Abstract
Background
Prolonged opioid administration leads to tolerance characterised by reduced analgesic potency. Pain management is additionally compromised by the hedonic effects of opioids, the cause of their misuse. The multifunctional protein β-arrestin2 regulates the hedonic effects of morphine and participates in tolerance. These actions might reflect μ opioid receptor up-regulation through reduced endocytosis. β-Arrestin2 also recruits kinases to μ receptors. We explored the role of Src kinase in morphine analgesic tolerance, locomotor stimulation and reinforcement in C57BL/6 mice.
Methods
Analgesic (tail withdrawal latency; % maximum possible effect (MPE), n = 8-16), locomotor (distance travelled, n = 7-8) and reinforcing (conditioned place preference, n = 7-8) effects of morphine were compared in wild type, μ+/-, μ-/- and β-arrestin2-/- mice. The influence of c-Src inhibitors, dasatinib (n = 8) and PP2 (n = 12), were examined.
Results
Analgesia in morphine treated wild type mice exhibited tolerance, declining by day 10 to a median of 62% MPE (interquartile range (IQR): 29-92). Tolerance was absent from mice receiving dasatinib. Tolerance was enhanced in μ+/- mice (34% MPE; IQR: 5-52 on day 5); dasatinib attenuated tolerance (100% MPE; IQR: 68-100) as did PP2 (91% MPE; IQR: 78-100). By contrast, c-Src inhibition neither affected morphine-evoked locomotor stimulation nor reinforcement. Remarkably, dasatinib not only attenuated tolerance, but also reversed established tolerance in μ+/- mice.
Conclusions
The ability of c-Src inhibitors to inhibit tolerance, thereby restoring analgesia, without altering the hedonic effect of morphine, make c-Src inhibitors promising candidates as adjuncts to opioid analgesics.
While estimates vary, 9% of Americans and 19% of Europeans (11 – 55% in developing countries) are reported to experience moderate to severe persistent pain.1 Many pain sufferers receive prolonged opioid administration. Unfortunately however, tolerance develops leading to the requirement for increasing opioid doses for adequate pain control.1,2 Opioid tolerance is associated with the development of dependence and unpleasant withdrawal when treatment stops. Additional complications of opioid analgesics include constipation and, at higher doses, respiratory depression.1 The requirement for escalating doses to maintain analgesia increases the potential for prescription opioid misuse, diversion and overdose.3,4 Despite intensive attempts to develop alternative analgesics, there are currently none to replace opioids in the treatment of severe pain. An alternative is to improve opioid analgesia, minimising activation of pathways responsible for their detrimental effects such as tolerance, either by seeking agonists biased against such pathways, or by inhibiting them with adjunct agents.5,6
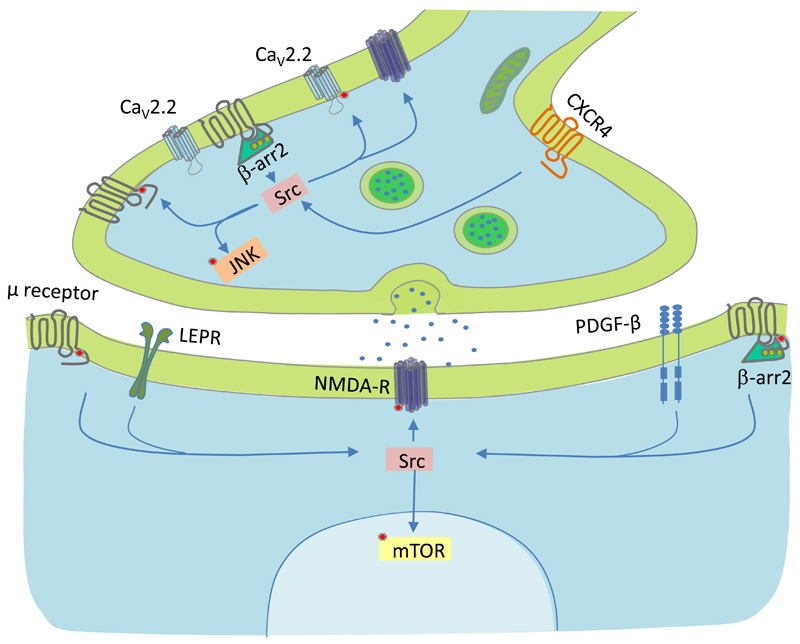
μ Opioid receptors mediate both the beneficial and the adverse effects of analgesic opioids.7 μ Receptors are G protein coupled receptors (GPCRs) that also recruit β-arrestin2, which participates in desensitisation, endocytosis and signalling through various kinases, including extracellular signal-regulated kinase (ERK) and the non-receptor tyrosine kinase, c-Src.2,8 Mice lacking β-arrestin2 (β-arr2-/- mice) exhibit reduced morphine tolerance and increased μ-receptor mediated basal nociception.9,10 The inhibition of several pathways that converge on c-Src also reduces morphine tolerance, implicating the tyrosine kinase as a potential hub for this process (fig. 1).
Fig. 1.
Pathways implicated in tolerance that converge on Src. Neurons contain high levels of c-Src.21 Recent studies have identified several pathways that converge on Src, their inhibition reduces morphine tolerance,6,38–42 potentially implicating the non-receptor tyrosine kinase as a hub in this process. Red spots represent targets of Src-mediated phosphorylation.24,25,43 μ Receptors (grey), the chemokine receptor type 4 (CXCR4), the leptin receptor (LEPR), N-methyl D-aspartate receptors (NMDA-R) and platelet derived growth factor receptor β (PDGF-β) are depicted in grey, red, green, dark blue and light blue, respectively. β-Arrestin2 (β-arr2), Src kinase (Src), c-Jun N-terminal kinase (JNK), mechanistic target of rapamycin (mTOR) and N-type Ca2+ channels (CaV2.2) are depicted in green, pink, orange, yellow and light blue, respectively.
The μ receptor-mediated activation of c-Src in primary afferent neurons requires β-arrestin2 and inhibition of c-Src causes reductions in μ receptor endocytosis and opioid-induced desensitisation.11,12 These observations led us to hypothesise that c-Src contributes to morphine tolerance. Given the evidence for a role of β-arrestin2 in the locomotor and reinforcing effects of morphine, we further hypothesised that c-Src also participates in these behaviours.13,14 Our findings suggest that c-Src inhibition suppresses tolerance without altering the locomotor or reinforcing effects of analgesic opioids.
Materials and Methods
Animals
In this study we used μ+/-, μ-/- and β-arr2-/- mice maintained on the C57BL/6J background, in the Ninewells Hospital Medical Resource Unit in accordance with the local ethics committee and UK Home Office regulations with an appropriate project license. They had access to food and water ad libitum with 12 hour cycles of light and dark, the temperature was maintained between 19 and 21°C. All experiments were performed in the light phase. Mice used in experiments were genotyped by Transnetyx (Cordova, TN, USA).
Behavioural Tests
Prior to each experiment, mice (aged 7-24 weeks, both sexes) were habituated. All experiments took place during the light phase. Drug doses were calculated using individual body weight and maximum volume administered in a single injection was 200 μL. Drug administration: Morphine sulphate (Sigma-Aldrich, Dorset, UK) was diluted in 0.9% NaCl in an aseptic environment and filtered using a 0.2 μm syringe filter prior to use. Morphine was administered subcutaneously (s.c.). For experiments involving c-Src inhibition, dasatinib (Bristol Myers Squibb, NY, USA), PP2 (Tocris, Bristol, UK) and PP3 (Tocris, Bristol, UK) were reconstituted in DMSO and Kolliphor EL (Sigma-Aldrich, Dorset, UK) and diluted in a 0.9% saline solution. Dasatinib (5 mg/Kg), its vehicle, PP2 (5 mg/Kg) and PP3 (5 mg/Kg) were administered via the intraperitoneal route (i.p.). Mice were randomly assigned to vehicle- or drug-treated groups while balancing the proportion of males and females. All samples were included for analysis with one exception: a mouse incorrectly assigned as μ-/-, which was omitted due to the initial genotyping error. During tail withdrawal assays the individual measuring the latency was blinded to the condition of the animal. Conditioned place preference and locomotor data were collected by CCTV and footage was analysed automatically by AnyMaze software (Stoelting Europe, Dublin, Ireland). Sample sizes were chosen based on our previous experience.10
Tail Withdrawal Assay
Morphine analgesia was assessed by measuring the latency for tail withdrawal from 48°C water 30 min after s.c. administration. Maximum exposure time to 48°C water was 15 s. We used an electronic thermostatic circulating water bath (Thermo Fisher, Loughborough, UK) to maintain water temperature within ± 0.1°C. Baseline tail withdrawal latencies were measured prior to the start of each experiment.
