ABSTRACT
Protein farnesylation refers to the addition of a 15-carbon farnesyl isoprenoid to the cysteine residue of the CaaX motif at the carboxy terminus of target proteins. In spite of its known roles in plant development and abiotic stress tolerance, how these processes are precisely regulated by farnesylation had remained elusive. We recently showed that CYP85A2, the cytochrome P450, which converts castasterone to brassinolide in the last step of brassinosteroid synthesis must be farnesylated in order to function in this pathway. Lack of either CYP85A2 or the farnesylation motif of CYP85A2 resulted in reduced brassinolide accumulation, hypersensitivity to ABA, and increased plant drought tolerance. In this study, we have assessed the influence of the N-terminal secretory signal and the C-terminal CaaX motif of CYP85A2 in mediating CYP85A2 function and targeting to endomembrane compartments. We show that CaaX motif could still target CYPA85A2 in the absence of an intact N-terminal secretory signal to the respective membrane compartments and partially rescue cyp85a2-2 phenotypes. However, in the absence of both the CaaX motif and the secretory signal, CYP85A2 is not targeted to the membranes and becomes unstable.
KEYWORDS: Abscisic acid (ABA), brassinosteroids, drought tolerance, farnesylation, seed germination, sub-cellular localization, secretory signal
Post-translational modification of proteins is a well-known mechanism across eukaryotes which affect protein structure, activity, stability and subcellular location to regulate various functions1. These modifications also improve protein-protein and protein-membrane interactions during different cellular and developmental processes.2 One of the most common post-translational modifications of proteins found in eukaryotic system is the addition of lipids such as fatty acids, glycosyl-phosphatidyl inositol anchors, or addition of the isoprenes, farnesyl diphosphate (FPP) and geranylgeranyl diphosphate (GGPP).3,4 In animals, plants and fungi, prenylation is a form of lipidation of proteins, where either a 15-carbon farnesyl or a 20-carbon geranylgeranyl isoprenoid is transferred to a conserved cysteine located in the carboxy-terminal of the protein called the ‘CaaX’ motif.5,6 Protein farnesyl transferase (PFT) and protein geranylgeranyl transferase (PGGT-I) have several targets in plants and mutation in these enzymes results in various developmental defects.7 In Arabidopsis, these enzymes form heterodimers with a common α subunit known as PLURIPETALA (PLP) which is shared between PFT and PGGT-I.8 Mutation in the α subunit (plp) results in severe developmental defects during shoot meristem and floral organ formation.8 Similar phenotypes can also be observed in the β subunit mutant of PFT, the era1 mutant (enhanced response to abscisic acid 1) along with hypersensitivity to ABA and enhanced drought tolerance.9-12 Despite this, the target of ERA1 that is farnesylated to negatively regulate ABA responses had remained unknown.
We recently showed that, the brassinosteroid biosynthetic enzyme CYP85A2 is a downstream target of ERA1 and that farnesylation of CYP85A2 is required for regulating brassinosteroid biosynthesis, which suppresses ABA sensitivity and drought tolerance.13 The T-DNA knockout mutant of CYP85A2, SALK_129352, designated as cyp85a2-2 resembled era1 with round shaped rosette leaves, shorter petioles and protruding carpels, and showed hypersensitivity to ABA like era1.13 Using this mutant background, we were able to show that both loss of function or lack of farnesylation of CYP85A2 resulted in reduced accumulation of active BR brassinolide (BL), enhanced hypersensitivity to ABA and plant drought tolerance. Mutation of the CaaX motif of CYP85A2 failed to rescue phenotypic defects (round and darker green leaves with short petioles) and ABA hypersensitivity of cyp85a2-2 mutant, indicating that CYP85A2 needs to be farnesylated to participate in BR biosynthesis.
