Abstract
Objective
Tourette syndrome (TS) and autism spectrum disorder (ASD) share clinical features and possibly an overlapping etiology. This study: 1) examined ASD symptom rates in participants with TS and 2) characterized the relationships between ASD symptom patterns and TS, obsessive-compulsive disorder (OCD), and attention-deficit/hyperactivity disorder (ADHD).
Method
Participants with TS (n = 535) and their family members (n =234) recruited for genetic studies reported TS, OCD, and ADHD symptoms and completed the Social Responsiveness Scale Second Edition (SRS), which was used to characterize ASD symptoms.
Results
SRS scores in participants with TS were similar to those observed in other clinical samples but lower than in ASD samples (mean SRS total raw score = 51; SD=32.4). More children with TS met cut-off criteria for ASD (22.8%) than adults with TS (8.7%). The elevated rate in children was primarily due to high scores on the SRS Repetitive and Restricted Behaviors (RRB) subscale. Total SRS scores were correlated with TS (r=0.27), OCD (r=0.37), and ADHD (r=0.44) and were higher among individuals with OCD symptom-based phenotypes than for those with tics alone.
Conclusion
Higher observed rates of ASD among children affected by TS may in part be due to difficulty in discriminating complex tics and OCD symptoms from ASD symptoms. Careful examination of ASD-specific symptom patterns (social communication vs. repetitive behaviors) is essential. Independent of ASD, the SRS may be a useful tool for identifying patients with TS with impairments in social communication, potentially putting them at risk for bullying and other negative sequelae.
Keywords: Tourette syndrome, autism, obsessive-compulsive disorder, attention-deficit/hyperactivity disorder, heritability
INTRODUCTION
With recognition that chronic tic disorders, including Tourette syndrome (TS) and autism spectrum disorder (ASD), frequently co-occur and share clinical and behavioral features,1,2 researchers are investigating potential overlapping pathophysiologies and genetic etiologies of these disorders.1,3–6 However, the rates of ASD and tic disorder comorbidities are somewhat asymmetrical, depending on which is considered the “primary disorder,” suggesting that at least some of the observed comorbidity may represent phenocopies rather than shared etiologies. Among those with a primary ASD diagnosis (typically ascertained in autism specialty clinics), the rate of co-occurring chronic tic disorders is reported to be 10–25%, compared to the population prevalence of 0.3–2.9%.4,7–11 However, among those with a primary TS diagnosis, rates of ASD are reported to be 5–15%, compared to a population prevalence of 1.9%.12–16 Additionally, chronic tic disorders and ASD have many common features, including stereotyped repetitive behaviors that are aggravated or worsened by heightened emotional states (tics and stereotypies), and higher prevalence in males than females.17 However, the presence and profile of autism spectrum symptoms in individuals affected with TS has not been systematically assessed in a large sample.
This study employed a population-based quantitative assessment, the Social Responsiveness Scale, Second Edition (SRS),18 to investigate the overlap between ASD symptoms and TS in TS-affected individuals and their putatively unaffected family members. Self-report quantitative assessments such as the SRS can be efficiently administered to a large group of individuals, and can be used to assign probable ASD diagnoses (although not necessarily formal DSM-based diagnoses). Assessing quantitative ASD symptom patterns in a large TS sample may also be informative in helping to determine whether some of the observed overlap between TS and ASD is due to symptom (rather than etiological) overlap between the two disorders, and for identifying TS subtypes based on ASD symptomatology. If identified, such subtypes may be useful for studies aimed at better understanding the etiology of TS, including the underlying biology and neural circuitry that account for the frequent co-occurrence of developmental neuropsychiatric disorders like ASD in patients with chronic tic disorders. Importantly, the inclusion of family members allows for heritability studies of specific symptom profiles, including examination of the genetic correlations between ASD symptoms and TS. While symptom-based endophenotypes have been pursued for ASD19,20 and TS21–24 separately, efforts to identify endophenotypes that cross the diagnostic boundaries of TS and ASD have just begun.5
The aims of the present study were to examine the prevalence and patterns of ASD symptoms in relation to TS and other common comorbid developmental neuropsychiatric disorders (i.e., obsessive-compulsive disorder [OCD] and attention-deficit/hyperactivity disorder [ADHD]) in individuals with TS and their first-degree family members. We hypothesized that 1) rates of provisional ASD diagnoses would be higher in this sample than in the general population, 2) these rates would be comparable to other clinical, non-ASD samples, and 3) at least some of the increased prevalence would be due to increased repetitive and restricted behaviors commonly seen in TS (e.g., would represent ASD phenocopies).
METHOD
Sample
The sample included individuals diagnosed with TS and their first-degree family members recruited by Tourette Syndrome Association International Consortium for Genetics (TSAICG) for genetic studies from TS specialty clinics in the US and from the Tourette Association of America. The methods for the parent study are described in detail elsewhere.25,26 Briefly, the inclusion criteria for probands (children with TS identified for the genetic study) included: age 6 years or older, established TS diagnosis, and availability of living parents for family-based genetic analyses. The exclusion criteria were known developmental disability and tics caused by neurological disorders other than TS (e.g., epilepsy, head trauma). Data collection for the parent study spanned multiple years, and the SRS was added at a later time point; therefore only a subset of the sample was available for these analyses. 846 individuals were included in the current sample. The majority were male (64.5%) and had a mean age of 30.9 (SD=18.2).
Procedures
Participants underwent a semi-structured interview and completed self-report measures regarding tic and other neuropsychiatric symptoms (those relevant for this study are described below). Interviews were conducted in person for most participants; for a few individuals, interviews were completed by Skype or over the phone, and self-report measures were returned via mail. The measure of ASD symptoms (SRS) was included for a subset of the participants: the earliest SRS was administered on 4/7/2006, and the most recent SRS was administered on 4/15/2014. All participants provided informed consent (written assent was also obtained for individuals younger than 18 years) and the study was approved by the institutional review boards of each participating site.
