Abstract
Histopathological interpretation of proliferative nodules occurring in association with congenital melanocytic nevi can be very challenging due to their similarities with congenital malignant melanoma and malignant melanoma arising in association with congenital nevi. We hereby report a diagnostically challenging case of congenital melanocytic nevus with proliferative nodules and ulcerations, which was originally misdiagnosed as congenital malignant melanoma. A subsequent histopathological examination in consultation by one of the authors (RL) and Mass Spectrometry Imaging analysis rendered a diagnosis of congenital melanocytic nevus with proliferative nodules. In this case, Mass Spectrometry Imaging, a novel method capable of distinguishing benign from malignant melanocytic lesions on a proteomic level, was instrumental in making the diagnosis of a benign nevus. We emphasize the importance of this method as an ancillary tool in the diagnosis of difficult melanocytic lesions.
Introduction
Congenital melanocytic nevi are present in 1% to 2% of newborns. Changes in its morphology are common and are usually divided into two main groups. The first group, which includes changes in coloration and hypertrichosis, has benign implications. However, the second group, which comprises development of nodular melanocytic lesions and ulcerations within the congenital melanocytic nevi, could signify de novo malignant melanoma arising within the nevus.(1–3) Proliferative nodules are sometimes difficult to differentiate from malignant melanoma due to the many clinical and histopathologic similarities.(4, 5)
Several recent studies have shown that mass spectrometry imaging can be used to differentiate between histologically difficult lesions. While there may be morphological features that look similar between benign or malignant lesions (6, 7) or between diseases of similar tissue origin (8, 9), the underlying biology including proteomics between these samples is different. Mass spectrometry imaging is a natural match for pathology as the same type of thin tissue sections used for pathology are used for mass spectrometry imaging to determine the protein composition in the tissue with the spatial information within the sample being preserved. In a recent study, we determined that five proteins were differentially expressed in the melanocytes of Spitz nevi and Spitzoid melanomas.(6) In a subsequent large study, mass spectrometry imaging proved to be helpful in the classification of diagnostically challenging atypical Spitzoid neoplasms when the previously determined molecular signature was applied.(10) Furthermore, the diagnosis rendered by mass spectrometry imaging correlated better with the clinical outcome than the histopathologic diagnosis. Mass spectrometry imaging determines differences in the expression of proteins within benign and malignant melanocytic lesions of any type, which allows for their correct classification and diagnosis.
Case Report
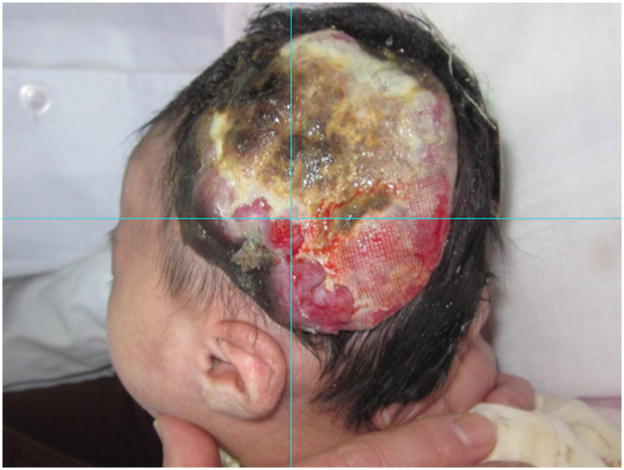
The patient was a 50-day-old healthy Chinese girl, born at term by cesarean section to a 30-year old mother. There was no family history of melanoma. At birth, the patient had a 4.7 x 4.2 cm well-demarcated, violaceous-to-hyperpigmented plaque over the left temporo-parietal area. Over a 7-week period the lesion increased in size and became ulcerated and hemorrhagic. Physical examination revealed a 10 x 9 cm multinodular, well-demarcated, focally ulcerated plaque with variegated color and necrotic areas (Figure 1).
Figure 1.
Ulcerated, multi-nodular, hemorrhagic plaque displaying variable colors, involving the left temporo-parietal scalp.
