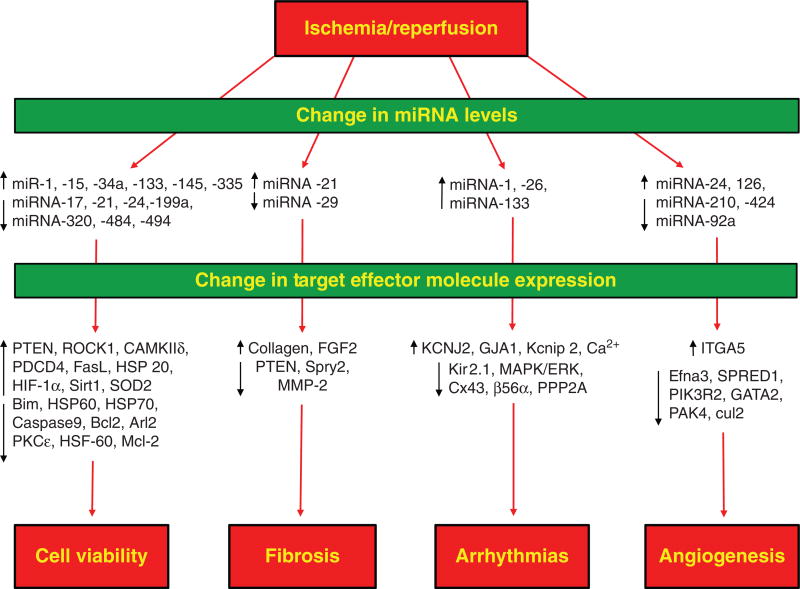
Abstract
Ischemic disorders, such as myocardial infarction, stroke, and peripheral vascular disease, are the most common causes of debilitating disease and death in westernized cultures. The extent of tissue injury relates directly to the extent of blood flow reduction and to the length of the ischemic period, which influence the levels to which cellular ATP and intracellular pH are reduced. By impairing ATPase-dependent ion transport, ischemia causes intracellular and mitochondrial calcium levels to increase (calcium overload). Cell volume regulatory mechanisms are also disrupted by the lack of ATP, which can induce lysis of organelle and plasma membranes. Reperfusion, although required to salvage oxygen-starved tissues, produces paradoxical tissue responses that fuel the production of reactive oxygen species (oxygen paradox), sequestration of proinflammatory immunocytes in ischemic tissues, endoplasmic reticulum stress, and development of postischemic capillary no-reflow, which amplify tissue injury. These pathologic events culminate in opening of mitochondrial permeability transition pores as a common end-effector of ischemia/reperfusion (I/R)-induced cell lysis and death. Emerging concepts include the influence of the intestinal microbiome, fetal programming, epigenetic changes, and microparticles in the pathogenesis of I/R. The overall goal of this review is to describe these and other mechanisms that contribute to I/R injury. Because so many different deleterious events participate in I/R, it is clear that therapeutic approaches will be effective only when multiple pathologic processes are targeted. In addition, the translational significance of I/R research will be enhanced by much wider use of animal models that incorporate the complicating effects of risk factors for cardiovascular disease.
Introduction
Although myocardial necrosis and severe coronary atherosclerotic disease were recognized in autopsies performed in the 1800s, thrombi were not typically observed in the coronary arteries supplying the infarcted region of the myocardium. The latter observation, coupled with the fact that the extent of coronary atherosclerosis was highly variable in the autopsied hearts, made clinicians of this era reluctant to conclude that an interruption of the arterial inflow was a causative factor in myocardial infarction (380). Even though experimental occlusion of major coronary arteries was shown to produce myocardial infarction in the affected regions of dog hearts in the 1880s, it was not until 100 years later, when DeWood and co-workers (191) demonstrated that patients with early signs of myocardial infarction almost always presented with an thrombotic occlusion of the artery supplying the affected region of their hearts. Importantly, thrombolysis not only restored arterial inflow in these catheterized patients, many of the clinical and electrocardiographics signs of developing infarcts were also reversed. These studies not only established that coronary ischemia was indeed a causative factor inmyocardial infarction but also suggested that endothelial fibrinolysins dissolved the clot that caused the infarction in autopsied patients who died 24 h after the onset of symptoms.
Well before the advent of thrombolytic therapy, it was discovered that reestablishing the blood supply, which is required to salvage previously ischemic tissue that had not progressed to irreversible injury, could paradoxically exacerbate tissue injury. First suggested by Jennings et al. (382) in 1960, the existence of reperfusion injury has been the subject of intense debate, with some investigators suggesting that reperfusion acts to worsen damage already sustained by cells exposed to ischemia (59, 482). This controversy relates to the inability to determine necrotic progress during the transition from tissue ischemia to reperfusion. However, the ability of interventions initiated when the blood supply is reestablished to reduce cellular damage and infarct size to levels below the protection afforded by reperfusion alone strongly supports the concept of lethal reperfusion injury (405, 881).
Recognition that pathologic events occurring during both ischemia and reperfusion contribute to tissue injury led to accelerated efforts to identify the mechanisms of ischemia/reperfusion (I/R) injury, with the hope for identifying novel treatments that might limit injury induced by the reduction in blood flow and/or damage produced iatrogenically by reperfusion. A remarkable series of impressive findings have been reported in the past 40 years, owing to a rapidly growing repertoire of sophisticated new techniques. From this work, it is now clear that ischemia impairs ATPase-dependent ion transport and disrupts cell volume regulatory mechanisms, which can lead to lysis of organelle and plasma membranes. In addition, new work has uncovered multiple death modalities that contribute to I/R-induced cell death, many of which occur by programmed sequences of events that may be amenable to pharmacologic intervention. Moreover, reperfusion produces paradoxical tissue responses that fuel the production of reactive oxygen and nitrogen species and promotes sequestration of proinflammatory immunocytes in ischemic tissues, endoplasmic reticulum stress, and development of postischemic capillary no-reflow, which amplify tissue injury. The aforementioned pathologic events culminate in opening of mitochondrial permeability transition pores (MPTPs) as a common end-effector of I/R-induced cell lysis and death. In addition to these mechanisms, much recent attention has focused on the influence of the intestinal microbiome, fetal exposure to stressors, epigenetic alterations in gene expression, proteolytic digestion products, and microparticles in the pathogenesis of I/R. In this review, we will summarize our current understanding of this plethora of pathologic contributors to the genesis of I/R injury, but will focus most of our attention on the reperfusion component of total tissue injury, since this segment is the most amenable to therapeutic intervention.
General Characteristics of Ischemia/Reperfusion
Both the degree to which blood flow is reduced and length of the ischemic period influence the extent of cell dysfunction, injury, and/or death (2, 113, 380, 405) (Fig. 1). This fact underscores the importance of rapid blood flow restoration as the mainstay of all therapies to limit ischemic injury. In this regard, it is important to note different organ systems display differential vulnerability to ischemia. Furthermore, in any given organ, cells can survive short periods of ischemia (duration dependent on the organ), with a variable proportion of those cells able to withstand longer ischemic bouts than others. This is termed reversible injury. With increasing durations of ischemia, a growing number of cells die, sustaining injuries that are irreversible and are characterized by loss of structural integrity in affected tissues.
Figure 1.
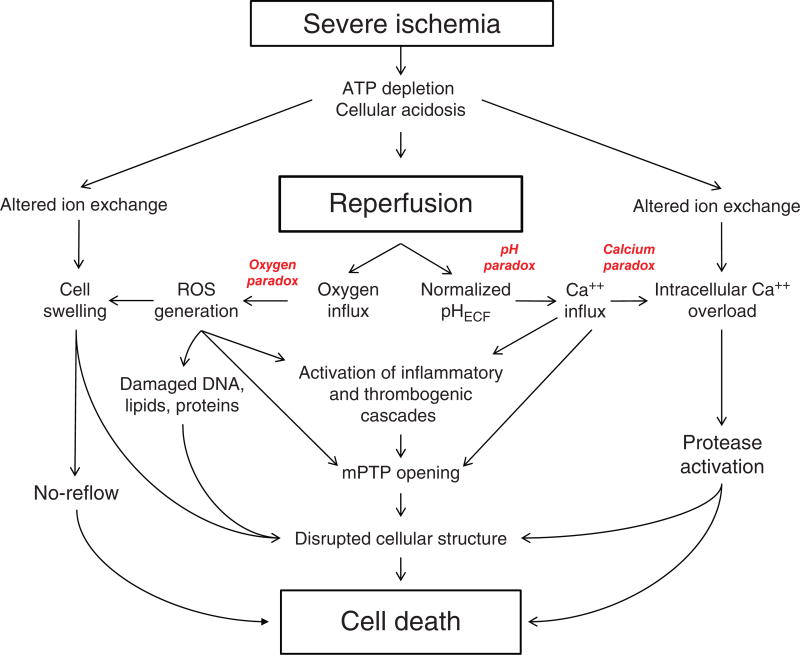
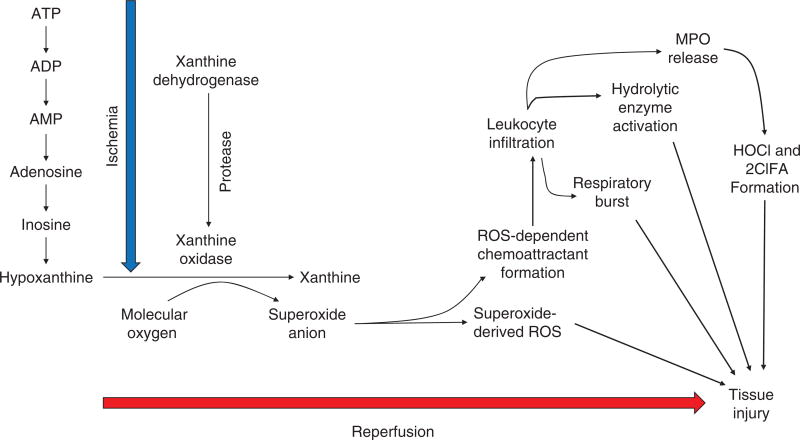
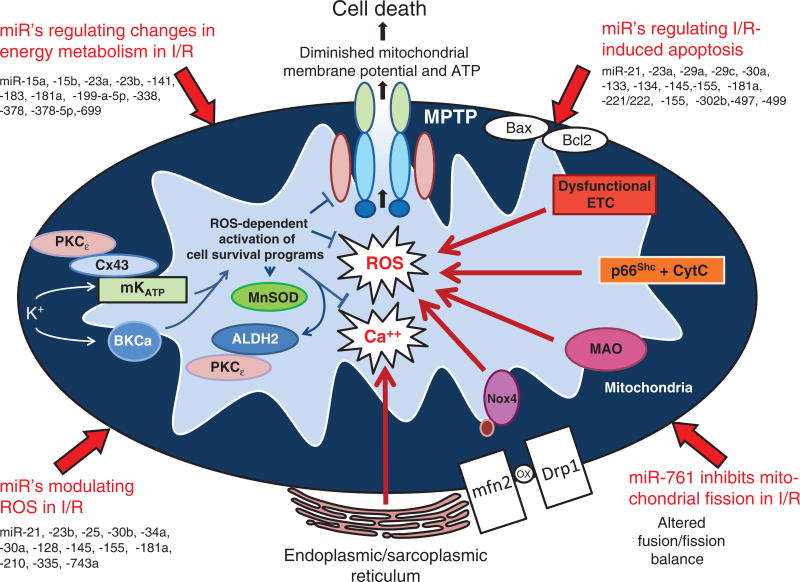
Major pathologic events contributing to ischemia/reperfusion injury. When the blood supply is markedly reduced or absent, ischemic cells switch to anaerobic metabolism to provide ATP. However, this results in cellular acidosis and insufficient ATP production to meet metabolic demand. As a consequence, ATPases are inactivated, while active Ca2+ efflux and Ca2+ reuptake by the endoplasmic reticulum are markedly reduced, with the net effect of this abherent ion transport producing Ca2+ overload in the cell. In addition, xanthine dehydrogenase is converted to XO during ischemia (see Fig. 7), coincident with accumulation of hypoxanthine, one of the substrates required to drive its enzymatic activity. On reperfusion, the delivery of oxygen and substrates required for aerobic ATP generation is restored as is extracellular pH via washout of accumulated H+ (pH paradox). The latter event promotes additional Ca2+ influx (calcium paradox), while the influx of oxygen fuels XO-driven production of ROS (oxygen paradox) (see Fig. 7). ROS produced by this and other mechanisms can damage virtually every biomolecule found in cells, promote opening of mitochondrial PTPs, and activate inflammatory and thrombogenic cascades to exacerbate cell injury. The latter events are further amplified by release of danger signals (e.g., ATP) and other proinflammatory and thrombogenic mediators from damaged cells (see text for further explanation). The ensuing massive influx of immunocytes at previously ischemic sites contribute to cell injury via the NADPH oxidase-driven respiratory burst, release of hydrolytic enzymes, and production of MPO-derived hypochlorous acid and N-chloramines. The development of the capillary no-reflow phenomenon during reperfusion results in nutritive perfusion impairment by mechanisms outlined in Figure 11.
It now seems clear that recanalization of occluded vessels, although necessary to reestablish oxygen and nutrient delivery necessary for cell function and for abstraction of cellular metabolites from the ischemic region, can provoke activation of deleterious processes that damage previously unaffected cells and also exacerbate injury due to ischemia per se. However, the situation is further complicated by metabolite/mediator transit into the blood draining reperfused tissues, which then travel to distant organs via the bloodstream to produce injury. Finally, short bouts of I/R (ischemic conditioning), which were long thought of as innocuous, are now recognized to activate cell survival programs that allow tissues to better withstand the onslaught of pathogenetic events invoked by lethal I/R. Thus, the response to I/R is bimodal, with short bouts conferring cardioprotection, while longer periods provoke cell dysfunction and death. These issues will be discussed in greater detail later.
Mechanistically distinct pathologic processes are invoked during the phases of ischemia and reperfusion
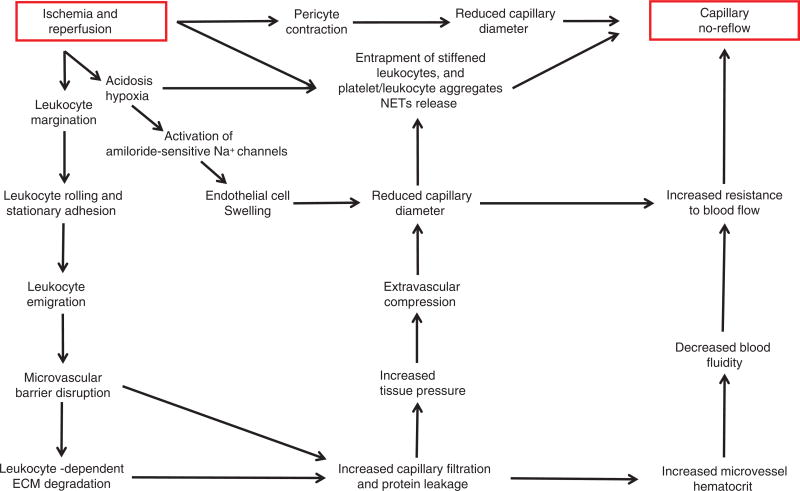
When the blood supply is reduced secondary to thrombosis, cells switch to anaerobic metabolism resulting in reductions in cell pH and ATP production (Fig. 1). As a consequence, the Na+/H+ exchanger (NHE) extrudes accumulating hydrogen ions in exchange for sodium ions (683). The lack of oxygen delivery forces cells to manufacture ATP anaerobically but this production occurs at levels insufficient to maintain the function of ATPases (e.g., Na+/K+ ATPase). This results in cellular calcium overload as a consequence of reduced active Ca++ reuptake into the endoplasmic reticulum and ATPase-dependent Ca++ efflux across the plasmalemma. Disruption of mitochondrial architecture occurs simultaneously, with prominent features being swelling, disorganized cristae, and the appearance of fuzzy osmophilic densities in the matrix space. In addition, mitochondrial membrane potential is dissipated secondary to opening of mitochondrial PTPs and inner membrane anion channels, which further impairs ATP production. In the heart, hypercontracture and contracture band necrosis are produced by myofibrillar damage secondary to ischemia-induced activation of intracellular proteases (e.g., calpains). Disruption of the plasma membrane as well as subcellular organelle membranes, secondary to swelling and altered ion movements allows intracellular components to leak into the extracellular fluid and disrupts energy metabolism (Fig. 1). Damage to capillary endothelial cells in the ischemic region occurs on a slower time scale, reflecting their lower requirement for energy. However, these cells swell during ischemia thereby reducing capillary lumenal diameter, which facilitates impaction of neutrophils in these microvessels. Development of this capillary no-reflow phenomenon worsens during reperfusion, limiting the delivery of arterial blood and pharmacologic agents into the ischemic region as well as preventing washout of accumulating metabolic wastes. In the brain, ischemia-induced contraction of pericytes surrounding microvessels also contributes to no-reflow. Microvascular permeability changes also occur as a consequence of I/R, leading to edema formation and increased interstitial fluid pressure. Edema formation increases the diffusion distance for oxygen and nutrients while the rise in tissue pressure contributes to no-reflow by physically compressing microvessels. The latter mechanism is especially important for organs that cannot readily increase their interstitial fluid spaces such as the brain, kidneys, and specific skeletal muscles where expansion is limited by the cranial vault, renal capsule, or encasement in tight fascial sheaths, respectively.
Prompt restoration of blood flow removes hydrogen ions that accumulated in the extracellular space during ischemia and also provides oxygen and substrates required for aerobic ATP generation. However, as first reported by Jennings et al. (380, 382) over 50 years ago, reperfusion is not without peril. In this landmark study, it was noted that reperfusion accelerated the development of myocardial necrosis. This led to the concept that reperfusion was not entirely beneficial, but rather produced injury by pathologic events associated with reestablishing the blood supply that had not occurred during the preceding ischemic period. Indeed, subsequent work showed that cell death can continue for up to 3 days after blood flow is restored to ischemic tissues (909, 915). Perhaps the strongest support for the reperfusion injury concept was provided by a large number of studies showing that interventions, given only at the time of reperfusion, attenuated, or abolished damage in previously ischemic tissues (881).
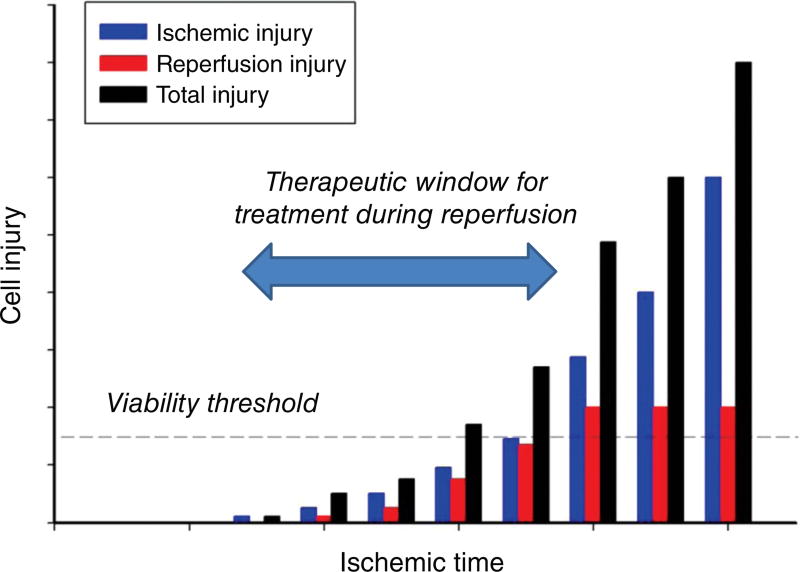
As depicted in Figure 1, it is now clear that a large number of pathologic processes underlie reperfusion injury. First, molecular oxygen is reintroduced to the tissues via arterial blood flowing into the previously ischemic tissue, thereby providing the missing substrate for generation of cytotoxic reactive oxygen species. Defects in the plasma membrane, endoplasmic reticulum, and mitochondria allow calcium to accumulate (calcium overload) in the cytosol and mitochondria, with rapid formation of hydroxyapatite crystals in the latter. The opening of the mPTP, endothelial dysfunction, appearance of a prothrombogenic phenotype, development of capillary no-reflow, and pronounced inflammatory responses also play major roles in the development of reperfusion injury (881). Understanding these mechanistically distinct pathologic processes that are invoked during ischemia and reperfusion allows for development of targeted therapies to reduce I/R injury. Moreover, such treatment modalities may prolong the ischemic time that a tissue can withstand before irreversible injury occurs (Fig. 2), thereby increasing the temporal window for organ transplantation, cardiopulmonary bypass, and surgical procedures to be conducted in a bloodless field.
Figure 2.
Total injury sustained by a tissue subjected to ischemia followed by reperfusion (I/R) (black bars) is attributable to ischemia per se (blue bars) and a component that is due to reestablishing the blood supply (red bars). At the onset of prolonged ischemia two separate general pathologic processes are initiated. The first are processes of tissue injury that are due to ischemia per se. The second are biochemical changes that occur during ischemia that contribute to the surge in generation of reactive oxygen species and infiltration of proinflammatory neutrophils and other immunocytes when molecular oxygen is reintroduced to the tissues during reperfusion. For a treatment to be effective in reducing cellular dysfunction and/or death when administered at the onset of reperfusion (therapeutic window), reestablishing the blood supply must occur before damage attributable to ischemia per se exceeds the viability threshold for irreversible damage. Concepts from Bulkley, 1987 (100).
Tissue responses to I/R are bimodal
Every tissue and organ is able to endure short interruptions in their blood supply without detectable deficits in function or cellular damage (Fig. 2). However, once the duration of ischemia exceeds a critical level, which varies by cell type and organ, the end-result is injury, perturbed function, and/or cell death. Thus, the extent of tissue injury, dysfunction and cell death varies in accord with the duration of ischemia. As a consequence, it is essential to reestablish the blood supply as soon as is possible after the onset of ischemia to limit the progression in severity of cell injury.
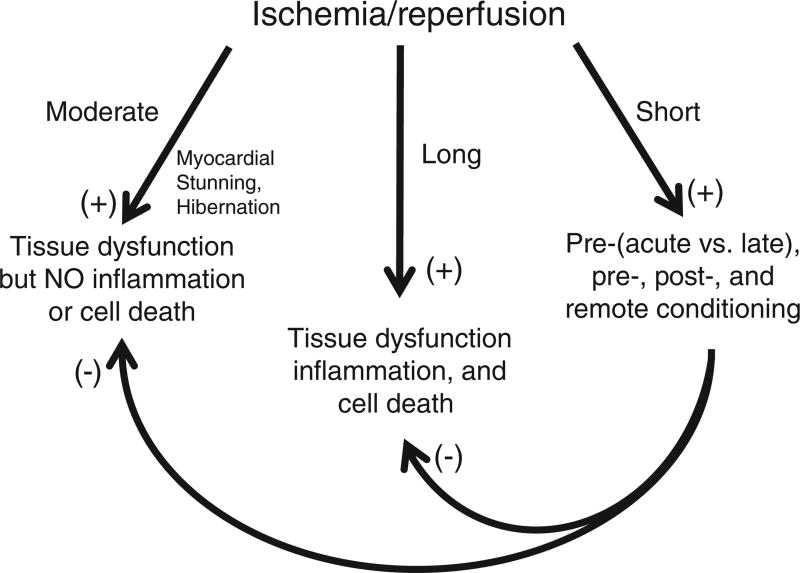
It was long thought that responses to ischemia were inconsequential if the duration was short, while extending ischemic times resulted in progressive increases in the extent of cell injury and reductions in tissue function. However, in 1986, this view was altered by the discovery that prior exposure of the heart to intermittent short bouts of ischemia and reperfusion (ischemic preconditioning), at durations (<5 min) previously thought to be without effect, exerted powerful infarct-sparing effects in hearts subsequently exposed to prolonged reductions in coronary blood flow (576). This breakthrough discovery provided the first evidence that tissue responses to ischemia are not invariably deleterious, but are actually bimodal (Fig. 3). That is, longer periods of ischemia induce cell dysfunction and/or death that is exacerbated by reperfusion, while short cycles of conditioning ischemia appear to activate intrinsic cell-survival programs that render tissues resistant to the deleterious effects invoked by subsequent exposure to prolonged ischemia followed by reperfusion (Fig. 3). This seminal discovery led to an explosion of interest aimed at identifying the underlying mechanisms, which in turn might be exploited to identify new treatment modalities to reduce negative outcomes after adverse ischemic events.
Figure 3.
Tissue responses to ischemia/reperfusion are bimodal (trimodal in the heart), depending on the duration and magnitude of ischemia. Prolonged and severe ischemia induces cell damage that progresses to infarction, with reperfusion paradoxically exacerbating tissue injury by invoking inflammatory responses. In the heart, shorter bouts of ischemia (5–20 min duration) induce myocardial stunning, wherein contractile function is initially impaired on reperfusion, but slowly improves, without progression to infarction and in the absence of significant inflammation. On the other hand, prolonged exposure to subacute levels of ischemia without reperfusion may induce myocardial hibernation, wherein cardiac cells modify their metabolic phenotype to survive but with a cost of reduced mechanical function. The third mode of response is exemplified by the tissue response to short periods of ischemia (<5 min) followed by reperfusion (ischemic conditioning) that do not produce detectable injury or dysfunction. Far from being innocuous and functionally inert, the response of all organs to such conditioning ischemia is characterized by activation of cell survival programs that confer tolerance to the deleterious effects induced by subsequent exposure to prolonged I/R such that postischemic injury is dramatically reduced. Cardioprotective effects are invoked when tissues are exposed to short bouts of conditioning I/R prior to (ischemic preconditioning) or during (ischemic per-conditioning) prolonged ischemia or at the onset of reperfusion after prolonged cessation of blood flow (ischemic postconditioning). Tolerance to prolonged I/R in one organ can also be activated by subjecting distant organs to conditioning I/R, a remote effect that can also magnify the beneficial actions of local conditioning.
Moderate I/R can induce myocardial stunning or hibernation
It was once thought that persistent myocardial contractile dysfunction resulted only after irreversible cellular damage occurred. However, in the heart (and perhaps other tissues), mechanical abnormalities can persist after restoration of coronary blood flow to normal or near normal levels in the absence of permanent damage if the ischemic phase is moderate (84, 184, 185) (Fig. 3). Indeed, it does not appear that myocardial stunning is caused by a primary deficit in reperfusion, but rather results from processes initiated by reestablishing the blood supply. One of the most important events appears to be related to reactive oxygen species formation (oxygen paradox). In addition, decreased responsiveness of contractile elements to calcium occurs in the face of transient calcium overload (calcium paradox). Calpain-induced proteolysis of myofibrils contributes to depressed contractile function, while rapid restoration of extracellular pH (pH paradox) leads to altered membrane ion channel activity. It has been proposed that stunning-induced contractile deficits may enhance the likelihood of cell survival by limiting the impact of the harsh ischemic milieu on myocyte injury progression toward irreversible damage during reperfusion (84, 184, 185).
Another type of adaptive response that may limit myocardial injury and death in hearts exposed to prolonged moderate or repetitive intermittent ischemia is adoption of a glycolytic phenotype by ischemic myocytes, reminiscent of the metabolic profile in neonatal myocardium. This phenomenon is termed myocardial hibernation and is associated with reduced contractile function and energy demands (184, 185, 725) (Fig. 3). This allows the hibernating myocardium to tolerate limited oxygen and nutrient delivery during periods of subacute ischemia, thereby preventing irreversible cardiomyocyte injury. When stimulated to develop the hibernating phenotype, myocardial cells upregulate the expression of stress and angiogenic proteins and reprogram metabolic pathways to reduce energy use. Survival of hibernating viable cells in ischemic organs is prolonged by eliminating nonfunctional cells via autophagic and apoptotic mechanisms (184, 185, 725).
Tissues and organs differ in their susceptibility to I/R
A fundamentally important concept of I/R is that the magnitude of injury and progression to irreversible damage after reperfusion is directly related to the duration of ischemia (2, 100) (Fig. 2). As a consequence, restoration of the blood supply as rapidly as possible is a major therapeutic goal to limit the extension of damage and cell death. In addition, there are common characteristic responses to I/R in all organs, including reactive oxygen species (ROS) generation, release of cytokines, chemokines, and other mediators from activated endothelium and tissue-resident macrophages and mast cells, endothelial vasodilator dysfunction in arterioles and endothelial barrier disruption in capillaries and postcapillary venules, neutrophil sequestration and activation, and development of a prothrombogenic phenotype. However, tissues and organs differ in their susceptibility to I/R that are due in part to variations in basal metabolic requirements amongst the tissues. This provides the rationale for the well-known effect of reductions in cellular metabolism secondary to tissue cooling to slow the progression of cellular damage in all organs. This approach can be used during revascularization procedures and to extend the preservation time for organs destined for transplant (57, 770).
Before examining differences amongst organs in their vulnerability to I/R, it is important to note that susceptibility of a given organ varies with the species being studied. For example, hearts isolated from rabbits, hamsters, ferrets, gerbils, rats, mice, and guinea pigs subjected to identical I/R protocols exhibit marked differences in their vulnerability to injury (255). Strain differences in susceptibility to I/R have also been reported (49, 50, 101, 199). Thus, care must be taken in extrapolating findings in one species or strain to another, let alone across all organ systems.
Of the body organs, the brain exhibits the highest sensitivity to ischemia. Although strokes can occur secondary to cerebral hemorrhage (which raises interstitial pressure and compresses vessels), focal cerebral ischemia (termed ischemic stroke) arising in a specific vascular territory secondary to thromboembolic or atherothrombotic vaso-occlusion represents the most common clinical presentation. While detectable irreversible damage occurs within 20 min after the onset of ischemia (606), the temporal window for initiating treatment is longer because cells exhibit differential susceptibility to a given degree of ischemia. Thus, significant restoration of function is observed if revascularization (e.g., thrombolytic therapy) occurs within 90 min to 4.5 h of the onset of symptoms (79, 313, 606).
The unique sensitivity of the brain to reductions in its blood supply relates to the fact that this organ has the highest metabolic activity per unit weight of any organ (451, 478). Moreover, the brain requires constant delivery of glucose as an absolute requirement for its metabolic demands. In addition, the brain has insignificant carbohydrate stores relative to other other organs like muscle or liver, and thus cannot operate anaerobically (451, 478). Another feature that accounts for the brains’ unique susceptibility to ischemia is the significantly lower levels of protective antioxidant enzymes [e.g., superoxide dismutase (SOD), catalase, glutathione peroxidase, and heme oxygenase-1] than are present in heart, liver, kidney, and lung (5, 171). Brain mitochondria also contain lower levels of cytochrome c oxidase than those in other organs, which would contribute to enhanced superoxide spillover from the mitochondrial electron transport chain (5). Cerebral cellular membranes also have high levels of polyunsaturated fatty acids, making the brain more susceptible to oxidative damage (5). Lastly, excessive neuronal release of glutamate and dopamine is induced by cerebral I/R, which cause neuronal calcium overload and subsequent cytotoxicity owing to ischemia-induced subversion of downstream signaling (142, 478, 618).
Like the brain, the myocardium is also exquisitely sensitive to ischemia. However, the time window before the onset of irreversible damage is slightly longer, and begins to appear after about 20 min of ischemia in both humans and animal models. As in the brain or any other organ, the sooner the affected coronary arteries are reopened, the better for salvage of viable cardiomyocytes. Treatment within two hours after the onset of ischemia is desirable (82). However, intervention initiated within 12 h has beneficial effects (471). The sensitivity of neonatal hearts to I/R depends on age, with the immature myocardium being highly susceptible to ischemia (849), whereas enhanced tolerance is seen shortly thereafter (401, 563, 611, 642, 849).
In the heart, fibrosis occurs after I/R by processes invoked by fibroblasts and mast cells and modulated by T cell subsets (249, 651, 844). How mast cells contribute to this process is unclear, but fibroblasts transdifferentiate to myofibroblasts, which exuberantly secrete matrix proteins to cause fibrosis and myocardial dysfunction. Depending on their polarization into Th1 versus Th2 cells, CD4+ T lymphocytes modulate the activity of fibroblasts to influence fibrosis and scar formation (651). In contrast, fibrosis does not occur in postischemic brain. Instead, I/R results in glial cell activation and release of matrix metalloproteases, which degrade extracellular matrix components (196). As a conquence, astrocytes and endothelial cells detach from basal lamina, resulting in disruption of the blood-brain-barrier. Detachment from the underlying basal lamina also results in glial and endothelial cell apoptosis (848).
After the heart and brain, the kidneys are the next most susceptible organ system to reductions in their blood supply, with permanent damage not appearing until the duration of ischemia exceeds 30 min in humans (549), while even longer ischemic times are tolerable in animal models (365). Renal cortical cells are the most sensitive to ischemia because renal oxygen levels are highest in this region of the kidney and progressively decrease from the outer medulla to the depths of the papillae. After induction of ischemia, outer medullary cells transition to anaerobic metabolism, allowing them to better survive in the hypoxic environment. Cells in the inner medulla and papillae normally rely on anaerobic glycolysis to generate ATP and are thus much less sensitive to ischemia.
Although the critical time periods of ischemia that the brain, heart, and kidneys can withstand before irreversible cell injury occurs are clearly demarcated, the time window for cell rescue by successful revascularization is much harder to assess for intestinal ischemia. This is due in large part to the difficulty in determining the onset of ischemia because the clinical symptoms of intestinal ischemia are often subtle in the early stages. However, in animal models, histologic evidence of mucosal injury appears 30 min after the onset of ischemia, with more conspicuous villous destruction evident at 60 min (122, 366). After revascularization, sloughed villi are rapidly replaced by cell migration (restitution), even after 90 min of ischemia (622). In humans, survival after acute mesenteric ischemia is approximately 50% if recognized and treated aggressively within 24 h after the onset of symptoms. However, progressive reductions in survival occur as this time interval is extended (412).
Superior mesenteric artery (SMA) occlusion produces a gradient of ischemia along the length of the bowel. The severity of ischemia is greatest in distal portions of the small intestine and proximal colon, where the reductions in blood flow are confined to the mucosal and submucosal layers and spare the muscularis/serosal layers (122, 651). Blood flow to the middle and distal colon is largely unaffected by SMA occlusion. Even with total SMA occlusion, there is limited perfusion (~25% of control) of the intestinal wall via collateral vessels (641). In sharp contrast, the blood supply to jejunum, ileum and colon is completely abolished after total SMA occlusion in neonates (1 day to 1 month old), responses that may contribute to neonatal necrotizing enterocolitis (163).
Intestinal I/R disrupts tight junctions between epithelial cells of the mucosal layer, which may allow movement of bacteria or enterotoxins from the lumen to the interstitial space of the affected bowel. From there, the translocated enterotoxin or bacteria can move to lymph nodes and the bloodstream. If the magnitude of ischemia is severe or the volume of ischemic mesenteric tissue is large, sepsis and multiple organ failure can ensue (423, 735). In support of this concept, depletion of gut commensal bacteria by antibiotic treatment was reported to be effective in reducing intestinal mucosal and lung injury induced by bowel ischemia (733, 890). Interestingly, antibiotic depletion of the intestinal microbiome also reduced infarct size after myocardial I/R and stroke (63, 466, 640, 733, 830, 890), which is discussed in greater detail later.
The skin and skeletal muscle tolerate much longer durations of ischemia than other organs. Indeed, the resistance of these organs to ischemia has long been appreciated since acute arterial injuries involving the extremities often require emergency application of tourniquets, sometimes for hours, with little injury to the affected tissues, especially if the compression is released for a short period of time after the first 1.5 to 2 h and then reapplied (687). Indeed, arthroscopic procedures and other surgical interventions for extremity trauma benefit from this resistance to ischemia because tourniquet application allows operation in a bloodless field. Importantly, skeletal muscles contain satellite cells which can regenerate muscle tissue even after wide-spread injury, while the heart, brain and kidneys cannot (818).
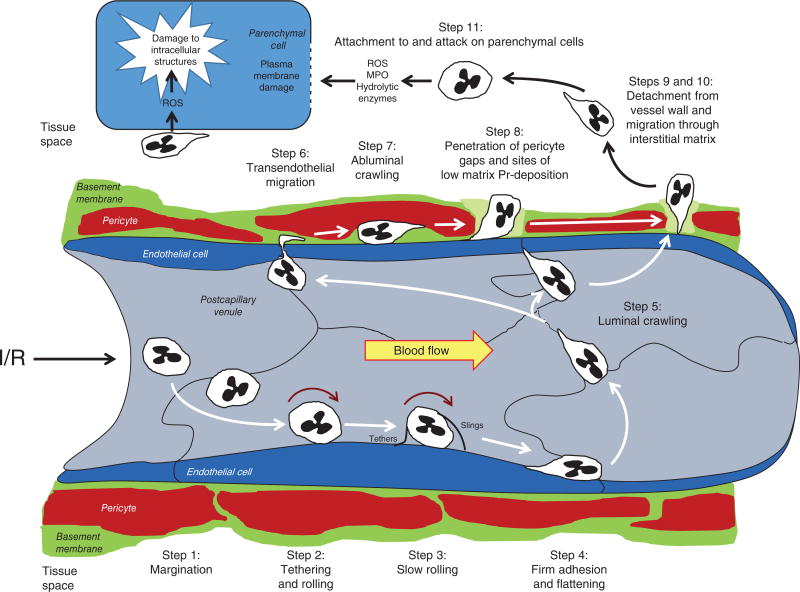
Although the microvasculatures of different organs exhibit significant structural and functional differences, the processes leading to postischemic leukosequestration in ischemic tissue regions are remarkably consistent (please see Fig. 10 and the section entitled “Inflammation Plays a Prominent Role in the Reperfusion Component of Total Tissue Injury in I/R,” for more detailed information). However, leukocyte recruitment in the liver is one important exception. Since hepatic sinusoids do not support selectin-mediated rolling or integrin-dependent adhesion as occurs in other microvascular beds, it has been suggested that sinusoidal leukocyte accumulation may be influenced more by physical factors such as low hydrodynamic forces and a vessel diameter that is close to that of leukocytes themselves (511). The sequestration of leukocytes in the lungs is also unusual compared to other organs in that postischemic PMN adhesion and migration occur primarily in alveolar capillaries and to a lesser extent in arterioles. In most other organs subjected to I/R, these adhesive interactions are largely confined to postcapillary venules, although arteriolar adhesion has also been noted in the coronary microcirculation (104). Moreover, the primacy of neutrophils in acute I/R injury is not clear in some tissues. For example, lymphocytes and monocytes may play a more important role in mediating injury responses in kidney (400) and brain (885).
Figure 10.
Neutrophil trafficking to ischemic sites occurs during reperfusion of ischemic tissues and involves 11 distinct steps. See text for further explanation. Figure modified from Refs. 923a and 816a.
Gut Microbiome and I/R
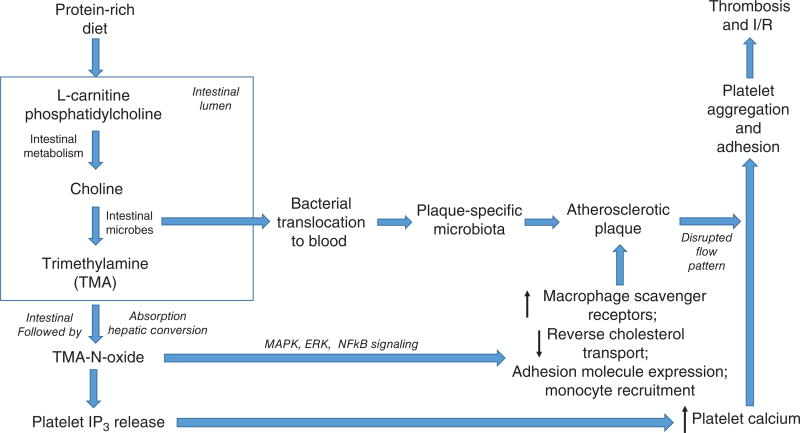
Our gastrointestinal tract (as well as the mucosal surfaces of the genitalia and respiratory system) harbors 100 trillion bacteria which interact with host cells in a symbiotic relationship to exchange nutrients, metabolites, and signaling molecules to influence a wide variety of physiologic processes ranging from energy regulation to cognitive function, with homeostasis maintained by the immune system (225, 226, 373). It is now appreciated that imbalances in the constituent populations of the intestinal microbiome, termed dysbiosis, contribute to causing or exacerbating disease states. Dysbiosis can be readily induced by environmental factors such as dietary changes and antibiotic regimens to modify intestinal microflora and thus influence disease states. One important cardiovascular consequence is contributions of the microbiome to atheromatous plaque formation via hepatic production of proatherogenic trimethylamine-N-oxide from trimethylamine (TMA) produced by gut microflora from phosphatidylcholine/choline and/or L-carnitine contained in red meat (434) (this work is discussed in greater detail later, under the section entitled “Genomic/Proteomic/Metabolomic Insights”). Similarly, changes in the oral microbiota associated with periodontitis influence atherosclerotic and thrombolytic processes and increase the risk for myocardial infarction (313).
With regard to I/R, depletion of gut commensal bacteria by antibiotic treatment was reported to be effective in attenuating intestinal (890) and lung injury induced by bowel ischemia (640, 733, 830), reduced infarct size after myocardial I/R (466), and limited brain injury after stroke (63). Antibiotic-induced depletion of the gut microbiome also reduced the expression of TNF, IL-6, and COX-2, and toll-like receptors (TLRs) 2 and 4, attenuated the recruitment of immunocytes such as B cells, and decreased complement and immunoglobulin deposition (890). However, the interpretation of studies using antibiotics to deplete intestinal bacteria is complicated by the types of microbes they target, which alters the balance in existing microbial populations and allows resistant organisms to bloom. In addition, antibiotics can disrupt mucosal barrier function and reduce type 17 helper T cells, a lymphocyte subpopulation that contributes to intestinal I/R (209, 373, 807). Thus, germ-free mice have been used as an approach to avoid these issues. Germ-free mice exhibited decreased expression of proinflammatory cytokines, reduced neutrophil sequestration, and decreased intestinal and pulmonary injury following intestinal I/R compared to that noted in mice with an intact intestinal microflora (735). Interestingly, these germ-free mice also demonstrate an increased expression of IL-10. When treated with a function-blocking antibody directed against this anti-inflammatory cytokine, mice lacking intestinal microflora exhibited I/R-induced inflammation and injury similar to that noted in conventional mice. Earlier work indicated that germ-free mice were protected from tissue injury induced by hemorrhagic shock (239). Based on recent work comparing antibiotic-treated to nondepleted mice, Benakis et al. (63) showed that intestinal bacteria participate in the trafficking of effector T cells from the small intestine to the leptomeninges in the brain where they exacerbate stroke-induced neuroinflammation secondary to secretion of IL-17. As a consequence, chemokine production is enhanced in the brain, leading to subsequent infiltration of neutrophils and other cytotoxic immune cells.
While the aforementioned work supports a role for the intestinal microbiota in exacerbating I/R injury, results of recent studies indicated that conventionally derived mice with an intact commensal bacterial population exhibited less injury after intestinal or renal I/R when compared to that noted in germ-free mice (379, 632, 846, 847). The protection against intestinal I/R injury was abrogated in mice that were genetically deficient in nucleotide-binding oligomerization domain-containing protein 2 (Nod2), an intracellular pattern recognition receptor (PPR) that induces autophagy on detection of the microbial cell wall component, muramyl dipeptide (632). Treatment of Nod2−/− with the autophagy inducer rapamycin protected against I/R injury. Taken together, these observations were interpreted to support the concept that the presence of an intact intestinal flora confers protection against intestinal I/R by a mechanism that involves Nod2 signaling and induction of the autophagy response. The microbiota may also benefit the host to limit I/R injury through the production of protective short chain fatty acids (e.g., acetate, proprionate, and butyrate) that exert anti-inflammatory actions and inhibit histone deacetylases (HDACs) (6, 23). On the other hand, Winek et al. (846) reported that the presence of the conventional gut microbiota does not influence stroke infarct volume but may protect the intestine from the development of severe colitis after stroke.
It is not clear why some reports described earlier indicate a protective effect of the intestinal microbiome in I/R, while others report detrimental effects. Even though the gut of healthy humans contains a fairly stable community of microorganisms, the composition of the enteric microbiota may be highly variable between individuals (433). Enteric microbial diversity is influenced by diet, genetic factors, environment, and gender, among other factors, and can change very rapidly. Thus, it would seem that the most likely explanation for the discrepant findings described earlier relates to differences in composition of the enteric microbiota amongst laboratories. It has also been shown that stroke alters the bacterial composition in the cecum, with specific changes correlating with extent of injury by a mechanism that may involve altered autonomic nervous system function (354). This appears to be due to I/R-induced central nervous system (CNS) lesions that result in disrupted signaling via the autonomic nervous system and hypothalamic-pituitary axis. In turn, this results in an altered intestinal microenvironment secondary to depressed intestinal motility and disrupted immune responses that favors bacterial synthesis of metabolic signals, immune modulators, neurotransmitters, neuromodulators and their precursors to modify systemic and gut immune responses, disrupt cellular metabolism, alter central hemodynamics, and reduce cerebral blood flow, actions which culminate in further depression of CNS function (354). Thus, changes in CNS function secondary to brain I/R modifies the intestinal microbiota, which may affect recovery and treatment outcomes. These points emphasize the importance of characterizing the enteric micro-biome in each study, which is now more practical with the development of new methods for low-error amplicon sequencing of bacterial 16S ribosomal RNA genes in combination with whole genome sequencing (226). Indeed, recent work indicates that intestinal microbial variation is a useful predictor of early acute rejection after organ transplantation (655).
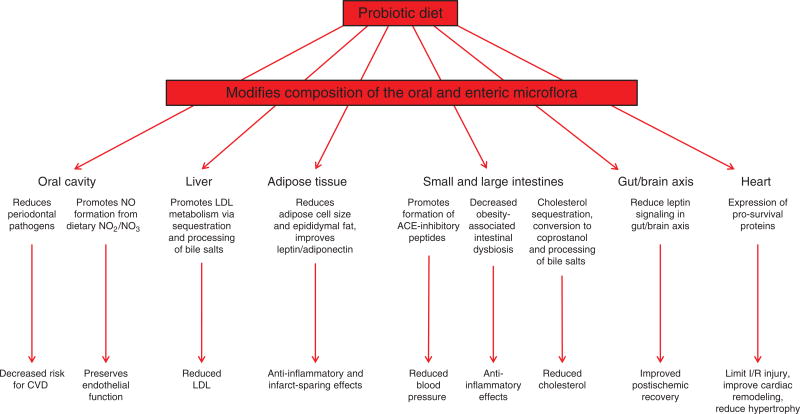
Interestingly, peroral colonizing of the intestine with the probiotics Lactobacillus plantarum, or bifidobacteria (containing Bifidobacterium longum, Bifidobacterium bifidum, and Bifidobacterium adolescentis) for 2 weeks reduced bacterial translocation from the gut lumen to remote sites, decreased cytokine levels, and attenuated mucosal disruption induced by mesenteric I/R (824, 825, 829) (Fig. 4). Similarly, animals fed a probiotic product containing L. plantarum prior to induction of myocardial I/R exhibited reductions in infarct size, improved contractile function, and attenuated the rise of blood leptin levels compared to untreated animals (466) while rats subjected to 6 weeks of sustained coronary artery ligation and concomitantly treated with Lactobacillus rhamnosis exhibited reduced myocardial hypertrophy, improved systolic and diastolic left ventricular function, and reductions in leptin/adiponectin ratio relative to untreated animals (259). Postischemic liver injury is also reduced by peroral administration of Lactobacillus paracasei F19 (582). Gavaging mice with VSL#3, a commercially available probiotic mixture containing B. longum, Bifidobacterium breve, Bifidobacterium infantis, Lactobacillus acidophilus, Lactobacillus casei, Lactobacillus bulgaricus, and Streptococcus thermophilus, was associated with significant reductions in local and systemic inflammatory markers after intestinal I/R (680). Taken together, the results of the studies described in this section of the review emphasize a critical role for commensal bacteria in local and remote I/R injury, effects that can be modulated by altering the constituency of the intestinal microflora with probiotic feeding or other dietary/drug manipulations (18, 225, 422, 423) (Fig. 4).
Figure 4.
Ingestion of probiotic diets modifies the composition profile of the oral and enteric microbiome to limit I/R via microflora-dependent alterations that decrease risk for cardiovascular disease via reductions in blood pressure, oral pathogens, blood LDL and total cholesterol, preservation of endothelium-dependent vasodilator mechanisms, activation of anti-inflammatory and infarct-sparing cell survival programs, and improved postischemic tissue remodeling.
Local I/R Can Induce Remote Organ Injury and Multiple Organ Failure
Injury to other organs often occurs on reperfusion after localized tissue ischemia, a phenomenon referred to as remote organ injury (ROI). Distant site injury can arise after I/R of the small or large intestines, lung, liver, kidney, skeletal muscle, or after aortic cross-clamping (114, 224, 331, 341, 686, 733, 792, 803, 813, 837). This ROI can progress to acute respiratory distress syndrome (ARDS) and systemic inflammatory response syndrome (SIRS) that are central to the pathogenesis of multiple organ dysfunction syndrome (MODS), especially if the volume of tissue affected by initial organ ischemia is large. Cardiac I/R may result in MODS if pump function is impaired to a degree that does not allow adequate perfusion of other organs. Pulmonary function is especially vulnerable to the deleterious effects of distant organ ischemia, particularly after bowel and/or liver I/R (114, 331, 733). Indeed, ARDS almost always precedes the development of multiple organ failure (114, 331, 686). Preexisting chronic inflammatory states likely exaggerate both local and remote cellular responses to exacerbate injury after reperfusion of the primary ischemic site (161).
In addition to pulmonary dysfunction, disruption of the intestinal mucosal barrier plays an important role in the development of MODS by allowing bacteria to enter the intestinal interstitium and lymphatics from the gut lumen. Bacterial translocation across the gut wall initiates and perpetuates the production of local inflammatory mediators that can gain access to distant organs via diffusing into the stream of reperfusing blood and via lymphatics draining the intestine (178, 740). Circulation of intestinal lymph back to the systemic circulation at the thoracic duct may be the primary route for entry of cytokines and other proinflammatory mediators after intestinal I/R, since mesenteric lymph duct ligation can prevent ROI (179). Lymphatics function in every postischemic organ to remove accumulating fluid, noxious antigens, cytokines, cellular debris and released macromolecules and provide a pathway for inflammatory cell efflux during repair of postischemic tissues (31). Moreover, cardiac lymphatic vessels demonstrate an impressive lymphangiogenic response that facilitates myocardial function after infarction via resolution of inflammation and accelerated healing (429).
Inflammatory leukocytes play a major role in the pathophysiology of ROI after localized I/R. A key event appears to be activation of neutrophils as they pass through the reperfused organ, which primes these granulocytes for sequestration in distant tissues (114). This involves not only the expression of adhesion molecules on the primed neutrophils, but also requires activation of endothelial cells in distant tissues (150). The latter step involves the release of inflammatory mediators into the stream of blood draining the reperfused organ and carried to distant sites by the circulation to promote adhesion molecule expression on postcapillary venules, as well as systemic complement activation. These mediators are derived from parenchymal cells, monocytes, and neutrophils in the ischemic tissue. Xanthine oxidase (XO), an enzyme that generates superoxide and hydrogen peroxide, has also been implicated in the pathogenesis of remote injury to cardiac muscle, lungs, and liver in response to intestinal I/R (114). However, its role in ROI is complex, involving ROS formation by a circulating form of the enzyme which is released from the surface of endothelial cells following I/R at the primary locus, as well as by XO-dependent formation of chemotactic factors at the local site, which subsequently promote recruitment of leukocytes to organs remote from the initial injury. In addition, reverse migration of neutrophils in the abluminal to luminal direction occurs in postischemic skeletal muscle, an event that is associated with enhanced ability to generate ROS by these cells. It appears that these reverse migrating cells can contribute to the development of reperfusion injury in the lung (851).
Neurogenic signals also contribute to inflammatory responses accompanying ROI (67, 91, 734). The proinflammatory phenotype produced locally and in the lung by intestinal I/R is largely prevented by capsaicin-induced neurotransmitter depletion in sensory neurons and by administration of tachykinin receptor antagonists (734). Calcitonin gene-related peptide and substance P, neuropeptides released from both sensory nerve endings and inflammatory cells (526, 648) are the most likely mediators for these effects. Neurokin-independent signaling may contribute to local and remote I/R-induced inflammatory responses via direct effects as well as release of TNFα (111, 734).
Risk Factors for I/R
Thromboembolic or atherothrombotic vasoocclusive diseases account for most ischemic episodes seen clinically in the westernized cultures. Advancing age, sex, and hereditary factors are risk factors for such events but there is little that can be done to prevent their effects per se (Fig. 5). Certain sex-specific risk factors, including early onset menopause and complications of pregnancy, such as preeclampsia or gestational diabetes, are associated with increased incidence of heart disease, but can be modified (371). Similarly, other important co-morbidities, including use of tobacco products and some recreational drugs (e.g., cocaine), occasional binge drinking, chronic overconsumption of alcohol (>3–4 drinks per day), hyperlipidemia, folate deficiency and hyperhomocysteinemia, hypertension, sedentary lifestyle, sleep disorders, such as obstructive sleep apnea, obesity, metabolic syndrome, and diabetes mellitus can be mitigated or controlled (81, 216, 236, 306, 664). It is now recognized that many comorbidities occur more frequently, exert more profound effects, and are/or more strongly associated with incidence of cardiovascular disease and myocardial infarction in women, including lupus, rheumatoid arthritis, diabetes mellitus, depression, and acute stress (669).
Figure 5.
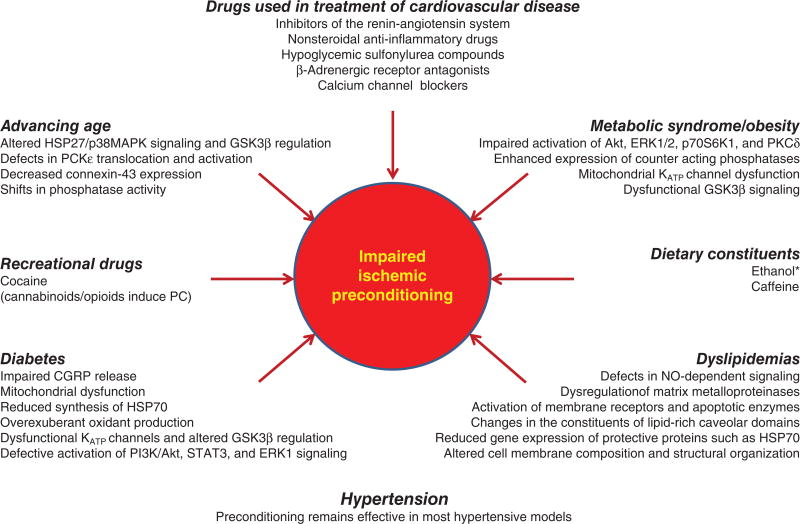
The presence of coexisting risk factors including metabolic syndrome, obesity, diabetes, advancing age, smoking, and dyslipidemias not only increase the likelihood for cardiovascular disease, but also worsen the outcome for those individuals who do suffer a heart attack or stroke. Interestingly, while ischemic and pharmacologic conditioning strategies are remarkably effective in young, healthy subjects, the presence of the aforementioned comorbid factors reduces their cardioprotective effects. The mechanisms underlying the impaired efficacy of conditioning is listed below each of the italicized co-morbid risk factors in the figure. Surprisingly little attention has been devoted to the effect of cigarette smoking to limit the efficacy of conditioning or with regard to the mechanisms by which this impairment occurs. Caffeine consumption also reduces the effectiveness preconditioning, as does the ingestion of alcoholic beverages at high levels, an effect that disappears as the absorbed ethanol is metabolized and eliminated from the blood. While use of some recreational drugs (eg, cocaine) abolishes ischemic preconditioning, morphine (or other opioids) injections or smoking marijuana may induce preconditioned phenotypes via activation opioid and cannabinoid receptors, respectively. It is also important to note that many of the drugs commonly used in the therapeutic management of patients with cardiovascular disease who are at high risk for myocardial infarction or stroke reduce or abolish the effectiveness of preconditioning stimuli by affecting their underlying signaling mechanisms. Reproduced from Ref. 449, with permission.
Unfortunately, most preclinical I/R work has been and continues to be conducted in young, healthy animals, where ischemia is acutely produced by ligating a vessel of interest or by placement of vascular clamps. Clearly, these models are not representative of relevant human patient populations, where thromboembolic or atherothrombotic vasoocclusive disease are the precipitating events and occur in an inflammatory environment not present in young, healthy subjects with minimal or no risk factors. In addition, cardioprotective drugs and experimental maneuvers such as ischemic preconditioning that are effective in limiting I/R injury in young and healthy animals, often fail to confer protection in the presence of comorbid risk factors (Fig. 5). The mechanisms underlying the impaired efficacy of conditioning is listed below each of the italicized comorbid risk factors in the figure. Surprisingly little attention has been devoted to the effect of cigarette smoking to limit the efficacy of conditioning or with regard to the mechanisms by which this impairment occurs (Fig. 5).
Caffeine consumption also reduces the effectiveness preconditioning, as does the ingestion of alcoholic beverages, an effect that disappears as the absorbed ethanol is metabolized and eliminated from the blood. While use of some recreational drugs (e.g., cocaine) abolishes ischemic preconditioning, morphine (or other opioids) injections, or smoking marijuana may induce preconditioned phenotypes via activation of opioid and cannabinoid receptors, respectively (Fig. 5). It is also important to note that many of the drugs commonly used in the therapeutic management of patients with cardiovascular disease who are at high risk for myocardial infarction or stroke reduce or abolish the effectiveness of preconditioning stimuli by affecting their underlying signaling mechanisms (Fig. 5). Importantly, recent studies have shown that caloric restriction, consumption of alcoholic beverages at low levels (1–2 drinks per day, with beneficial effect present only once ethanol has been metabolized), and exercise increase ischemic tolerance in hearts and other organs in the presence of comorbidities, even those risk factors that are irreversible (advancing age) or cannot be controlled (male sex, genetic factors) (81, 450) (Fig. 5).
Fetal Programming, Transgenerational Inheritance, and Susceptibility to Ischemic Vascular Disease
It is now clear that a variety of factors may be encountered in fetal life that are associated with increased risk for cardiovascular disease in adults (14, 660). Work conducted by Barker and coworkers (46–48, 536) provided the first evidence that decreased full term birth weights from approximately 9 to 5.5 pounds, as an index of poor intrauterine nutrition, was associated with increased incidence and mortality from ischemic disease in adults. He hypothesized that this association was driven by a failure of fetal nutritional supply to meet demands, causing the fetus to undergo physiologic adaptions to survive in utero by modifying blood flow distribution and thus nutrient delivery to spare the most vital organs at the expense of other organs (314). Subsequent work demonstrated a similar correlation in adults who had higher than average birthweights (>9.5 pounds) (536). This “U”-shaped relation between birth weight and cardiac disease has been recapitulated in a large number of studies supporting the concept that a broad range of environmental cues (maternal stress, age, obesity, cocaine, ethanol or tobacco smoke exposure, hemodynamic effects, growth factors, preeclampsia, gestational diabetes, oxygen, and nutrient availability) can influence placental growth and initiate programs that enhance the myocardial susceptibility to ischemic disease later in life (468, 657).
The maladaptive responses to intrauterine stresses that lead to increased disease risk in adults have been termed fetal programming or fetal origins of disease. Indeed, low birth weight is also associated with increased incidence of hypertension, obesity, type 2 diabetes, and chronic renal disease later in life (657, 782). These chronic disease states represent major risk factors for cardiovascular disorders, again suggesting that fetal programming is an important contributor to the prevalence of ischemic disease in westernized cultures. In addition, recent work indicates that prenatal exposure to hypoxia or cocaine inhibit the infarct-sparing effects of ischemic preconditioning later in adult life by a mechanism involving irreversible fetal reprogramming of protein kinase C epsilon expression (561, 626). A large number of studies have provided evidence that adult animals that were exposed to hypoxia, glucocorticoids or maternal low protein diet or obesity in fetal life demonstrate an increased susceptibility to I/R in the early postnatal period (108a, 211a, 211b, 268a, 268b, 327a, 390a, 491a, 491b, 626, 626a, 626b, 660, 675b, 676a, 782, 863a, 864a, 864b, 864c). These results support the notion that a fetus developing in adverse conditions becomes an adult who is susceptible to enhanced I/R injury.