Measures
The Social Responsiveness Scale Second Edition (SRS)18 is a 65-item quantitative measure of autism spectrum symptoms that assesses an individual’s ability to engage in emotionally appropriate reciprocal social interactions. It has a normal distribution in the general population, and is highly heritable, with heritability estimates ranging from 56–95%.20,27–29 It has good test-retest reliability (r=0.83) and validity when compared with the gold standard for autism diagnoses, the Autism Diagnostic Interview, Revised (ADI-R; r=0.64 and higher depending on the ADI-R algorithm)30 and the ADOS (ADOS; r=0.26–0.43).31 The SRS contains five treatment subscales derived for clinical utility, including the following domains: social awareness, social cognition, social communication, social motivation, and restricted interests and repetitive behaviors. Factor analysis has identified a two-factor solution that mirrors DSM-5 criteria for ASD (social communication and interaction deficits [SCI] and restricted interests and repetitive behaviors [RRB]).18
The SRS-2 comes in different versions, one for each of three different age ranges, and modified based on whether the respondent is rating their own behavior or another’s. The current study employed the parent report for individuals younger than 18 years old and the adult self-report for individuals age 18 and above (one participating site in this study asked adults to find a relative or someone who know them well to rate them rather than self-report, n=3). Scoring procedures for this study followed those outlined in the SRS-2 administration manual: a case was excluded if 7 or more responses were missing and, if less than 7 were missing, the missing values were replaced with the median score. Total and subscale raw scores were computed, and when possible, age and gender-normed T-scores were calculated for each individual.
Based on previous studies, the following ranges of raw scores are associated with different characteristics: in unaffected samples, SRS raw scores range from 23–53; in mixed or non-ASD clinical samples, SRS raw scores range from 40–75; and in ASD-affected samples, SRS raw scores range from 86–116.18 For the current study, a total SRS raw score of ≥80 was used to identify individuals who likely meet criteria for ASD (e.g., had a provisional ASD diagnosis). Previous research has found a range of cut-off scores (65–85) provide good sensitivity and specificity in various samples for provisional ASD.18 We chose a cut-off of ≥80 based on recommendations from the SRS manual; while a cut-off of 70 is recommended for general screening and 85 is recommended for ASD clinical settings, we wanted a cut-off that would not be as stringent as that used in ASD-specific clinical settings and not as loose as that used in general populations. T-scores (normed based on age and sex distributions) were also examined for both the total score and treatment subscales. For the current study, an age-corrected T-score of >75 was used to identify individuals with a probable autism spectrum disorder (ASD).30
The TSAICG Tic and Comorbid Symptom (TICS) Inventory25,32 was administered by trained clinicians using a semi-structured interview format to assess tic, obsessive-compulsive, and attention-deficit/hyperactivity symptoms. Data from the TICS Inventory, in conjunction with other available clinical data (e.g., medical records), were used by raters to determine diagnostic status for TS, OCD, and ADHD using a best-estimate process.33 Second, individual symptom responses were used to derive rates of TS, OCD, and ADHD symptom endorsement. The TICS Inventory includes a measure of tic severity derived from the Yale Global Tic Severity Scale, comprised of worst-ever tic frequency, severity, and interference ratings; tic complexity was not included in the severity measure. The OCD and ADHD assessments are modified from the Yale Brown Obsessive Compulsive Scale (YBOCS)34 and Swanson, Nolan and Pelham Questionnaire (SNAP-IV).25,32,35
Statistical Analyses
Descriptive statistics and correlation analyses were conducted in SPSS version 19. Using SRS raw scores and a cut-off score of 80, we examined the relationship between provisional ASD diagnoses and tic severity, TS, OCD, and ADHD diagnoses, as well as examining how these relationships differed by gender and age. Using SRS T-scores (cut-off score of 75), we examined the distributions of the treatment subscales and DSM-5 factor-derived subscales among individual affected and unaffected with TS, both children and adults.
A previous study employing latent class analysis (LCA) in this sample identified the following classes of individuals based on their likelihood of endorsing TS, OCD, and ADHD symptoms on the TICS inventory: TS+OCD+ADHD, OCD Symmetry, TS+ADHD, Tics only, and unaffected.36 For the current study, we used the SRS data as an auxiliary variable in the LCA (Mplus version 7.1) to examine the pattern of SRS scores (total scores and DSM-5 subscale scores) in each previously derived latent class. This test is similar to a chi-square test but also takes into account the probability of an individual’s class membership. This analysis was conducted on a restricted sample, as only 415 of the individuals (196 TS affected, 219 TS unaffected; 63% male; age 5–77 years, mean=32.04, SD=18.86) in the original LCA had SRS data available.
Familiality estimates
Familiality estimates were calculated for scores on DSM-5 subscales (SCI and RRB) using the Sequential Oligogenic Linkage Analysis Routine (SOLAR) statistical package. SOLAR employs a variance components approach using information from all available family members across generations and calculates kinship coefficients (since the sample consisted of parent–child trios, we could not separate out the effects of shared genetics and shared environment in these analyses). Age, sex, and sex*age were used as covariates for all familiality analyses.
