeks of incubation and VvCDKA after 3 weeks (Fig. 2 a). The same occurred with cyclin genes, VvCYCD3.2a increased their expression level after 1 week of incubation, while VvCYCA2, VvCYCB and VvCYCD3.1 after 2 weeks of incubation (Fig. 2 b). The increase in the expression of these genes coincided with the increase of Aux and CK biosynthesis genes, and is consistent with the above results indicating that Aux and CK upregulated the expression of these genes in GSE.
Figure 2.
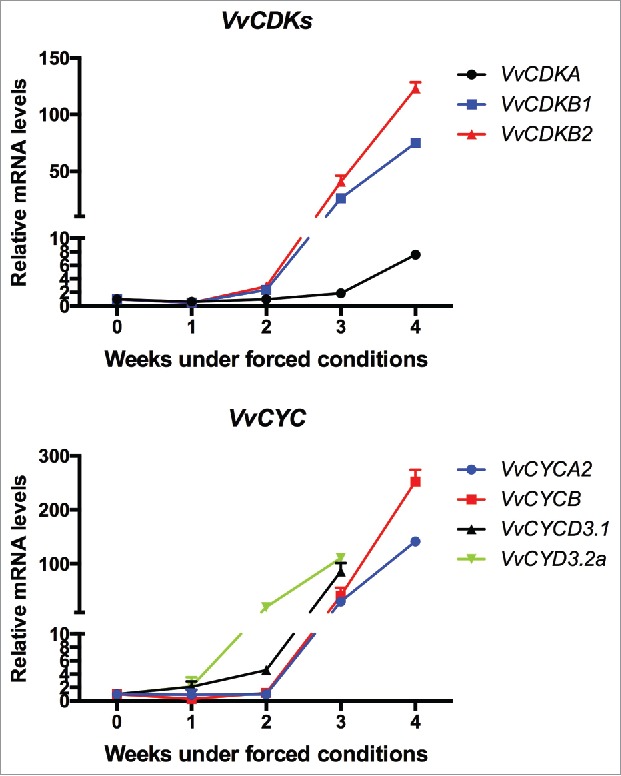
Temporal expression of cell cycle genes in single-bud cuttings during endodormancy release. Temporal expression analysis of (a) cyclin dependent-kinase VvCDKA, VvCDKB1 and VvCDKB2 and (b) cyclins VvCYCA2, VvCYCB, VvCYCD3.1 and VvCYCD3.2a was performed in single-bud cuttings of Thompson seedless grapevines under forced growing conditions in the growth chamber. Gene expression analysis was performed by RT-qPCR normalized to VvUBIQUITIN. Values are referred to control samples (time = 0) and correspond to the average of 3 biologic replicates, bars represent s.d.
Temporal expression of respiration genes
A substantial amount of mitochondrial energy is required for cell-cycle progression. High rate of cellular division requires high mitochondrial ATP production,10,11,12 and the mETC genes such as cytochrome c (CYTc) and subunits of cytochrome c oxidase (COX) have been associated with cell proliferation and high ATP levels.13,14 Temporal expression analysis of mETC genes, VvCITC and subunits 6 of cytochrome oxidase VvCOX6, increased their expression level after 3 weeks of incubation in single-bud cuttings of grapevines exposed to forced growing conditions (Fig. 3). These results indicate that during the release of the bud of ED, the resumption of cell division precedes the increase in mitochondrial respiration. Increasing evidence suggests that cell cycle regulatory proteins are involved in mitochondrial activity. In mammalian cells, cyclin D1 coordinates the bioenergetics of the mitochondria during the G1/S progression,15 while cyclin B1/cdk1 phosphorylates subunits of the complex I increasing mitochondria respiration.12
Figure 3.
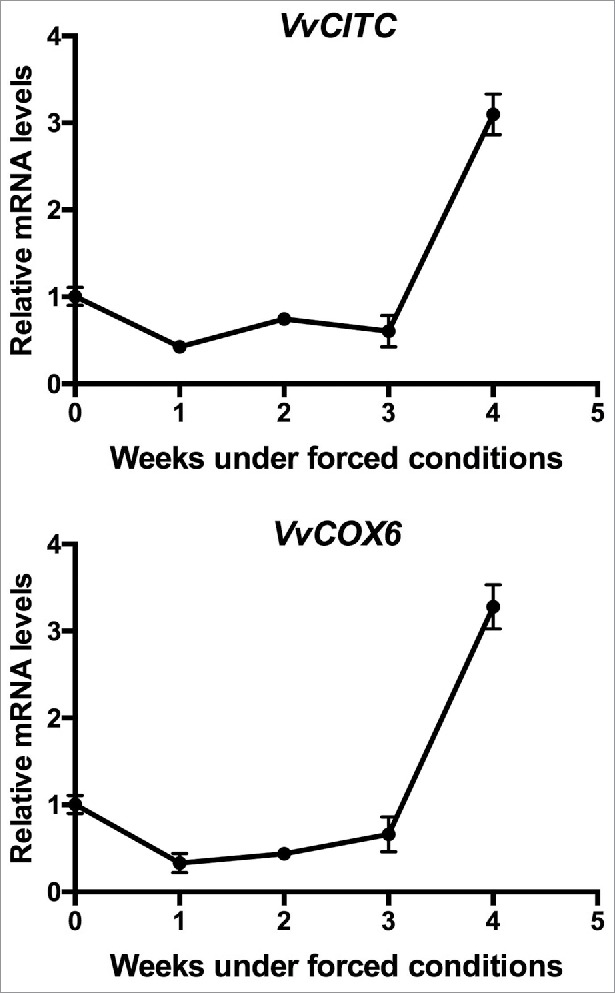
Temporal expression of respiratory genes in single-bud cuttings during endodormancy release. Temporal expression analysis of mETC genes VvCITC and VvCOX6 was performed in single-bud cuttings of Thompson seedless grapevines under forced growing conditions in the growth chamber. Gene expression analysis was performed by RT-qPCR normalized to VvUBIQUITIN. Values are referred to control samples (time = 0) and correspond to the average of 3 biologic replicates, bars represent s.d.