The mechanisms contributing to the development of adult cardiovascular disease after fetal stress in utero are only now being uncovered. Barker’s group originally proposed fetal malnutrition induced persistent glucose-preserving adaptations that ultimately contribute to the development of insulin resistance and type 2 diabetes in later life (314). The development of this so-called thrifty phenotype in response to fetal malnutrition was proposed to confer a competitive advantage by preparing the newborn for an anticipated deficient nutritional environment at birth (53). While potentially advantageous to the newborn, placental insufficiency is ultimately maladaptive, because it contributes to the appearance of adult cardiovascular disease secondary to the effect of intrauterine programming to limit fetal growth (323, 782). With regard to adult susceptibility to I/R after fetal stress, there is a growing body of evidence indicating that reductions in the expression of cardioprotective genes such as protein kinase Cε (PKCε), endothelial nitric oxide synthase (eNOS), adenosine monophosphate (AMP) kinase, and heat-shock protein70 by a ROS-mediated, but nicotinamide adenine dinucleotide phosphate (NADPH) oxidase-independent epigenetic repression mechanism may play a role (211a, 268b, 390a, 491a, 491b, 626, 626a, 626b, 864a). In addition, fetal hypoxia induces changes in extracellular matrix and myofibrillar architecture, oxidative stress, diastolic dysfunction, reduced capillary density, sympathetic dominance, glucocorticoid receptor deficiency, and altered endothelium-dependent vasodilator function in adult hearts, which may contribute to this enhanced susceptibility to I/R (108a, 211b, 268a, 268b, 327a, 327b, 675a, 675b, 675c, 675d, 676a, 857, 863a).
Fetal malnutrition restricts intrauterine growth and is associated with oxidative and nitrosative stress, altered gene expression related to nutrient metabolism, angiogenesis, inflammatory cytokine expression, and decreased placental growth factor expression. Although it remains to be determined whether these changes are causal in nature, an emerging body of evidence supports the idea that intrauterine glucocorticoid overexposure may explain the relation between low birth weight and increased risk for the development of obesity, hypertension, type 2 diabetes, altered renal function, and ischemic disease in later life (56, 468, 568, 657). It is almost certain that the link between fetal growth and adult onset disease involves changes in gene expression, which most likely involve epigenetic phenomena (468, 657, 814, 815). In this regard, gestational hypoxia induces epigenetic repression of the glucocorticoid receptor gene in the developing heart, which results in increased susceptibility to myocardial I/R injury after birth (857). Epigenetic changes also likely contribute to transgenerational programming (10, 14, 21, 814, 815). The intrauterine environment also influences the functions of adipose tissue and the innate immune system, which may ultimately enhance the likelihood for development of cardiovascular disease in later life (182, 600, 757). More recently, it has become appreciated that early bacterial colonization of the neonatal gut influences the occurrence of cardiovascular and other diseases later in life, suggesting a role of the microbiome in fetal programming (440).
Several Modes of Cell Death Are Induced by I/R
I/R-induced cell death was long thought to occur solely by necrosis, an unregulated and irreversible process that is characterized by mitochondrial swelling, cytoplasmic vacuolization, and swelling of the nucleus and cytoplasm (oncosis) secondary to energy failure brought on by the reduction in the blood supply. Loss of plasma membrane integrity allows release of toxic cellular molecules to trigger a pronounced inflammatory response, with both processes exacerbating destruction of the affected cell and collateral damage to adjacent cells (Fig. 6). While it was also once thought that reducing the duration of ischemia and the magnitude of reperfusion injury were the only therapeutic options to prevent this fatal injury, the discovery that necrotic cell death could be reduced by ischemic preconditioning, coupled with an understanding of the underlying cardioprotective mechanisms, has led to the concept that activation of survival kinases and inhibition of the mitochondrial PTP are therapeutic modalities worth pursuing into clinical trials as a means to limit necrosis. Very recent work indicates that the mitochondrial PTP can undergo transient low conductance openings termed MitoWinks that function to promote mitochondrial and cell survival by allowing resetting of individual mitochondria to limit matrix calcium overload with little energetic cost (518). This raises the intriguing possibility that MitoWinks may be involved in the ROS generation that activates the RISK pathway of cardio-protection in ischemic preconditioning, as has been shown for opening of mKATP channels.
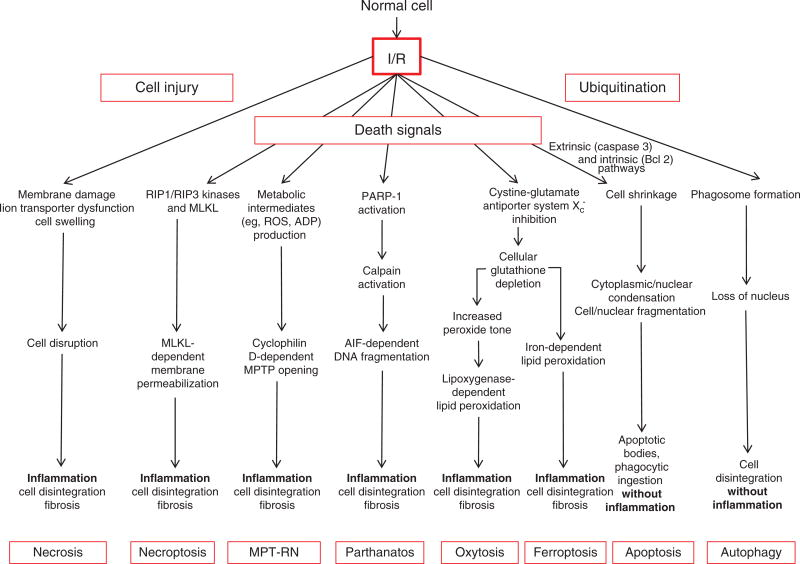
Figure 6.
Cell death modalities in ischemia/reperfusion (I/R). I/R-induced necrosis generally occurs as a result of dysfunctional ion transport mechanisms, which causes cells to swell and eventually burst, effects that are exacerbated by plasma membrane damage. Release of proinflammatory mediators and damaged biomolecules initiates the influx of inflammatory cells such as neutrophils, which disrupt the extracellular matrix and cause damage to parenchyal cells by release of cytotoxic oxidants and hydrolytic enzymes. Apopotosis is a regulated form of cell death that causes cell shrinkage and condensation of the cytosol and nucleus, which eventually form apoptotic bodies. Because they are surrounded by cell membranes, apoptotic bodies can be engulfed and digested by phagocytes without evoking an inflammatory response. Autophagy provides a mechanism to remove damaged or senescent protein aggregates and organelles by enclosing them in membrane-lined vesicles called proteasomes which fuse with lysosomes containing enzymes that degrade the ingested material, usually without evoking an inflammatory response. While normally performing this “housekeeping” function, autophagy may also provide cells with a survival mechanism to withstand the deleterious effects of ischemia, by generating amino acids and fatty acids for cell function. However, when uncontrolled, autophagy contributes to ischemic cell death. While necrosis was once believed to occur from non-specific trauma or injury as a result of I/R, it now appears that postischemic infarction may also be attributable to programmed events that require a dedicated molecular circuitry that has been termed programmed necrosis or necroptosis. Necroptosis is initiated by TNF-like cytokines that activate RIP kinases to mediate necrosis via increased production of reactive oxygen species and calcium overload, which in turn modulate the mitochondrial permeability transition pore (MPTP), leading to dissipation of the proton electrochemical gradient, with subsequent ATP depletion, further ROS production, and swelling and rupture of mitochondrial membranes. Recent genetic studies have suggested that the MPTP is predominantly involved in a second form of regulated necrosis that is designated MPT-RN that is critically dependent on cyclophilin D. Parthanotos can be distinguished from other forms of programmed cell death by its requirement for poly-ADP-ribose polymerase activation. Two newly described cell death modalities have been implicated in I/R, ferroptosis and oxytosis. Both involve inhibition of the cytine-glutamate antiporter Xc−, but differ in their modes of lipid peroxidation, being iron dependent and lipoxygenase dependent, respectively.
Importantly, it is now recognized that cells subjected to I/R also die in a programmed manner via apoptosis and autophagy, processes that are regulated by coordinated cellular signaling mechanisms (442, 454, 599, 832) (Fig. 6). Furthermore, it now appears that the apparently random, unregulated and irreversible events that lead to I/R-induced necrosis may, under certain circumstances, involve the activation and orchestration of specific signaling mechanisms in yet another death pathway termed programmed necrosis or necroptosis (253, 442, 599, 832) (Fig. 6). Thus, it may be possible to salvage ischemic cells undergoing regulated cell death by apoptosis, autophagy, and necroptosis by interfering with the signaling pathways involved (see 599 for review). In subsections below, we describe the basic mechanisms underlying each of these distinct but overlapping cell death modalities and the evidence they contribute in to the pathogenesis of lethal I/R injury.
Apoptosis
Apoptosis is characterized morphologically by membrane blebbing, cell shrinkage, nuclear fragmentation and chromatin condensation, while activation of caspases is the distinguishing biochemical feature (453) (Fig. 6). The cell signaling mechanisms underlying apoptotic cell death can occur via the extrinsic (or death receptor) and intrinsic (mitochondrial) pathways (Fig. 6), although there multiple biochemical and functional linkages between the two (94, 442, 453, 832, 843).
The extrinsic or death receptor pathway involves binding of ligands, such as Fas, TRAIL, and TNFα, to proinflammatory receptors, resulting in their trimerization. This promotes recruitment of death domain-containing adapter proteins (e.g., FADD and TRADD) to complete the death-inducing signaling complex. Once assembled, this receptor complex activates caspase-8, a protease that activates caspase-3 by a cleavage-dependent mechanism. Caspase-3 hydrolyzes many cellular proteins to bring about apoptosis (94, 169, 383, 442, 453, 843).
In response to cytotoxic stimuli such as oxidative stress, the intrinsic or mitochondrial pathway is activated (Fig. 6). This involves incorporation of Bcl2 protein family members such as Bax and Bak into the outer mitochondrial membrane (94, 453, 843). While not well understood from a mechanistic standpoint, these prodeath proteins enable the release of pro-apoptotic proteins cytochrome c, Smac/DIABLO, Omi/HtrA2, and endonuclease-G (endo-G) from the inter-membrane space by acting to permeabilize the outer membrane. Cytochrome c binds to the cytosolic protein APAF1 to stimulate assembly the apoptosome, a multi-protein complex in which caspase-9 and 3 protease system is activated, resulting in cellular protein cleavage. Caspase activation also occurs by mechanisms dependent on Smac/DIABLO and Omi/HtrA2, but these prodeath proteins do so by sequestering or digesting caspase-inhibitory proteins. Lastly, DNA fragmentation characteristic of this form of apoptosis is mediated by endo-G (94, 453, 843).
Apoptotic cell death is induced by I/R, although the extent of cells dying via this modality is significantly lower than necrosis. Upregulation and activation of prodeath Bcl2 proteins (e.g., Bax, Bak, Bid, BNIP3, and Puma) and their translocation and integration into mitochondrial membranes occurs in ischemic cells (199, 320, 389, 560, 836, 852). However, ischemia per se is not sufficient for activation of Bcl2 proteins because many are redox sensitive, requiring the oxidative stress that is evoked by reperfusion. The observations that Bax-, Bid-, BNIP3-, or Puma-deficient animals demonstrate reduced apoptotic cell death clearly support their contribution to the progression of postischemic tissue injury (64, 199, 788, 836, 852). Surprisingly, the degree of protection noted in these knockout animals was greater than would be predicted from the extent of postischemic apoptosis, suggesting that these prodeath Bcl2 proteins may have effects that are independent of their role in apoptotic signaling during I/R. Alternatively, genetic knockout of proapoptotic Bcl2 proteins, may produce compensatory alterations in antiapoptotic proteins, which also influence Ca2+ homeostasis, thereby modifying the extent of I/R injury (701).
A number of apoptogenic factors are released from mitochondria during I/R, including the archetypal cytochrome c. Other apoptogens that are released during I/R and likely contribute to I/R injury include the caspase activators Omi/HtrA2 and Smac/DIABLO. Indeed, pharmacologic or genetic inhibition of Omi/HtrA2 attenuates postischemic death by apoptotic mechanisms (419, 590), but such approaches have not yet been used to more clearly define a causal role for Smac/DIABLO in I/R injury. Although cerebral ischemia induces release of mitochondrial endonuclease G (588), mice genetically deficient in this inducer of nuclear DNA fragmentation during apoptosis retained their sensitivity to prolonged I/R (864).
I/R-induced cell death is reduced in animals treated with pan-caspase inhibitors, providing additional support for the notion that apoptosis contributes to death of cardiac myocytes (169, 350, 360, 870, 875). Similar observations were observed after genetic deletion or knockdown of specific caspases participating in the extrinsic and intrinsic pathways (152, 473). While such observations might lead to the proposal that targeting caspases may be an important therapeutic means to reduce I/R injury, caspase inhibition may not be ideal because other aspects of mitochondrial function will still be adversely affected. As a consequence, caspase inhibition may at best only delay the inevitable, but at worst, may instead drive the cell to necrotic death (812).
Mice deficient in Fas, TNF receptor 1, or TRAF1 exhibit smaller infarcts than wild-type mice, suggesting that death receptor pathway activation contributes (383, 480, 519, 910, 911). On the other hand, smaller infarcts are also noted in Bax knockout mice or mice treated with a small molecule inhibitor of Omi/Htr2, suggesting that the mitochondrial pathway is also activated to produce apoptosis in I/R (343, 344, 519).
Autophagy
Autophagy occurs under normal conditions where it functions as a mechanism for disposal of damaged or obsolete organelles and protein aggregates by a process involving packaging into autophagosomes that are transferred to lysosomes for elimination from the cell. This process is also activated by conditions associated with I/R (e.g., energy deprivation, oxidative stress, and ER stress), where it acts to promote cell survival by generating amino acids and fatty acids for maintenance of cell function or by removing damaged organelles, oxidized proteins, and protein aggregates (115, 331, 390, 539, 540, 698) (Fig. 6). Furthermore, since ischemia basically starves cells of nutrients (and oxygen), the autophagic breakdown of cellular components might promote cellular survival by providing substrates for maintaining cellular energy levels. In support of the latter concept, inhibition of autophagy has been shown to amplify I/R-induced damage (390, 762), while pharmacologic stimulation of autophagy confers protection against I/R (115, 116, 359). However, if the ischemic period is prolonged, the extent of autophagic degradation of critical cellular constituents contributes to postischemic damage and thus is a death modality in I/R (320, 326, 539, 540, 700, 805, 900).
Morphologically, autophagy begins with assembly of the phagophore, an isolation membrane that expands around the cell constituents to be processed (284, 330, 489). As this isolation membrane expands to fully encase the cell compartment/organelle, it forms the vesicular autophagosome. Fusion of this structure with a lysosome permits degradation of the enveloped materials. Thus, autophagy performs a housekeeping function in normal cells. Autophagy is regulated principally by the mammalian target of rapamycin (mTOR), which inhibits the process. However, this negative regulation is disinhibited (i.e., mTOR is inactivated) under conditions associated with I/R, such as nutrient withdrawal or oxidative stress (284, 330, 489, 540). This derepresses several kinases (Atg1, Atg13, and Atg17) that initiate the formation of the phagophore by a process that involves activation of Vps34, a class III phosphatidyl inositol 3 kinase, which in turn binds to Vps150, Atg14, and beclin-1. This complex functions to recruit other regulatory proteins which are essential for the expansion of the isolation membrane to form the mature vesicular autophagosome. Fusion of the autophagosome with a lysosome occurs by a mechanism dependent on the small GTPase Rab7 and the lysosomal membrane protein LAMP2 (284, 330, 489).
Mitophagy is a cargo-specific form of autophagy that selectively targets mitochondria for degradation. It is further differentiated from autophagy by its cellular signaling mechanisms, which involve parkin and PINK1, which facilitate sequestration of damaged mitochondria into autophagosomes. I/R is associated with reductions in parkin protein levels after stroke (558), suggesting that decreased mitophagy may allow accumulation of damaged mitochondria and ensuing cell death. Moreover, the protective effects of ischemic and pharmacologic preconditioning appear to require mitophagy to selectively eliminate mitochondria damaged by subsequent exposure to I/R (24, 283, 284, 342, 457). This may leave behind a population of mitochondria that have a high threshold for opening of the mitochondrial PTP, thereby reducing the likelihood of I/R-induced cell death in preconditioned tissues (283, 327, 457). This notion is further supported by the observation that stimulation of mitophagy by overexpression of regulator of calcineurin 1-1L protects hypoxic cardiomyocytes from apoptosis (867).
Necrosis versus regulated necrosis
Necrosis is characterized morphologically by swelling of cells and their constituent organelles, mitochondrial disruption, absence of nuclear fragmentation, plasma membrane rupture, and leakage of intracellular contents, which leads to the demise of the cell. In contrast to the genetically encoded nature of apoptosis and autophagy, where cell signaling programs are activated to produce cell death, necrosis is often referred to as accidental cell death because it was believed to occur by random, uncontrolled processes that led to expiration of the cell in response to overwhelming stress. However, it is now recognized that programmed (which occurs in a physiological setting to preserve tissue homeostasis) or regulated (which occurs in pathologic conditions) necrosis occurs in embryonic development and in pathologic states, especially I/R, respectively (Fig. 6). While these cell death modalities share features with necrosis, they can be inhibited by pharmacologic and genetic interventions directed at signaling elements that ultimately result in death of the cell. This implies that regulated and programmed necrosis rely on distinct molecular mechanisms that are under tight control versus the random, uncontrolled events typifying necrotic cell death that occurs in response to very harsh environmental pertubations that cannot be delimited. Cells can be driven to regulated necrosis by I/R via activation of at least three separate signaling pathways, which are delineated by the terms necroptosis, mitochondrial permeability transition-dependent regulated necrosis (MPT-RN), and parthanatos (22, 231, 233, 258, 599) (Fig. 6).
Necroptosis is activated by cell stress or ligation of death receptors, such as TNF receptor 1 or Fas receptor, by their ligands and leads to mobilization and activation of a group of serine/threonine kinases called receptor interacting protein kinases (RIPKs) (Fig. 6). Once activated, two particular RIPKs, RIPK1, and RIPK3, coordinate their activities to increase oxidative stress via stimulation of NADPH oxidases or mitochondrial oxidant production via a complex signaling path that results in cell death (566, 567, 727, 812), RIP3 also phosphorylates the pseudokinase mixed lineage kinase domain-like protein (MLKL) to cause necroptosis. The finding that necrostatin-1 reduces TNFα- and I/R-induced cell death through inhibition of RIP1 kinase activity while RIP3-deficient mice are protected from I/R supports the concept that necroptosis occurs via receptor-induced, well-regulated cellular processes (130, 175, 207, 234, 503–508, 593, 727, 861, 913). Further confirmation of the necroptotic pathway in I/R-induced cell death will come from use of the recently described MLKL knockout mouse (853).
One potential mitochondrial target for RIP-mediated necrosis is the MPT pore. This large, nonspecific channel in the inner mitochondrial membrane is normally closed, but opens during I/R in response to overexuberant ROS production and excessive increases in mitochondrial matrix Ca2+ levels (35–38, 316, 453). As a result, the permeability of the inner membrane suddenly increases, which dissipates the proton electrochemical gradient (ΔΨm). This results in ATP depletion, further ROS production, and ultimately swelling and rupture of the organelle. This death modality is critically dependent on cyclophilin D and constitutes a second form of regulated necrosis that is designated MPT-dependent regulated necroptosis (MPT-RN) (Fig. 6) (13, 35–38, 258, 316, 453).
The cell death modality designated parthanatos represents a third form of regulated necrosis (Fig. 6). Parthanatos is activated by genotoxic stresses such as oxidants and alkylating agents as well as I/R, which in turn leads to an overstimulation of the DNA repair enzyme poly(ADP-ribose) polymerase-1 (PARP1) (231). PARP1 activates calpain, a cysteine protease that acts to promote the release of the perhaps misnamed apoptosis-inducing factor (AIF) from the mitochondria. AIF then moves to the nucleus and degrades DNA (90, 827). There is some evidence that PARP1-mediated cell death may require RIPK 1 (863), but how this kinase or the MPT integrates in the parthanatos signaling cascade is unclear.
Two newly described and related forms of regulated, non-apoptotic cell death—ferroptosis and oxytosis—have also been recently implicated in I/R (Fig. 6). Both types share common trigger (inhibition of the cystine-glutamate antiporter system Xc− and resulting depletion of glutathione) and execution (lipid peroxidation) mechanisms, but differ in their requirement for lipoxygenases, which participate in oxytosis (110, 151). As the name implies, ferroptosis is a cell death modality that is characterized by iron-dependent lipid peroxidation. Ferroptosis inhibitors such as liproxstatin-1 and third-generation ferrostatins have been shown to decrease liver and kidney injury after I/R (110, 117, 250, 455). On the other hand, oxytosis is characterized by increased peroxide tone that is associated with translocation of 12- and 15-lipoxygenases to cellular membranes, particularly in mitochondria, resulting in peroxidation of lipid components (151). This form of programmed cell death has been shown to occur in stroke and myocardial infarction via use of lipoxygenase inhibitors or genetic ablation of 15-lipoxygenase (117, 392, 617, 618, 731, 811, 883).
From the foregoing discussion, it is clear that cells die in the ischemic zone by necrosis, apoptosis, autophagy, and at least five forms of regulated necrosis (necroptosis, MPT-RN, parthanatos, ferroptosis, and oxytosis). However, the relative contributions of each death modality to overall infarction induced by I/R remain unclear. Nor is it known which tissues are most sensitive to each death mechanism. Given that inhibitor studies specific for each death modality provide only partial protection, while combination therapies can be synergistic, it would appear that there is a strong degree of interconnectivity and overlap in the signaling transduction elements mediating programmed cell death (678).
Mechanisms Underlying I/R Cell Injury and Death
The net result of the multiple, often interactive mechanisms that contribute to the pathogenesis of I/R injury is damage to proteins, lipids, carbohydrate moieties, and DNA in cells and tissues. The cumulative toll of these harsh insults, if sufficiently severe, results in cell death by necrosis and programmed cell death by mechanisms described earlier. The next several sections summarizes major sequelae to I/R and what is known about the underlying mechanisms.
Calcium overload
When the blood supply to an organ is reduced, the cells in the affected region switch to anaerobic glycolysis to generate ATP. This leads to lactate, H+, and NADH+ accumulation and cytosolic pH decreases. In an attempt to reestablish acid-base balance, the plasmolemmal NHE is activated and transports H+ ions out of the cytosol in exchange for Na+ (35–38, 572). The influx of Na+ ions in turn activates the plasmolemmal Na+/Ca2+ exchanger which exchanges Na+ for Ca2+ (Fig. 1). Upon reperfusion, the activity of the NHE exchanger is accelerated by the washout of the extracellular H+ ions that accumulated during ischemia, which increases the proton gradient across the plasmolemma and further increases cytosolic Ca2+ (35, 36, 572, 683, 766). In addition, Ca2+ handling by the endoplasmic/sarcoplasmic reticulum (ER/SR) SERCA ATPase is impaired by I/R, which limits Ca2+ reuptake from the cytosol. On the other hand, Ca2+ release from the ER/SR via ryanodine receptors is enhanced (683, 759, 766). These perturbations in ER/SR calcium handling further exacerbate the lethal elevations in cytoplasmic Ca2+ levels (Fig. 1). The massive increases in intracellular Ca+ concentrations activate a variety of processes, described later, which contribute to cell death following I/R.
In an attempt to deal with the enormous alterations in cytosolic Ca2+ levels induced by I/R, transport via the mitochondrial Ca2+ uniporter is increased. Using the negative ΔΨm created by I/R, this transporter drives movement of the positively charged Ca2+ ions into the mitochondrial matrix (153, 759, 766) (Fig. 1). While this reduces cytosolic Ca2+, elevations in mitochondrial Ca2+ trigger the MPT. Pathological activation of calpains, a family of cysteine proteases that target a variety of cytoskeletal, ER, and mitochondrial proteins, as well as depressed activity of Ca2+/calmodulin-dependent protein kinases (CaMKs) also occur in response to I/R-induced elevations Ca2+ (160, 164, 583). Ischemic preconditioning appears to prevent the I/R-induced depression of SR CaMK II activity (609) while pharmacological inhibition of calpains exerts protective effects in variety of organs (119, 129, 138, 336, 436, 484, 537, 629, 791). In addition, calpastatin, an endogenous calpain inhibitor, can be degraded during I/R, thereby enhancing calpain activity (715, 732). Therapeutic transfer of the calpastatin gene reduces myocardial contractile dysfunction and infarction induced by I/R (525).
Another pathologic process that is driven by increased intracellular Ca2+ during I/R is the formation of calcium pyrophosphate complexes and uric acid. These entities both constitute danger signals that activate inflammasomes to initially generate IL-1β and TNFα to exacerbate I/R injury. These cytokines in turn activate transcription factors such as NFkB to increase expression of additional cytokines and chemokines, thereby fueling a cytokine storm in a vicious cycle to provoke further cell injury by profound inflammation (7, 44, 239).
Oxidative/nitrosative stress
As noted above, the influx of oxygen that occurs when the blood supply to an ischemic organ is reestablished fuels the excessive production of ROS, creating the paradox that reperfusion can induce injury (oxygen paradox) because these highly reactive species can modify proteins, lipids, nucleic acids, and sugars in cells and tissues to dysfunction and cell death. While the primary importance of ROS production in the pathophysiology of I/R injury was first identified 35 years ago (293) (Fig. 10), it is now appreciated that redox molecules derived from NO [referred to as reactive nitrogen species (RNS)] also contribute to I/R injury via oxidative and nitrosative reactions with virtually every biomolecule found in cells (see 204, 243, 290, 292, 463, 615, 649, 682 for review). However, depending on their concentrations, both NO and ROS can either participate directly in I/R or in conferring protection by ischemic and pharmacological preconditioning and postconditioning (244). RNOS contribute to the deleterious effects of I/R by modifying macromolecule structure and function, disrupting/activating signaling cascades, stimulating the production/release of proinflammatory mediators by various cell types, inducing the expression of adhesion molecules that mediate leukocyte-endothelial cell adhesive interactions, and decreasing the bioavailability of protective NO (222, 243, 290, 292, 463, 615, 649).
Reactive oxygen species in I/R
Oxidant and nitrosative stress during I/R (and other conditions) involves direct damage by RNOS to nucleic acids, proteins, lipids, and carbohydrates (292–294). In addition, cell signaling dysfunction is produced by “indirect” effects that are mediated by RNOS-dependent alterations in thiol redox circuits or by direct effects via covalent, oxidative, or nitrosative modification of key regulatory proteins in cell signaling cascades (92, 154, 273, 498). A cornerstone piece of evidence that oxidants play an important role in I/R is derived from application of ROS detection methods, such as redox-sensitive green fluorescent protein, 2′,7′-dihydorodichorofluorescein, and MitoSox Red, among others, to postischemic tissues. Data obtained using such methods does not allow determination of which ROS is being produced, but are widely accepted as specific markers for oxidative stress in I/R. However, very recent work that these methods also detect reactive sulfide species (RSS) and indeed are more sensitive to RSS than ROS, suggesting that such methods cannot distinguish ROS from RSS (181). As a consequence, the production of ROS in particular states may be overestimated, while potential contributions of RSS to the outcome may not be appreciated. This is more likely a problem for ROS assessment under normal conditions, where oxidants and H2S are both produced, probably in similar amounts (181), to subserve signaling functions, whereas the oxidant production is clearly elevated in I/R as assessed by electron paramagnetic resonance spectroscopy (462, 481, 924), while it is likely that production of cardioprotective H2S is reduced.
Superoxide and other ROS
The univalent reduction of molecular oxygen produces the superoxide anion radical (O2−), which is the initial reactive oxygen species produced during I/R. Support for this concept was first presented by Granger and coworkers, who showed that IRI was reduced significantly by treatment with SOD (293) (Fig. 7). Subsequent work demonstrating that less IRI occurred in animals treated with SOD mimetics and in genetic models where the cytoplasmic or mitochondrial isoforms of SOD were overexpressed (132).
Figure 7.
Mechanism for XO-dependent production of ROS at the onset of reperfusion. During the period of ischemia, ATP is step-wise catabolized to hypoxanthine, which accumulates in the tissues because the lack of blood flow does not wash out metabolites from the tissues. Coincident with these changes, xanthine dehydrogenase is converted to XO by a proteolytic mechanism. Thus, a requisite substrate (hypoxanthine) and the activated enzyme (XO) are present in excess in ischemic tissues, but the oxidation of hypoxanthine to xanthine and uric acid cannot proceed, owing to the lack of molecular oxygen that is required to fuel the reaction. On reperfusion, this requisite substrate is suddenly resupplied to the tissue, which fuels the rapid overproduction of ROS. ROS-induced formation of chemoattractants promotes leukocyte infiltration, neutrophils in particular, which in turn exacerbate cellular injury via NADPH oxidase-dependent respiratory burst, MPO-mediated formation of hypochlorous acid, N-chloramines, and 2-chloro fatty acids (2ClFA), and release and activation of hydrolytic enzymes that target every type of biomolecule found in cells and tissues.
Superoxide per se is not particularly toxic in vivo because this radical species undergoes rapid, spontaneous (i.e., non-catalytic) dismutation to hydrogen peroxide (H2O2), a conversion accelerated about ten thousand-fold by SOD. This rapid conversion effectively prevents the reaction of O2− with other biomolecules in cells unless generation of O2− is in very close proximity to potential reactants. On the other hand, H2O2 derived from the dismutation of superoxide can be directly cytotoxic when produced at high concentrations and can also fuel the generation of the highly reactive hydroxyl radical (HO·) via the iron- or copper-catalyzed Fenton reaction. Phagocytic cells use an NADPH oxidase isoform to generate superoxide, which dismutates to H2O2, a damaging oxidant in its own right but which is also a substrate for myeloperoxidase (MPO)-catalyzed formation of hypochlorous acid. Moreover, superoxide can be converted to another highly reactive species, the hydroperoxyl radical (HOO•), in the acidic environment that typifies ischemic tissues (11). Superoxide-dependent cell toxicity can also be initiated by its interaction with NO, which leads to the formation of peroxynitrite (ONOO−) and other damaging RNOS. Peroxynitrite can be protonated to form peroxynitrous acid (ONOOH), itself a strong and highly cytotoxic oxidant. Peroxynitrite also serves as precursor to •OH generation, being more effective in this regard than the reaction of reduced iron with H2O2 to produce hydroxyl radicals. Finally, if produced within several molecular diameters of target reactants, especially enzymes with iron-sulfur centers such as aconitase, fumarase, NADH dehydrogenase, creatine kinase, and calcineurin, superoxide can oxidatively inactivate their catalytic functions (649). Interestingly, circadian variations in the expression of calcineurin as well as its regulator RCan1.4, confer time-of-day changes in the susceptibility of the myocardium to IRI (673). Whether oxidants play a role in this response has not been evaluated, but it is clear that there is an interplay between the circadian clock and cellular redox status (17, 26, 554).
Sources of superoxide
Superoxide is produced by number of cellular enzymes, as well as via the electron transport chain in mitochondria. The major enzymatic sources are XO, NADPH oxidases, cytochrome P450 oxidases, and uncoupled NOS. The relative importance of each of these enzymes in the development of oxidative stress in I/R varies depending upon the species and tissue examined, time after the onset of I/R, or the experimental protocol used to produce IRI. For example, endothelial XO plays a major role in ROS generation early on, while leukocyte NADPH oxidase may be more quantitavely significant in the later phases in a model of bowel I/R (289). While ROS-induced apoptotic cell death in neurons exposed to anoxia-reoxygenation also appears to involve a temporal shift in oxidant sources, a transient increase in mitochondrial ROS production occurs early during hypoxia that progresses to a XO-dependent second phase, while enhanced NADPH oxidase activity predominates as a ROS source upon reoxygenation (4).
Although expressed in many tissues, particularly high levels of XO have been reported in hepatocytes, intestinal enterocytes, and capillary endothelial cells (624). Under hypoxic conditions such as ischemia, XO is formed from xanthine dehydrogenase, while one of its substrates, hypoxanthine, accummulates in the tissues secondary to ATP catabolism and lack of washout because the flow of blood is interrupted. This sets the stage for a burst of O2− production when the other required substrate, molecular oxygen, floods the tissues on reperfusion (Fig. 7). The importance of XO-derived O2− in I/R is shown by the decreases in Ca2+ overload, markers of oxidant stress, leukocyte infiltration, and tissue injury that occur when the enzyme is inhibited or depleted prior to I/R (290, 463, 649). XO bound to the surface of endothelial cells is released during local tissue I/R, resulting in increased plasma concentrations of the enzyme, which can circulate to remote organs to instigate oxidant-dependent distant site injury. It is of interest to note that XO catalyzes the conversion of nitrite to NO (278), providing mechanistic insight regarding the effectiveness of nitrite treatment for ischemic disorders (719).
In addition to XO, I/R-induced oxidative stress also involves superoxide generated by two general forms of the NADPH oxidases (NOX), the prototypical NOX of phagocytic leukocytes and isoforms expressed by a variety of non-phagocytic cells. Phagocytic NOX is quantitatively most significant in terms of total superoxide generated in response to I/R. Normally, this NOX isoform is inactive, but can be stimulated to participate in host defense on exposure to bacteria as well as by inflammatory mediators released in sterile inflammatory conditions such as I/R. These stimuli provoke a massive respiratory burst characterized by superoxide release extracellularly or into phagolysosomes (463, 649). Phagocyte NOX-derived O2− rapidly dismutates to hydrogen peroxide, which fuels hydroxyl radical generation via the Fenton reactions as well as MPO-catalyzed production of hypochlorous acid.
As noted above, a second general class of NOX isoforms are expressed in nonphagocytic cells, expecially fibroblasts, vascular smooth muscle, and endothelial cells comprising the vascular wall (391, 463, 649). These vascular isoforms differ from the phagocytic NOX in that they are constitutively active, produce low levels of superoxide, and participate in cell signaling via effects on kinases and phosphatases (391). However, their activity can be upregulated by proinflammatory mediators, with maximal rates of O2− production that are approximately 10% of those achieved during the respiratory burst by leukocyte NOX. Superoxide production at this rate, although relatively low, is sufficient to contribute to I/R-induced oxidant stress (208, 261).
Phagocytic NOX is not constitutively active under normal conditions because its regulatory subunits are sequestered in separate subcellular compartments. Holoenzyme assembly of activated NOX is driven by exposure to proinflammatory mediators, which results in recruitment of these cytosolic moieties to the catalytic subunit residing in the plasma membrane (463, 649). In contrast to phagocytic NOX, vascular cells appear to maintain a portion of total NOX that is fully assembled and active in cell membranes, accounting for low level constitutive production of the ROS that subserve signaling functions, while a second pool is associated with cytoskeletal elements. Similar to phagocytic NOX, a third pool of vascular NOX subunits is not constitutively active because the enzyme constituents are sequestered in different cellular compartments until stimulation, which promotes their translocation and assembly into the holoenzyme (463, 649). A large body of evidence supports a role for both vascular wall and leukocyte NOXs in postischemic injury to cells of the vascular wall and organ parenchyma after exposure to I/R or anoxia-reoxygenation (463, 649).
The microsomal mixed function oxidase cytochrome P450 (CYP) enzymes are best known for their role in xenobiotic metabolism by the liver, as well as catalyzing the univalent oxidation or reduction of some lipids (e.g., arachidonic acid), vitamins, steroids, and cholesterol. However, it is now known that endothelial cells also express members of this family of membrane-bound, heme-containing oxidases (282, 296). Vascular CYPs catalyze the formation of eicosanoid derivatives from arachidonic acid, some of which produce vasoconstriction [20-hydroxyeicosatetraenoic acid 20-HETE)], while others produce vasodilation and/or exert anti-inflammatory effects [epoxyeicosatrienoic acids (EETs)]. Thus, the role and importance of distinct CYP-derived products in I/R is complex, since these enzymes catalyze production of both anti-inflammatory EETs as well as harmful vasoconstrictors and ROS (180). Indeed, CYP-derived 20-HETE may contribute to I/R injury in the heart and brain, by a mechanism that involves formation of ROS and dihydroxydecanoic acid (131, 210, 302, 874). In sharp contrast, CYP-derived EETs have been shown to attenuate postischemic inflammatory responses (180, 302, 862).
NOS exists as three isoforms designated endothelial, neuronal, and inducible NOS. Each of these isotypes are dual-function oxidoreductase enzymes that require tetrahydrobiopterin (BH4) as an essential cofactor to shuttle electrons from molecular oxygen to L-arginine (L-arg), a reaction that produces L-citrulline and NO. All NOSs can become uncoupled from NO production when BH4 or L-arg are absent, producing O2− instead of NO (212, 663). This uncoupling can also occur by dissociation of NOS from associated proteins (e.g., HSP90) that are necessary for coupled function, oxidation of BH4 secondary to overproduction of O2− or ONOO−, oxidation of the zinc-thiolate complex that stabilizes the NOS homodimer, or via S-glutathionylation (663). NOS uncoupling appears to be an important contributor to I/R injury, and can be reversed by administration of L-arg, BH4, or the BH4 precurser sepiaterin in animal models (212, 663, 708, 864).
In normal cells, mitochondria represent the major intracellular source of O2−, mainly due to “electron leak” at complex I (NADH ubiquinone oxidoreductase) and complex III (ubiquinone/cytochrome c reductase) in the electron transport chain (477, 633). It also appears that a significant proportion of I/R-induced ROS formation is derived from mitochondria (Fig. 8) because postischemic oxidant production, vascular dysfunction, and parenchymal cell injury are attenuated by treatment with inhibitors specific for steps in the electron transport chain, targeted delivery of antioxidants to mitochondria, and transgenic overexpression of mitochondrial versus cytosol-specific isoforms of antioxidant enzymes (e.g., MnSOD vs. CuZnSOD, respectively) (145, 146, 362, 477, 633, 745, 868). Recent work indicates that ischemic accumulation of succinate is a metabolic signature of ischemia and acts as an electron store in the absence of oxygen. This accumulation is propelled by purine nucleotide breakdown to fumarate during ischemia, which drives succinate dehydrogenase to operate in reverse, generating succinate, an effect that is magnified by partial reversal of the malate/aspartate shuttle in ischemia. Upon reperfusion, the accumulated succinate fuels ROS generation by reverse electron transport at mitochondrial complex I (145, 146). In addition to increased postischemic ROS production by this organelle, the bioavailability of mitochondrial oxidants is enhanced by I/R-induced reductions in mitochondrial antioxidant activities, which reduces the disposal or scavenging of ROS as they are produced (745).
Figure 8.
Generation of ROS by mitochondria (mitoROS) is a nexus for both activation of cell survival programs that mediate the effect of conditioning stimuli to enhance tolerance to I/R and serves as a focal point for overexuberant ROS-induced ROS release that contributes to the pathogenesis of cell injury in I/R. On the one hand, ROS triggers the activation of cell survival programs in responses to a number of mildly noxious stimuli, such as short bouts of ischemia or antecedent ethanol exposure or pharmacologic agents (activators of mitochondrial ATP-sensitive potassium (mKATP) or large conductance, calcium-activated potassium (BKCa) channels. The enhanced tolerance to ischemia invoked by these mitoROS-dependent conditioning stimuli, which can be delivered before (preconditioning), during (perconditioning) or at the onset of reperfusion (postconditioning), activate protective protein kinases, such as PKCε, the expression of prosurvival genes (e.g., heme oxygenase-1) and mitochondrial antioxidant defenses (e.g., MnSOD, aldehyde dehydrogenase-1, or ALDH2), as well as targeting the MPTP to maintain the channel in a closed state. On the other hand, overexuberant ROS generation at the onset of reperfusion, driven by ROS-induced ROS release that is fueled by electron transport chain dysfunction, especially at complexes I and III, and enhanced activities of p66Shc, MAO, and NADPH oxidase-4 (Nox4) in mitochondria, causes the MPTP to open, leading to swelling, cell disruption, and death. Not depicted is the effect of oxidants to alter the balance of mitochondrial fission and fusion in conditioning and I/R, which emerging evidence has implicated as contributory to both processes. The dual nature of ROS as protective vs damaging species relates to the type of ROS produced, their concentrations, and/or the compartmental localization for their production. MIcroRNAs (miR’s) formed during conditioning and during I/R produce changes in mitochondrial energy metabolism, apoptosis, mitochondrial fission/fusion balance, and ROS production. See text for further explanation. Modified from Refs. 406 and 730.
Recent evidence indicates that sources other than the electron transport chain may be quantitatively important sources of mitochondrial ROS production in conditions characterized by oxidative stress. These include two enzymes localized to the mitochondrial outer membrane, p66Shc and monoamine oxidase (MAO) (118, 194, 367, 790) (Fig. 8). The redox adaptor protein p66Shc is activated during reperfusion by phosphorylation of Ser 36, which promotes p66Shc translocation to the mitochondrial outer membrane, where it oxidizes cytochrome c, producing ROS in the process (29, 790, 868). Prevention of p66Shc phosphorylation and translocation to the outer mitochondrial membrane reduces postischemic myocardial damage (868). Intravenous injection of small interfering RNA targeting p66Shc reduced cerebral lesion volumes, preserved blood-brain-barrier integrity, decreased neurological deficits and improved survival in a murine stroke model and preserved claudin-5 levels in primary human brain microvascular endothelial cells exposed to hypoxia/reoxygenation (737). Genetic deletion of p66Shc also effectively reduces tissue injury and oxidative stress induced by hindlimb, myocardial, and cerebral I/R (118, 609, 738, 897). Interestingly, the results of a more recent study indicates that larger infarct sizes occurred in mice genetically deficient in p66Shc and in p66Shc-silenced wild-type mice, as long as coronary occlusions did not exceed 30 min and infarct sizes were small (12). For longer occlusions, that p66Shc ablation did not confer protection.
The MAOs are another outer mitochondrial membrane enzyme that appears to be a prominent source of oxidants (Fig. 8). These enzymes normally function to oxidatively deaminate monoamine neurotransmitters and dietary tyramines, producing aldehydes and hydrogen peroxide (193, 194, 205). However, there is an emerging body of evidence indicating that MAOs also contribute to oxidative stress in cardiac I/R injury (70, 367, 408, 409, 817).
Nitrosative stress in I/R
The formation of NO• is catalyzed by the action of NOS. This radical species can also be produced via the enzymatic reduction of nitrite or nitrate by XO (278, 831). Under hypoxic conditions, mitochondrial cytochrome c oxidase also serves as a source of NO• (120). The half-life of this molecule is very short (a few seconds), owing to its high reactivity. Its evancescent nature, when coupled with its ability to readily cross cell membranes and the fact that the endothelial isoform of NOS (eNOS) produces NO• in relatively low quantities allows this radical species to be ideal signaling molecule under physiological conditions. As such, NO• plays an important regulatory role in the vasculature, where it acts as a vasodilator, modulates platelet aggregation and adhesion, prevents adhesive interactions between marginating leukocytes/platelets and the endothelium, and regulates angiogenesis (339, 615, 706). However, the physiology of NO• is quite complex because its high reactivity permits its interactions with a large number of biomolecules (97, 299, 463, 498, 509, 615, 806).
NO• exerts both direct and indirect effects, depending on the rate of its production (299, 463). Direct effects occur when this radical species is produced at low concentrations or fluxes, allowing NO to subserve a signaling function via interactions with its classic target guanylate cyclase as well as participating in the formation of nitrosylated lipids and proteins that regulate/modify cell function, and prevention of iron-dependent formation of ferryl-heme radicals by H2O2 (719). Indirect effects are the result of interaction of NO with O2 or O2−, which leads to the formation of dinitrogen trioxide (N2O3) or peroxynitrite (ONOO−), respectively. When generated at low concentrations, these secondarily derived RNOS participate in cell signaling. On the other hand, when N2O3 and ONOO− are produced at high rates during I/R pathophysiological nitrosative stress ensues.
Biologic targets of oxidative/nitrosative stress in I/R
As noted above, ROS and RNOS can attack cell proteins, lipids, and nucleic acids in postischemic tissues. However, work conducted in the last 15 years has led to the concept that simple damage to the structure of these macromolecules does not fully explain the deleterious effects of I/R-induced oxidative/nitrosative stress on cellular function. Indeed, it has become apparent that postischemic ROS/RNOS influence the function of regulatory and effector proteins involved in the response to I/R induces dysregulation of the network of thiol redox circuits in cells, as discussed later.
Cellular redox signaling in I/R
While the generation of ROS, RNS, and RNOS were once thought to be invariably deleterious, it is now abundantly clear that cellular mechanisms have evolved to exploit these reactive species in signaling cascades. Because of their potential for relatively indiscriminate reactivity with virtually all biomolecules in cells, their signaling specificity in radical-mediated control systems occurs by mechanisms very different from the classical ligand-receptor signaling paradigm (D’Autréaux, 2007). For example, it is now known that H2O2 can influence specific signaling pathways by targeting thioldisulfide redox switches on thioredoxin proteins or by interacting with other regulatory/effector proteins (168, 398, 414). It has been proposed that influencing the switching of key redox-active cysteines between reduced thiol and oxidized disulfide forms on proteins that are compartmentalized allows H2O2 to participate in discrete, spatially and kinetically distinct signaling cascades (168, 246, 273, 274, 334, 338, 398, 414).
Like H2O2, NO or RNOS can also react with cysteine or reduced glutathione, with this interaction producing S-nitrosothiols as the major path to influence cell signaling independent of the classical sGC-mediated path (498). Interestingly, when proteins are S-nitrosylated, they typically produce protective responses via targeting a variety of cell surface receptors, the transcription factor NFκB, IκB kinase, PKC, apoptotic enzymes, PTPs, MnSOD, cytoskeletal actin, and mitochondrial complex I (248, 249, 275, 337, 338, 498, 531, 643, 753, 842). A recently recognized system of denitrosylases (e.g., S-nitrosoglutathione reductases and the thioredoxin system) provides exquisite control of the extent of S-nitrosylation, modulating the impact of interactions between NO or NO derivatives with thiols as well as redox signaling in general (65). The extent to which dysregulated S-nitrosylation/denitrosylation participate in I/R has not yet been examined.
Results of a recent study have challenged the widely held view that ROS production by mitochondria or other intracellular sources plays a key role in the pathogenesis of I/R injury (534). In an elegant series of experiments, it was shown that treatment with synthetic copolymer-based sarcolemmal stabilizers such as poloxamer 188 reduced postischemic necrosis, apoptosis, hypercontracture, and mitochondrial dysfunction without disrupting intracellular oxidative stress or lipid peroxidation. Because the copolymers do not cross the sarcolemma, these findings suggest that intracellular targets of reactive oxygen species are not sufficiently disrupted to affect cell death when sarcolemmal integrity is preserved by synthetic stabilizers. As such, the results are consistent with the concept that postischemic oxidative stress is uncoupled from postischemic myocyte injury and supports the notion that I/R destabilizes the sarcolemma, resulting in disrupted regulation of intracellular Ca2+, as a major mechanism underlying postischemic myocardial injury. Further, these observations provide an attractive explanation for the failure of antioxidant therapies to limit postischemic tissue injury in clinical trials.
Endoplasmic reticulum stress in I/R
The ER is a complex membranous organelle that participates in regulating calcium homeostasis, lipid synthesis, and the folding of proteins (564). ER dysfunction occurs in response to a wide variety of insults and results in protein misfolding and unfolding in the organelle, a state referred to as ER stress. As these misfolded/unfolded proteins accumulate, they activate transmembrane receptors to induce the unfolded protein response (UPR) (564). The UPR functions to mitigate the accumulation of unfolded proteins by accelerating their degradation and by increasing the expression of chaperones in ER and promoting the formation of new proteins. However, cell death occurs by apoptosis when the UPR fails to relieve ER stress.
Proapoptotic pathways of the UPR are activated during reperfusion of ischemic tissues secondary to ROS generation and the liberation of proinflammatory cytokines (288, 564, 736, 783, 785). On the other hand, the UPR activating transcription factor (ATF)6 system plays a role in cardioprotective effects. Indeed, cardiac-specific upregulation of ATF6 results in improved function and reduced cell death by necrosis and apoptosis following myocarcadial infarction, effects that were associated with increased expression of ER-resident chaperones GRP78 and −98, (533). In sharp contrast, inhibitors targeting ATF6 function have been shown to exacerbate postischemic cardiac contractile dysfunction and increased mortality (785). Mechanistically, ATF6 participates in the activation of several genes, including mesencephalic astrocyte-derived neurotrophic factor (MANF) and the ER stress response gene, Derlin-3, to produce these effects (61, 62, 760). Treatment with recombinant MANF reduced injury in cultured cardiomyocytes subjected to simulated I/R, while knockdown of MANF expression produced opposite effects (760). Similar protection was noted after overexpression of the Derlin-3 gene and other components of the UPR, and also appear to participate in the ER-stress reducing and infarct-sparing effects of ischemic pre- and postconditioning (61, 186, 514, 527). It is of interest to note that in addition to their other better-known therapeutic activities, AMPK activators (e.g., metformin), ischemic preconditioning and/or statins exert some of their cardioprotective effects via influencing the UPR (135, 198, 773).
Mitochondrial dysfunction contributes to I/R
Oxygen lack during low blood flow states inhibits the flow of electrons through the mitochondrial respiratory chain. As a result, ADP phosphorylation to ATP by the F1F0 ATPase cannot occur (192, 303). Indeed, under these conditions of inhibited electron transfer, the ATP synthase actually operates in a reverse mode (ATP hydrolase), hydrolyzing the little remaining ATP in an attempt to maintain Δψm (192, 572). As consequence of these two events, ATP levels decrease very rapidly when ischemia is induced. Interestingly, selective inhibition of mitochondrial F1F0 ATPase slows the rate of ATP loss during ischemia but improves restoration of cellular ATP levels on reperfusion and limits infarct size (303). Impaired oxidative phosphorylation induced by ischemia also inhibits the breakdown and/or oxidation of fatty acids (707). As toxic fatty acids accumulate within affected cells, they fuel inflammatory arachidonic acid metabolite generation (810) and promote mitochondrial PTP opening (195). Because mitochondrial bioenergetic changes are essential early warning signs for impending ischemic conditions, monitoring patient bioenergetic health index shows potential as a new biomaker (123).
As previously mentioned, mitochondria are important sources of oxidative stress in I/R, with excessive ROS being generated by the electron transport chain and the mitochondrial outer membrane proteins p66Shc and MAOs, and several other mitochondrial proteins including mitochondrial NOX4 (172, 406) (Fig. 8). Superoxide produced under physiologic conditions via complexes I and III of the electron transport chain under physiologic conditions is neutralized by SOD. However, the increased leakage of superoxide during ischemia, especially at complex I, overwhelms cellular antioxidant defenses (172, 477, 628, 729). Restoration of oxygen delivery when blood flow is reestablished further compounds these sequelae.
Several other mitochondrial ROS sources have been described, including α-ketoglutarate dehydrogenase (αKGDH), electron-transfer flavoprotein, pyruvate dehydrogenase, glycerophosphate dehydrogenase, and ROS modulator 1 (Romo1). There is evidence that αKGDH can be a major source of oxidants when the NADH/NAD ratio is high, as occurs in I/R. Indeed, increased phosphorylation of this enzyme has been reported in female rats, which reduces ROS generation by αKGDH by mitochondria and cardiomyocytes isolated from these animals after A/R, providing a potential explanation for the lower risk for cardiovascular disease in premenopausal females (464, 628). Roles for the other mitochondrial proteins mentioned above in I/R-induced ROS generation have not yet been studied. However, given the central role of TNF in I/R injury, it is tempting to speculate that ROS modulator 1 may play an important role in postischemic ROS generation by mitochondria because this recently described protein has been shown to link TNF signaling to apoptotic cell death via mitochondrial oxidant production (419, 420).
As described above, I/R-induced opening of the mitochondrial PTP is a final end effector in the plethora of events in the progression to cell death during reperfusion. This pore is quiescent during ischemia because it is inhibited by acidotic conditions. However, I/R-induced mitochondrial Ca2+ overload coupled with the enormous increase in ROS production associated with the reintroduction of molecular oxygen cause the mitochondrial PTP to open (192–194, 603) (Fig. 8). Because of the open mitochondrial PTP is large, readily allowing molecules up to 1.5 kD in size to cross the channel, a massive flux of H+ ions pass back into the mitochondrial matrix, thereby dissipating the Δψm, uncoupling the electron transport chain and inhibiting ATP synthesis (37, 315). At the same time, water is driven osmotically into the organelle, causing excessive swelling that can proceed to rupture.
Although the protein constituents of the mitochondrial PTP and how they interact to control its permeability have not been definitely identified, adenine nucleotide translocase, mitochondrial phosphate carrier, and cyclophilin-D are leading structural candidates (13, 35, 36, 316, 723). Despite this uncertainty, the development of cyclophilin-D inhibitors has allowed examination of the role of the mitochondrial PTP in I/R injury. Inhibition of this putative pore constituent has been shown to mitigate I/R-induced cell death (13, 149, 192, 570, 646, 690, 723). The development of CypD-deficient mice and their use in I/R studies has confirmed the aforementioned pharmacologic inhibitor studies (39, 187, 693). Mitochondrial DNA is another target in I/R (77).
Mitochondria form intercommunicating tubular networks that are linked to the cytoskeleton and are tethered to the endoplasmic reticulum via a network of membrane contact sites. Recent work indicates that mitochondrial tubular networks provide a conductive pathway dependent on proton-motive force for ultrarapid energy distribution within the cell (269). In mammalian cells, the endoplasmic reticulum membrane contact sites with mitochondria are closely apposed (gap distance of 6–15 nm between ER and mitochondrial membranes), cover about 2% to 5% of mitochondrial surface area, and function to establish tethering domains that enable exchange of signals or metabolites (e.g., Ca2+ and lipids) between these organelles (635). These dynamic organelles also undergo cycles of division (fission) and fusion, processes that can become unbalanced in pathologic states to produce alterations in mitochondrial morphology and function (137). Large networks of fused mitochondria appear with loss of fission. On the other hand, excessive fission produces small, fragmented mitochondria, a requisite step for extrinsic apoptotic cell death. Since ischemia-induced reductions in ATP levels and increased mitochondrial ROS production promote fission of these organelles, the ensuing fragmentation of mitochondria contribute to postischemic apoptotic cell death. Moreover, inhibition of mitochondrial fission reduces I/R-induced mitochondrial fragmentation and prevents opening of the mitochondrial PTP (605). Postischemic endothelial dysfunction may also involve this mechanism, since H/R induces mitochondrial fission and fragmentation in cultured endothelial cells (268). It is interesting to note that ER membrane contact sites define the location of fission on mitochondria by controlling where fission machinery assembles (635). Although not yet explored in I/R, these results suggest that therapeutic targeting of membrane contacts sites to modulate mitochondrial fission/fusion may represent a novel approach for clinical cardioprotection in I/R.
Protein kinases play critical roles in the pathogenesis of I/R injury
These include the mitogen-activated protein kinases (MAPKs), protein kinase Cδ (PKCδ), calcium calmodulin protein kinases (CaMK), and receptor-interacting protein (RIP) kinases. The MAPKs are a family of heterogeneous serine/threonine kinases that participate in cell growth, proliferation, survival, and death and include the extracellular signal-regulated kinases (ERKs), c-Jun N-terminal kinases (JNKs), and the p38 MAPKs, with several isoforms and splice variants existing within each group. Since abundant evidence consistently supports a protective role for ERKs in the setting of I/R (327), we will focus on the roles of JNKs and p38 MAPKs in this section.
Evidence supporting a role of JNK in I/R is provided by the observations that: (i) JNK activation occurs in postischemic tissues, (ii) treatment with the selective JNK inhibitors or ablation of the Jnk2 and Jnk3 genes attenuate I/R injury in liver and brain, respectively, and (iii) JNK1- or JNK2-deficient mice exhibited infarct-sparing effects following I/R relative to wildtype animals (83, 404, 456, 571, 601, 778, 828, 850, 919). Although these data strongly support a critical role for JNKs in I/R injury, their participation in postischemic damage is complex and controversial because JNK activation has been shown to be just as protective in the heart as inactivation of the kinase (404). In addition, JNK inhibition has been shown to worsen I/R injury in the liver (479). The reasons underlying these discrepant observations are unclear.
Similar to JNKs, evidence in favor of a role for p38MAPK in I/R is equivocal. Supporting evidence includes the observations that p38 MAPKs are activated by I/R, while treatment with pharmacological p38 MAPKs inhibitors effectively reduces I/R-induced cell death (324, 431, 491, 494, 571, 637, 763, 917). However, it is also clear that preconditioning interventions confer protection against I/R injury by a mechanism that is dependent on p38 MAPK activation (328, 896). The most likely explanation for these incongruent findings may lie in differential p38 MAPK isoform activation in I/R vs preconditioning since lethal I/R results in activation of the p38α isotype while preconditioning interventions activate the cytoprotective β isoform of p38 MAPK (173, 307, 403).
JNK and p38 MAPK activation also contribute to the pathogenesis of I/R injury through their effects to upregulate inflammatory cytokines (421, 684). These kinases, as well as PKCδ (see later), localize to mitochondria to activate death pathways (40, 305, 435, 919). This occurs by a mechanism involving p38- and JNK-dependent phosphorylation and inactivation of the antiapoptotic Bcl2 protein (174, 227) and phosphorylation-mediated activation of several other prodeath Bcl2 proteins (93, 200, 419, 483, 530, 560, 574, 724, 922).
The PKC family includes at least 10 different isoforms (α, β1, β2, γ, δ, ε, η, θ, ζ, ι/, and λ). Of these diverse serine-threonine kinase isotypes, PKCδ and PKCε are the most important in the context of I/R, with the former playing an important role in I/R injury, while the latter is a major contributor in the cardioprotective mechanism underlying pre-and postconditioning (148, 172, 449, 721, 746). With regard to the role of PKCδ, translocation and activation of this isoform occurs in response to I/R (305, 443, 746). More importantly, Mochly-Rosen and coworkers designed peptide activators and inhibitors that specifically target PKCδ to dissect out the role of this specific isoform in the pathogenesis of reperfusion injury. Using these agents, this group and others demonstrated that I/R injury was ameliorated by targeted inhibition of PKCδ (93, 99, 144, 368, 447, 574), while transgenic expression of a PKCδ activator enhanced ischemic damage (136). It is of interest to note that PKCδ appears to play a role in myocardial, α1-adrenergic, and exercise preconditioning in the rat, but not in some other species (99, 552).
A role for CaMK in postischemic myocardial injury was postulated because I/R results in cellular calcium overload and large increases in cytosolic Ca2+ can activate these enzymes. Thus, it was not surprising that activation and translocation of CaMK-II isoform was noted in ischemic hearts (583, 798). Subsequent and indeed more compelling work showed that inhibition of CaMK-II attenuates cell dysfunction and death induced by I/R (816, 909).
The RIP kinases (briefly discussed earlier under section “Necrosis versus regulated necrosis”) normally function to regulate the NF-kB and ERK signaling pathways that are activated by ligation of receptors in the TNF family (240) but are also critical for the progression of regulated necrotic death in response to stressful stimuli (141, 175, 176, 234, 349). With regard to I/R, treatment with a specific inhibitor of RIP1 called necrostatin was shown to reduce postischemic infarct size (175, 176, 593, 670, 726). Similar results were recently demonstrated in RIP3-deficient mice, where it was also shown that RIP3 induced the activation of CaMKII via phosphorylation at Thr287 in response to I/R (909). This study also established that I/R-induced, RIP3-dependent, CaMKII activation to induce necroptosis occurred by a ROS-dependent mechanism involving oxidation of CaMKII at Met281 and Met282 (221). Cerebral I/R also involves RIP3 activation by a mechanism that involves NMDA receptor-mediated calcium influx to activate nNOS (562). Consequent NO production results in RIP3 S-nitrosylation at Cys119 to facilitate its activation to promote necroptosis of cerebral neurons after I/R.