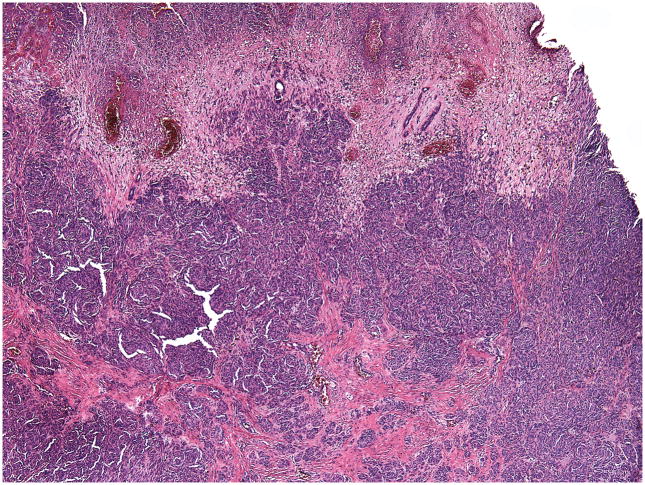
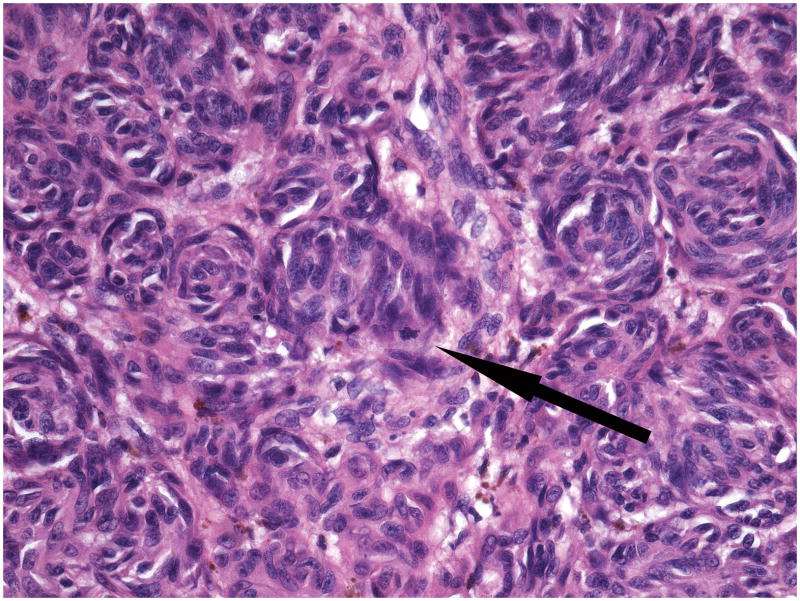
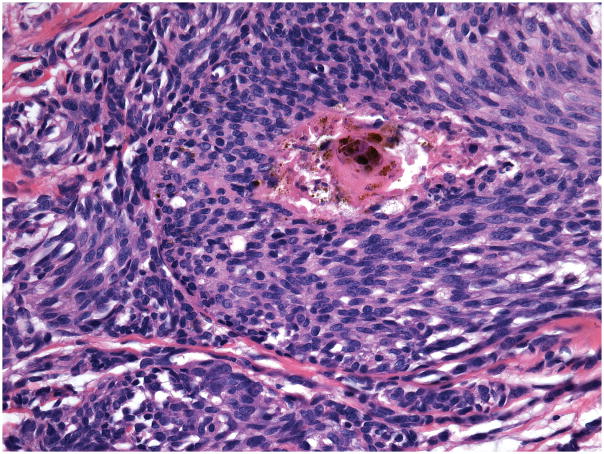
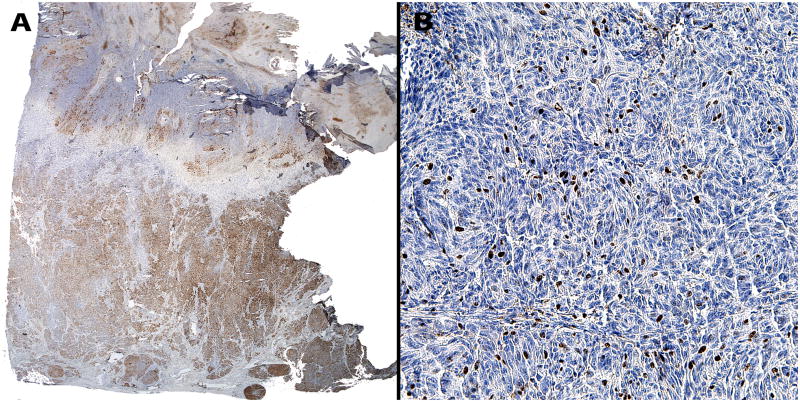
A complete excision of the entire lesion with 1-cm margin followed by a full-thickness skin graft was performed. Initial histopathological examination of the excised lesion at two Chinese hospitals rendered a diagnosis of malignant melanoma. When the patient was 9 months old the case was sent for consultation to one of the authors (RL). The lesion showed conflicting histopathological criteria. In favor of the diagnosis of benign nevus were the presence of dense melanocytic proliferation throughout the dermis extending into the subcutaneous fat, whorled nested pattern, and monomorphous melanocytes. However, there was also prominent ulceration (Figure 2), lack of maturation, mitotic figures (Figure 3) and melanocytes above the dermal- epidermal junction. Focal necrosis (Figure 4) and patchy positivity of the entire lesion with HMB-45 were also seen (Figure 5A). The proliferative marker Ki-67 was positive in approximately 15% of the melanocytic nuclei (Figure 5B).
