Abstract
Purpose
Docetaxel is standard of care for androgen-independent prostate cancer (AIPC). Doxercalciferol (1α-hydroxyvitamin D2) had modest activity in phase I/II trials. Preclinical data support combining vitamin D analogues with docetaxel to treat AIPC.
Experimental Design
Chemotherapy-naive men with metastatic AIPC were randomized 1:1to receive, on a 4-week cycle, docetaxel (35 mg/m2 i.v., days1, 8, and15) with or without doxercalciferol (10 μg orally, days 1–28). The primary end point was prostate-specific antigen (PSA) response. Secondary end points were progression-free survival, overall survival, objective response, and toxicity. Survival was analyzed as intent to treat.
Results
Seventy patients were randomized. Median follow-up was 17.6 months (range, 3.3–45.2). PSA response rate was 46.7% [95% confidence interval (95% CI), 30–64] in the doxercalciferol arm and 39.4% (95% CI, 25–56) with placebo (P = 0.560). Median progression-free survival in the doxercalciferol arm was 6.17 months (95% CI, 4.20–10.7) versus 6.20 months (95% CI, 4.83–9.07) with placebo (P = 0.764). Median overall survival in the doxercalciferol arm was 17.8 months (95% CI, 14.9–23.6) versus 16.4 months (95% CI, 11.9–23.8) with placebo (P = 0.383). Twenty-four patients in the doxercalciferol arm and 23 in the placebo arm were evaluable for objective response. No complete responses were observed. Partial objective response rate was 12.5% with doxercalciferol versus 8.7% with placebo (P = 0.672). Rate of grade ≥3 toxicity was 46% with doxercalciferol versus 42% with placebo (P = 0.785).
Conclusions
Daily doxercalciferol with weekly docetaxel did not enhance PSA response rate or survival. Toxicity was similar between arms. Despite the disappointing results of this study, other vitamin D analogues remain under active investigation.
Prostate adenocarcinoma will be diagnosed in ~219,000 men and result in 27,000 deaths in the United States in 2007 (1). Although androgen deprivation therapy is effective against advanced disease, eventually all patients develop androgenindependent prostate cancer (AIPC). Docetaxel (Taxotere, sanofiaventis) is standard of care for AIPC (2); however, the median improvement in survival over mitoxantrone is ~2.5 months, underscoring the need for better therapies.
Prostate cancer pathogenesis is complex and with areas still to be elucidated. Based on epidemiologic evidence, Schwartz and Hulka (3) hypothesized in 1990 that vitamin D maintains the differentiated phenotype of prostate cells and that low levels of vitamin D allow subclinical prostate cancers to progress pathologically. Vitamin D analogues, such as calcitriol (1α,25-dihydroxyvitamin D3), inhibit prostate cancer cell growth in vitro (4–9) and in vivo (9–11) through the induction of apoptosis and inhibition of angiogenesis and invasiveness. The effects of vitamin D analogues are felt, in part, to be mediated through their interaction with the vitamin D receptor (6, 12–14). Interestingly, vitamin D receptors are expressed in many human tissues, including prostate cancer cells (15). Calcitriol has been extensively explored as a treatment for prostate cancer. However, hypercalcemia and its sequelae have complicated its clinical evaluation, spawning the search for a less calcemic alternative.
Doxercalciferol (1α-dihydroxyvitamin D2, Hectorol, Genzyme), an inactive prohormone, undergoes hepatic conversion to its active metabolites, 1α,25-dihydroxyvitamin D2 and 1α,24-dihydroxyvitamin D2 (16–19). Doxercalciferol is less calcemic than calcitriol in vivo (20) and safely tolerated over extended time in humans (21). Because of this and its ability to bypass renally mediated 1α-hydroxylation, it is Food and Drug Administration approved for the treatment of secondary hyperparathyroidism in hemodialysis patients. 1α,24-Dihydroxyvitamin D2 inhibits growth and induces differentiation markers as well as calcitriol in prostate cancer cell lines (22). Phase I and II testing of single-agent doxercalciferol showed modest activity against AIPC (23, 24) as evidenced by objective responses and prolonged stable disease. Main toxicities included reversible mild hypercalcemia with resulting renal insufficiency and constipation.