Downstream molecular targets of CaMKII and RIP kinases that couple I/R to cell death are not well defined. However, it is known that L-type Ca2+ channels and Na+ channels are phosphorylated and thus activated by CaMK-II (303, 819, 820). These effects enhance the huge influx of Ca2+ associated with I/R. CaMK also acts to enable Ca2+ release from cardiac SR (835), thereby contributing to calcium overload in myocardial I/R. In contrast, RIP kinases induce ROS production (562, 567) and increase intracellular levels of the death-inducing lipid, ceramide (781).
Epigenetic Changes Contribute to I/R Injury
Epigenetic changes refer to transmissible variations in phenotypic traits that are caused by external or environmental factors that switch genes on and off and affect how cells read genes instead of being caused by changes in DNA sequence of the genes. In the next three sections, we review the three main ways by which genes are epigenetically regulated—DNA methylation, histone modification, and noncoding RNAs, and how epigenetic changes contribute to the pathogenesis of I/R.
DNA methylation
DNA methylation is enzymatically catalyzed by DNA methyl-transferases and causes chromatin condensation, which interferes with transcriptional activator binding to the DNA and silencing of transcription (147, 579). DNA demethylation can also occur, resulting in enhanced gene expression. One of the first studies to implicate DNA methylation in I/R was conducted by Endres and colleagues (217, 218), who showed that stroke increased the content of methylated DNA in affected brain. In subsequent work, the same group showed that reductions in DMNT levels conferred protection in cerebral I/R injury (216). More recently, it has been shown that the temporal profiles of DNA methylation with respect to chromatin hyper- and hypomethylation following various ischemic conditions are highly dynamic (553). A catalog of specific gene targets for methylation in I/R is emerging. As one example, ischemia-induced DNA methylation silenced endothelial thrombospondin-1 gene expression (356). Another report indicates that the cardioprotective PKCε gene is silenced by chronic cocaine exposure-induced methylation, which may account for enhanced I/R injury associated with abuse of this substance (561). Ischemia can also lead to demethylation and thus activation of genes that contribute to injury. For example, stroke-induced demethylation of the Na+-K+-2Cl− cotransporter type 1 gene (476) and increased expression of this transporter may contribute to postischemic brain edema (402).
Histone modifications
Epigenetic gene expression is also regulated by histone modifications, which can occur by methylation, acetylation, phosphorylation, ubiquitination, and sumoylation (147, 920). Acetylation-induced histone modification is a prominent regulator of gene expression in I/R. It is catalyzed by histone acetyltransferases and reversed by HDACs. This was first reported over 30 years ago in a study noting that cardiac ischemia caused a large decrease in histone acetylation, which was most prominent in histones H3 and H4 (771). Similar reductions have been detected in ischemic brain (654). Subsequent work demonstrated that ischemia induces HDAC activity, providing a mechanistic underpinning for the observed reduction in H3/4 acetylation. Based on such observations, it was hypothesized that maintaining histone acetylation would exert cardioprotective effects. Indeed, HDACs inhibition increased histone acetylation, resulting in reduced I/R injury (165, 289, 418, 654, 754, 771, 854). Similarly, treatment of cultured cardiac myocytes with siRNA targeting HDAC4 confers protection in an in vitro model of ischemic injury (288). Initial work has identified several cardioprotective genes that are upregulated by histone acetylation to induce protection including heat shock proteins, hypoxia-inducible factor-1α, caspases, Bcl2, and several survival kinases (Akt, ERK, AMPK, and p21) (9, 33, 229, 288, 418, 586). An increasing body of evidence implicates another HDAC, silent information regulator 1 or SIRT1, as a protective protein in cerebral ischemia (871). Although it remains unclear whether histone ubiquitination or sumolyation play important roles in modifying gene expression in response to I/R, it has been shown that aortic cross-clamping in humans increased phosphorylation of histone H2AX (157).
Noncoding RNAs
Several specific families of noncoding RNAs (ncRNA) have been described, including long ncRNAs, piwi-interacting RNAs (piRNA), and short RNAs, especially microRNAs (miRNA) (147, 159). Long ncRNAs are largely directed at genetic imprinting while piRNAs exert effects that are primarily involved in genomic maintenance, and thus will not be discussed here. Rather, we focus attention on short RNAs, especially microRNAs (miRNA) that are too short (19–25 nucleotides) in length to encode proteins. However, they function to censor gene expression by repressing mRNA translation or by inducing mRNA degradation. As a consequence, miRNAs have the potential to participate in the pathogenesis of I/R injury (86, 162, 277, 281, 297, 492, 517, 688, 691, 866, 887, 894). In addition to regulating the expression of mRNAs and thus protein expression within cells, miRNAs can also be secreted to the extracellular compartment, as can ribosomal RNA. These extracellular RNAs adversely affect outcomes in I/R by acting as damage-associated molecular patterns (DAMPs) and as cofactors for activation of inflammatory cascades and thrombosis (899). Indeed, myocardial I/R is associated with significant release of RNA, including several miRNAs, primarily by cardiac myocytes, although endothelial cells, fibroblasts, and vascular smooth muscle cells also contribute (105, 133). Importantly, these studies also showed that treatment with RNAase1 reduced TNFα shedding, oxidant production, cardiomyocyte hypercontracture, LDH release, and infarct size in hearts subjected to I/R. Another general characteristic of ischemia-induced miRNAs is that their expression profiles are highly variable, depending on the duration of ischemia (preconditioning vs. index ischemia), cell type, or at what time point after blood flow is re-established their expression pattern is studied (1, 230, 252, 459, 695, 767, 879).
Recent work has revealed that the expression of key molecules involved in cell survival and apoptosis (e.g., Bcl-2, FasL, HSP20, HSP60, HSP70, LRRFIP1, Mcl-1, Pdcd4, PI3K, PTEN, and SIRT-1) are altered by miRNA expression in I/R (Fig. 9). MiRNA expression profiling has been used to uncover differential regulation of several miRNAs in different organs following I/R to modulate gene function involved in cardiac cell death, arrhythmogenesis, fibrosis and extracellular matrix remodeling, and angiogenesis (276, 296, 388, 674, 858, 894). As this emerging body of evidence implicates causal roles of specific miRNAs in postischemic tissue injury, approaches to block miRNA function are being avidly pursued. One such approach to interfere with miRNA function relates to the development of single stranded RNA analogs that are complementary to miRNAs but have been modified to confer stability and enhance delivery, while retaining their target specificity (455).
Figure 9.
Functional roles and target genes for miRNAs implicated in ischemia/reperfusion and preconditioning. See text for further explanation.
Ischemia or hypoxia markedly increase the expression of miRNA-1 which acts to promote apoptosis (by targeting Bcl-2, HSP-60, and HSP-70 gene expression) and arrhythmias (through effects on the expression of the potassium channel subunit kir2.1 and connexin-43, a major component of gap junctions) (68, 230, 252, 459, 550, 613, 695, 767, 879) (Fig. 9). Use of short, locked nucleic acid-based antimiRs has uncovered roles for miRNA-15 and themiRNA-34 family in cardiac necrosis induced by I/R (66, 363). On the other hand, downregulation of prosurvival miRNAs (e.g., miRNA–21) also contributes to cell death during ischemia. However, miRNA-21 recovers within two days of reperfusion where it functions to enhance MMP-2 expression and promote fibrosis. Collagen deposition in postischemic heart also occurs at this time point in reperfusion, an effect regulated by downregulated expression of miRNA-29 expression. Overexpression of miRNAs targeting prosurvival genes, such as the anti-apoptotic miRNA-378, limits myocyte necrosis after ischemia (228). As summarized in Figure 9, the expression of a plethora of other miRNAs occurs during I/R, where they influence a number of pathologic processes in postischemic tissues (43, 86, 417, 688, 703, 767, 877, 887).
In addition to their demonstrated role in cell death, arrhythmogenesis, stroke, acute kidney injury, extracellular matrix remodeling, and angiogenesis, miRNAs also influence development of atherosclerotic lesions, oxidative stress levels, inflammatory processes, and endothelial senescence (364, 524, 547, 647, 801, 908). An emerging body of evidence indicates that miRNA-mediated gene silencing also contributes to endothelial dysfunction, platelet dysfunction, and leukocyte recruitment in tissues exposed to I/R, with differential expression patterns varying with duration and extent of ischemia, reperfusion, and the presence of coexisting risk factors (355, 517, 550, 613, 888). What is not yet clear is how changes in epigenetics, bioenergetics, and microRNA expression profiles link to coordinately reprogram the temporal expression of inflammatory genes during the phase shifts (recognition, initiation, adaptation, and resolution) that occur over the course of inflammatory responses in the setting of I/R.
Because miRNAs are present in the blood after I/R, there is growing interest in their use as biomarkers for cardiovascular disease (1, 280, 459, 492, 517). Circulating miRNAs may arise as a result of release from dead cells or by active secretion as protein-miRNA complexes or in membrane-bound apoptotic bodies, exosomes, and/or microvesicles that protect the noncoding miRNAs and account for their remarkable stability in plasma. Expression profiling of circulating miRNAs will likely reveal patterns that improve assessment of risk, diagnosis, and prognosis in persons suffering from ischemic disorders. Indeed, increases in cardiac troponin I, a classic marker for cardiac ischemic injury, correlate very well with alterations in miRNA-208a. Unlike other miRNAs that increase after cardiac I/R, miRNA-208a does not increase after kidney or skeletal muscle damage, pointing to use of this non-coding mRNA as a specific marker of myocardial I/R. In addition, because circulatingmiRNA-208a is not eliminated by the kidney, whereas cardiac troponin I undergoes renal excretion, this particular miRNA may be better biomarker for acute myocardial infarction in patients with concomitant end-stage renal disease. With regard to risk stratification, analysis of a unique signature pattern of 20 miRNAs from whole genome miRNA expression profiles predicted acutemyocardial infarction with a specificity of 96%, a sensitivity of 90%, and an accuracy of 93% (862). Several recent studies also point to the potential utility of miRNA expression patterns for prognostic utility after MI (188, 189, 280, 541, 542, 923). However, we are not aware that any attempt to identify unique miRNA biosignatures relevant for prediction of responses to particular therapies in individual patients has been attempted. Nonetheless, the enormous strides in the application of precision medicine to inform prognosis and guide treatment in cancer patients should provide a strong impetus for discovering biomarker signatures that allow for therapeutic management of ischemic disease that is tailored to the individual patient.
Inflammation Plays a Prominent Role in the Reperfusion Component of Total Tissue Injury in I/R
Because I/R in most organs occurs in the absence of microorganisms, the invoked inflammatory response has been termed sterile inflammation. However, the inflammatory responses to I/R are similar to those invoked by invading pathogens. Both are characterized by recruitment of neutrophils and other leukocytes to the affected tissue site, coincident with the production of cytokines, chemokines, and other proinflammatory stimuli that serve as directional cues (249, 310, 372, 446, 463). Leukocyte trafficking to ischemic sites occurs primarily during reperfusion and involves 11 distinct steps (594–596, 816a, 923a, see Fig. 10). Step 1: Margination. As neutrophils exit capillaries and enter the larger diameter postcapillary venular segment of the microcirculation, hydrodynamic forces move granulocytes from the center stream of flowing blood to the endothelial wall (margination). Step 2: Tethering and rolling. If appropriate adhesion molecules are expressed on activated endothelial cells and the marginated neutrophil, as occurs during I/R, granulocytes are captured (tethered) by adhesion molecules expressed on both cell types that mediate rolling of the leukocyte along the vessel wall. Step 3: Slow rolling. The rolling neutrophil monitors its local environment for the presence of activating factors that promote adhesion molecule expression, thereby enabling the leukocyte to further slow its rolling behavior by forming more weak adhesive interactions that are mediated by the selectins. As the cells roll along and interact with P-selectin on the endothelial surface, excess membrane on the surface of neutrophils is pulled out into long nanotubes (microvilli) that form tethers at the rear of the rolling leukocyte. These tethers eventually detach as the cell continues to roll, but do not retract. Instead, they persist and are slung in front of the rolling leukocyte, to interact again with P-selectin. The membranous nanotube is now referred to as a sling, which the neutrophil rolls over to again be retarded in its movement down the vessel wall as the sling transitions to a tethering function. Step 4: Firm or stationary adhesion. By establishing strong adhesive interactions mediated by integrin-dependent interactions with endothelial ICAM-1 that are upregulated by chemokines, the slowly rolling leukocyte progresses to firm (stationary) adhesion. Step 5: Luminal crawling. Integrin-ICAM-1-dependent adhesion activates intracellular signaling pathways that induce cytoskeletal changes and polarization of the cell that lead to luminal motility. The crawling neutrophils move preferentially along interendothelial junctions in search of preferred routes for diapedesis and are often observed moving against the direction of flow in this exploration. Step 6: Transendothelial cell migration. Neutrophils cross the endothelial cell barrier by traversing interendothelial junctions at preferential sites that overlie areas in the basement membrane that exhibit low matrix protein (Pr−) deposition. Occasionally, inflammatory phagocytes cross the endothelial barrier by moving through cells in a transcellular route. Step 7: Abluminal crawling. Once through the endothelium, the diapedesing neutrophils crawl abluminally along pericyte processes, interacting with basement membrane structures at the same time, with both attachment events mediated by adhesion molecules. Step 8: Penetration of pericyte gaps and regions of low matrix protein expression in the basement membrane. Abluminally crawling neutrophils breach the pericyte layer where gaps between these cells exist, which coincide with regions of low matrix protein deposition (depicted as a lighter shade of green) in the basement membrane. These steps occur more or less simultaneously and use different adhesion molecules to propel the leukocyte through these barriers. Steps 9 and 10: Detachment from the vessel wall and migration through the interstital matrix. Continued migration of the diapedesing leukocyte into the tissue space requires its detachment from components of the vessel wall, which involves release of pseudopodial extensions from their sites of attachment to basement membrane components and adhesion receptors on pericytes and endothelial cells. Once this is achieved, the leukocyte follows directional cues provided by a chemotactic gradient and migrates through the tissue space toward inflammatory foci. Step 11: Attachment to and attack on parenchymal cells. Migrating neutrophils release their cytotoxic arsenal (ROS, MPO, and hydrolytic enzymes) in proximity to both functional and damaged parenchymal cells to cause injury. These phagocytic cells can also establish adhesive interactions with parenchymal cells, which is associated with increased detection of oxidants in the intracellular compartment where they produce injury in a highly compartmented manner.
Leukocyte sequestration increases markedly during reperfusion, when oxygen and blood-borne immune cells are reintroduced to the tissues. While required to support cellular metabolism, which should rescue ischemic tissue, the influx of oxygen in the reperfusing blood serves as a requisite substrate for enzymatic ROS formation by XO and NADPH oxidase. Neutrophil sequestration into previously ischemic tissues occurs secondary to release of danger signals from necrotic cells and formation of mediators that promote adhesive interactions between these innate immune cells and postcapillary venules followed by their emigration into the tissues. Once sequestered in ischemic tissues, activated neutrophils produce a NADPH oxidase-dependent respiratory burst, release hydrolytic enzymes, generate highly injurious hypochlorous acid and N-chloramines via the enzymatic activity of MPO, and secrete pore-forming molecules to produce extensive collateral damage to vascular and parenchymal cells. By these mechanisms, infiltrating neutrophils induce reperfusion injury that amplifies the cellular damage initiated by ischemia (87, 245, 249, 290, 310, 463).
A large number of pathologic processes contribute to I/R-induced leukocyte infiltration. For example, proinflammatory mediator formation and adhesion molecule expression on the surface of leukocytes and postcapillary venular endothelium are provoked by the postischemic oxidant generation. In addition to these effects, NO, a potent anti-adhesive signaling molecule, is quenched by I/R-induced ROS formation. The bioavailabilty of NO is further reduced by the decline in endothelial NOS activity associated with I/R. Postischemic oxidation of NO’s primary downstream signaling target, soluble guanylyl cylase, renders it less responsive to this antiadhesive molecule (397). At the same time, activation of tissue-resident mast cells and macrophages occurs and these inflammatory cells release a variety of other mediators (e.g., TNFα IL-1 and other cytokines, angiotensin II, PAF, and LTB4) to further promote leukocyte-endothelial cell adhesive interactions. Platelet activation is also induced by I/R, which also plays an important role in facilitating leukosequestration in postischemic tissues.
As the adherent leukocytes emigrate into the tissues, they disrupt microvascular barrier function during their transit, leading to edema formation and increased diffusion distance for oxygen and nutrients. The latter problem is exacerbated by the development of postischemic capillary no-reflow, a leukocyte-dependent nutritive perfusion failure, and endothelium-dependent vasoregulatory dysfunction in arterioles. It is important to emphasize that leukocyte-endothelial cell adhesive interactions, which precipitate the microvascular complications and tissue injury induced by reperfusion, are one of the earliest signs of tissue dysfunction and injury elicited by I/R (310, 463).
Humoral mediators, cytokines, and complement in I/R
Activation of the complement cascade, a complex reaction scheme that involves approximately 30 soluble and membrane-bound proteins, occurs via classical, alternative, and mannose-binding lectin pathways. Each of these three distinct pathways for complement activation participate in the pathogenesis of I/R injury by directing the formation of a membrane attack complex in plasma membranes that leads to cell lysis. In addition, complement activation provides signals that recruit and activate neutrophils and macrophages to ischemic tissues. Indeed, the observation that chemotactic activity noted in untreated aliquots of lymph (a surrogate for interstitial fluid) draining postischemic tissues can be abolished by addition of a neutralizing antibody directed against C5a strongly supports the notion that complement activation promotes neutrophil infiltration in I/R (73, 202).
That I/R could trigger activation of the complement cascade was first described by Hill and Ward (340) almost 50 years ago. Subsequent studies demonstrated increased expression for all of the classical complement cascade proteins in ischemic tissues (804, 876). Postischemic complement activation is triggered by release of subcellular membrane consistuents secondary to cell necrosis (127, 248, 461, 672). Complement depletion or treatment with function-blocking antibodies that target specific proteins in the cascade have proven to be very effective in limiting postischemic inflammation and tissue injury, and constitute some of the strongest evidence supporting a role for complement cascade in I/R (76, 167, 251, 522, 530, 739, 795, 838, 845, 904). The lectin pathway for complement activation has also been implicated in the pathogenesis of myocardial, cerebral, and renal I/R (281, 627). Interestingly, mice deficient in the alternative pathway protein factor B or treated with a chemical alternative pathway inhibitor demonstrated improved outcomes in a stroke model, as did mice deficient in both the classical and lectin pathways (213). However, mice deficient in C6, a component of the membrane attack complex were not protected. These observations led to the conclusion that activation of the alternative pathway in stroke participates in amplifying the complement cascade to propagate cerebral injury but does so by a mechanism that does not involve the membrane attack complex (213).
In contrast to the invariably deleterious effects of complement activation in I/R, cytokines may exert pro- or anti-inflammatory effects in postischemic tissues, the net effect being related to what particular mediators are produced, where they are released and at what time during ischemia or reperfusion they appear, and how much of each mediator is produced. Although an enormous number of studies have been directed at uncovering the expression profiles for cytokines produced during the various phases of I/R, tumor necrosis factor-α (TNFα), and to a lesser extent interleukin-1, have emerged at center stage in the orchestration of mediator responses to I/R in most tissues. Macrophages are a major source of TNFα in I/R, but this cytokine is also produced by a number of other cell types. This cytokine acts as a paracrine meditor in eliciting localized responses but also enters the circulation at sufficient concentrations to exert effects at distant sites. TNFα binding to its specific receptors results in activation of NFκB and other transcription factors, chemokine expression, ROS formation, and the expression of adhesion molecules. These activities stimulate the recruitment and activation of neutrophils in postischemic tissues.
Endogenous danger signals and I/R
When ischemic cells undergo necrosis, cell membranes rupture, releasing ATP, heat-shock proteins, S100 proteins, and other stimuli that are normally shielded from the immune system by their intracellular location. The release of these danger signals, also collectively referred to as damage-associated molecular patterns (DAMPs), into the extracellular compartment is detected by other proteins called pattern recognition receptors (PRRs), [one important example being the toll-like receptors (TLRs)]. When DAMPs bind to cell surface TLRs, a number of transcription factors (e.g., NF-κB) are activated to promote the upregulation of proteins involved in activation of innate immune responses (496, 543, 548, 764). As an example, release of intracellular ATP from necrotic cells activates the NLRP3 inflammasome, which in turn functions to induce neutrophil infiltration into ischemic tissues (548, 764).
Cell Types Involved in Postischemic Inflammation
In addition to parenchymal cells, endothelial cells, vascular smooth muscle cells, neurons, platelets, and several immune cells subtypes are also affected by and contribute to the pathophysiology of I/R injury. In patients surviving acute ischemic events, fibroblasts play a role in remodeling the extracellular matrix and scar formation in the affected organ, but this will not be reviewed herein. Because inflammatory responses contribute to much of the reperfusion component of total tissue injury in I/R, we focus on the endothelium and immunocytes, both circulating and and those residing in the tissues, and how these cells interact to produce postischemic damage.
Endothelial cells
The endothelial cells lining blood vessels walls constitute a significant proportion of the different cell types in each tissue, representing 3% to 5% of myocardial volume but outnumbering (~45% of the total cells) other cell types in murine ventricles, as well as comprising more than 60% of the nonmyocytes in the myocardium (576). This abundance suggests that endothelial cells play an even larger role in normal cardiac physiology and the heart’s response to injury than assumed previously. I/R compromises endothelial barrier function, promotes adhesion of immune cells to and emigration across postcapillary venular endothelium, disrupts endothelium-dependent vasodilation, and control of endothelial hemostasic mechanisms. In addition, narrowing the capillary lumens occurs secondary to I/R-induced endothelial cell swelling, which facilitates the entrapment of neutrophils in these minute vessels, thereby contributing to the development of postischemic no-reflow. The mechanisms underlying each of these deficits in endothelial cell function will be summarized next, with the exception of leukocyte/endothelial cell adhesive interactions, as they were discussed in the section on inflammation earlier.
Microvascular permeability changes in I/R
A single layer of endothelial cells are arranged circumferentially around the inner lining of all blood vessels. These cells comprise an effective barrier to the movement of solutes and fluid from the blood stream into underlying tissues of most organs, while production of NO and other anti-adhesive molecules (H2S, CO, and adenosine) maintain a nonthrombogenic and anti-inflammatory phenotype under normal conditions. The integrity of intercellular junctional complexes between adjacent endothelial cells determine the porosity of this barrier. I/R disrupts both tight and adherens junctions in endothelial cells (460, 551). An impressive variety of proinflammatory mediators are released during I/R including ROS, chemokines (e.g., RANTES (regulated upon activation, normal T-cell expressed, and secreted), cytokines (e.g., TNF and IL-1), tachykinins (e.g., substance P), growth factors [e.g., vascular endothelial growth factor (VEGF)], and proteases (e.g., MMPs) as well as low molecular weight factors (e.g., histamine, PAF, and LTB4). These mediators activate cell signaling mechanisms to provoke phosphorylation of junctional components, their internalization/degradation, and/or their connections to cytoskeletal elements, with the end result being dissolution of intercellular junctions and increased microvascular permeability, events associated with leukosequestration (8, 15, 460, 551, 662, 774). Recent work indicates that chemoattractants increase microvascular permeability by inducing TNF release from adherent neutrophils when they are in close proximity to endothelial junctions (241). The formation of intercellular junctional gaps involve calcium-dependent phosphorylation of myosin light chain kinase and cytoskeletal contraction (460) and also arise via influences on a redox sensor, tricellulin, that is localized to tight junction elements at three-cell endothelial contacts (158). In addition to granulocytes, CD4+ T lymphocytes are also capable of inducing endothelial barrier disruption and increased permeability, which appears to be related to their participation in neutrophil recruitment (512).
Leukocyte/endothelial cell interactions in postcapillary venules
The sequestration and infiltration into the tissues by PMNs and other immunocytes is a hallmark of I/R. Endothelial cells play a central role in this process, influencing a sequence of events that are both complex and highly dynamic. Leukosequestration begins with white cells forming adhesive interactions with the endothelium lining postcapillary venules, followed by their egress through the endothelium and subjacent basement membrane, and subsequent migration into the tissue spaces as they track directional cues released from damaged cells and accessory immunocytes such as mast cells. The mechanisms underlying these characteristic responses to I/R were depicted in Figure 4 and described in detail earlier.
I/R disrupts endothelium-dependent vasomotor control mechanisms in arterioles
I/R induces endothelium-dependent vasomotor control dysfunction as well as activation of vasoconstrictor mechanisms (286, 908). Endothelium-dependent vasodilator responses are impaired in postischemic tissues because the bioavailability of NO is reduced by several pathologic mechanisms. First, expression and activity of eNOS are reduced (908). Second, NO is effectively scavenged as a result of postischemic increases in TNFα-induced ROS production (908). A third cause of reduced bioavailability of NO relates to increased competition for the eNOS substrate, arginine, by arginase (335). Fourth, reductions in the eNOS cofactor dihydrobiopterin impairs NO production (784). The latter results in eNOS uncoupling, wherein the enzyme produces superoxide instead of NO.
On the other hand, endothelin production by the endothelium is enhanced by I/R, leading to vasoconstriction, inhibition of NO production, capillary no-reflow, and activation of inflammatory cells (28, 439, 821). Through these effects and other mechanisms, endothelin contributes to postischemic cell death and is arrhythmogenic in the heart. In view of these actions, it is surprising that exogenous administration of this vasoconstrictor prior to I/R is cardioprotective. This is consistent with the concept of hormesis, wherein mildly noxious stimuli induce preconditioning, while greater intensity provocations induce injury.
I/R induces a thrombogenic phenotype in endothelial cells
Under normal conditions, the endothelium participates in the control of hemostasis, maintaining an antithrombotic state, via effects to prevent platelet adhesion and aggregation. However, this is reversed after I/R owing to reductions in bioavailability of endothelial NO (see earlier) and production of prothrombogenic mediators such as PAF. As a consequence, vasoconstriction ensues as does platelet activation, which culminates in increased adhesion of platelets to the endothelium and to each other or to leukocytes to form homotypic or heterotypic aggregates, respectively. The adhesion and aggegretory effects are due to increased surface expression of P-selectin, loss of NO/cGMP-mediated regulation of platelet calcium levels, and ligation of platelet surface integrin glycoprotein (GP) IIb–IIIa with fibrinogen, resulting in increased platelet aggregation (51, 499, 638). Increased surface expression of endothelial tissue factor is also induced by I/R. This pathologic event activates clotting factors and the formation of microthrombi, which may participate in the development of capillary no-reflow (51, 589, 780).
Neutrophils
Ischemia is associated with relatively modest increases in the numbers of leukocytes interacting with the blood vessel wall, which progressively increase with ischemic duration. Within minutes of reestablishing the blood supply to an ischemic organ, the number of rolling and adherent leukocytes, which are primarily neutrophils, increases dramatically. These adhesive events occur almost exclusively in postcapillary venules and precipitate enhanced transmigration of these inflammatory phagocytes into the tissue spaces (310, 460, 463). Upon becoming firmly adherent, the flowing blood exerts a directional force that causes the leukocyte to adopt a more flattened, tear-drop morphology, which allows the adhesive cell to better withstand the anti-adhesive effects imposed by the flowing blood as well as increasing the surface area for contact between the immunocyte and the endothelium. This is accompanied by a directional redistribution and polarization of signaling, adhesion, cytoskeletal and receptor proteins toward the leading edge of the cells, permitting the leukocyte to extend pseudopods. The immunocytes use these structures to crawl along the endothelium, usually along the interendothelial junctions. Recent work has shown that adherent neutrophils migrate along the endothelium following a gradient of directional cues provided by chemoattractants derived from damaged and dying cells. This allows intravascular homing of the leukocyte to foci of injury before they are allowed to diapedese (548). In addition, it now appears that crawling leukocytes can sense that they have encountered sites along the junction that are permissive for transmigration between endothelial cells, and diapedesis occurs. These permissive sites occur at regions of low expression of basement membrane constituents which also overlie gaps in the pericyte layer surrounding postcapillary venules. Once they cross these barriers and are recruited to the tissue space, PMNs secrete a host of factors known to contribute to tissue injury. These include ROS, MPO-generated hypochlorous acid, a large number of cytokines and chemokines, proteases such as MMPs and elastase, and lipid mediators such as LTB4 and perhaps chlorinated fatty acids (662).
Transmigration across the endothelium may occur by paracellular movement between adjacent endothelial cells or transcellular progression through individual endothelial cells, with the former pathway representing the most common route in I/R (117, 548, 594, 595). Recent advances in the use of multicolor fluorescence spinning disk and multiphoton confocal intravital microscopic imaging have shown that once the inflammatory phagocytes pierce the endothelial barrier and subendothelial matrix, they migrate along pericytes to exit the vascular wall at regions of low matrix deposition in the basement membrane that are also characterized by gaps between pericytes (594, 595, 644, 741). Once in the subendothelial space, neutrophils are guided by pericyte cues to enter and move through the interstitium by a mechanism dependent on actin polymerization and on matrix metalloproteinase (MMP)activity but without degradation of pericellular collagen (486). Reverse migration of neutrophils away from sites of injury and inflammation has also been described, but the pathophysiologic relevance of this response has not been evaluated in I/R (98, 596).
Postischemic tissue injury is significantly reduced by neutrophil depletion, treatment with immunoneutralizing antibodies directed against adhesion molecules, and in mice genetically deficient in adhesion molecules, results which provide the strongest evidence for a role for neutrophils in I/R (309, 335, 378, 385, 446, 602, 658, 667). Although neutrophils appear to be the major immunocyte contributing to injury occurring in the first hours of reperfusion, other inflammatory cells, such as macrophages, lymphocytes, mast cells, and platelets are now known to influence neutrophil sequestration (662).
Lymphocytes
For many years, work directed at uncovering the role of inflammation in I/R injury was directed almost exclusively at components of innate immunity including neutrophils, the complement system, postischemic release of proinflammatory cytokines, chemokines and other mediators, and tissue-resident sentinels such as mast cells. This emphasis on innate immune contributions was probably driven by the acute nature of most experimental models of I/R. However, recent unequivocal demonstrations of the importance of T and B lymphocytes [and dendritic cells (DCs), see later] in postischemic tissue injury has established a role for the adaptive immune system in I/R (102, 347, 348, 502, 886). This is consistent with the emerging concept that the innate and adaptive immune systems share reciprocal regulatory activities (410).
There is significant accumulation of CD4+ T cells in postischemic tissues, suggesting that these immunocytes may participate in the pathogenesis of I/R injury (107, 322, 407, 532, 610, 692, 710, 797, 873). More compelling support for this concept was provided by studies using pharmacological inhibitors that target T cell activation, migration, proliferation, and adhesion, while studies conducted in immunodeficient mice and in murine genetic knockout models lacking specific T cell types or T cell-derived effectors combined with adoptive immunotransfer of various T cell subsets illustrate the specific role for CD4+ T cells in I/R (458, 502, 512, 873, 886). In most organs tested, T cell/endothelial cell adhesive interactions are mediated by endothelial ICAM-1 (32, 85, 438, 859), VCAM-1 (438) and P-selectin (32, 318, 859). On the other hand, transendothelial migration of these immunocytes is dependent on ICAM-1 (859), CD44 (861), and CD47 (742). It is important to note that this is not the case for stroke, where adhesion of T cells to cerebral microvasculature is not observed (886).
Two general subsets of CD4+ T lymphocytes, Th1 and Th2 cells have been described. Th1 cells are proinflammatory, secreting IL-2, IL-12, IFNγ, and TNFα. On the other hand, Th2 cells secrete primarily the anti-inflammatory cytokines IL-4, IL-5, IL-10, and IL-13. Consistent with these observations, recent studies using knockout mice have revealed a protective role for Th2 cells, while Th1 exert deleterious effects in I/R (711, 859, 889), suggesting that a balance between Th1 and Th2 activities influences the magnitude of postischemic tissue injury. Another subset of T helper lymphocytes, Th17 cells, appears to participate in organ rejection after I/R associated with transplantation and in lung I/R (139, 321, 758). DCs induce naïve CD4+ T cells to differentiate into both Th1 and Th17 cells in an in vitro anoxia-reoxygenation model (873).
Occuring in a sterile environment, I/R is typified by the absence of foreign antigens (exceptions may be in cases of organ transplantation or epithelial barrier dysfunction induced by intestinal I/R). Thus, the mechanisms underlying CD4+ T cell activation and production of tissue injury after I/R are unclear. In light of these considerations, it is surprising that several studies have demonstrated antigen-dependent T cell activation in postischemic tissues (458, 516, 692). In contrast, several other studies provide evidence indicating that CD4+ T cells contribute to hepatic and renal I/R via antigen-independent mechanisms (322, 621, 713, 797, 873). This conundrum is likely explained by the danger model of immune regulation (543), described earlier. According to this model, danger or alarm antigenic signals liberated from dying cells are responsible for activating antigen presenting cells. High-mobility group box 1 (HMGB1) has been shown to mediate hepatic I/R, suggesting that release of this nuclear protein involved in DNA binding and gene expression may be a candidate danger signal (794). In addition, antigen-independent activation of CD4+ T lymphocytes by Kuppfer cells has been noted after hepatic I/R, an effect attributed to postischemic ROS, TNFα, and IL-6 release (322).
Another mechanism whereby CD4+ T cells may promote postischemic tissue injury is via enhancing neutrophil infiltration into ischemic sites. Depletion or genetic deficiency of CD4+ T lymphocytes is associated with reductions in neutrophil sequestration and injury after I/R (107, 532, 610, 621, 709, 797, 873). This postischemic T cell-mediated neutrophil infiltration has been attributed to secretion of IL-17 by these lymphocytes (107, 709), although it seems likely that other T-cell-derived factors that are known to promote neutrophil sequestration, such as IL-1 and TNFα, may also participate. T cells contribute to postischemic renal injury by mechanisms dependent on IFNγ secretion and engagement of co-stimulatory molecules CD28 and B7 that does not require neutrophils (610). Still other studies implicate interactions between CD4+ lymphocytes with tissue-resident macrophages (i.e., Kupffer cells), an effect that may also involve platelets (417) and is mediated by CD40/CD154 binding (710).
B cells and other lymphocytes (e.g., CD8+, Treg, and NK cells) also appear to participate in I/R. Indeed, several studies using B cell-deficient mice or inhibiting NK cells have demonstrated significant reductions in postischemic tissue injury (102, 134, 469, 476, 710, 715, 845, 902, 904). The mechanisms underlying I/R injury by this lymphocyte subset have been explored using mice deficient in components of the complement system that interact with B cell receptors, with the results supporting the concept that I/R may involve B cell-derived IgM and complement system activation (102, 476, 845, 902, 904).
There is also some evidence for subsets of CD8+ T cells, T-regulatory cells, γδ T cells and natural killer cells in modulating the responses to I/R injury, especially in the setting of transplantation (41, 60, 125, 347, 348, 469, 512, 573, 610, 717), but some reports are contradictory (101–103, 458, 873). Treg cells accumulate 3–7 days into reperfusion and are involved in resolution and repair of tissue injury induced by I/R by a mechanism that involves IL-10 secretion (134, 260, 424, 425, 448).
Dendritic cells
DCs are the major antigen-presenting cells of the immune system and participate in I/R injury via their ability to activate T and B cells. As a consequence of I/R, DCs accumulate and are activated by TLR ligands such as interferon-γ, matrix components, and molecules liberated from necrotic cells (360a, 379a). In turn, DCs provide two signals to activate T cells, including antigen-specific signals arising from T cell receptor binding of peptides presented by MHC and a second signal provided by costimulatory molecules such CD40, CD80, and CD86. Of course, T cells can also be activated by antigen-independent pathways by cytokines, ROS and other pro-inflammatory molecules such as TNFα and CGRP that are formed during I/R, as outlined above. In the setting of transplantation-induced I/R, danger signals released from dying cells are recognized by PRRs of the innate immune system with subsequent activation of inflammatory cells, including DCs, which are directed to a more mature phenotype (379a, 639a). After lymph node homing, mature DCs present antigens to T cells causing their differentiation toward Th1 and Th17 effector cells, which can contribute to graft failure. There are also reports indicating that resident DCs confer protective responses to limit I/R injury and damage induced by allotransplantation (420a, 639a). Although the non-stressed brain lacks DCs or functional counterparts that mediate antigen uptake and presentation, these cells do appear in brain parenchyma within 1 hr after induction of ischemia, where they act to exacerbate stroke-induced infarction (235a).
Platelets
Platelets normally circulate in an inactive state, owing to constitutive presence of inhibitory factors elaborated by quiescent endothelial cells (466a, 662, 664a). However, I/R-induced reductions in the bioavailability of NO, prostacyclin and other antiadhesive molecules, coupled with release of proinflammatory mediators such ROS, PAF, and other factors, results in platelet activation, which provokes not only their aggregation, but also platelet adherence to the endothelium and circulating and tissue-resident immunocytes. These interactions are mediated by platelet-expressed P-selectin and its ligands, PSGL-1 and GPIbα as well as several integrin receptors, notably αIIbβ3 (25, 155, 156, 465, 466a, 499, 634, 662, 664a, 671, 676, 681).
Of the platelets adhering to the vascular wall during I/R, 75% are attached to leukocytes that are tethered to endothelial cells, with the remainder being bound directly by the endothelium (156, 416, 466a, 662, 664a). This raises the possibility for significant cross-talk amongst the three cell types to modulate adhesive-dependent inflammatory and thrombogenic events (466a, 662, 664a). Indeed, activated platelets release a number of proinflammatory and mitogenic molecules (e.g., IL-1β, RANTES, and soluble CD154), cytotoxic agents (e.g., hydrogen peroxide), proapoptotic molecules (calpain and TGFβ), and microvesicles (223, 262, 263, 416, 466a, 662, 664a). Some off these changes may account for the ability of platelets to participate in I/R-induced endothelial apoptosis (34, 623) and to cause NETosis (466a, 662, 664a). Clearly, platelet-induced promotion of leukocyte activation and adhesion is an important mechanism whereby thrombocytes contribute to postischemic tissue injury (416, 466a, 662, 664a, 676, 912).
Mast cells
Mast cells are tissue-resident inflammatory cells that are strategically localized close to blood vessels and neurons of most organs, being especially numerous in connective tissues and at mucosal surfaces of the airways and GI tract, allowing them to perform a sentinel function (170, 659, 749, 869). They contain numerous cytoplasmic granules that are filled with a large number of preformed proinflammatory mediators, including proteases, histamine, and serotonin, angiotensin II, and cytokines such as TNFα that are released on degranulation (170). Within minutes of activation, mast cells also synthesize and release arachidonate-derived lipid mediators (170). Mast cell degranulation and mediator release induces vascular fluid and protein leakage into the interstitium, resulting in the formation of edema, and promotes leukocyte homing to sites of inflammation (560a). Although it was long-held that mast cell degranulation resulted in indiscriminate release of all preformed mediators, it is now clear that certain constituent molecules can be expressed selectively, depending upon the nature of the stimulus and other prevailing tissue conditions (170, 206, 257, 394, 472, 749, 777).
I/R promotes mast cell degranulation secondary to postischemic ROS production, complement activation, protease-dependent cleavage of extracellular matrix proteins and resulting exposure/release of matricryptic factors, and release of PAF, LTB4, CXC chemokines and calcitonin gene-related peptide (170, 395, 472, 659, 685, 869). If bacterial translocation occurs in response to severe gut ischemia, bacterial toxins also contribute to mast cell activation. Studies employing pharmacologic mast cell stabilizers, which act to prevent activation or degranulation, mast cell deficient animals, and knockout mouse models lacking mast cell surface receptors for specific mediators have provided compelling support for the role of mast cells in I/R (3, 69, 88, 254, 277, 394, 395, 472, 659, 668, 685, 747, 748, 869). There is also evidence for ROI to the lung by local mast cells that are induced to degranulate by pathologic events initiate by distant site I/R (277).
Many reports have provided evidence that mast cell activation is associated with increased neutrophil infiltration into ischemic organs where these extravasated sentinels contribute to injury (65, 254, 256, 277, 395, 495, 685, 747, 748, 841, 907). Mast cells are an important source of angiotensin II, via expression of renin and chymase, which contributes to postischemic leukosequestration by a mechanism dependent on angiotensin II type I and type II receptor activation, NADPH oxidased-derived ROS, and the release of calcitonin gene-related peptide (895). However, there is a leukocyte-independent component of mast cell-degranulation-induced I/R (88, 472). A similar pattern occurs with regard to I/R-induced, mast cell-dependent disruption of endothelial barrier function, with increases in vascular permeability exhibiting both leukocyte-dependent and independent mechanisms (495). As an additional contributory mechanism to ischemic stroke, mast cell release of proteases such as tissue plasminogen activator as well as heparin, promotes thrombolysis, and hemorrhage (749).
The aforementioned work strongly supports use of mast cell inhibitors or stabilizers as an important therapy in ischemic disorders (88, 394, 411, 747–749). However, mast cell-derived proteinase-activated receptor-2 (PAR-2) appears to modulate the deleterious effects of intestinal I/R (121), as does mast cell release of the anti-inflammatory cytokine IL-10 (298) and proteases that target endothelin-1 for degradation (439, 544). The latter observations support the need for continued investigation of this expansive range of mast cell biology in I/R.
Monocytes, macrophages, and Kupffer cells
Kupffer cells are tissue-resident macrophages that are associated with hepatic sinusoidal epithelium, which places them in a strategic location to capture and clear bacteria and potentially injurious agents, such as endotoxin, arising from the bowel (71). Like mast cells, the participation of Kupffer cells in I/R is complicated by the fact that these phagocytic cells can function to either limit or promote inflammation, the net effect of which depends on ischemic duration and at what time point their functional role is assessed after reperfusion is instituted, which will be discussed below (204).
Proinflammatory actions
Kupffer cell activation occurs by two distinct, yet complementary mechanisms. TLR-dependent signaling mediates the first while the second occurs by complement activation. Hepatocyte injury after I/R liberates an inflammatory ligand for TLR-4 designated HMGB1, by ROS- and TLR-4-dependent mechanism (793, 794). HMGB1 engagement of TLR-4 on Kupffer cells establishes a positive feedback for sustaining inflammatory responses after liver I/R that involves downstream expression of inflammatory cytokines occurring secondary to stimulation of NFκB-dependent transcription (71, 793, 794). The mechanism for complement activation involves the formation of C3a and C5a. Ligation of C3a and C5a receptors on Kupffer cells stimulates phospholipase C to produce diacylglycerol (DAG) and inositol 3-phosphate (IP3). DAG stimulates protein kinase C (PKC)-dependent ROS production by NADPH oxidase (648a). ROS production is further reinforced by the effect of IP3 to stimulate Ca2+ mobilization from internal stores and uptake from the extracellular compartment. This IP3-dependent increase in Ca2+ also stimulates eicosanoid synthesis in Kupffer cells by the enzymatic activity of phospholiase A2-dependent cyclooxygenase (374).
Once activated by the aforementioned mechanisms in I/R, overexuberant production of oxidants, cytokines, PAF, and other proinflammatory mediators by Kupffer cells contribute to postischemic liver injury and remodeling (58, 108, 351, 374, 375, 378, 648a). MMP-9 released from Kupffer cells, hepatic stellate cells and sinusoidal endothelial cells also contributes to the pathogenesis of hepatic I/R in the first 24 h of reperfusion but then switch roles at later times to facilitate liver recovery (235, 519). Gadolinium chloride (GdCl3), which causes Kupffer cell depletion in the liver and also produces a loss of macrophages in other organs, has been used to demonstrate the role for these inflammatory phagocytes in postischemic neutrophil sequestration and tissue injury (252, 267, 493, 513). These studies indicated that Kupffer cells per se were not only directly responsible for early tissue damage after I/R, but also participated indirectly in later stages of injury which are primarily neutrophil dependent, since their recruitment occurred by Kupffer cell-dependent mechanisms (513). In addition to promoting neutrophil recruitment, Kupffer cells also activate CD4+ T-lymphocytes in liver I/R (322).
Anti-inflammatory actions
As noted earlier, Kupffer cells play dual roles in hepatic I/R, contributing to inflammation and cell injury early on during reperfusion, while exerting several actions that promote liver repair and healing at later stages. First, Kupffer cells induce apoptosis of PMNs and can also phagocytize these inflammatory cells, thereby acting as a brake to limit their injurious effects (96, 578, 713). Second, I/R often results in erythrocyte damage that results in increased plasma hemoglobin levels. As a consequence, heme-mediated oxidative injury occurs. Kuppfer cells function to limit this oxidative stress by clearing free hemoglobin from the plasma via the CD163 scavenger receptor, which is then followed by heme oxygenase-1 (HO-1)-dependent heme degradation (54, 55, 190, 211, 264, 274, 432, 452, 677, 751, 787). Indeed, the success of hepatic transplantion correlates with donor liver HO-1 expression before surgery (264). It is of interest to further note that Kupffer cells expressing high levels of HO-1 liberate proinflammatory cytokines at lower rates while their release of anti-inflammatory mediators is increased (211, 431).
Monocytes/macrophages also play pro- and anti-inflammatory roles in postischemic heart, demonstrating phenotypic plasticity that allow them to exacerbate injury and to participate in regenerative processes (206a, 249, 250, 717a). In the early stages of reperfusion, necrosis activates macrophages to extend ischemic injury in a fashion similar to Kupffer cell activation in the liver. In later stages, these inflammatory cells function to remove debris and clear injured myocardium of dead cells and facilitate healing. Microglia are the brain’s resident macrophages and like cardiac macrophages and hepatic Kupffer cells exhibit a biphasic polarization during reperfusion, first contributing to tissue injury and then facilitating repair (524a). Similar divergent roles for macrophages have been reported in organ transplantation (679a).
Plasma Membrane-Derived Microparticles
Following activation by I/R, apoptotic signaling, thrombin, or TNF, a number of cell types including platelets, erythrocytes, leukocytes, and endothelial cells, release small (0.1–1 µm diameter) membrane vesicles called microparticles (27, 353, 487, 545, 652). Although originally dismissed as cellular debris, it is now clear that microparticles display strong procoagulant activity and encapsulate a number of molecular entities that contribute to the pathogenesis of I/R by shuttling signaling agents amongst cell types (27, 353, 487, 545, 652, 772). These include bioactive lipids, chemokines, cytokines, tissue factor, arachidonic acid, integrins, receptors, RNA, proteases, growth factors, and caspases. Microparticle derived PAF may be of particular importance in I/R since this powerful mediator activates platelets to promote their aggregation, adhesion to endothelial cells and leukocytes, and leukocyte sequestration in postischemic tissues. Calpain, a protease implicated in I/R, is also carried on these vesicular structures, which protects the enzyme from plasma inhibitors (413, 584).
Because microparticles derived from membranes of activated endothelium, platelets, lymphocytes and leukocytes are liberated into the blood flow reperfusing ischemic sites, they can travel via the circulation to distant organs to cause ROI secondary to distant thrombogenic and proinflammatory effects (140, 265). Moreover, I/R-induced microparticle release from endothelial cells, platelets, NK cells, and CD8+ T cells activate JNK and NFκB and increase the expression of endothelial cell adhesion molecules (772). Exciting recent work indicates that adherent platelets form extremely long (up to 250 µm), negatively charged protrusions (FLIPRs) under conditions of flow. As neutrophils roll over FLIPRs, they retrieve fragments of these platelet membrane strands as microparticles on their surface, which in turn activate these phagocytic cells (775). While the role of this neutrophil activating mechanism has not been evaluated in I/R, it is tempting to speculate that this could be very important given the strong association among neutrophils, platelets, and the endothelium in postischemic tissues.
Protein Cleavage Products and other Degradation Products in I/R
The pathologic processes induced by I/R results in damage to and degradation of intracellular proteins. This is accompanied by impaired function of the ubiquitin-proteasome system (UPS), the tightly-regulated catalytic machinery that normally functions to degrade damaged/dysfunctional intracellular proteins. The end result of both processes is disrupted signaling and accumulation of toxic protein aggregates in postischemic tissues (106, 166, 306). Ischemic preconditioning preserves postischemic UPS function by a mechanism that preserves signaling molecules, such PKCε and PKCδ. Although the latter observation supports the view that therapeutic approaches targeted at preserving UPS function may enhance cell viability in I/R, reports of the efficacy of proteasome inhibition as a treatment for myocardial ischemia have yielded discordant results, with some studies describing reductions in infarct size, while others show deleterious effects on cardiac function (893). Since the cardiac UPS is comprised of distinct subpopulations which differ in subunit composition, associating partners, and posttranslational modifications, differential activation of protective versus antiprotective UPS subpopulations may account for the incongruous data (106, 166, 201, 892).
Two important protease systems that are now recognized to contribute to I/R injury are the calpain-calpastatin system and MMPs (336, 649). Pharmacological calpain or MMP inhibition reduced I/R injury in the myocardium, kidneys, and brain (119, 128, 339, 415, 523, 872). The DNA repair enzyme poly (ADP-ribose) polymerase-1 (PARP-1) is a preferred substrate of several so-called “suicide” proteases, which include the calpains and MMPs (124). The activity of these proteases on PARP-1 produces cleavage fragments that serve as signature biomarkers for specific patterns of protease activity in cell death programs.
While calpain knockout mice are not commercially available, use of knockout models targeting specific MMP isoforms has established roles for MMP-2 and MMP-9 as important contributors to I/R injury. I/R-induced formation of H2O2 and ONOO- activates these MMPs and their enzymatic activity results in endothelial and contractile dysfunction in the heart in the absence of discernable cell death (649). In addition to intracellular MMPs, activation of MMPs in the extracellular space (e.g., following their release from tissue-resident immune cells) target the extracellular matrix proteins (ECMs) to expose matricryptic sites or release matricryptins that are normally hidden within the three-dimensional conformational structure of the ECM proteins. As a consequence, cleavage products derived from the ECM can produce cytotoxic effects in I/R (339). Some small peptide fragments that are released from the ECM after MMP-mediated proteolytic cleavage function as “matrikines,” interacting with specific cell receptors to alter cell function, including induction of neutrophil chemotaxis (840), which could potentially contribute to postischemic leukosequestration. In addition to protein degradation that contributes to postischemic injury at early stages, MMP expression is transcriptionally upregulated during later myocardial remodeling and repair. The importance of MMPs in the production of both injury and in repair complicates the potential use of MMP inhibitors in the treatment of I/R.
Membrane lipid degradation and fatty acid oxidation pathways are also altered by I/R and contribute to postischemic tissue injury. Pharmacologic inhibition of fatty acid oxidation exerts infarct-sparing effects and improves cardiac function after I/R (707). The mechanism underlying this protective effect is not yet clear but may be due to the resulting shift from fatty acid oxidation to glucose utilization for ATP production under ischemic conditions as well as to prevention of fatty acid metabolite accumulation. On the other hand, I/R results in increased phospholipase activation, which liberates arachidonic acid from cell membranes. Thus, it is not surprising that phospholipase A2 inhibition is protective in stroke models, limiting infarct size and improving neurologic outcomes (345). Since ROS production is also a consequence of lipid degradation, it is likely that lipooxidative mediators contribute to secondary injury in the brain after stroke (491, 636).
I/R Induces Capillary No-Reflow, a Nutritive Perfusion Impairment
I/R is associated with the development of the no-reflow phenomenon, a failure of nutritive perfusion characterized by a decrease in the number of perfused capillaries (309, 310, 697). This postischemic impairment in capillary reflow was first described in the brain by Ames and co-workers (19) and was subsequently shown to occur in postischemic skeletal muscle, heart, kidney, and small intestine (384–387, 396, 428, 697, 750, 752). While it was once thought that microvascular thrombus formation was an important contributing mechanism underlying this nutritive perfusion impairment, microvessel thrombosis is rarely observed in intravital microscopic studies or after light and electron microscopic examination of reperfused tissues (309, 310). Moreover, postischemic capillary no-reflow is not improved by heparin treatment. An exception to this conclusion is distal embolization induced by percutaneous coronary intervention (PCI), causing capillary no-reflow following myocardial ischemia which can also occur in regions not exposed to ischemia before this procedure. This nutritive perfusion impairment occurs in 5% to 50% of patients undergoing PCI and is an independent predictor of adverse outcome (585).
Rather than obstruction by platelet or fibrin thrombi, it appears that leukocyte-capillary plugging contributes to the no-reflow-induced microvascular perfusion impairment associated with I/R (218, 309, 310, 750) (Fig. 11). Indeed, studies conducted in the heart noted the presence of leukocytes in a very high proportion of capillaries exhibiting no-reflow after reperfusion. Physical impaction of leukocytes in capillary lumens occurs because neutrophils are large (8 µm diameter), stiff cells that become even less deformable when exposed to the acidic environment that exists in ischemic tissues, making it more difficult to traverse the smaller diameter (4 µm) capillaries. In the face of this increased stiffness and because blood pressure driving flow through capillaries is reduced during ischemia, these large cells are more likely to become entrapped in capillaries, thereby blocking perfusion. Capillary plugging by neutrophils is further compounded by I/R-induced disruption of endothelial cell volume regulatory mechanisms, which causes endothelial cells to swell, thereby narrowing the capillary lumen (396, 546) (Fig. 11). Treatment with hypertonic, hyperosmotic saline/dextran solutions limits endothelial cell swelling and reduces capillary no-reflow (385, 546, 555).
Figure 11.
Mechanisms underlying the development of the postischemic capillary no-reflow phenomenon. See text for explanation.
I/R-induced, neutrophil-dependent increases in microvascular permeability also contribute to postischemic no-reflow in some tissues, such as skeletal muscle (309, 310, 386) (Fig. 11). The postischemic disruption of endothelial barrier function results in fluid and protein efflux across exchange microvessels. The accumulation of edema fluid in ischemic tissues coupled with parenchymal cell swelling increases tissue pressure. The resulting decrease in transmural pressure causes capillaries and postcapillary venules to collapse, preventing blood flow through their narrowed lumens. This edemagenic mechanism to explain postischemic nutritive perfusion impairments is most relevant to organs that cannot readily expand when fluid accumulates in the interstitial spaces because they are encased by nonelastic structures. For example, the cranial vault, fascial sheathes, and renal capsule surround the brain, many skeletal muscles, and kidneys, respectively, to limit their expansion as edema forms. The movement of fluid from the plasma to the interstitial space (interstitial edema) and into parenchymal cells (cell edema) induced by ischemia results in increased microvessel hematocrit and blood viscosity, which may act to further impair capillary perfusion in postischemic tissues by raising the resistance to blood flow. Degradation of the coronary vasculature after I/R contributes to disrupted capillary integrity, leading to hemorrhagic infarctions (termed vascular rhexis) that also contribute to the no-reflow phenomenon (898).
Neutrophil depletion virtually abolishes this perfusion impairment in reperfused tissues, as does pretreatment with function blocking antibodies directed against CD11/CD18 on leukocytes and ICAM-1 or P-selectin on the endothelium (385–387). ROS are also involved since antioxidant therapy prevents postischemic leukocyte/endothelial cell adhesion and restores capillary perfusion (556, 557). Very recent work has implicated neutrophil extracellular traps (NETs), web-like constructs of decondensed chromatin and anti-microbial proteins released by these phagocytic cells in response to platelet and/or endothelial activation, in myocardial no-reflow (263) (Fig. 11). These NETs facilitate activation of the coagulation cascade and platelets, as well as by actions to promote cleavage of tissue factor pathway inhibitor, thereby limiting endogenous anticoagulation (263, 285). Neutrophilic NETosis may be facilitated by platelets adhering to their surface (466a, 662, 664a).
Pericytes surround almost all capillaries, extending processes along and around the vessel to cover greater than 95% of their abluminal surface in some organs. These perivascular cells also surround small arterioles and venules, but their density is reduced in these vessels. Since pericytes are contractile and responsive to mediators implicated in I/R, it has been suggested that they may modulate postischemic nutritive perfusion (238, 321, 598) (Fig. 11). Moreover, because CNS capillaries are more richly invested with pericytes than in other areas of the body, they may be particularly important in stroke. Indeed, pericyte contracture reduces capillary lumenal diameter in retinal or cerebral ischemia. Even after reperfusion, these pericapillary cells remain contracted by an endothelium-dependent, peroxynitrite-mediated mechanism (317, 631, 882). Stroke-induced pericyte contracture was followed by pericyte death in rigor, which produced irreversible nutritive perfusion impairment (317).
Assessment of the principal mechanisms contributing to the microvascular perfusion impairment in each patient is now being explored to tailor the selection of treatment strategies (anti-platelet therapy vs vasodilators vs embolic protection devices versus and pharmacologic pre- and postconditioning strategies) to limit reperfusion injury (586, 587, 625, 697).
Genomic/Proteomic/Metabolomic Insights
Genome-wide association studies have been used to examine myocardial gene expression signatures, identifying approximately 152 genomic loci and over 300 genes that may contribute to enhanced risk for coronary artery disease and myocardial infarction (52, 74, 75, 112, 597, 765, 855). This genomic analysis has also uncovered chromosomal locations associated with ischemic stroke and peripheral artery disease, with some loci in common to those associated with coronary disease and heart attack. The latter observation suggests that these organs share overlapping genetic contributions to ischemic disease risk. An interesting outcome of this genomic analysis has been identification of chromosomal regions that lack genes that had been linked to ischemic disease risk and infarction in previous work. This formulates the basis for uncovering novel mechanisms by which genes in these previously underappreciated loci contribute to ischemic disease. Genomic studies are also being pursued for precision medicine, based on the enormous potential for uncovering specific chromosomal variations that may account for differential patient responses to cardiovascular (and other) drugs, thereby allowing design of personalized therapeutic regimens related to specific genotypes.
Similarly, data obtained from application of metabolomic, proteomic, and lipidomic profiling approaches have provided a wealth of data that have great potential to identify novel biological indicators and new pathologic pathways that contribute to cardiovascular disease risk and infarction (30, 80, 143, 266, 501, 779). This requires the development of innovative technological platforms and computational models to allow use of shared high-powered computational resources for systematic data mining, processing, and integration to identify complex data patterns and infer relations amongst protein, lipid and metabolite expression in individual patients to their cardiovascular disease phenotype. Even small scaleomics studies have led to remarkable insights. As an example, Wang and co-workers (829) used metabolomic approaches to identify phospholipid-associated molecules that contribute to plaque formation in arteries that depends on dietary phosphotidylcholine intake, metabolism of this phospholipid to trimethylamine (TMA) by commensal bacteria in the gastrointestinal tract (Fig. 12). Following intestinal absorption, TMA is transported to the liver where the enzymatic activity of flavin monooxygenases metabolizes this precursor to trimethylamine N-oxide (TMAO). TMAO facilitates the buildup of arterial plaque by promoting a proatherogenic transformation in macrophage phenotype (829). Based on subsequent work, it was suggested that TMAO may increase macrophage scavenger receptors while reducing reverse cholesterol transport, thereby promoting foam cell formation and development of atherosclerosis (434). This was followed by the demonstration that TMAO promotes the activation and recruitment of leukocytes to aortic endothelial cells concident with activation of MAPK, ERK, and NFkB signaling cascades and expression of inflammatory genes (702).
Figure 12.
Overnutrition via consumption of a Western diet rich in red meats, lipids, and fructose and lacking fermentable fiber may contribute to a shift in the intestinal microbiome to a profile that favors enhanced risk for myocardial infarction, stroke, and peripheral vascular disease. As one example, consumption of protein-rich diets containing red meats leads to the liberation of carnitine and phosphatidyl choline as the bolus of ingested food is digested in the small bowel, which are converted to TMA by colonic microbes. Upon absorption, TMA is metabolized to the proatherogenic TMAO by the liver to promote the formation of atherosclerotic plaques. The problem is exacerbated by diet-induced dysbiosis, which favors the translocation of bacteria into the bloodstream and promotion of plaque formation. Interestingly, a blood-like microbiome exists in plaques that resembles the patient’s oral and intestinal microbial profiles, with some of the bacterial species correlating with plasma cholesterol levels. TMAO also contributes to a prothrombogenic phenotype via effects to increase platelet IP3 levels, thereby elevating intracellular calcium levels to promote platelet aggregration and adhesion to injured endothelium.