Conditioned Place Preference
We used a two compartment model of conditioned place preference to investigate morphine reinforcement in mice. One chamber had wall covering of black and white horizontal stripes and the other black and white vertical stripes. The compartments are contained within an operant box. These boxes are soundproofed and allow light levels to be controlled at approximately 70 lumens. The temperature was maintained between 21 and 23°C. Mice were habituated to the testing environment and allowed free access to both chambers prior to experiments. Mouse activity was recorded using a CCTV camera and parameters such as time spent in each chamber and distance travelled were acquired using AnyMaze software. During the 4-day conditioning period, all mice received s.c. injections of 0.9% saline (volume matched to that of the morphine injection) in either chamber. Four hours later the mice received an s.c. injection of morphine sulphate in the opposite chamber. After each injection they were confined to the corresponding chamber for 30 min. Between conditioning sessions mice were returned to their home cages. On day five mice were allowed free access to both chambers for 15 min. The time spent in each chamber was recorded using AnyMaze software.
Cell Culture and Western Blots
SW620 human colon cancer cells were maintained in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin (Invitrogen) at 37°C and 5% CO2.15 Cell lysis was performed in RIPA buffer (Thermo-Fisher, Loughborough, UK). Proteins were separated using denaturing SDS-PAGE and transferred onto nitrocellulose membranes. c-Src and phosphorylated c-Src proteins were probed with rabbit anti-Src and anti-p-Src antibodies, respectively (both from New England Biolabs, Hitchin, UK). Mouse anti-actin antibody (Abcam, Cambridge, UK) was used as a loading control. The primary antibodies were visualised with enhanced chemiluminescence reactions (ECL Prime, GE Life Sciences, Bucks, UK) using the appropriate horse radish peroxidase conjugated secondary antibodies.
Data Analysis
Tail withdrawal latencies were calculated as a percentage of maximal possible effect (MPE). The %MPE is calculated using the following equation in which the maximum exposure time (MET) is 15 s and the basal latency was the time for tail withdrawal from 48°C water in the absence of drug administration:
Comparison of conditioned place preference was done using preference scores, calculated by subtracting the time spent in the saline paired chamber from the time spent in the morphine paired chamber.
Morphine dose-response relationships were fitted with a logistic equation to determine EC50 values, using GraphPad Prism software (La Jolla, CA, USA).
Statistics
Non-parametric % MPE values for tolerance studies, which do not conform to the normal distribution are expressed as median ± interquartile range (IQR). All other data are expressed as mean ± standard deviation (SD). Statistical comparisons of the development of tolerance (% MPE values) were analysed using the Kruskall-Wallis test. Pairwise analyses within genotypes (vs day 1) and between genotypes (on the same days) were compared using the Dunn’s multiple comparison correction. Other pair-wise statistical comparisons on parametric data (i.e. distance and time) were made using two-tailed t-tests (paired or unpaired, as indicated). Three or more groups were compared using one-way or two-way ANOVA, as appropriate. Repeated-measures ANOVA was used when data were acquired over multiple days. Post hoc pair-wise testing was performed using either the Dunnett’s test (one-way ANOVA), or the Bonferroni test (two-way ANOVA). P values of less than 0.05 were considered statistically significant. Statistical testing was performed using Graphpad Prism software.
Results
Src Inhibition Attenuates Morphine Analgesic Tolerance
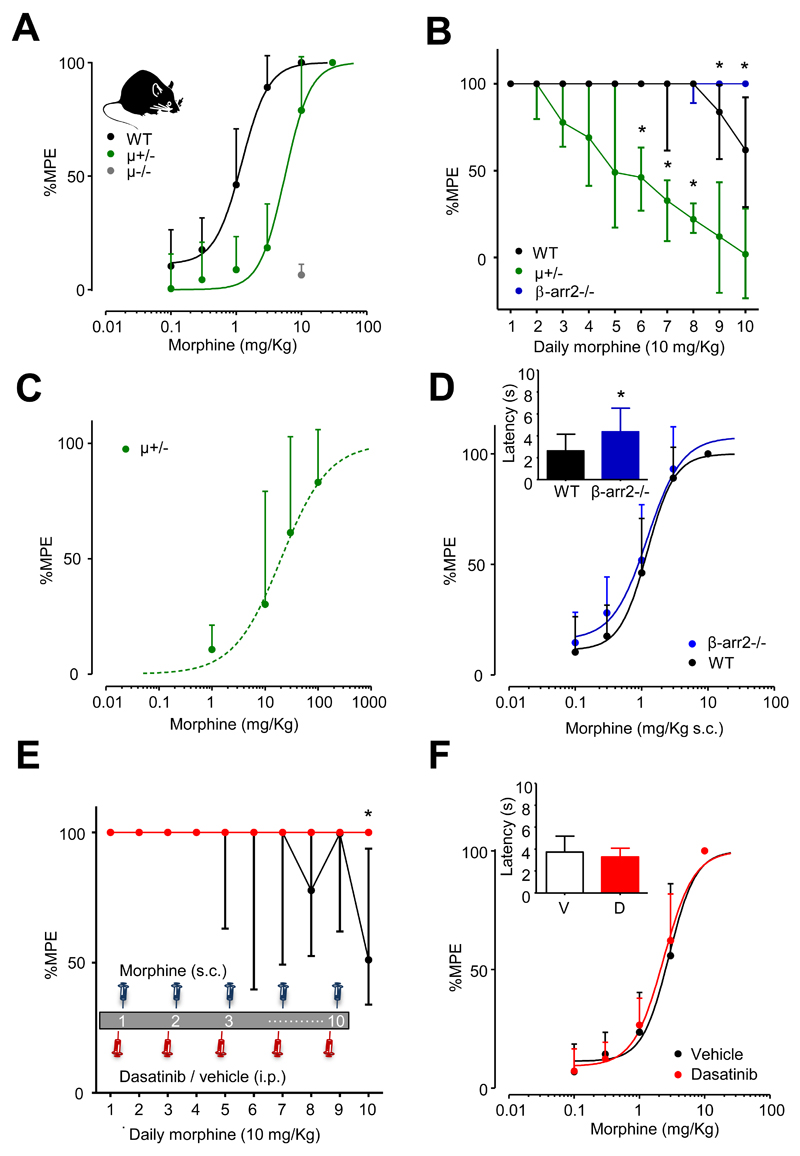
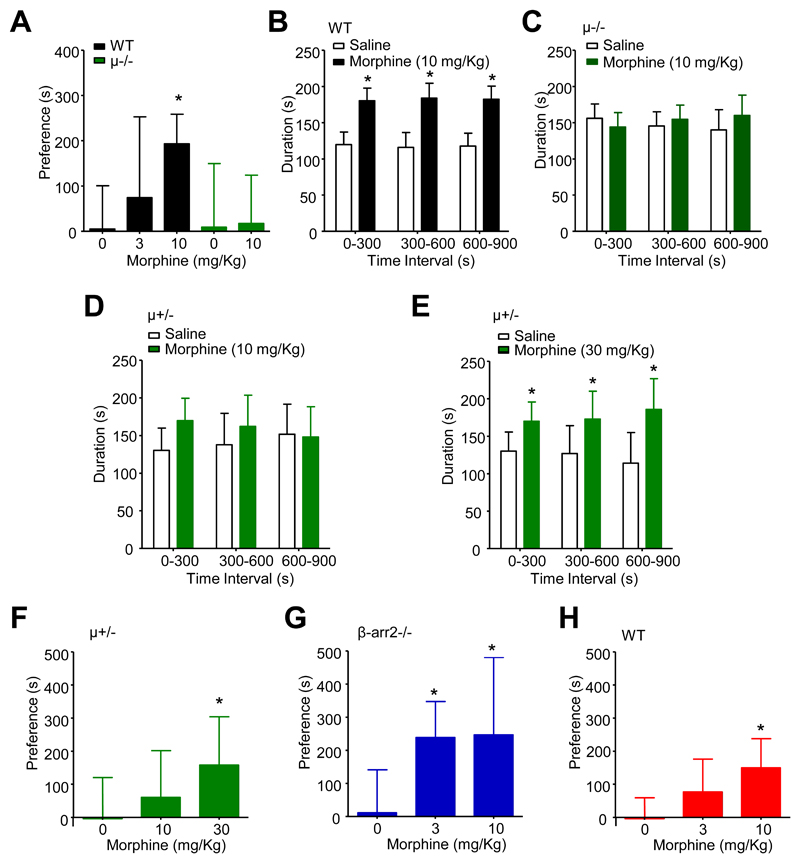
The subcutaneous administration of morphine caused a dose-dependent analgesia, prolonging tail withdrawal by C57BL/6 mice from 48°C water (fig. 2A). Consistent with a previous report,7 this effect depended on μ receptor expression, as evidenced by a lack of morphine (10 mg/Kg) analgesia in μ-/- mice (fig. 2A). Furthermore, morphine was less potent in μ+/- mice, which lack 50% of the full complement of μ receptors,7 without alteration of maximal efficacy (fig. 2A, and see Table 1).
Fig. 2.