Preclinical (25–29) and clinical (30–32) data support combining vitamin D analogues with chemotherapy, including docetaxel, to treat prostate cancer. We hypothesized that continuous vitamin D receptor binding to the active metabolites of doxercalciferol (afforded by daily administration of this relatively less calcemic vitamin D2 analogue) may be necessary for optimal anticancer activity. Therefore, we conducted a randomized phase II trial of weekly docetaxel with or without daily doxercalciferol in chemotherapy-naive men with AIPC. The primary end point was prostate-specific antigen (PSA) response as defined by the PSA Working Group consensus criteria (33). Secondary end points were median progression-free survival (PFS), median overall survival (OS), toxicity, and radiographic tumor response.
Materials and Methods
Patient selection
Eligibility criteria at baseline included ≥18 y of age, histologic diagnosis of prostate adenocarcinoma, radiographic evidence of metastasis, chemotherapy naive (although immunotherapy and experimental therapies were allowed if given ≥4 wk before), Eastern Cooperative Oncology Group performance status (34) of ≤2, life expectancy of ≥3 mo, and written informed consent. Inclusion criteria were adequate major organ function [WBC count ≥3,000/μL, absolute neutrophil count >1,500/μL, platelet count ≥100,000/μL, hemoglobin ≥10 g/dL, total bilirubin below the institutional upper limit of normal, creatinine ≤1.8 mg/dL, alanine and aspartate transaminases <2.5 times the upper limit of normal, serum calcium ≤10.2 mg/dL, and serum phosphorus ≤5.0 mg/dL]; discontinuation, at least 4 wk before, of PC-SPES, saw palmetto, or other herbal supplements used as treatment for prostate cancer; peripheral neuropathy grade ≤1; and prior treatment with bilateral orchiectomy or other primary hormonal therapy with subsequent treatment failure. Patients without a history of orchiectomy at baseline were required to continue luteinizing hormone–releasing hormone agonist therapy and have a serum testosterone level of <50 ng/dL. Sexually active patients agreed to use contraception while on and for 6 mo after stopping treatment.
Antiandrogen therapy was discontinued before enrollment. This entailed cessation of flutamide, nilutamide, or ketoconazole at least 4 wk before and of biclutamide at least 6 wk before registration, at which point evidence for PSA progression was required. Use of low-dose megestrol acetate for amelioration of hot flashes was allowed. Use of bisphosphonates was allowed if started at least 4 wk before study entry and accompanied by a rising PSA.
Patients with bone as their only site of metastasis were required to have a serum PSA of ≥10 ng/mL and at least one metastatic lesion detectable by bone scan. The demonstration of progressive disease (PD) via the appearance of at least one new lesion on bone scan, increase in the size or number of measurable lesions or two or more rising PSA measurements at least 2 wk apart, was required at baseline.
Exclusion criteria at baseline were as follows: second malignancy within 5 y (excluding basal or squamous cell carcinoma of the skin treated curatively); brain metastasis; nephrolithiasis within 10 y; chronic hypercalcemia (i.e., serum calcium >1.0 mg/dL the upper limit of normal); chronic gastrointestinal disease (i.e., malabsorption, surgery affecting absorption, and chronic ulcerative colitis); urinary protein >4 g/24 h; urinary calcium ≥500 mg/24 h; active angina, New York Heart Association class II–IV heart failure, or history of myocardial infarction within 6 mo; uncontrolled infection; or hypersensitivity to polysorbate 80. Use of digitalis, thiazide diuretics, calcium supplements, anticonvulsants, fluoride, and lithium was not allowed. Use of steroids was permitted unless taken for prostate cancer. Patients treated with suramin, strontium, or other therapeutic radioisotopes or radiotherapy within 4 wk were excluded.