More directly relevant to I/R, recent work by Zhu et al. (921) uncovered a strong association between plasma TMAO levels and thrombotic events (Fig. 12). They further demonstrated that TMAO promoted platelet aggregation in response to various agonists, enhanced platelet adhesion to collagen, and decreased time to occlusive thrombosis in a carotid injury model. Similar results were obtained in platelets obtained from animals fed TMAO or choline. These effects were absent in mice treated with antibiotics and in germ-free animals. Because washed platelets obtained from TMAO-treated animals were not hyperresponsive, Zhu et al. (921) suggested that plasma TMAO directly activated platelets to produce a prothrombogenic phenotype. Support for the latter contention was provided by the observation that TMAO exposure caused platelet IP3 levels to increase and trigger Ca2+ release (Fig. 12).
It is of interest to note that the effects of the gut microbiota on hepatic TMAO production and potential for atherogenic lesion and thrombosis formation were transmissible, inasmuch as fecal transplants from animals with high TMAO levels to germ-free mice produced a proatherogenic and prothrombogenic phenotype (297, 921). This same group has also shown that treatment with a structural analog of choline nonlethally inhibited TMA production from polymicrobial cultures and reduced TMAO levels, macrophage foam cell formation, and atherosclerotic lesion development in atheroprone mice fed high choline or L-carnitine diets (830). Thus, it appears that new therapies targeting gut commensal bacterial production of TMA may reduce the risk for thrombosis. Other avenues include dietary restriction of foods rich in TMA precursors, gut microbiome manipulation by pre- and probiotics, use of drugs that target the hepatic conversion of TMA to TMAO, and blocking the ability of TMAO to elicit its biologic effects via development of agents that that bind and assist in eliminating TMA or TMAO or antagonists that target as yet unidentified TMAO receptors (95). With regard to the notion that reductions in TMAO levels by restricting intake of TMA precursers or TMAO may attenuate the likelihood of adverse cardiovascular events, it is curious to note that seafood constuents of the cardioprotective Mediterranean diet are rich in TMAOs (799). This suggests that a much greater understanding of TMAO metabolism is required to advance therapeutic approaches related to the gut microbiome as a driver of cardiometabolic vascular disease.
Concluding Remarks and Perspectives
Age-adjusted cardiovascular mortality has declined dramatically over the past several decades. This has been accompanied by major improvements in discharge disposition, decreases in the likelihood of readmission, and an impressive reduction in hospitalization rates for patients with or at risk for cardiovascular disease and stroke. Advances in pharmaceuticals (e.g., thrombolytic agents, antiplatelet drugs, beta blockers, and angiotensin converting enzyme inhibitors and receptor blockers), aggressive management of risk factors for cardiovascular disease, development of approaches to restore tissue perfusion (e.g., PCI and cardiopulmonary bypass), improved patient education and awareness, enhancements in quality of care (via more rapid risk stratification, timeliness of treatment, and hospital process performance analysis to ensure appropriate application of proven interventions), the discovery of sensitive blood indicators, and development of sophisticated imaging methodologies to detect subclinical disease years before symptomatic presentation all have contributed to this success. During this same period, intensive research has uncovered several major concepts regarding the mechanisms of I/R including: (i) the discovery that short bouts of I/R activate cell survival programs (ischemic conditioning) that limit lethal I/R injury (and indicating that there is a bimodal or hormetic response to I/R), (ii) reperfusion paradoxically amplifies cell injury and death, which occurs by mechanisms that are distinct from those induced by ischemia per se, (iii) uncovering of multiple death modalities that contribute to I/R-induced cell death, many of which occur by programmed sequences of events, (iv) fetal exposure to stressors incur programming events that enhance the susceptibility to cardiovascular disease and I/R syndromes later in life, and (v) numerous, complex, and highly interactive mechanisms underlie the pathogenesis of I/R. These include, but are not limited to, disruption in ion transport mechanisms that result in cellular calcium overload, overexuberant production of ROS and RNOS, inflammation, protein kinase activation, development of ER and mitochondrial dysfunction, epigenetic alterations in gene expression, formation of protein cleavage products, development of no-reflow, roles for the gut microbiome, and genomic/metabolomic/lipidomic contributions to clinical phenotypes.
Despite this enhanced mechanistic understanding of I/R injury from preclinical studies, there has been very little success in transforming these discoveries into new adjuvant therapies with proven efficacy in relevant patient populations (89, 109, 399, 427, 474, 565, 597, 614, 645, 809). Indeed, no new treatment to reduce infarct size has emerged since the advent of thrombolysis and angioplasty. This disappointing translation of mechanistic findings relates in part to the limitations imposed by conducting evaluative trials in patients with advanced disease that may be beyond salvage, which also imposes a short time window for improving outcomes. Perhaps more importantly, much of the preclinical work conducted to date has been accomplished in young, healthy animals, whereas patients usually present with coexisting risk factors. Another factor, which is only now emerging as a consideration, is contributions from the host’s microbiome. Because the microbiome is influenced by a host of factors that are rarely controlled for, microbiota composition differences may contribute in a major way to discrepant findings in the literature. A fourth explanation relates to therapeutic focus on a single contributory mechanism in the setting of multi-factorial pathological processes that sum to produce tissue injury and death. Indeed, the large number of contributory factors in the pathogenesis of I/R injury argues against the concept of single drug intervention that has characterized the approaches adopted in basic research, by the pharmaceutical industry, and in clinical trials. On the other hand, studies investigating the mechanisms underlying the protective actions of ischemic preconditioning have shown that this intervention targets multiple pathologic processes in I/R, raising the hope that pharmacologic agents that mimic its powerful cardioprotective effects might prove more effective. In addition, since conditioning activates cell signaling programs to upregulate the expression of several survival proteins, gene therapy approaches based on these discoveries have also shown promise. However, while success of initial small scale trials indicated that such conditioning-based approaches might improve clinical outcomes (16, 126, 823, 834), larger trials have failed to confirm efficacy (399, 614, 645, 796, 881). This led to proposals to better standardize protocols for therapeutic intervention by conditioning approaches, more extensive and rigorous experimental validation of new targets in preclinical work before patient testing is considered, and to design clinical trials with an eye toward decreasing patient heterogeneity with regard to cardiovascular disease phenotype (399, 881). Until only the last decade or so, I/R studies in general and work focused on conditioning mechanisms was conducted largely in young, healthy animals. This experimental focus, when coupled with the growing body of evidence indicating that the cardioprotective effects of conditioning strategies are subverted by the presence of comorbid risk factors, indicates that the design of preclinical studies should include models that better mirror patient phenotype in those suffering adverse cardiovascular events. Another point of this discussion is to recognize that ischemic disorders have complex, multifactorial etiologies that are pathophysiologically heterogeneous but highly interactive. As such, prevailing paradigms have to be constantly evaluated, updated, and adjusted by new evidence, with care taken not to become entrenched or biased by current dogma.
The application of stem cell therapy holds great promise because it targets repair and regenerative processes in postischemic tissues, thereby avoiding the issues described above that have contributed to the failure of treatments directed at mechanisms directly causing injury in I/R. Indeed, injection of adult stem cells into hearts and brains damaged by I/R was shown to improve function and facilitate beneficial remodeling (78, 607, 786, 906). While it was presumed that these effects were due to differentiation of the engrafted stem cells into cardiac myocytes and vascular cells, other studies failed to demonstrate such plasticity (42, 577). In addition, adult stem cells show poor survivability after injection into the harsh milieu present in postischemic tissues (270). Moreover, sufficient numbers of cardiac myocytes cannot be generated from the relatively small numbers of stem cells that are injected (346). Based on this information, it was recently suggested that adult stem cells improve function and promote reparative remodelling via their release of paracrine mediators such as growth factors and chemokines (346). The beneficial actions of such paracrine factors appear to be related to release of cytoprotective molecules, immunomodulatory effects, promotion of cardiomyocyte proliferation, alterations in ECM remodeling that limit fibrosis, stimulation of angiogenesis, activation of tissue resident stem cells to differentiate into cardiac myocytes, and may involve release of exosomes and microvesicles (346). Because of the complex multifactorial processes that participate in postischemic healing, it is clear that these paracrine mechanisms are highly dynamic and require exquisite temporal and spatial organization to effect repair and regeneration. Understanding these multifaceted, dynamically phased, and highly pleiotropic mechanisms will be a major focus of future research to improve stem cell-mediated tissue repair and regeneration after I/R.
Acknowledgments
The authors’ work was supported by grants from the National Institutes of Health (HL-092327, HL-094404, HL-116525, AA-022108, GM-115553, and HL-095486).
References
- 1.Abdellatif M. Differential expression of microRNAs in different disease states. Circ Res. 2012;110:638–650. doi: 10.1161/CIRCRESAHA.111.247437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abela CB, Homer-Vanniasinkham S. Clinical implications of ischaemia-reperfusion injury. Pathophysiology. 2003;9:229–240. doi: 10.1016/s0928-4680(03)00025-7. [DOI] [PubMed] [Google Scholar]
- 3.Abonia JP, Friend DS, Austen WG, Jr, Moore FD, Jr, Carroll MC, Chan R, Afnan J, Humbles A, Gerard C, Knight P, Kanaoka Y, Yasuda S, Morokawa N, Austen KF, Stevens RL, Gurish MF. Mast cell protease 5 mediates ischemia-reperfusion injury of mouse skeletal muscle. J Immunol. 2005;174:7285–7291. doi: 10.4049/jimmunol.174.11.7285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abramov AY, Scorziello A, Duchon M. Three distinct mechanisms generate oxygen free radicals in neurons and contribute to cell death during anoxia and reoxygenation. J Neurosci. 2007;27:1129–1138. doi: 10.1523/JNEUROSCI.4468-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adibhatha RM, Hatche JF. Lipid oxidation and peroxidation in CNS health and disease: From molecular mechanisms to therapeutic opportunities. Antiox Redox Signal. 2010;12:125–169. doi: 10.1089/ars.2009.2668. [DOI] [PubMed] [Google Scholar]
- 6.Aguilar-Nascimento JE, Salomao AB, Nochi RJ, Jr, Nascimento M, Neves S. Intraluminal injection of short chain fatty acids diminishes intestinal mucosa injury in experimental ischemia-reperfusion. Acta Cir Bras. 2006;21:21–25. doi: 10.1590/s0102-86502006000100006. [DOI] [PubMed] [Google Scholar]
- 7.Ahern PP, Izcue A, Maloy KJ, Powrie F. The interleukin-23 axis in intestinal inflammation. Immunol Rev. 2008;226:147–159. doi: 10.1111/j.1600-065X.2008.00705.x. [DOI] [PubMed] [Google Scholar]
- 8.Ahluwalia A, De Felipe C, O’Brien J, Hunt SP, Perretti M. Impaired IL-1beta-induced neutrophil accumulation in tachykinin NK1 receptor knockout mice. Br J Pharmacol. 1998;124:1013–1015. doi: 10.1038/sj.bjp.0701978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aikawa R, Komuro I, Yamazaki T, Zou Y, Kudoh S, Tanaka M, Shiojima I, Hiroi Y, Yazaki Y. Oxidative stress activates extracellualar signal-regulated kinases through Src and Ras in cultured cardiac myocytes of neonatal rats. J Clin Invest. 1997;100:1813–1821. doi: 10.1172/JCI119709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aiken CE, Ozanne SE. Transgenerational developmental programming. Hum Reprod Update. 2014;20:63–75. doi: 10.1093/humupd/dmt043. [DOI] [PubMed] [Google Scholar]
- 11.Aikens J, Dix TA. Perhydroxyl radical (HOO.) initiated lipid peroxidation. The role of fatty acid hydroperoxides. J Biol Chem. 1991;266:15091–15098. [PubMed] [Google Scholar]
- 12.Akhmedov A, Montecucco F, Braunersreuther V, Camici GG, Jakob P, Reiner MF, Glanzmann M, Burger F, Paneni F, Galan K, Pelli G, Vuilleumier N, Belin A, Vallee JP, Mach F, Luscher TF. Genetic deletion of the adaptor protein p66Shc increases susceptibility to short-term ischaemic myocardial injury via intracellular salvage pathways. Eur Heart J. 2015;36:516–526. doi: 10.1093/eurheartj/ehu400. [DOI] [PubMed] [Google Scholar]
- 13.Alam MR, Baetz D, Ovize M. Cyclophilin D and myocardial ischemia-reperfusion injury: A fresh perspective. J Mol Cell Cardiol. 2015;78:80–89. doi: 10.1016/j.yjmcc.2014.09.026. [DOI] [PubMed] [Google Scholar]
- 14.Alexander BT, Dasinger JH, Intapad S. Fetal programming and cardiovascular pathology. Compr Physiol. 2015;5:997–1025. doi: 10.1002/cphy.c140036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alexander JS, Elrod JW. Extracellular matrix, junctional integrity and matrix metalloproteinase interactions in endothelial permeability regulation. J Anat. 2002;200:561–574. doi: 10.1046/j.1469-7580.2002.00057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ali ZA, Callaghan CJ, Lim E, Ali AA, Nouraei SA, Akthar AM, Boyle JR, Varty K, Kharbanda RK, Dutka DP, Gaunt ME. Remote ischemic preconditioning reduces myocardial and renal injury after elective abdominal aortic aneurysm repair: A randomized controlled trial. Circulation. 2007;116:I98–105. doi: 10.1161/circulationaha.106.679167. [DOI] [PubMed] [Google Scholar]
- 17.Allen CL, Bayraktutan U. Oxidative stress and its role in the pathogenesis of ischaemic stroke. Int J Stroke. 2009;4:461–470. doi: 10.1111/j.1747-4949.2009.00387.x. [DOI] [PubMed] [Google Scholar]
- 18.Alverdy JC, Chang EB. The re-emerging role of the intestinal microflora in critical illness and inflammation: Why the gut hypothesis of sepsis syndrome will not go away. J Leukoc Biol. 2008;83:461–466. doi: 10.1189/jlb.0607372. [DOI] [PubMed] [Google Scholar]
- 19.Ames A, III, Wright RL, Kowada M, Thurston JM, Majno G. Cerebral ischemia. II. The no-reflow phenomenon. Am J Pathol. 1968;52:437–453. [PMC free article] [PubMed] [Google Scholar]
- 20.Anand SS, Islam S, Rosengren A, Franzosi MG, Steyn K, Yusufali AH, Keltai M, Diaz R, Rangarajan S, Yusuf S. Risk factors for myocardial infarction in women and men: Insights from the INTERHEART study. Eur Heart J. 2008;29:932–940. doi: 10.1093/eurheartj/ehn018. [DOI] [PubMed] [Google Scholar]
- 21.Anderson CM, Lopez F, Zimmer A, Benoit JN. Placental insufficiency leads to developmental hypertension and mesenteric artery dysfunction in two generations of Sprague-Dawley rat offspring. Biol Reprod. 2006;74:538–544. doi: 10.1095/biolreprod.105.045807. [DOI] [PubMed] [Google Scholar]
- 22.Andrabi SA, Dawson TM, Dawson VL. Mitochondrial and nuclear cross talk in cell death: Parthanatos. Ann N Y Acad Sci. 2008;1147:233–241. doi: 10.1196/annals.1427.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Andrade-Oliveira V, Amano MT, Correa-Costa M, Castoldi A, Felizardo RJ, de Almeida DC, Bassi EJ, Moraes-Vieira PM, Hiyane MI, Rodas AC, Peron JP, Aguiar CF, Reis MA, Ribeiro WR, Valduga CJ, Curi R, Vinolo MA, Ferreira CM, Camara NO. Gut bacteria products prevent AKI induced by ischemia-reperfusion. J Am Soc Nephrol. 2015;26:1877–1888. doi: 10.1681/ASN.2014030288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Andres AM, Hernandez G, Lee P, Huang C, Ratliff EP, Sin J, Thornton CA, Damasco MV, Gottlieb RA. Mitophagy is required for acute cardioprotection by simvastatin. Antioxid Redox Signal. 2014;21:1960–1973. doi: 10.1089/ars.2013.5416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andrews RK, Berndt MC. Platelet physiology and thrombosis. Thromb Res. 2004;114:447–453. doi: 10.1016/j.thromres.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 26.Anea CB, Zhang M, Chen F, Ali MI, Hart CM, Stepp DW, Kovalenkov YO, Merloiu AM, Pati P, Fulton D, Rudic RD. Circadian clock control of Nox4 and reactive oxygen species in the vasculature. PLoS One. 2013;8:e78626. doi: 10.1371/journal.pone.0078626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Angelillo-Scherrer A. Leukocyte-derived microparticles in vascular homeostasis. Circ Res. 2011;110:356–369. doi: 10.1161/CIRCRESAHA.110.233403. [DOI] [PubMed] [Google Scholar]
- 28.Antonopoulos A, Kyriacou C, Kazianis G. Significance of endothelin-1 in myocardial infarction. Hellenic J Cardiol. 2007;48:161–164. [PubMed] [Google Scholar]
- 29.Arany I, Faisa A, Clark JS, Vera T, Baliga R, Nagamine Y. p66Shc-mediated mitochondrial dysfunction in renal proximal tubule cells during oxidative injury. Am J Physiol. 2010;298:F1214–F1221. doi: 10.1152/ajprenal.00639.2009. [DOI] [PubMed] [Google Scholar]
- 30.Arrell DK, Elliott ST, Kane LA, Guo Y, Ko YH, Pedersen PL, Robinson J, Murata M, Murphy AM, Marban E, Van Eyk JE. Proteomic analysis of pharmacological preconditioning: Novel protein targets converge to mitochondrial metabolism pathways. Circ Res. 2006;99:706–714. doi: 10.1161/01.RES.0000243995.74395.f8. [DOI] [PubMed] [Google Scholar]
- 31.Aspelund A, Robciuc MR, Karaman S, Makinen T, Alitalo K. Lymphatic system in cardiovascular medicine. Circ Res. 2016;118:515–530. doi: 10.1161/CIRCRESAHA.115.306544. [DOI] [PubMed] [Google Scholar]
- 32.Atarashi K, Hirata T, Matsumoto M, Kanemitsu N, Miyasaka M. Rolling of Th1 cells via P-selectin glycoprotein ligand-1 stimulates LFA-1-mediated cell binding to ICAM-1. J Immunol. 2005;174:1424–1432. doi: 10.4049/jimmunol.174.3.1424. [DOI] [PubMed] [Google Scholar]
- 33.Aune SE, Herr DJ, Kutz CJ, Menick DR. Histone deacetylases exert class-specific roles in conditioning the brain and heart against acute ischemic injury. Front Neurol. 2015;6:145. doi: 10.3389/fneur.2015.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Azad N, Iyer A, Vallyathan V, Wang L, Castranova V, Stehlik C, Rojanaskul Y. Role of oxidative/nitrosative stress-mediated Bcl-2 regulation in apoptosis and malignant transformation. Ann NY Acad Sci. 2010;1203:1–6. doi: 10.1111/j.1749-6632.2010.05608.x. [DOI] [PubMed] [Google Scholar]
- 35.Baines CP. The mitochondrial permeability transition pore and ischemia-reperfusion injury. Basic Res Cardiol. 2009;104:181–188. doi: 10.1007/s00395-009-0004-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baines CP. The molecular composition of the mitochondrial permeability transition pore. J Mol Cell Cardiol. 2009;46:850–857. doi: 10.1016/j.yjmcc.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baines CP. The cardiac mitochondrion: Nexus of stress. Annu Rev Physiol. 2010;72:61–80. doi: 10.1146/annurev-physiol-021909-135929. [DOI] [PubMed] [Google Scholar]
- 38.Baines CP. How and when do myocytes die during ischemia and reperfusion: The late phase. J Cardiovasc Pharmacol Ther. 2011;16:239–243. doi: 10.1177/1074248411407769. [DOI] [PubMed] [Google Scholar]
- 39.Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, Brunskill EW, Sayen MR, Gottlieb RA, Dorn GW, Robbins J, Molkentin JD. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature. 2005;434:658–662. doi: 10.1038/nature03434. [DOI] [PubMed] [Google Scholar]
- 40.Baines CP, Zhang J, Wang GW, Zheng YT, Xiu JX, Cardwell EM, Bolli R, Ping P. Mitochondrial PKCe and MAPK form signaling modules in the murine heart: Enhanced mitochondrial PKCe-MAPK interactions and differential MAPK activation in PKCe-induced cardioprotection. Circ Res. 2002;90:390–7. doi: 10.1161/01.res.0000012702.90501.8d. [DOI] [PubMed] [Google Scholar]
- 41.Baldwin WM, III, Su CA, Shroka TM, Fairchild RL. Experimental models of cardiac transplantation: Design determines relevance. Curr Opin Organ Transplant. 2014;19:525–530. doi: 10.1097/MOT.0000000000000113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Balsam LB, Wagers AJ, Christensen JL, Kofidis T, Weissman IL, Robbins RC. Haematopoietic stem cells adopt mature haematopoietic fates in ischaemic myocardium. Nature. 2004;428:668–673. doi: 10.1038/nature02460. [DOI] [PubMed] [Google Scholar]
- 43.Bang C, Fiedler J, Thum T. Cardiovascular importance of the microRNA-23/27/24 family. Microcirculation. 2012;19:208–214. doi: 10.1111/j.1549-8719.2011.00153.x. [DOI] [PubMed] [Google Scholar]
- 44.Barchowsky A, Munro SR, Morana SJ, Vincenti MP, Treadwell M. Oxidant-sensitive and phosphorylation-dependent activation of NF-kappa B and AP-1 in endothelial cells. Am J Physiol. 269:L829–836. doi: 10.1152/ajplung.1995.269.6.L829. [DOI] [PubMed] [Google Scholar]
- 45.Barker DJ. Fetal origins of coronary heart disease. BMJ. 1995;311:171–174. doi: 10.1136/bmj.311.6998.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barker DJ, Osmond C. Infant mortality, childhood nutrition, and ischaemic heart disease in England and Wales. Lancet. 1986;1:1077–1081. doi: 10.1016/s0140-6736(86)91340-1. [DOI] [PubMed] [Google Scholar]
- 47.Barker DJ, Osmond C. Low birth weight and hypertension. BMJ. 1988;297:134–135. doi: 10.1136/bmj.297.6641.134-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barker DJ, Thornburg KL. The obstetric origins of health for a lifetime. Clin Obstet Gynecol. 2013;56:511–9. doi: 10.1097/GRF.0b013e31829cb9ca. [DOI] [PubMed] [Google Scholar]
- 49.Barnabei MS, Palpant NJ, Metzger JM. Influence of genetic background on ex vivo and in vivo cardiac function in several commonly used inbred mouse strains. Physiol Genomics. 2010;42:103–113. doi: 10.1152/physiolgenomics.00071.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Barone FC, Knudsen DJ, Nelson AH, Feuerstein GZ, Willette RN. Mouse strain differences in susceptibility to cerebral ischemia are related to cerebral vascular anatomy. J Cereb Blood Flow Metab. 1993;13:683–692. doi: 10.1038/jcbfm.1993.87. [DOI] [PubMed] [Google Scholar]
- 51.Barrabes JA, Inserte J, Mirabet M, Quiroga A, Hernando V, Figueras J, Garcia-Dorado D. Antagonism of P2Y12 or GPIIb/IIIa receptors reduces platelet-mediated myocardial injury after ischaemia and reperfusion in isolated rat hearts. Thromb Haemost. 2010;104:128–135. doi: 10.1160/TH09-07-0440. [DOI] [PubMed] [Google Scholar]
- 52.Barth AS, Tomaselli GF. Gene scanning and heart attack risk. Trends Cardiovasc Med. 2016;26:260–265. doi: 10.1016/j.tcm.2015.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bateson P, Gluckman P, Hanson M. The biology of developmental plasticity and the predictive adaptive response hypothesis. J Physiol. 2014;592:2357–2368. doi: 10.1113/jphysiol.2014.271460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bauer I, Wanner GA, Rensing H, Alte C, Miescher EA, Wolf B, Pannen BH, Clemens MG, Bauer M. Expression pattern of heme oxygenases 1 and 2 in normal and stress-exposed rat liver. Hepatology. 1998;27:829–838. doi: 10.1002/hep.510270327. [DOI] [PubMed] [Google Scholar]
- 55.Bauer M, Bauer I. Heme oxygenase-1: Redox regulation and role in the hepatic response to oxidative stress. Antioxid Redox Signal. 2002;4:749–758. doi: 10.1089/152308602760598891. [DOI] [PubMed] [Google Scholar]
- 56.Baum M. Role of the kidney in the prenatal and early postnatal programming of hypertension. Am J Physiol Renal Physiol. 2010;29:F235–F247. doi: 10.1152/ajprenal.00288.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Baumgartner WA, Williams GM, Fraser CD, Jr, Cameron DE, Gardner TJ, Burdick JF, Augustine S, Gaul PD, Reitz BA. Cardiopulmonary bypass with profound hypothermia. An optimal preservation method for multiorgan procurement. Transplantation. 1989;47:123–127. doi: 10.1097/00007890-198901000-00027. [DOI] [PubMed] [Google Scholar]
- 58.Bautista AP, Meszaros K, Bojta J, Spitzer JJ. Superoxide anion generation in the liver during the early stage of endotoxemia in rats. J Leukoc Biol. 1990;48:123–128. doi: 10.1002/jlb.48.2.123. [DOI] [PubMed] [Google Scholar]
- 59.Baxter GF. The neutrophil as a mediator of myocardial ischemia-reperfusion injury: Time to move on. Basic Res Cardiol. 2002;97:268–275. doi: 10.1007/s00395-002-0366-7. [DOI] [PubMed] [Google Scholar]
- 60.Beldi G, Banz Y, Kroemer A, Sun X, Wu Y, Graubardt N, Rellstab A, Nowak M, Enjyoji K, Li X, Junger WG, Candinas D, Robson SC. Deletion of CD39 on natural killer cells attenuates hepatic ischemia/reperfusion injury in mice. Hepatology. 2010;51:1702–1711. doi: 10.1002/hep.23510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Belmont PJ, Chen WJ, San Pedro MN, Thuerauf DJ, Gellings Lowe N, Gude N, Hilton B, Wolkowicz R, Sussman MA, Glembotski CC. Roles for endoplasmic reticulum-associated degradation and the novel endoplasmic reticulum stress response gene Derlin-3 in the ischemic heart. Circ Res. 2010;106:307–316. doi: 10.1161/CIRCRESAHA.109.203901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Belmont PJ, Chen WJ, Thuerauf DJ, Glembotski CC. Regulation of microRNA expression in the heart by the ATF6 branch of the ER stress response. J Mol Cell Cardiol. 2012;53:259–267. doi: 10.1016/j.yjmcc.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Benakis C, Brea D, Caballero S, Faraco G, Moore J, Murphy M, Sita G, Racchumi G, Ling L, Pamer EG, Iadecola C, Anrather J. Commensal microbiota affects ischemic stroke outcome by regulating intestinal gammadelta T cells. Nat Med. 2016;22:516–523. doi: 10.1038/nm.4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ben-Ari Z, Pappo O, Cheporko Y, Yasovich N, Offen D, Shainberg A, Leshem D, Sulkes J, Vidne BA, Hochhauser E. Bax ablation protects against hepatic ischemia/reperfusion injury in transgenic mice. Liver Transpl. 2007;13:1181–1188. doi: 10.1002/lt.21221. [DOI] [PubMed] [Google Scholar]
- 65.Benhar M, Forrester MT, Stamler JS. Protein denitrosylation: Enzymatic mechanisms and cellular functions. Nat Rev Cell Mol Biol. 2009;10:21–732. doi: 10.1038/nrm2764. [DOI] [PubMed] [Google Scholar]
- 66.Bernardo BC, Gao XM, Winbanks CE, Boey EJ, Tham YK, Kiriazis H, Gregorevic P, Obad S, Kauppinen S, Du XJ, Lin RC, McMullen JR. Therapeutic inhibition of the miR-34 family attenuates pathological cardiac remodeling and improves heart function. Proc Natl Acad Sci U S A. 2012;109:17615–17620. doi: 10.1073/pnas.1206432109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bhatia M, Saluja AK, Hofbauer B, Frossard JL, Lee HS, Castagliuolo I, Wang CC, Gerard N, Pothoulakis C, Steer ML. Role of substance P and the neurokinin 1 receptor in acute pancreatitis and pancreatitis-associated lung injury. Proc Natl Acad Sci U S A. 1998;95:4760–4765. doi: 10.1073/pnas.95.8.4760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bhatt K, Kato M, Natarajan R. Mini-review: Emerging roles of microRNAs in the pathophysiology of renal diseases. Am J Physiol Renal Physiol. 2016;310:F109–118. doi: 10.1152/ajprenal.00387.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bhattacharya K, Farwell K, Huang M, Kempuraj D, Donelan J, Papaliodis D, Vasiadi M, Theoharides TC. Mast cell deficient W/Wv mice have lower serum IL-6 and less cardiac tissue necrosis than their normal littermates following myocardial ischemia-reperfusion. Int J Immunopathol Pharmacol. 2007;20:69–74. doi: 10.1177/039463200702000108. [DOI] [PubMed] [Google Scholar]
- 70.Bianchi P, Kunduzova O, Masini E, Cambon C, Bani D, Raimondi L, Seguelas MH, Nistri S, Colucci W, Leducq N, Parini A. Oxidative stress by monoamine oxidase mediates receptor-independent cardiomyocyte apoptosis by serotonin and postischemic myocardial injury. Circulation. 2005;112:3297–3305. doi: 10.1161/CIRCULATIONAHA.104.528133. [DOI] [PubMed] [Google Scholar]
- 71.Bilzer M, Roggel F, Gerbes AL. Role of Kupffer cells in host defense and liver disease. Liver Int. 2006;26:1175–1186. doi: 10.1111/j.1478-3231.2006.01342.x. [DOI] [PubMed] [Google Scholar]
- 72.Bindoli A, Rigobello MP. Principles in redox signaling: From chemistry to functional significance. Antioxid Redox Signal. 2013;18:1557–1593. doi: 10.1089/ars.2012.4655. [DOI] [PubMed] [Google Scholar]
- 73.Birdsall HH, Green DM, Trial J, Youker KA, Burns AR, MacKay CR, LaRosa GJ, Hawkins HK, Smith CW, Michael LH, Entman ML, Rossen RD. Complement C5a, TGF-beta 1, and MCP-1, in sequence, induce migration of monocytes into ischemic canine myocardium within the first one to five hours after reperfusion. Circulation. 1997;95:684–692. doi: 10.1161/01.cir.95.3.684. [DOI] [PubMed] [Google Scholar]
- 74.Bjorkegren JL, Kovacic JC, Dudley JT, Schadt EE. Genome-wide significant loci: How important are they? Systems genetics to understand heritability of coronary artery disease and other common complex disorders. J Am Coll Cardiol. 2015;65:830–845. doi: 10.1016/j.jacc.2014.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Black M, Wang W. Ischemic stroke: From next generation sequencing and GWAS to community genomics? OMICS. 2015;19:451–460. doi: 10.1089/omi.2015.0083. [DOI] [PubMed] [Google Scholar]
- 76.Bless NM, Warner RL, Padgaonkar VA, Lentsch AB, Czermak BJ, Schmal H, Friedl HP, Ward PA. Roles for C-X-C chemokines and C5a in lung injury after hindlimb ischemia-reperfusion. Am J Physiol. 1999;276:L57–63. doi: 10.1152/ajplung.1999.276.1.L57. [DOI] [PubMed] [Google Scholar]
- 77.Bliksoen M, Baysa A, Eide L, Bjoras M, Suganthan R, Vaage J, Stenslokken KO, Valen G. Mitochondrial DNA damage and repair during ischemia-reperfusion injury of the heart. J Mol Cell Cardiol. 2015;78:9–22. doi: 10.1016/j.yjmcc.2014.11.010. [DOI] [PubMed] [Google Scholar]
- 78.Bliss TM, Kelly S, Shah AK, Foo WC, Kohli P, Stokes C, Sun GH, Ma M, Masel J, Kleppner SR, Schallert T, Palmer T, Steinberg GK. Transplantation of hNT neurons into the ischemic cortex: Cell survival and effect on sensorimotor behavior. J Neurosci Res. 2006;83:1004–1014. doi: 10.1002/jnr.20800. [DOI] [PubMed] [Google Scholar]
- 79.Bluhmki E, Chamorro A, Davalos A, Machnig T, Sauce C, Wahlgren N, Wardlaw J, Hacke W. Stroke treatment with alteplase given 3.0–4.5 h after onset of acute ischaemic stroke (ECASS III): Additional outcomes and subgroup analysis of a randomised controlled trial. Lancet Neurol. 2009;8:1095–1102. doi: 10.1016/S1474-4422(09)70264-9. [DOI] [PubMed] [Google Scholar]
- 80.Bodi V, Marrachelli VG, Husser O, Chorro FJ, Vina JR, Monleon D. Metabolomics in the diagnosis of acute myocardial ischemia. J Cardiovasc Transl Res. 2013;6:808–815. doi: 10.1007/s12265-013-9505-9. [DOI] [PubMed] [Google Scholar]
- 81.Boengler K, Schulz R, Heusch G. Loss of cardioprotection with ageing. Cardiovasc Res. 2009;83:247–261. doi: 10.1093/cvr/cvp033. [DOI] [PubMed] [Google Scholar]
- 82.Boersma E, Maas AC, Deckers JW, Simoons ML. Early thrombolytic treatment in acute myocardial infarction: Reappraisal of the golden hour. Lancet. 1996;348:771–775. doi: 10.1016/S0140-6736(96)02514-7. [DOI] [PubMed] [Google Scholar]
- 83.Bogoyevitch MA, Gillespie-Brown J, Ketterman AJ, Fuller SJ, Ben-Levy R, Ashworth A, Marshall CJ, Sugden PH. Stimulation of the stress-activated mitogen-activated protein kinase subfamilies in perfused heart. p38/RK mitogen-activated protein kinases and c-Jun N-terminal kinases are activated by ischemia/reperfusion. Circ Res. 1996;79:162–73. doi: 10.1161/01.res.79.2.162. [DOI] [PubMed] [Google Scholar]
- 84.Bolli R, Marban E. Molecular and cellular mechanisms of myocardial stunning. Physiol Rev. 1999;79:609–663. doi: 10.1152/physrev.1999.79.2.609. [DOI] [PubMed] [Google Scholar]
- 85.Bonder CS, Norman MU, Macrae T, Mangan PR, Weaver CT, Bullard DC, McCafferty DM, Kubes P. P-selectin can support both Th1 and Th2 lymphocyte rolling in the intestinal microvasculature. Am J Pathol. 2005;167:1647–1660. doi: 10.1016/S0002-9440(10)61248-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Boon RA, Dimmeler S. MicroRNAs in myocardial infarction. Nat Rev Cardiol. 2015;12:135–142. doi: 10.1038/nrcardio.2014.207. [DOI] [PubMed] [Google Scholar]
- 87.Boros P, Bromberg JS. New cellular and molecular immune pathways in ischemia/reperfusion injury. Am J Transplant. 2006;6:652–658. doi: 10.1111/j.1600-6143.2005.01228.x. [DOI] [PubMed] [Google Scholar]
- 88.Bortolotto SK, Morrison WA, Han X, Messina A. Mast cells play a pivotal role in ischaemia reperfusion injury to skeletal muscles. Lab Invest. 2004;84:1103–1111. doi: 10.1038/labinvest.3700126. [DOI] [PubMed] [Google Scholar]
- 89.Botker HE, Kharbanda R, Schmidt MR, Bottcher M, Kaltoft AK, Terkelsen CJ, Munk K, Andersen NH, Hansen TM, Trautner S, Lassen JF, Christiansen EH, Krusell LR, Kristensen SD, Thuesen L, Nielsen SS, Rehling M, Sorensen HT, Redington AN, Nielsen TT. Remote ischaemic conditioning before hospital admission, as a complement to angioplasty, and effect on myocardial salvage in patients with acute myocardial infarction: A randomised trial. Lancet. 2010;375:727–34. doi: 10.1016/S0140-6736(09)62001-8. [DOI] [PubMed] [Google Scholar]
- 90.Boujrad H, Gubkina O, Robert N, Krantic S, Susin SA. AIF-mediated programmed necrosis: A highly regulated way to die. Cell Cycle. 2007;6:2612–9. doi: 10.4161/cc.6.21.4842. [DOI] [PubMed] [Google Scholar]
- 91.Bozic CR, Lu B, Hopken UE, Gereard C, Berard NP. Neurogenic amplification of immune complex inflammation. Science. 1996;273:1722–1725. doi: 10.1126/science.273.5282.1722. [DOI] [PubMed] [Google Scholar]
- 92.Braunersreuther V, Jaquet V. Reactive oxygen species in myocardial reperfusion injury: From physiopathology to therapeutic approaches. Curr Pharm Biotechnol. 2012;13:97–114. doi: 10.2174/138920112798868782. [DOI] [PubMed] [Google Scholar]
- 93.Bright R, Raval AP, Dembner JM, Pérez-Pinzón MA, Steinberg GK, Yenari MA, Mochly-Rosen D. Protein kinase C d mediates cerebral reperfusion injury in vivo. J Neurosci. 2004;24:6880–6888. doi: 10.1523/JNEUROSCI.4474-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Broughton BR, Reutens DC, Sobey CG. Apoptotic mechanisms after cerebral ischemia. Stroke. 2009;40:e331–e339. doi: 10.1161/STROKEAHA.108.531632. [DOI] [PubMed] [Google Scholar]
- 95.Brown KE, Brunt EM, Heinecke JW. Immunohistochemical detection of myeloperoxidase and its oxidation products in Kupffer cells of human liver. Am J Pathol. 2001;159:2081–2088. doi: 10.1016/S0002-9440(10)63059-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Brown JM, Hazen SL. The gut microbial endocrine organ: Bacterially derived signals driving cardiometabolic diseases. Annu Rev Med. 2015;66:343–359. doi: 10.1146/annurev-med-060513-093205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bryan NS, Grisham MB. Methods to detect nitric oxide and its metabolites in biological samples. Free Radic Biol Med. 2007;43:645–657. doi: 10.1016/j.freeradbiomed.2007.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Buckley CD, Ross EA, McGettrick HM, Osborne CE, Haworth O, Schmutz C, Stone PC, Salmon M, Matharu NM, Vohra RK, Nash GB, Rainger GE. Identification of a phenotypically and functionally distinct population of long-lived neutrophils in a model of reverse endothelial migration. J Leukoc Biol. 2006;79:303–311. doi: 10.1189/jlb.0905496. [DOI] [PubMed] [Google Scholar]
- 99.Budas GR, Churchill EN, Mochly-Rosen D. Cardioprotective mechanisms of PKC isozyme-selective activators and inhibitors in the treatment of ischemia-reperfusion injury. Pharmacol Res. 2007;55:523–536. doi: 10.1016/j.phrs.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 100.Bulkley GB. Free radical-mediated reperfusion injury: A selective review. Br J Cancer Suppl. 1987;8:66–73. [PMC free article] [PubMed] [Google Scholar]
- 101.Burne MJ, Haq M, Matsuse H, Mohapatra S, Rabb H. Genetic susceptibility to renal ischemia reperfusion injury revealed in a murine model. Transplantation. 2000;69:1023–1025. doi: 10.1097/00007890-200003150-00065. [DOI] [PubMed] [Google Scholar]
- 102.Burne-Taney MJ, Ascon DB, Daniels F, Racusen L, Baldwin W, Rabb H. B cell deficiency confers protection from renal ischemia reperfusion injury. J Immunol. 2003;171:3210–3215. doi: 10.4049/jimmunol.171.6.3210. [DOI] [PubMed] [Google Scholar]
- 103.Burne-Taney MJ, Yokota-Ikeda N, Rabb H. Effects of combined T- and B-cell deficiency on murine ischemia reperfusion injury. Am J Transplant. 2005;5:1186–1193. doi: 10.1111/j.1600-6143.2005.00815.x. [DOI] [PubMed] [Google Scholar]
- 104.Burns AR, Smith CW, Walker DC. Unique structural features that influence neutrophil emigration into the lung. Physiol Rev. 2003;83:309–336. doi: 10.1152/physrev.00023.2002. [DOI] [PubMed] [Google Scholar]
- 105.Cabrera-Fuentes HA, Ruiz-Meana M, Simsekyilmaz S, Kostin S, Inserte J, Saffarzadeh M, Galuska SP, Vijayan V, Barba I, Barreto G, Fischer S, Lochnit G, Ilinskaya ON, Baumgart-Vogt E, Boning A, Lecour S, Hausenloy DJ, Liehn EA, Garcia-Dorado D, Schluter KD, Preissner KT. RNase1 prevents the damaging interplay between extracellular RNA and tumour necrosis factor-alpha in cardiac ischaemia/reperfusion injury. Thromb Haemost. 2014;112:1110–1119. doi: 10.1160/TH14-08-0703. [DOI] [PubMed] [Google Scholar]
- 106.Caldeira MV, Salazar IL, Curcio M, Canzoniero LM, Duarte CB. Role of the ubiquitin-proteasome system in brain ischemia: Friend or foe? Prog Neurobiol. 2014;112:50–69. doi: 10.1016/j.pneurobio.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 107.Caldwell CC, Okaya T, Matignoni A, Husted T, Schuster R, Lentsch AB. Divergent functions of CD4+ T lymphocytes in acute liver inflammation and injury after ischemia-reperfusion. Am J Physiol. 2005;289:G969–G976. doi: 10.1152/ajpgi.00223.2005. [DOI] [PubMed] [Google Scholar]
- 108.Caldwell-Kenkel JC, Currin RT, Tanaka Y, Thurman PG, Lemasters JJ. Kupffer cell activation and endothelial cell damage aftrer storage of rat livers: Effect of reperfusion. Hepatology. 1991;13:83–95. [PubMed] [Google Scholar]
- 108a.Calvert JW, Lefer DJ, Gundewar S, Poston L, Coetzee WA. Developmental programming resulting from maternal obesity in mice: Effects on myocardial ischaemia-reperfusion injury. Exp Physiol. 2009;94:805–814. doi: 10.1113/expphysiol.2009.047183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Candilio L, Hausenloy DJ, Yellon DM. Remote ischemic conditioning: A clinical trial’s update. J Cardiovasc Pharmacol Ther. 2011;16:304–312. doi: 10.1177/1074248411411711. [DOI] [PubMed] [Google Scholar]
- 110.Cao JY, Dixon SJ. Mechanisms of ferroptosis. Cell Mol Life Sci. 2016;73:2195–2209. doi: 10.1007/s00018-016-2194-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cao T, Pinter E, Al-Rasheed S, Gerard N, Hoult JR, Brain SD. Neurokinin-1 receptor agonists are involved in mediating neutrophil accumulation in the inflamed, but not normal, cutaneous microvasculature: An in vivo study using neurokinin-1 receptor knockout mice. J Immunol. 2000;164:5424–5429. doi: 10.4049/jimmunol.164.10.5424. [DOI] [PubMed] [Google Scholar]
- 112.Cappola TP, Margulies KB. Functional genomics applied to cardiovascular medicine. Circulation. 2011;124:87–94. doi: 10.1161/CIRCULATIONAHA.111.027300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Carati CJ. Changes in macromolecular permeability of microvessels in rat small intestine after total occlusion ischaemia/reperfusion. Microcirc Endothelium Lymphatics. 1988;4:69–86. [PubMed] [Google Scholar]
- 114.Carden DL, Granger DN. Pathophysiology of ischemia-reperfusion injury. J Pathol. 2000;190:255–266. doi: 10.1002/(SICI)1096-9896(200002)190:3<255::AID-PATH526>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 115.Cardinal J, Pan P, Tsung A. Protective role of cisplatin in ischemic liver injury through induction of autophagy. Autophagy. 2009;5:1211–2. doi: 10.4161/auto.5.8.9972. [DOI] [PubMed] [Google Scholar]
- 116.Carloni S, Girelli S, Scopa C, Buonocore G, Longini M, Balduini W. Activation of autophagy and Akt/CREB signaling play an equivalent role in the neuroprotective effect of rapamycin in neonatal hypoxiaischemia. Autophagy. 2010;6:366–77. doi: 10.4161/auto.6.3.11261. [DOI] [PubMed] [Google Scholar]
- 117.Carman CV, Springer TA. A transmigratory cup in leukocyte diapedesis both through individual vascular endothelial cells and between them. J Cell Biol. 2004;167:377–388. doi: 10.1083/jcb.200404129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Carpi A, Menabò R, Kaludercic N, Pelicci P, Di Lisa F, Giorgio M. The cardioprotective effects elicited by p66(Shc) ablation demonstrate the crucial role of mitochondrial ROS formation in ischemia/reperfusion injury. Biochim Biophys Acta. 2009;1787:774–780. doi: 10.1016/j.bbabio.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 119.Carragher NO. Calpain inhibition: A therapeutic strategy targeting multiple disease states. Curr Pharm Des. 2006;12:615–638. doi: 10.2174/138161206775474314. [DOI] [PubMed] [Google Scholar]
- 120.Castello PR, David PS, McClure T, Crook Z, Poyton RO. Mitochondrial cytochrome oxidase produces nitic oxide under hypoxic conditions: Implications for oxygen sensing and hypoxic sensing in eukaryotes. Cell Metab. 2006;3:277–287. doi: 10.1016/j.cmet.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 121.Cattaruzza F, Cenac N, Barocelli E, Impicciatore M, Rushbrook JI, Zhang M. Protective effect of proteinase-activated receptor 2 activation on motility impairment and tissue damage induced by intestinal ischemia/reperfusion in rodents. Am J Pathol. 2013;169:177–188. doi: 10.2353/ajpath.2006.051098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Cerqueira NF, Hussni CA, Yoshida WB. Pathophysiology of mesenteric ischemia/reperfusion: A review. Acta Cir Bras. 2005;20:336–343. doi: 10.1590/s0102-86502005000400013. [DOI] [PubMed] [Google Scholar]
- 123.Chacko BK, Kramer PA, Ravi S, Benavides GA, Mitchell T, Dranka BP, Ferrick D, Singal AK, Ballinger SW, Bailey SM, Hard RW, Zhang J, Zhi D, Darley-Usmar VM. The Bioenergetic Health Index: A new concept in mitochondrial translational research. Clin Sci (Lond) 2014;127:367–373. doi: 10.1042/CS20140101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chaitanya GV, Steven AJ, Babu PP. Molecular PARP-1 cleavage fragments: Signatures of cell-death proteases in neurodegeneration. Cell Commun Signal. 2010;8:31. doi: 10.1186/1478-811X-8-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chamorro A, Meisel A, Planas AM, Urra X, van de Beek D, Veltkamp R. The immunology of acute stroke. Nat Rev Neurol. 2012;8:401–410. doi: 10.1038/nrneurol.2012.98. [DOI] [PubMed] [Google Scholar]
- 126.Chan MT, Boet R, Ng SC, Poon WS, Gin T. Effect of ischemic preconditioning on brain tissue gases and pH during temporary cerebral artery occlusion. Acta Neurochir Suppl. 2005;95:93–96. doi: 10.1007/3-211-32318-x_20. [DOI] [PubMed] [Google Scholar]
- 127.Charlagorla P, Liu J, Patel M, Rushbrook JI, Zhang M. Loss of plasma membrane integrity, complement response and formation of reactive oxygenspecies during early myocardial ischemia/reperfusion. Mol Immunol. 2013;56:507–512. doi: 10.1016/j.molimm.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chatterjee PK, Brown PA, Cuzzocrea S, Zacharowski K, Stewart KN, Mota-Filipe H, McDonald MC, Thiemermann C. Calpain inhibitor-1 reduces renal ischemia/reperfusion injury in the rat. Kidney Int. 2001;59:2073–2083. doi: 10.1046/j.1523-1755.2001.00722.x. [DOI] [PubMed] [Google Scholar]
- 129.Chatterjee PK, Todorovic Z, Sivarajah A, Mota-Filipe H, Brown PA, Stewart KN, Mazzon E, Cuzzocrea S, Thiemermann C. Inhibitors of calpain activation (PD150606 and E-64) and renal ischemia-reperfusion injury. Biochem Pharmacol. 2005;69:1121–1131. doi: 10.1016/j.bcp.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 130.Chavez-Valdez R, Martin LJ, Flock DL, Northington FJ. Necrostatin-1 attenuates mitochondrial dysfunction in neurons and astrocytes following neonatal hypoxia-ischemia. Neuroscience. 2012;219:192–203. doi: 10.1016/j.neuroscience.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Chehal MK, Granville DJ. Cytochrome p450 2C (CYP2C) in ischemic heart injury and vascular dysfunction. Can J Physiol Pharmacol. 2006;84:15–20. doi: 10.1139/y05-139. [DOI] [PubMed] [Google Scholar]
- 132.Chen C, Feng Y, Zou L, Wang L, Chen HH, Cai JY, Xu JM, Sosnovik DE, Chao W. Role of extracellular RNA and TLR3-Trif signaling in myocardial ischemia-reperfusion injury. J Am Heart Assoc. 2014;3:e000683. doi: 10.1161/JAHA.113.000683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Chen EP, Bittner HB, Davis RD, Folz RJ, Van Trigt P. Extracellular superoxide dismutase transgene overexpression preserves postischemic myocardial function in isolated murine hearts. Circulation. 1996;94:II412–II417. [PubMed] [Google Scholar]
- 134.Chen J, Crispín JC, Tedder TF, Dalle Lucca J, Tsokos GC. B cells contribute to ischemia/reperfusion-mediated injury. J Autoimmunity. 2009;32:195–200. doi: 10.1016/j.jaut.2009.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Chen JC, Wu ML, Huang KC, Lin WW. HMG-CoA reductase inhibitors activate the unfolded protein response and induce cytoprotective GRP78 expression. Cardiovasc Res. 2008;80:138–150. doi: 10.1093/cvr/cvn160. [DOI] [PubMed] [Google Scholar]
- 136.Chen L, Hahn H, Wu G, Chen CH, Liron T, Schechtman D, Cavallaro G, Banci L, Guo Y, Bolli R, Dorn GW, II, Mochly-Rosen D. Opposing cardioprotective actions and parallel hypertrophic effects of delta PKC and epsilon PKC. Proc Natl Acad Sci U S A. 2001;98:11114–11119. doi: 10.1073/pnas.191369098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Chen L, Knowlton AA. Mitochondria and heart failure: New insights into an energetic problem. Minerva Cardioangiol. 2010;58:213–229. [PMC free article] [PubMed] [Google Scholar]
- 138.Chen M, Won DJ, Krajewski S, Gottlieb RA. Calpain and mitochondria in ischemia/reperfusion injury. J Biol Chem. 2002;277:29181–29186. doi: 10.1074/jbc.M204951200. [DOI] [PubMed] [Google Scholar]
- 139.Chen Y, Wood KJ. Interleukin-23 and Th17 cells in transplantation immunity: Does 23+17 equal rejection? Transplant. 2007;84:1071–1074. doi: 10.1097/01.tp.0000287126.12083.48. [DOI] [PubMed] [Google Scholar]
- 140.Chironi GN, Boulanger CM, Simon A, Dignat-George F, Freyssinet JM, Tedgui A. Endothelial microparticles in diseases. Cell Tissue Res. 2009;335:143–151. doi: 10.1007/s00441-008-0710-9. [DOI] [PubMed] [Google Scholar]
- 141.Cho YS, Challa S, Moquin D, Genga R, Ray TD, Guildford M, Chan FK. Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell. 2009;137:1112–23. doi: 10.1016/j.cell.2009.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Choi DW. The role of glutamate neurotoxicity in hypoxic-ischemic neuronal death. Annu Rev Neurosci. 1990;13:171–182. doi: 10.1146/annurev.ne.13.030190.001131. [DOI] [PubMed] [Google Scholar]
- 143.Chou HC, Cehn YW, Lee TR, Wu FS, Chan HT, Lyu PC, Timms JF, Chan HL. Proteomics study of oxidative stress and Src kinase inhibition in H9C2 cardiomyocytes: A cell model of heart ischemia-reperfusion injury and treatment. Free Radic Biol Med. 2010;49:96–108. doi: 10.1016/j.freeradbiomed.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 144.Chou WH, Choi DS, Zhang H, Mu D, McMahon T, Kharazia VN, Lowell CA, Ferriero DM, Messing RO. Neutrophil protein kinase Cdelta as a mediator of stroke-reperfusion injury. J Clin Invest. 2004;114:49–56. doi: 10.1172/JCI21655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Chouchani ET, Pell VR, Gaude E, Aksentijevic D, Sundier SY, Robb EL, Logan A, Nadtochiy SM, Ord EN, Smith AC, Eyassu F, Shirley R, Hu CH, Dare AJ, James AM, Rogatti S, Hartley RC, Eaton S, Costa AS, Brookes PS, Davidson SM, Duchen MR, Saeb-Parsy K, Shattock MJ, Robinson AJ, Work LM, Frezza C, Krieg T, Murphy MP. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature. 2014;515:431–435. doi: 10.1038/nature13909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Chouchani ET, Pell VR, James AM, Work LM, Saeb-Parsy K, Frezza C, Krieg T, Murphy MP. A Unifying Mechanism for Mitochondrial Superoxide Production during Ischemia-Reperfusion Injury. Cell Metab. 2016;23:254–263. doi: 10.1016/j.cmet.2015.12.009. [DOI] [PubMed] [Google Scholar]
- 147.Choudhuri S, Cui Y, Klaassen CD. Molecular targets of epigenetic regulation and effectors of environmental influences. Toxicol Appl Pharmacol. 2010;245:378–393. doi: 10.1016/j.taap.2010.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Churchill EN, Mochly-Rosen D. The roles of PKCdelta and epsilon isoenzymes in the regulation of myocardial ischaemia/reperfusion injury. Biochem Soc Trans. 2007;35:1040–1042. doi: 10.1042/BST0351040. [DOI] [PubMed] [Google Scholar]
- 149.Clarke SJ, McStay GP, Halestrap AP. Sanglifehrin A acts as a potent inhibitor of the mitochondrial permeability transition and reperfusion injury of the heart by binding to cyclophilin-D at a different site from cyclosporin A. J Biol Chem. 2002;277:34793–34799. doi: 10.1074/jbc.M202191200. [DOI] [PubMed] [Google Scholar]
- 150.Colletti LM, Cortis A, Lukacs N, Kunkel SL, Green M, Strieter RM. Tumor necrosis factor up-regulates intercellular adhesion molecule 1, which is important in the neutrophil-dependent lung and liver injury associated with hepatic ischemia and reperfusion in the rat. Shock. 1998;10:182–191. doi: 10.1097/00024382-199809000-00006. [DOI] [PubMed] [Google Scholar]
- 151.Conrad M, Angeli JP, Vandenabeele P, Stockwell BR. Regulated necrosis: Disease relevance and therapeutic opportunities. Nat Rev Drug Discov. 2016;15:348–366. doi: 10.1038/nrd.2015.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Contreras JL, Vilatoba M, Eckstein C, Bilbao G, Anthony Thompson J, Eckhoff DE. Caspase-8 and caspase-3 small interfering RNA decreases ischemia/reperfusion injury to the liver in mice. Surgery. 2004;136:390–400. doi: 10.1016/j.surg.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 153.Contreras L, Drago I, Zampese E, Pozzan T. Mitochondria: The calcium connection. Biochim Biophys Acta. 2010;1797:607–618. doi: 10.1016/j.bbabio.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 154.Coombes JS, Powers SK, Hamilton KL, Demirel HA, Shanely RA, Zergerolglu MA, Sen CK, Packer L, Ji LL. Improved cardiac performance after ischemia in aged rats supplemented with vitamin E and alpha-lipoic acid. Am J Physiol. 2000;279:R2149–R2155. doi: 10.1152/ajpregu.2000.279.6.R2149. [DOI] [PubMed] [Google Scholar]
- 155.Cooper D, Chitman KD, Williams MC, Granger DN. Time-dependent platelet-vessel wall interactions induced by ischemia-reperfusion. Am J Physiol. 2003;284:G1027–1033. doi: 10.1152/ajpgi.00457.2002. [DOI] [PubMed] [Google Scholar]
- 156.Cooper D, Russell J, Chitman KD, Williams MC, Wolf RE, Granger DN. Leukocyte dependence of platelet adhesion in postcapillary venules. Am J Physiol. 2004;286:H1895–H1900. doi: 10.1152/ajpheart.01000.2003. [DOI] [PubMed] [Google Scholar]
- 157.Corbucci GG, Perrino C, Donato G, Ricchi A, Lettieri B, Troncone G, Indolfi C, Chiariello M, Avvedimento EV. Transient and reversible deoxyribonucleic acid damage in human left ventricle under controlled ischemia and reperfusion. J Am Coll Cardiol. 2004;43:1992–1999. doi: 10.1016/j.jacc.2004.01.040. [DOI] [PubMed] [Google Scholar]
- 158.Cording J, Gunther R, Vigolo E, Tscheik C, Winkler L, Schlattner I, Lorenz D, Haseloff RF, Schmidt-Ott KM, Wolburg H, Blasig IE. Redox regulation of cell contacts by tricellulin and occludin: Redox-sensitive cysteine sites in tricellulin regulate both tri- and bicellular junctions in tissue barriers as shown in hypoxia and ischemia. Antioxid Redox Signal. 2015;23:1035–1049. doi: 10.1089/ars.2014.6162. [DOI] [PubMed] [Google Scholar]