CYP85A2 has an interesting domain organization with a CaaX motif at the C-terminus and a secretory signal peptide at the N-terminus (Fig. 1a). In our previous study, for sub-cellular localizations, we inserted an RFP tag internally after the signal peptide to preserve both ends of the protein to maintain protein function so that the requirement of CaaX motif could be evaluated. We observed that CYP85A2 farnesylation was required for its precise localization to the ER,13 but the requirement of the secretory signal peptide for this response was not examined. To investigate this, we fused RFP to the N-terminus of CYP85A2 to mask the secretory signal peptide of CYP85A2 and used it to complement cyp85a2-2 mutant (Fig. 1b). When transgenic plants were observed, CypPro::RFP-CYP85A2(CSPY) was unable to fully rescue the cyp85a2-2 phenotypes, although moderate extension in leaf petiole length was observed compared to cyp85a2-2. (Fig. 2a). However, our previous study showed a complete rescue when cyp85a2-2 mutant was transformed with a CYP85A2 construct with an intact signal peptide before RFP (CypPro::SP-RFP-CYP85A2-CSPY).13 This indicated that an N-terminal secretory signal, in addition to the CaaX motif is essential for CYP85A2 function and thus masking of the secretory signal could impede precise targeting of RFP-CYP85A2. As expected, farnesyl motif mutated CypPro::RFP-CYP85A2(SSPY) construct with a masked N-terminus completely failed to rescue cyp85a2-2 phenotypes (Fig. 2a).
Figure 1.
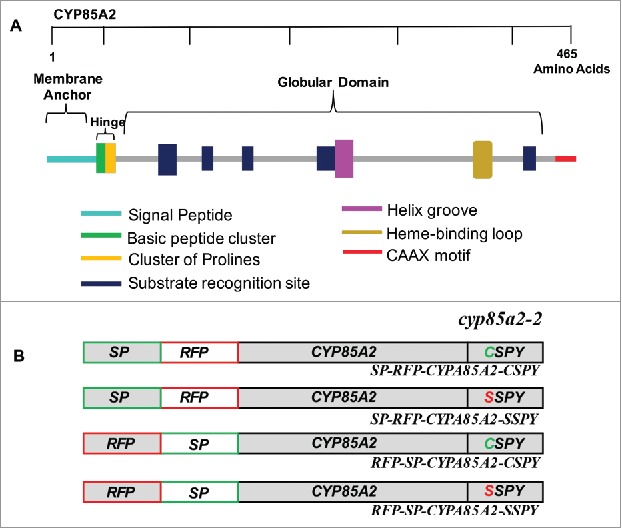
Domain organization of CY85A2. A. Organization of the various domains of CYP85A2 and their respective functions are indicated. B. Schematic diagram of constructs used to rescue cyp85a2-2 in the previous study (top two) and in current study (bottom two) are shown.
Figure 2.
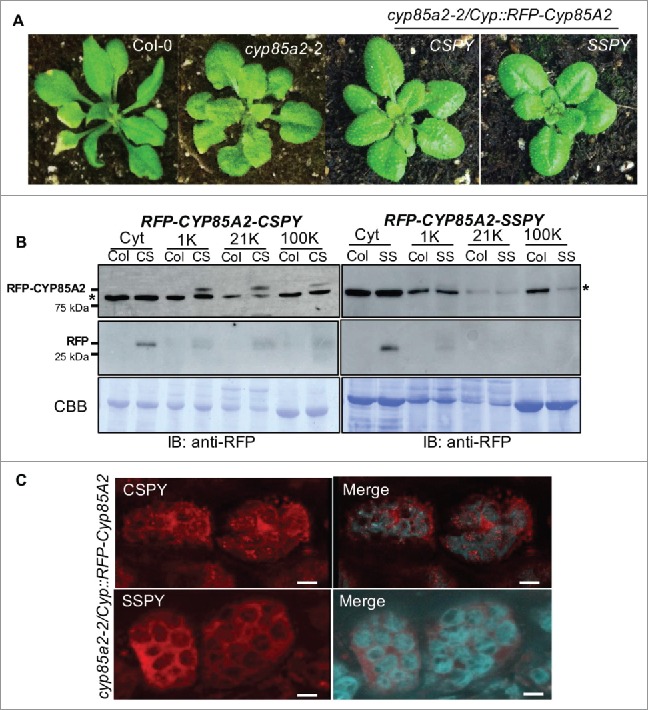
N-terminal signal peptide is required for rescue of cyp85a2-2 mutant phenotype. A. Rosette leaf morphology of Col-0, cyp85a2-2, Cyp::RFPCYP85A2-CSPY/cyp85a2-2 and Cyp::RFPCYP85A2-SSPY/cyp85a2-2. B. Western blot showing RFP-CYP85A2 accumulation in Col-0 controls, Cyp::RFPCYP85A2-CSPY/cyp85a2-2 and Cyp::RFPCYP85A2-SSPY/cyp85a2-2 in different membrane fractions. * Non-specific signal was detected with anti-RFP. C. Bundle sheath cells expressing RFP–CYP85A2 in either CSPY (top) or SSPY (bottom) seedlings. Chloroplast autofluorescence can be seen in blue; merge indicates merged channel between the RFP fluorescence and chloroplast autofluorescence. Scale bars = 10 μm
To determine the influence of perturbing either the signal peptide or farnesylation motif on the intracellular localization of CYP85A2, solubilized microsomal fractions were extracted from 10-day-old seedlings and subjected to Western blotting using anti-RFP antibodies. In the transgenic plants expressing an intact CSPY motif with a masked N-terminus (CypPro::RFP-CYP85A2-CSPY), RFP-CYP85A2(CSPY) accumulated in microsomal membrane fractions of 21K and 100K, as well as the 1000 g fractions which sediments nuclear