Figure 2.
Ulcerated epidermis and completely necrotic upper dermis. Dense melanocytic proliferation is seen throughout the entire dermis.
Figure 3.
Large, slightly pleomorphic melanocytes in whorled nests and a mitotic figure (arrow).
Figure 4.
Area of necrosis in the center of a melanocytic nest.
Figure 5.
(A) Positive patchy immunohistochemical staining with HMB-45 throughout the entire lesion. (B) High proliferative index with Ki-67 positive in approximately 10–15% of melanocytes.
Materials and Methods
We have previously used Mass Spectrometry Imaging (MSI) to differentiate Spitz nevi from Spitzoid melanomas on a proteomic level.(6) Subsequently, we studied a melanoma from a mother and two atypical pigmented lesions from her newborn baby by MSI to determine whether these were metastases or atypical nevi.(11) In the current case we used MSI to aid our diagnosis by distinguishing on a proteomic level between malignant melanoma and a nodular proliferation within a benign congenital nevus. In brief, 5μm-thick formalin-fixed, paraffin-embedded tissue sections were used after de-paraffinization. One of the sections was placed on an indium-tin oxide glass slide for mass spectral analysis and a serial section on a standard glass slide for hematoxylin and eosin (H&E) staining. A digital microscopy image of the H&E stained section was acquired using a Leica SCN 400 digital microscope scanner. The image was annotated using custom software for areas of interest for analysis. The microscopy image was then merged with an image of the MSI slide using Adobe Photoshop software, and coordinates of the annotations were determined. Trypsin and matrix were applied to these locations using a Portrait 630 acoustic robotic microspotter. Mass spectra were acquired from the designated locations using a Bruker AutoFlex Speed mass spectrometer operated in reflectron positive ion mode. Twenty four areas of interest from the melanocytic component of the lesion, each measuring 300 μm in diameter, were mapped on each slide. The mass spectral profile was acquired for each one of these areas of interest. In this case we applied the very same algorithm, classification method and criteria that we used in our original study.(6) The resultant spectra were compared to the previously described proteomic signature composed of 5 proteins, and each area of interest was designated as either consistent with nevus or consistent with melanoma.(6) For the entire case to be diagnosed as either benign (nevus) or malignant (melanoma), an overwhelming number of the areas of interest (>66%) had to be designated by the instrument as such. A detailed description of this method and the instruments used can be found elsewhere.(6, 12)
In a recent study we compared banal melanocytic nevi with conventional malignant melanomas and determined proteomic differences, which can distinguish between the two.[(13)] We also applied that new algorithm to our case to ascertain whether on a proteomic level we can distinguish between malignant melanoma and a proliferative nodule within a benign congenital nevus.
Results
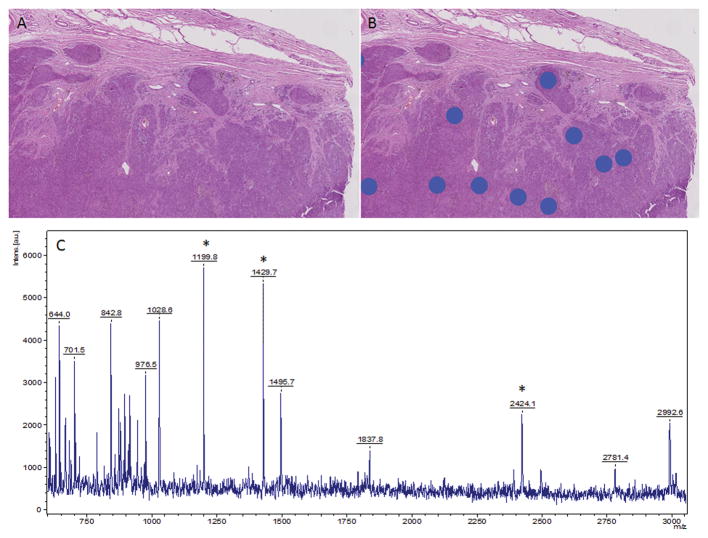
When using the algorithm for differentiating between Spitz nevus and Spitzoid melanoma 17/24 (71%) spectra were in favor of a nevus and the lesion was classified as benign. When we used the new algorithm for discriminating between banal nevi and conventional melanomas in this case, all 24 spectra (24/24 – 100%) classified the lesion as benign nevus (Figure 6). The patient is currently healthy and with no evidence of disease at 3.5 years of age (Figure 7).