- 159.Costa FF. Non-coding RNAs: Meet thy masters. Bioessays. 2010;32:599–608. doi: 10.1002/bies.200900112. [DOI] [PubMed] [Google Scholar]
- 160.Couchonnal L, Anderson ME. The role of calmodulin kinase II in myocardial physiology and disease. Physiology. 2008;23:151–159. doi: 10.1152/physiol.00043.2007. [DOI] [PubMed] [Google Scholar]
- 161.Courties G, Moskowitz MA, Nahrendorf M. The innate immune system after ischemic injury: Lessons to be learned from the heart and brain. JAMA Neurol. 2014;71:233–236. doi: 10.1001/jamaneurol.2013.5026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Creemers EE, Tijsen AJ, Pinto YM. Circulating MicroRNAs: Novel biomarkers and extracellular communicators in cardiovascular disease? Circ Res. 2011;110:483–495. doi: 10.1161/CIRCRESAHA.111.247452. [DOI] [PubMed] [Google Scholar]
- 163.Crissinger KD, Granger DN. Characterization of intestinal collateral blood flow in the developing piglet. Pediatr Res. 1988;24:473–476. doi: 10.1203/00006450-198810000-00011. [DOI] [PubMed] [Google Scholar]
- 164.Croall DE, Ersfeld K. The calpains: Modular designs and functional diversity. Genome Biol. 2007;8:218. doi: 10.1186/gb-2007-8-6-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Crosson CE, Mani SK, Husain S, Alsarraf O, Menick DR. Inhibition of histone deacetylase protects the retina from ischemic injury. Invest Ophthalmol Vis Sci. 2010;51:3639–3645. doi: 10.1167/iovs.09-4538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Cui Z, Scruggs SB, Gilda JE, Ping P, Gomes AV. Regulation of cardiac proteasomes by ubiquitination, SUMOylation, and beyond. J Mol Cell Cardiol. 2014;71:32–42. doi: 10.1016/j.yjmcc.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Czermak BJ, Sarma V, Pierson CL, Warner RL, Huber-Lang M, Bless NM, Schmal H, Friedl HP, Ward PA. Protective effects of C5a blockade in sepsis. Nat Med. 1999;5:788–792. doi: 10.1038/10512. [DOI] [PubMed] [Google Scholar]
- 168.D’Autréaux B, Toledano MB. ROS as signaling molecules: Mechanisms that generate specificity in ROS homeostasis. Nat Mol Cell Biol. 2007;8:813–824. doi: 10.1038/nrm2256. [DOI] [PubMed] [Google Scholar]
- 169.Daemen MA, van’t Veer C, Denecker G, Heemskerk VH, Wolfs TG, Clauss M, Vandenabeele P, Buurman WA. Inhibition of apoptosis induced by ischemia-reperfusion prevents inflammation. J Clin Invest. 1999;104:541–9. doi: 10.1172/JCI6974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Dai H, Korthuis RJ. Mast cell proteases and inflammation. Drug Discov Today Dis Models. 2011;8:47–55. doi: 10.1016/j.ddmod.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Damle SS, Moore EE, Babu AN, Meng X, Fullerton DA, Banerjee A. Hemoglobin-based oxygen carrier induces heme oxygenase-1 in the heart and lung but not brain. J Am Coll Surg. 2009;208:592–598. doi: 10.1016/j.jamcollsurg.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 172.Dan Dunn J, Alvarez LA, Zhang X, Soldati T. Reactive oxygen species and mitochondria: A nexus of cellular homeostasis. Redox Biol. 2015;6:472–485. doi: 10.1016/j.redox.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Das M, Cui J, Das DK. Generation of survival signal by differential interaction of p38MAPKalpha and p38MAPKbeta with caveolin-1 and caveolin-3 in the adapted heart. J Mol Cell Cardiol. 2007;42:206–213. doi: 10.1016/j.yjmcc.2006.08.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.De Chiara G, Marcocci ME, Torcia M, Lucibello M, Rosini P, Bonini P, Higashimoto Y, Damonte G, Armirotti A, Amodei S, Palamara AT, Russo T, Garaci E, Cozzolino F. Bcl-2 Phosphorylation by p38 MAPK: Identification of target sites and biologic consequences. J Biol Chem. 2006;281:21353–21361. doi: 10.1074/jbc.M511052200. [DOI] [PubMed] [Google Scholar]
- 175.Degterev A, Huang Z, Boyce M, Li Y, Jagtap P, Mizushima N, Cuny GD, Mitchison TJ, Moskowitz MA, Yuan J. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol. 2005;1:112–119. doi: 10.1038/nchembio711. [DOI] [PubMed] [Google Scholar]
- 176.Degterev A, Linkermann A. Generation of small molecules to interfere with regulated necrosis. Cell Mol Life Sci. 2016;73:2251–2267. doi: 10.1007/s00018-016-2198-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Deitch EA. Gut lymph and lymphatics: A source of factors leading to organ injury and dysfunction. Ann N Y Acad Sci. 2010;1207(Suppl 1):E103–111. doi: 10.1111/j.1749-6632.2010.05713.x. [DOI] [PubMed] [Google Scholar]
- 178.Deitch EA. Gut-origin sepsis: Evolution of a concept. Surgeon. 2012;10:350–356. doi: 10.1016/j.surge.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Deitch EA, Xu D, Kaise VL. Role for the gut in the development of injury- and shock-induced SIRS and MODS: The gut-lymph hypothesis, a review. Front Biosci. 2006;11:520–528. doi: 10.2741/1816. [DOI] [PubMed] [Google Scholar]
- 180.Deng Y, Theken KN, Lee CR. Cytochrome P450 epoxygeneases, soluble hydrolase, and the regulation of cardiovascular inflammation. J Mol Cell Cardiol. 2010;48:331–341. doi: 10.1016/j.yjmcc.2009.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.DeLeon ER, Gao Y, Huang E, Arif M, Arora N, Divietro A, Patel S, Olson KR. A case of mistaken identity: Are reactive oxygen species actually reactive sulfide species? Am J Physiol Regul Integr Comp Physiol. 2016 doi: 10.1152/ajpregu.00455.2015. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.de Moura EG, Lisboa PC, Passos MC. Neonatal programming of neuroimmunomodulation—role of adipocytokines and neuropeptides. Neuroimmunomodulation. 2008;15:176–188. doi: 10.1159/000153422. [DOI] [PubMed] [Google Scholar]
- 183.De Pascali F, Hemann C, Samons K, Chen CA, Zweier JL. Hypoxia and reoxygenation induce endothelial nitric oxide synthase uncoupling in endothelial cells through tetrahydrobiopterin depletion and s-glutathionlyation. Biochemistry. 2014;53:3679–3688. doi: 10.1021/bi500076r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Depre C, Park JY, Shen YT, Zhao X, Qiu H, Yan L, Tian B, Vatner SF, Vatner DE. Molecular mechanisms mediating preconditioning following chronic ischemia differ from those in classical second window. Am J Physiol Heart Circ Physiol. 2010;299:H752–H762. doi: 10.1152/ajpheart.00147.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Depre C, Vatner SF. Cardioprotection in stunned and hibernating myocardium. Heart Fail Rev. 2007;12:307–317. doi: 10.1007/s10741-007-9040-3. [DOI] [PubMed] [Google Scholar]
- 186.Depre C, Vatner SF. Mechanisms of cell survival in myocardial hibernation. Trends Cardiovasc Med. 2005;15:101–110. doi: 10.1016/j.tcm.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 187.Devalaraja-Narashimha K, Diener AM, Padanilam BJ. Cyclophilin D gene ablation protects mice from ischemic renal injury. Am J Physiol Renal Physiol. 2009;297:F749–F759. doi: 10.1152/ajprenal.00239.2009. [DOI] [PubMed] [Google Scholar]
- 188.Devaux Y, Mueller M, Haaf P, Goretti E, Twerenbold R, Zangrando J, Vausort M, Reichlin T, Wildi K, Moehring B, Wagner DR, Mueller C. Diagnostic and prognostic value of circulating microRNAs in patients with acute chest pain. J Intern Med. 2015;277:260–271. doi: 10.1111/joim.12183. [DOI] [PubMed] [Google Scholar]
- 189.Devaux Y, Stammet P, Friberg H, Hassager C, Kuiper MA, Wise MP, Nielsen N. MicroRNAs: New biomarkers and therapeutic targets after cardiac arrest? Crit Care. 2015;19:54. doi: 10.1186/s13054-015-0767-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Devey L, Ferenbach D, Mohr E, Sangster K, Bellamy CO, Hughes J, Wigmore SJ. Tissue-resistant macrophages protect the liver from ischemia reperfusion injury via a heme oxygenase-1-dependent mechanism. Mol Ther. 2009;17:65–72. doi: 10.1038/mt.2008.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.DeWood MA, Spores J, Notske R, Mouser LT, Burroughs R, Golden MS, Lang HT. Prevalence of total coronary occlusion during the early hours of transmural myocardial infarction. N Engl J Med. 1980;303:897–902. doi: 10.1056/NEJM198010163031601. [DOI] [PubMed] [Google Scholar]
- 192.Di Lisa F, Canton M, Menabò R, Kaludercic N, Bernardi P. Mitochondria and cardioprotection. Heart Fail Rev. 2007;12:249–60. doi: 10.1007/s10741-007-9028-z. [DOI] [PubMed] [Google Scholar]
- 193.Di Lisa F, Giorgio M, Ferdinandy P, Schulz R. New aspects of p66Shc in ischemia reperfusion injury and cardiovascular diseases. Br J Pharmacol. 2016 doi: 10.1111/bph.13478. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Di Lisa F, Kaludercic N, Carpi A, Menabo R, Giorgio M. Mitochondria and vascular pathology. Pharmacol Rep. 2009;61:123–130. doi: 10.1016/s1734-1140(09)70014-3. [DOI] [PubMed] [Google Scholar]
- 195.Di Lisa F, Kaludercic N, Carpi A, Menabò R, Giorgio M. Mitochondrial pathways for ROS formation and myocardial injury: The relevance of p66(Shc) and monoamine oxidase. Basic Res Cardiol. 2009;104:131–139. doi: 10.1007/s00395-009-0008-4. [DOI] [PubMed] [Google Scholar]
- 196.Di Paola M, Lorusso M. Interaction of free fatty acids with mitochondria: Coupling, uncoupling and permeability transition. Biochim Biophys Acta. 2006;1757:1330–1337. doi: 10.1016/j.bbabio.2006.03.024. [DOI] [PubMed] [Google Scholar]
- 197.Dinagl U, Iadecola C, Moskowitz MA. Pathobiology of ischemic stroke: An integrated view. Trends Neurosci. 1999;22:391–397. doi: 10.1016/s0166-2236(99)01401-0. [DOI] [PubMed] [Google Scholar]
- 198.Divald A, Kivity S, Wang P, Hochhauser E, Roberts B, Teichberg S, Gomes AV, Powell SR. Myocardial ischemic preconditioning preserves postischemic function of the 26S proteasome through diminished oxidative damage to 19S regulatory particle subunits. Circ Res. 2010;106:1829–1838. doi: 10.1161/CIRCRESAHA.110.219485. [DOI] [PubMed] [Google Scholar]
- 199.Diwan A, Krenz M, Syed FM, Wansapura J, Ren X, Koesters AG, Li H, Kirshenbaum LA, Hahn HS, Robbins J, Jones WK, Dorn GW. Inhibition of ischemic cardiomyocytes apoptosis through targeted ablation of Bnip3 restrains postinfarction remodeling in mice. J Clin Invest. 2007;117:2825–2833. doi: 10.1172/JCI32490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Dodd-o JM, Hristopoulos ML, Welsh-Servinsky LE, Tankersley CG, Pearse DB. Strain-specific differences in sensitivity to ischemia-reperfusion lung injury in mice. J Appl Physiol. 2006;100:1590–1595. doi: 10.1152/japplphysiol.00681.2005. [DOI] [PubMed] [Google Scholar]
- 201.Donovan N, Becker EB, Konishi Y, Bonni A. JNK phosphorylation and activation of BAD couples the stress-activated signaling pathway to the cell death machinery. J Biol Chem. 2002;277:40944–40949. doi: 10.1074/jbc.M206113200. [DOI] [PubMed] [Google Scholar]
- 202.Drews O, Wildgruber R, Zong C, Sukop U, Nissum M, Weber G, Gomes AV, Ping P. Mammalian proteasome subpopulations with distinct molecular compositions and proteolytic activities. Mol Cell Proteomics. 2007;6:2021–2031. doi: 10.1074/mcp.M700187-MCP200. [DOI] [PubMed] [Google Scholar]
- 203.Dreyer WJ, Michael LH, Nguyen T, Smith CW, Anderson DC, Entman ML, Rossen RD. Kinetics of C5a release in cardiac lymph of dogs experiencing coronary artery ischemia-reperfusion injury. Circ Res. 1992;71:1518–1524. doi: 10.1161/01.res.71.6.1518. [DOI] [PubMed] [Google Scholar]
- 204.Duan J, Kasper DL. Oxidative depolymerization of polysaccharides by reactive oxygen/nitrogen species. Glycobiology. 2011;21:401–409. doi: 10.1093/glycob/cwq171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Duffield JS, Forbes SJ, Constantinou CM, Clay S, Partolina M, Vuthoori S, Wu SJ, Wang R, Iredale JP. Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J Clin Invest. 2005;115:56–65. doi: 10.1172/JCI22675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Duquesnes N, Lezoualc’h F, Crozatier B. PKC-delta and PKC-epsilon: Foes of the same family or strangers? J Mol Cell Cardiol. 2011;51:665–673. doi: 10.1016/j.yjmcc.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 206a.Dutta P, Nahrendorf M. Monocytes in myocardial infarction. Arterioscler Thromb Vasc Biol. 2015;35:1066–1070. doi: 10.1161/ATVBAHA.114.304652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Dvorak AM. Basophils and mast cells: Piecemeal degranulation in situ and ex vivo: A possible mechanism for cytokine-induced function in disease. Immunol Ser. 1992;57:169–271. [PubMed] [Google Scholar]
- 208.Dvoriantchikova G, Degterev A, Ivanov D. Retinal ganglion cell (RGC) programmed necrosis contributes to ischemia-reperfusion-induced retinal damage. Exp Eye Res. 2014;123:1–7. doi: 10.1016/j.exer.2014.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Dworakowski R, Alom-Ruiz SP, Shah AM. NADPH oxidase-derived reactive oxygen species in the regulation of endothelial phenotype. Pharmacol Rep. 2008;60:21–28. [PubMed] [Google Scholar]
- 210.Edgerton C, Crispin JC, Moratz CM, Bettelli E, Oukka M, Simovic M, Zacharia A, Egan R, Chen J, Dalle Lucca JJ, Juang YT, Tsokos GC. IL-17 producing CD4+ T cells mediate accelerated ischemia/reperfusion-induced injury in autoimmunity-prone mice. Clin Immunol. 2009;130:313–321. doi: 10.1016/j.clim.2008.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Edin ML, Wang Z, Bradbury JA, Graves JP, Lih FH, DeGraff LM, Foley JF, Torphy R, Ronnekleiv OK, Tomer KB, Lee CR, Zeldin DC. Endothelial expression of human cytochrome P450 epoxygenase CYP2C8 increases susceptibility to ischemia-reperfusion injury in isolated mouse heart. FASEB J. 2011;25:3436–3447. doi: 10.1096/fj.11-188300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211a.Elmes MJ, Gardner DS, Langley-Evans SC. Fetal exposure to a maternal low-protein diet is associated with altered left ventricular pressure response to ischaemia-reperfusion injury. Br J Nutr. 2007;98:93–100. doi: 10.1017/S000711450769182X. [DOI] [PubMed] [Google Scholar]
- 211b.Elmes MJ, McMullen S, Gardner DS, Langley-Evans SC. Prenatal diet determines susceptibility to cardiac ischaemia-reperfusion injury following treatment with diethylmaleic acid and N-acetylcysteine. Life Sci. 2008;82:149–155. doi: 10.1016/j.lfs.2007.10.022. [DOI] [PubMed] [Google Scholar]
- 212.Ellett JD, Atkinson C, Evans ZP, Amani Z, Balish E, Schmid MG, von Rooijen N, Schnellmann RG, Chavin KD. Murine Kupffer cells are protective in toal hepatic ischemia/reperfusion injury with bowel congestion through IL-10. J Immunol. 2010;184:5849–5858. doi: 10.4049/jimmunol.0902024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Elrod JW, Duranski MR, Langston W, Greer JJ, Tao L, Dugas TR, Kevil CG, Champion HC, Lefer DJ. eNOS gene therapy exacerbates hepatic ischemia-reperfusion injury in diabetes: A role for eNOS uncoupling. Circ Res. 2006;99:78–85. doi: 10.1161/01.RES.0000231306.03510.77. [DOI] [PubMed] [Google Scholar]
- 214.Elvington A, Atkinson C, Zhu H, Yu J, Takahashi K, Stahl GL, Kindy MS, Tomlinson S. The alternative complement pathway propagates inflammation and injury in murine ischemic stroke. J Immunol. 2012;189:4640–4647. doi: 10.4049/jimmunol.1201904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Elzey BD, Tian J, Jensen RJ, Swanson AK, Lees JR, Lentz SR, Stein CS, Neiswandt B, Wang Y, Davidson BL, Ratliff TL. Platelet-mediated modulation of adaptive immunity. A communication link between innate and adaptive immune compartments. Immunity. 2003;19:9–19. doi: 10.1016/s1074-7613(03)00177-8. [DOI] [PubMed] [Google Scholar]
- 216.Endres M, Ahmadi M, Kruman I, Biniszkiewicz D, Meisel A, Gertz K. Folate deficiency increases postischemic brain injury. Stroke. 2005;36:321–325. doi: 10.1161/01.STR.0000153008.60517.ab. [DOI] [PubMed] [Google Scholar]
- 217.Endres M, Fan G, Meisel A, Dirnagl U, Jaenisch R. Effects of cerebral ischemia in mice lacking DNA methyltransferase 1 in post-mitotic neurons. Neuroreport. 2001;12:3763–6. doi: 10.1097/00001756-200112040-00032. [DOI] [PubMed] [Google Scholar]
- 218.Endres M, Meisel A, Biniszkiewicz D, Namura S, Prass K, Ruscher K, Lipski A, Jaenisch R, Moskowitz MA, Dirnagl U. DNA methyltransferase contributes to delayed ischemic brain injury. J Neurosci. 2000;20:3175–3181. doi: 10.1523/JNEUROSCI.20-09-03175.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Engler RL, Schmid-Schonbein GW, Pavelec RS. Leukocyte capillary plugging in myocardial ischemia and reperfusion in the dog. Am J Pathol. 1983;111:98–111. [PMC free article] [PubMed] [Google Scholar]
- 220.Entman ML, Youker K, Shoji T, Kukielka G, Shappell SB, Taylor AA, Smith CW. Neutrophil induced oxidative injury of cardiac myocytes. A compartmented system requiring CD11b/CD18-ICAM-1 adherence. J Clin Invest. 1992;90:1335–1345. doi: 10.1172/JCI115999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Erickson JR, Joiner MA, Guan X, Kurtschke W, Yang J, Oddis CV, Bartlett RK, Lowe JS, O’Donnell SE, Aykin-Burns N, Zimmerman MC, Zimmerman K, Ham A-JL, Weiss RM, Spitz DR, Shea MA, Colbran RJ, Mohler PJ, Anderson ME. A dynamic pathway for calciumin-dependent activation of CaMKII by methionine oxidation. Cell. 2008;133:462–474. doi: 10.1016/j.cell.2008.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Erusalimsky JD, Moncada S. Nitric oxide and mitochondrial signaling: From physiology to pathophysiology. Arteroscler Thromb Vasc Biol. 2007;27:2524–2531. doi: 10.1161/ATVBAHA.107.151167. [DOI] [PubMed] [Google Scholar]
- 223.Esch JS, Jurk K, Knoefel WT, Roeder G, Voss H, Tustas RY, Schmelzle M, Krieg A, Eisenberger CF, Topp S, Rogiers X, Fischer L, Aken HV, Kehrel BE. Platelet activation and increased tissue factor expression on monocytes in reperfusion injury following orthotopic liver transplantation. Platelets. 2010;21:348–359. doi: 10.3109/09537101003739897. [DOI] [PubMed] [Google Scholar]
- 224.Esme H, Fidan H, Koken T, Solak O. Effect of lung ischemia-reperfusion on oxidative stress parameters of remote tissues. Eur J CardioCardio-Thoracic Surg. 2006;29:294–298. doi: 10.1016/j.ejcts.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 225.Ettinger G, MacDonald K, Reid G, Burton JP. The influence of the human microbiome and probiotics on cardiovascular Health. Gut Microbes. 2014;5:719–728. doi: 10.4161/19490976.2014.983775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Fagundes CT, Amaral FA, Teixeira AL, Souza DG, Teixeira MM. Adapting to environmental stresses: The role of the microbiota in controlling innate immunity and behavioral responses. Immunol Rev. 2012;245:250–264. doi: 10.1111/j.1600-065X.2011.01077.x. [DOI] [PubMed] [Google Scholar]
- 227.Faith JJ, Guruge JL, Charbonneau M, Subramanian S, Seedorf H, Goodman AL, Clemente JC, Knight R, Heath AC, Leibel RL, Rosenbaum M, Gordon JI. The long-term stability of the human gut microbiota. Science. 2013;341:1237439. doi: 10.1126/science.1237439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Fan M, Du L, Stone AA, Gilbert KM, Chambers TC. Modulation of mitogen-activated protein kinases and phosphorylation of Bcl-2 by vinblastine represent persistent forms of normal fluctuations at G2-M1. Cancer Res. 2000;60:6403–6407. [PubMed] [Google Scholar]
- 229.Fang J, Song XW, Tian J, Chen HY, Li DF, Wang JF, Ren AJ, Yuan WJ, Lin L. Overexpression of microRNA-378 attenuates ischemia-induced apoptosis by inhibiting caspase-3 expression in cardiac myocytes. Apoptosis. 2012;17:410–423. doi: 10.1007/s10495-011-0683-0. [DOI] [PubMed] [Google Scholar]
- 230.Faraco G, Pancani T, Formentini L, Mascagni P, Fossati G, Leoni F, Moroni F, Chiarugi A. Pharmacological inhibition of histone deacetylases by suberoylanilide hydroxamic acid specifically alters gene expression and reduces ischemic injury in the mouse brain. Mol Pharmacol. 2006;70:1876–1884. doi: 10.1124/mol.106.027912. [DOI] [PubMed] [Google Scholar]
- 231.Fasanaro P, Greco S, Ivan M, Capogrossi MC, Martelli F. microRNA: Emerging therapeutic targets in acute ischemic diseases. Pharmacol Ther. 2010;125:92–104. doi: 10.1016/j.pharmthera.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 232.Fatokun AA, Dawson VL, Dawson TM. Parthanatos: Mitochondrial-linked mechanisms and therapeutic opportunities. Br J Pharmacol. 2014;171:2000–2016. doi: 10.1111/bph.12416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Fauman EB, Saper MA. Structure and function of the protein tyrosine phosphatases. Trends Biochem Sci. 1996;21:413–417. doi: 10.1016/s0968-0004(96)10059-1. [DOI] [PubMed] [Google Scholar]
- 234.Fayaz SM, Suvanish Kumar VS, Davis CK, Rajanikant GK. Novel RIPK3 inhibitors discovered through a structure-based approach exert post-ischemic neuroprotection. Mol Divers. 2016;20:719–728. doi: 10.1007/s11030-016-9663-1. [DOI] [PubMed] [Google Scholar]
- 235.Fayaz SM, Suvanish Kumar VS, Rajanikant GK. Necroptosis: Who knew there were so many interesting ways to die? CNS Neurol Disord Drug Targets. 2014;13:42–51. doi: 10.2174/18715273113126660189. [DOI] [PubMed] [Google Scholar]
- 235a.Felger JC, Abe T, Kaunzner UW, Gottfried-Blackmore A, Gal-Toth J, McEwen BS, Iadecola C, Bulloch K. Brain dendritic cells in ischemic stroke: Time course, activation state, and origin. Brain Behav Immun. 2010;24:724–737. doi: 10.1016/j.bbi.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Feng M, Wang H, Wang Q, Guan W. Matrix metalloprotease 9 promotes liver recovery from ischemia and reperfusion injury. J Surg Res. 2013;180:156–161. doi: 10.1016/j.jss.2012.09.042. [DOI] [PubMed] [Google Scholar]
- 237.Ferdinandy P, Schulz R, Baxter GF. Interaction of cardiovascular risk factors with myocardial ischemia/reperfusion injury, preconditioning, and postconditioning. Pharmacol Rev. 2007;59:418–458. doi: 10.1124/pr.107.06002. [DOI] [PubMed] [Google Scholar]
- 238.Fernandez-Klett F, Offenhauser N, Dirnagl U, Priller J, Lindauer U. Pericytes in capillaries are contractile in vivo, but arterioles mediate functional hyperemia in the mouse brain. Proc Natl Acad Sci USA. 2010;107:22290–22295. doi: 10.1073/pnas.1011321108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Ferran C, Millan MT, Csizmadia V, Cooper JT, Brostjan C, Bach FH, Winkler H. Inhibition of NF-kappa B by pyrrolidine dithiocarbamate blocks endothelial cell activation. Biochem Biophys Res Comm. 1995;214:212–223. doi: 10.1006/bbrc.1995.2277. [DOI] [PubMed] [Google Scholar]
- 240.Ferraro FJ, Rush BF, Jr, Simonian GT, Bruce CJ, Murphy TF, Hsieh JT, Klein K, Condon M. A comparison of survival at different degrees of hemorrhagic shock in germ-free and germ-bearing rats. Shock. 1995;4:117–120. doi: 10.1097/00024382-199508000-00007. [DOI] [PubMed] [Google Scholar]
- 241.Festjens N, Vanden Berghe T, Cornelis S, Vandenabeele P. RIP1, a kinase on the crossroads of a cell’s decision to live or die. Cell Death Differ. 2007;14:400–410. doi: 10.1038/sj.cdd.4402085. [DOI] [PubMed] [Google Scholar]
- 242.Finsterbusch M, Voisin MB, Beyrau M, Williams TJ, Nourshargh S. Neutrophils recruited by chemoattractants in vivo induce microvascular plasma protein leakage through secretion of TNF. J Exp Med. 2014;211:1307–1314. doi: 10.1084/jem.20132413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Fleming I, Michaelis UR, Bredenkötter D, Fisslthaler B, Dehghani F, Brandes RP, Busse R. Endothelium-derived hyperpolarizing factor synthase (cytochrome P450 2C9) is a functionally significant source of reactive oxygen species in coronary arteries. Circ Res. 2001;88:44–51. doi: 10.1161/01.res.88.1.44. [DOI] [PubMed] [Google Scholar]
- 244.Folino A, Losano G, Rastaldo R. Balance of nitric oxide and reactive oxygen species in myocardial reperfusion injury and protection. J Cardiovasc Pharmacol. 2013;62:567–575. doi: 10.1097/FJC.0b013e3182a50c45. [DOI] [PubMed] [Google Scholar]
- 245.Ford DA. Lipid oxidation by hypochlorous acid: Chlorinated lipids in atherosclerosis and myocardial ischemia. Clin Lipidol. 2010;5:835–852. doi: 10.2217/clp.10.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Forman HJ, Fukoto JM, Miller T, Zhang H, Rinna A, Levy S. The chemistry of cell signaling by reactive oxygen and nitrogen species and 4-hydroxynonenal. Arch Biochem Biophys. 2008;477:183–195. doi: 10.1016/j.abb.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Forman HJ, Torre M, Fukoto J. Redox signaling. Mol Cell Biochem. 2002;234:49–62. [PubMed] [Google Scholar]
- 248.Foster MW, Hess DT, Stamler JS. Protein S-nitrosylation in health and disease: A current perspective. Trends Mol Med. 2009;15:391–404. doi: 10.1016/j.molmed.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Frangogiannis NG. Inflammation in cardiac injury, repair and regeneration. Curr Opin Cardiol. 2015;30:240–245. doi: 10.1097/HCO.0000000000000158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Frangogiannis NG. The immune system and cardiac repair. Pharmacol Res. 2008;58:88–111. doi: 10.1016/j.phrs.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Friedmann Angeli JP, Schneider M, Proneth B, Tyurina YY, Tyurin VA, Hammond VJ, Herbach N, Aichler M, Walch A, Eggenhofer E, Basavarajappa D, Radmark O, Kobayashi S, Seibt T, Beck H, Neff F, Esposito I, Wanke R, Forster H, Yefremova O, Heinrichmeyer M, Bornkamm GW, Geissler EK, Thomas SB, Stockwell BR, O’Donnell VB, Kagan VE, Schick JA, Conrad M. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat Cell Biol. 2014;16:1180–1191. doi: 10.1038/ncb3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Fritzinger DC, Hew BE, Lee JQ, Newhouse J, Alam M, Ciallella JR, Bowers M, Gorsuch WB, Guikema BJ, Stahl GL, Vogel CW. Derivatives of human complement component C3 for therapeutic complement depletion: A novel class of therapeutic agents. Adv Exp Med Biol. 2008;632:293–307. [PubMed] [Google Scholar]
- 253.Frost RJ, van Rooij E. miRNAs as therapeutic targets in ischemic heart disease. J Cardiovasc Transl Res. 2010;3:280–289. doi: 10.1007/s12265-010-9173-y. [DOI] [PubMed] [Google Scholar]
- 254.Fulda S. Regulation of necroptosis signaling and cell death by reactive oxygen species. Biol Chem. 2016;397:657–660. doi: 10.1515/hsz-2016-0102. [DOI] [PubMed] [Google Scholar]
- 255.Gaboury JP, Johnston B, Niu X, Kubes P. Mechanisms nderlying acute mast cell-induced leukocyte rolling and adhesion in vivo. J Immunol. 1995;154:804–813. [PubMed] [Google Scholar]
- 256.Galinanes M, Hearse DJ. Species differences in susceptibility to ischemic injury and responsiveness to myocardial protection. Cardioscience. 1990;1:127–143. [PubMed] [Google Scholar]
- 257.Galli SJ, Gordon JR, Wershil BK. Cytokine production by mast cells and basophils. Curr Opin Immunol. 1991;3:865–872. doi: 10.1016/s0952-7915(05)80005-6. [DOI] [PubMed] [Google Scholar]
- 258.Galli SJ, Kalesnikoff J, Grimbaldeston A, Piliponsky A, Williams C, Tsai M. Mast cellsare “tunable’ effector and immunoregulatory cells: Recent advances. Annu Rev Immunol. 2005;23:749–786. doi: 10.1146/annurev.immunol.21.120601.141025. [DOI] [PubMed] [Google Scholar]
- 259.Galluzzi L, Kepp O, Krautwald S, Kroemer G, Linkermann A. Molecular mechanisms of regulated necrosis. Semin Cell Dev Biol. 2014;35:24–32. doi: 10.1016/j.semcdb.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 260.Gan XT, Ettinger G, Huang CX, Burton JP, Haist JV, Rajapurohitam V, Sidaway JE, Martin G, Gloor GB, Swann JR, Reid G, Karmazyn M. Probiotic administration attenuates myocardial hypertrophy and heart failure after myocardial infarction in the rat. Circ Heart Fail. 2014;7:491–499. doi: 10.1161/CIRCHEARTFAILURE.113.000978. [DOI] [PubMed] [Google Scholar]
- 261.Gandalfo MT, Jang HR, Bagnasco SM, Ko GJ, Agreda P, Satpute SR, Crow MT, King LS, Rabb H. Foxp3 +regulatory T cells participate in repair of ischemic acute kidney injury. Kidney Int. 2009;76:717–729. doi: 10.1038/ki.2009.259. [DOI] [PubMed] [Google Scholar]
- 262.Gao X, Zhang H, Belmadani S, Wu J, Xu X, Elford H, Potter BJ, Zhang C. Role of TNF-alpha-induced reactive oxygen species in endothelial dysfunction during reperfusion injury. Am J Physiol Heart Circ Physiol. 2008;295:H2242–2249. doi: 10.1152/ajpheart.00587.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Gawaz M. Role of platelets in coronary thrombosis and reperfusion of ischemic myocardium. Cardiovasc Res. 2004;61:498–511. doi: 10.1016/j.cardiores.2003.11.036. [DOI] [PubMed] [Google Scholar]
- 264.Ge L, Zhou X, Ji WJ, Lu RY, Zhang Y, Zhang YD, Ma YQ, Zhao JH, Li YM. Neutrophil extracellular traps in ischemia-reperfusion injury-induced myocardial no-reflow: Therapeutic potential of DNase-based reperfusion strategy. Am J Physiol Heart Circ Physiol. 2015;308:H500–H509. doi: 10.1152/ajpheart.00381.2014. [DOI] [PubMed] [Google Scholar]
- 265.Genken E, Buis CI, Visser DS, Blokzijl H, Moshage H, Nemes B, Leuvenink HG, de Jong KP, Peeters PM, Slooff MJ, Porte RJ. Expression of heme oxygenase-1 in human livers before transplantation correlates with graft injury and function after transplantation. Am J Transplant. 2005;5:1875–1885. doi: 10.1111/j.1600-6143.2005.00960.x. [DOI] [PubMed] [Google Scholar]
- 266.George FD. Microparticles in vascular diseases. Thromb Res. 2008;122:555–559. doi: 10.1016/S0049-3848(08)70020-3. [DOI] [PubMed] [Google Scholar]
- 267.Gerszten RE, Asnani A, Carr SA. Status and prospects for discovery and verification of new biomarkers of cardiovascular disease by proteomics. Circ Res. 2011;109:463–474. doi: 10.1161/CIRCRESAHA.110.225003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Giakoustidis DE, Iliadis S, Tsantilas D, Papageorgiou G, Kontos N, Kostopoulou E, Botsoglou NA, Gerasimidis T, Dimitriadou A. Blockade of Kupffer cells by gadolinium chloride reduces lipid peroxidation and protects liver from ischemia/reperfusion injury. Hepatogastroenterology. 2003;50:1587–1592. [PubMed] [Google Scholar]
- 268a.Giussani DA, Camm EJ, Niu Y, Richter HG, Blanco CE, Gottschalk R, Blake EZ, Horder KA, Thakor AS, Hansell JA, Kane AD, Wooding FB, Cross CM, Herrera EA. Developmental programming of cardiovascular dysfunction by prenatal hypoxia and oxidative stress. PLoS One. 2012;7:e31017. doi: 10.1371/journal.pone.0031017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268b.Giussani DA, Davidge ST. Developmental programming of cardiovascular disease by prenatal hypoxia. J Develop Origins Health Dis. 2013;4:328–337. doi: 10.1017/S204017441300010X. [DOI] [PubMed] [Google Scholar]
- 269.Giedt RJ, Yang C, Zweier JL, Matzavinos A, Alevriadou BR. Mitochondrial fission in endothelial cells after simulated ischemia/reperfusion: Role of nitric oxide and reactive oxygen species. Free Radic Biol Med. 2012;52:348–356. doi: 10.1016/j.freeradbiomed.2011.10.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Glancy B, Hartnell LM, Malide D, Yu ZX, Combs CA, Connelly PS, Subramaniam S, Balaban RS. Mitochondrial reticulum for cellular energy distribution in muscle. Nature. 2015;523:617–620. doi: 10.1038/nature14614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008;103:1204–1219. doi: 10.1161/CIRCRESAHA.108.176826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Go YM, Jones DP. Intracellular proatherogenic events and cell adhesion modulated by extracellular thiol/disulfide redox state. Circulation. 2005;111:2973–2980. doi: 10.1161/CIRCULATIONAHA.104.515155. [DOI] [PubMed] [Google Scholar]
- 273.Go YM, Jones DP. Redox compartmentalization in eukaryotic cells. Biochim Biophys Acta. 2008;1780:1273–1290. doi: 10.1016/j.bbagen.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Go YM, Park H, Koval M, Orr M, Reed M, Liang Y, Smith D, Pohl J, Jones DP. A key role for mitochondria in endothelial signaling by plasma cysteine/cystine redox potential. Free Radic Biol Med. 2010;48:275–283. doi: 10.1016/j.freeradbiomed.2009.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Goda N, Suzuki K, Naito M, Takeoka S, Tsuchida E, Ishimura Y, Tamatani T, Suematsu M. Distribution of heme oxygenase isoforms in rat liver. Topographic basis for carbon monoxide-mediated microvascular relaxation. J Clin Invest. 1998;101:604–612. doi: 10.1172/JCI1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Godoy LC, Moretti AI, Jurado MC, Oxer D, Janiszewski M, Ckless K, Velasco IT, Laurindo FR, Souza HP. Loss of CD40 endogenous S-nitrosylation during inflammatory response in endotoxemic mice and patients with sepsis. Shock. 2010;33:626–633. doi: 10.1097/SHK.0b013e3181cb88e6. [DOI] [PubMed] [Google Scholar]
- 277.Godwin JG, Ge X, Stephan K, Jurisch A, Tullius SG, Iacomini J. Identification of a microRNA signature of renal ischemia reperfusion injury. Proc Natl Acad Sci U S A. 2010;107:14339–14344. doi: 10.1073/pnas.0912701107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Goldman G, Welbourn R, Klausne JM, Kobzik L, Valeri CR, Shepro D, Hechtman HB. Mast cells and leukotrienes mediate neutrophil sequestration and lung edema after remote ischemia in rodents. Surgery. 1992;12:578–586. [PubMed] [Google Scholar]
- 279.Golwala NH, Hodenette C, Murthy SN, Nossaman BD, Kadowitz PJ. Vascular responses to nitrite are mediated by xanthine oxidoreductase and mitochondrial aldehyde dehydrogenase in the rat. Can J Physiol Pharmacol. 2009;87:1095–1101. doi: 10.1139/Y09-101. [DOI] [PubMed] [Google Scholar]
- 280.Gonzalez LM, Moeser AJ, Blikslager AT. Animal models of ischemia-reperfusion-induced intestinal injury: Progress and promise for translational research. Am J Physiol Gastrointest Liver Physiol. 2015;308:G63–75. doi: 10.1152/ajpgi.00112.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Goretti E, Wagner DR, Devaux Y. miRNAs as biomarkers of myocardial infarction: A step forward towards personalized medicine? Trends Mol Med. 2014;20:716–725. doi: 10.1016/j.molmed.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 282.Gorsuch WB, Chrysanthou E, Schwaeble WJ, Stahl GL. The complement system in ischemia-reperfusion injuries. Immunobiology. 2012;217:1026–1033. doi: 10.1016/j.imbio.2012.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Gottlieb RA. Cytochrome P450: Major player in reperfusion injury. Arch Biochem Biophys. 2003;420:262–267. doi: 10.1016/j.abb.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 284.Gottlieb RA, Gustafsson AB. Mitochondrial turnover in the heart. Biochim Biophys Acta. 2011;1813:1295–1301. doi: 10.1016/j.bbamcr.2010.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Gottlieb RA, Mentzer RM. Autophagy during cardiac stress: Joys and frustrations of autophagy. Annu Rev Physiol. 2010;72:45–59. doi: 10.1146/annurev-physiol-021909-135757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Gould TJ, Vu TT, Swystun LL, Dwivedi DJ, Mai SH, Weitz JI, Liaw PC. Neutrophil extracellular traps promote thrombin generation through platelet-dependent and platelet-independent mechanisms. Arterioscler Thromb Vasc Biol. 2014;34:1977–1984. doi: 10.1161/ATVBAHA.114.304114. [DOI] [PubMed] [Google Scholar]
- 287.Gourdin MJ, Bree B, De Kock M. The impact of ischaemia-reperfusion on the blood vessel. Eur J Anaesthesiol. 2009;26:537–547. doi: 10.1097/EJA.0b013e328324b7c2. [DOI] [PubMed] [Google Scholar]
- 288.Gracanin M, Lam MA, Morgan PE, Rodgers KJ, Hawkins CL, Davies MJ. Amino acid, peptide, and protein hydroperoxides and their decomposition products modify the activity of the 26S proteasome. Free Radic Biol Med. 2011;50:389–399. doi: 10.1016/j.freeradbiomed.2010.11.019. [DOI] [PubMed] [Google Scholar]
- 289.Granger A, Abdullah I, Huebner F, Stout A, Wang T, Huebner T, Epstein JA, Gruber PJ. Histone deacetylase inhibition reduces myocardial ischemia-reperfusion injury in mice. FASEB J. 2008;22:3549–3560. doi: 10.1096/fj.08-108548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Granger DN. Role of xanthine oxidase and granulocytes in ischemia-reperfusion injury. Am J Physiol. 1988;255:H1269–H1275. doi: 10.1152/ajpheart.1988.255.6.H1269. [DOI] [PubMed] [Google Scholar]
- 291.Granger DN. Ischemia-reperfusion: Mechanisms of microvascular dysfunction and the influence of risk factors for cardiovascular disease. Microcirculation. 1999;6:167–178. [PubMed] [Google Scholar]
- 292.Granger DN, Korthuis RJ. Physiologic mechanisms of postischemic tissue injury. Ann Rev Physiol. 1996;57:311–332. doi: 10.1146/annurev.ph.57.030195.001523. [DOI] [PubMed] [Google Scholar]
- 293.Granger DN, Kvietys PR. Reperfusion injury and reactive oxygen species: The evolution of a concept. Redox Biol. 2015;6:524–551. doi: 10.1016/j.redox.2015.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Granger DN, Rutili G, McCord JM. Superoxide radicals in feline intestinal ischemia. Gastroenterology. 1981;81:22–29. [PubMed] [Google Scholar]
- 295.Granger DN, Stokes KY, Shigematsu T, Cerwinka WH, Tailor A, Krieglstein CF. Splanchnic ischaemia-reperfusion injury: Mechanistic insights provided by mutant mice. Acta Physiol Scand. 2001;173:83–91. doi: 10.1046/j.1365-201X.2001.00888.x. [DOI] [PubMed] [Google Scholar]
- 296.Granville DJ, Tashakkor B, Takeuchi C, Gustafsson ÅB, Sayen MR, Wentworth P, Jr, Yeager M, Gottlieb RA. Reduction of ischemia and reperfusion-induced myocardial damage by cytochrome P450 inhibitors. Proc Natl Acad Sci U S A. 2004;101:1321–1326. doi: 10.1073/pnas.0308185100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Greco S, De Simone M, Colussi C, Zaccagnini G, Fasanaro P, Pescatori M, Cardani R, Perbellini R, Isaia E, Sale P, Meola G, Capogrossi MC, Gaetano C, Martelli F. Common micro-RNA signature in skeletal muscle damage and regeneration induced by Duchenne muscular dystrophy and acute ischemia. FASEB J. 2009;23:3335–3346. doi: 10.1096/fj.08-128579. [DOI] [PubMed] [Google Scholar]
- 298.Gregory JC, Buffa JA, Org E, Wang Z, Levison BS, Zhu W, Wagner MA, Bennett BJ, Li L, DiDonato JA, Lusis AJ, Hazen SL. Transmission of atherosclerosis susceptibility with gut microbial transplantation. J Biol Chem. 2015;290:5647–5660. doi: 10.1074/jbc.M114.618249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Grimbaldeston MA, Nakae S, Kalesnikoff J, Tsai M, Galli S. Mast cell-derived interleukin 10 limits skin pathology in contact dermatitis and chronic irradiation with ultraviolet B. Nature Immunol. 2007;8:1095–1104. doi: 10.1038/ni1503. [DOI] [PubMed] [Google Scholar]
- 300.Grisham MB, Jourd’Heuil D, Wink DA. Nitric oxide. I. Physiological chemistry of nitric oxide and its metabolites: Implications in inflammation. Am J Physiol. 1999;276:G315–G321. doi: 10.1152/ajpgi.1999.276.2.G315. [DOI] [PubMed] [Google Scholar]
- 301.Grootjans J, Lenaerts K, Derikx JP, Matthijsen RA, de Bruine AP, van Bijnen AA, van Dam RM, Dejong CH, Buurman WA. Human intestinal ischemia-reperfusion-induced inflammation characterized: Experiences from a new translational model. Am J Pathol. 2010;176:2283–2291. doi: 10.2353/ajpath.2010.091069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Gross GJ, Falk JR, Gross ER, Isbell M, Moore J, Nithipatikom K. Cytochrome P450 and arachidonic acid metabolites: Role in myocardial ischemia/reperfusion injury revisited. Cardiovasc Res. 2005;68:18–25. doi: 10.1016/j.cardiores.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 303.Grover GJ, Atwal KS, Sleph PG, Wang FL, Monshizadegan H, Monticello T, Green DW. Excessive ATP hydrolysis in ischemic myocardium by mitochondrial F1F0-ATPase: Effect of selective pharmacological inhibition of mitochondrial ATPase hydrolase activity. Am J Physiol Heart Circ Physiol. 2004;287:H1747–H1755. doi: 10.1152/ajpheart.01019.2003. [DOI] [PubMed] [Google Scholar]
- 304.Grueter CE, Abiria SA, Dzhura I, Wu Y, Ham AJ, Mohler PJ, Anderson ME, Colbran RJ. L-type Ca2+ channel facilitation mediated by phosphorylation of the b subunit by CaMKII. Mol Cell. 2006;23:641–650. doi: 10.1016/j.molcel.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 305.Guan QH, Pei DS, Zhang QG, Hao ZB, Xu TL, Zhang GY. The neuroprotective action of SP600125, a new inhibitor of JNK, on transient brain ischemia/reperfusion-induced neuronal death in rat hippocampal CA1 via nuclear and non-nuclear pathways. Brain Res. 2005;1035:51–59. doi: 10.1016/j.brainres.2004.11.050. [DOI] [PubMed] [Google Scholar]
- 306.Gundewar S, Calvert JW, Elrod JW, Lefer DJ. Cytoprotective effects of N,N,N-trimethylsphingosine during ischemia- reperfusion injury are lost in the setting of obesity and diabetes. Am J Physiol Heart Circ Physiol. 2007;293:H2462–H2471. doi: 10.1152/ajpheart.00392.2007. [DOI] [PubMed] [Google Scholar]
- 307.Guo G, Bhat NR. p38a MAP kinase mediates hypoxia-induced motor neuron cell death: A potential target of minocycline’s neuroprotective action. Neurochem Res. 2007;32:2160–6. doi: 10.1007/s11064-007-9408-8. [DOI] [PubMed] [Google Scholar]
- 308.Guo Y, Flaherty MP, Wu WJ, Tan W, Zhu X, Li Q, Bolli R. Genetic background, gender, age, body temperature, and arterial blood pH have a major impact on myocardial infarct size in the mouse and need to be carefully measured and/or taken into account: Results of a comprehensive analysis of determinants of infarct size in 1,074 mice. Basic Res Cardiol. 2012;107:288. doi: 10.1007/s00395-012-0288-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Gurusamy N, Lekli I, Gherghiceanu M, Popescu LM, Das DK. BAG-1 induces autophagy for cardiac cell survival. Autophagy. 2009;5:120–121. doi: 10.4161/auto.5.1.7303. [DOI] [PubMed] [Google Scholar]
- 310.Gute DC, Ishida T, Yarimizu K, Korthuis RJ. Inflammatory responses to ischemia and reperfusion in skeletal muscle. Mol Cell Biochem. 1998;179:169–187. doi: 10.1023/a:1006832207864. [DOI] [PubMed] [Google Scholar]
- 311.Gute D, Korthuis RJ. Role of leukocyte adherence in reperfusion-induced microvascular dysfunction and tissue injury. In: Granger DN, Schmid-Schönbein GW, editors. Leukocyte Adhesion. New York, NY: Oxford Uuniversity Press; 1995. [Google Scholar]
- 312.Gysembergh A, Margonari H, Loufoua J, Ovize A, Andre-Fouet X, Minaire Y, Ovize M. Stretch-induced protection shares a common mechanism with ischemic preconditioning in rabbit heart. Am J Physiol. 1998;274:H955–H964. doi: 10.1152/ajpheart.1998.274.3.H955. [DOI] [PubMed] [Google Scholar]
- 313.Hacke W, Donnan G, Fieschi C, Kaste M, von Kummer R, Broderick JP, Brott T, Frankel M, Grotta JC, Haley EC, Jr, Kwiatkowski T, Levine SR, Lewandowski C, Lu M, Lyden P, Marler JR, Patel S, Tilley BC, Albers G, Bluhmki E, Wilhelm M, Hamilton S. Association of outcome with early stroke treatment: Pooled analysis of ATLANTIS, ECASS, and NINDS rt-PA stroke trials. Lancet. 2004;363:768–74. doi: 10.1016/S0140-6736(04)15692-4. [DOI] [PubMed] [Google Scholar]
- 314.Haheim LL, Olsen I, Ronningen KS. Oral infection, regular alcohol drinking pattern, and myocardial infarction. Med Hypotheses. 2012;79:725–730. doi: 10.1016/j.mehy.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 315.Hales CN, Barker DJ. The thrifty phenotype hypothesis. Br Med Bull. 2001;60:5–20. doi: 10.1093/bmb/60.1.5. [DOI] [PubMed] [Google Scholar]
- 316.Halestrap AP. A pore way to die: The role of mitochondria in reperfusion injury and cardioprotection. Biochem Soc Trans. 2010;38:841–860. doi: 10.1042/BST0380841. [DOI] [PubMed] [Google Scholar]
- 317.Halestrap AP. What is the mitochondrial permeability transition pore? J Mol Cell Cardiol. 2009;46:821–31. doi: 10.1016/j.yjmcc.2009.02.021. [DOI] [PubMed] [Google Scholar]
- 318.Hall CN, Reynell C, Gesslein B, Hamilton NB, Mishra A, Sutherland BA, O’Farrell FM, Buchan AM, Lauritzen M, Attwell D. Capillary pericytes regulate cerebral blood flow in health and disease. Nature. 2014;508:55–60. doi: 10.1038/nature13165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Haller H, Kunzendorf U, Sacherer K, Lindschau C, Walz G, Distler A, Luft FC. T cell adhesion to P-selectin induces tyrosine phosphorylation of pp125 focal adhesion kinase and other substrates. J Immunol. 1997;158:1061–1067. [PubMed] [Google Scholar]
- 320.Hamacher-Brady A, Brady NR, Logue SE, Sayen MR, Jinno M, Kirshenbaum LA, Gottlieb RA, Gustafsson AB. Response to myocardial ischemia/reperfusion injury involves Bnip3 and autophagy. Cell Death Differ. 2007;14:146–157. doi: 10.1038/sj.cdd.4401936. [DOI] [PubMed] [Google Scholar]
- 321.Hamilton NB, Attwell D, Hall CN. Pericyte-mediated regulation of capillary diameter: A component of neurovascular coupling in health and disease. Front Neuroenergetics. 2010:5. doi: 10.3389/fnene.2010.00005. pii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Hanidziar D, Koulmanda M. Inflammation and the balance of Treg and Th17 cells in transplant rejection and tolerance. Curr Opin Organ Transplant. 2010;15:411–415. doi: 10.1097/MOT.0b013e32833b7929. [DOI] [PubMed] [Google Scholar]
- 323.Hanschen M, Zahler S, Krombach F, Khandoga A. Reciprocal activation between CD4+ T cells and Kupffer cells during hepatic ischemia-reperfusion. Transplant. 2008;86:710–718. doi: 10.1097/TP.0b013e3181821aa7. [DOI] [PubMed] [Google Scholar]
- 324.Hanson MA, Gluckman PD. Early developmental conditioning of later health and disease: Physiology or pathophysiology? Physiol Rev. 2014;94:1027–1076. doi: 10.1152/physrev.00029.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Harding SJ, Browne GJ, Miller BW, Prigent SA, Dickens M. Activation of ASK1, downstream MAPKK and MAPK isoforms during cardiac ischaemia. Biochim Biophys Acta. 2010;1802:733–740. doi: 10.1016/j.bbadis.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Hariharan N, Zhai P, Sadoshima J. Oxidative stress stimulates autophagic flux during ischemia/reperfusion. Antioxid Redox Signal. 2011;14:2179–2190. doi: 10.1089/ars.2010.3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Hausenloy DJ, Yellon DM. Survival kinases in ischemic preconditioning and postconditioning. Cardiovasc Res. 2006;70:240–253. doi: 10.1016/j.cardiores.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 327a.Hauton D. Hypoxia in early pregnancy induces cardiac dysfunction in adult offspring of Rattus norvegicus, a non-hypoxia-adapted species. Comp Biochem Physiol A Mol Integr Comp Physiol. 2012;163:278–285. doi: 10.1016/j.cbpa.2012.07.020. [DOI] [PubMed] [Google Scholar]
- 327b.Hauton D, Ousley V. Prenatal hypoxia induces increased cardiac contractility on a background of decreased capillary density. BMC Cardivasc Disord. 2009;9:1. doi: 10.1186/1471-2261-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Hausenloy DJ, Yellon DM. Myocardial ischemia-reperfusion injury: A neglected therapeutic target. J Clin Invest. 2013;123:92–100. doi: 10.1172/JCI62874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Hausenloy DJ, Yellon DM, Mani-Babu S, Duchen MR. Preconditioning protects by inhibiting the mitochondrial permeability transition. Am J Physiol Heart Circ Physiol. 2004;287:H841–849. doi: 10.1152/ajpheart.00678.2003. [DOI] [PubMed] [Google Scholar]
- 330.He B, Xiao J, Ren AJ, Zhang YF, Zhang H, Chen M, Xie B, Gao XG, Wang YW. Role of miR-1 and miR-133a in myocardial ischemic postconditioning. J Biomed Sci. 2011;18:22. doi: 10.1186/1423-0127-18-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.He C, Klionsky DJ. Regulation mechanisms and signaling pathways of autophagy. Annu Rev Genet. 2009;43:67–93. doi: 10.1146/annurev-genet-102808-114910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.He GZ, Dong LG, Chen XF, Zhou KG, Shu H. Lymph duct ligation during ischemia/reperfusion prevents pulmonary dysfunction in a rat model with omega-3 polyunsaturated fatty acid and glutamine. Nutrition. 2011;27:604–614. doi: 10.1016/j.nut.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 333.He S, Wang L, Miao L, Wang T, Du F, Zhao L, Wang X. Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-alpha. Cell. 2009;137:1100–11. doi: 10.1016/j.cell.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 334.Hehner SP, Breitkreutz R, Shubinsky G, Unsoeld H, Shulze-Osthoff K, Schmitz ML, Dröge W. Enhancement of T cell receptor signaling by a mild oxidative shift in the intracellular thiol pool. J Immunol. 2000;165:4319–4328. doi: 10.4049/jimmunol.165.8.4319. [DOI] [PubMed] [Google Scholar]
- 335.Hein TW, Zhang C, Wang W, Chang CI, Thengchaisri N, Kuo L. Ischemia-reperfusion selectively impairs nitric oxide-mediated dilation in coronary arterioles: Counteracting role of arginase. FASEB J. 2003;17:2328–2330. doi: 10.1096/fj.03-0115fje. [DOI] [PubMed] [Google Scholar]
- 336.Hernandez LA, Grisham MB, Twohig B, Arfors KE, Harlan JM, Granger DN. Role of neutrophils in ischemia-reperfusion-induced microvascular injury. Am J Physiol. 1987;253:H699–H703. doi: 10.1152/ajpheart.1987.253.3.H699. [DOI] [PubMed] [Google Scholar]
- 337.Hernando V, Inserte J, Sartório CL, Parra VM, Poncelas-Nozal M, Garcia-Dorado D. Calpain translocation and activation as pharmacological targets during myocardial ischemia/reperfusion. J Mol Cell Cardiol. 2010;49:271–279. doi: 10.1016/j.yjmcc.2010.02.024. [DOI] [PubMed] [Google Scholar]
- 338.Hess DT, Stamler JS. Regulation by S-nitrosylation of protein posttranslational modification. J Biol Chem. 2012;287:4411–4418. doi: 10.1074/jbc.R111.285742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Hickey MJ, Kubes P. Role of nitric oxide in regulation of leucocyte-endothelial cell interactions. Exp Physiol. 1997;82:339–348. doi: 10.1113/expphysiol.1997.sp004029. [DOI] [PubMed] [Google Scholar]
- 340.Hill JW, Nemoto EM. Matrix-derived inflammatory mediator N-acetyl proline-glycine-proline is neurotoxic and upregulated in brain after ischemic stroke. J Neuroinflammation. 2015;12:214. doi: 10.1186/s12974-015-0428-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Hill JH, Ward PA. The phlogistic role of C3 leukotactic fragments in myocardial infarcts of rats. J Exp Med. 1971;133:885–900. doi: 10.1084/jem.133.4.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Hirsch J, Niemann CU, Hansen KC, Choi SJN, Su X, Frank JA, Fang X, Hirose R, Theodore P, Sapru A, Burlingame AL, Matthay MA. Alterations in the proteome of pulmonary alveolar type II cells in the rat after hepatic ishchemia-reperfusion. Crit Care Med. 2008;36:1846–1854. doi: 10.1097/CCM.0b013e31816f49cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Hochhauser E, Cheporko Y, Yasovich N, Pinchas L, Offen D, Barhum Y, Pannet H, Tobar A, Vidne BA, Birk E. Bax deficiency reduces infarct size and improves long-term function after myocardial infarction. Cell Biochem Biophys. 2007;47:11–20. doi: 10.1385/cbb:47:1:11. [DOI] [PubMed] [Google Scholar]
- 344.Hochhauser E, Kivity S, Offen D, Maulik N, Otani H, Barhum Y, Pannet H, Shneyvays V, Shainberg A, Goldshtaub V, Tobar A, Vidne BA. Bax ablation protects against myocardial ischemia-reperfusion injury in transgenic mice. Am J Physiol Heart Circ Physiol. 2003;284:H2351–H2359. doi: 10.1152/ajpheart.00783.2002. [DOI] [PubMed] [Google Scholar]
- 345.Hoda MN, Singh I, Singh AK, Khan M. Reduction of lipoxidative load by secretory phospholipase A2 inhibition protects against neurovascular injury following experimental stroke in rat. J Neuroinflammation. 2009;6:21. doi: 10.1186/1742-2094-6-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Hodgkinson CP, Bareja A, Gomez JA, Dzau VJ. Emerging concepts in paracrine mechanisms in regenerative cardiovascular medicine and biology. Circ Res. 2016;118:95–107. doi: 10.1161/CIRCRESAHA.115.305373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Hofmann U, Frantz S. Role of lymphocytes in myocardial injury, healing, and remodeling after myocardial infarction. Circ Res. 2015;116:354–367. doi: 10.1161/CIRCRESAHA.116.304072. [DOI] [PubMed] [Google Scholar]
- 348.Hofmann U, Frantz S. Role of T-cells in myocardial infarction. Eur Heart J. 2015;37:873–879. doi: 10.1093/eurheartj/ehv639. [DOI] [PubMed] [Google Scholar]
- 349.Holler N, Zaru R, Micheau O, Thome M, Attinger A, Valitutti S, Bodmer JL, Schneider P, Seed B, Tschopp J. Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nat Immunol. 2000;1:489–95. doi: 10.1038/82732. [DOI] [PubMed] [Google Scholar]
- 350.Holly TA, Drincic A, Byun Y, Nakamura S, Harris K, Klocke FJ, Cryns VL. Caspase inhibition reduces myocyte cell death induced by myocardial ischemia and reperfusion in vivo. J Mol Cell Cardiol. 1999;31:1709–1715. doi: 10.1006/jmcc.1999.1006. [DOI] [PubMed] [Google Scholar]
- 351.Horie Y, Ishii H. Liver dysfunction elicited by gut ischemia-reperfusion. Pathophysiology. 2001;8:11–20. doi: 10.1016/s0928-4680(01)00063-3. [DOI] [PubMed] [Google Scholar]
- 352.Horie Y, Wolf R, Russell J, Shanley TP, Granger DN. Role of Kupffer cells in gut ischemia/reperfusion-induced hepatic microvascular dysfunction in mice. Hepatology. 1997;26:1499–1505. doi: 10.1002/hep.510260617. [DOI] [PubMed] [Google Scholar]