Dasatinib attenuates morphine tolerance. (A) The dose-dependence of morphine prolongation of latency for tail withdrawal from noxious heat in wild type (n = 29) and μ+/- mice (n = 15). The ED50 was significantly greater in μ+/- mice (Table 1). Morphine (10 mg/Kg) had no effect on tail withdrawal latency when applied to μ-/- mice (n = 15). (B) The development of morphine (10 mg/Kg) analgesic tolerance in wild type (n = 16), μ+/- (n = 15) and β-arr2-/- (n = 15) mice. Data identified with asterisks were significantly different from equivalent wild type data (*p < 0.05, Kruskall-Wallis test, post hoc Dunn’s correction). (C) The dose-dependence of morphine prolongation of latency for tail withdrawal from noxious heat in μ+/- mice, in which tolerance was induced by 4 once daily injections of morphine (10 mg/Kg s.c.). The morphine dose-response relationship was examined on day 5 (n = 8). Compared to naïve MOP+/- mice, tolerance caused a reduction in the analgesic potency of morphine (Table 1). (D) The dose-response relationship for morphine in β-arr2-/- (n = 16) was similar to that of wild type mice (Table 1). Inset, tail withdrawal latency was longer for β-arr2-/- compared to WT mice (*p < 0.001, student’s t-test). (E) Dasatinib (5 mg/Kg i.p.), applied 30 mins before morphine (10 mg/Kg), reduced morphine tolerance in wild type mice (n = 8) compared to vehicle treated controls (n = 8). Inset, the schematic represents the dosing regimen. Data identified with asterisks were significantly different from equivalent vehicle data (*p < 0.01, Kruskall-Wallis test, post hoc Dunn’s correction). (F) Dasatinib neither affected the morphine dose-response relationship nor the time for tail withdrawal measured in the absence of morphine (inset bar graph). Data points in (B) and (E) represent median values and error bars are ± IQR. All other data are expressed as mean ± SD.
Table 1.
The influence of genotype and tolerance on morphine analgesia ED50.
| Genotype | ED50 morphine (mg/Kg) | n number |
|---|---|---|
| WT | 1.2 ± 0.1 | 29 |
| MOP+/- opioid naive | 5.9 ± 0.8* | 15 |
| MOP+/- opioid tolerant | 38 ± 14# | 8 |
| β-arr2-/- | 1.5 ± 0.4 | 16 |
Opioid tolerance was established in MOP+/- mice by 4 days of morphine (10 mg/Kg s.c.) injections.
p < 0.05 (unpaired t test vs WT);
p < 0.05 (unpaired t test vs MOP+/- opioid naïve).
We examined the development of tolerance to repeated once daily injections of morphine (10 mg/Kg s.c.). Using this paradigm the analgesic effect declined in wild type mice over several days reflecting the gradual development of morphine tolerance (fig. 2B). There was a significant reduction of the prolongation of tail withdrawal, with respect to that recorded on day 1, on days 9 and 10 of morphine administration (P < 0.05; Kruskall-Wallis test; post hoc Dunn’s correction). Tolerance involves reduced μ receptor reserve through desensitization and endocytosis.2 Consistent with this, μ+/- mice demonstrated a significant reduction, by day 6 (P < 0.05; Kruskall-Wallis test; post hoc Dunn’s correction) in the morphine-evoked prolongation of tail withdrawal latency, with respect to that recorded on day 1. Pair-wise comparisons of tail withdrawal latencies revealed μ+/- mice displayed significantly more tolerance than wild type mice on days 6, 7 and 8 (P < 0.05; Kruskall-Wallis test; post hoc Dunn’s correction; fig. 2B). Examination of the morphine dose-response relationship in μ+/- mice on day 4 revealed a significant (Student t-test, p < 0.05) reduction in analgesic potency compared to morphine-naïve μ+/- mice (fig. 2C; Table 1).
The multifunctional anchoring protein, β-arrestin2, participates in opioid receptor endocytosis and its absence leads to an up-regulation of μ receptors at the cell surface of primary afferent neurons.12 Mice lacking β-arrestin2 (β-arr2-/- mice) also exhibit reduced morphine tolerance.9 Consistent with these findings, β-arr2-/- mice, when treated once daily with morphine (10 mg/Kg s.c.), exhibited less tolerance on days 9 and 10 than did wild type mice (P < 0.05; Kruskall-Wallis test; post hoc Dunn’s correction; fig. 2B). β-arr2-/- mice had an unaltered morphine dose-response relationship compared to wild type mice (Table 1) and increased basal latencies for tail withdrawal from 48°C water (student’s t-test p < 0.001; fig. 2D).
The activation of μ receptors in primary afferent neurons leads to a β-arrestin2-dependent stimulation of c-Src activation.12 We tested the hypothesis that c-Src contributes to the development of morphine analgesic tolerance using the anti-leukaemia c-Src inhibitor dasatinib, which crosses the blood brain barrier in mice.15 When administered once daily to wild type mice, 30 min before morphine (10 mg/Kg s.c.), dasatinib (5 mg/Kg i.p.) reduced the development of analgesic tolerance (fig. 2E). As previously observed (fig. 2B), morphine tolerance developed slowly in WT mice, but was nevertheless diminished by dasatinib (fig. 2E). The attenuation of morphine tolerance was significant on day 10 (P < 0.01; Kruskall-Wallis test; post hoc Dunn’s correction; fig. 2E). On day 10 vehicle treated mice exhibited morphine (10 mg/Kg s.c.) analgesia that had declined to a median of 51% MPE (IQR: 34-94; n = 8) of that on day 1. By contrast, dasatinib treated mice maintained full analgesia on day 10. The attenuation of morphine analgesic tolerance by dasatinib occurred without alteration of either the morphine dose-response relationship or basal nociception (fig. 2F).
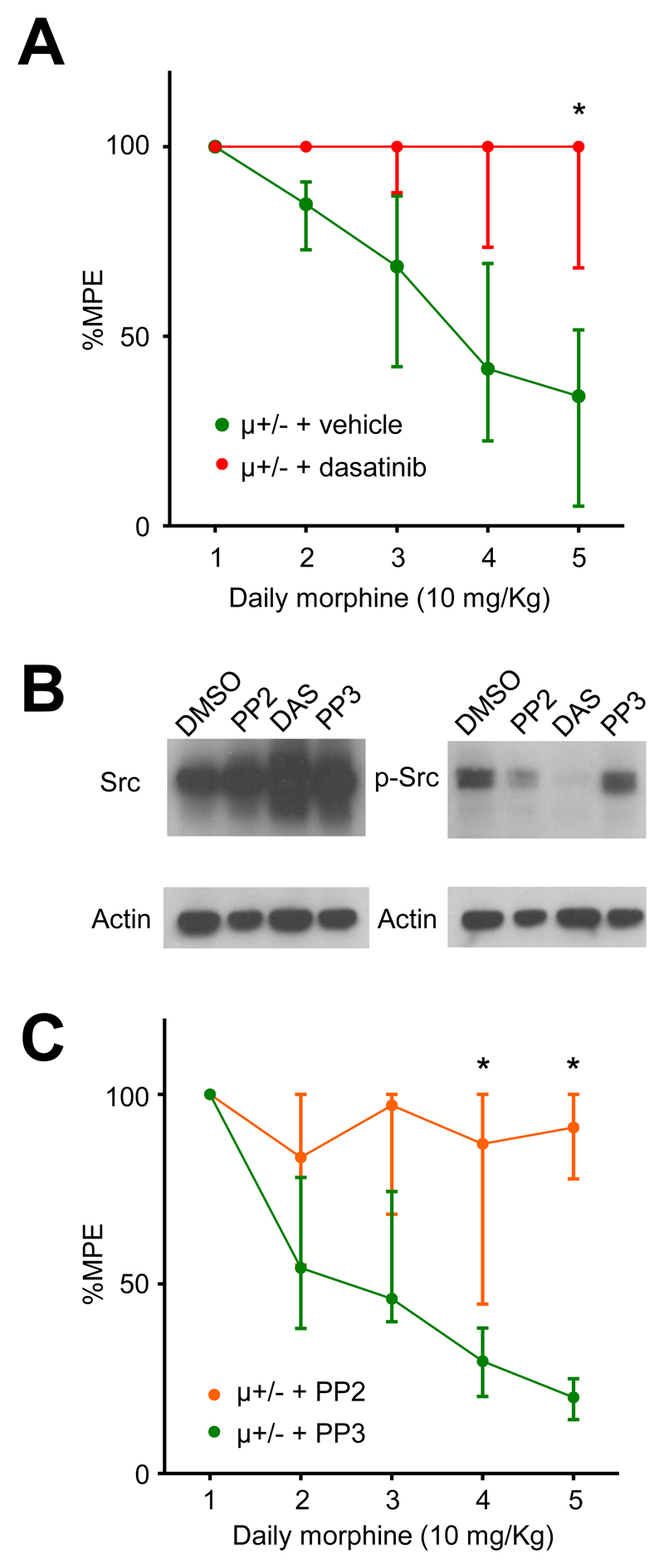
Due to the slow development of tolerance in wild type mice we examined the effects of dasatinib in μ+/- in which tolerance develops faster (fig. 2B). Vehicle treated μ+/- mice developed morphine tolerance from day 4 (p < 0.05; Kruskall-Wallis test; post hoc Dunn’s correction; fig. 3A). Dasatinib reduced morphine tolerance in μ+/- mice, the attenuation was significant on day 5 compared to vehicle treated μ+/- mice (P < 0.05; Kruskall-Wallis test; post hoc Dunn’s correction; fig. 3A). By day 5 of daily morphine administration analgesia in μ+/- mice receiving vehicle i.p. had declined to a median of 34% MPE (IQR: 5-52; n = 8) of that seen on day 1. By contrast, on day 5, μ+/- mice receiving dasatinib (5 mg/Kg i.p.) 30 minutes prior to morphine, maintained a median of 100% MPE (IQR: 68-100; n = 8) analgesia (fig. 3A).