RESULTS
Rates of Autism Symptoms and Probable ASD
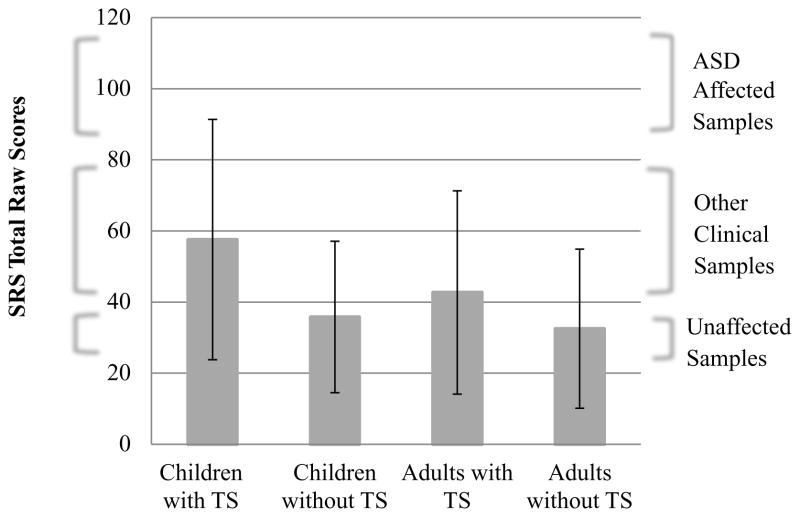
Among the 846 individuals (participants with TS n=535, participants without TS n=234), approximately 12% met criteria for probable ASD (SRS total raw score M=44.8, SD=30.4; see Table 1 and Figure 1). Of those with TS, 18% (n=96) met cutoff criteria for probable ASD, while 3% (n=8) of individuals without TS met cutoff criteria. The SRS demonstrated acceptable internal consistency in the current sample for the total scale score (Cronbach’s α=0.96) and the DSM-5-related subscales (SCI, α=0.95 and RRB, α=0.88). The average SRS scores for those with TS were similar to those observed in other published clinical samples of children but lower than in published ASD samples (mean SRS total raw score=51; SD=32.4; see Figure 1).18 Rates of probable ASD were the highest among participants with TS and, among these, more children than adults met the cut-off criteria for ASD (22.8% vs. 8.7%; due to limited data on children without TS, this subgroup was not included in subsequent analyses).
Table 1.
Sample Characteristics
| All Children | Children With TS | Children Without TS | All Adults | Adults With TS | Adults Without TS | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | 313 | 294 | 10 | 533 | 241 | 224 | ||||||
| n | % | n | % | n | % | n | % | n | % | n | % | |
| Male | 251 | 80.2 | 240 | 81.6 | 6 | 60 | 295 | 55.3 | 161 | 66.8 | 100 | 44.6 |
| M | SD | M | SD | M | SD | M | SD | M | SD | M | SD | |
| Age | 11.4 | 3.6 | 11.5 | 3.7 | 9.7 | 2.9 | 42.4 | 12.8 | 37.6 | 15.0 | 46.9 | 9.2 |
| SRS | 56.5 | 33.7 | 57.6 | 33.8 | 35.8 | 21.3 | 37.8 | 25.8 | 42.7 | 28.6 | 32.5 | 22.4 |
Note: SRS = Social Responsiveness Scale; TS = Tourette Syndrome.
Figure 1.
Social Responsiveness Scales (SRS) raw score distribution among adults and children affected and unaffected by Tourette syndrome (TS). Note: Range of SRS total raw scores for unaffected samples is 23–35, for mixed or non-autism spectrum disorder (ASD) clinical samples, 40–75, and for ASD-affected samples, 86–116, taken from the published literature.
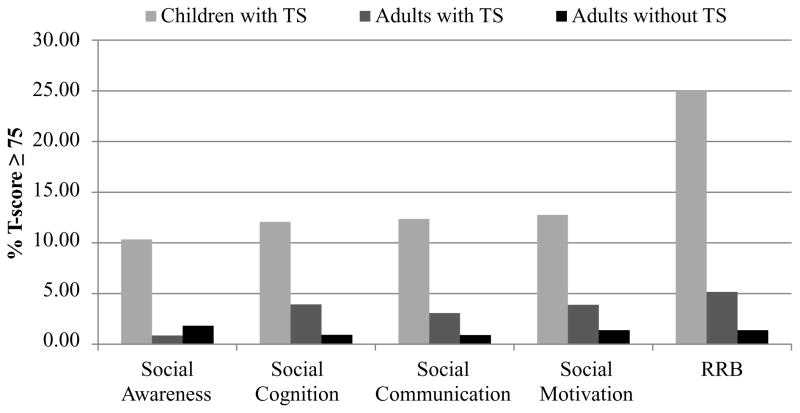
Next we examined the rates of individuals who met the cut-off score for the treatment subscales (Figure 2). The distribution of SRS T-scores for the total scale and subscales among children with TS, adults with TS, and adults without TS can be seen in Figure S1, available online. Overall, only children with TS met ASD cut-off score criteria across all the subscales at elevated rates; the rate of those meeting the cut-off for the four SCI subscales were 10.0–12.7%, while the proportion at or above the ASD cut-off on the restricted interests and repetitive behaviors (RRB) subscale was much higher (25%) (Figure S1, available online). In contrast, only 0.9–5.2% of adults with TS and 0.9–1.8% of adults without TS met cut-off criteria for any of the subscales.
Figure 2.
Percent of those meeting Social Responsiveness Scale cut-off score (T-score ≥75) on treatment subscales among adults and children affected and unaffected by Tourette syndrome (TS). Note: Children without TS were excluded due to small numbers (n=10). RRB = restricted interests and repetitive behaviors.
Associations Between ASD and Other Disorders
Significant correlations were observed between the SRS total raw score and tic severity (r=0.32; p<.001) and TS diagnosis (r=0.27; p<.001), but not OCD (r=0.04; p=.23) or ADHD (r=0.06; p=.11). We also examined differences in gender, OCD, and ADHD rates between those who did and did not meet the cut-off for probable ASD (SRS total raw score >80) among individuals with TS. Chi-square analyses indicated no significant differences in gender (3.9% of TS+ASD participants were female, compared to 21.2% of TS–ASD participants; χ2=1.53, p=.25). However, rates of comorbid OCD (83.3% vs. 52.3%; χ2=30.78, p<.001) and ADHD (78.5% vs. 38.3%; χ2=49.20, p<.001) were significantly higher among individuals with TS who also met criteria for ASD than among those who did not. In comparison, among participants with TS, 7.5% of individuals without OCD and 7.4% of individuals without ADHD met cut-off criteria for probable ASD.