- 353.Horstman LL, Jy W, Bidot CJ, Nordberg ML, Minagar A, Alexander JS, Kelley RE, Ahn YS. Potential roles of cell-derived microparticles in ischemic brain disease. Neurol Res. 2009;31:799–806. doi: 10.1179/016164109X12445505689526. [DOI] [PubMed] [Google Scholar]
- 354.Houlden A, Goldrick M, Brough D, Vizi ES, Lenart N, Martinecz B, Roberts IS, Denes A. Brain injury induces specific changes in the caecal microbiota of mice via altered autonomic activity and mucoprotein production. Brain Behav Immun. 2016;57:10–20. doi: 10.1016/j.bbi.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Howangyin KY, Silvestre JS. Diabetes mellitus and ischemic diseases: Molecular mechanisms of vascular repair dysfunction. Arterioscler Thromb Vasc Biol. 2014;34:1126–1135. doi: 10.1161/ATVBAHA.114.303090. [DOI] [PubMed] [Google Scholar]
- 356.Hu CJ, Chen SD, Yang DI, Lin TN, Chen CM, Huang TH, Hsu CY. Promoter region methylation and reduced expression of thrombospondin-1 after oxygen-glucose deprivation in murine cerebral endothelial cells. J Cereb Blood Flow Metab. 2006;26:1519–26. doi: 10.1038/sj.jcbfm.9600304. [DOI] [PubMed] [Google Scholar]
- 357.Hu Y, Deng H, Xu S, Zhang J. MicroRNAs regulate mitochondrial function in cerebral ischemia-reperfusion injury. Int J Mol Sci. 2015;16:24895–24917. doi: 10.3390/ijms161024895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Huang C, Andres AM, Ratliff EP, Hernandez G, Lee P, Gottlieb RA. Preconditioning involves selective mitophagy mediated by Parkin and p62/SQSTM1. PLoS One. 2011;6:e20975. doi: 10.1371/journal.pone.0020975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Huang C, Liu W, Perry CN, Yitzhaki S, Lee Y, Yuan H, Tsukada YT, Hamacher-Brady A, Mentzer RM, Jr, Gottlieb RA. Autophagy and protein kinase C are required for cardioprotection by sulfaphenazole. Am J Physiol Heart Circ Physiol. 2010;298:H570–579. doi: 10.1152/ajpheart.00716.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Huang JQ, Radinovic S, Rezaiefar P, Black SC. In vivo myocardial infarct size reduction by a caspase inhibitor administered after the onset of ischemia. Eur J Pharmacol. 2000;402:139–142. doi: 10.1016/s0014-2999(00)00477-5. [DOI] [PubMed] [Google Scholar]
- 360a.Huang Y, Rabb H, Womer KL. Ischemia-reperfusion and immediate T cell responses. Cell Immunol. 2007;248:4–11. doi: 10.1016/j.cellimm.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Huang H, Tohme S, Al-Khafaji AB, Tai S, Loughran P, Chen L, Wang S, Kim J, Billiar T, Wang Y, Tsung A. Damage-associated molecular pattern-activated neutrophil extracellular trap exacerbates sterile inflammatory liver injury. Hepatology. 2015;62:600–614. doi: 10.1002/hep.27841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Hughes BG, Schulz R. Targeting MMP-2 to treat ischemic heart injury. Basic Res Cardiol. 2014;109:424. doi: 10.1007/s00395-014-0424-y. [DOI] [PubMed] [Google Scholar]
- 363.Hullinger TG, Montgomery RL, Seto AG, Dickinson BA, Semus HM, Lynch JM, Dalby CM, Robinson K, Stack C, Latimer PA, Hare JM, Olson EN, van Rooij E. Inhibition of miR-15 protects against cardiac ischemic injury. Circ Res. 2011;110:71–81. doi: 10.1161/CIRCRESAHA.111.244442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Hulsmans M, De Keyzer D, Holvoet P. MicroRNAs regulating oxidative stress and inflammation in relation to obesity and atherosclerosis. FASEB J. 2011;25:2515–2527. doi: 10.1096/fj.11-181149. [DOI] [PubMed] [Google Scholar]
- 365.Humphreys MR, Castle EP, Lohse CM, Sebo TJ, Leslie KO, Andrews PE. Renal ischemia time in laparoscopic surgery: An experimental study in a porcine model. Int J Urol. 2009;16:105–109. doi: 10.1111/j.1442-2042.2008.02173.x. [DOI] [PubMed] [Google Scholar]
- 366.Ikeda H, Suzuki Y, Suzuki M, Koike M, Tamura J, Tong J, Nomura M, Itoh G. Apoptosis is a major mode of cell death caused by ischaemia and ischaemia/reperfusion injury to the rat intestinal epithelium. Gut. 1998;42:530–537. doi: 10.1136/gut.42.4.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Inagaki T, Akiyama T, Du CK, Zhan DY, Yoshimoto M, Shirai M. Monoamine oxidase-induced hydroxyl radical production and cardiomyocyte injury during myocardial ischemia-reperfusion in rats. Free Radic Res. 2016;50:645–653. doi: 10.3109/10715762.2016.1162300. [DOI] [PubMed] [Google Scholar]
- 368.Inagaki K, Chen L, Ikeno F, Lee FH, Imahashi K, Bouley DM, Rezaee M, Yock PG, Murphy E, Mochly-Rosen D. Inhibition of delta-protein kinase C protects against reperfusion injury of the ischemic heart in vivo. Circulation. 2003;108:2304–2307. doi: 10.1161/01.CIR.0000101682.24138.36. [DOI] [PubMed] [Google Scholar]
- 369.Inagaki K, Churchill E, Mochly-Rosen D. Epsilon protein kinase C as a potential therapeutic target for the ischemic heart. Cardiovasc Res. 2006;70:222–230. doi: 10.1016/j.cardiores.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 370.Inderbitzen D, Beldi G, Avital I, Vinci G, Candinas D. Local and remote ischemia-reperfusion injury is mitigated in mice overexpressing human C1 inhibitor. Eur J Surg Res. 2004;36a:142–147. doi: 10.1159/000077255. [DOI] [PubMed] [Google Scholar]
- 371.Report Brief. Institute of Medicine. Committee on Women’s Health Research, National Academies Press; Washington, DC: 2010. Women’s Health Research: Progress, Pitfalls, and Promise. [PubMed] [Google Scholar]
- 372.Ioannou A, Dalle Lucca J, Tsokos GC. Immunopathogenesis of ischemia/reperfusion-associated tissue damage. Clin Immunol. 2011;141:3–14. doi: 10.1016/j.clim.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 373.Ivanov II, Frutos Rde L, Manel N, Yoshinaga K, Rifkin DB, Sartor RB, Finlay BB, Littman DR. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell Host Microbe. 2008;4:337–349. doi: 10.1016/j.chom.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Jaeschke H, Bautista AP, Spolarics Z, Farhood A, Spitzer JJ. Proceedings of the 5th International Congress on Oxygen Radicals. Elsevier Science Publishers; Amsterdam: 1993. Enhanced generation of superoxide by complement-stimulated Kupffer cells and priming of neutrophils during the initial reperfusion phase after hepatic ischemia. [Google Scholar]
- 375.Jaeschke H, Bautista AP, Spolarics Z, Spitzer JJ. Superoxide generation by neutrophils and Kupffer cells during in vivo reperfusion after hepatic ischemia in rats. J Leukoc Biol. 1992;52:377–382. doi: 10.1002/jlb.52.4.377. [DOI] [PubMed] [Google Scholar]
- 376.Jaeschke H, Farhood A, Fisher MA, Smith CW. Sequestration of neutrophils in the hepatic vasculature during endotoxemia is independent of beta 2 integrins and intercellular adhesion molecule-1. Shock. 1996;6:351–356. doi: 10.1097/00024382-199611000-00009. [DOI] [PubMed] [Google Scholar]
- 377.Jaeschke H, Farhood A, Kupffer Cell activation after no-flow ischemia versus hemorrhagic shock. Free Rad Biol Med. 2002;33:210–219. doi: 10.1016/s0891-5849(02)00867-5. [DOI] [PubMed] [Google Scholar]
- 378.Jaeschke H, Farhood A, Smith CW. Neutrophils contribute to ischemia-reperfusion injury in rat liver in vivo. FASEB J. 1990;4:3355–3359. [PubMed] [Google Scholar]
- 379.Jang HR, Gandolfo MT, Ko GJ, Satpute S, Racusen L, Rabb H. Early exposure to germs modifies kidney damage and inflammation after experimental ischemia-reperfusion injury. Am J Physiol Renal Physiol. 2009;297:F1457–F1465. doi: 10.1152/ajprenal.90769.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379a.Jang HR, Rabb H. Immune cells in experimental acute kidney injury. Nat Rev Nephrol. 2015;11:88–101. doi: 10.1038/nrneph.2014.180. [DOI] [PubMed] [Google Scholar]
- 380.Jennings RB. Historical perspective on the pathology of myocardial ischemia/reperfusion injury. Circ Res. 2013;113:428–438. doi: 10.1161/CIRCRESAHA.113.300987. [DOI] [PubMed] [Google Scholar]
- 381.Jennings RB, Reimer KA. The cell biology of acute myocardial ischemia. Annu Rev Med. 1991;42:225–246. doi: 10.1146/annurev.me.42.020191.001301. [DOI] [PubMed] [Google Scholar]
- 382.Jennings RB, Sommers HM, Smyth GA, Flack HA, Linn H. Myocardial necrosis induced by temporary occlusion of a coronary artery in the dog. Arch Pathol. 1960;70:68–78. [PubMed] [Google Scholar]
- 383.Jeremias I, Kupatt C, Martin-Villalba A, Habazettl H, Schenkel J, Boekstegers P, Debatin KM. Involvement of CD95/Apo1/Fas in cell death after myocardial ischemia. Circulation. 2000;102:915–920. doi: 10.1161/01.cir.102.8.915. [DOI] [PubMed] [Google Scholar]
- 384.Jerome SN, Akimitsu T, Gute DC, Korthuis RJ. Ischemic preconditioning attenuates capillary no-reflow induced by prolonged ischemia and reperfusion. Am J Physiol. 1995;266:H1316–H1321. doi: 10.1152/ajpheart.1995.268.5.H2063. [DOI] [PubMed] [Google Scholar]
- 385.Jerome SN, Akimitsu T, Korthuis RJ. Leukocyte adhesion, edema, and development of postischemic capillary no-reflow. Am J Physiol. 1994;267:H1329–1336. doi: 10.1152/ajpheart.1994.267.4.H1329. [DOI] [PubMed] [Google Scholar]
- 386.Jerome SN, Dore M, Paulson JC, Smith CW, Korthuis RJ. P-selectin and ICAM-1-dependent adherence reactions: Role in the genesis of postischemic no-reflow. Am J Physiol. 1994;266:H1316–H1321. doi: 10.1152/ajpheart.1994.266.4.H1316. [DOI] [PubMed] [Google Scholar]
- 387.Jerome SN, Smith CW, Korthuis RJ. CD18-dependent adherence reactions play an important role in the development of the no-reflow phenomenon. Am J Physiol. 1993;264:H479–483. doi: 10.1152/ajpheart.1993.264.2.H479. [DOI] [PubMed] [Google Scholar]
- 388.Jeyaseelan K, Lim KY, Armugam A. MicroRNA expression in the blood and brain of rats subjected to transient focal ischemia by middle cerebral artery occlusion. Stroke. 2008;39:959–966. doi: 10.1161/STROKEAHA.107.500736. [DOI] [PubMed] [Google Scholar]
- 389.Ji X, Luo Y, Ling F, Stetler RA, Lan J, Cao G, Chen J. Mild hypothermia diminishes oxidative DNA damage and pro-death signaling events after cerebral ischemia: A mechanism for neuroprotection. Front Biosci. 2007;12:1737–1747. doi: 10.2741/2185. [DOI] [PubMed] [Google Scholar]
- 390.Jiang M, Liu K, Luo J, Dong Z. Autophagy is a renoprotective mechanism during in vitro hypoxia and in vivo ischemia-reperfusion injury. Am J Pathol. 2010;176:1181–1192. doi: 10.2353/ajpath.2010.090594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390a.Jiang X, Ma H, Li C, Cao Y, Wang Y, Zhang Y, Liu Y. Effects of neonatal dexamethasone administration on cardiac recovery ability under ischemia-reperfusion in 24-wk-old rats. Pediatr Res. 2016;80:128–135. doi: 10.1038/pr.2016.54. [DOI] [PubMed] [Google Scholar]
- 391.Jiang M, Zhang Y, Dusting GJ. NADPH oxidase-mediated redox signaling: Roles in cellular stress response, stress tolerance, and tissue repair. Pharmacol Rev. 2011;63:218–242. doi: 10.1124/pr.110.002980. [DOI] [PubMed] [Google Scholar]
- 392.Jin G, Arai K, Murata Y, Wang S, Stins MF, Lo EH, van Leyen K. Protecting against cerebrovascular injury: Contributions of 12/15-lipoxygenase to edema formation after transient focal ischemia. Stroke. 2008;39:2538–2543. doi: 10.1161/STROKEAHA.108.514927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Jin R, Yang G, Li G. Inflammatory mechanisms in ischemic stroke: Role of inflammatory cells. J Leukoc Biol. 2010;87(5):779–789. doi: 10.1189/jlb.1109766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Jin Y, Silverman AJ, Vannucci SJ. Mast cell stabilization limits hypoxic-ischemic brain damage in the immature rat. Dev Neurosci. 2007;9:373–384. doi: 10.1159/000105478. [DOI] [PubMed] [Google Scholar]
- 395.Jin Y, Silverman AJ, Vannucci SJ. Mast cells are early responders after hypoxia-ischemia in immature rat brain. Stroke. 2009;40:3107–3112. doi: 10.1161/STROKEAHA.109.549691. [DOI] [PubMed] [Google Scholar]
- 396.Johnston WH, Latta H. Glomerular mesangial and endothelial cell swelling following temporary renal ischemia and its role in the no-reflow phenomenon. Am J Pathol. 1977;89:153–166. [PMC free article] [PubMed] [Google Scholar]
- 397.Jones AW, Durante W, Korthuis RJ. Heme oxygenase-1 deficiency leads to alteration of soluble guanylate cylcase redox regulation. J Pharmacol Exp Therap. 2010;335:85–91. doi: 10.1124/jpet.110.169755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Jones DP, Sies H. The redox code. Antioxid Redox Signal. 2015;23:734–746. doi: 10.1089/ars.2015.6247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Jones SP, Tang XL, Guo Y, Steenbergen C, Lefer DJ, Kukreja RC, Kong M, Li Q, Bhushan S, Zhu X, Du J, Nong Y, Stowers HL, Kondo K, Hunt GN, Goodchild TT, Orr A, Chang CC, Ockaili R, Salloum FN, Bolli R. The NHLBI-sponsored Consortium for preclinicAl assESsment of cARdioprotective therapies (CAESAR): A new paradigm for rigorous, accurate, and reproducible evaluation of putative infarct-sparing interventions in mice, rabbits, and pigs. Circ Res. 2015;116:572–586. doi: 10.1161/CIRCRESAHA.116.305462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Jong HR, Ko GJ, Wasowska BA, Rabb H. The interaction between ischemia-reperfusion and immune responses in the kidney. J Mol Med. 2009;87:859–864. doi: 10.1007/s00109-009-0491-y. [DOI] [PubMed] [Google Scholar]
- 401.Julia PL, Kofsky ER, Buckberg GD, Young HH, Bugyi HI. Studies of myocardial protection in the immature heart. I. Enhanced tolerance of immature versus adult myocardium to global ischemia with reference to metabolic differences. J Thorac Cardiovasc Surg. 1990;100:879–887. [PubMed] [Google Scholar]
- 402.Kahle KT, Simard JM, Staley KJ, Nahed BV, Jones PS, Sun D. Molecular mechanisms of ischemic cerebral edema: Role of electroneutral ion transport. Physiology (Bethesda) 2009;24:257–265. doi: 10.1152/physiol.00015.2009. [DOI] [PubMed] [Google Scholar]
- 403.Kaiser RA, Bueno OF, Lips DJ, Doevendans PA, Jones F, Kimball TF, Molkentin JD. Targeted inhibition of p38 mitogen-activated protein kinase antagonizes cardiac injury and cell death following ischemia-reperfusion in vivo. J Biol Chem. 2004;279:15524–15530. doi: 10.1074/jbc.M313717200. [DOI] [PubMed] [Google Scholar]
- 404.Kaiser RA, Liang Q, Bueno O, Huang Y, Lackey T, Klevitsky R, Hewett TE, Molkentin JD. Genetic inhibition or activation of JNK1/2 protects the myocardium from ischemia-reperfusion-induced cell death in vivo. J Biol Chem. 2005;280:32602–32608. doi: 10.1074/jbc.M500684200. [DOI] [PubMed] [Google Scholar]
- 405.Kalogeris T, Baines C, Krenz M, Korthuis RJ. Cell biology of ischemia/reperfusion injury. Int Rev Cell Mol Biol. 2012;298:229–318. doi: 10.1016/B978-0-12-394309-5.00006-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Kalogeris TJ, Bao Y, Korthuis RJ. Mitochondrial reactive oxygen species: A double-edged sword in ischemia/reperfusion versus preconditioning. Redox Biol. 2014;2:702–714. doi: 10.1016/j.redox.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Kaludercic N, Carpi A, Menabo R, Di Lisa F, Paolocci N. Monoamine oxidases (MAO) in the pathogenesis of heart failure and ischemia/reperfusion injury. Biochim Biophys Acta. 2011;1813:1323–1332. doi: 10.1016/j.bbamcr.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Kaludercic N, Mialet-Perez J, Paolocci N, Parini A, Di Lisa F. Monoamine oxidases as sources of oxidants in the heart. J Mol Cell Cardiol. 2014;73:34–42. doi: 10.1016/j.yjmcc.2013.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Kaludercic N, Takimoto E, Nagayama T, Lai E, Chen K, Shih JC, Pacak K, Kass DA, Di Lisa F, Paolocci N. Lack of monoamine oxidase A and B activity prevents heart failure in pressure-overloaded mice. J Mol Cell Cardiol. 2010;48:S13. [Google Scholar]
- 410.Karp CL. Links between innate and adaptive immunity. In: Serhan CN, Ward PA, Gilroy DW, editors. Fundamentals of Inflammation. New York, NY: Cambridge University Press; 2010. pp. 28–37. [Google Scholar]
- 411.Karra L, Berent-Maoz B, Ben-Zaimra M, Levi-Schaffer F. Are we ready to downregulate mast cells? Curr Opin Immunol. 2009;21:708–714. doi: 10.1016/j.coi.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 412.Kassahun WT, Schulz T, Richter O, Hauss J. Unchanged high mortality rates from acute occlusive intestinal ischemia: Six year review. Langenbecks Arch Surg. 2008;393:163–171. doi: 10.1007/s00423-007-0263-5. [DOI] [PubMed] [Google Scholar]
- 413.Kelton JG, Warkentin TE, Hayward CP, Murphy WG, Moore JC. Calpain activity in patients with thrombotic thrombocytopenic purpura is associated with platelet microparticles. Blood. 1992;80:2246–2251. [PubMed] [Google Scholar]
- 414.Kemp M, Go YM, Jones DP. Nonequilibrium thermodynamics of thiol/disulfide redox systems: A perspective on redox systems biology. Free Radic Biol Med. 2008;44:921–937. doi: 10.1016/j.freeradbiomed.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Khalil PN, Neuhof C, Huss R, Pollhammer M, Khalil MN, Neuhof H, Fritz H, Siebeck M. Calpain inhibition reduces infarct size and improves global hemodynamics and left ventricular contractility in a porcine myocardial ischemia/reperfusion model. Eur J Pharmacol. 2005;528:124–131. doi: 10.1016/j.ejphar.2005.10.032. [DOI] [PubMed] [Google Scholar]
- 416.Khandoga A, Biberthaler P, Enders G, Axmann S, Hatter J, Messmer K, Krombach F. Platelet adhesion mediated by fibrinogen-intercellular adhesion molecule-1 binding induces tissue injury in the postischemic liver in vivo. Tansplantation. 2002;74:681–688. doi: 10.1097/00007890-200209150-00016. [DOI] [PubMed] [Google Scholar]
- 417.Khandoga A, Hanschen M, Kessler JS, Kronbach F. CD4+ T cells contribute to postischemic liver injury in mice by interacting with sinusoidal endothelium and platelets. Hepatology. 2006;43:306–315. doi: 10.1002/hep.21017. [DOI] [PubMed] [Google Scholar]
- 418.Kim HJ, Rowe M, Ren M, Hong JS, Chen PS, Chuang DM. Histone deacetylase inhibitors exhibit anti-inflammatory and neuroprotective effects in a rat permanent ischemic model of stroke: Multiple mechanisms of action. J Pharmacol Exp Ther. 2007;321:892–901. doi: 10.1124/jpet.107.120188. [DOI] [PubMed] [Google Scholar]
- 419.Kim J, Kim DS, Park MJ, Cho HJ, Zervos AS, Bonventre JV, Park KM. Omi/HtrA2 protease is associated with tubular cell apoptosis and fibrosis induced by unilateral ureteral obstruction. Am J Physiol Renal Physiol. 2010;298:F1332–F1340. doi: 10.1152/ajprenal.00737.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Kim JJ, Lee SB, Park JK, Yoo YD. TNF-alpha-induced ROS production triggering apoptosis is directly linked to Romo1 and Bcl-X(L) Cell Death Differ. 2010;17:1420–1434. doi: 10.1038/cdd.2010.19. [DOI] [PubMed] [Google Scholar]
- 420a.Kim MG, Boo CS, Ko YS, Lee HY, Cho WY, Kim HK, Jo SK. Depletion of kidney CD11c+ F4/80+ cells impairs the recovery process in ischaemia/reperfusion-induced acute kidney injury. Nephrol Dial Transplant. 2010;25:2908–2921. doi: 10.1093/ndt/gfq183. [DOI] [PubMed] [Google Scholar]
- 421.King LA, Toledo AH, Rivera-Chavez FA, Toledo-Pereyra LH. Role of p38 and JNK in liver ischemia and reperfusion. J Hepatobiliary Pancreat Surg. 2009;16:763–770. doi: 10.1007/s00534-009-0155-x. [DOI] [PubMed] [Google Scholar]
- 422.Kinross JM, Darzi AW, Nicholson JK. Gut microbiome-host interactions in health and disease. Genome Med. 2011;3:14. doi: 10.1186/gm228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Kinross J, Warren O, Basson S, Holmes E, Silk D, Darzi A, Nicholson JK. Intestinal ischemia/reperfusion injury: Defining the role of the gut microbiome. Biomark Med. 2009;3:175–192. doi: 10.2217/bmm.09.11. [DOI] [PubMed] [Google Scholar]
- 424.Kinsey GR, Huang L, Vergis AL, Li L, Okusa MD. Regulatory T cells contribute to the protective effect of ischemic preconditioning in the kidney. Kidney Int. 2010;77:771–780. doi: 10.1038/ki.2010.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Kinsey GR, Sharma R, Huang L, Li L, Vergis AL, Ye H, Ju ST, Okusa MD. Regulatory T cells suppress innate immunity in kidney ischemia-reperfusion injury. J Am Soc Nephrol. 2009;20:1744–1753. doi: 10.1681/ASN.2008111160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Kiray M, Bagriyanik HA, Pekcetin C, Ergur BU, Uysal N. Protective effects of deprenyl in transient cerebral ischemia in rats. Chin J Physiol. 2008;51:275–281. [PubMed] [Google Scholar]
- 427.Kloner RA. Current state of clinical translation of cardioprotective agents for acute myocardial infarction. Circ Res. 2013;113:451–463. doi: 10.1161/CIRCRESAHA.112.300627. [DOI] [PubMed] [Google Scholar]
- 428.Kloner RA, Ganote CE, Jennings RB. The “no-reflow” phenomenon after temporary coronary occlusion in the dog. J Clin Invest. 1974;54:1496–1508. doi: 10.1172/JCI107898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429.Klotz L, Norman S, Vieira JM, Masters M, Rohling M, Dube KN, Bollini S, Matsuzaki F, Carr CA, Riley PR. Cardiac lymphatics are heterogeneous in origin and respond to injury. Nature. 2015;522:62–67. doi: 10.1038/nature14483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430.Kobayashi A, Imamura H, Isobe M, Matsuyama Y, Soeda J, Matsunaga K, Kawasaki S. Mac-1 (CD11b/CD18) and intercellular adhesion molecule-1 in ischemia-reperfusion injury of rat liver. Am J Physiol Gastrointest Liver Physiol. 2001;281:G577–585. doi: 10.1152/ajpgi.2001.281.2.G577. [DOI] [PubMed] [Google Scholar]
- 431.Kobayashi M, Takeyoshi I, Yoshinari D, Matsumoto K, Morishita Y. P38 mitogen-activated protein kinase inhibition attenuates ischemia-reperfusion injury of the rat liver. Surgery. 2002;131:344–349. doi: 10.1067/msy.2002.121097. [DOI] [PubMed] [Google Scholar]
- 432.Kobayasi T, Hirano K, Yamamoto T, Hasegawa G, Hatakeyama K, Suematsu M, Naito M. The protective role of Kupffer cells in the ischemia-reperfused rat liver. Arch Histol Cytol. 2002;65:251–261. doi: 10.1679/aohc.65.251. [DOI] [PubMed] [Google Scholar]
- 433.Koboziev I, Reinoso Webb C, Furr KL, Grisham MB. Role of the enteric microbiota in intestinal homeostasis and inflammation. Free Radic Biol Med. 2014;68:122–133. doi: 10.1016/j.freeradbiomed.2013.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, Britt EB, Fu X, Wu Y, Li L, Smith JD, DiDonato JA, Chen J, Li H, Wu GD, Lewis JD, Warrier M, Brown JM, Krauss RM, Tang WH, Bushman FD, Lusis AJ, Hazen SL. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013;19:576–585. doi: 10.1038/nm.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435.Kohda Y, Gemba M. Cephaloridine induces translocation of protein kinase C d into mitochondria and enhances mitochondrial generation of free radicals in the kidney cortex of rats causing renal dysfunction. J Pharmacol Sci. 2005;98:49–57. doi: 10.1254/jphs.fp0040926. [DOI] [PubMed] [Google Scholar]
- 436.Kohli V, Madden JF, Bentley RC, Clavien PA. Calpain mediates ischemic injury of the liver through modulation of apoptosis and necrosis. Gastroenterology. 1999;116:168–78. doi: 10.1016/s0016-5085(99)70241-6. [DOI] [PubMed] [Google Scholar]
- 437.Kokura S, Wolf RE, Yoshikawa T, Granger DN, Aw TY. Molecular mechanisms of neutrophil-endothelial cell adhesion induced by redox imbalance. Circ Res. 1999;84:516–524. doi: 10.1161/01.res.84.5.516. [DOI] [PubMed] [Google Scholar]
- 438.Kokura S, Wolf RE, Yoshikawa T, Ichikawa H, Granger DN, Aw TY. Endothelial cells exposed to anoxia/reoxygenation are hyperadhesive to T-lymphocytes: Kinetics and molecular mechanisms. Microcirculation. 2000;7:13–23. [PubMed] [Google Scholar]
- 439.Kolettis TM, Barton M, Langleben D, Matsumura Y. Endothelin in coronary artery disease and myocardial infarction. Cardiol Rev. 2013;21:249–256. doi: 10.1097/CRD.0b013e318283f65a. [DOI] [PubMed] [Google Scholar]
- 440.Koleva PT, Kim JS, Scott JA, Kozyrskyj AL. Microbial programming of health and disease starts during fetal life. Birth Defects Res C Embryo Today Rev. 2015;105:265–277. doi: 10.1002/bdrc.21117. [DOI] [PubMed] [Google Scholar]
- 441.Kondo T, Reaume AG, Huang TT, Carlson E, Murakami K, Chen SF, Hoffman EK, Scott RW, Epstein CJ, Chan PH. Reduction of CuZn-superoxide dismutase activity exacerbates neuronal cell injury and edema formation after transient focal cerebral ischemia. J Neurosci. 1997;17:4180–4189. doi: 10.1523/JNEUROSCI.17-11-04180.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442.Konstantinidis K, Whelan RS, Kitsis RN. Mechanisms of cell death in heart disease. Arterioscler Thromb Vasc Biol. 2012;32:1552–1562. doi: 10.1161/ATVBAHA.111.224915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443.Koponen S, Goldsteins G, Keinänen R, Koistinaho J. Induction of protein kinase Cd subspecies in neurons and microglia after transient global brain ischemia. J Cereb Blood Flow Metab. 2000;20:93–102. doi: 10.1097/00004647-200001000-00013. [DOI] [PubMed] [Google Scholar]
- 444.Korichneva I. Redox regulation of cardiac protein kinase C. Exp Clin Cardiol. 2005;10:256–261. [PMC free article] [PubMed] [Google Scholar]
- 445.Kornfeld OS, Hwang S, Disatnik MH, Chen CH, Qvit N, Mochly-Rosen D. Mitochondrial reactive oxygen species at the heart of the matter: New therapeutic approaches for cardiovascular diseases. Circ Res. 2015;116:1783–1799. doi: 10.1161/CIRCRESAHA.116.305432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446.Korthuis RJ, Anderson DC, Granger DN. Role of neutrophil-endothelial cell adhesion in inflammatory disorders. J Crit Care. 1994;9:47–71. doi: 10.1016/0883-9441(94)90032-9. [DOI] [PubMed] [Google Scholar]
- 447.Korzick DH, Kostyak JC, Hunter JC, Saupe KW. Local delivery of PKCepsilon-activating peptide mimics ischemic preconditioning in aged hearts through GSK-3beta but not F1-ATPase inactivation. Am J Physiol Heart Circ Physiol. 2007;293:H2056–2063. doi: 10.1152/ajpheart.00403.2007. [DOI] [PubMed] [Google Scholar]
- 448.Kosieradzki M, Rowiński W. Ischemia/reperfusion injury in kidney transplantation: Mechanisms and prevention. Transplant Proc. 2008;40:3279–3288. doi: 10.1016/j.transproceed.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 449.Krenz M, Baines C, Kalogeris T, Korthuis RJ. Cell Survival Programs and Ischemia/Reperfusion: Hormesis, Preconditioning, and Cardiovascular Protection. In: Granger DN, Granger JP, editors. Integrated Systems Physiology: Molecules to Function eBook series. Morgan-Claypool: 2013. [Google Scholar]
- 450.Krenz M, Korthuis RJ. Moderate ethanol ingestion and cardiovascular protection: From epidemiologic associations to cellular mechanisms. J Mol Cell Cardiol. 2012;52:93–104. doi: 10.1016/j.yjmcc.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451.Kristián T. Metabolic stages, mitochondria and calcium in hypoxic/ischemic brain damage. Cell Calcium. 2004;36:221–233. doi: 10.1016/j.ceca.2004.02.016. [DOI] [PubMed] [Google Scholar]
- 452.Kristiansen M, Graverson JH, Jacobsen C, Sonne O, Hoffman HJ, Law SK, Moestrup SK. Identification of the haemoglobin scavenger receptor. Nature. 2001;409:198–201. doi: 10.1038/35051594. [DOI] [PubMed] [Google Scholar]
- 453.Kroemer G, Galluzzi L, Brenner C. Mitochondrial membrane permeabilization in cell death. Physiol Rev. 2007;87:99–163. doi: 10.1152/physrev.00013.2006. [DOI] [PubMed] [Google Scholar]
- 454.Kroemer G, Galluzzi L, Vandenabeele P, Abrams J, Alnemri ES, Baehrecke EH, Blagosklonny MV, El-Deiry WS, Golstein P, Green DR, Hengartner M, Knight RA, Kumar S, Lipton SA, Malorni W, Nuñez G, Peter ME, Tschopp J, Yuan J, Piacentini M, Zhivotovsky B, Melino G. Classification of cell death: Recommendations of the Nomenclature Committee on Cell Death. Cell Death Differ. 2009;16:3–11. doi: 10.1038/cdd.2008.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 455.Krutzfeldt J, Rajewsky N, Braich R, Rajeev KG, Tuschl T, Manoharan M, Stoffel M. Silencing of microRNAs in vivo with ‘antagomirs’. Nature. 2005;438:685–689. doi: 10.1038/nature04303. [DOI] [PubMed] [Google Scholar]
- 456.Kuan CY, Whitmarsh AJ, Yang DD, Liao G, Schloemer AJ, Dong C, Bao J, Banasiak KJ, Haddad GG, Flavell RA, Davis RJ, Rakic P. A critical role of neural-specific JNK3 for ischemic apoptosis. Proc Natl Acad Sci U S A. 2003;100:15184–15189. doi: 10.1073/pnas.2336254100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 457.Kubli DA, Gustafsson AB. Mitochondria and mitophagy: The yin and yang of cell death control. Circ Res. 2012;111:1208–1221. doi: 10.1161/CIRCRESAHA.112.265819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 458.Kuboki S, Sakai N, Tschöp J, Edwards MJ, Lentsch AB, Caldwell CC. Distinct contributions of CD4+ T cell subsets in hepatic ischemia/reperfusion injury. Am J Physiol. 2009;296:G1054–G1059. doi: 10.1152/ajpgi.90464.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 459.Kukreja RC, Yin C, Salloum FN. MicroRNAs: New players in cardiac injury and protection. Mol Pharmacol. 2011;80:558–564. doi: 10.1124/mol.111.073528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 460.Kumar P, Shen Q, Pivetti CD, Lee ES, Wu MH, Yuan SY. Molecular mechanisms of endothelial hyperpermeability: Implications in inflammation. Expert Rev Mol Med. 2009;11:e19. doi: 10.1017/S1462399409001112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 461.Kume M, Yamamoto Y, Saad S, Gomi T, Kimoto S, Shimabukuro T, Yagi T, Nakagami M, Takada Y, Morimoto T, Yamaoka Y. Ischemic preconditioning of the liver in rats: Implications of heat shock protein induction to increase tolerance of ischemia-reperfusion injury. J Lab Clin Med. 1996;128:251–258. doi: 10.1016/s0022-2143(96)90026-8. [DOI] [PubMed] [Google Scholar]
- 462.Kuppusamy P, Zweier JL. Cardiac applications of EPR imaging. NMR Biomed. 2004;17:226–239. doi: 10.1002/nbm.912. [DOI] [PubMed] [Google Scholar]
- 463.Kvietys PR, Granger DN. Role of reactive oxygen and nitrogen species in the vascular responses to inflammation. Free Radic Biol Med. 2012;52:556–592. doi: 10.1016/j.freeradbiomed.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 464.Lagranha CJ, Deschamps A, Aponte A, Steenbergen C, Murphy E. Sex differences in the phosphorylation of mitochondrial proteins result in reduced production of reactive oxygen species and cardioprotection in females. Circ Res. 2010;106:1681–1691. doi: 10.1161/CIRCRESAHA.109.213645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 465.Lam FW, Burns AR, Smith CW, Rumbaut RE. Platelets enhance neutrophil transendothelial migration via P-selectin glycoprotein ligand-1. Am J Physiol Heart Circ Physiol. 2011;300:H468–H475. doi: 10.1152/ajpheart.00491.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 466.Lam V, Su J, Koprowski S, Hsu A, Tweddell JS, Rafiee P, Gross GJ, Salzman NH, Baker JE. Intestinal microbiota determine severity of myocardial infarction in rats. FASEB J. 2012;26:1727–1735. doi: 10.1096/fj.11-197921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 466a.Lam FW, Vijayan KV, Rumbaut RE. Platelets and their interactions with other immune cells. Compr Physiol. 2015;5:1265–1280. doi: 10.1002/cphy.c140074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 467.Langley-Evans SC. Hypertension induced by foetal exposure to a maternal low-protein diet, in the rat, is prevented by pharmacological blockade of maternal glucocorticoid synthesis. J Hypertens. 1997;15:537–544. doi: 10.1097/00004872-199715050-00010. [DOI] [PubMed] [Google Scholar]
- 468.Langley-Evans SC, McMullen S. Developmental origins of adult disease. Med Princ Pract. 2010;19:87–98. doi: 10.1159/000273066. [DOI] [PubMed] [Google Scholar]
- 469.Lappas CM, Day YJ, Marshall MA, Engelhardt VH, Linden J. Adenosine A2A receptor activation reduces hepatic ischemia reperfusion injury by inhibiting CD1d-dependent NKT activation. J Exp Med. 2006;203:2639–2648. doi: 10.1084/jem.20061097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 470.Lassègue B, Clempus RE. Vascular NAD(P)H oxidases: Specific features, expression, and regulation. Am J Physiol. 2003;285:R277–R297. doi: 10.1152/ajpregu.00758.2002. [DOI] [PubMed] [Google Scholar]
- 471.LATE_Study_Group. Late Assessment of Thrombolytic Efficacy (LATE) study with alteplase 6–24 hours after onset of acute myocardial infarction. Lancet. 1993;342:759–66. [PubMed] [Google Scholar]
- 472.Lazarus B, Messina A, Barker JE, Hurley JV, Romeo R, Morrison WA, Knight KR. The role of mast cells in schaemia-reperfusion injury in murine skeletal muscle. J Pathol. 2000;191:443–448. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH666>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 473.Le DA, Wu Y, Huang Z, Matsushita K, Plesnila N, Augustinack JC, Hyman BT, Yuan J, Kuida K, Flavell RA, Moskowitz MA. Caspase activation and neuroprotection in caspase-3-deficientmice after in vivo cerebral ischemia and in vitro oxygen glucose deprivation. Proc Natl Acad Sci U S A. 2002;99:15188–15193. doi: 10.1073/pnas.232473399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 474.Lecour S, Botker HE, Condorelli G, Davidson SM, Garcia-Dorado D, Engel FB, Ferdinandy P, Heusch G, Madonna R, Ovize M, Ruiz-Meana M, Schulz R, Sluijter JP, Van Laake LW, Yellon DM, Hausenloy DJ. ESC working group cellular biology of the heart: Position paper: Improving the preclinical assessment of novel cardioprotective therapies. Cardiovasc Res. 2014;104:399–411. doi: 10.1093/cvr/cvu225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 475.Lee H, Green D, Lai L, Hou YJ, Jensenius JC, Liu D, Cheong C, Park CG, Zhang M. Early complement factors in the local tissue immunocomplex generated during intestinal ischemia/reperfusion injury. Mol Immunol. 2010;47:972–981. doi: 10.1016/j.molimm.2009.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 476.Lee HA, Hong SH, Kim JW, Jang IS. Possible involvement of DNA methylation in NKCC1 gene expression during postnatal development and in response to ischemia. J Neurochem. 2010;114:520–9. doi: 10.1111/j.1471-4159.2010.06772.x. [DOI] [PubMed] [Google Scholar]
- 477.Lee H-L, Chen C-L, Yeh ST, Zweier JL, Chen Y-R. Biphasic modulation of the mitochondrial electron transport chain in myocardial ischemia and reperfusion. Am J Physiol. 2012;302:H1410–H1422. doi: 10.1152/ajpheart.00731.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 478.Lee JM, Grabb MC, Zipfel GJ, Choi DW. Brain tissue responses to ischemia. J Clin Invest. 2000;106:723–731. doi: 10.1172/JCI11003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 479.Lee KH, Kim SE, Lee YS. SP600125, a selective JNK inhibitor, aggravates hepatic ischemia-reperfusion injury. Exp Mol Med. 2006;38:408–16. doi: 10.1038/emm.2006.48. [DOI] [PubMed] [Google Scholar]
- 480.Lee P, Sata M, Lefer DJ, Factor SM, Walsh K, Kitsis RN. Fas pathway is a critical mediator of cardiac myocyte death and MI during ischemia-reperfusion in vivo. Am J Physiol Heart Circ Physiol. 2003;284:H456–H463. doi: 10.1152/ajpheart.00777.2002. [DOI] [PubMed] [Google Scholar]
- 481.Lee MC, Velayutham M, Komatsu T, Hille R, Zweier JL. Measurement and characterization of superoxide generation from xanthine dehydrogenase: A redox-regulated pathway of radical generation in ischemic tissues. Biochemistry. 2014;53:6615–6623. doi: 10.1021/bi500582r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 482.Lefer DJ. Do neutrophils contribute to myocardial reperfusion injury? Basic Res Cardiol. 2002;97:263–267. doi: 10.1007/s00395-002-0363-x. [DOI] [PubMed] [Google Scholar]
- 483.Lei K, Davis RJ. JNK phosphorylation of Bim-related members of the Bcl2 family induces Bax-dependent apoptosis. Proc Natl Acad Sci U S A. 2003;100:2432–7. doi: 10.1073/pnas.0438011100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 484.Lejay A, Fang F, John R, Van JA, Barr M, Thaveau F, Chakfe N, Geny B, Scholey JW. Ischemia reperfusion injury, ischemic conditioning and diabetes mellitus. J Mol Cell Cardiol. 2016;91:11–22. doi: 10.1016/j.yjmcc.2015.12.020. [DOI] [PubMed] [Google Scholar]
- 485.Lemay S, Rabb H, Postler G, Singh AK. Prominent and sustained up-regulation of gp130-signaling cytokines and the chemokine MIP-2 in murine renal ischemia-reperfusion injury. Transplant. 2000;69:959–963. doi: 10.1097/00007890-200003150-00049. [DOI] [PubMed] [Google Scholar]
- 486.Lerchenberger M, Uhl B, Stark K, Zuchtriegel G, Eckart A, Miller M, Puhr-Westerheide D, Praetner M, Rehberg M, Khandoga AG, Lauber K, Massberg S, Krombach F, Reichel CA. Matrix metalloproteinases modulate ameboid-like migration of neutrophils through inflamed interstitial tissue. Blood. 2013;122:770–780. doi: 10.1182/blood-2012-12-472944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 487.Leroyer AS, Anfosso F, Lacroix R, Sabatier F, Simoncini S, Njock SM, Jourde N, Brunet P, Camoin-Jau L, Sampol J, Dignat-George F. Endothelial-derived microparticles: Biological conveyors at the crossroad of inflammation, thrombosis and angiogenesis. Thromb Haemost. 2010;104:456–463. doi: 10.1160/TH10-02-0111. [DOI] [PubMed] [Google Scholar]
- 488.Lesnefsky EJ, Chen Q, Moghaddas S, Hassan MO, Tandler B, Hoppel CL. Blockade of electron transport during ischemia protects cardiac mitochondria. J Biol Chem. 2004;279:47961–47967. doi: 10.1074/jbc.M409720200. [DOI] [PubMed] [Google Scholar]
- 489.Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 490.Levonen AL, Dickinson DA, Moellering DR, Mulcahy RT, Forman HJ, Darley-Usmar VM. Biphasic effects of 15-deoxy-delta (12,14)-prostaglandin J(2) on glutathione induction and apoptosis in human endothelial cells. Arterioscler Thromb Vasc Biol. 2001;21:1846–1851. doi: 10.1161/hq1101.098488. [DOI] [PubMed] [Google Scholar]
- 491.Lewen A, Matz P, Chan PH. Free radical pathways in CNS injury. J Neurotrauma. 2000;17:871–890. doi: 10.1089/neu.2000.17.871. [DOI] [PubMed] [Google Scholar]
- 491a.Li G, Xiao Y, Estrella JL, Ducsay CA, Gilbert RD, Zhang L. Effect of fetal hypoxia on heart susceptibility to ischemia and reperfusion injury in the adult rat. J Soc Gynecol Investig. 2003;10:265–274. doi: 10.1016/s1071-5576(03)00074-1. [DOI] [PubMed] [Google Scholar]
- 491b.Li G, Bae S, Zhang L. Effect of prenatal hypoxia on heat stress-mediated cardioprotection in adult rat heart. Am J Physiol Heart Circ Physiol. 2004;286:H1712–H1719. doi: 10.1152/ajpheart.00898.2003. [DOI] [PubMed] [Google Scholar]
- 492.Li J, Xu J, Cheng Y, Wang F, Song Y, Xiao J. Circulating microRNAs as mirrors of acute coronary syndromes: MiRacle or quagMire? J Cell Mol Med. 2013;17:1363–1370. doi: 10.1111/jcmm.12148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 493.Li JY, Gu X, Zhang WH, Jia S, Zhou Y. GdCl3 abates hepatic ischemia-reperfusion injury by inhibiting apoptosis in rats. Hepatobil Panceat Dis Int. 2009;8:518–523. [PubMed] [Google Scholar]
- 494.Li R, Ding T, Liu X, Li C. Influence of SB203580 on cell apoptosis and P38MAPK in renal ischemia/reperfusion injury. J Huazhong Univ Sci Technolog Med Sci. 2006;26:50–52. doi: 10.1007/BF02828037. [DOI] [PubMed] [Google Scholar]
- 495.Liao L, Harris NR, Granger DN. Oxidized low-density lipoproteins and microvascular responses to ischemia-reperfusion. Am J Physiol. 1996;271:H2508–H2514. doi: 10.1152/ajpheart.1996.271.6.H2508. [DOI] [PubMed] [Google Scholar]
- 496.Liesz A, Dalpke A, Mracsko E, Antoine DJ, Roth S, Zhou W, Yang H, Na SY, Akhisaroglu M, Fleming T, Eigenbrod T, Nawroth PP, Tracey KJ, Veltkamp R. DAMP signaling is a key pathway inducing immune modulation after brain injury. J Neuroscience. 2015;35:583–598. doi: 10.1523/JNEUROSCI.2439-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 497.Liesz AL, Suri-Payer E, Veltkamp C, Doerr H, Sommer C, Rivest S, Giese T, Veltkamp R. Regulatory T cells are key cerebroprotective immunomodulators in acute experimental stroke. Nature Med. 2009;15:192–199. doi: 10.1038/nm.1927. [DOI] [PubMed] [Google Scholar]
- 498.Lima B, Forrester MT, Hess DT, Stamler JS. S-nitrosylation in cardiovascular signaling. Circ Res. 2010;106:633–646. doi: 10.1161/CIRCRESAHA.109.207381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 499.Lindemann S, Klingel B, Fisch A, Meyer J, Darius H. Increased platelet sensitivity toward platelet inhibitors during physical exercise in patients with coronary artery disease. Thromb Res. 1999;93:51–59. doi: 10.1016/s0049-3848(98)00155-8. [DOI] [PubMed] [Google Scholar]
- 500.Lindsay T, Romaschin A, Walker PM. Free radical mediated damage in skeletal muscle. Microcirc Endothelium Lymphatics. 1989;5:157–170. [PubMed] [Google Scholar]
- 501.Lindsey ML, Mayr M, Gomes AV, Delles C, Arrell DK, Murphy AM, Lange RA, Costello CE, Jin YF, Laskowitz DT, Sam F, Terzic A, Van Eyk J, Srinivas PR. Transformative impact of proteomics on cardiovascular health and disease: A scientific statement from the American Heart Association. Circulation. 2015;132:852–872. doi: 10.1161/CIR.0000000000000226. [DOI] [PubMed] [Google Scholar]
- 502.Linfert D, Austen WG, Jr, Rabb H. Lymphocytes and ischemia-reperfusion injury. Transplant Rev. 2009;23:1–10. doi: 10.1016/j.trre.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 503.Linkermann A, Brasen JH, Darding M, Jin MK, Sanz AB, Heller JO, De Zen F, Weinlich R, Ortiz A, Walczak H, Weinberg JM, Green DR, Kunzendorf U, Krautwald S. Two independent pathways of regulated necrosis mediate ischemia-reperfusion injury. Proc Natl Acad Sci U S A. 2013;110:12024–12029. doi: 10.1073/pnas.1305538110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 504.Linkermann A, Brasen JH, Himmerkus N, Liu S, Huber TB, Kunzendorf U, Krautwald S. Rip1 (receptor-interacting protein kinase 1) mediates necroptosis and contributes to renal ischemia/reperfusion injury. Kidney Int. 2012;81:751–761. doi: 10.1038/ki.2011.450. [DOI] [PubMed] [Google Scholar]
- 505.Linkermann A, De Zen F, Weinberg J, Kunzendorf U, Krautwald S. Programmed necrosis in acute kidney injury. Nephrol Dial Transplant. 2012;27:3412–3419. doi: 10.1093/ndt/gfs373. [DOI] [PubMed] [Google Scholar]
- 506.Linkermann A, Hackl MJ, Kunzendorf U, Walczak H, Krautwald S, Jevnikar AM. Necroptosis in immunity and ischemia-reperfusion injury. Am J Transplant. 2013;13:2797–2804. doi: 10.1111/ajt.12448. [DOI] [PubMed] [Google Scholar]
- 507.Linkermann A, Heller JO, Prokai A, Weinberg JM, De Zen F, Himmerkus N, Szabo AJ, Brasen JH, Kunzendorf U, Krautwald S. The RIP1-kinase inhibitor necrostatin-1 prevents osmotic nephrosis and contrast-induced AKI in mice. J Am Soc Nephrol. 2013;24:1545–1557. doi: 10.1681/ASN.2012121169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 508.Linkermann A, Skouta R, Himmerkus N, Mulay SR, Dewitz C, De Zen F, Prokai A, Zuchtriegel G, Krombach F, Welz PS, Green DR. Necroptosis. N Engl J Med. 370:455–465. [Google Scholar]
- 509.Lira VA, Brown DL, Lira AK, Kavazis AN, Soltow QA, Zeanah EH, Criswell DS. Nitric oxide and AMPK cooperatively regulate PGC-1 in skeletal muscle cells. J Physiol. 2010;588:3551–3566. doi: 10.1113/jphysiol.2010.194035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 510.Liu D, Gan X, Huang P, Chen X, Ge M, Hei Z. Inhibiting tryptase after ischemia limits small intestinal ischemia-reperfusion injury through protease-activated receptor 2 in rats. J Trauma Acute Care Surg. 2012;73:1138–1144. doi: 10.1097/TA.0b013e318265d08d. [DOI] [PubMed] [Google Scholar]
- 511.Liu L, Kubes P. Molecular mechanisms of leukocyte recruitment: Organ-specific mechanisms of action. Thromb Haemost. 2003;89:213–220. [PubMed] [Google Scholar]
- 512.Liu M, Chien CC, Grigoryev DN, Gandalfo MT, Colvin RB, Rabb H. Effect of T cells on vascular permeability in early ischemic acute kidney injury in mice. Microvasc Res. 2009;77:340–347. doi: 10.1016/j.mvr.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 513.Liu P, McGuire PM, Fisher MA, Farhood A, Smith CW, Jaeschke H. Activation of Kupffer cells and neutrophils for reactive oxygen formation is responsible for endotoxin-enhanced liver injury after hepatic ischemia. Shock. 1995;3:56–62. [PubMed] [Google Scholar]
- 514.Liu XH, Zhang ZY, Sun S, Wu XD. Ischemic postconditioning protects myocardium from ischemia/reperfusion injury through attenuating endoplasmic reticulum stress. Shock. 2008;30:422–427. doi: 10.1097/SHK.0b013e318164ca29. [DOI] [PubMed] [Google Scholar]
- 515.Liu Y, Kato H, Nakata N, Kogure K. Protection of rat hippocampus against ischemic neuronal damage by pretreatment with sublethal ischemia. Brain Res. 1992;586:121–124. doi: 10.1016/0006-8993(92)91380-w. [DOI] [PubMed] [Google Scholar]
- 516.Loi P, Paular F, Pajak B, Nagy N, Salmon I, Moser M, Goldman M, Flamand V. The fate of dendritic cells in a mouse model of liver ischemia/reperfusion injury. Transplant Proc. 2004;36:1275–1279. doi: 10.1016/j.transproceed.2004.05.052. [DOI] [PubMed] [Google Scholar]
- 517.Lorenzen JM. Vascular and circulating microRNAs in renal ischaemia-reperfusion injury. J Physiol. 2015;593:1777–1784. doi: 10.1113/JP270318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 518.Lu X, Kwong JQ, Molkentin JD, Bers DM. Individual Cardiac Mitochondria Undergo Rare Transient Permeability Transition Pore Openings. Circ Res. 2016;118:834–841. doi: 10.1161/CIRCRESAHA.115.308093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 519.Lu YY, Li ZZ, Jiang DS, Wang L, Zhang Y, Chen K, Zhang XF, Liu Y, Fan GC, Chen Y, Yang Q, Zhou Y, Zhang XD, Liu DP, Li H. TRAF1 is a critical regulator of cerebral ischaemia-reperfusion injury and neuronal death. Nat Commun. 2013;4:2852. doi: 10.1038/ncomms3852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 520.Lu X, Li N, Shushakova N, Schmitt R, Menne J, Susnik N, Meier M, Leitges M, Haller H, Gueler F, Rong S. C57BL/6 and 129/Sv mice: Genetic difference to renal ischemia-reperfusion. J Nephrol. 2012;25:738–743. doi: 10.5301/jn.5000053. [DOI] [PubMed] [Google Scholar]
- 521.Lu X, Li N, Shushakova N, Schmitt R, Menne J, Susnik N, Meier M, Leitges M, Haller H, Gueler F, Rong S, LueddeLuedde M, Lutz M, Carter N, Sosna J, Jacoby C, Vucur M, Gautheron J, Roderburg C, Borg N, Reisinger F, Hippe HJ, Linkermann A, Wolf MJ, Rose-John S, Lullmann-Rauch R, Adam D, Flogel U, Heikenwalder M, Luedde T, Frey N. RIP3, a kinase promoting necroptotic cell death, mediates adverse remodelling after myocardial infarction. Cardiovasc Res. 2014;103:206–216. doi: 10.1093/cvr/cvu146. [DOI] [PubMed] [Google Scholar]
- 522.Lucchesi BR, Kilgore KS. Complement inhibitors in myocardial ischemia/reperfusion injury. Immunopharmacology. 1997;38:27–42. doi: 10.1016/s0162-3109(97)00060-x. [DOI] [PubMed] [Google Scholar]
- 523.Ma M. Role of calpains in the injury-induced dysfunction and degeneration of the mammalian axon. Neurobiol Dis. 2013;60:61–79. doi: 10.1016/j.nbd.2013.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 524.Ma X, Becker Buscaglia LE, Barker JR, Li Y. MicroRNAs in NF-kappaB signaling. J Mol Cell Biol. 2011;3:159–166. doi: 10.1093/jmcb/mjr007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 524a.Ma Y, Wang J, Wang Y, Yang GY. The biphasic function of microglia in ischemic stroke. Prog Neurobiol. 2016 doi: 10.1016/j.pneurobio.2016.01.005. pii: S0301-0082(15)30070-8. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 525.Maclean D, Fishbein MC, Braunwald E, Maroko PR. Long-term preservation of ischemic myocardium after experimental coronary artery occlusion. J Clin Invest. 1978;61:541–551. doi: 10.1172/JCI108965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 526.Maekawa A, Lee JK, Nagaya T, Kamiya K, Yasui K, Horiba M, Miwa K, Uzzaman M, Maki M, Ueda Y, Kodama I. Overexpression of calpastatin by gene transfer prevents troponin I degradation and ameliorates contractile dysfunction in rat hearts subjected to ischemia/reperfusion. J Mol Cell Cardiol. 2003;35:1277–84. doi: 10.1016/s0022-2828(03)00238-4. [DOI] [PubMed] [Google Scholar]
- 527.Maggi CA. The effects of tachykinin on inflammatory and immune cells. Regul Petides. 1997;70:75–90. doi: 10.1016/s0167-0115(97)00029-3. [DOI] [PubMed] [Google Scholar]
- 528.Mao XR, Crowder CM. Protein misfolding induces hypoxic preconditioning via a subset of the unfolded protein response machinery. Mol Cell Biol. 2010;30:5033–5042. doi: 10.1128/MCB.00922-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 529.Mariappan N, Soorappan RN, Haque M, Sriramula S, Francis J. TNF-α-induced mitochondrial stress and cardiac dysfunction: Restoration by superoxide dismutase mimetic Tempol. Am J Physiol. 2007;293:H2776–H2737. doi: 10.1152/ajpheart.00376.2007. [DOI] [PubMed] [Google Scholar]
- 530.Maroko PR, Carpenter CB, Chiariello M, Fishbein MC, Radvany P, Knostman JD, Hale SL. Reduction by cobra venom factor of myocardial necrosis after coronary artery occlusion. J Clin Invest. 1978;61:661–670. doi: 10.1172/JCI108978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 531.Marshall HE, Merchant K, Stamler JS. Nitrosation and oxidation in the regulation of gene expression. FASEB J. 2000;14:1889–1900. doi: 10.1096/fj.00.011rev. [DOI] [PubMed] [Google Scholar]
- 532.Martin M, Mory C, Piescher A, Wittekind C, Fiedler M, Uhlmann D. Protective effects of early CD4 +T cell reduction in hepatic ischemia/reperfusion injury. J Gastrointest Surg. 2010;14:511–519. doi: 10.1007/s11605-009-1104-3. [DOI] [PubMed] [Google Scholar]
- 533.Martindale JJ, Fernandez R, Thuerauf D, Whittaker R, Gude N, Sussman MA, Glembotski CC. Endoplasmic reticulum stress gene induction and protection from ischemia/reperfusion injury in the hearts of transgenic mice with a tamoxifen-regulated form of ATF6. Circ Res. 2006;98:1186–1193. doi: 10.1161/01.RES.0000220643.65941.8d. [DOI] [PubMed] [Google Scholar]
- 534.Martindale JJ, Metzger JM. Uncoupling of increased cellular oxidative stress and myocardial ischemia reperfusion injury by directed sarcolemma stabilization. J Mol Cell Cardiol. 2014;67:26–37. doi: 10.1016/j.yjmcc.2013.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 535.Martinez D, Pentinat T, Ribo S, Daviaud C, Bloks VW, Cebria J, Villalmanzo N, Kalko SG, Ramon-Krauel M, Diaz R, Plosch T, Tost J, Jimenez-Chillaron JC. In utero undernutrition in male mice programs liver lipid metabolism in the second-generation offspring involving altered Lxra DNA methylation. Cell Metab. 2014;19:941–951. doi: 10.1016/j.cmet.2014.03.026. [DOI] [PubMed] [Google Scholar]
- 536.Martyn CN, Hales CN, Barker DJ, Jespersen S. Fetal growth and hyperinsulinaemia in adult life. Diabet Med. 1998;15:688–694. doi: 10.1002/(SICI)1096-9136(199808)15:8<688::AID-DIA649>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 537.Marzocco S, Di Paola R, Autore G, Mazzon E, Pinto A, Caputi AP, Thiemermann C, Cuzzocrea S. Calpain inhibitor I reduces intestinal ischemia-reperfusion injury in the rat. Shock. 2004;21:38–44. doi: 10.1097/01.shk.0000095056.62263.b2. [DOI] [PubMed] [Google Scholar]
- 538.Massberg S, Enders G, Matos FC, Tomic LI, Leiderer R, Eisenmenger S, Messmer K, Krombach F. Fibrinogen deposition at the postischemic vessel wall promotes platelet adhesion during ischemia-reperfusion in vivo. Blood. 1999;94:3829–3838. [PubMed] [Google Scholar]
- 539.Matsui Y, Kyoi S, Takagi H, Hsu CP, Hariharan N, Ago T, Vatner SF, Sadoshima J. Molecular mechanisms and physiological significance of autophagy during myocardial ischemia and reperfusion. Autophagy. 2008;4:409–415. doi: 10.4161/auto.5638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 540.Matsui Y, Takagi H, Qu X, Abdellatif M, Sakoda H, Asano T, Levine B, Sadoshima J. Distinct roles of autophagy in the heart during ischemia and reperfusion: Roles of AMP-activated protein kinase and Beclin 1 in mediating autophagy. Circ Res. 2007;100:914–922. doi: 10.1161/01.RES.0000261924.76669.36. [DOI] [PubMed] [Google Scholar]
- 541.Matsumoto S, Sakata Y, Nakatani D, Suna S, Mizuno H, Shimizu M, Usami M, Sasaki T, Sato H, Kawahara Y, Hamasaki T, Nanto S, Hori M, Komuro I. A subset of circulating microRNAs are predictive for cardiac death after discharge for acute myocardial infarction. Biochem Biophys Res Commun. 2012;427:280–284. doi: 10.1016/j.bbrc.2012.09.039. [DOI] [PubMed] [Google Scholar]
- 542.Matsumoto S, Sakata Y, Suna S, Nakatani D, Usami M, Hara M, Kitamura T, Hamasaki T, Nanto S, Kawahara Y, Komuro I. Circulating p53-responsive microRNAs are predictive indicators of heart failure after acute myocardial infarction. Circ Res. 2013;113:322–326. doi: 10.1161/CIRCRESAHA.113.301209. [DOI] [PubMed] [Google Scholar]
- 543.Matzinger P. The danger model: A renewed sense of self. Science. 2002;296:301–305. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- 544.Maurer M, Wedemeyer J, Metz M, Piliponsky AM, Welle rK, Chatterjea D, Clouthier DE, Yanagisawa MM. Mast cells promote homeostasis by limiting endothelin-1-induced toxicity. Nature. 2004;432:512–516. doi: 10.1038/nature03085. [DOI] [PubMed] [Google Scholar]
- 545.Mause SF, Weber C. Microparticles: Protagonists of a novel communication network for intercellular information exchange. Circ Res. 2010;107:1047–1057. doi: 10.1161/CIRCRESAHA.110.226456. [DOI] [PubMed] [Google Scholar]