Fig. 3.
Inhibition of c-Src attenuates morphine tolerance. (A) Dasatinib (n = 8) reduced tolerance in μ+/- mice compared to vehicle injections (n = 8). Data identified with asterisks were significantly different from equivalent vehicle data (*p < 0.05, Kruskall-Wallis test, post hoc Dunn’s correction). (B) Western blot showing total c-Src (left panel) and phosphorylated c-Src (right panel) extracted from SW620 colon cancer cells treated with either DMSO (vehicle), PP2 (10 μM), dasatinib (10 μM) or PP3 (10 μM). β-Actin was used as a loading control. PP2 and dasatinib reduced phosphorylated c-Src levels, while PP3 had no effect relative to vehicle. (C) The relatively selective c-Src inhibitor, PP2 (5 mg/Kg i.p.), also attenuated the development of morphine tolerance, while the inactive analogue, PP3 (5 mg/Kg i.p.) did not. Data identified with asterisks were significantly different from equivalent PP3 data (*p < 0.01, Kruskall-Wallis test, post hoc Dunn’s correction). Data are presented as median ± IQR.
While c-Src inhibition is considered responsible for its clinical efficacy, dasatinib also inhibits other tyrosine kinases.16 By comparison, PP2 is more specific and has the advantage of the inactive analogue PP3, which can be used as a comparator.12 We tested the inhibitory effects of dasatinib, PP2 and PP3 in SW620 colon cancer cells, which have high levels of basal c-Src activity.17 Consistent with their reported properties, dasatinib and PP2 inhibited c-Src when administered to colon cancer cells, while PP3 was inactive (fig. 3B). We administered PP2 or PP3 (5 mg/Kg i.p.) to μ+/- mice, once daily 30 min before morphine (10 mg/Kg s.c.). PP3 had no effect; the development of morphine tolerance was similar to that seen in vehicle treated mice. However, PP2 attenuated tolerance from day 4 when compared to PP3 treated mice (P < 0.05; Kruskall-Wallis test; post hoc Dunn’s correction; fig. 3C). Morphine analgesia in μ+/- mice declined to a median of 20% MPE (IQR: 14-25; n = 12) of its level on day 1 in mice receiving PP3. By contrast, on day 5 μ+/- mice receiving PP2 maintained 91% MPE (IQR: 78-100; n = 12) of the analgesia seen on day 1. Neither dasatinib nor PP2 (or PP3) affected basal tail withdrawal when applied to μ+/- mice in the absence of morphine (data not shown).
Src Inhibition Does Not Affect The Psychomotor Effects Of Morphine
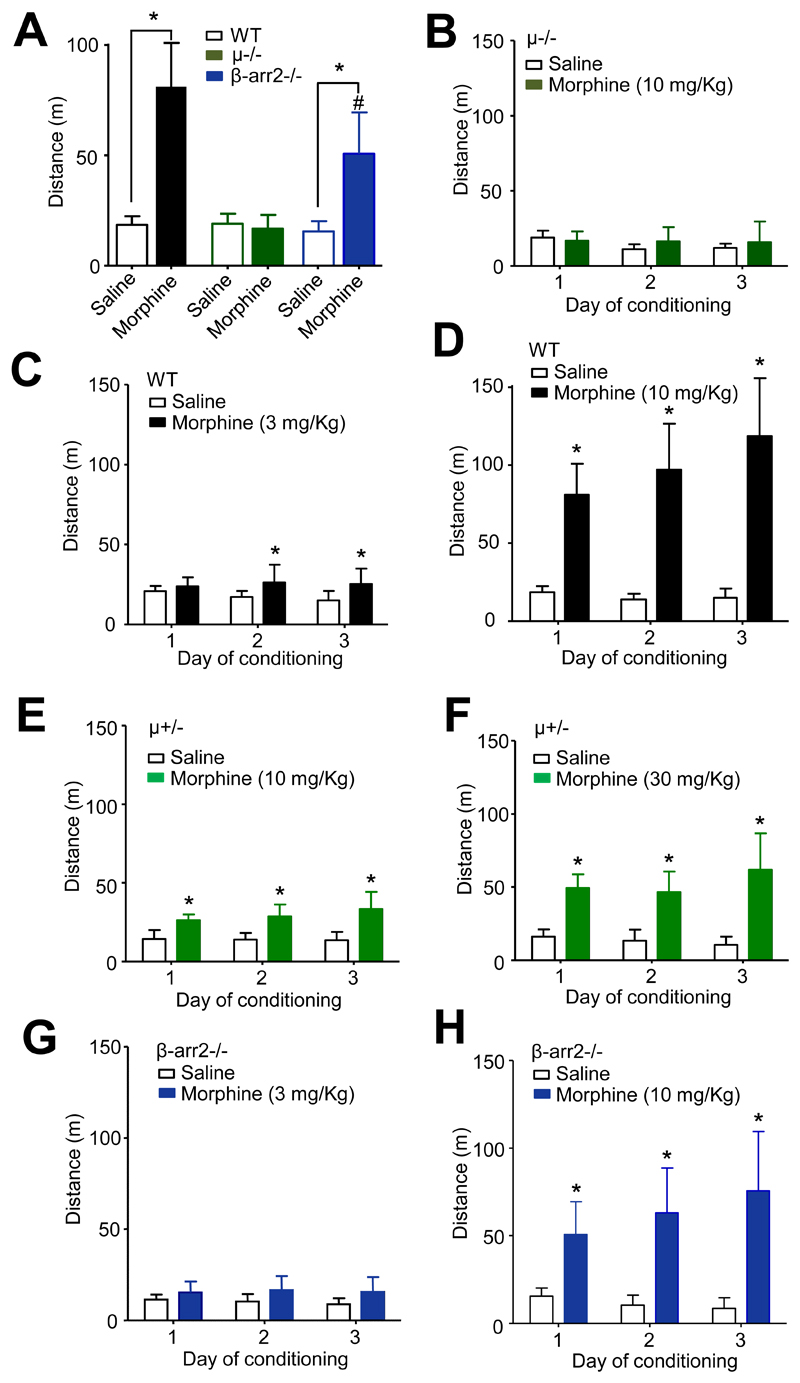
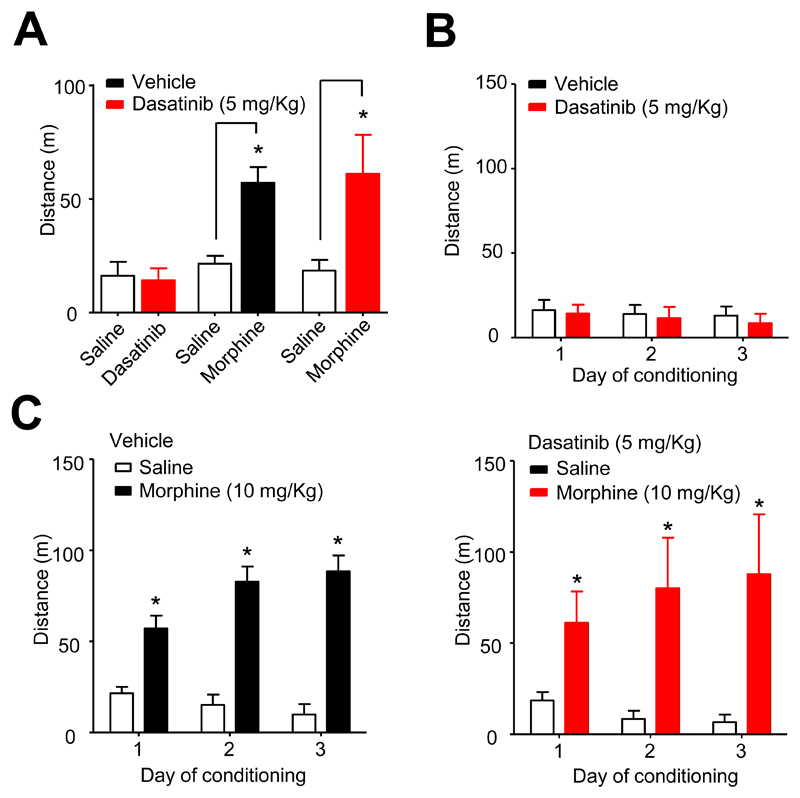
In addition to analgesia, morphine evokes psychomotor-stimulatory and reinforcing effects in mice.13,14 Compared to saline injections (19 ± 4 m travelled on day 1, 30 min following injection), WT mice, administered morphine (10 mg/Kg s.c.), exhibited dramatically increased locomotor activity (81 ± 20 m, n = 8, travelled on day 1, 30 min following injection), quantified by analysis of video tracking (p < 0.05, paired t-test; fig. 4A). By contrast, locomotor stimulation was absent from μ-/- mice administered morphine (10 mg/Kg), in which the average distances travelled were 19 ± 5 m and 17 ± 6 m on day 1, following saline and morphine injections (n = 7), respectively (fig. 4A). Morphine (10 mg/Kg) was without an effect on locomotion in μ-/- mice on all three days of administration (fig. 4B). By contrast, morphine (3 mg/Kg) caused a modest enhancement of locomotion in wild type mice (fig. 4C). However, there was neither an effect of time per se, nor a significant interaction of morphine and time (drug F1,28 = 5.8, p < 0.05, time F2,28 = 0.98, p = 0.4, interaction F2,28 = 2.9, p = 0.07; two-way ANOVA). By contrast, repeated daily morphine (10 mg/Kg) administration caused sensitisation of locomotor stimulation in WT mice (drug F1,28 = 72, time F2,28 = 12, interaction F2,28 = 17, all p < 0.0005; two-way ANOVA; fig. 4D).