and chloroplastic compartments (Fig. 2b). This observation is interesting since either absence or masking of secretory signal should have resulted in cytosolic localization of RFP-CYP85A2(CSPY). Therefore, we could presume that the membrane localization of RFP-CYP85A2(CSPY) was primarily due to farnesylation of the CSPY motif. Alternatively, the masked internal secretory signal could still target a subset of RFP-CYP85A2 to the ER (Fig. 2b). In the cytosol, we also found processed RFP-CYP85A2(CSPY) around ∼ 28 kDa range which is the expected size for the RFP tag. This suggests that the cytosolic fraction of RFP-CYP85A2(CSPY) could be unstable and turned over rapidly (Fig. 2b).
In contrast, transgenic plants deficient in both the N-terminus and the farnesylation motif (CypPro::RFP-CYP85A2-SSPY), RFP-CYP85A2(SSPY) could not be detected in the microsomal fractions indicating that an intact farnesylation motif and an N-terminal signal peptide are required for efficient localization of the whole pool of CYP85A2. When the cytosolic fraction was examined, only the processed form of RFP-CYP85A2(SSPY) was found accumulating, indicating that, when CYP85A2 is not targeted to the ER compartments, it is subjected to rapid degradation (Fig. 2b). Given that no membrane targeting was seen with RFP-CYP85A2-SSPY also eliminates the alternative possibility that CYP85A2 could still be targeted to the ER by the secretory signal in spite of masking the N-terminal signal peptide.
We further verified these observations through sub-cellular localization of these two variants in the petioles of the transgenic seedlings. Confocal imaging revealed that the RFP-CYP85A2(CSPY) could be observed in the cytosol although a significant subset of RFP-CYP85A2(CSPY) was found associating with ER compartments of bundle sheath cells, in close association with the chloroplasts (Fig. 2c). This was similar to the functionally intact SP-RFP-CYP85A2(CSPY) protein which was shown previously.13 This localization pattern is consistent with the Western blotting data and also shows that the cytosolic fraction detected through imaging is likely just the RFP tag remaining after degradation of RFP-CYP85A2(CSPY). When similar imaging was performed using transgenic lines expressing RFP-CYP85A2(SSPY), we detected complete cytosolic localization (Fig. 2c) consistent with the Western blotting which showed accumulation of processed RFP in the cytosol. Based on these observations, we could infer that an intact farnesylation motif is required for precise subcellular localization of CYP85A2, even in the absence of an N-terminal secretory signal. In spite of localization to ER compartments, RFP-CYP85A2(CSPY) was not able to completely rescue the morphological defects of cyp85a2-2. This raises the possibility that RFP-CYP85A2(CSPY) only partially restores BL production in these transgenic lines and full restoration will require an intact N-terminal secretory signal peptide. Estimation of BL levels in these lines would allow us to examine this possibility.