Before implementation, this study was approved by the Institutional Review Board of the University of Wisconsin-Madison and of participating clinical sites. All patients gave written informed consent before study entry.
Pretreatment evaluation and follow-up studies
Evaluations done at baseline and on day 1 of every 28-d cycle included a physical exam with an assessment of weight, vital signs, and Eastern Cooperative Oncology Group performance status; complete blood count including a platelet count with differential; blood urea nitrogen; electrolytes; total bilirubin; aspartate aminotransferase; lactate dehydrogenase; alkaline phosphatase; and serum PSA. Additional studies completed at baseline included serum calcium and phosphorus levels; computed tomography scan of the abdomen and pelvis; whole body bone scan; and, for patients using luteinizing hormone–releasing hormone agonists, a serum testosterone level. A 24-h urine collection for assessment of calcium, phosphorus, and protein was collected at baseline and then on day 1 of cycles 2 and 3. Thereafter, a 24-h urine collection for assessment of calcium and phosphorus was collected every 8 wk. Baseline imaging scans were required within the 4 wk before initiation of treatment. Other prestudy evaluations were required within the 2 wk before the first treatment. A complete blood count with differential and platelet count was evaluated every week during treatment. Serum phosphorus and calcium levels were evaluated every week during treatment for the first 4 wk and then every 4 wk thereafter. Computed tomography scan and bone scan were done every three cycles (i.e., 12 wk). Patients were followed until death to assess survival.
Study design and randomization
We used a randomized, placebo-controlled, double-blinded phase II study design. The University of Wisconsin Hospital and Clinics Pharmaceutical Research Center generated the randomization list. Patients were randomized with an allocation ratio of 1:1 without stratification by any variable or study site.
Drug administration
Docetaxel was supplied commercially. Patients received, on a 28-d cycle, 35 mg/m2 docetaxel i.v. on days 1, 8, and 15 over 1 h. Weekly docetaxel was used for this trial because at the time of its implementation every-3-week docetaxel was not Food and Drug Administration approved for AIPC and weekly docetaxel was presumed to be as effective as the every-3-week formulation with a more favorable toxicity profile. Patients were instructed to take 4 mg of dexamethasone the evening before, morning of, and evening of the day they received docetaxel. Granulocyte colony-stimulating growth factors were not routinely used but were given at the treating physician’s discretion in cycles after febrile neutropenia.
Doxercalciferol was supplied by Genzyme as 2.5 μg soft gel capsules. Patients took 10 μg (i.e., four capsules of 2.5 μg) of doxercalciferol or placebo (equal in weight to, and containing only the inactive ingredients found in, the doxercalciferol capsules), orally on days 1 to 28. Patients were asked to take the study drugs before breakfast and at the same time. Patients were given a medication diary, which was reviewed at each clinic visit.
Toxicity and dose modifications
Toxicities were graded according to the National Cancer Institute Common Toxicity Criteria version 2.0. All treated patients were considered evaluable for toxicity.
Docetaxel
A first dose reduction (i.e., dose level -1) for docetaxel constituted a 25% lowering compared with the initial dose (i.e., dose level 0). A second dose reduction (i.e., dose level -2) for docetaxel was defined by a 50% lowering from the initial dose. Patients requiring a third dose reduction were removed from treatment.
Docetaxel was held for 1 wk for an absolute neutrophil count of ≤1,000/mm3 or a platelet count of ≤100,000/m3 and discontinued if counts did not recover within 14 d. The dose of docetaxel was reduced by one level for grade 4 thrombocytopenia, grade 4 neutropenia lasting ≥7 d, or grade 4 febrile neutropenia. The dose of docetaxel was not reduced for grade ≤3 neutropenia or grade 4 neutropenia lasting ≤7 d.