SRS and TS Subphenotypes
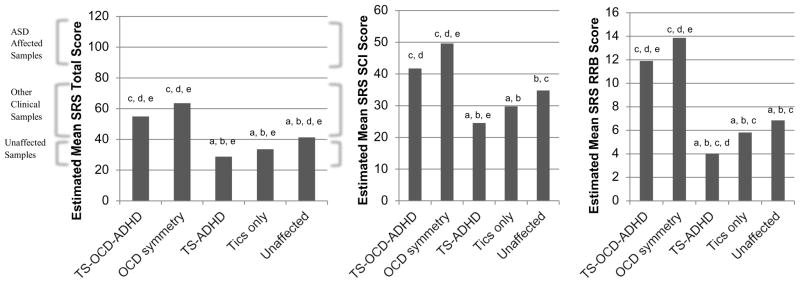
Finally, we examined the relationship between the SRS total scores and previously identified TS sub-phenotypes.36 These sub-phenotypes (TS+OCD+ADHD, OCD symmetry, TS+ADHD, tics only, and unaffected) were empirically derived using latent class analysis (LCA) from the larger set of TS families; the names indicate the predominant symptom patterns seen in each group.36 For example, individuals in the OCD symmetry class had predominantly OCD symmetry symptoms (e.g., evening-up); 20% also had simple motor tics. There were significant differences in SRS scores among the different classes (χ2=128.41, df=4, p<.01). The highest SRS scores were found in the two classes who endorsed OCD symptoms (TS+OCD+ADHD; OCD symmetry; see Figure 3), and were the only classes with mean SRS total scores in the range of non-ASD clinical samples. The overall tests for the association between DSM-5 SRS subscales and LCA classes were also significant (SCI: χ2=39.31, df=4, p<.01; RRB: χ2=186.02, df=4, p<.01). Again, the two classes who endorsed OCD symptoms at high rates had significantly higher SCI and RRB scores than other classes, with the same patterns seen when those with and without OCD symptoms were compared.
Figure 3.
Social Responsiveness Scale (SRS) Total score and DSM-5 subscale scores among Tourette syndrome (TS) sub-phenotypes. Note: letters indicate significant differences between 2 classes (p<.01; a=TS-OCD-ADHD, b=OCD symmetry, c=TS-ADHD, d=tics only, e=unaffected). ADHD = attention-deficit/hyperactivity disorder; ASD = autism spectrum disorder; OCD = obsessive-compulsive disorder; RRB = restricted interests and repetitive behaviors; SCI = social communication and interaction deficits.
Familiality Analyses
The familiality estimates for the SCI and RRB subscales were both significant (Table 2); familiality was higher for the SCI subscale (h2=0.44) than for the RRB subscale (h2=0.30). Additionally, the fit of both models was improved by including TS and OCD as covariates; including these covariates in the models resulted in lower familiality estimates for the SCI and RRB subscales, and the log likelihood statistics showed a significant difference between the models with and without TS and OCD, suggesting that both SRS subscales have a genetic relationship with TS and with OCD.
Table 2.
Familiality Analyses and Model Comparisons for Social Responsiveness Scale DSM-5 Subscales
| N | Familiality Estimate | SD | |
|---|---|---|---|
| SCI | |||
| Base model | 489 | 0.44** | 0.09 |
| w/TS and OCD | 275 | 0.30** | 0.12 |
| RRB | |||
| Base model | 490 | 0.30** | 0.07 |
| w/TS and OCD | 276 | 0.20** | 0.09 |
| Loglikelihood | Chi-square | Df | |
| SCI | |||
| All covariates | −495.40 | ||
| w/o age | −495.55 | 0.30 | 1 |
| w/o sex | −495.72 | 0.65 | 1 |
| w/o age* sex | −496.63 | 2.47 | 1 |
| w/o TS | −497.07 | 3.35* | 1 |
| w/o OCD | −497.70 | 4.61* | 1 |
| w/o TS and OCD | −501.11 | 11.42* | 2 |
| RRB | |||
| All covariates | −497.73 | ||
| w/o age | −498.13 | 0.79 | 1 |
| w/o sex | −497.74 | 0.03 | 1 |
| w/o age* sex | −497.82 | 0.17 | 1 |
| w/o TS | −500.71* | 5.96* | 1 |
| w/o OCD | −503.78* | 12.10* | 1 |
| w/o TS and OCD | −510.32* | 25.18* | 2 |
Note: All models included age, sex and age*sex as covariates. OCD = obsessive-compulsive disorder diagnostic status; RRB = Restricted Interests and Repetitive Behaviors subscale; SCI = Social Communication and Interaction Deficits subscale; SRS = Social Responsiveness Scale; TS = Tourette syndrome diagnostic status.
p≤.1 significance level to screen covariates;
p≤.01
DISCUSSION
This study was spurred by the increasing recognition and interest in the relationship of autism symptoms in the TS population. Using a large TS family-based sample, we explored the prevalence and patterns of ASD symptoms in relation to TS and other common comorbid disorders (i.e., OCD and ADHD). We were particularly interested in determining whether the rates of probable ASD diagnoses were elevated in individuals with TS, as has been previously reported, and if so, if this elevation could be fully or partially explained by the presence of TS (or OCD) related symptoms that mimicked ASD symptoms or by shared genetic etiology.