Fig. 4.
Either fewer μ receptors or the absence of β-arrestin2 diminishes psychomotor stimulation by morphine. (A) Morphine (10 mg/Kg s.c.) stimulated locomotor activity in WT mice averaged over the 3 days of conditioning (*p < 0.001, n = 8, paired t-test compared to saline). This effect was not seen in μ-/- mice in which morphine had no effect on the averaged locomotion (n = 7). By contrast, morphine stimulated locomotion in β-arr2-/- mice (*p < 0.01, paired t-test, n = 8), but the average distance travelled was less than that of WT mice (#p < 0.01, unpaired t-test). (B) Morphine (10 mg/Kg) was without effect on distance travelled by μ-/- mice (n = 7) on all days of conditioning. (C) Morphine (3 mg/Kg) administration to WT mice (n = 8) showed modest increase on distance travelled on days 2 and 3 of conditioning (*p < 0.05, two-way ANOVA, post hoc Bonferroni test). (D) At a higher dose morphine (10 mg/Kg s.c.) increased distance travelled on all 3 days and this effect exhibited sensitization (*p < 0.0001, two-way ANOVA, post hoc Bonferroni test; n = 8). (E) The locomotor effect of morphine (10 mg/Kg) was diminished in μ+/- mice (n = 8) and there was no sensitisation (*p < 0.01 on day 1, p < 0.0001 on day 2 and 3, two-way ANOVA, post hoc Bonferroni test). (F) A higher dose of morphine (30 mg/Kg) enhanced locomotion, but there was no effect of time over the 3 days of conditioning (*p < 0.0001, two-way ANOVA, post hoc Bonferroni test; n = 8). Morphine (3 mg/Kg) had no effect on distance travelled by β-arr2-/- mice on days 1-3 (n = 8). (G) At a higher dose morphine (10 mg/Kg) increased distance travelled by β-arr2-/- mice on all 3 days and this effect exhibited sensitization (*p < 0.01 on day 1, p < 0.0001 on day 2 and 3, two-way ANOVA, post hoc Bonferroni test; n = 8).
By contrast to WT mice, morphine (10 mg/Kg) locomotor stimulation was modest in μ+/- mice and did not exhibit sensitisation (drug F1,28 = 36, p < 0.0001, time F2,28 = 1.7, p = 0.2, interaction F2,28 = 2.5, p = 0.1; two-way ANOVA; fig. 4E). A higher dose of morphine (30 mg/Kg) evoked a more robust locomotor stimulation accompanied by sensitisation (drug F1,28 = 45, p < 0.0001, time F2,28 = 3.1, p = 0.06, interaction F2,28 = 8.4, p < 0.001; two-way ANOVA; fig. 4 F).
The psychomotor effect of morphine is also influenced by β-arrestin2 expression.13,14 In keeping with previous reports, β-arr2-/- mice displayed a diminished morphine (10 mg/Kg s.c.) locomotor stimulatory response compared to WT mice (fig. 4A). Morphine increased (p < 0.01, paired t-test) the average distance travelled by β-arr2-/- mice to 51 ± 19 m (n = 8) compared to 16 ± 5 m (n = 8) in β-arr2-/- mice receiving vehicle. When compared to the locomotor stimulation by morphine (10 mg/Kg) exhibited by wild type mice, the effect of morphine in β-arr2-/- mice was significantly (p < 0.01, Student t-test) diminished. While there was no significant effect of the lower dose of morphine (3 mg/Kg) on locomotion in β-arr2-/- mice (fig. 4G), mice receiving 10 mg/Kg morphine exhibited increased locomotion and sensitization (drug F1,28 = 31, time F2,28 = 6.7, interaction F2,28 = 21, all p < 0.005; two-way ANOVA; fig. 4H).
The requirement for β-arrestin2 for the full locomotor stimulatory response to morphine (fig. 4A) has been linked to its role in recruiting phospho-ERK to D1 receptors in the striatum. Inhibition of mitogen-activated protein kinase/ERK kinase, which phosphorylates and thereby activates ERK, reduces morphine locomotor stimulation.14 We used dasatinib to determine whether c-Src is also involved in the stimulation of locomotion by morphine. When administered daily (5 mg/Kg, i.p.) either alone (n = 8) or 30 min prior to morphine (n = 8), dasatinib neither affected the average basal locomotion nor the average morphine locomotor stimulation compared to mice receiving vehicle (fig. 5A). Dasatinib had no effect on locomotion on any of the three days of its sole administration (fig. 5B). Furthermore, morphine (10 mg/Kg) caused locomotor stimulation and sensitization when administered on days 1-3 either after vehicle (drug F1,28 = 561, time F2,28 = 18, interaction F2,28 = 72, all p < 0.0001; two-way ANOVA) or dasatinib (drug F1,28 = 64, p < 0.0001, time F2,28 = 1.5, p = 0.24, interaction F2,28 = 11, p = 0.0003; two-way ANOVA; fig. 5C).
Fig. 5.
Dasatinib does not affect psychomotor stimulation by morphine. (A) The graph of locomotion averaged over 3 days of conditioning with dasatinib and/or morphine. Dasatinib had no effect on locomotion when administered alone to wild type mice (n = 8). Dasatinib (n = 8) also had no effect compared to vehicle (n = 8) on the stimulation of locomotion by morphine, which was significant in both cases (*p < 0.001, paired t-test). (B) Dasatinib (5 mg/Kg) was without effect on distance travelled by mice (n = 8) on all 3 days of conditioning. (C) Morphine caused locomotor stimulation with sensitization over the 3 days of conditioning when mice were administered either vehicle (left panel) or dasatinib (right panel) i.p (*p < 0.0001, two-way ANOVA, post hoc Bonferroni test).
Src Inhibition Does Not Affect Morphine Reinforcement
Conditioned place preference represents drug reinforcement, an important component of human substance misuse.18 While there was no preference on day 1 of conditioning, WT mice exhibited a clear preference for the environment that was paired with morphine (10 mg/Kg s.c.) administration on day 5 after conditioning (p < 0.01, one way ANOVA with post hoc Dunnett’s test vs no morphine; fig. 6A). Increased time spent in the morphine (10 mg/Kg) paired environment was evident throughout the 15 min testing period with no influence of time (drug F1,42 = 143, p < 0.0001, time F2,42 < 0.0001, p = 1.0, interaction F2,42 = 0.2, p = 0.86; two-way ANOVA; fig. 6B). In confirmation of the essential role for μ receptors in this reinforcing effect, μ-/- mice lacked preference for the morphine (10 mg/Kg) paired environment (fig. 6A and C). μ-/- mice spent equal times in the environments paired with either saline or morphine at all stages during testing (fig. 6C). Similarly, μ+/- mice exhibited no preference for the morphine (10 mg/Kg) paired environment (fig. 6D). Morphine preference did however become apparent throughout the 15 min period in μ+/- mice receiving the higher dose of 30 mg/Kg morphine (drug F1,28 = 19, p < 0.0005, time F2,28 < 0.0001, p = 1.0, interaction F2,28 = 1.2, p = 0.32; two-way ANOVA; fig. 6E). Comparison of the dose-dependence of morphine preference in μ+/- mice reveals an apparent dextral shift compared to wild type mice (fig. 6F versus fig. 6A) with morphine (30 mg/Kg) causing a significant preference (p < 0.01, one way ANOVA with post hoc Dunnett’s test vs no morphine).
Fig. 6.