When these transgenic plants were tested for ABA sensitivity CypPro::RFP-CYP85A2-CSPY were able to completely rescue ABA hypersensitivity of cyp85a2-2 mutant (Fig. 3), while CypPro::RFP-CYP85A2-SSPY lines were similar to cyp85a2-2 in their ABA sensitivity. This suggests that even partial accumulation of BL in the CypPro::RFP-CYP85A2-CSPY lines could be sufficient to reduce the ABA sensitivity of cyp85a2-2, further confirming brassinolide as a potent inhibitor of ABA responses.
Figure 3.
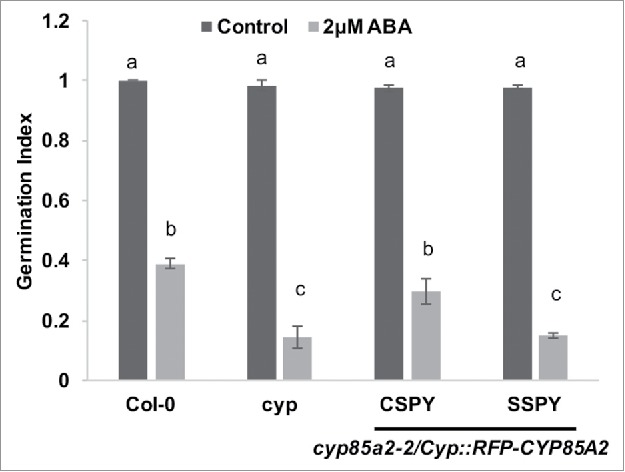
CYP85A2 with a masked N-terminal signal peptide rescues ABA, hypersensitivity of cyp85a2-2. Graph represents the mean germination index of Col-0, cyp85a2-2, Cyp::RFPCYP85A2-CSPY/cyp85a2-2, and Cyp::RFPCYP85A2-SSPY/cyp85a2-2 seeds plated on control (0.5 × MS) and 2 μM ABA plates. Indices calculated by standardizing germination percentages of the various lines to that of Col-0. Values are means of three technical replicates (bars represent ± Standard Error of Mean). Different letters over bars indicate significant differences based on Tukey's multiple comparison test (P < 0.05).
Taken together, with observations from our previous work and this study, we propose that both the secretory signal at the N-terminus and the farnesylation motif at the C-terminus are required for complete functionality of CYP85A2, although, an intact N-terminus alone in the absence of the CaaX motif failed to rescue any of the cyp85a2-2 phenotypes, pointing to the essential nature of farnesylation of CYP85A2 for its function.13 The requirement of the N-terminus for P450 localization to the ER has been studied previously.14 Through in vitro transcription and translation assays in the presence of ER compartments, it was shown that the N-terminal hydrophobic domain of the human cytochrome P450c21 is required for its precise membrane insertion.14 Deletion of the hydrophobic domain also resulted in accumulation of the truncated forms in the soluble fraction and rapid degradation of P450c21, suggesting that the protein could unfold in the absence of the N-terminus-mediated membrane targeting.14 Therefore, when the N-terminal signal peptide is masked, co-translational import of RFP-CYP85A2 into the ER will not occur and the ensuing cytosolic RFP-CYP85A2 should get farnesylated in order to be targeted to appropriate ER compartments to partially participate in BL biosynthesis. We consistently found the cytosolic pool of RFP-CYP85A2 to be unstable indicating that it has to be targeted to the ER compartment for it to be stable and protected. In conclusion, this work has uncovered the significance of the various targeting domains for CYP85A2 functionality and further reinforces the importance of CYP85A2 farnesylation for its targeting to participate in BR biosynthesis.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgment
This work was supported by the Strategic Partnership Grants for Projects from Natural Sciences and Engineering Research Council of Canada for Marcus A. Samuel and Fellowship from the Alberta Crop Industry Development Fund for Muhammad Jamshed.