- 546.Mazzoni MC, Borgstrom P, Warnke KC, Skalak TC, Intaglietta M, Arfors KE. Mechanisms and implications of capillary endothelial swelling and luminal narrowing in low-flow ischemias. Int J Microcirc Clin Exp. 1995;15:265–270. doi: 10.1159/000179028. [DOI] [PubMed] [Google Scholar]
- 547.McCall CE, El Gazzar M, Liu T, Vachharajani V, Yoza B. Epigenetics, bioenergetics, and microRNA coordinate gene-specific reprogramming during acute systemic inflammation. J Leukoc Biol. 2011;90:439–446. doi: 10.1189/jlb.0211075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 548.McDonald B, Pittman K, Menezes GB, Hirota SA, Slaba I, Waterhouse CCM, Beck PL, Muruve DA, Kubes P. Intravascular danger signals guide neutrophils to sites of sterile inflammation. Science. 2010;330:362–366. doi: 10.1126/science.1195491. [DOI] [PubMed] [Google Scholar]
- 549.McDougal WS. Renal perfusion/reperfusion injuries. J Urol. 1988;140:1325–30. doi: 10.1016/s0022-5347(17)42037-4. [DOI] [PubMed] [Google Scholar]
- 550.McManus DD, Freedman JE. MicroRNAs in platelet function and cardiovascular disease. Nat Rev Cardiol. 2015;12:711–717. doi: 10.1038/nrcardio.2015.101. [DOI] [PubMed] [Google Scholar]
- 551.Mehta D, Malik AB. Signaling mechanisms regulating endothelial permeability. Physiol. Rev. 2006;86:279–367. doi: 10.1152/physrev.00012.2005. [DOI] [PubMed] [Google Scholar]
- 552.Meldrum DR, Cleveland JC, Jr, Meng X, Sheridan BC, Gamboni F, Cain BS, Harken AH, Banerjee A. Protein kinase C isoform diversity in preconditioning. J Surg Res. 1997;69:183–187. doi: 10.1006/jsre.1997.5072. [DOI] [PubMed] [Google Scholar]
- 553.Meller R, Pearson A, Simon RP. Dynamic changes in DNA methylation in ischemic tolerance. Front Neurol. 2015;6:102. doi: 10.3389/fneur.2015.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 554.Mendez I, Vazquez-Martinez O, Hernandez-Munoz R, Valente-Godinez H, Diaz-Munoz M. Redox regulation and pro-oxidant reactions in the physiology of circadian systems. Biochimie. 2016;124:178–186. doi: 10.1016/j.biochi.2015.04.014. [DOI] [PubMed] [Google Scholar]
- 555.Menger MD, Thierjung C, Hammersen F, Messmer K. Dextran vs. hydroxyethylstarch in ischemic tolerance. Frontiersinhibitioninhibition of postischemic leukocyte adherence in neurology 6, 102 striated-striated muscle. Circ Shock. 1993;41:248–255. [PubMed] [Google Scholar]
- 556.Menger MD, Pelikan S, Steiner D, Messmer K. Microvascular ischemia-reperfusion injury in striated muscle: Significance of “reflow paradox”. Am J Physiol. 1992;263:H1901–1906. doi: 10.1152/ajpheart.1992.263.6.H1901. [DOI] [PubMed] [Google Scholar]
- 557.Menger MD, Steiner D, Messmer K. Microvascular ischemia-reperfusion injury in striated muscle: Significance of “no reflow”. Am J Physiol. 1992;263:H1892–1900. doi: 10.1152/ajpheart.1992.263.6.H1892. [DOI] [PubMed] [Google Scholar]
- 558.Mengesdorf T, Jensen PH, Mies G, Aufenberg C, Paschen W. Down-regulation of parkin protein in transient focal cerebral ischemia: A link between stroke and degenerative disease? Proc Natl Acad Sci U S A. 2002;99:15042–15047. doi: 10.1073/pnas.232588799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 559.Menini S, Amadio L, Oddi G, Ricci C, Pesci C, Pugliese F, Giorgio M, Migliaccio E, Pelicci P, Iacobini C, Pugliese G. Deletion of p66Shc longevity gene protects against experimental diabetic glomerulopathy by preventing diabetes-induced oxidative stress. Diabetes. 2006;55:1642–1650. doi: 10.2337/db05-1477. [DOI] [PubMed] [Google Scholar]
- 560.Metukuri MR, Beer-Stolz D, Namas RA, Dhupar R, Torres A, Loughran PA, Jefferson BS, Tsung A, Billiar TR, Vodovotz Y, Zamora R. Expression and subcellular localization of BNIP3 in hypoxic hepatocytes and liver stress. Am J Physiol Gastrointest Liver Physiol. 2009;296:G499–509. doi: 10.1152/ajpgi.90526.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 560a.Metz M, Maurer M. Mast cells-key effector cells in immune responses. Trends Immunol. 2007;28:234–241. doi: 10.1016/j.it.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 561.Meyer K, Zhang H, Zhang L. Direct effect of cocaine on epigenetic regulation of PKCe gene repression in the fetal rat heart. J Mol Cell Cardiol. 2009;47:504–511. doi: 10.1016/j.yjmcc.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 562.Miao W, Qu Z, Shi K, Zhang D, Zong Y, Zhang G, Hu S. RIP3 S-nitrosylation contributes to cerebral ischemic neuronal injury. Brain Res. 2015;1627:165–176. doi: 10.1016/j.brainres.2015.08.020. [DOI] [PubMed] [Google Scholar]
- 563.Milerova M, Charvatova Z, Skarka L, Ostadalova I, Drahota Z, Fialova M, Ostadal B. Neonatal cardiac mitochondria and ischemia/reperfusion injury. Mol Cell Biochem. 2010;335:147–153. doi: 10.1007/s11010-009-0251-x. [DOI] [PubMed] [Google Scholar]
- 564.Minamino T, Komuro I, Kitakaze M. Endoplasmic reticulum stress as a therapeutic target in cardiovascular disease. Circ Res. 2010;107:1071–1082. doi: 10.1161/CIRCRESAHA.110.227819. [DOI] [PubMed] [Google Scholar]
- 565.Miura T, Miki T. Limitation of myocardial infarct size in the clinical setting: Current status and challenges in translating animal experiments into clinical therapy. Basic Res Cardiol. 2008;103:501–513. doi: 10.1007/s00395-008-0743-y. [DOI] [PubMed] [Google Scholar]
- 566.Moquin D, Chan FK. The molecular regulation of programmed necrotic cell injury. Trends Biochem Sci. 2010;35:434–441. doi: 10.1016/j.tibs.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 567.Morgan MJ, Kim YS, Liu ZG. TNFa and reactive oxygen species in necrotic cell death. Cell Res. 2008;18:343–349. doi: 10.1038/cr.2008.31. [DOI] [PubMed] [Google Scholar]
- 568.Moritz KM, De Matteo R, Dodic M, Jefferies AJ, Arena D, Wintour EM, Probyn ME, Bertram JF, Singh RR, Zanini S, Evans RG. Prenatal glucocorticoid exposure in the sheep alters renal development in utero: Implications for adult renal function and blood pressure control. Am J Physiol Regul Integr Comp Physiol. 2011;301:R500–R509. doi: 10.1152/ajpregu.00818.2010. [DOI] [PubMed] [Google Scholar]
- 569.Muller FL, Song W, Liu Y, Chaudhuri A, Pieke-Dahl S, Strong R, Huang TT, Epstein CJ, Roberts LJ, Jr, Csete M, Faulkner JA, Van Remmen H. Absence of CUZn superoxide dismutase leads to elevated oxidative stress and acceleration of age-dependent skeletal muscle atrophy. Free Radic Biol Med. 2006;40:1993–2004. doi: 10.1016/j.freeradbiomed.2006.01.036. [DOI] [PubMed] [Google Scholar]
- 570.Muramatsu Y, Furuichi Y, Tojo N, Moriguchi A, Maemoto T, Nakada H, Hino M, Matsuoka N. Neuroprotective efficacy of FR901459, a novel derivative of cyclosporin A, in in vitro mitochondrial damage and in vivo transient cerebral ischemia models. Brain Res. 2007;1149:181–190. doi: 10.1016/j.brainres.2007.02.036. [DOI] [PubMed] [Google Scholar]
- 571.Murayama T, Tanabe M, Matsuda S, Shimazu M, Kamei S, Wakabayashi G, Kawachi S, Matsumoto K, Yamazaki K, Matsumoto K, Koyasu S, Kitajima M. JNK (c-Jun NH2 terminal kinase) and p38 during ischemia reperfusion injury in the small intestine. Transplantation. 2006;81:1325–1330. doi: 10.1097/01.tp.0000209167.48030.6b. [DOI] [PubMed] [Google Scholar]
- 572.Murphy E, Steenbergen C. Ion transport and energetics during cell death and protection. Physiology (Bethesda) 2008;23:115–123. doi: 10.1152/physiol.00044.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 573.Murphy SP, Porrett PM, Turka LA. Innate immunity in transplant tolerance and rejection. Immunol Rev. 2011;241:39–48. doi: 10.1111/j.1600-065X.2011.01009.x. [DOI] [PubMed] [Google Scholar]
- 574.Murriel CL, Churchill E, Inagaki K, Szweda LI, Mochly-Rosen D. Protein kinase Cd activation induces apoptosis in response to cardiac ischemia and reperfusion damage: A mechanism involving BAD and the mitochondria. J Biol Chem. 2004;279:47985–47991. doi: 10.1074/jbc.M405071200. [DOI] [PubMed] [Google Scholar]
- 575.Murriel CL, Mochly-Rosen D. Opposing roles of delta and epsilonPKC in cardiac ischemia and reperfusion: Targeting the apoptotic machinery. Arch Biochem Biophys. 2003;420:246–254. doi: 10.1016/j.abb.2003.08.038. [DOI] [PubMed] [Google Scholar]
- 576.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: A delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 577.Murry CE, Soonpaa MH, Reinecke H, Nakajima H, Nakajima HO, Rubart M, Pasumarthi KB, Virag JI, Bartelmez SH, Poppa V, Bradford G, Dowell JD, Williams DA, Field LJ. Haematopoietic stem cells do not transdifferentiate into cardiac myocytes in myocardial infarcts. Nature. 2004;428:664–668. doi: 10.1038/nature02446. [DOI] [PubMed] [Google Scholar]
- 578.Muschen M, Warskulat U, Peters-Regehr T, Bode JG, Kubitz R, Haussinger D. Involvement of CD95 (Apo-1/Fas) ligand expressed by rat Kupffer cells in hepatic immunoregulation. Gastroenterol. 1999;116:666–677. doi: 10.1016/s0016-5085(99)70189-7. [DOI] [PubMed] [Google Scholar]
- 579.Muthusamy V, Bosenberg M, Wajapeyee N. Redefining regulation of DNA methylation by RNA interference. Genomics. 2010;96:191–198. doi: 10.1016/j.ygeno.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 580.Nakahira K, Hisata S, Choi AM. The roles of mitochondrial damage-associated molecular patterns in diseases. Antioxid Redox Signal. 2015;23:1329–1350. doi: 10.1089/ars.2015.6407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 581.Nakano Y, Kondo T, Matsuo R, Hashimoto I, Kawasaki T, Kohno K, Myronovych A, Tadano S, Hisakura K, Ikeda O, Watganabe M, Murata S, Fukunaga K, Ohkohchi N. Platelet dynamics in the early phase of postischemic liver in vivo. J Surg Res. 2008;149:192–198. doi: 10.1016/j.jss.2007.09.016. [DOI] [PubMed] [Google Scholar]
- 582.Nardone G, Compare D, Liguori E, Di Mauro V, Rocco A, Barone M, Napoli A, Lapi D, Iovene MR, Colantuoni A. Protective effects of Lactobacillus paracasei F19 in a rat model of oxidative and metabolic hepatic injury. Am J Physiol Gastrointest Liver Physiol. 2010;299:G669–G676. doi: 10.1152/ajpgi.00188.2010. [DOI] [PubMed] [Google Scholar]
- 583.Netticadan T, Temsah R, Osada M, Dhalla NS. Status of Ca2+/calmodulin protein kinase phosphorylation of cardiac SR proteins in ischemia-reperfusion. Am J Physiol. 1999;277:C384–C391. doi: 10.1152/ajpcell.1999.277.3.C384. [DOI] [PubMed] [Google Scholar]
- 584.Neuhof C, Neuhof H. Calpain system and its involvement in myocardial ischemia and reperfusion injury. World J Cardiol. 2014;6:638–652. doi: 10.4330/wjc.v6.i7.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 585.Ngoh GA, Facundo HT, Hamid T, Dillmann W, Zachara NE, Jones SP. Unique hexosaminidase reduces metabolic survival signal and sensitizes cardiac myocytes to hypoxia/reoxygenation injury. Circ Res. 2009;104:41–49. doi: 10.1161/CIRCRESAHA.108.189431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 586.Niccoli G, Burzotta F, Galiuto L, Crea F. Myocardial no-reflow in humans. J Am Coll Cardiol. 2009;54:281–292. doi: 10.1016/j.jacc.2009.03.054. [DOI] [PubMed] [Google Scholar]
- 587.Niccoli G, Cosentino N, Spaziani C, Minelli S, Fracassi F, Crea F. New strategies for the management of no-reflow after primary percutaneous coronary intervention. Expert Rev Cardiovasc Ther. 2011;9:615–630. doi: 10.1586/erc.11.49. [DOI] [PubMed] [Google Scholar]
- 588.Nielsen M, Zimmer J, Diemer NH. Endonuclease G expression in thalamic reticular nucleus after global cerebral ischemia. Exp Brain Res. 2008;190:81–89. doi: 10.1007/s00221-008-1452-3. [DOI] [PubMed] [Google Scholar]
- 589.Nieswandt B, Pleines I, Bender M. Platelet adhesion and activation mechanisms in arterial thrombosis and ischaemic stroke. J Thromb Haemost. 2011;9(Suppl 1):92–104. doi: 10.1111/j.1538-7836.2011.04361.x. [DOI] [PubMed] [Google Scholar]
- 590.Nilakantan V, Liang HL, Rajesh S, Mortensen J, Chandran K. Time-dependant protective effects of mangenese(III) tetrakis (1-methyl-4-pyridyl) porphyrin on mitochondrial function following renal ischemia-reperfusion injury. Free Radic Res. 2010;44:773–782. doi: 10.3109/10715761003786164. [DOI] [PubMed] [Google Scholar]
- 591.Nordente A, Martorana GE, Miggianno GA, Petitti T, Giardina B, Littaru GP, Santini SA. Free radical production by activated haem proteins: Protective effect of coenzyme Q. Mol Aspects Med. 1994;15(Suppl):S109–S115. doi: 10.1016/0098-2997(94)90020-5. [DOI] [PubMed] [Google Scholar]
- 592.Norman MU, Zbytnuik L, Kubes P. Interferon-γ limits lymphocyte adhesion to inflamed endothelium: A nitric oxide regulatory feedback mechanism. Eur J Immunol. 2008;38:1368–1380. doi: 10.1002/eji.200737847. [DOI] [PubMed] [Google Scholar]
- 593.Northington FJ, Chavez-Valdez R, Graham EM, Razdan S, Gauda EB, Martin LJ. Necrostatin decreases oxidative damage, inflammation, and injury after neonatal HI. J Cereb Blood Flow Metab. 2011;31:178–189. doi: 10.1038/jcbfm.2010.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 594.Nourshargh S, Alon R. Leukocyte migration into inflamed tissues. Immunity. 2014;41:694–707. doi: 10.1016/j.immuni.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 595.Nourshargh S, Hordijk PL, Sixt M. Breaching multiple barriers: Leukocyte motility through venular walls and the interstitium. Nat Rev Mol Cell Biol. 2010;11:366–378. doi: 10.1038/nrm2889. [DOI] [PubMed] [Google Scholar]
- 596.Nourshargh S, Renshaw SA, Imhof BA. Reverse migration of neutrophils: Where, when how, and why? Trends Immunol. 2016;37:273–286. doi: 10.1016/j.it.2016.03.006. [DOI] [PubMed] [Google Scholar]
- 597.O’Donnell CJ, Nabel EG. Genomics of cardiovascular disease. N Engl J Med. 2011;365:2098–2109. doi: 10.1056/NEJMra1105239. [DOI] [PubMed] [Google Scholar]
- 598.O’Farrell FM, Attwell D. A role for pericytes in coronary no-reflow. Nat Rev Cardiol. 2014;11:427–432. doi: 10.1038/nrcardio.2014.58. [DOI] [PubMed] [Google Scholar]
- 599.Oerlemans MI, Koudstaal S, Chamuleau SA, de Kleijn DP, Doevendans PA, Sluijter JP. Targeting cell death in the reperfused heart: Pharmacological approaches for cardioprotection. Int J Cardiol. 2013;165:410–422. doi: 10.1016/j.ijcard.2012.03.055. [DOI] [PubMed] [Google Scholar]
- 600.Ojha S, Fainberg HP, Sebert S, Budge H, Symonds ME. Maternal health and eating habits: Metabolic consequences and impact on child health. Trends Mol Med. 2015;21:126–133. doi: 10.1016/j.molmed.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 601.Okuno S, Saito A, Hayashi T, Chan PH. The c-Jun N-terminal protein kinase signaling pathway mediates Bax activation and subsequent neuronal apoptosis through interaction with Bim after transient focal cerebral ischemia. J Neurosci. 2004;24:7879–7887. doi: 10.1523/JNEUROSCI.1745-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 602.Oliver MG, Specian RD, Perry MA, Granger DN. Morphologic assessment of leukocyte-endothelial cell interactions in mesenteric venules subjected to ischemia and reperfusion. Inflammation. 1991;15:331–346. doi: 10.1007/BF00917350. [DOI] [PubMed] [Google Scholar]
- 603.Ong SB, Gustafsson AB. New roles for mitochondria in cell death in the reperfused myocardium. Cardiovasc Res. 2012;94:190–193. doi: 10.1093/cvr/cvr312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 604.Ong SB, Samangouei P, Kalkhoran SB, Hausenloy DJ. The mitochondrial permeability transition pore and its role in myocardial ischemia reperfusion injury. J Mol Cell Cardiol. 2015;78:23–34. doi: 10.1016/j.yjmcc.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 605.Ong SB, Subrayan S, Lim SY, Yellon DM, Davidson SM, Hausenloy DJ. Inhibiting mitochondrial fission protects the heart against ischemia/reperfusion injury. Circulation. 2010;121:2012–2022. doi: 10.1161/CIRCULATIONAHA.109.906610. [DOI] [PubMed] [Google Scholar]
- 606.Ordy JM, Wengenack TM, Bialobok P, Coleman PD, Rodier P, Baggs RB, Dunlap WP, Kates B. Selective vulnerability and early progression of hippocampal CA1 pyramidal cell degeneration and GFAP-positive astrocyte reactivity in the rat four-vessel occlusion model of transient global ischemia. Exp Neurol. 1993;119:128–139. doi: 10.1006/exnr.1993.1014. [DOI] [PubMed] [Google Scholar]
- 607.Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, Pickel J, McKay R, Nadal-Ginard B, Bodine DM, Leri A, Anversa P. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–705. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 608.Orsini F, Moroni M, Contursi C, Yano M, Pelicci P, Giorgio M, Migliaccio E. Regulatory effects of the mitochondrial energetic status on mitochondrial p66Shc. Biol Chem. 2006;387:1405–1410. doi: 10.1515/BC.2006.176. [DOI] [PubMed] [Google Scholar]
- 609.Osada M, Netticadan T, Kawabata K, Tamura K, Dhalla NS. Ischemic preconditioning prevents I/R-induced alterations in SR calcium-calmodulin protein kinase II. Am J Physiol. 2000;278:H1791–H1798. doi: 10.1152/ajpheart.2000.278.6.H1791. [DOI] [PubMed] [Google Scholar]
- 610.Osman M, Russell J, Granger DN. Lymphocyte-derived interferon-γ mediates ischemia-reperfusion-induced leukocyte and platelet adhesion in intestinal microcirculation. Am J Physiol. 2009;296:G659–G663. doi: 10.1152/ajpgi.90495.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 611.Ostadal B, Ostadalova I, Kolar F, Sedmera D. Developmental determinants of cardiac sensitivity to hypoxia. Can J Physiol Pharmacol. 2014;92:566–574. doi: 10.1139/cjpp-2013-0498. [DOI] [PubMed] [Google Scholar]
- 612.Ostadal B, Ostadalova I, Kolar F, Sedmera D. The use of microRNAs to modulate redox and immune response to stroke. Antioxid Redox Signal. 2015;22:187–202. doi: 10.1089/ars.2013.5757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 613.OuyangOuyang YB, Stary CM, White RE, Giffard RG. Developmental determinants of cardiac sensitivity to hypoxia. Can J Physiol Pharmacol. 2015;92:566–574. doi: 10.1139/cjpp-2013-0498. [DOI] [PubMed] [Google Scholar]
- 614.Ovize M, Thibault H, Przyklenk K. Myocardial conditioning: Opportunities for clinical translation. Circ Res. 2013;113:439–450. doi: 10.1161/CIRCRESAHA.113.300764. [DOI] [PubMed] [Google Scholar]
- 615.Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 616.Palladini G, Ferrigno A, Richelmi P, Perlini S, Vairetti M. Role of matrix metalloproteinases in cholestasis and hepatic ischemia/reperfusion injury: A review. World J Gastroenterol. 2015;21:12114–12124. doi: 10.3748/wjg.v21.i42.12114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 617.Pallast S, Arai K, Pekcec A, Yigitkanli K, Yu Z, Wang X, Lo EH, van Leyen K. Increased nuclear apoptosis-inducing factor after transient focal ischemia: A 12/15-lipoxygenase-dependent organelle damage pathway. J Cereb Blood Flow Metab. 2010;30:1157–1167. doi: 10.1038/jcbfm.2009.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 618.Pallast S, Arai K, Wang X, Lo EH, van Leyen K. 12/15-Lipoxygenase targets neuronal mitochondria under oxidative stress. J Neurochem. 2009;111:882–889. doi: 10.1111/j.1471-4159.2009.06379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 619.Paradies G, Petrosillo G, Paradies V, Ruggiero FM. Role of cardiolipin peroxidation and Ca2+ in mitochondrial dysfunction and disease. Cell Calcium. 2009;45:643–650. doi: 10.1016/j.ceca.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 620.Parks DA, Bulkley GB, Granger DN, Hamilton SR, McCord JM. Ischemic injury in the cat small intestine: Role of superoxide radicals. Gastroenterology. 1982;82:9–15. [PubMed] [Google Scholar]
- 621.Park P, Haas M, Cunningham PN, Bao L, Alexander JJ, Quigg RJ. Injury in renal ischemia-reperfusion is independent from immunoglobulins and T-lymphocytes. Am J Physiol. 2002;282:F352–F357. doi: 10.1152/ajprenal.00160.2001. [DOI] [PubMed] [Google Scholar]
- 622.Park PO, Haglund U. Regeneration of small bowel mucosa after intestinal ischemia. Crit Care Med. 1992;20:135–139. doi: 10.1097/00003246-199201000-00026. [DOI] [PubMed] [Google Scholar]
- 623.Park S, Kondo T, Nakano Y, Murata S, Fukunaga K, Oda T, Sasakik R, Ohkohchi N. Platelet adhesion in the sinusoid caused hepatic injury by neutrophils after hepatic ischemia reperfusion. Platelets. 2010;21:282–288. doi: 10.3109/09537101003637265. [DOI] [PubMed] [Google Scholar]
- 624.Parks DA, Granger DN. Xanthine oxidase: Biochemistry, distribution and physiology. Acta Physiol Scand. 1986;548:87–99. [PubMed] [Google Scholar]
- 625.Patel B, Fisher M. Therapeutic advances in myocardial microvascular resistance: Unravelling the enigma. Pharmacol Ther. 2010;127:131–147. doi: 10.1016/j.pharmthera.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 626.Patterson AJ, Chen M, Xue Q, Xiao D, Zhang L. Chronic prenatal hypoxia induces epigenetic programming of PKC {epsilon} gene repression in rat hearts. Circ Res. 2010;107:365–373. doi: 10.1161/CIRCRESAHA.110.221259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 626a.Patterson AJ, Zhang L. Hypoxia and fetal heart development. Curr Mol Med. 2010;10:653–666. doi: 10.2174/156652410792630643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 626b.Patterson AJ, Xiao D, Xiong F, Dixon B, Zhang L. Hypoxia-derived oxidative stress mediates epigenetic repression of PKCε gene in foetal rat hearts. Cardiovasc Res. 2012;93:302–310. doi: 10.1093/cvr/cvr322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 627.Pavlov VI, Tan YS, McClure EE, La Bonte LR, Zou C, Gorsuch WB, Stahl GL. Human mannose-binding lectin inhibitor prevents myocardial injury and arterial thrombogenesis in a novel animal model. Am J Pathol. 2015;185:347–355. doi: 10.1016/j.ajpath.2014.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 628.Pell VR, Chouchani ET, Murphy MP, Brookes PS, Krieg T. Moving forwards by blocking back-flow: The Yin and Yang of MI Therapy. Circ Res. 2016;118:898–906. doi: 10.1161/CIRCRESAHA.115.306569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 629.Peng S, Kuang Z, Zhang Y, Xu H, Cheng Q. The protective effects and potential mechanism of Calpain inhibitor Calpeptin against focal cerebral ischemia-reperfusion injury in rats. Mol Biol Rep. 2011;38:905–912. doi: 10.1007/s11033-010-0183-2. [DOI] [PubMed] [Google Scholar]
- 630.Pepe S. Mitochondrial function in ischaemia and reperfusion of the ageing heart. Clin Exp Pharmacol Physiol. 2000;27:745–750. doi: 10.1046/j.1440-1681.2000.03326.x. [DOI] [PubMed] [Google Scholar]
- 631.Peppiatt CM, Howarth C, Mobbs P, Attwell D. Bidirectional control of CNS capillary diameter by pericytes. Nature. 2006;443:700–704. doi: 10.1038/nature05193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 632.Perez-Chanona E, Muhlbauer M, Jobin C. The microbiota protects against ischemia/reperfusion-induced intestinal injury through nucleotide-binding oligomerization domain-containing protein 2 (NOD2) signaling. Am J Pathol. 2014;184:2965–2975. doi: 10.1016/j.ajpath.2014.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 633.Perrelli M-G, Pagliaro P, Penna C. Ischemia/reperfusion injury and cardioprotective mechanisms: Role of mitochondria and reactive oxygen species. World J Cardiol. 2011;3:186–200. doi: 10.4330/wjc.v3.i6.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 634.Peters MJ, Dixon G, Kotowicz KT, Hatch DJ, Heyderman RS, Klein NJ. Circulating platelet-neutrophil complexes represent a subpopulation of activated neutrophils primed for adhesion, phagocytosis and intracellular killing. Br J Haematol. 1999;106:391–399. doi: 10.1046/j.1365-2141.1999.01553.x. [DOI] [PubMed] [Google Scholar]
- 634a.Petrovic-Djergovic D, Goonewardena SN, Pinsky DJ. Inflammatory disequilibrium in stroke. Circ Res. 2016;119:142–158. doi: 10.1161/CIRCRESAHA.116.308022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 635.Phillips MJ, Voeltz GK. Structure and function of ER membrane contact sites with other organelles. Nat Rev Mol Cell Biol. 2016;17:69–82. doi: 10.1038/nrm.2015.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 636.Phillis JW, O’Regan MH. The role of phospholipases, cyclooxygenases, and lipoxygenases in cerebral ischemic/traumatic injuries. Crit Rev Neurobiol. 2003;15:61–90. doi: 10.1615/critrevneurobiol.v15.i1.30. [DOI] [PubMed] [Google Scholar]
- 637.Piao CS, Kim JB, Han PL, Lee JK. Administration of the p38 MAPK inhibitor SB203580 affords brain protection with a wide therapeutic window against focal ischemic insult. J Neurosci Res. 2003;73:537–544. doi: 10.1002/jnr.10671. [DOI] [PubMed] [Google Scholar]
- 638.Pigazzi A, Heydrick S, Folli F, Benoit S, Michelson A, Loscalzo J. Nitric oxide inhibits thrombin receptor-activating peptide-induced phosphoinositide 3-kinase activity in human platelets. J Biol Chem. 1999;274:14368–14375. doi: 10.1074/jbc.274.20.14368. [DOI] [PubMed] [Google Scholar]
- 639.Pinto AR, Ilinykh A, Ivey MJ, Kuwabara JT, D’Antoni ML, Debuque R, Chandran A, Wang L, Arora K, Rosenthal NA, Tallquist ML. Revisiting cardiac cellular composition. Circ Res. 2016;118:400–409. doi: 10.1161/CIRCRESAHA.115.307778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 639a.Podestà MA, Cucchiari D, Ponticelli C. The diverging roles of dendritic cells in kidney allotransplantation. Transplant Rev. 2015;29:114–120. doi: 10.1016/j.trre.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 639b.Prabhu SD, Frangogiannis NG. The biological basis for cardiac repair after myocardial infarction: From inflammation to fibrosis. Circ Res. 2016;119:91–112. doi: 10.1161/CIRCRESAHA.116.303577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 640.Prakash A, Sundar SV, Zhu YG, Tran A, Lee JW, Lowell C, Hellman J. Lung ischemia-reperfusion is a sterile inflammatory process influenced by commensal microbiota in mice. Shock. 2015;44:272–279. doi: 10.1097/SHK.0000000000000415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 641.Premen AJ, Banchs V, Womack WA, Kvietys PR, Granger DN. Importance of collateral circulation in the vascularly occluded feline intestine. Gastroenterology. 1987;92:1215–1219. doi: 10.1016/s0016-5085(87)91080-8. [DOI] [PubMed] [Google Scholar]
- 642.Pridjian AK, Levitsky S, Krukenkamp I, Silverman NA, Feinberg H. Developmental changes in reperfusion injury. A comparison of intracellular cation accumulation in the newborn, neonatal, and adult heart. J Thorac Cardiovasc Surg. 1987;93:428–433. [PubMed] [Google Scholar]
- 643.Prime TA, Blaikie FH, Evans C, Nadtochiy SM, James AM, Dahm CC, Vitturi DA, Patel RP, Hiley CR, Abakumova I, Requejo R, Chouchani ET, Hurd TR, Garvey JF, Taylor CT, Brookes PS, Smith RA, Murphy MP. A mitochondria-targeted S-nitrosothiol modulates respiration, nitrosates thiols, and protects against ischemia-reperfusion injury. Proc Natl Acad Sci. 2009;106:10764–10769. doi: 10.1073/pnas.0903250106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 644.Proebstl D, Voisin MB, Woodfin A, Whiteford J, D’Acquisto F, Jones GE, Rowe D, Nourshargh S. Pericytes support neutrophil subendothelial cell crawling and breaching of venular walls in vivo. J Exp Med. 2012;209:1219–1234. doi: 10.1084/jem.20111622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 645.Przyklenk K. Ischaemic conditioning: Pitfalls on the path to clinical translation. Br J Pharmacol. 2015;172:1961–1973. doi: 10.1111/bph.13064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 646.Puglisi RN, Strande L, Santos M, Schulte G, Hewitt CW, Whalen TV. Beneficial effects of cyclosporine and rapamycin in small bowel ischemic injury. J Surg Res. 1996;65:115–118. doi: 10.1006/jsre.1996.0352. [DOI] [PubMed] [Google Scholar]
- 647.Qin B, Yang H, Xiao B. Role of microRNAs in endothelial inflammation and senescence. Mol Biol Rep. 2012;39:4509–4518. doi: 10.1007/s11033-011-1241-0. [DOI] [PubMed] [Google Scholar]
- 648.Quartara L, Maggi CA. The tachykinin NK1 receptor. Part II: Distribution and pathophysiological roles. Neuropeptides. 1998;32:1–49. doi: 10.1016/s0143-4179(98)90015-4. [DOI] [PubMed] [Google Scholar]
- 648a.Quesnelle KM, Bystrom PV, Toledo-Pereyra LH. Molecular responses to ischemia and reperfusion in the liver. Arch Toxicol. 2015;89:651–657. doi: 10.1007/s00204-014-1437-x. [DOI] [PubMed] [Google Scholar]
- 649.Raedschelders K, Ansley DM, Chen DDY. The cellular and molecular origin of reactive oxygen species generation during myocardial ischemia and reperfusion. Pharmacol Therap. 2012;133:230–255. doi: 10.1016/j.pharmthera.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 650.Rahimtoola SH. The hibernating myocardium. Am Heart J. 1989;117:211–221. doi: 10.1016/0002-8703(89)90685-6. [DOI] [PubMed] [Google Scholar]
- 651.Ramos G, Hofmann U, Frantz S. Myocardial fibrosis through the lenses of T-cell biology. J Mol Cell Cardiol. 2016;92:41–45. doi: 10.1016/j.yjmcc.2016.01.018. [DOI] [PubMed] [Google Scholar]
- 652.Rautou PE, Vion AC, Amabile N, Chironi G, Simon A, Tedgui A, Boulanger CM. Microparticles, vascular function, and atherothrombosis. Circ Res. 2011;109:593–606. doi: 10.1161/CIRCRESAHA.110.233163. [DOI] [PubMed] [Google Scholar]
- 653.Ren M, Leng Y, Jeong M, Leeds PR, Chuang DM. Valproic acid reduces brain damage induced by transient focal cerebral ischemia in rats: Potential roles of histone deacetylase inhibition and heat shock protein induction. J Neurochem. 2004;89:1358–1367. doi: 10.1111/j.1471-4159.2004.02406.x. [DOI] [PubMed] [Google Scholar]
- 654.Ren XP, Wu J, Wang X, Sartor MA, Qian J, Jones K, Nicolaou P, Pritchard TJ, Fan GC. MicroRNA-320 is involved in the regulation of cardiac ischemia/reperfusion injury by targeting heat-shock protein 20. Circulation. 2009;119:2357–2366. doi: 10.1161/CIRCULATIONAHA.108.814145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 655.Ren Z, Jiang J, Lu H, Chen X, He Y, Zhang H, Xie H, Wang W, Zheng S, Zhou L. Intestinal microbial variation may predict early acute rejection after liver transplantation in rats. Transplantation. 2014;98:844–852. doi: 10.1097/TP.0000000000000334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 656.Reynaert NL, Guala AS, Wouters EF, van der Vliet A, Janssen-Heininger YM. Nitric oxide represses inhibitory κB kinase through S-nitrosylation. Proc Natl Acad Sci U S A. 2004;101:8945–8950. doi: 10.1073/pnas.0400588101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 657.Reynolds RM. Corticosteroid-mediated programming and the pathogenesis of obesity and diabetes. J Steroid Biochem Mol Biol. 2010;122:3–9. doi: 10.1016/j.jsbmb.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 658.Riaz AA, Wan MX, Schaefer T, Schramm R, Ekberg H, Menger MD, Jeppsson B, Thorlacius H. Fundamental and distinct roles of P-selectin and LFA-1 in ischemia/reperfusion-induced leukocyte-endothelium interactions in the mouse colon. Ann Surg. 2002;236:777–784. doi: 10.1097/00000658-200212000-00010. discussion 784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 659.Ribatti D. The crucial role of mast cells in blood-brain barrier alterations. Exp Cell Res. 2015;338:119–125. doi: 10.1016/j.yexcr.2015.05.013. [DOI] [PubMed] [Google Scholar]
- 660.Roberts VH, Frias AE, Grove KL. Impact of maternal obesity on fetal programming of cardiovascular disease. Physiology (Bethesda) 2015;30(3):224–231. doi: 10.1152/physiol.00021.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 661.Rocha-Ferreira E, Phillips E, Francesch-Domenech E, Thei L, Peebles DM, Raivich G, Hristova M. The role of different strain backgrounds in bacterial endotoxin-mediated sensitization to neonatal hypoxic-ischemic brain damage. Neuroscience. 2015;311:292–307. doi: 10.1016/j.neuroscience.2015.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 662.Rodriguez SF, Granger DN. Role of blood cells in ischemia-reperfusion-induced endothelial barrier failure. Cardiovasc Res. 2010;87:291–299. doi: 10.1093/cvr/cvq090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 663.Roe ND, Ren J. Nitric oxide synthase uncoupling: A therapeutic target in cardiovascular diseases. Vascul Pharmacol. 2012;57:168–172. doi: 10.1016/j.vph.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 664.Roerecke M, Rehm J. Alcohol consumption, drinking patterns, and ischemic heart disease: A narrative review of meta-analyses and a systematic review and meta-analysis of the impact of heavy drinking occasions on risk for moderate drinkers. BMC Med. 2014;12:182. doi: 10.1186/s12916-014-0182-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 664a.Rossaint J, Zarbock A. Platelets in leucocyte recruitment and function. Cardiovasc Res. 2015;107:386–395. doi: 10.1093/cvr/cvv048. [DOI] [PubMed] [Google Scholar]
- 665.Roy S, Khanna S, Hussain SR, Biswas S, Azad A, Rink C, Gnyawali S, Shilo S, Nuovo GJ, Sen CK. MicroRNA expression in response to murine myocardial infarction: miR-21 regulates fibroblast metalloprotease-2 via phosphatase and tensin homologue. Cardiovasc Res. 2009;82:21–29. doi: 10.1093/cvr/cvp015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 666.Romagnani S. Regulation of the T cell response. Clin Exptl Allergy. 2006;36:1357–1366. doi: 10.1111/j.1365-2222.2006.02606.x. [DOI] [PubMed] [Google Scholar]
- 667.Romson JL, Hook BG, Kunkel SL, Abrams GD, Schork MA, Lucchesi BR. Reduction of the extent of ischemic myocardial injury by neutrophil depletion in the dog. Circulation. 1983;67:1016–1023. doi: 10.1161/01.cir.67.5.1016. [DOI] [PubMed] [Google Scholar]
- 668.Rork TH, Hadzimichalis NM, Kappil MA, Merrill GF. Acetaminophen attenuates peroxynitrite-activated matrix metalloprotease-2-mediated troponin I cleavage in the isolated guinea pig myocardium. J Mol Cell Cardiol. 2006;40:553–561. doi: 10.1016/j.yjmcc.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 669.Rosen SE, Henry S, Bond R, Pearte C, Mieres JH. Sex-specific disparities in risk factors for coronary heart disease. Curr Atheroscler Rep. 2015;17(8):523. doi: 10.1007/s11883-015-0523-8. [DOI] [PubMed] [Google Scholar]
- 670.Rosenbaum DM, Degterev A, David J, Rosenbaum PS, Roth S, Grotta JC, Cuny GD, Yuan J, Savitz SI. Necroptosis, a novel form of caspase-independent cell death, contributes to neuronal damage in a retinal ischemia-reperfusion injury model. J Neurosci Res. 2010;88:1569–1576. doi: 10.1002/jnr.22314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 671.Rossaint J, Zarbock A. Platelets in leucocyte recruitment and function. Cardiovasc Res. 2015;107:386–395. doi: 10.1093/cvr/cvv048. [DOI] [PubMed] [Google Scholar]
- 672.Rossen RD, Michael LH, Hawkins HK, Youker K, Dreyer WJ, Baughn RE, Entman ML. Cardiolipin-protein complexes and initiation of complement activation after coronary artery occlusion. Circ Res. 1994;75:546–555. doi: 10.1161/01.res.75.3.546. [DOI] [PubMed] [Google Scholar]
- 673.Rotter D, Grinsfelder DB, Parra V, Pedrozo Z, Singh S, Sachan N, Rothermel BA. Calcineurin and its regulator, RCAN1, confer time-of-day changes in susceptibility of the heart to ischemia/reperfusion. J Mol Cell Cardiol. 2014;74:103–111. doi: 10.1016/j.yjmcc.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 674.Roy S, Khanna S, Hussain SR, Biswas S, Azad A, Rink C, Gnyawali S, Shilo S, Nuovo GJ, Sen CK. MicroRNA expression in response to murine myocardial infarction: miR-21 regulates fibroblast metalloprotease-2 via phosphatase and tensin homologue. Cardiovasc Res. 2009;82:21–29. doi: 10.1093/cvr/cvp015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 675.Rudolf V, Freeman BA. Cardiovascular consequences when nitric oxide and lipid signaling converge. Circ Res. 2009;105:511–512. doi: 10.1161/CIRCRESAHA.109.202077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 675a.Rueda-Clausen CF, Morton JS, Davidge ST. Effects of hypoxia-induced intrauterine growth restriction on cardiopulmonary structure and function during adulthood. Cardiovasc Res. 2009;81:713–722. doi: 10.1093/cvr/cvn341. [DOI] [PubMed] [Google Scholar]
- 675b.Rueda-Clausen CF, Morton JS, Lopaschuk GD, Davidge ST. Long-term effects of intrauterine growth restriction on cardiac metabolism and susceptibility to ischaemia/reperfusion. Cardiovasc Res. 2011;90:285–294. doi: 10.1093/cvr/cvq363. [DOI] [PubMed] [Google Scholar]
- 675c.Rueda-Clausen CF, Morton JS, Dolinsky VW, Dyck JR, Davidge ST. Synergistic effects of prenatal hypoxia and postnatal high-fat diet in the development of cardiovascular pathology in young rats. Am J Physiol Regul Integr Comp Physiol. 2012;303:R418–R426. doi: 10.1152/ajpregu.00148.2012. [DOI] [PubMed] [Google Scholar]
- 675d.Rueda-Clausen CF, Morton JS, Oudit GY, Kassiri Z, Jiang Y, Davidge ST. Effects of hypoxia-induced intrauterine growth restriction on cardiac siderosis and oxidative stress. J Dev Orig Health Dis. 2012;3(5):350–357. doi: 10.1017/S2040174412000219. [DOI] [PubMed] [Google Scholar]
- 676.Russell J, Cooper D, Tailor A, Stokes KY, Ganger DN. Low venular shear rates promote leukocyte-dependent recruitment of adherent platelets. Am J Physiol. 2003;284:G123–G129. doi: 10.1152/ajpgi.00303.2002. [DOI] [PubMed] [Google Scholar]
- 676a.Ryan KJ, Elmes MJ, Langley-Evans SC. The effects of prenatal protein restriction on β-adrenergic signalling of the adult rat heart during ischaemia reperfusion. J Nutr Metab. 2012:397389. doi: 10.1155/2012/397389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 677.Ryter SW, Alam J, Choi AMK. Heme oxygenase-1/carbon monoxide: From basic science to therapeutic applications. Physiol Rev. 2006;86:583–650. doi: 10.1152/physrev.00011.2005. [DOI] [PubMed] [Google Scholar]
- 678.Ryter SW, Mizumura K, Choi AM. The impact of autophagy on cell death modalities. Int J Cell Biol. 2014;2014:502676. doi: 10.1155/2014/502676. Epub 2014 Feb 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 679.Saitoh M, Nishitoh H, Fuji M, Takeda K, Tobiume K, Sawada Y, Kawabata M, Miyazono K, Ichijo H. Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO J. 1998;17:2596–2606. doi: 10.1093/emboj/17.9.2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 679a.Salehi S, Reed EF. The divergent roles of macrophages in solid organ transplantation. Curr Opin Organ Transplant. 2015;20:446–453. doi: 10.1097/MOT.0000000000000209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 680.Salim SY, Young PY, Lukowski CM, Madsen KL, Sis B, Churchill TA, Khadaroo RG. VSL#3 probiotics provide protection against acute intestinal ischaemia/reperfusion injury. Benef Microbes. 2013;4:357–365. doi: 10.3920/BM2013.0026. [DOI] [PubMed] [Google Scholar]
- 681.Salter JW, Krieglstein CF, Isserkutz AC, Granger DN. Platelets modulate ischemia/reperfusion-induced leukocyte recruitment in the mesenteric circulation. Am J Physiol. 2001;281:G1432–G1439. doi: 10.1152/ajpgi.2001.281.6.G1432. [DOI] [PubMed] [Google Scholar]
- 682.Salvemini D, Cazzocrea S. Superoxide, superoxide dismutase and ischemic injury. Curr Opin Invest Drugs. 2002;3:886–895. [PubMed] [Google Scholar]
- 683.Sanada S, Komuro I, Kitakaze M. Pathophysiology of myocardial reperfusion injury: Preconditioning, postconditioning, and translational aspects of protective measures. Am J Physiol. 2011;301:H1723–H1741. doi: 10.1152/ajpheart.00553.2011. [DOI] [PubMed] [Google Scholar]
- 683a.Sansbury BE, Spite M. Resolution of acute inflammation and the role of resolvins in immunity, thrombosis, and vascular biology. Circ Res. 2016;119:113–130. doi: 10.1161/CIRCRESAHA.116.307308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 684.Sant00E9;n S, Mihaescu A, Laschke MW, Menger MD, Wang Y, Jeppsson B, Thorlacius H. p38 MAPK regulates ischemia-reperfusion-induced recruitment of leukocytes in the colon. Surgery. 2009;145:303–12. doi: 10.1016/j.surg.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 685.Santen S, Wang Y, Menger MD, Jeppsson B, Thorlacius H. Mast-cell-dependent secretion of CXC chemokines regulates ischemia-reperfusion-induced leukocyte recruitment in the colon. Int J Colorectal Dis. 2008;23:527–534. doi: 10.1007/s00384-007-0436-2. [DOI] [PubMed] [Google Scholar]
- 686.Santora RJ, Lie ML, Grigoryev DN, Nasir O, Moore FA, Hassoun HT. Therapeutic distant organ effects of regional hypothermia during mesenteric ischemia-reperfusion injury. J Vasc Surg. 2010;52:1003–1014. doi: 10.1016/j.jvs.2010.05.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 687.Sapega AA, Heppenstall RB, Chance B, Park YS, Sokolow D. Optimizing tourniquet application and release times in extremity surgery. A biochemical and ultrastructural study. J Bone Joint Surg Am. 1985;67:303–314. [PubMed] [Google Scholar]
- 688.Saugstad JA. Non-coding RNAs in stroke and neuroprotection. Front Neurol. 2015;6:50. doi: 10.3389/fneur.2015.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 689.Savas C, Ozogul C, Karaoz E, Delibas N, Ozguner F. Splenectomy reduces remote organ damage after intestinal ischaemia-reperfusion injury. Acta Chir Belg. 2003;103:315–320. doi: 10.1080/00015458.2003.11679431. [DOI] [PubMed] [Google Scholar]
- 690.Saxton NE, Barclay JL, Clouston AD, Fawcett J. Cyclosporin A pretreatment in a rat model of warm ischaemia/reperfusion injury. J Hepatol. 2002;36:241–247. doi: 10.1016/s0168-8278(01)00248-3. [DOI] [PubMed] [Google Scholar]
- 691.Sayed D, Abdellatif M. MicroRNAs in development and disease. Physiol Rev. 2011;91:827–887. doi: 10.1152/physrev.00006.2010. [DOI] [PubMed] [Google Scholar]
- 692.Saztpute SR, Park JM, Jang HR, Agreda P, Liu M, Gandalfo MT, Racusen L, Rabb H. The role for T cell repertoire/antigen-specific interactions in experimental kidney ischemia reperfusion injury. J Immunol. 2009;183:984–992. doi: 10.4049/jimmunol.0801928. [DOI] [PubMed] [Google Scholar]
- 693.Schinzel AC, Takeuchi O, Huang Z, Fisher JK, Zhou Z, Rubens J, Hetz C, Danial NN, Moskowitz MA, Korsmeyer SJ. Cyclophilin D is a component of mitochondrial permeability transition and mediates neuronal cell death after focal cerebral ischemia. Proc Natl Acad Sci U S A. 2005;102:12005–12010. doi: 10.1073/pnas.0505294102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 694.Schofield ZV, Woodruff TM, Halai R, Wu MC, Cooper MA. Neutrophils–a key component of ischemia-reperfusion injury. Shock. 2013;40:463–470. doi: 10.1097/SHK.0000000000000044. [DOI] [PubMed] [Google Scholar]
- 695.Schroen B, Heymans S. Small but smart–microRNAs in the centre of inflammatory processes during cardiovascular diseases, the metabolic syndrome, and ageing. Cardiovasc Res. 2012;93:605–6013. doi: 10.1093/cvr/cvr268. [DOI] [PubMed] [Google Scholar]
- 696.Schulz C, Massberg S. Platelets in atherosclerosis and thrombosis. Handb Exp Pharmacol. 2012:111–133. doi: 10.1007/978-3-642-29423-5_5. [DOI] [PubMed] [Google Scholar]
- 697.Schwartz BG, Kloner RA. Coronary no reflow. J Mol Cell Cardiol. 2012;52:873–882. doi: 10.1016/j.yjmcc.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 698.Sciarretta S, Yee D, Ammann P, Nagarajan N, Volpe M, Frati G, Sadoshima J. Role of NADPH oxidase in the regulation of autophagy in cardiomyocytes. Clin Sci (Lond) 2015;128:387–403. doi: 10.1042/CS20140336. [DOI] [PubMed] [Google Scholar]
- 699.Sciarretta S, Zhai P, Shao D, Maejima Y, Robbins J, Volpe M, Condorelli G, Sadoshima J. Rheb is a critical regulator of autophagy during myocardial ischemia: Pathophysiological implications in obesity and metabolic syndrome. Circulation. 2012;125:1134–1146. doi: 10.1161/CIRCULATIONAHA.111.078212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 700.Sciarretta S, Zhai P, Volpe M, Sadoshima J. Pharmacological modulation of autophagy during cardiac stress. J Cardiovasc Pharmacol. 2012;60:235–241. doi: 10.1097/FJC.0b013e3182575f61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 701.Scorrano L, Oakes SA, Opferman JT, Cheng EH, Sorcinelli MD, Pozzan T, Korsmeyer SJ. BAX and BAK regulation of endoplasmic reticulum Ca2+: A control point for apoptosis. Science. 2003;300:135–139. doi: 10.1126/science.1081208. [DOI] [PubMed] [Google Scholar]
- 702.Seldin MM, Meng Y, Qi H, Zhu W, Wang Z, Hazen SL, Lusis AJ, Shih DM. Trimethylamine N-oxide promotes vascular inflammation through signaling of mitogen-activated protein kinase and nuclear factor-kappaB. J Am Heart Assoc. 2016;5 doi: 10.1161/JAHA.115.002767. pii: e002767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 703.Sepramaniam S, Armugam A, Lim KY, Karolina DS, Swaminathan P, Tan JR, Jeyaseelan K. MicroRNA 320a functions as a novel endogenous modulator of aquaporins 1 and 4 as well as a potential therapeutic target in cerebral ischemia. J Biol Chem. 2010;285:29223–29230. doi: 10.1074/jbc.M110.144576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 704.Serino M, Blasco-Baque V, Nicolas S, Burcelin R. Far from the eyes, close to the heart: Dysbiosis of gut microbiota and cardiovascular consequences. Curr Cardiol Rep. 2014;16:540–547. doi: 10.1007/s11886-014-0540-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 705.Seronde MF, Vausort M, Gayat E, Goretti E, Ng LL, Squire IB, Vodovar N, Sadoune M, Samuel JL, Thum T, Solal AC, Laribi S, Plaisance P, Wagner DR, Mebazaa A, Devaux Y. Circulating microRNAs and outcome in patients with acute heart failure. PloS One. 2015;10:e0142237. doi: 10.1371/journal.pone.0142237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 706.Sessa WC. Molecular control of blood flow and angiogenesis: Role of nitric oxide. J Thromb Haemost. 2009;7(Suppl 1):35–37. doi: 10.1111/j.1538-7836.2009.03424.x. [DOI] [PubMed] [Google Scholar]
- 707.Sesti C, Simkhovich BZ, Kalvinsh I, Kloner RA. Mildronate, a novel fatty acid oxidation inhibitor and antianginal agent, reduces myocardial infarct size without affecting hemodynamics. J Cardiovasc Pharmacol. 2006;47:493–499. doi: 10.1097/01.fjc.0000211732.76668.d2. [DOI] [PubMed] [Google Scholar]
- 708.Settergren M, Böhm F, Malmström RE, Chancon KM, Pernow J. L-arginine and tetrahydrobiopterin protects against ischemia/reperfusion-induced endothelial dysfunction in patients with type 2 diabetes mellitis and coronary artery disease. Atherosclerosis. 2009;204:73–78. doi: 10.1016/j.atherosclerosis.2008.08.034. [DOI] [PubMed] [Google Scholar]
- 709.Sharma AK, Laubach VE, Ramos SI, Zhao Y, Stukenborg G, Linden J, Kron IL, Yang Z. Adenosine A2A receptor activation on CD4 +T lymphocytes and neutrophils attenuates lung ischemia-reperfusion injury. J Thorac Cardiovasc Surg. 2010;139:474–482. doi: 10.1016/j.jtcvs.2009.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 710.Shen X, Wang Y, Gao F, Ren F, Busuttil RW, Kupiec-Weglinski JW, Zhai Y. CD4+ T cells promote tissue inflammation via CD40 signaling without de novo activation in a murine model of liver ischemia/reperfusion injury. Hepatology. 2009;50:1537–1546. doi: 10.1002/hep.23153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 711.Shen XD, Ke B, Zhai Y, Gao F, Anselmo D, Lassman CR, Busuttil RW, Kupiec-Weglinski JC. Stat4 and Stat6 signaling in hepatic ischemia/reperfusion injury in mice: HO-1 dependence of Stat4 disruption-mediated cytoprotection. Hepatology. 2003;37:269–303. doi: 10.1053/jhep.2003.50066. [DOI] [PubMed] [Google Scholar]
- 712.Sheu EG, Oakes SM, Ahmadi-Yazdi C, Afnan J, Carroll MC, Moore FD. Restoration of skeletal muscle ischemia-reperfusion injury in humanized immunodeficient mice. Surgery. 2009;146:340–346. doi: 10.1016/j.surg.2009.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 713.Shi J, Fujiedo H, Kokubo Y, Wake K. Apoptosis of neutrophils and their elimination by Kupffer cells in rat liver. Hepatology. 1996;24:1256–1263. doi: 10.1053/jhep.1996.v24.pm0008903407. [DOI] [PubMed] [Google Scholar]
- 714.Shi J, Gilbert GE, Kokubo Y, Ohashi T. Role of the liver in regulating numbers of circulating neutrophils. Blood. 2001;98:1226–1230. doi: 10.1182/blood.v98.4.1226. [DOI] [PubMed] [Google Scholar]
- 715.Shi Y, Melnikov VY, Schrier RW, Edelstein CL. Downregulation of the calpain inhibitor protein calpastatin by caspases during renal ischemia-reperfusion. Am J Physiol Renal Physiol. 2000;279:F509–F517. doi: 10.1152/ajprenal.2000.279.3.F509. [DOI] [PubMed] [Google Scholar]
- 716.Shichita T, Sugiyama Y, Ooboshi H, Sugimori H, Nakagawa R, Takeda I, Iwaki T, Okada Y, Iida M, Cua OJ, Iwakura Y, Yoshimura A. Pivotal role of cerebral interleukin-17-producing γδT cells in the delayed phase of ischemic brain injury. Nat Med. 2009;15:946–951. doi: 10.1038/nm.1999. [DOI] [PubMed] [Google Scholar]
- 717.Shimamura K, Kawamura H, Nagara T, Kato T, Naito T, Kameyama H, Hatakeyama K, Abo T. Association of NKT cells and granulocytes with liver injury after reperfusion of the portal vein. Cell Immunol. 2005;234:31–38. doi: 10.1016/j.cellimm.2005.04.022. [DOI] [PubMed] [Google Scholar]
- 717a.Shinagawa H, Frantz S. Cellular immunity and cardiac remodeling after myocardial infarction: Role of neutrophils, monocytes, and macrophages. Curr Heart Fail Rep. 2015;12:247–254. doi: 10.1007/s11897-015-0255-7. [DOI] [PubMed] [Google Scholar]
- 718.Shiotani S, Shimada M, Taketomi A, Soejima Y, Yoshizumi T, Hashimoto K, Shimokawa H, Maehara Y. Rho-kinase as a novel gene therapeutic target in treatment of cold ischemia/reperfusion-induced acute lethal liver injury: Effect on hepatocellular NADPH oxidase system. Gene Ther. 2007;14:1425–1433. doi: 10.1038/sj.gt.3303000. [DOI] [PubMed] [Google Scholar]
- 719.Shiva S, Sack MN, Greer JJ, Duranski M, Ringwoood LA, Burwell L, Wang X, MacArthur PH, Shoja A, Raghavachari N, Calvert JW, Brookes PS, Lefer DJ, Gladwin MT. Nitrite augments tolerance to ischemia/reperfusion injury via the modulation of mitochondrial electron transfer. J Exp Med. 2007;204:2089–2102. doi: 10.1084/jem.20070198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 720.Sies H. Oxidative stress: Introductory remarks. In: Sies H, editor. Oxidative Stress. London: Academic Press; 1985. pp. 1–8. [Google Scholar]
- 721.Simkhovich BZ, Przyklen kK, Kloner RA. Role of protein kinase C in ischemic “conditioning”: From first evidence to current perspectives. J Cardiovasc Pharmacol Ther. 2013;18:525–532. doi: 10.1177/1074248413494814. [DOI] [PubMed] [Google Scholar]
- 722.Singbartl K, Forlow SB, Ley K. Platelet, but not endothelial, P-selectin is critical for neutrophil-mediated acute postischemic renal failure. FASEB J. 2001;15:2337–2344. doi: 10.1096/fj.01-0199com. [DOI] [PubMed] [Google Scholar]
- 723.Singh D, Chander V, Chopra K. Cyclosporine protects against ischemia/reperfusion injury in rat kidneys. Toxicology. 2005;207:339–347. doi: 10.1016/j.tox.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 724.Sitailo LA, Tibudan SS, Denning MF. Bax activation and induction of apoptosis in human keratinocytes by the protein kinase C delta catalytic domain. J Invest Dermatol. 2004;123:434–443. doi: 10.1111/j.0022-202X.2004.23403.x. [DOI] [PubMed] [Google Scholar]
- 725.Slezak J, Tribulova N, Okruhlicova L, Dhingra R, Bajaj A, Freed D, Singal P. Hibernating myocardium: Pathophysiology, diagnosis, and treatment. Can J Physiol Pharmacol. 2009;87:252–265. doi: 10.1139/Y09-011. [DOI] [PubMed] [Google Scholar]
- 726.Smith CC, Davidson SM, Lim SY, Simpkin JC, Hothersall JS, Yellon DM. Necrostatin: A potentially novel cardioprotective agent? Cardiovasc Drugs Ther. 2007;21:227–233. doi: 10.1007/s10557-007-6035-1. [DOI] [PubMed] [Google Scholar]
- 727.Smith CC, Yellon DM. Necroptosis, necrostatins and tissue injury. J Cell Mol Med. 2011;15:1797–1806. doi: 10.1111/j.1582-4934.2011.01341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 728.Smith VA, Johnson T. Evaluation of an animal product-free variant of MegaCell MEM as a storage medium for corneas destined for transplantation. Ophthalmic Res. 2010;43:33–42. doi: 10.1159/000246576. [DOI] [PubMed] [Google Scholar]
- 729.Solaini G, Harris DA. Biochemical dysfunction in heart mitochondria exposed to ischaemia and reperfusion. Biochem J. 2005;390:377–394. doi: 10.1042/BJ20042006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 730.Song MA, Paradis AN, Gay MS, Shin J, Zhang L. Differential expression of microRNAs in ischemic heart disease. Drug Disc Today. 2015;20:223–235. doi: 10.1016/j.drudis.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 731.Song L, Yang H, Wang HX, Tian C, Liu Y, Zeng XJ, Gao E, Kang YM, Du J, Li HH. Inhibition of 12/15 lipoxygenase by baicalein reduces myocardial ischemia/reperfusion injury via modulation of multiple signaling pathways. Apoptosis. 2014;19:567–580. doi: 10.1007/s10495-013-0946-z. [DOI] [PubMed] [Google Scholar]
- 732.Sorimachi Y, Harada K, Saido TC, Ono T, Kawashima S, Yoshida K. Downregulation of calpastatin in rat heart after brief ischemia and reperfusion. J Biochem. 1997;122:743–748. doi: 10.1093/oxfordjournals.jbchem.a021818. [DOI] [PubMed] [Google Scholar]
- 733.Sorkine P, Szold O, Halpern P, Gutman M, Greemland M, Rudick V, Goldman G. Gut decontamination reduces bowel ischemia-induced lung injury in rats. Chest. 1997;112:491–495. doi: 10.1378/chest.112.2.491. [DOI] [PubMed] [Google Scholar]