Unlike the absence of β-arrestin2, which enhances reinforcement by morphine, dasatinib had no effect. (A) Morphine (3 and 10 mg/Kg s.c.) caused a dose-dependent preference of wild type mice (n = 8) for the paired environment (*p < 0.01, one-way ANOVA, post hoc Dunnett’s test). Morphine preference was lacking in μ-/- mice (n = 7). (B) Following conditioning, the duration of occupancy of the morphine-paired environment increased significantly compared to the saline-paired environment in wild type mice at all three 5 min intervals (*p < 0.0001, two-way ANOVA, post hoc Bonferroni test; n = 8). (C) μ-/- mice (n = 7) exhibited no morphine conditioned place preference at any stage during the 15 min test period. (D) μ+/- mice (n = 8) also exhibited no morphine (10 mg/Kg) conditioned place preference. (E) By contrast, μ+/- mice (n = 8) exhibited conditioned place preference to a higher dose of morphine (30 mg/Kg) (*p < 0.05 for 300-600 s, p < 0.001 for 600-900 s, two-way ANOVA, post hoc Bonferroni test). (F) Morphine (10 and 30 mg/Kg) caused a dose-dependent preference of μ+/- mice (n = 8) for the paired environment (*p < 0.05, one way ANOVA, post hoc Dunnett’s test). (G) Morphine preference occurred at a lower dose in β-arr2-/- mice (*p < 0.01, one way ANOVA, post hoc Dunnett’s test; n = 8). (H) By contrast, dasatinib had no effect morphine preference (*p < 0.01, one way ANOVA, post hoc Dunnett’s test; n = 8).
A previous study demonstrated that an absence of β-arrestin2 enhances the rewarding properties of morphine.13 In agreement with this we found that β-arr2-/- mice exhibited an increased sensitivity to morphine conditioned place preference (fig. 6G). Unlike wild type mice that lacked a significant response to 3 mg/Kg morphine, the same dose caused a robust conditioned place preference in β-arr2-/- mice (p < 0.01, one way ANOVA with post hoc Dunnett’s test vs no morphine), which was similar to that associated with 10 mg/Kg morphine (fig. 6G). These findings suggest that inhibition of a β-arrestin2-mediated signalling pathway may increase reward. We next examined whether dasatinib (5 mg/Kg i.p.) causes a similar increase in morphine conditioned place preference. Comparison of the dose-dependence for conditioned place preference reveals that, unlike the absence of β-arrestin2, which enhanced the sensitivity to morphine preference in β-arr2-/- mice, the dose-dependence in dasatinib treated wild type mice resembles that seen in untreated wild type mice, with morphine preference observed at the 10 mg/Kg dose (p < 0.01, one way ANOVA with post hoc Dunnett’s test vs no morphine; fig. 6H).
Dasatinib Reverses Morphine Analgesic Tolerance
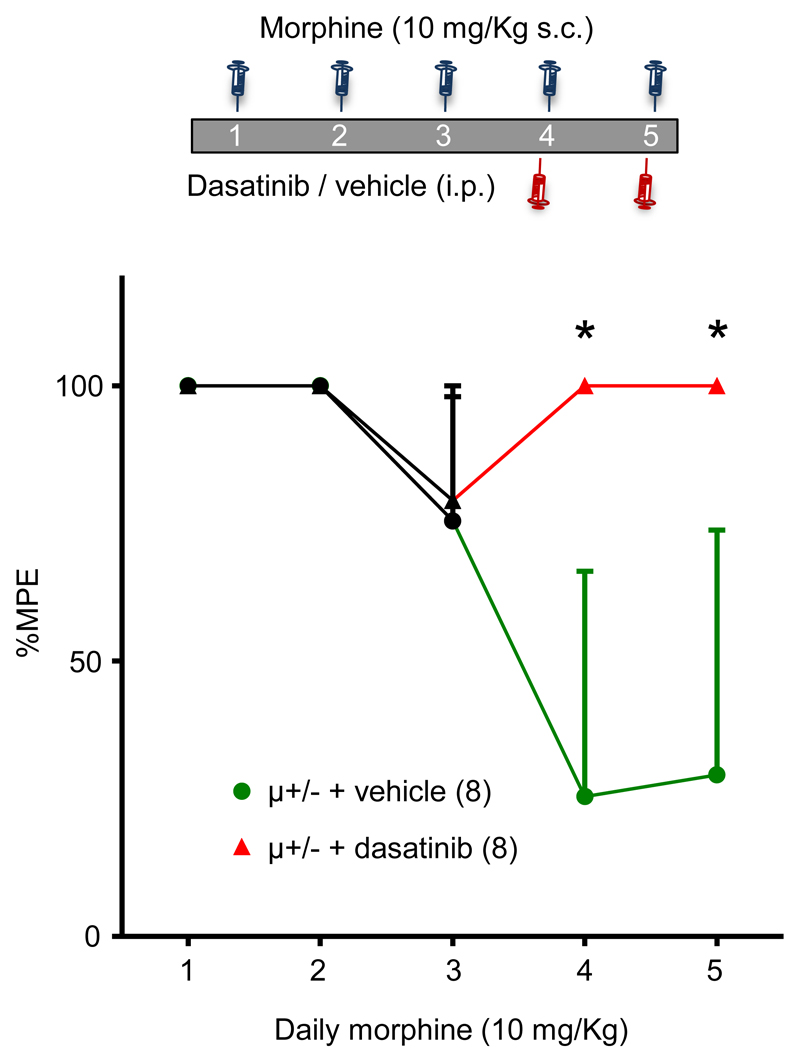
Having established that dasatinib inhibits morphine tolerance without affecting reward, we explored whether dasatinib influences tolerance in mice in which it had already developed. μ+/- mice were given morphine (10 mg/Kg) daily to initiate the development of tolerance and on day 4 received either dasatinib or vehicle 30 min prior to morphine administration (fig. 7). Tolerance continued to develop in vehicle treated mice. However, dasatinib caused an immediate reversal of tolerance and attenuated its further development. Comparisons of analgesia on days 4 and 5 between vehicle and dasatinib treated mice revealed a statistically significant difference (P < 0.05; Kruskall-Wallis test; post hoc Dunn’s correction; fig. 7).
Fig. 7.
Reversal of morphine tolerance by dasatinib. The diagram depicts the morphine, dasatinib/vehicle injection schedule on days 1 - 5. Data in the graph are average tail withdrawal latencies expressed as percentage of maximal possible effect (%MPE). μ+/- mice injected with vehicle (n = 8) 30 min prior to morphine on days 4 and 5 continued to develop tolerance. By contrast, μ+/- mice receiving dasatinib (n = 8) 30 min prior to morphine on days 4 and 5 exhibited reversal of analgesic tolerance. Data identified with asterisks were significantly different from equivalent vehicle data (*p < 0.05, Kruskall-Wallis test, post hoc Dunn’s correction). Data are presented as median ± IQR.
Discussion
This study reveals a requirement for c-Src activity for morphine analgesic tolerance and identifies c-Src inhibitors as agents that promote sustained analgesia. The c-Src inhibitor, dasatinib not only attenuated tolerance, but when administered before morphine also rapidly restored analgesia that had diminished during the preceding days. These effects occurred without altered psychomotor or reinforcing effects of morphine, suggesting that inhibitors of c-Src reduce opioid tolerance, without increasing reward.
The c-Src inhibitors alone had no effect on nociception and dasatinib did not influence the dose dependence of analgesia by morphine. These findings suggest that tolerance is required for c-Src inhibitors to enhance morphine analgesia. However, it remains to be determined whether c-Src activity is necessary for the expression and/or the development of tolerance. It is challenging to derive mechanistic insights from behavioral experiments. However, in future it would be worthwhile investigating whether the reversal of morphine analgesic tolerance persists after elimination of the c-Src inhibitor as has been demonstrated in the case of an NMDA receptor antagonist.19 Such an effect would be consistent with a requirement for c-Src activity for the development of tolerance.
The non-receptor tyrosine kinase v-Src was the first retroviral oncogene to be discovered.20 Subsequent research identified its cellular counterpart, c-Src, in vertebrates in which it is highly enriched at the synapse implying a role for the kinase in regulating neurotransmission.21 G protein coupled receptors, including μ receptors, couple to c-Src through mechanisms that are either independent (such as a PKC-mediated mechanism) or dependent on β-arrestins.12,22–24 μ Receptor-mediated activation of c-Src in DRG neurones, which is dependent on β-arrestin2, contributes to inhibition of presynaptic voltage-activated Ca2+ channels through phosphorylation of a specific alternatively spliced isoform of the N-type channel.12,25 In addition to its immediate role in μ receptor-mediated signal transduction, β-arrestin2-dependent c-Src activity also participates in μ receptor endocytosis and desensitization. The c-Src inhibitor, PP2, increases surface expression of μ receptors in DRG neurons and decreases opioid-induced heterologous desensitization in locus ceruleus neurons.11,12 These mechanisms may contribute to the attenuation of morphine analgesic tolerance by c-Src inhibitors in vivo.