Our results suggest that, although ASD rates are likely to be elevated in individuals with TS, some of the previously observed elevations in other TS samples may be due to confounding of tic or OCD symptoms for ASD symptoms (or vice versa). The rates of probable ASD using SRS cutoff scores in our current sample are similar to those found in mixed, non-ASD clinical samples—higher than in non-clinical samples and lower than in ASD samples.18 Previous studies have shown that children with mood and anxiety disorders have elevated rates of ASD based on SRS cutoff criteria, suggesting that some of the elevation in SRS scores may reflect underlying psychiatric impairment rather than being specific for ASD. Our finding that 23% of TS-affected participants met cutoff criteria for probable ASD (83% of whom also met criteria for OCD) is in line with this work; in previous studies, 25% of children with anxiety disorders and 38% of children with depression had SRS scores that exceeded the suggested cutoff criteria.37 However, this hypothesis requires testing by validating SRS-derived ASD diagnoses in participants with TS using current gold-standard assessments for ASD (e.g., the Autism Diagnostic Interview-Revised [ADI-R]). Our findings also suggest a role for shared genetic background between social impairment as measured by the SRS and TS and OCD, which may also partially explain the observed elevations in probable ASD rates in our sample. However, a recent study examining genetic correlations between neuropsychiatric disorders found no genetic relationships between TS and ASD, thus this relationship also requires further investigation in additional samples.38
Our other analyses provide additional support for the hypothesis that some of the elevation in SRS scores may be due to general psychiatric impairment. For example, among individuals with TS in our sample, children were more likely to meet ASD criteria than adults (22.8% vs. 8.7%), suggesting that as tics recede in adulthood, ASD-like symptoms may also be less apparent (although we acknowledge that in individuals with high-functioning ASD, social impairment can also improve substantially in adulthood39). Similarly, the rates of probable ASD in individuals with TS alone were comparable to the rates in individuals without TS, and the majority of the elevation in probable ASD rates was seen in participants with TS who also had comorbid OCD or ADHD. Finally, SRS total and subscale scores were substantially higher for participants who had prominent OCD symptoms in the LCA analysis than they were for individuals with tics but no or few OCD symptoms. The fact that scores on the RRB subscale were higher for individuals who endorsed symmetry and/or other OCD symptoms suggests that this SRS subscale may in fact be tapping into common repetitive behaviors seen in individuals with TS and/or OCD (e.g., evening up behaviors), which could be confused with the stereotypies seen in ASD. This is also supported by previous research using factor analysis in children with TS, where items measuring repetitive behaviors in autism loaded onto a factor with OCD-related items rather than onto a factor with social communication items.5
Similarly, the repetitive behaviors (RRB) subscale was particularly elevated for children (but not adults) with TS. Given lower ASD rates in adults with TS in our sample, these higher RRB scores might represent, at least in part, a “false comorbidity” (i.e., the endorsement of similar items on TS and ASD scales that represent the same behavior) rather than separate symptoms from the two different disorders. The lower rate of ASD among adults with TS might be explained if repetitive behaviors on the SRS were endorsed in reference to tics rather than true ASD symptoms, as the majority of tics decrease in adulthood,40 although as noted above, there is evidence to suggest that social skills deficits in ASD also improve with age.39
Taken together, the results of this study suggest that although ASD rates appear to be somewhat elevated over population rates, at least some of the ASD-like symptoms, particularly repetitive behaviors, are more strongly related to comorbid symptoms, in particular, OCD symptoms in this TS sample. Previous research on OCD and autism has been mixed; some studies have found higher rates of symmetry-type OCD symptoms in those with comorbid ASD, while others have not found this difference.41,42,43 In the current study, there was not a significant correlation between the total SRS score and the presence of comorbid OCD, or ADHD. However, when analyses were restricted to participants with TS, higher rates of comorbid OCD and ADHD were found among those that met criteria for ASD. Additionally, the results of the LCA analyses suggest that comorbid OCD is strongly related to the presence of ASD symptoms in individuals with TS. The highest ASD symptom scores were associated with the TS sub-phenotypes that included OCD symptoms; this pattern held for the total SRS scores as well as for both the SCI and RRB subscale scores. Future research will be needed to examine how much of the relationship between OCD and ASD symptoms is due to false comorbidity and how much represents an underlying, possibly genetic relationship between TS, OCD, and ADHD.
The familiality estimates in our current study provide some interesting preliminary information about the genetic relationships underlying the elevation of ASD symptoms in our TS families. RRB scores, and SCI scores, which are less likely to be confounded with tics or symmetry symptoms than RRB scores, were both elevated in our sample, and our analyses indicated higher familiality estimates for the SCI subscale than for the RRB subscale. Interestingly, both familiality estimates were lower when TS and OCD were included in the models. These revised estimates (h2r=0.30 for SCI and 0.20 for RRB) represent the true familiality of each subscale and are not confounded by the familiality of TS or OCD. However, our work cannot determine whether the familiality and phenotypic variance of the SRS subscales that remains after controlling for TS and OCD is due to a true autism-spectrum phenotype, or whether it represents impairment in social interactions due to other causes (i.e., subclinical mood and/or anxiety symptoms). Future research along this line should use clinical assessment instruments specifically designed to identify individuals with autism spectrum disorder to arrive at a true estimate of the comorbidity between TS and ASD and to examine the familiality of ASD in TS families. Similarly, additional focus on social communication symptoms, independent of ASD diagnoses, in participants with TS is warranted.
There are some limitations to this work. In particular, the small sample size available for the familiality estimates and the fact that most families were parent—child trios rather than extended families limits the interpretation of these results. Similarly, the analyses examining the relationship between SRS scores and previously derived latent classes were conducted on a subsample based on available data. Additionally, the observed difference in rates of ASD symptoms between children and adults with TS may be due to an ascertainment bias, in that some of the adults with TS in our sample were parents of children with TS. Also, the SRS was completed by caregivers for children, but the majority of SRS for adults were self-reported; whether this caused the differences between children and adult ratings cannot be ruled out. Finally, it should be noted that we do not have clinical assessments of ASD symptoms or ASD diagnoses or measures of intellectual or language functioning in our sample. We relied on self or parent reports using the SRS, which is a commonly used instrument both in research, and more recently, because if its ease of administration and established cut-offs, in clinical practice.44 Therefore, future research will be needed replicate and extend our findings.
Nevertheless, this work has important implications for clinicians who diagnose or treat individuals with TS. In such individuals, particularly if there is concern about possible ASD, a careful clinical examination of symptom patterns that are specific to ASD is essential, with a particular focus on social communication deficits, as measured by the SCI component of the SRS, for example, rather than repetitive behaviors, as measured by the RRB component, as an indication of ASD. Supplementary measures such as the Social Communication Questionnaire (SCQ) may also be useful in screening for ASD in TS-affected children.45 In addition, even in the absence of ASD, the SRS may be a very useful tool for identifying children with TS who have such social communication deficits, potentially putting them at increased risk for negative sequelae among their peers.
Supplementary Material
Distributions of Social Responsiveness Scale DSM-5 subscale T-scores. Note: RRB = restricted interests and repetitive behaviors; SCI = social communication and interaction deficits; TS = Tourette syndrome.