Figure 6.
Histology directed mass spectral analysis. Hematoxylin and eosin stained section of the tissue without (A) and with (B) annotations for areas of mass spectral data collection. C) Representative mass spectra taken from a region under one of the blue dots in (B). *Highlight selected peaks are part of the classification algorithm.
Figure 7.
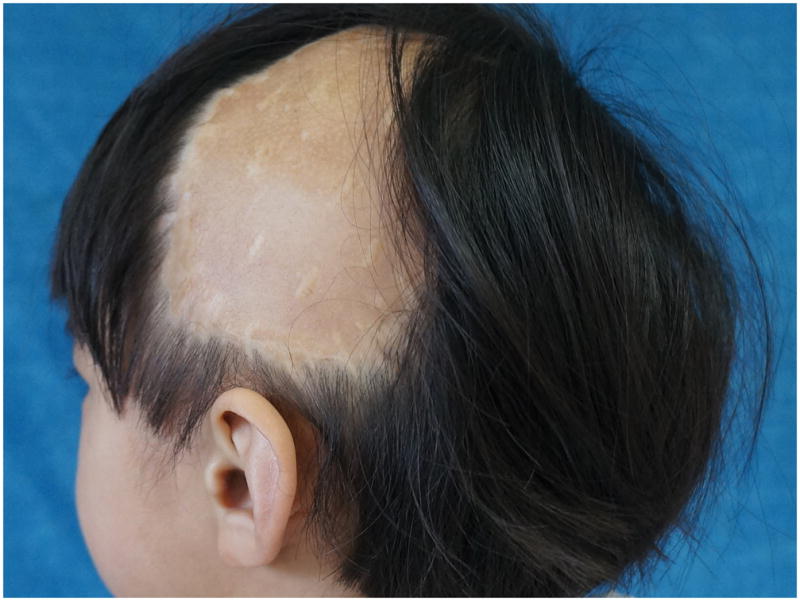
The patient at the age of 2.5 years, alive and without evidence of disease.
Discussion
Despite the rarity of malignant melanoma arising in congenital nevi, it is always a concern in rapidly enlarging, ulcerated, and hemorrhagic lesions, and in lesions with a proliferative nodular pattern.(14, 15) Ulcerations and erosions, usually associated with melanoma, are generally due to skin fragility in the newborns, a mechanism not completely understood. Giam et al(1) proposed two possible hypotheses to explain the erosive skin changes seen in 10 patients with congenital melanocytic nevi: 1) melanocytes in the nevi possibly secrete a factor that affects the formation of basement membrane components with subsequent skin fragility; 2) there is weakening of the attachment of the basement membrane to the papillary dermis secondary to the proliferation of nevic cells at the dermal-epidermal junction.(1, 16, 17) The latter hypothesis corresponds most with our case.