Tolerance is arguably the most problematic aspect of opioid analgesia. The phenomenon leads to a requirement for escalating doses in patients suffering from persistent pain. Those on weak opioids often progress to stronger options and the continuing proliferation of prescriptions for strong opioids has led to their increased availability for diversion and misuse.1,4 The demonstration of a role for β-arrestin2 in tolerance and other side effects of opioids triggered the search for μ receptor agonists biased in favour of G protein stimulation.5,26–31 The first was herkinorin, which activates G proteins without recruitment of β-arrestin2 and produces analgesia in rats with markedly decreased tolerance compared to that of morphine.28,29 Herkinorin also caused less respiratory depression and constipation, μ receptor mediated side effects of morphine that are dependent on β-arrestin2 expression.29,32 The discovery of herkinorin was followed by TRV130 and PZM21, additional analgesic μ receptor agonists biased against β-arrestin2 recruitment.26,27 While the relative tendency for these agonists to cause tolerance remains unreported, both cause negligible β-arrestin2 recruitment and less respiratory depression and constipation than is associated with morphine. TRV130 performed well as an acute pain medication during bunionectomy in a phase 2 clinical trial.31 However, no biased μ receptor agonist has yet been tested in patients suffering from persistent pain. Furthermore, the extent that G protein bias plays in the apparently superior analgesic profiles of these new molecules compared to morphine remains unclear. An alternative explanation may be partial efficacy.33
An alternative to developing agonists biased against β-arrestin2 is to inhibit downstream components of the pathway, such as c-Src; our findings suggest that this is an effective approach for attenuating opioid tolerance. It is advantageous that c-Src inhibition, unlike deletion of β-arrestin2, does not influence the reinforcing or psychomotor effects of morphine. This suggests that c-Src inhibitors are unlikely to increase the hedonic effects of opioids. While ERK has been implicated in mediating the influence of β-arrestin2 on psychomotor activation,14 the cause of the enhanced sensitivity to morphine reinforcement in β-arr2-/- mice remains unknown. It is possible that this reflects an upregulation of surface μ receptors and/or dopamine receptors in the reward pathway in the absence of β-arrestin2-dependent endocytosis. If so, our findings suggest that this does not involve c-Src. Additional work is required to establish whether c-Src participates in other side effects of morphine such as constipation and respiratory depression.
While c-Src inhibitors were not anti-nociceptive in the acute pain model used in our study, c-Src activity has been implicated in persistent inflammatory, neuropathic and bone cancer pain, in which c-Src inhibitors reduce hyperalgesia.34–37 Hyperalgesia is associated with Src-mediated phosphorylation of the NMDA receptor, which leads to enhanced excitatory transmission in spinal neurones.34,36 Several parallels can be drawn between hyperalgesia and morphine tolerance, including a common requirement for NMDA receptor activity;38 the involvement of c-Src in both processes provides a potentially unifying mechanism. The ability of c-Src inhibitors to inhibit hyperalgesia and reverse tolerance, thereby restoring analgesia, makes them promising candidates as adjuncts to opioid analgesics.
Acknowledgements
We thank Lianne Strachan, Ph.D., (University of Dundee Behavioural Neuroscience Core Facility) for assistance with behavioural assays, Robert Lefkowitz, M.D., (Duke University) for β-arr2-/- mice and Brigitte Kieffer, Ph.D., (McGill University) for μ-/- mice.
Research Support: This work was supported by NIAA/BJA grant number WKRO-2014-0052 (T.G.H.) and a Tenovus Scotland grant number T15/54 (T.G.H. & F.A.B) and a Wellcome Trust PhD Fellowship grant number 100674/Z/12/A (F.A.B).
Footnotes
Author Contributions: F.A.B., D.T.B-H., C.S. and L.W. performed experiments and collected data. F.A.B., W.W. and T.G.H. conceived the study and designed the experiments. F.A.B. and T.G.H. analyzed and interpreted the data, prepared the figures and wrote the manuscript.
Competing Interests: The authors declare no competing financial interests.
Contributor Information
Wendy Walwyn, Shirley and Stefan Hatos Center for Neuropharmacology, University of California, Los Angeles, California 90095, USA
Tim G. Hales, Institute of Academic Anaesthesia, Division of Neuroscience, School of Medicine, Ninewells Hospital, University of Dundee, Dundee, DD1 9SY, UK
References
- 1.Noble M, Treadwell JR, Tregear SJ, Coates VH, Wiffen PJ, Akafomo C, Schoelles KM. Long-term opioid management for chronic noncancer pain. Cochrane Database Syst Rev. 2010 doi: 10.1002/14651858.CD006605.pub2. CD006605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Williams JT, Ingram SL, Henderson G, Chavkin C, von Zastrow M, Schulz S, Koch T, Evans CJ, Christie MJ. Regulation of mu-opioid receptors: desensitization, phosphorylation, internalization, and tolerance. Pharmacol Rev. 2013;65:223–54. doi: 10.1124/pr.112.005942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Webster LR, Fine PG. Review and critique of opioid rotation practices and associated risks of toxicity. Pain Med. 2012;13:562–70. doi: 10.1111/j.1526-4637.2012.01357.x. [DOI] [PubMed] [Google Scholar]
- 4.Zacny J, Bigelow G, Compton P, Foley K, Iguchi M, Sannerud C. College on Problems of Drug Dependence taskforce on prescription opioid non-medical use and abuse: position statement. Drug Alcohol Depend. 2003;69:215–32. doi: 10.1016/s0376-8716(03)00003-6. [DOI] [PubMed] [Google Scholar]
- 5.Manglik A, Lin H, Aryal DK, McCorvy JD, Dengler D, Corder G, Levit A, Kling RC, Bernat V, Hubner H, Huang XP, et al. Structure-based discovery of opioid analgesics with reduced side effects. Nature. 2016;537:185–190. doi: 10.1038/nature19112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu JT, Zhao JY, Zhao X, Ligons D, Tiwari V, Atianjoh FE, Lee CY, Liang L, Zang W, Njoku D, Raja SN, et al. Opioid receptor-triggered spinal mTORC1 activation contributes to morphine tolerance and hyperalgesia. J Clin Invest. 2014;124:592–603. doi: 10.1172/JCI70236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matthes HW, Maldonado R, Simonin F, Valverde O, Slowe S, Kitchen I, Befort K, Dierich A, Le Meur M, Dolle P, Tzavara E, et al. Loss of morphine-induced analgesia, reward effect and withdrawal symptoms in mice lacking the mu-opioid-receptor gene. Nature. 1996;383:819–23. doi: 10.1038/383819a0. [DOI] [PubMed] [Google Scholar]
- 8.Smith JS, Rajagopal S. The beta-Arrestins: Multifunctional Regulators of G Protein-coupled Receptors. J Biol Chem. 2016;291:8969–77. doi: 10.1074/jbc.R115.713313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bohn LM, Lefkowitz RJ, Gainetdinov RR, Peppel K, Caron MG, Lin FT. Enhanced morphine analgesia in mice lacking beta-arrestin 2. Science. 1999;286:2495–8. doi: 10.1126/science.286.5449.2495. [DOI] [PubMed] [Google Scholar]
- 10.Lam H, Maga M, Pradhan A, Evans CJ, Maidment NT, Hales TG, Walwyn W. Analgesic tone conferred by constitutively active mu opioid receptors in mice lacking beta-arrestin 2. Mol Pain. 2010;7:24. doi: 10.1186/1744-8069-7-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dang VC, Chieng BC, Christie MJ. Prolonged stimulation of mu-opioid receptors produces beta-arrestin-2-mediated heterologous desensitization of alpha(2)-adrenoceptor function in locus ceruleus neurons. Mol Pharmacol. 2012;82:473–80. doi: 10.1124/mol.112.079350. [DOI] [PubMed] [Google Scholar]