Acknowledgments
Funding
This work was supported by the National Institutes of Health, grant numbers R01MH096767 (PI: Carol Mathews), U01NS040024 (PI: David Pauls/Jeremiah Scharf), K23MH085057 (PI: Jeremiah Scharf), K02MH00508 (PI: David Pauls), and R01NS016648 (PI: David Pauls), and from the Tourette Association of America.
Dr. Kevin Delucchi served as the statistical expert.
The authors acknowledge the participants of the study, the Tourette Association of America, and the members of the Tourette Syndrome Association International Genetics Consortium. The authors would also like to acknowledge John N. Constantino, MD, of Washington University, one of the developers of the Social Responsiveness Scale, who provided guidance on the analytic approach used in this manuscript.
Footnotes
Disclosures
Dr. Darrow has received grant funding from the NIH.
Dr. Grados has received grant funding from the International Obsessive Compulsive Disorder Foundation (IOCDF), the Tourette Association of America (TAA), and the NIH.
Dr. Sandor has received Unrestricted Educational Grants in support of Conferences he organized from Purdue and Shire and CME speaker fee from Purdue. He was paid for work on Data Safety Monitoring Committee by Psyadon.
Dr. Hirschtritt has received funding through a training grant administered by the National Institute of Mental Health (R25 MH060482).
Dr. Dion has received research support from the Tourette Association of America (TAA).
Dr. King has received research support from the Tourette Association of America (TAA).
Dr. Budman has received research support from the Tourette Association of America (TAA). She reports funding for clinical research studies from Neurocrine Pharmaceuticals, Psyadon Pharmaceuticals, Otsuka Pharmaceuticals, Synchroneuron Pharmaceuticals, Teva Pharmaceuticals, and Auspex Pharmaceuticals. She has also been a speaker for the TAA and the Center for Disease Control Partnership and a consultant to Bracket.
Dr. Cath has received grant funding from ZONMW, Fonds Psychische Gezondheid, Hersenstichting, Fonds Nuts Ohra, Tourette Syndrome Association USA, and Marie Curie Initial Training Networks (ITN); call: FP7-PEOPLE-2012-ITN.
Dr. Lyon has served on advisory boards for GenePeeks, Inc., Omicia, Inc., and Seven Bridges Genomics, Inc., is a consultant to Genos, Inc., and previously served as a consultant to Good Start Genetics, Inc.
Dr. McMahon is a paid consultant for Pathways to Careers, an employment program for adults with disabilities and a co-inventor of a patent for a DNA based test for autism screening at the University of Utah in collaboration with LineaGen, a for profit company.
Dr. Delucchi has received grant funding from the NIH.
Dr. Scharf is a member of the Scientific Advisory Board of the Tourette Association of America (TAA) and the TLC Foundation for Body-Focused Repetitive Behaviors (BFRBs). He has received travel and grant support from the TAA and the TLC Foundation for BFRBs, honoraria from the Centers for Disease Control and Prevention on behalf of the TAA, and consulting fees from Nuvelution, Pharma and Abide Pharmaceuticals.
Dr. Mathews is the co-chair of the Scientific Advisory Board of the Tourette Association of America (TAA). She has received grant funding, travel reimbursement, and speakers honoraria from the TAA. She has also received grant funding from the International Obsessive Compulsive Disorder Foundation (IOCDF) and the NIH.
Drs. Illmann, Pauls, Greenberg, Lee and Ms. Osiecki report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Dr. Sabrina M. Darrow, University of California, San Francisco.
Dr. Marco Grados, Johns Hopkins University School of Medicine, Baltimore.
Dr. Paul Sandor, University of Toronto and University Health Network, and Youthdale Treatment Centers, Ontario, Canada.
Dr. Matthew E. Hirschtritt, University of California, San Francisco.
Dr. Cornelia Illmann, Psychiatric and Neurodevelopmental Genetics Unit, Massachusetts General Hospital, Boston.
Ms. Lisa Osiecki, Psychiatric and Neurodevelopmental Genetics Unit, Massachusetts General Hospital, Boston.
Dr. Yves Dion, University of Montreal, Quebec, Canada.
Dr. Robert King, Yale Child Study Center, Yale University School of Medicine, New Haven, CT.
Dr. David Pauls, Psychiatric and Neurodevelopmental Genetics Unit, Massachusetts General Hospital, Boston.
Dr. Cathy L. Budman, North Shore/Long Island Jewish Health System, Feinstein Institute for Medical Research, Manhasset, NY.
Dr. Danielle C. Cath, University of Groningen, University Medical Center Groningen; Utrecht University; and Drenthe Mental Health Institution, Assen, the Netherlands.
Dr. Erica Greenberg, Psychiatric and Neurodevelopmental Genetics Unit, Massachusetts General Hospital, Boston.
Dr. Gholson J. Lyon, Stanley Institute for Cognitive Genomics, Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
Dr. William M. McMahon, University of Utah, Salt Lake City.
Dr. Paul C. Lee, Tripler Army Medical Center, Honolulu.
Dr. Kevin L. Delucchi, University of California, San Francisco.
Dr. Jeremiah M. Scharf, Psychiatric and Neurodevelopmental Genetics Unit, Massachusetts General Hospital, Boston. Massachusetts General and Brigham and Women’s Hospitals, Boston.
Dr. Carol A. Mathews, University of Florida, Gainesville.