Histopathological interpretation and clinical management of atypical proliferative nodules in congenital melanocytic nevi pose significant challenges due to their similarities with melanoma. (4, 5, 14) In these cases treatment options are uncertain. Proliferative nodules show a wide spectrum of changes including findings sometimes overlapping with those found in malignant melanoma.(17, 18)
Nodular proliferations in nevi should be interpreted as benign proliferative nodules in association with a nevus if they demonstrate high cellularity, low or absent nuclear atypia, absence of necrosis and atypical mitoses, no ascent of melanocytes in the epidermis, no destructive expansive growth, a smooth transition and blending between the nodular proliferation and the adjacent nevus cells, and lack of inflammatory infiltrate.(14, 15, 18, 19) On the other hand, nodules with two or more of the following histopathological features are considered atypical: sharp demarcation, expansive growth, epidermal effacement, focal pagetoid spread, variable pleomorphism, one or more mitotic figures per high power field, and atypical mitoses.(15, 20) Malignant melanoma should be suspected when there are large zones of necrosis, extremely atypical melanocytes, and numerous mitoses.(17, 18)
In our case, there were conflicting histopathological criteria. Features suggestive of melanoma included the extensive necrosis not only of the epidermis but also of the upper half of the reticular dermis; pagetoid scatter of melanocytes in the epidermis; lack of maturation; areas of necrosis within melanocytic nests; markedly increased proliferative index with approximately 10–15% of the melanocytes labeling with Ki-67; and HMB-45 patchy positivity throughout the entire lesion. In contrast, the nested pattern of melanocytic proliferation, the presence of monomorphous melanocytes lacking pleomorphism, and absence of cytologic atypia and atypical mitoses were features of a benign nevus. There are small numbers of reported cases in the literature of diagnostically challenging proliferative nodules within congenital melanocytic nevi (Table 1). Proliferative nodules vary in size from a few millimeters up to 10 centimeters. They present with variegated color and may be ulcerated.(4, 5, 14, 21) Histopathologically, the proliferation of melanocytes is seen in the dermis and often extends to the subcutis.(4, 5, 14, 15, 21, 22) An intraepidermal component may be present.(21–23) The melanocytes are larger than those in the adjacent nevus, monomorphous and densely packed in hypercellular areas. In rare cases there was cytologic atypia.(15) Maturation may or may not be present. Mitoses are almost invariably seen and, in some cases, may be as high as 27 per mm2.(2, 4, 24–26) Although necrosis was not identified in any of the reported cases, our case demonstrated small foci of necrosis. Between 5–20% of melanocytes within proliferative nodules labeled positively with the proliferative marker Ki-67.(4, 5, 15, 22) Phadke et al compared Ki-67 and phosphohistone H3 (PPH3) expression levels in 18 benign and 25 atypical proliferative nodules with that of background congenital nevi and showed that Ki-67 and PPH3 scores were significantly higher in atypical proliferative nodules.(20) Most reported cases of proliferative nodules within congenital nevi stained negative for HMB-45. In a few cases the melanocytes of the proliferative nodules expressed HMB-45 either diffusely or focally.(15, 23, 24) In our case there was diffuse patchy positivity with HMB-45 throughout the lesion.
Table 1.
Proliferative nodules in association with congenital melanocytic nevi
| Case # | Author/ Ref # |
Age | Sex | Anatomical Site |
Clinical presentation |
Histopathological diagnosis |
Histopathological findings |
Location of melanocytic proliferation |
Maturation | Mitoses per mm2 |
Atypia | Necrosis | Proliferative index |
CGH/ FISH |
IPOX | Re- excision |
Follow up |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Murphy et al; Ref# 18 | 3 mos | M | Popliteal fossa | 2 x 1.2 cm brown patch with rapidly growing 1 x 0.8 cm brown-black nodule | Compound combined melanocytic nevus with features of both, congenital melanocytic nevus and deep penetrating nevus | Large monomorphous melanocytes with abundant basophilic cytoplasm with fine melanin pigment | Epidermis and dermis | No | 3–5 | NO | NO | 5–10% | Neg | NK | YES | 15 months – no recurrence |
| 2 | Scalvenzi et al; Ref# 5 | 1 week | M | Back | 8 x 11 cm multicolored, multinodular plaque with focal ulceration | Giant congenital melanocytic nevus with proliferative dermal nodules | Hypercellular growth of densely packed, uniform melanocytes with small nucleus and no pleomorphism | Dermis | NK | NK | NO | NO | 10% | S-100 pos; HMB-45 neg | YES | 2 years – alive and well | |
| 3 | Borbujo et al; Ref# 10 | Newborn | F | Bathing suit distribution | 2 x 2 cm ulcerated hemorrhagic nodule within giant congenital melanocytic nevus | Proliferative nodules within a giant congenital nevus | Diffuse growth of melanocytes with hyperchromatic nuclei and several mitoses; epidermal erosion present | Dermis | NK | Present | NO | NO | NK | NK | NO | 1 year – the nodule disappeared | |