References
- 1.Nalivaeva NN, Turner AJ. Post-translational modifications of proteins: acetylcholinesterase as a model system. Proteomics. 2001;1:735–47. doi: 10.1002/1615-9861(200106)1:6%3c735::AID-PROT735%3e3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 2.Duan G, Walther D. The Roles of Post-translational Modifications in the Context of Protein Interaction Networks. PLoS Comput Biol. 2015;11(2). doi: 10.1371/journal.pcbi.1004049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thompson GA, Okuyama H. Lipid-linked proteins of plants. Prog. Lipid Res. 2000;39:19–39. doi: 10.1016/S0163-7827(99)00014-4. PMID:10729606 [DOI] [PubMed] [Google Scholar]
- 4.Yalovsky S, Rodríguez-Concepción M, Gruissem W. Lipid modifications of proteins - Slipping in and out of membranes. Trends Plant Sci. 1999;4:439–45. doi: 10.1016/S1360-1385(99)01492-2. [DOI] [PubMed] [Google Scholar]
- 5.Andrews M, Huizinga DH, Crowell DN. The CaaX specificities of Arabidopsis protein prenyltransferases explain era1 and ggb phenotypes. BMC Plant Biol. 2010;10:118. doi: 10.1186/1471-2229-10-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hancock JF, Cadwallader K, Paterson H, Marshall CJ. A CAAX or a CAAL motif and a second signal are sufficient for plasma membrane targeting of ras proteins. EMBO J. 1991;10:4033–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Galichet A, Gruissem W. Protein farnesylation in plants - Conserved mechanisms but different targets. Curr Opin Plant Biol. 2003;6:530–5. doi: 10.1016/j.pbi.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 8.Running MP, Lavy M, Sternberg H, Galichet A, Gruissem W, Hake S, Ori N, Yalovsky S. Enlarged meristems and delayed growth in plp mutants result from lack of CaaX prenyltransferases. Proc Natl Acad Sci U S A. 2004;101:7815–20. doi: 10.1073/pnas.0402385101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cutler S, Ghassemian M, Bonetta D, Cooney S, McCourt P. A protein farnesyl transferase involved in abscisic acid signal transduction in Arabidopsis. Science. 1996;273:1239–41. doi: 10.1126/science.273.5279.1239. [DOI] [PubMed] [Google Scholar]
- 10.Pei Z. Role of Farnesyltransferase in ABA Regulation of Guard Cell Anion Channels and Plant Water Loss. Science. 1998;282:287–90. doi: 10.1126/science.282.5387.287. [DOI] [PubMed] [Google Scholar]
- 11.Yalovsky S, Kulukian A, Rodríguez-Concepción M, Young CA, Gruissem W. Functional requirement of plant farnesyltransferase during development in Arabidopsis. Plant Cell. 2000;12:1267−78. doi: 10.1105/tpc.12.8.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bonetta D, Bayliss P, Sun S, Sage T, McCourt P. Farnesylation is involved in meristem organization in Arabidopsis. Planta. 2000;211:182–90. doi: 10.1007/s004250000283. [DOI] [PubMed] [Google Scholar]
- 13.Northey JGB, Liang S, Jamshed M, Deb S, Foo E, Reid JB, McCourt P, Samuel MA. Farnesylation mediates brassinosteroid biosynthesis to regulate abscisic acid responses. Nat Plants. 2016;2:16114. doi: 10.1038/nplants.2016.114. [DOI] [PubMed] [Google Scholar]
- 14.Hsu LC, Hu MC, Cheng HC, Lu JC, Chung BC. The N-terminal hydrophobic domain of P450c21 is required for membrane insertion and enzyme stability. J Biol Chem. 1993;268:14682–6. [PubMed] [Google Scholar]