References
- 1.Clarke RA, Lee S, Eapen V. Pathogenetic model for Tourette syndrome delineates overlap with related neurodevelopmental disorders including Autism. Transl Psychiatry. 2012;2:e158. doi: 10.1038/tp.2012.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robertson MM. A personal 35 year perspective on Gilles de la Tourette syndrome: prevalence, phenomenology, comorbidities, and coexistent psychopathologies. The lancet Psychiatry. 2015;2(1):68–87. doi: 10.1016/S2215-0366(14)00132-1. [DOI] [PubMed] [Google Scholar]
- 3.Scharf JM, Mathews CA. Copy number variation in Tourette syndrome: another case of neurodevelopmental generalist genes? Neurology. 2010;74(20):1564–1565. doi: 10.1212/WNL.0b013e3181e24161. [DOI] [PubMed] [Google Scholar]
- 4.Canitano R, Vivanti G. Tics and Tourette syndrome in autism spectrum disorders. Autism. 2007;11(1):19–28. doi: 10.1177/1362361307070992. [DOI] [PubMed] [Google Scholar]
- 5.Huisman-van Dijk HM, Schoot Rvd, Rijkeboer MM, Mathews CA, Cath DC. The relationship between tics, OC, ADHD and autism symptoms: A cross- disorder symptom analysis in Gilles de la Tourette syndrome patients and family-members. Psychiatry Research. 2016;237:138–146. doi: 10.1016/j.psychres.2016.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fernandez TV, Sanders SJ, Yurkiewicz IR, et al. Rare copy number variants in tourette syndrome disrupt genes in histaminergic pathways and overlap with autism. Biol Psychiatry. 2012;71(5):392–402. doi: 10.1016/j.biopsych.2011.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rapin I. Autism spectrum disorders: relevance to Tourette syndrome. Adv Neurol. 2001;85:89–101. [PubMed] [Google Scholar]
- 8.Baron-Cohen S, Scahill VL, Izaguirre J, Hornsey H, Robertson MM. The prevalence of Gilles de la Tourette syndrome in children and adolescents with autism: a large scale study. Psychol Med. 1999;29(5):1151–1159. doi: 10.1017/s003329179900896x. [DOI] [PubMed] [Google Scholar]
- 9.Scharf JM, Miller LL, Gauvin CA, Alabiso J, Mathews CA, Ben-Shlomo Y. Population prevalence of Tourette syndrome: a systematic review and meta-analysis. Mov disord. 2015;30:221–228. doi: 10.1002/mds.26089. [DOI] [PubMed] [Google Scholar]
- 10.Simonoff E, Pickles A, Charman T, Chandler S, Loucas T, Baird G. Psychiatric disorders in children with autism spectrum disorders: prevalence, comorbidity, and associated factors in a population-derived sample. J Am Acad Child Adolesc Psychiatry. 2008;47:921–9. doi: 10.1097/CHI.0b013e318179964f. [DOI] [PubMed] [Google Scholar]
- 11.Lanzi G, Zambrino CA, Termine C, et al. Prevalence of tic disorders among primary school students in the city of Pavia, Italy. Arch Dis Child. 2004;89:45–47. [PMC free article] [PubMed] [Google Scholar]
- 12.Khalifa N, von Knorring AL. Psychopathology in a Swedish population of school children with tic disorders. J Am Acad Child Adolesc Psychiatry. 2006;45:1346–1353. doi: 10.1097/01.chi.0000251210.98749.83. [DOI] [PubMed] [Google Scholar]
- 13.Burd L, Li Q, Kerbeshian J, Klug MG, Freeman RD. Tourette syndrome and comorbid pervasive developmental disorders. J Child Neurol. 2009;24(2):170–175. doi: 10.1177/0883073808322666. [DOI] [PubMed] [Google Scholar]
- 14.Kim YS, Leventhal BL, Koh YJ, et al. Prevalence of autism spectrum disorders in a total population sample. Am J Psychiatry. 2011;168:904–912. doi: 10.1176/appi.ajp.2011.10101532. [DOI] [PubMed] [Google Scholar]
- 15.Kadesjo B, Gillberg C. Tourette’s disorder: epidemiology and comorbidity in primary school children. J Am Acad Child Adolesc Psychiatry. 2000;39:548–555. doi: 10.1097/00004583-200005000-00007. [DOI] [PubMed] [Google Scholar]
- 16.Cath D, Ludolph AG. Other psychiatric comorbidities in tourette syndrome. In: Martino D, Leckman JF, editors. Tourette Syndrome. New York: Oxford University Press; 2012. pp. 74–106. [Google Scholar]
- 17.Zinner SH, Coffey BJ. Developmental and behavioral disorders grown up: Tourette’s disorder. J Dev Behav Pediatr. 2009;30:560–573. doi: 10.1097/DBP.0b013e3181bd7f3e. [DOI] [PubMed] [Google Scholar]
- 18.Constantino JN, Gruber CP. Social Responsiveness Scale. 2. Torrance, CA: Western Psychologial Services; 2012. [Google Scholar]
- 19.Constantino JN, Todd RD. Intergenerational transmission of subthreshold autistic traits in the general population. Biol Psychiatry. 2005;57:655–660. doi: 10.1016/j.biopsych.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 20.Constantino JN, Todd RD. Autistic traits in the general population: a twin study. Arch Gen Psychiatry. 2003;60:524–530. doi: 10.1001/archpsyc.60.5.524. [DOI] [PubMed] [Google Scholar]
- 21.Cavanna AE, Critchley HD, Orth M, Stern JS, Young MB, Robertson MM. Dissecting the Gilles de la Tourette spectrum: a factor analytic study on 639 patients. J Neurol Neurosurg Psychiatry. 2011;82:1320–1323. doi: 10.1136/jnnp.2010.225029. [DOI] [PubMed] [Google Scholar]