| 4 | Park et al; Ref# 11 | Newborn | F | Back | 15 cm in diameter ulcerated plaque with multiple nodules varying between 1 – 8 cm | Proliferative nodules in a giant congenital melanocytic nevus | Cellular nodule composed of slightly atypical melanocytes; smooth border between the nodule and adjacent congenital melanocytic nevus | Dermis and subcutis | NK | Present | Slight | NO | 10–15% | S-100, HMB-45 and Melan A - pos | NK | Benign course; no details | |
| 5 | Hernandez-Martin et al; Ref# 17 | Newborn | F | Trunk and genitalia | Large plaque with variable pigmentation, nodularity, and ulcerated areas | Ulcerated sclerotic giant congenital melanocytic nevus | Melanocytic proliferation in the dermis; nests and solitary units of melanocytes surrounded by compact collagen bundles with a few intermingled fibroblasts | Epidermis, dermis, and subcutis | NK | Absent | Present | NO | NK | NK | NO | 2 years; the nevus faded and softened | |
| 6 | Aoyagi et al; Ref# 19 | Newborn | F | Bathing suit distribution | ~ 20 black, smooth proliferative nodules up to 3.5 cm in diameter | Proliferative nodules associated with a congenital melanocytic nevus | Plump, oval, heavily pigmented melanocytes with large vesicular nuclei in the papillary dermis gradually maturing into small, round melanocytes | Epidermis, dermis, and subcutis | YES | Absent | NO | NO | NK | HMB-45 pos in papillary dermis | YES; Over a period of 6 months several excisions of some nodules | 3 years; good health; some nodules resolved spontaneously | |
| 7 | van Houtenet et al; Ref# 4 | 1 week | M | Bathing suit distribution | Multi-nodular dark brown plaque with variable pigmentation | Proliferative nodules in a giant congenital melanocytic nevus | Dense, hypercellular proliferation of moderately large melanocytes; smooth transition between the nodule and the non-nodular part of the nevus | Dermis | YES | 15 | NO | NO | 20% | NK | Excision of the nodule | 1 year; good health | |
| 8 | Nguyen TLT et al; Ref# 20 | 10-day | M | Neck, upper chest and upper back | Large hyperpigmented plaque with multiple nodues | Proliferative nodules in a giant congenital melanocytic nevus | Hypercellular nonexpansile dermal nodule composed of densely packed uniform melanocytes | Dermis | NO | 27 | NO | NO | 60% | Tetraploidy by FISH and no chromosomal abberations | HMB-45 and Mart-1 - pos | NO | 13 months; no evidence of recurrence |
| 9 | De Vooght A et al; Ref# 22 | Newborn | M | Bathing suit distribution | Large bathing trunk type nevus with a nodule | Proliferative nodule in a giant congenital melanocytic nevus | Nodule in the deep dermis and subcutis of high cellularity, blending with surrounding nevus | Dermis | NO | High | NO | NO | NK | MAGE genes 1,3 and 10 neg | Melan A and Tyrosinase pos, HMB-45 +/− | NO | 1 month, recurrence of nodule but histologically bland |
| 10 | Angelucci D et al; Ref# 21 | Newborn | M | Right lower limb and gluteus | Large nevus with papules, nodules and ulceration | Proliferative nodules in a giant congenital melanocytic nevus | Nodule of atypical, pleomorphic melanocytes | Epidermis and dermis | NO | High | YES | NO | NK | Tyrosinase pos | 2 years; no evidence of malignancy | ||
| 11 | Our case | 7 weeks | F | Scalp | 10 x 9 cm nodular, well-demarcated, focally ulcerated plaque with variegated color and necrotic areas | Proliferative nodules in a congenital melanocytic nevus | Dense, hypercellular proliferation of monomorphous melanocytes arranged in nests with whorled pattern | Epidermis, dermis, and subcutis | NO | 15 | NO | YES | 15% | HMB-45 patchy and diffusely positive | YES | 3.5 years; alive and well; no recurrence |
Prior studies have demonstrated that melanomas and proliferative nodules, which have developed in association with congenital melanocytic nevi, show distinct patterns of genomic gains and/or losses as evaluated by comparative genomic hybridization (CGH) that can be used in their differentiation.(19, 22, 24) In proliferative nodules arising within congenital melanocytic nevi Bastian et al (19) described a predominant pattern of gain or loss of entire chromosomes. This differed from the aberration pattern observed in melanoma, in which most cases have aberrations involving only partial chromosomes.(18) Murphy et al found no chromosomal aberrations by CGH in a proliferative nodule, which developed within a congenital melanocytic nevus in a 3-month-old infant.(22) Nguyen et al reported a case of a 10-day-old boy with a giant congenital melanocytic nevus with multiple nodules.(24) The authors documented polyploidy by FISH in a proliferative nodule and warned against its misinterpretation as FISH-positive result.(24) Multiple whole chromosomal copy number gains by CGH were reported in a case of metastatic melanoma in association with a giant congenital melanocytic nevus in an adult.(27)
We applied MSI analysis to differentiate benign proliferative nodules from melanoma in association with a large congenital nevus. The case was classified as benign. The patient is well and without recurrence or evidence of melanoma.