Cascade of events that precedes bud-break in grapevines
Based on the above results, we proposed that the cascade of events that occur during the release of buds from the ED and that precede its sprouting, would started by a fall in ABA levels, followed by an increase in Aux and CK content which would induce cell cycle activation, which in turn, would produce an increase in mitochondrial respiration.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.Boss PK, Buckeridge EJ, Poole A, Thomas MR. New insights into grapevine flowering. Funct Plant Biol. 2003;30:593–606. doi: 10.1071/FP02112. [DOI] [PubMed] [Google Scholar]
- 2.Mullins MG, Bouquet A, Williams LE. Biology of the grapevine. Cambridge University Press, Cambridge, UK; 1992. [Google Scholar]
- 3.Boss PK, Thomas MR. Association of dwarfism and floral induction with grape green revolution mutation. Nature. 2002;416:847–8. doi: 10.1038/416847a. [DOI] [PubMed] [Google Scholar]
- 4.Kühn N, Ormeño-Nuñez J, Jaque-Zamora G, Pérez FJ. Photoperiod modifies the diurnal expression profile of VvPHYA and VvPHYB transcripts in field-grown grapevine leaves. J Plant Physiol. 2009;166:1172–80. doi: 10.1016/j.jplph.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 5.Grant TNL, Gargrave J, Dami IE. (2013). Morphological, physiological, and biochemical changes in Vitis genotypes in response to photoperiod regimes. Am J Enol Vitic. 2013;64:466–75. doi: 10.5344/ajev.2013.13060. [DOI] [Google Scholar]
- 6.Carmona MJ, Chaib J, Martínez-Zapater JM, Thomas MR. A molecular genetic perspective of reproductive development in grapevine. J Exp Bot. 2008;59:2579–96. doi: 10.1093/jxb/ern160. [DOI] [PubMed] [Google Scholar]
- 7.Lebon G, Wojnarowiez G, Holzapfel B, Fontaine F, Vaillant-Gaveau N, Clément C. Sugars and flowering in the grapevine (Vitis vinifera L). J Exp Bot. 2008;59:2565–78. doi: 10.1093/jxb/ern135. [DOI] [PubMed] [Google Scholar]
- 8.Noriega X, Pérez FJ. ABA biosynthesis genes are down-regulated while auxin and cytokinin biosynthesis genes are up-regulated during the release of grapevine from endodormancy. J Plant Growth Regul. 2017. doi: 10.1007/s00344-017-9685-7. [DOI] [Google Scholar]
- 9.del Pozo JC, Lopez-Matas MA, Ramirez-Parra E, Gutierrez C. Hormonal control of the plant cell cycle. Physiol Plant. 2005;123:173–85. doi: 10.1111/j.1399-3054.2004.00420.x. [DOI] [Google Scholar]
- 10.Schieke SM, McCoy JP, Frinkel T. Coordination of mitochondrial bioenergetics with G1 phase cell cycle progression. Cell Cycle. 2008;7:1782–7. doi: 10.4161/cc.7.12.6067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mitra K, Wunder C, Roysam B, Lippincott-Scwartz J. A hyperfused mitochondrial state achieved at G1-S regulate cyclin E buildup and entry into S phase. Proc Natl Acad Sci USA. 2009;106:11960–5. doi: 10.1073/pnas.0904875106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Z, Fang M, Candas D, Zhang T, Qin L, Elridge A, Waschman-Hogiu S, Ahmed KM, Chromy BA, Nantajit D, et al.. CyclinB1/cdka1 coordinates mitochondrial respiration for cell-cycle G2M/progression. Dev Cell. 2014;29:217–32. doi: 10.1016/j.devcel.2014.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Owusu-Ansah E, Yavari A, Mandal S, Banerjee U. Distinct mitochondrial retrograde signals control the G1-S cell cycle checkpoint. Nat Genet. 2008;40:356–61. doi: 10.1038/ng.2007.50. [DOI] [PubMed] [Google Scholar]
- 14.Hamasaki H, Yoshizumi T, Takahashi N, Higuchi M, Kuromori T, Imura Y, Shimada H, Matsui M. SD3, an Arabidopsis thaliana homolog of TIM21, affects intracellular ATP levels and seedling development. Mol Plant. 2012;5:461–71. doi: 10.1093/mp/ssr088. [DOI] [PubMed] [Google Scholar]
- 15.Sakamaki T, Casimiro MC, Ju X, Quong AA, Katiyar S, Liu M, Jiao X, Li A, Zhang X, Lu Y. CyclinD1 determines mitochondrial function in vivo. Mol Cell Biol. 2006;26:5449–69. doi:https://doi.org/ 10.1128/MCB.02074-05. [DOI] [PMC free article] [PubMed] [Google Scholar]