- 12.Walwyn W, Evans CJ, Hales TG. Beta-arrestin2 and c-Src regulate the constitutive activity and recycling of mu opioid receptors in dorsal root ganglion neurons. J Neurosci. 2007;27:5092–104. doi: 10.1523/JNEUROSCI.1157-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bohn LM, Gainetdinov RR, Sotnikova TD, Medvedev IO, Lefkowitz RJ, Dykstra LA, Caron MG. Enhanced rewarding properties of morphine, but not cocaine, in beta(arrestin)-2 knock-out mice. J Neurosci. 2003;23:10265–73. doi: 10.1523/JNEUROSCI.23-32-10265.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Urs NM, Daigle TL, Caron MG. A dopamine D1 receptor-dependent beta-arrestin signaling complex potentially regulates morphine-induced psychomotor activation but not reward in mice. Neuropsychopharmacology. 2011;36:551–8. doi: 10.1038/npp.2010.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Porkka K, Koskenvesa P, Lundan T, Rimpilainen J, Mustjoki S, Smykla R, Wild R, Luo R, Arnan M, Brethon B, Eccersley L, et al. Dasatinib crosses the blood-brain barrier and is an efficient therapy for central nervous system Philadelphia chromosome-positive leukemia. Blood. 2008;112:1005–12. doi: 10.1182/blood-2008-02-140665. [DOI] [PubMed] [Google Scholar]
- 16.Bain J, Plater L, Elliott M, Shpiro N, Hastie CJ, McLauchlan H, Klevernic I, Arthur JS, Alessi DR, Cohen P. The selectivity of protein kinase inhibitors: a further update. Biochem J. 2007;408:297–315. doi: 10.1042/BJ20070797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baker AM, Cox TR, Bird D, Lang G, Murray GI, Sun XF, Southall SM, Wilson JR, Erler JT. The role of lysyl oxidase in SRC-dependent proliferation and metastasis of colorectal cancer. J Natl Cancer Inst. 2011;103:407–24. doi: 10.1093/jnci/djq569. [DOI] [PubMed] [Google Scholar]
- 18.Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–9. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- 19.Tiseo PJ, Inturrisi CE. Attenuation and reversal of morphine tolerance by the competitive N-methyl-D-aspartate receptor antagonist, LY274614. J Pharmacol Exp Ther. 1993;264:1090–6. [PubMed] [Google Scholar]
- 20.Martin GS. The hunting of the Src. Nat Rev Mol Cell Biol. 2001;2:467–75. doi: 10.1038/35073094. [DOI] [PubMed] [Google Scholar]
- 21.Brugge JS, Cotton PC, Queral AE, Barrett JN, Nonner D, Keane RW. Neurones express high levels of a structurally modified, activated form of pp60c-src. Nature. 1985;316:554–7. doi: 10.1038/316554a0. [DOI] [PubMed] [Google Scholar]
- 22.Luttrell LM, Ferguson SS, Daaka Y, Miller WE, Maudsley S, Della Rocca GJ, Lin F, Kawakatsu H, Owada K, Luttrell DK, Caron MG, et al. Beta-arrestin-dependent formation of beta2 adrenergic receptor-Src protein kinase complexes. Science. 1999;283:655–61. doi: 10.1126/science.283.5402.655. [DOI] [PubMed] [Google Scholar]
- 23.Moyers JS, Bouton AH, Parsons SJ. The sites of phosphorylation by protein kinase C and an intact SH2 domain are required for the enhanced response to beta-adrenergic agonists in cells overexpressing c-src. Mol Cell Biol. 1993;13:2391–400. doi: 10.1128/mcb.13.4.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang L, Zhao H, Qiu Y, Loh HH, Law PY. Src phosphorylation of micro-receptor is responsible for the receptor switching from an inhibitory to a stimulatory signal. J Biol Chem. 2009;284:1990–2000. doi: 10.1074/jbc.M807971200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raingo J, Castiglioni AJ, Lipscombe D. Alternative splicing controls G protein-dependent inhibition of N-type calcium channels in nociceptors. Nat Neurosci. 2007;10:285–92. doi: 10.1038/nn1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen XT, Pitis P, Liu G, Yuan C, Gotchev D, Cowan CL, Rominger DH, Koblish M, Dewire SM, Crombie AL, Violin JD, et al. Structure-activity relationships and discovery of a G protein biased mu opioid receptor ligand, [(3-methoxythiophen-2-yl)methyl]({2-[(9R)-9-(pyridin-2-yl)-6-oxaspiro-[4.5]decan- 9-yl]ethyl})amine (TRV130), for the treatment of acute severe pain. J Med Chem. 2013;56:8019–31. doi: 10.1021/jm4010829. [DOI] [PubMed] [Google Scholar]
- 27.DeWire SM, Yamashita DS, Rominger DH, Liu G, Cowan CL, Graczyk TM, Chen XT, Pitis PM, Gotchev D, Yuan C, Koblish M, et al. A G protein-biased ligand at the mu-opioid receptor is potently analgesic with reduced gastrointestinal and respiratory dysfunction compared with morphine. J Pharmacol Exp Ther. 2013;344:708–17. doi: 10.1124/jpet.112.201616. [DOI] [PubMed] [Google Scholar]
- 28.Groer CE, Tidgewell K, Moyer RA, Harding WW, Rothman RB, Prisinzano TE, Bohn LM. An opioid agonist that does not induce mu-opioid receptor--arrestin interactions or receptor internalization. Mol Pharmacol. 2007;71:549–57. doi: 10.1124/mol.106.028258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lamb K, Tidgewell K, Simpson DS, Bohn LM, Prisinzano TE. Antinociceptive effects of herkinorin, a MOP receptor agonist derived from salvinorin A in the formalin test in rats: new concepts in mu opioid receptor pharmacology: from a symposium on new concepts in mu-opioid pharmacology. Drug Alcohol Depend. 2012;121:181–8. doi: 10.1016/j.drugalcdep.2011.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raehal KM, Schmid CL, Groer CE, Bohn LM. Functional selectivity at the mu-opioid receptor: implications for understanding opioid analgesia and tolerance. Pharmacol Rev. 2011;63:1001–19. doi: 10.1124/pr.111.004598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Viscusi ER, Webster L, Kuss M, Daniels S, Bolognese JA, Zuckerman S, Soergel DG, Subach RA, Cook E, Skobieranda F. A randomized, phase 2 study investigating TRV130, a biased ligand of the mu-opioid receptor, for the intravenous treatment of acute pain. Pain. 2016;157:264–72. doi: 10.1097/j.pain.0000000000000363. [DOI] [PubMed] [Google Scholar]
- 32.Raehal KM, Walker JK, Bohn LM. Morphine side effects in beta-arrestin 2 knockout mice. J Pharmacol Exp Ther. 2005;314:1195–201. doi: 10.1124/jpet.105.087254. [DOI] [PubMed] [Google Scholar]
- 33.Thompson GL, Kelly E, Christopoulos A, Canals M. Novel GPCR paradigms at the mu-opioid receptor. Br J Pharmacol. 2015;172:287–96. doi: 10.1111/bph.12600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guo W, Zou S, Guan Y, Ikeda T, Tal M, Dubner R, Ren K. Tyrosine phosphorylation of the NR2B subunit of the NMDA receptor in the spinal cord during the development and maintenance of inflammatory hyperalgesia. J Neurosci. 2002;22:6208–17. doi: 10.1523/JNEUROSCI.22-14-06208.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lai CY, Lin TB, Hsieh MC, Chen GD, Peng HY. SIRPalpha1-SHP2 Interaction Regulates Complete Freund Adjuvant-Induced Inflammatory Pain via Src-Dependent GluN2B Phosphorylation in Rats. Anesth Analg. 2016;122:871–81. doi: 10.1213/ANE.0000000000001116. [DOI] [PubMed] [Google Scholar]
- 36.Liu XJ, Gingrich JR, Vargas-Caballero M, Dong YN, Sengar A, Beggs S, Wang SH, Ding HK, Frankland PW, Salter MW. Treatment of inflammatory and neuropathic pain by uncoupling Src from the NMDA receptor complex. Nat Med. 2008;14:1325–32. doi: 10.1038/nm.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.De Felice M, Lambert D, Holen I, Escott KJ, Andrew D. Effects of Src-kinase inhibition in cancer-induced bone pain. Mol Pain. 2016;12 doi: 10.1177/1744806916643725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Trujillo KA, Akil H. Inhibition of morphine tolerance and dependence by the NMDA receptor antagonist MK-801. Science. 1991;251:85–7. doi: 10.1126/science.1824728. [DOI] [PubMed] [Google Scholar]
- 39.Hu F, Cui Y, Guo R, Chen J, Shen N, Hua X, Mo L, Feng J. Spinal leptin contributes to the development of morphine antinociceptive tolerance by activating the STAT3-NMDA receptor pathway in rats. Mol Med Rep. 2014;10:923–30. doi: 10.3892/mmr.2014.2250. [DOI] [PubMed] [Google Scholar]
- 40.Marcus DJ, Zee M, Hughes A, Yuill MB, Hohmann AG, Mackie K, Guindon J, Morgan DJ. Tolerance to the antinociceptive effects of chronic morphine requires c-Jun N-terminal kinase. Mol Pain. 2015;11:34. doi: 10.1186/s12990-015-0031-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rivat C, Sebaihi S, Van Steenwinckel J, Fouquet S, Kitabgi P, Pohl M, Melik Parsadaniantz S, Reaux-Le Goazigo A. Src family kinases involved in CXCL12-induced loss of acute morphine analgesia. Brain Behav Immun. 2014;38:38–52. doi: 10.1016/j.bbi.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 42.Wang Y, Barker K, Shi S, Diaz M, Mo B, Gutstein HB. Blockade of PDGFR-beta activation eliminates morphine analgesic tolerance. Nat Med. 2012;18:385–7. doi: 10.1038/nm.2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kuhar JR, Bedini A, Melief EJ, Chiu YC, Striegel HN, Chavkin C. Mu opioid receptor stimulation activates c-Jun N-terminal kinase 2 by distinct arrestin-dependent and independent mechanisms. Cell Signal. 2015;27:1799–806. doi: 10.1016/j.cellsig.2015.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]