Our case demonstrates that Mass Spectrometry Imaging, as an objective ancillary method using molecular and protein biomarkers, is a promising diagnostic tool in challenging and difficult melanocytic lesions. Further determining the value of this test will require larger series of cases.
Footnotes
Conflict of Interest Disclosure: None
Funding sources: None
References
- 1.Giam YC, Williams ML, Leboit PE, Orlow SJ, Eichenfield LF, Frieden IJ. Neonatal erosions and ulcerations in giant congenital melanocytic nevi. Pediatr Dermatol. 1999;16(5):354–8. doi: 10.1046/j.1525-1470.1999.00092.x. [DOI] [PubMed] [Google Scholar]
- 2.Rhodes AR. Pigmented birthmarks and precursor melanocytic lesions of cutaneous melanoma identifiable in childhood. Pediatr Clin North Am. 1983;30(3):435–63. doi: 10.1016/s0031-3955(16)34394-2. [DOI] [PubMed] [Google Scholar]
- 3.Lynch HT, Fusaro RM. National Institutes of Health consensus report on precursors to malignant melanoma. A difference in opinion. JAMA. 1984;252(20):2872–3. [PubMed] [Google Scholar]
- 4.van Houten AH, van Dijk MC, Schuttelaar ML. Proliferative nodules in a giant congenital melanocytic nevus-case report and review of the literature. J Cutan Pathol. 2010;37(7):764–76. doi: 10.1111/j.1600-0560.2009.01431.x. [DOI] [PubMed] [Google Scholar]
- 5.Scalvenzi M, Palmisano F, Cacciapuoti S, et al. Giant congenital melanocytic naevus with proliferative nodules mimicking congenital malignant melanoma: a case report and review of the literature of congenital melanoma. Case Rep Dermatol Med. 2013;2013:473635. doi: 10.1155/2013/473635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lazova R, Seeley EH, Keenan M, Gueorguieva R, Caprioli RM. Imaging mass spectrometry--a new and promising method to differentiate Spitz nevi from Spitzoid malignant melanomas. Am J Dermatopathol. 2012;34(1):82–90. doi: 10.1097/DAD.0b013e31823df1e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morgan TM, Seeley EH, Fadare O, Caprioli RM, Clark PE. Imaging the clear cell renal cell carcinoma proteome. J Urol. 2013;189(3):1097–103. doi: 10.1016/j.juro.2012.09.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seeley EH, Washington MK, Caprioli RM, M’Koma AE. Proteomic patterns of colonic mucosal tissues delineate Crohn’s colitis and ulcerative colitis. Proteomics Clin Appl. 2013;7(7–8):541–9. doi: 10.1002/prca.201200107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fata CR, Seeley EH, Desouki MM, et al. Are clear cell carcinomas of the ovary and endometrium phenotypically identical? A proteomic analysis. Hum Pathol. 2015;46(10):1427–36. doi: 10.1016/j.humpath.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 10.Lazova R, Seeley EH, Kutzner H, et al. Imaging mass spectrometry assists in the classification of diagnostically challenging atypical Spitzoid neoplasms. J Am Acad Dermatol. 2016;75(6):1176–86. e4. doi: 10.1016/j.jaad.2016.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alomari AK, Glusac EJ, Choi J, et al. Congenital nevi versus metastatic melanoma in a newborn to a mother with malignant melanoma - diagnosis supported by sex chromosome analysis and Imaging Mass Spectrometry. J Cutan Pathol. 2015;42(10):757–64. doi: 10.1111/cup.12523. [DOI] [PubMed] [Google Scholar]
- 12.Aerni HR, Cornett DS, Caprioli RM. Automated acoustic matrix deposition for MALDI sample preparation. Anal Chem. 2006;78(3):827–34. doi: 10.1021/ac051534r. [DOI] [PubMed] [Google Scholar]
- 13.Lazova R, Seeley EH. Mass Spectrometry Imaging - an objective and reliable method to differentiate between benign melanocytic nevi and malignant melanomas. J Cutan Pathol. 2016;43:e74–75. [Google Scholar]