- 22.Davis LK, Yu D, Keenan CL, et al. Partitioning the heritability of Tourette syndrome and obsessive compulsive disorder reveals differences in genetic architecture. PLoS genetics. 2013;9(10):e1003864. doi: 10.1371/journal.pgen.1003864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Haan MJ, Delucchi KL, Mathews CM, Cath DC. Tic symptom dimensions and their heritabilities in Tourette’s syndrome. Psychiatr Genet. 2015;25(3):112–118. doi: 10.1097/YPG.0000000000000084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grados MA, Mathews CA. Latent Class Analysis of Gilles de la Tourette Syndrome Using Comorbidities: Clinical and Genetic Implications. Biol Psychiatry. 2008;64:219–25. doi: 10.1016/j.biopsych.2008.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Darrow SM, Illmann C, Gauvin C, et al. Web-based phenotyping for Tourette Syndrome: Reliability of common co-morbid diagnoses. Psychiatry Res. 2015;228:816–825. doi: 10.1016/j.psychres.2015.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scharf JM, Yu D, Mathews CA, et al. Genome-wide association study of Tourette’s syndrome. Molecular psychiatry. 2013;18:721–728. doi: 10.1038/mp.2012.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Constantino JN, Przybeck T, Friesen D, Todd RD. Reciprocal social behavior in children with and without pervasive developmental disorders. J Dev Behav Pediatr. 2000;21:2–11. doi: 10.1097/00004703-200002000-00002. [DOI] [PubMed] [Google Scholar]
- 28.Colvert E, Tick B, McEwen F, et al. Heritability of Autism Spectrum Disorder in a UK Population-Based Twin Sample. JAMA psychiatry. 2015;72:415–423. doi: 10.1001/jamapsychiatry.2014.3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Freitag CM. The genetics of autistic disorders and its clinical relevance: a review of the literature. Molecular psychiatry. 2007;12:2–22. doi: 10.1038/sj.mp.4001896. [DOI] [PubMed] [Google Scholar]
- 30.Constantino JN, Davis SA, Todd RD, et al. Validation of a brief quantitative measure of autistic traits: comparison of the social responsiveness scale with the autism diagnostic interview-revised. J Autism Dev Disord. 2003;33:427–433. doi: 10.1023/a:1025014929212. [DOI] [PubMed] [Google Scholar]
- 31.Constantino JN, Lavesser PD, Zhang Y, Abbacchi AM, Gray T, Todd RD. Rapid quantitative assessment of autistic social impairment by classroom teachers. J Am Acad Child Adolesc Psychiatry. 2007;46:1668–1676. doi: 10.1097/chi.0b013e318157cb23. [DOI] [PubMed] [Google Scholar]
- 32.Tourette Syndrome Association International Consortium for G. Genome scan for Tourette disorder in affected-sibling-pair and multigenerational families. Am J Hum Genet. 2007;80:265–272. doi: 10.1086/511052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leckman JF, Sholomskas D, Thompson WD, Belanger A, Weissman MM. Best estimate of lifetime psychiatric diagnosis: a methodological study. Arch Gen Psychiatry. 1982;39:879–883. doi: 10.1001/archpsyc.1982.04290080001001. [DOI] [PubMed] [Google Scholar]
- 34.Goodman WK, Price LH, Rasmussen SA, et al. The Yale-Brown Obsessive Compulsive Scale. I. Development, use, and reliability. Arch Gen Psychiatry. 1989;46:1006–1011. doi: 10.1001/archpsyc.1989.01810110048007. [DOI] [PubMed] [Google Scholar]
- 35.Swanson JM. School-Based Assessments and Interventions for ADD Students. Irvine, CA: KC Publishing; 1992. [Google Scholar]
- 36.Darrow SM, Hirschtritt ME, Davis LK, et al. Symmetry and disinhibition are heritable cross-disorder endophenotypes for Tourette Syndrome [published online ahead of print November 2016] Am J Psychiatry. doi: 10.1176/appi.ajp.2016.16020240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pine DS, Guyer AE, Goldwin M, Towbin KA, Leibenluft E. Autism spectrum disorder scale scores in pediatric mood and anxiety disorders. J Am Acad Child Adolesc Psychiatry. 2008;47:652–661. doi: 10.1097/CHI.0b013e31816bffa5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Anttila V, Bulik-Sullivan B, Finucane HK, et al. Analysis of shared heritability in common disorders of the brain [published online ahead of print May 2016] bioRxiv. doi: 10.1101/048991. [DOI] [Google Scholar]
- 39.Seltzer MM, Krauss MW, Shattuck PT, Orsmond G, Swe A, Lord C. The symptoms of autism spectrum disorders in adolescence and adulthood. J Autism Dev Disord. 2003;33:565–581. doi: 10.1023/b:jadd.0000005995.02453.0b. [DOI] [PubMed] [Google Scholar]
- 40.Bruun RD, Budman CL. The course and prognosis of Tourette syndrome. Neurologic Clinics. 1997;15(2):291–298. doi: 10.1016/s0733-8619(05)70313-3. [DOI] [PubMed] [Google Scholar]
- 41.Kano Y, Ohta M, Nagai Y, Pauls DL, Leckman JF. Obsessive-compulsive symptoms in parents of Tourette syndrome probands and autism spectrum disorder probands. Psychiatry and Clinical Neurosciences. 2004;58(4):348–352. doi: 10.1111/j.1440-1819.2004.01266.x. [DOI] [PubMed] [Google Scholar]
- 42.Cath DC, Ran N, Smit JH, van Balkom AJLM, Comijs HC. Symptom overlap between autism spectrum disorder, generalized social anxiety disorder and obsessive-compulsive disorder in adults: A preliminary case-controlled study. Psychopathol. 2008;41:101–110. doi: 10.1159/000111555. [DOI] [PubMed] [Google Scholar]
- 43.Lewin AB, Wood JJ, Gunderson S, Murphy TK, Storch EA. Phenomenology of comorbid autism spectrum and obsessive-compulsive disorders among children. J Dev Phys Disabil. 2011;23:543–553. [Google Scholar]
- 44.Aldridge FJ, Gibbs VM, Schmidhofer K, Williams M. Investigating the clinical usefulness of the Social Responsiveness Scale (SRS) in a tertiary level, autism spectrum disorder specific assessment clinic. J Autism Dev Disord. 2012;42:294–300. doi: 10.1007/s10803-011-1242-9. [DOI] [PubMed] [Google Scholar]
- 45.Stewart E, Cancilliere MK, Freeman J, et al. Elevated autism spectrum disorder traits in young children with OCD. Child Psychiatry Hum Dev. 2016;47:993–1001. doi: 10.1007/s10578-016-0629-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Distributions of Social Responsiveness Scale DSM-5 subscale T-scores. Note: RRB = restricted interests and repetitive behaviors; SCI = social communication and interaction deficits; TS = Tourette syndrome.