- 734.Souza DG, Mendonça VA, de A Castro MS, Poole S, Teixeira MM. Role of tachykinin NK receptors on the local and remote injuries following ischaemia and reperfusion of the superior mesenteric artery in the rat. Br J Pharmacol. 2002;135:303–312. doi: 10.1038/sj.bjp.0704464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 735.Souza DG, Vieira AT, Soares AC, Pinho V, Nicoli JR, Vieira LQ, Teixeira MM. The essential role of the intestinal microbiota in facilitating acute inflammatory responses. J Immunol. 2004;173:4137–4146. doi: 10.4049/jimmunol.173.6.4137. [DOI] [PubMed] [Google Scholar]
- 736.Sozen E, Karademir B, Ozer NK. Basic mechanisms in endoplasmic reticulum stress and relation to cardiovascular diseases. Free Radic Biol Med. 2015;78:30–41. doi: 10.1016/j.freeradbiomed.2014.09.031. [DOI] [PubMed] [Google Scholar]
- 737.Spescha RD, Klohs J, Semerano A, Giacalone G, Derungs RS, Reiner MF, Rodriguez Gutierrez D, Mendez-Carmona N, Glanzmann M, Savarese G, Krankel N, Akhmedov A, Keller S, Mocharla P, Kaufmann MR, Wenger RH, Vogel J, Kulic L, Nitsch RM, Beer JH, Peruzzotti-Jametti L, Sessa M, Luscher TF, Camici GG. Post-ischaemic silencing of p66Shc reduces ischaemia/reperfusion brain injury and its expression correlates to clinical outcome in stroke. Eur Heart J. 2015;36:1590–1600. doi: 10.1093/eurheartj/ehv140. [DOI] [PubMed] [Google Scholar]
- 738.Spescha RD, Shi Y, Wegener S, Keller S, Weber B, Wyss MM, Lauinger N, Tabatabai G, Paneni F, Cosentino F, Hock C, Weller M, Nitsch RM, Luscher TF, Camici GG. Deletion of the ageing gene p66(Shc) reduces early stroke size following ischaemia/reperfusion brain injury. Eur Heart J. 2013;34:96–103. doi: 10.1093/eurheartj/ehs331. [DOI] [PubMed] [Google Scholar]
- 739.Stahl GL, Xu Y, Hao L, Miller M, Buras JA, Fung M, Zhao H. Role for the alternative complement pathway in ischemia/reperfusion injury. Am J Pathol. 2003;162:449–455. doi: 10.1016/S0002-9440(10)63839-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 740.Stallion A, Kou TD, Latfi SQ, Miller KA, Dahms BB, Dudgeon DL, Levine AD. Ischemia/reperfusion: A clinically relevant model of intestinal injury yielding systemic inflammation. J Pediatr Surg. 2005;40:470–477. doi: 10.1016/j.jpedsurg.2004.11.045. [DOI] [PubMed] [Google Scholar]
- 741.Stark K, Eckart A, Haidari S, Tirniceriu A, Lorenz M, von Bruhl ML, Gartner F, Khandoga AG, Legate KR, Pless R, Hepper I, Lauber K, Walzog B, Massberg S. Capillary and arteriolar pericytes attract innate leukocytes exiting through venules and ‘instruct’ them with pattern-recognition and motility programs. Nat Immunol. 2013;14:41–51. doi: 10.1038/ni.2477. [DOI] [PubMed] [Google Scholar]
- 742.Stefanidakis M, Newton G, Lee WY, Parkos CA, Luscinskas FW. Endothelial CD47 interaction with SIRPγ is required for human T-cell transendothelial migration under shear flow conditions in vitro. Blood. 2008;112:1280–1289. doi: 10.1182/blood-2008-01-134429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 743.Stocker R, Yamamoto Y, McDonagh A, Glazer A, Ames BN. Bilirubin is an antioxidant of possible physiological importance. Science. 1987;235:1043–1045. doi: 10.1126/science.3029864. [DOI] [PubMed] [Google Scholar]
- 744.Stokes KY, Granger DN. Platelets: A critical link between inflammation and microvascular dysfunction. J Physiol. 2012;590:1023–1034. doi: 10.1113/jphysiol.2011.225417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 745.Stowe DF, Camara KS. Mitochondrial reactive oxygen species production in excitable cells: Modulators of mitochondrial and cell function. Antiox Redox Signal. 2009;11:1373–1414. doi: 10.1089/ars.2008.2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 746.Strasser RH, Simonis G, Schön SP, Braun MU, Ihl-Vahl R, Weinbrenner C, Marquetant R, Kübler W. Two distinct mechanisms mediate a differential regulation of protein kinase C isozymes in acute and prolonged myocardial ischemia. Circ Res. 1999;85:77–87. doi: 10.1161/01.res.85.1.77. [DOI] [PubMed] [Google Scholar]
- 747.Strbian D, Karailainen-Lindsberg ML, Kovanen PT, Tatlisumak T, Lindsberg PJ. Mast cell stabilization reduces hemorrhage formation and mortality after administration of thrombolytics in experimental ischemic stroke. Circulation. 2007;116:411–418. doi: 10.1161/CIRCULATIONAHA.106.655423. [DOI] [PubMed] [Google Scholar]
- 748.Strbian D, Karialainen-Lindsberg ML, Tatlisumak T, Lindsberg PJ. Cerebral mast cells regulate early ischemic brain swelling and neutrophil accumulation. J Cereb Blood Flow Metab. 2006;26:605–612. doi: 10.1038/sj.jcbfm.9600228. [DOI] [PubMed] [Google Scholar]
- 749.Strbian D, Kovanen PT, Karajalainen-Lindsberg ML, Tatlisumak T, Lindsberg PJ. An emerging role of mast cells in cerebral ischemia and hemorrhage. Annals Med. 2009;41:438–450. doi: 10.1080/07853890902887303. [DOI] [PubMed] [Google Scholar]
- 750.Strock PE, Majno G. Vascular responses to experimental tourniquet ischemia. Surg Gynecol Obstet. 1969;129:309–318. [PubMed] [Google Scholar]
- 751.Suematsu M, Ishimura Y. The heme oxygenase-carbon monoxide system: A regulator of hepatobiliary functions. Hepatology. 2000;31:3–6. doi: 10.1002/hep.510310102. [DOI] [PubMed] [Google Scholar]
- 752.Summers WK, Jamison RL. The no reflow phenomenon in renal ischemia. Lab Invest. 1974;25:635–643. [PubMed] [Google Scholar]
- 753.Sun J, Murphy E. Protein s-nitrosylation and cardioprotection. Circ Res. 2010;106:285–296. doi: 10.1161/CIRCRESAHA.109.209452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 754.Sun J, Wu Q, Sun H, Qiao Y. Inhibition of histone deacetylase by butyrate protects rat liver from ischemic reperfusion injury. Int J Mol Sci. 2014;15:21069–21079. doi: 10.3390/ijms151121069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 755.Sutherland BA, Rahman RMA, Apleton I. Mechanisms of action of green tea catechins, with a focus on ischemia-induced neurodegeneration. J Nutr Biochem. 2006;17:291–306. doi: 10.1016/j.jnutbio.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 756.Swank GM, Deitch EA. Role of the gut in multiple organ failure: Bacterial translocation and permeability changes. World J Surg. 1996;20:411–417. doi: 10.1007/s002689900065. [DOI] [PubMed] [Google Scholar]
- 757.Symonds ME, Pope M, Sharkey D, Budge H. Adipose tissue and fetal programming. Diabetologia. 2012;55:1597–1606. doi: 10.1007/s00125-012-2505-5. [DOI] [PubMed] [Google Scholar]
- 758.Syrjälä SO, Keränen MA, Tuuminen R, Nykänen AI, Tammi M, Krebs R, Lemström KB. Increased Th17 rather than Th1 alloimmune response is associated with cardiac allograft vasculopathy after hypothermic presentation in the rat. J Heart Lung Transplant. 2010;29:1047–1057. doi: 10.1016/j.healun.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 759.Szydlowska K, Tymianski M. Calcium, ischemia and excitotoxicity. Cell Calcium. 2010;47:122–129. doi: 10.1016/j.ceca.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 760.Tadimalla A, Belmont PJ, Thuerauf DJ, Glassy MS, Martindale JJ, Gude N, Sussman MA, Glembotski CC. Mesencephalic astrocyte-derived neurotrophic factor is an ischemia-inducible secreted endoplasmic reticulum stress response protein in the heart. Circ Res. 2008;103:1249–1258. doi: 10.1161/CIRCRESAHA.108.180679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 761.Tailor A, Cooper D, Granger DN. Platelet-vessel wall interactions in the microcirculation. Microcirc. 2005;12:275–285. doi: 10.1080/10739680590925691. [DOI] [PubMed] [Google Scholar]
- 762.Takagi H, Matsui Y, Hirotani S, Sakoda H, Asano T, Sadoshima J. AMPK mediates autophagy during myocardial ischemia in vivo. Autophagy. 2007;3:405–407. doi: 10.4161/auto.4281. [DOI] [PubMed] [Google Scholar]
- 763.Takagi Y, Nozaki K, Sugino T, Hattori I, Hashimoto N. Phosphorylation of c-Jun NH(2)-terminal kinase and p38 mitogen-activated protein kinase after transient forebrain ischemia in mice. Neurosci Lett. 2000;294:117–120. doi: 10.1016/s0304-3940(00)01552-4. [DOI] [PubMed] [Google Scholar]
- 764.Takahashi M. NLRP3 in myocardial ischaemia-reperfusion injury: Inflammasome-dependent or -independent role in different cell types. Cardiovasc Res. 2013;99:4–5. doi: 10.1093/cvr/cvt142. [DOI] [PubMed] [Google Scholar]
- 765.Talukdar HA, Foroughi Asl H, Jain RK, Ermel R, Ruusalepp A, Franzen O, Kidd BA, Readhead B, Giannarelli C, Kovacic JC, Ivert T, Dudley JT, Civelek M, Lusis AJ, Schadt EE, Skogsberg J, Michoel T, Bjorkegren JL. Cross-tissue regulatory gene networks in coronary artery disease. Cell Systems. 2016;2:196–208. doi: 10.1016/j.cels.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 766.Talukder MA, Zweier JL, Periasamy M. Targeting calcium transport in ischaemic heart disease. Cardiovasc Res. 2009;84:345–352. doi: 10.1093/cvr/cvp264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 767.Tan JR, Koo YX, Kaur P, Liu F, Armugam A, Wong PT, Jeyaseelan K. microRNAs in stroke pathogenesis. Curr Mol Med. 2011;11:76–92. doi: 10.2174/156652411794859232. [DOI] [PubMed] [Google Scholar]
- 768.Tan L, Yu JT, Guan HS. Reservatrol exerts pharmacological preconditioning by activating PGC-1alpha. Med Hypoth. 2008;71:664–667. doi: 10.1016/j.mehy.2008.06.031. [DOI] [PubMed] [Google Scholar]
- 769.Tankersley CG, Moldobaeva A, Wagner EM. Strain variation in response to lung ischemia: Role of MMP-12. Respir Res. 2012;13:93. doi: 10.1186/1465-9921-13-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 770.Taylor MJ, Baicu SC. Current state of hypothermic machine perfusion preservation of organs: The clinical perspective. Cryobiology. 2010;60:S20–35. doi: 10.1016/j.cryobiol.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 771.Taylor PB, Young BG. Effect of myocardial ischemia on uridine incorporation and histone acetylation. Can J Physiol Pharmacol. 1982;60:313–318. doi: 10.1139/y82-043. [DOI] [PubMed] [Google Scholar]
- 772.Teoh NC, Ajamieh H, Wong HJ, Croft K, Mori T, Allison AC, Farrell GC. Microparticles mediate hepatic ischemia-reperfusion injury and are the targets of Diannexin (ASP8597) PloS One. 2014;9:e104376. doi: 10.1371/journal.pone.0104376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 773.Terai K, Hiramoto Y, Masaki M, Sugiyama S, Kuroda T, Hori M, Kawase I, Hirota H. AMP-activated protein kinase protects cardiomyocytes against hypoxic injury through attenuation of endoplasmic reticulum stress. Mol Cell Biol. 2005;25:9554–9575. doi: 10.1128/MCB.25.21.9554-9575.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 774.Terao S, Yilmaz G, Stokes KY, Russell J, Ishikawa M, Kawase T, Granger DN. Blood cell-derived RANTES mediates cerebral microvascular dysfunction, inflammation, and tissue injury after focal ischemia-reperfusion. Stroke. 2008;39:2560–257. doi: 10.1161/STROKEAHA.107.513150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 775.Tersteeg C, Heijnen HF, Eckly A, Pasterkamp G, Urbanus RT, Maas C, Hoefer IE, Nieuwland R, Farndale RW, Gachet C, de Groot PG, Roest M. FLow-induced PRotrusions (FLIPRs): A platelet-derived platform for the retrieval of microparticles by monocytes and neutrophils. Circ Res. 2014;114:780–791. doi: 10.1161/CIRCRESAHA.114.302361. [DOI] [PubMed] [Google Scholar]
- 776.Terui K, Enosawa S, Haga S, Zhang HQ, Kuroda H, Kouchi K, Matsunaga T, Yoshida H, Engelhardt JF, Irani K, Ohnuma N, Ozaki M. Stat3 confers resistance against hypoxia/reoxygenation-induced oxidative injury in hepatocytes through upregulation of Mn-SOD. J Hepatol. 2004;41:957–965. doi: 10.1016/j.jhep.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 777.Theoharidis TC, Kempuraj D, Tagen M, Conti P, Kalogeromitros D. Differential relased mast cell mediators and the pathogenesis of inflammation. Immunol Rev. 2007;217:65–78. doi: 10.1111/j.1600-065X.2007.00519.x. [DOI] [PubMed] [Google Scholar]
- 778.Theruvath TP, Snoddy MC, Zhong Z, Lemasters JJ. Mitochondrial permeability transition in liver ischemia and reperfusion: Role of c-Jun N-terminal kinase 2. Transplantation. 2008;85:1500–1504. doi: 10.1097/TP.0b013e31816fefb5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 779.Thomas A, Lenglet S, Chaurand P, Deglon J, Mangin P, Mach F, Steffens S, Wolfender JL, Staub C. Mass spectrometry for the evaluation of cardiovascular diseases based on proteomics and lipidomics. Thromb Haemost. 2011;106:20–33. doi: 10.1160/TH10-12-0812. [DOI] [PubMed] [Google Scholar]
- 780.Thomas WS, Mori E, Copeland BR, Yu JQ, Morrissey JH, del Zappo GJ. Tissue factor contributes to microvascular defects following cerebral ischemia. Stroke. 1993;24:847–853. doi: 10.1161/01.str.24.6.847. [DOI] [PubMed] [Google Scholar]
- 781.Thon L, Möhlig H, Mathieu S, Lange A, Bulanova E, Winoto-Morbach S, Schütze S, Bulfone-Paus S, Adam D. Ceramide mediates caspase-independent programmed cell death. FASEB J. 2005;19:1945–1956. doi: 10.1096/fj.05-3726com. [DOI] [PubMed] [Google Scholar]
- 782.Thornburg KL, O’Tierney PF, Louey S. Review: The placenta is a programming agent for cardiovascular disease. Placenta. 2010;31(Suppl):S54–S59. doi: 10.1016/j.placenta.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 783.Thuerauf DJ, Marcinko M, Gude N, Rubio M, Sussman MA, Glembotski CC. Activation of the unfolded protein response in infarcted mouse heart and hypoxic cultured cardiac myocytes. Circ Res. 2006;99:275–282. doi: 10.1161/01.RES.0000233317.70421.03. [DOI] [PubMed] [Google Scholar]
- 784.Tiefenbacher CP, Chilian WM, Mitchell M, Defily DV. Restoration of endothelium-dependent vasodilation after reperfusion injury by tetrahydrobiopterin. Circ. 1996;94:1423–1429. doi: 10.1161/01.cir.94.6.1423. [DOI] [PubMed] [Google Scholar]
- 785.Toko H, Takahashi H, Kayama Y, Okada S, Minamino T, Terasaki F, Kitaura Y, Komuro I. ATF6 is important under both pathological and physiological states in the heart. J Mol Cell Cardiol. 2010;49:113–120. doi: 10.1016/j.yjmcc.2010.03.020. [DOI] [PubMed] [Google Scholar]
- 786.Tomita S, Li RK, Weisel RD, Mickle DA, Kim EJ, Sakai T, Jia ZQ. Autologous transplantation of bone marrow cells improves damaged heart function. Circulation. 1999;100:II247–256. doi: 10.1161/01.cir.100.suppl_2.ii-247. [DOI] [PubMed] [Google Scholar]
- 787.Tomiyama K, Ikeda A, Ueki S, Nakao A, Stolz DB, Koike Y, Afrazi A, Gandhi C, Tokita D, Geller DA, Murase N. Inhibition of Kupffer cell-mediated early proinflammatory response with carbon monoxide in transplant-induced hepatic ischemia/reperfusion injury in rats. Hepatology. 2008;48:1608–1620. doi: 10.1002/hep.22482. [DOI] [PubMed] [Google Scholar]
- 788.Toth A, Jeffers JR, Nickson P, Min JY, Morgan JP, Zambetti GP, Erhardt P. Targeted deletion of Puma attenuates cardiomyocyte death and improves cardiac function during ischemia-reperfusion. Am J Physiol Heart Circ Physiol. 2006;291:H52–60. doi: 10.1152/ajpheart.01046.2005. [DOI] [PubMed] [Google Scholar]
- 789.Tracey KJ. Physiology and immunology of the cholinergic anti-inflammatory pathway. J Clin Invest. 2007;117:289–296. doi: 10.1172/JCI30555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 790.Trinei M, Migliaccio E, Bernardi P, Paolucci F, Pelicci P, Giorgio M. p66Shc, mitochondria, and the generation of reactive oxygen species. Methods Enzymol. 2013;528:99–110. doi: 10.1016/B978-0-12-405881-1.00006-9. [DOI] [PubMed] [Google Scholar]
- 791.Tsubokawa T, Yamaguchi-Okada M, Calvert JW, Solaroglu I, Shimamura N, Yata K, Zhang JH. Neurovascular and neuronal protection by E64d after focal cerebral ischemia in rats. J Neurosci Res. 2006;84:832–840. doi: 10.1002/jnr.20977. [DOI] [PubMed] [Google Scholar]
- 792.Tsukamoto T, Chanthaphavong RS, Pape HC. Current theories on the pathophysiology of multiple organ failure after trauma. Injury. 2010;41(1):21–26. doi: 10.1016/j.injury.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 793.Tsung A, Hoffman RA, Izuishi K, Critchlow ND, Nakao A, Chan MH, Lotze MT, Geller DA, Billiar TR. Hepatic ischemia-reperfusion involves functional TLR4 signaling in nonparenchymal cells. J Immunol. 2005;175:7661–7668. doi: 10.4049/jimmunol.175.11.7661. [DOI] [PubMed] [Google Scholar]
- 794.Tsung A, Sahai R, Tanaka H, Takao A, Fink MP, Lotze MT, Yang H, Li J, Tracey KJ, Geller DA, Billiar TR. The nuclear factor HMGB1 mediates hepatic injury after liver ischemia-reperfusion. J Exp Med. 2005;201:1135–1143. doi: 10.1084/jem.20042614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 795.Tuboly E, Futakuchi M, Varga G, Erces D, Tokes T, Meszaros A, Kaszaki J, Suzui M, Imai M, Okada A, Okada N, Boros M, Okada H. C5a inhibitor protects against ischemia/reperfusion injury in rat small intestine. Microbiol Immunol. 2016;60:35–46. doi: 10.1111/1348-0421.12338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 796.Turer AT, Hill JA. Pathogenesis of myocardial ischemia-reperfusion injury and rationale for therapy. Am J Cardiol. 2016;106:360–368. doi: 10.1016/j.amjcard.2010.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 797.Uchida Y, Ke B, Freitas MCS, Ji H, Zhao D, Benjamin ER, Najafsan N, Yagita H, Akiba H, Busuttil RW, Kupiec-Weglinski JW. The emerging role of T cell immunoglobulin mucin-1 in the mechanism of liver ischemia and reperfusion injury in the mouse. Hepatology. 2010;51:1363–1372. doi: 10.1002/hep.23442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 798.Uemura A, Naito Y, Matsubara T. Dynamics of Ca(2+)/calmodulin-dependent protein kinase II following acute myocardial ischemia-translocation and autophosphorylation. Biochem Biophys Res Commun. 2002;297:997–1002. doi: 10.1016/s0006-291x(02)02279-9. [DOI] [PubMed] [Google Scholar]
- 799.Ufnal M, Zadlo A, Ostaszewski R. TMAO: A small molecule of great expectations. Nutrition. 2015;31:1317–1323. doi: 10.1016/j.nut.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 800.Ulven T. Short-chain free fatty acid receptors FFA2/GPR43 and FFA3/GPR41 as new potential therapeutic targets. Front Endocrin. 2012;3:111. doi: 10.3389/fendo.2012.00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 801.Urbich C, Kuehbacher A, Dimmeler S. Role of microRNAs in vascular diseases, inflammation, and angiogenesis. Cardiovasc Res. 2008;79:581–588. doi: 10.1093/cvr/cvn156. [DOI] [PubMed] [Google Scholar]
- 802.Ussher JR, Lopaschuk GD, Arduini A. Gut microbiota metabolism of L-carnitine and cardiovascular risk. Atherosclerosis. 2013;231:456–461. doi: 10.1016/j.atherosclerosis.2013.10.013. [DOI] [PubMed] [Google Scholar]
- 803.Vaghasiya JD, Sheth NR, Bhalodia YS, Jivani NP. Exaggerated liver injury produced by renal ischemia reperfusion in diabetes: Effect of exenatide. Saudi J Gastroenterol. 2010;16:174–180. doi: 10.4103/1319-3767.65187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 804.Vakeva A, Meri S. Complement activation and regulator expression after anoxic injury of human endothelial cells. Acta Pathol Microbiol Immunol Scand. 1998;106:1149–1156. doi: 10.1111/j.1699-0463.1998.tb00271.x. [DOI] [PubMed] [Google Scholar]
- 805.Valentim L, Laurence KM, Townsend PA, Carroll CJ, Soond S, Scarabelli TM, Knight RA, Latchman DS, Stephanou A. Urocortin inhibits Beclin1-mediated autophagic cell death in cardiac myocytes exposed to ischaemia/reperfusion injury. J Mol Cell Cardiol. 2006;40:846–852. doi: 10.1016/j.yjmcc.2006.03.428. [DOI] [PubMed] [Google Scholar]
- 806.Valko M, Leibfritz D, Moncol J, Cronin MTD, Mazur M, Telser J. Free radicals and antioxidants in normal functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 807.van Ampting MT, Schonewille AJ, Vink C, Brummer RJ, van der Meer R, Bovee-Oudenhoven IM. Damage to the intestinal epithelial barrier by antibiotic pretreatment of salmonella-infected rats is lessened by dietary calcium or tannic acid. J Nutr. 2010;140:2167–2172. doi: 10.3945/jn.110.124453. [DOI] [PubMed] [Google Scholar]
- 808.Vanden Berghe T, Vandenabeele P, Pasparakis M, Bleich M, Weinberg JM, Reichel CA, Brasen JH, Kunzendorf U, Anders HJ, Stockwell BR, Green DR. Synchronized renal tubular cell death involves ferroptosis. Proc Nat Acad Sci. 2014;111:16836–16841. doi: 10.1073/pnas.1415518111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 809.Vander Heide RS, Steenbergen C. Cardioprotection and myocardial reperfusion: Pitfalls to clinical application. Circ Res. 2013;113:464–477. doi: 10.1161/CIRCRESAHA.113.300765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 810.Van der Vusse GJ, Reneman RS, van Bilsen M. Accumulation of arachidonic acid in ischemic/reperfused cardiac tissue: Possible causes and consequences. Prostaglandins Leukot Essent Fatty Acids. 1997;57:85–93. doi: 10.1016/s0952-3278(97)90497-x. [DOI] [PubMed] [Google Scholar]
- 811.van Leyen K, Kim HY, Lee SR, Jin G, Arai K, Lo EH. Baicalein and 12/15-lipoxygenase in the ischemic brain. Stroke. 2006;37:3014–3018. doi: 10.1161/01.STR.0000249004.25444.a5. [DOI] [PubMed] [Google Scholar]
- 812.Vandenabeele P, Declercq W, Van Herreweghe F, Vanden Berghe T. The role of the kinases RIP1 and RIP3 in TNF-induced necrosis. Sci Signal. 2010;3(115):re4. doi: 10.1126/scisignal.3115re4. [DOI] [PubMed] [Google Scholar]
- 813.Vega VL, Mardones L, Maldonado M, Nicovani S, Manriquez V, Roa J, Ward PH. Xanthine oxidase released from reperfused hind limbs mediate kupffer cell activation, neutrophil sequestration, and hepatic oxidative stress in rats subjected to tourniquet shock. Shock. 2000;14:565–571. doi: 10.1097/00024382-200014050-00012. [DOI] [PubMed] [Google Scholar]
- 814.Vickers MH. Developmental programming and transgenerational transmission of obesity. Ann Nutr Metab. 2014;64(Suppl 1):26–34. doi: 10.1159/000360506. [DOI] [PubMed] [Google Scholar]
- 815.Vickers MH. Early life nutrition, epigenetics and programming of later life disease. Nutrients. 2014;6(6):2165–2178. doi: 10.3390/nu6062165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 816.Vila-Petroff M, Salas MA, Said M, Valverde CA, Sapia L, Portiansky E, Hajjar RJ, Kranias EG, Mundiña-Weilenmann C, Mattiazzi A. CaMKII inhibition protects against necrosis and apoptosis in irreversible ischemia-reperfusion injury. Cardiovasc Res. 2007;73:689–698. doi: 10.1016/j.cardiores.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 816a.Voisin MB, Nourshargh S. Neutrophil transmigration: Emergence of an adhesive cascade within venular walls. J Innate Immun. 2013;5:336–347. doi: 10.1159/000346659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 817.Vuohelainen V, Hamalainen M, Paavonen T, Karlsson S, Moilanen E, Mennander A. Inhibition of monoamine oxidase A increases recovery after experimental cardiac arrest. Interact Cardiovasc Thorac Surg. 2015;21:441–449. doi: 10.1093/icvts/ivv175. [DOI] [PubMed] [Google Scholar]
- 818.Wagers AJ, Conboy IM. Cellular and molecular signatures of muscle regeneration: Current concepts and controversies in adult myogenesis. Cell. 2005;122:659–667. doi: 10.1016/j.cell.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 819.Wagner S, Dybkova N, Rasenack EC, Jacobshagen C, Fabritz L, Kirchhof P, Maier SK, Zhang T, Hasenfuss G, Brown JH, Bers DM, Maier LS. Ca2+/calmodulin-dependent protein kinase II regulates cardiac Na+ channels. J Clin Invest. 2006;116:3127–3138. doi: 10.1172/JCI26620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 820.Wagner S, Maier LS. Modulation of cardiac Na(+) and Ca(2+) currents by CaM and CaMKII. J Cardiovasc Electrophysiol. 2006;17(Suppl 1):S26–S33. doi: 10.1111/j.1540-8167.2006.00382.x. [DOI] [PubMed] [Google Scholar]
- 821.Wainwright CL, McCabe C, Kane KA. Endothelin and the ischaemic heart. Curr Vasc Pharmacol. 2005;3:333–341. doi: 10.2174/157016105774329417. [DOI] [PubMed] [Google Scholar]
- 822.Wall TM, Sheehy R, Hartman JC. Role of bradykinin in myocardial preconditioning. J Pharmacol Exp Ther. 1994;270:681–689. [PubMed] [Google Scholar]
- 823.Walsh SR, Boyle JR, Tang TY, Sadat U, Cooper DG, Lapsley M, Norden AG, Varty K, Hayes PD, Gaunt ME. Remote ischemic preconditioning for renal and cardiac protection during endovascular aneurysm repair: A randomized controlled trial. J Endovasc Ther. 2009;16:680–689. doi: 10.1583/09-2817.1. [DOI] [PubMed] [Google Scholar]
- 824.Wang B, Huang Q, Zhang W, Li N, Li J. Lactobacillus plantarum prevents bacterial translocation in rats following ischemia and reperfusion injury. Dig Dis Sci. 2011;56:3187–3194. doi: 10.1007/s10620-011-1747-2. [DOI] [PubMed] [Google Scholar]
- 825.Wang H, Zhang W, Zuo L, Zhu W, Wang B, Li Q, Li J. Bifidobacteria may be beneficial to intestinal microbiota and reduction of bacterial translocation in mice following ischaemia and reperfusion injury. Br J Nutr. 2013;109:1990–1998. doi: 10.1017/S0007114512004308. [DOI] [PubMed] [Google Scholar]
- 826.Wang K, Zhang J, Liu J, Tian J, Wu Y, Wang X, Quan L, Xu H, Wang W, Liu H. Variations in the protein level of Omi/HtrA2 in the heart of aged rats may contribute to the increased susceptibility of cardiomyocytes to ischemia/reperfusion injury and cell death: Omi/HtrA2 and aged heart injury. Age (Dordr) 2013;35:733–746. doi: 10.1007/s11357-012-9406-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 827.Wang Y, Dawson VL, Dawson TM. Poly(ADP-ribose) signals to mitochondrial AIF: A key event in parthanatos. Exp Neurol. 2009;218:193–202. doi: 10.1016/j.expneurol.2009.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 828.Wang Y, Ji HX, Xing SH, Pei DS, Guan QH. SP600125, a selective JNK inhibitor, protects ischemic renal injury via suppressing the extrinsic pathways of apoptosis. Life Sci. 2007;80:2067–2075. doi: 10.1016/j.lfs.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 829.Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, Feldstein AE, Britt EB, Fu X, Chung YM, Wu Y, Schauer P, Smith JD, Allayee H, Tang WH, DiDonato JA, Lusis AJ, Hazen SL. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 830.Wang Z, Roberts AB, Buffa JA, Levison BS, Zhu W, Org E, Gu X, Huang Y, Zamanian-Daryoush M, Culley MK, DiDonato AJ, Fu X, Hazen JE, Krajcik D, DiDonato JA, Lusis AJ, Hazen SL. Non-lethal inhibition of gut microbial trimethylamine production for the treatment of atherosclerosis. Cell. 2015;163:1585–1595. doi: 10.1016/j.cell.2015.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 831.Webb A, Bond R, McLean P, Uppal R, Benjamin N, Ahluwalia A. Reduction of nitrite to nitric oxide during ischemia protects against myocardial ischemia-reperfusion damage. Proc Natl Acad Sci U S A. 2004;101:13683–13688. doi: 10.1073/pnas.0402927101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 832.Webster KA. Mitochondrial membrane permeabilization and cell death during myocardial infarction: Roles of calcium and reactive oxygen species. Future Cardiol. 2012;8:863–884. doi: 10.2217/fca.12.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 833.Wegener S, Gottschalk B, Jovanovic V, Knab R, Fiebach JB, Schellinger PD, Kucinski T, Jungehulsing GJ, Brunecker P, Muller B, Banasik A, Amberger N, Wehrens XH, Lehnart SE, Reiken SR, Marks AR. Ca2+/calmodulin-dependent protein kinase II phosphorylation regulates the cardiac ryanodine receptor. Circ Res. 2014;94:e61–70. doi: 10.1161/01.RES.0000125626.33738.E2. [DOI] [PubMed] [Google Scholar]
- 834.Wegener S, Gottschalk B, Jovanovic V, Knab R, Fiebach JB, Schellinger PD, Kucinski T, Jungehulsing GJ, Brunecker P, Muller B, Banasik A, Amberger N, Wernecke KD, Siebler M, Rother J, Villringer A, Weih M. Transient ischemic attacks before ischemic stroke: Preconditioning the human brain? A multicenter magnetic resonance imaging study. Stroke. 2004;35:616–621. doi: 10.1161/01.STR.0000115767.17923.6A. [DOI] [PubMed] [Google Scholar]
- 835.Wehrens XH, Lehnart SE, Reiken SR, Marks AR. Ca2+/calmodulin-dependent protein kinase II phosphorylation regulates the cardiac ryanodine receptor. Circ Res. 2004;94:e61–70. doi: 10.1161/01.RES.0000125626.33738.E2. [DOI] [PubMed] [Google Scholar]
- 836.Wei Q, Yin XM, Wang MH, Dong Z. Bid deficiency ameliorates ischemic renal failure and delays animal death in C57BL/6 mice. Am J Physiol Renal Physiol. 2006;290:F35–42. doi: 10.1152/ajprenal.00184.2005. [DOI] [PubMed] [Google Scholar]
- 837.Weinbroum AA, Hochhauser E, Rudick V, Kluger Y, Sorkine P, Karchevsky E, Graf E, Boher P, Flaishon R, Fjodorov D, Niv D, Vidne BA. Direct induction of acute lung and myocardial dysfunction by liver ischemia and reperfusion. J Trauma. 1997;43:627–633. doi: 10.1097/00005373-199710000-00011. discussion 633-625. [DOI] [PubMed] [Google Scholar]
- 838.Weisman HF, Bartow T, Leppo MK, Boyle MP, Marsh HC, Jr, Carson GR, Roux KH, Weisfeldt ML, Fearon DT. Recombinant soluble CR1 suppressed complement activation, inflammation, and necrosis associated with reperfusion of ischemic myocardium. Trans Assoc Am Physicians. 1990;103:64–72. [PubMed] [Google Scholar]
- 839.Welborn MB, III, Moldawer LL, Seeger JM, Minter RM, Huber TS. Role of endogenous interleukin-10 in local and distant organ injury after visceral ischemia-reperfusion. Shock. 2003;20:35–40. doi: 10.1097/01.SHK.0000071062.67193.b6. [DOI] [PubMed] [Google Scholar]
- 840.Wells JM, Gaggar A, Blalock JE. MMP generated matrikines. Matrix Biol. 2015;44–46:122–129. doi: 10.1016/j.matbio.2015.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 841.Wershil BK, Wang ZS, Gordon JR, Galli SJ. Recruitment of neutrophils during IgE-dependent cutaneous late phase reactions in the mouse is mast cell-dependent. Partial inhibition of the reaction with antiserum against tumor necrosis factor-alpha. J Clin Invest. 1991;87:446–453. doi: 10.1172/JCI115016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 842.West JD, Marnett LJ. Endogenous reactive intermediates as modulators of cell signaling and cell death. Chem Res Toxicol. 2006;19:173–194. doi: 10.1021/tx050321u. [DOI] [PubMed] [Google Scholar]
- 843.Whelan RS, Kaplinskiy V, Kitsis RN. Cell death in the pathogenesis of heart disease: Mechanisms and significance. Annu Rev Physiol. 2010;72:19–44. doi: 10.1146/annurev.physiol.010908.163111. [DOI] [PubMed] [Google Scholar]
- 844.Willems IE, Havenith MG, DeMey JG, Chaponnier C, Brown RA. The alpha-smooth muscle actin-positive cells in healing human myocardial scars. Am J Pathol. 1994;145:868–875. [PMC free article] [PubMed] [Google Scholar]
- 845.Williams JP, Pechet TT, Weiser MR, Reid R, Kobzik L, Moore FD, Jr, Carroll MC, Hechtman HB. Intestinal reperfusion injury is mediated by IgM and complement. J Appl Physiol. 1997;86:938–942. doi: 10.1152/jappl.1999.86.3.938. [DOI] [PubMed] [Google Scholar]
- 846.Winek K, Engel O, Koduah P, Heimesaat MM, Fischer A, Bereswill S, Dames C, Kershaw O, Gruber AD, Curato C, Oyama N, Meisel C, Meisel A, Dirnagl U. Depletion of cultivatable gut microbiota by broad-spectrum antibiotic pretreatment worsens outcome after murine stroke. Stroke. 2016;47:1354–1363. doi: 10.1161/STROKEAHA.115.011800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 847.Winek K, Meisel A, Dirnagl U. Gut microbiota impact on stroke outcome: Fad or fact? J Cereb Blood Flow Metab. 2016;36:891–898. doi: 10.1177/0271678X16636890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 848.Winquist RJ, Kerr S. Cerebral ischemia-reperfusion injury and adhesion. Neurology. 1997;49:S23–S26. doi: 10.1212/wnl.49.5_suppl_4.s23. [DOI] [PubMed] [Google Scholar]
- 849.Wittnich C. Age-related differences in myocardial metabolism affects response to ischemia. Age in heart tolerance to ischemia. Am J Cardiovasc Pathol. 1992;4:175–180. [PubMed] [Google Scholar]
- 850.Wolf PS, Merry HE, Farivar AS, McCourtie AS, Mulligan MS. Stress-activated protein kinase inhibition to ameliorate lung ischemia reperfusion injury. J Thorac Cardiovasc Surg. 2008;135:656–665. doi: 10.1016/j.jtcvs.2007.11.026. [DOI] [PubMed] [Google Scholar]
- 851.Woodfin A, Voisin MB, Beyrau M, Colom B, Caille D, Diapouli FM, Nash GB, Chavakis T, Albelda SM, Rainger GE, Meda P, Imhof BA, Nourshargh S. The junctional adhesion molecule JAM-C regulates polarized transendothelial migration of neutrophils in vivo. Nat Immunol. 2011;12:761–769. doi: 10.1038/ni.2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 852.Wu B, Qiu W, Wang P, Yu H, Cheng T, Zambetti GP, Zhang L, Yu J. p53 independent induction of PUMA mediates intestinal apoptosis in response to ischaemia-reperfusion. Gut. 2007;56:645–654. doi: 10.1136/gut.2006.101683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 853.Wu SY, Tang SE, Ko FC, Wu GC, Huang KL, Chu SJ, Wu J, Huang Z, Ren J, Zhang Z, He P, Li Y, Ma J, Chen W, Zhang Y, Zhou X, Yang Z, Wu SQ, Chen L, Han J. Mlkl knockout mice demonstrate the indispensable role of Mlkl in necroptosis. Cell Res. 2013;23:994–1006. doi: 10.1038/cr.2013.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 854.Wu SY, Tang SE, Ko FC, Wu GC, Huang KL, Chu SJ. Valproic acid attenuates acute lung injury induced by ischemia-reperfusion in rats. Anesthesiology. 2015;122:1327–1337. doi: 10.1097/ALN.0000000000000618. [DOI] [PubMed] [Google Scholar]
- 855.Wung SF, Hickey KT, Taylor JY, Gallek MJ. Cardiovascular genomics. J Nurs. 2013;45:60–68. doi: 10.1111/jnu.12002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 856.Xie LH, Chen F, Karagueusian HS, Weiss JN. Oxidative stress-induced afterdepolarizations and calmodulin kinase II signaling. Circ Res. 2009;104:79–86. doi: 10.1161/CIRCRESAHA.108.183475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 857.Xiong F, Lin T, Song M, Ma Q, Martinez SR, Lv J, MataGreenwood E, Xiao D, Xu Z, Zhang L. Antenatal hypoxia induces epigenetic repression of glucocorticoid receptor and promotes ischemic-sensitive phenotype in the developing heart. J Mol Cell Cardiol. 2016;91:160–171. doi: 10.1016/j.yjmcc.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 858.Xu CF, Yu CH, Li YM. Regulation of hepatic microRNA expression in response to ischemic preconditioning following ischemia/reperfusion injury in mice. OMICS. 2009;13:513–520. doi: 10.1089/omi.2009.0035. [DOI] [PubMed] [Google Scholar]
- 859.Xu H, Manivannan A, Jiang HR, Liversidge J, Shazrp PF, Forester JV, Crane IJ. Recruitment of IFN-γ-producing (Th1-like) cells into the inflamed retina in vivo is preferentially regulated by p-selectin glycoprotein ligand 1: P/E-selectin interactions. J Immunol. 2004;172:3215–3224. doi: 10.4049/jimmunol.172.5.3215. [DOI] [PubMed] [Google Scholar]
- 860.Xu J, Zhao J, Evan G, Xiao C, Cheng Y, Xiao J. Circulating microRNAs: Novel biomarkers for cardiovascular diseases. J Mol Med. 2012;90:865–870. doi: 10.1007/s00109-011-0840-5. [DOI] [PubMed] [Google Scholar]
- 861.Xu X, Chua KW, Chua CC, Liu CF, Hamdy RC, Chua BH. Synergistic protective effects of humanin and necrostatin-1 on hypoxia and ischemia/reperfusion injury. Brain Res. 2010;1355:189–194. doi: 10.1016/j.brainres.2010.07.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 862.Xu X, Zhang XA, Wang DW. The role of CYP450 epoxygenases and metabolites, epoxysatrienoic acids, cardiovascular and malignant diseases. Adv Drug Deliv Rev. 2011;63:597–608. doi: 10.1016/j.addr.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 863.Xu Y, Huang S, Liu ZG, Han J. Poly(ADP-ribose) polymerase-1 signaling to mitochondria in necrotic cell death requires RIP1/TRAF2-mediated JNK1 activation. J Biol Chem. 2006;281:8788–8795. doi: 10.1074/jbc.M508135200. [DOI] [PubMed] [Google Scholar]
- 863a.Xu Y, Williams SJ, O’Brien D, Davidge ST. Hypoxia or nutrient restriction during pregnancy in rats leads to progressive cardiac remodeling and impairs postischemic recovery in adult male offspring. FASEB J. 2006;20:1251–1253. doi: 10.1096/fj.05-4917fje. [DOI] [PubMed] [Google Scholar]
- 864.Xu Z, Zhang J, David KK, Yang ZJ, Li X, Dawson TM, Dawson VL, Koehler RC. Endonuclease G does not play an obligatory role in poly(ADP-ribose) polymerase-dependent cell death after transient focal cerebral ischemia. Am J Physiol Regul Integr Comp Physiol. 2010;299:R215–R221. doi: 10.1152/ajpregu.00747.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 864a.Xue Q, Zhang L. Prenatal hypoxia causes a sex-dependent increase in heart susceptibility to ischemia and reperfusion injury in adult male offspring: Role of protein kinase C epsilon. J Pharmacol Exp Ther. 2009;330:624–632. doi: 10.1124/jpet.109.153239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 864b.Xue Q, Dasgupta C, Chen M, Zhang L. Foetal hypoxia increases cardiac AT(2)R expression and subsequent vulnerability to adult ischaemic injury. Cardiovasc Res. 2011;89:300–308. doi: 10.1093/cvr/cvq303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 864c.Xue Q, Chen P, Li X, Zhang G, Patterson AJ, Luo J. Maternal high-fat diet causes a sex-dependent increase in AGTR2 expression and cardiac dysfunction in adult male rat offspring. Biol Reprod. 2015;93:49. doi: 10.1095/biolreprod.115.129916. [DOI] [PubMed] [Google Scholar]
- 865.Yamashiro S, Noguchi K, Kuniyoshi Y, Koja K, Sakanashi M. Role of tetrahydrobiopterin on ischemia-reperfusion injury in isolated injury in isolated perfused rat hearts. J Cardiovasc Surg (Torino) 2003;44:37–49. [PubMed] [Google Scholar]
- 866.Yang JS, Lai EC. Alternative miRNA biogenesis pathways and the interpretation of core miRNA pathway mutants. Mol Cell. 2011;43:892–903. doi: 10.1016/j.molcel.2011.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 867.Yan L, Yang H, Li Y, Duan H, Wu J, Qian P, Li B, Wang S. Regulator of calcineurin 1-1L protects cardiomyocytes against hypoxia-induced apoptosis via mitophagy. J Cardiovasc Pharmacol. 2014;64:310–317. doi: 10.1097/FJC.0000000000000121. [DOI] [PubMed] [Google Scholar]
- 868.Yang M, Stowe DF, Udoh KB, Heisner JS, Camara AK. Reversible blockade of complex I or inhibition of PKCbeta reduces activation and mitochondria translocation of p66Shc to preserve cardiac function after ischemia. PLoS One. 2014;9:e113534. doi: 10.1371/journal.pone.0113534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 869.Yang MQ, Ma YY, Ding J, Li JY. The role of mast cells in ischemia and reperfusion injury. Inflamm Res. 2014;63:899–905. doi: 10.1007/s00011-014-0763-z. [DOI] [PubMed] [Google Scholar]
- 870.Yang W, Guastella J, Huang JC, Wang Y, Zhang L, Xue D, Tran M, Woodward R, Kasibhatla S, Tseng B, Drewe J, Cai SX. MX1013, a dipeptide caspase inhibitor with potent in vivo antiapoptotic activity. Br J Pharmacol. 2003;140:402–412. doi: 10.1038/sj.bjp.0705450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 871.Yang Y, Duan W, Li Y, Yan J, Yi W, Liang Z, Wang N, Yi D, Jin Z. New role of silent information regulator 1 in cerebral ischemia. Neurobiol Aging. 2013;34:2879–2888. doi: 10.1016/j.neurobiolaging.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 872.Yang Y, Rosenberg GA. Matrix metalloproteinases as therapeutic targets for stroke. Brain Res. 2015;1623:30–38. doi: 10.1016/j.brainres.2015.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 873.Yang Z, Sharma AK, Linden J, Kron IL, Laubach VE. CD4+ T lymphocytes mediate acute pulmonary ischemia-reperfusion injury. J Thorac Cardiovasc Surg. 2009;137:695–702. doi: 10.1016/j.jtcvs.2008.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 874.Yang ZJ, Carter EL, Kibler KK, Kwansa H, Crafa DA, Martin LJ, Roman RJ, Harder DR, Koehler RC. Attenuation of neonatal ischemic brain damage using a 20-HETE synthesis inhibitor. J Neurochem. 2012;121:168–179. doi: 10.1111/j.1471-4159.2012.07666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 875.Yaoita H, Ogawa K, Maehara K, Maruyama Y. Attenuation of ischemia/reperfusion injury in rats by a caspase inhibitor. Circulation. 1998;97:276–281. doi: 10.1161/01.cir.97.3.276. [DOI] [PubMed] [Google Scholar]
- 876.Yasojima K, Kilgore KS, Washington RA, Lucchesi BR, McGeer PL. Complement gene expression by rabbit heart: Upregulation by ischemia and reperfusion. Circ Res. 1998;82:1224–1230. doi: 10.1161/01.res.82.11.1224. [DOI] [PubMed] [Google Scholar]
- 877.Ye Y, Hu Z, Lin Y, Zhang C, Perez-Polo JR. Downregulation of microRNA-29 by antisense inhibitors and a PPAR-gamma agonist protects against myocardial ischaemia-reperfusion injury. Cardiovasc Res. 2010;87:535–544. doi: 10.1093/cvr/cvq053. [DOI] [PubMed] [Google Scholar]
- 878.Ye Y, Perez-Polo JR, Aguilar D, Birnbaum Y. The potential effects of anti-diabetic medications on myocardial ischemia-reperfusion injury. Basic Res Cardiol. 2011;106:925–952. doi: 10.1007/s00395-011-0216-6. [DOI] [PubMed] [Google Scholar]
- 879.Ye Y, Perez-Polo JR, Qian J, Birnbaum Y. The role of microRNA in modulating myocardial ischemia-reperfusion injury. Physiol Genomics. 2011;43:534–542. doi: 10.1152/physiolgenomics.00130.2010. [DOI] [PubMed] [Google Scholar]
- 880.Yellon DM, Downey JM. Preconditioning the myocardium: From cellular physiology to clinical cardiology. Physiol Rev. 2003;83:1113–1151. doi: 10.1152/physrev.00009.2003. [DOI] [PubMed] [Google Scholar]
- 881.Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med. 2007;357:1121–1135. doi: 10.1056/NEJMra071667. [DOI] [PubMed] [Google Scholar]
- 882.Yemisci M, Gursoy-Ozdemir Y, Vural A, Can A, Topalkara K, Dalkara T. Pericyte contraction induced by oxidative-nitrosative stress impairs capillary reflow despite successful opening of an occluded cerebral artery. Nat Med. 2009;15:1031–1038. doi: 10.1038/nm.2022. [DOI] [PubMed] [Google Scholar]
- 883.Yigitkanli K, Pekcec A, Karatas H, Pallast S, Mandeville E, Joshi N, Smirnova N, Gazaryan I, Ratan RR, Witztum JL, Montaner J, Holman TR, Lo EH, van Leyen K. Inhibition of 12/15-lipoxygenase as therapeutic strategy to treat stroke. Ann Neurol. 2013;73:129–135. doi: 10.1002/ana.23734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 884.Yilmaz G, Granger DN. Cell adhesion molecules and ischemic stroke. Neurol Res. 2008;30:783–793. doi: 10.1179/174313208X341085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 885.Yilmaz G, Granger DN. Cell adhesion molecules and ischemic stroke. Neurol Res. 2008;30:783–793. doi: 10.1179/174313208X341085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 886.Yilmaz G, Granger DN. Leukocyte recruitment and ischemic brain injury. Neuromol Med. 2010;12:193–204. doi: 10.1007/s12017-009-8074-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 887.Yin KJ, Deng Z, Huang H, Hamblin M, Xie C, Zhang J, Chen YE. miR-497 regulates neuronal death in mouse brain after transient focal cerebral ischemia. Neurobiol Dis. 2010;38:17–26. doi: 10.1016/j.nbd.2009.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 888.Yin KJ, Hamblin M, Chen YE. Non-coding RNAs in cerebral endothelial pathophysiology: Emerging roles in stroke. Neurochem Int. 2014;77:9–16. doi: 10.1016/j.neuint.2014.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 889.Yokota N, Burne-Taney M, Racusen L, Rabb H. Contrasting roles for STAT4 and STAT6 signal transduction pathways in murine renal ischemia-reperfusion injury. Am J Physiol. 2003;285:F319–F325. doi: 10.1152/ajprenal.00432.2002. [DOI] [PubMed] [Google Scholar]
- 890.Yoshiya K, Lapchak PH, Thai TH, Kannan L, Rani P, Lucca JJ, Tsokos GC. Depletion of gut commensal bacteria attenuates intestinal ischemia/reperfusion injury. Am J Physiol Gastrointest Liver Physiol. 2011;301:G1020–G1030. doi: 10.1152/ajpgi.00239.2011. [DOI] [PubMed] [Google Scholar]
- 891.Youle RJ, Narendra DP. Mechanisms of mitophagy. Nat Rev Mol Cell Biol. 2011;12:9–14. doi: 10.1038/nrm3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 892.Young GW, Wang Y, Ping P. Understanding proteasome assembly and regulation: Importance to cardiovascular medicine. Trends Cardiovasc Med. 2008;18:93–98. doi: 10.1016/j.tcm.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 893.Yu X, Kem DC. Proteasome inhibition during myocardial infarction. Cardiovasc Res. 2010;85:312–320. doi: 10.1093/cvr/cvp309. [DOI] [PubMed] [Google Scholar]
- 894.Yuan Y, Wang JY, Xu LY, Cai R, Chen Z, Luo BY. MicroRNA expression changes in the hippocampi of rats subjected to global ischemia. J Clin Neurosci. 2010;17:774–778. doi: 10.1016/j.jocn.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 895.Yusof M, Kamad K, Gaskin FS, Korthuis RJ. Angiotensin II mediates postischemic leukocyte-endothelial interactions: Role of calcitonin gene-related peptide. Am J Physiol Heart Circ Physiol. 2007;292:H3032–3037. doi: 10.1152/ajpheart.01210.2006. [DOI] [PubMed] [Google Scholar]
- 896.Yusof M, Kamada K, Kalogeris T, Gaskin FS, Korthuis RJ. Hydrogen sulfide triggers late-phase preconditioning in postischemic small intestine by an NO- and p38 MAPK-dependent mechanism. Am J Physiol Heart Circ Physiol. 2009;296:H868–H876. doi: 10.1152/ajpheart.01111.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 897.Zaccagnini G, Martelli F, Fasanaro P, Magenta A, Gaetano C, Di Carlo A, Biglioli P, Giorgio M, Martin-Padura I, Pelicci PG, Capogrossi MC. p66ShcA modulates tissue response to hindlimb ischemia. Circulation. 2004;109:2917–2923. doi: 10.1161/01.CIR.0000129309.58874.0F. [DOI] [PubMed] [Google Scholar]
- 898.Zaman AK, French CJ, Spees JL, Binbrek AS, Sobel BE. Vascular rhexis in mice subjected to non-sustained myocardial ischemia and its therapeutic implications. Exp Biol Med (Maywood) 2011;236:598–603. doi: 10.1258/ebm.2011.011026. [DOI] [PubMed] [Google Scholar]
- 899.Zernecke A, Preissner KT. Extracellular ribonucleic acids (RNA) enter the stage in cardiovascular disease. Circ Res. 2016;118:469–479. doi: 10.1161/CIRCRESAHA.115.307961. [DOI] [PubMed] [Google Scholar]
- 900.Zhai P, Sciarretta S, Galeotti J, Volpe M, Sadoshima J. Differential roles of GSK-3beta during myocardial ischemia and ischemia/reperfusion. Circ Res. 2011;109:502–511. doi: 10.1161/CIRCRESAHA.111.249532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 901.Zhang C. MicroRNAs in vascular biology and vascular disease. J Cardiovasc Transl Res. 2010;3:235–240. doi: 10.1007/s12265-010-9164-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 902.Zhang M, Alicot EM, Carroll MC. Human natural IgM can induce ischemia/reperfusion injury in amurine intestinal model. Mol Immunol. 2008;44:103–110. doi: 10.1016/j.molimm.2008.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 903.Zhang M, Austen WG, Jr, Chiu I, Alicot EM, Hung R, Ma M, Verna N, Xu M, Hechtman HB, Moore FD, Jr, Carroll MC. Identification of a specific self-reactive IgM antibody that initiates intestinal ischemia/reperfusion injury. Proc Nat Acad Sci. 2004;101:3886–3891. doi: 10.1073/pnas.0400347101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 904.Zhang M, Carroll MC. Natural antibody mediated innate autoimmune response. Mol Immunol. 2007;44:103–110. doi: 10.1016/j.molimm.2006.06.022. [DOI] [PubMed] [Google Scholar]
- 905.Zhang ZG, Chopp M. Neurorestorative therapies for stroke: Underlying mechanisms and translation to the clinic. Lancet Neurol. 2009;8:491–500. doi: 10.1016/S1474-4422(09)70061-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 906.Zhang Z, Liang D, Gao X, Zhao C, Qin X, Xu Y, Su T, Sun D, Li W, Wang H, Liu B, Cao F. Selective inhibition of inositol hexakisphosphate kinases (IP6Ks) enhances mesenchymal stem cell engraftment and improves therapeutic efficacy for myocardial infarction. Basic Res Cardiol. 2014;109:417. doi: 10.1007/s00395-014-0417-x. [DOI] [PubMed] [Google Scholar]
- 907.Zhang Y, Ramos BF, Jakschik BA. Neutrophil recruitment by tumor necrosis factor from mast cells in immune complex peritonitis. Science. 1992;258:1957–1959. doi: 10.1126/science.1470922. [DOI] [PubMed] [Google Scholar]
- 908.Zhang C, Wa J, Xu X, Potter BJ, Gao X. Direct relationship between levels of TNFa expression and endothelial dysfunction in reperfusion injury. Basic Res Cardiol. 2010;105:453–464. doi: 10.1007/s00395-010-0083-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 909.Zhang T, Zhang Y, Cui M, Jin L, Wang Y, Lv F, Liu Y, Zheng W, Shang H, Zhang J, Zhang M, Wu H, Guo J, Zhang X, Hu X, Cao CM, Xiao RP. CaMKII is a RIP3 substrate mediating ischemia- and oxidative stress-induced myocardial necroptosis. Nat Med. 2016;22:175–182. doi: 10.1038/nm.4017. [DOI] [PubMed] [Google Scholar]
- 910.Zhang XF, Zhang R, Huang L, Wang PX, Zhang Y, Jiang DS, Zhu LH, Tian S, Zhang XD, Li H. TRAF1 is a key mediator for hepatic ischemia/reperfusion injury. Cell Death Dis. 2014;5:e1467. doi: 10.1038/cddis.2014.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 911.Zhang Y, Zhao J, Lau WB, Jiao LY, Liu B, Yuan Y, Wang X, Gao E, Koch WJ, Ma XL, Wang Y. Tumor necrosis factor-alpha and lymphotoxin-alpha mediate myocardial ischemic injury via TNF receptor 1, but are cardioprotective when activating TNF receptor 2. PLoS One. 2013;8:e60227. doi: 10.1371/journal.pone.0060227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 912.Zhao BQ, Chauhan AK, Canau HM, Patten IS, Yang JJ, Dockal M, Scheiflinger F, Wagner DD. Von Willebrand factor-cleaving protease ADAMTS13 reduces ischemic brain injury in experimental stroke. Blood. 2009;114:3329–3334. doi: 10.1182/blood-2009-03-213264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 913.Zhao H, Ning J, Lemaire A, Koumpa FS, Sun JJ, Fung A, Gu J, Yi B, Lu K, Ma D. Necroptosis and parthanatos are involved in remote lung injury after receiving ischemic renal allografts in rats. Kidney Int. 2015;87:738–748. doi: 10.1038/ki.2014.388. [DOI] [PubMed] [Google Scholar]
- 914.Zhao H, Ren C, Chen X, Shen J. From rapid to delayed and remote postconditioning: The evolving concept of ischemic postconditioning in brain ischemia. Curr Drug Targets. 2012;13:173–187. doi: 10.2174/138945012799201621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 915.Zhao ZQ, Nakamura M, Wang NP, Velez DA, Hewan-Lowe KO, Guyton RA, Vinten-Johansen J. Dynamic progression of contractile and endothelial dysfunction and infarct extension in the late phase of reperfusion. J Surg Res. 2000;94:133–144. doi: 10.1006/jsre.2000.6029. [DOI] [PubMed] [Google Scholar]
- 916.Zhao ZQ, Nakamura M, Wang NP, Wilcox JN, Shearer S, Ronson RS, Guyton RA, Vinten-Johansen J. Reperfusion induces myocardial apoptotic cell death. Cardiovasc Res. 2000;45:651–660. doi: 10.1016/s0008-6363(99)00354-5. [DOI] [PubMed] [Google Scholar]
- 917.Zheng SY, Fu XB, Xu JG, Zhao JY, Sun TZ, Chen W. Inhibition of p38 mitogen-activated protein kinase may decrease intestinal epithelial cell apoptosis and improve intestinal epithelial barrier function after ischemia-reperfusion injury. World J Gastroenterol. 2005;11:656–660. doi: 10.3748/wjg.v11.i5.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 918.Zheng X, Zhang X, Sun H, Feng B, Li M, Chen G, Vladau C, Chen D, Suzuki M, Min L, Liu W, Zhong R, Garcia B, Jevnikar A, Min WP. Protection of renal ischemia injury using combination gene silencing of complement 3 and caspase 3 genes. Transplantation. 2006;82:1781–1786. doi: 10.1097/01.tp.0000250769.86623.a3. [DOI] [PubMed] [Google Scholar]
- 919.Zhou Q, Lam PY, Han D, Cadenas E. c-Jun N-terminal kinase regulates mitochondrial bioenergetics by modulating pyruvate dehydrogenase activity in primary cortical neurons. J Neurochem. 2008;104:325–335. doi: 10.1111/j.1471-4159.2007.04957.x. [DOI] [PubMed] [Google Scholar]
- 920.Zhu Q, Wani AA. Histone modifications: Crucial elements for damage response and chromatin restoration. J Cell Physiol. 2010;223:283–288. doi: 10.1002/jcp.22060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 921.Zhu W, Gregory JC, Org E, Buffa JA, Gupta N, Wang Z, Li L, Fu X, Wu Y, Mehrabian M, Sartor RB, McIntyre TM, Silverstein RL, Tang WH, DiDonato JA, Brown JM, Lusis AJ, Hazen SL. Gut microbial metabolite TMAO enhances platelet hyperreactivity and thrombosis risk. Cell. 2016;165:111–124. doi: 10.1016/j.cell.2016.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 922.Zhuang S, Demirs JT, Kochevar IE. p38 mitogen-activated protein kinase mediates bid cleavage, mitochondrial dysfunction, and caspase-3 activation during apoptosis induced by singlet oxygen but not by hydrogen peroxide. J Biol Chem. 2000;275:25939–25948. doi: 10.1074/jbc.M001185200. [DOI] [PubMed] [Google Scholar]
- 923.Zile MR, Mehurg SM, Arroyo JE, Stroud RE, DeSantis SM, Spinale FG. Relationship between the temporal profile of plasma microRNA and left ventricular remodeling in patients after myocardial infarction. Circ Cardiovasc Genet. 2011;4:614–619. doi: 10.1161/CIRCGENETICS.111.959841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 923a.Zuidema MY, Korthuis RJ. Intravital microscopic methods to evaluate anti-inflammatory effects and signaling mechanisms evoked by hydrogen sulfide. Methods Enzymol. 2015;555:93–125. doi: 10.1016/bs.mie.2014.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 924.Zweier JL, Flaherty JT, Weisfeldt ML. Direct measurement of free radical generation following reperfusion of ischemic myocardium. Proc Natl Acad Sci U S A. 1987;84:1404–1407. doi: 10.1073/pnas.84.5.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]