- 14.Borbujo J, Jara M, Cortes L, Sanchez de Leon L. A newborn with nodular ulcerated lesion on a giant congenital nevus. Pediatr Dermatol. 2000;17(4):299–301. doi: 10.1046/j.1525-1470.2000.01782.x. [DOI] [PubMed] [Google Scholar]
- 15.Park K, Fukumoto T, Kuki C, Asada H, Kuwahara M, Kasai T. A neonatal case of proliferative nodules in a giant congenital melanocytic nevus with histological evaluation. Int J Dermatol. 2014;53(4):e252–3. doi: 10.1111/ijd.12282. [DOI] [PubMed] [Google Scholar]
- 16.Reed RJ. Giant congenital nevi: a conceptualization of patterns. J Invest Dermatol. 1993;100(3):300S–12S. doi: 10.1111/1523-1747.ep12470191. [DOI] [PubMed] [Google Scholar]
- 17.Leech SN, Bell H, Leonard N, et al. Neonatal giant congenital nevi with proliferative nodules: a clinicopathologic study and literature review of neonatal melanoma. Arch Dermatol. 2004;140(1):83–8. doi: 10.1001/archderm.140.1.83. [DOI] [PubMed] [Google Scholar]
- 18.Feito-Rodriguez M, de Lucas-Laguna R, Bastian BC, et al. Nodular lesions arising in a large congenital melanocytic naevus in a newborn with eruptive disseminated Spitz naevi. Br J Dermatol. 2011;165(5):1138–42. doi: 10.1111/j.1365-2133.2011.10502.x. [DOI] [PubMed] [Google Scholar]
- 19.Bastian BC, Xiong J, Frieden IJ, et al. Genetic changes in neoplasms arising in congenital melanocytic nevi: differences between nodular proliferations and melanomas. Am J Pathol. 2002;161(4):1163–9. doi: 10.1016/S0002-9440(10)64393-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Phadke PA, Rakheja D, Le LP, et al. Proliferative nodules arising within congenital melanocytic nevi: a histologic, immunohistochemical, and molecular analyses of 43 cases. The American journal of surgical pathology. 2011;35(5):656–69. doi: 10.1097/PAS.0b013e31821375ea. [DOI] [PubMed] [Google Scholar]
- 21.Hernandez-Martin A, Torrelo A, Echevarria C, Contreras F. Ulcerated sclerotic giant congenital melanocytic naevus: case report and review of the literature. Clin Exp Dermatol. 2007;32(5):529–32. doi: 10.1111/j.1365-2230.2007.02433.x. [DOI] [PubMed] [Google Scholar]
- 22.Murphy MJ, Jen M, Chang MW, Grant-Kels JM, Makkar H. Molecular diagnosis of a benign proliferative nodule developing in a congenital melanocytic nevus in a 3-month-old infant. J Am Acad Dermatol. 2008;59(3):518–23. doi: 10.1016/j.jaad.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 23.Aoyagi S, Akiyama M, Mashiko M, Shibaki A, Shimizu H. Extensive proliferative nodules in a case of giant congenital naevus. Clin Exp Dermatol. 2008;33(2):125–7. doi: 10.1111/j.1365-2230.2007.02542.x. [DOI] [PubMed] [Google Scholar]
- 24.Nguyen TL, Theos A, Kelly DR, Busam K, Andea AA. Mitotically active proliferative nodule arising in a giant congenital melanocytic nevus: a diagnostic pitfall. Am J Dermatopathol. 2013;35(1):e16–21. doi: 10.1097/DAD.0b013e318265fe12. [DOI] [PubMed] [Google Scholar]
- 25.Angelucci D, Natali PG, Amerio PL, Ramenghi M, Musiani P. Rapid perinatal growth mimicking malignant transformation in a giant congenital melanocytic nevus. Hum Pathol. 1991;22(3):297–301. doi: 10.1016/0046-8177(91)90165-l. [DOI] [PubMed] [Google Scholar]
- 26.de Vooght A, Vanwijck R, Gosseye S, Bayet B. Pseudo-tumoral proliferative nodule in a giant congenital naevus. Br J Plast Surg. 2003;56(2):164–7. doi: 10.1016/s0007-1226(03)00090-0. [DOI] [PubMed] [Google Scholar]
- 27.Machan S, Molina-Ruiz AM, Fernandez-Acenero MJ, et al. Metastatic melanoma in association with a giant congenital melanocytic nevus in an adult: controversial CGH findings. Am J Dermatopathol. 2015;37(6):487–94. doi: 10.1097/DAD.0000000000000152. [DOI] [PubMed] [Google Scholar]