Treatment was discontinued for grade 3 or 4 neuropathy and grade 4 hypersensitivity reactions. Otherwise, for nonhematologic grade 3 and 4 toxicities and for grade 2 neuropathy, docetaxel was held until the toxicity resolved to grade ≤1 and restarted at the next lower dose level. The dose of docetaxel was reduced by one dose level for grade 2 stomatitis without treatment delay. Treatment was discontinued if withheld >14 d due to grade ≥3 nonhematologic toxicity.
Doxercalciferol
Dose levels for once-daily oral doxercalciferol were 10 μg (initial), 7.5 μg (dose level -1), 5.0 μg (dose level -2), and 2.5 μg (dose level -3). Doxercalciferol was held for nonhematologic grade ≥3 toxicities. It was resumed at the next lower dose level if toxicities resolved to grade ≤2 within 14 d but otherwise discontinued. Patients with grade ≥2 creatinine or grade ≥2 calcium were dose reduced by one level once the toxicity had resolved to grade ≤1. Patients with other grade 1 or 2 toxicities continued doxercalciferol without dose modification. Patients with grade ≥3 anemia received packed RBC transfusions as needed without dose modification.
Treatment plan and disease assessment
Treatment was continued until PD, withdrawn consent, or unacceptable toxicity occurred. Treatment was stopped if the treating physician deemed that continuing would be contrary to the patient’s best interests.
Only patients completing 12 wk of treatment were considered evaluable for objective response using WHO criteria (35) to interpret scans. For patients with bone-only disease, PD was defined as the appearance of any new lesion in the absence of a suspected “flare” response; additionally, the time requirement for stable disease was a minimum of 12 wk.
PSA response was defined by PSA Working Group consensus criteria (33). A response was defined as a minimal PSA decline of ≥50% from baseline for at least two successive measurements taken 4 wk apart without evidence of clinical or radiographic progression; stable disease was defined as neither a response nor PD for 12 wk; PD was not defined by PSA alone in this study; and PSA response duration was defined as time to 50% increase from PSA nadir.
Statistical methods
The primary efficacy end point was PSA response. Secondary end points were PFS, OS, objective response, and toxicity. The study was designed to detect an anticipated difference of 20% in PSA response rate between the two study arms with 80% power at the one-sided 10% significance level, assuming a true PSA response rate of 40% for placebo arm and 60% for doxercalciferol arm. The sample size required to achieve this was 66 per arm, for a total of 132 patients. An interim analysis was planned after at least 33 patients from each treatment group had completed the study.
Efficacy analyses of PFS and OS were conducted on an intention-to-treat basis (i.e., all randomized patients were considered evaluable for survival). Baseline characteristics were summarized using standard descriptive statistics. The χ2 test (or Fisher’s exact test) and the nonparametric Wilcoxon rank sum test were used to analyze differences in categorical and continuous variables, respectively. The comparisons of PSA and objective response rates between study arms were done using χ2 analysis. PFS and OS curves were calculated using Kaplan-Meier methodology, and the differences in survival curves between treatment arms were assessed with a log-rank test. All computations were done with Statistical Analysis System software (version 6.12; SAS Institute). All P values were calculated with two-sided tests of significance.
Results
Baseline patient characteristics
Seventy patients were enrolled and randomized 1:1 without stratification factors from October 2002 to July 2005. Table 1 summarizes patient characteristics at baseline, which were well balanced between arms with the exception of slightly greater body surface area in the doxercalciferol arm (P = 0.047). All patients had PD despite androgen deprivation therapy before study entry. One patient (in the placebo arm) had not been treated with a luteinizing hormone–releasing hormone agonist or antiandrogen therapy with biclutamide or flutamide but had undergone orchiectomy. Prior therapy in addition to hormone therapy in the treatment and placebo arms included surgery (excluding orchiectomy) in 38.9% versus 51.5%, radiotherapy (63.9% versus 48.5%), and immunotherapy (5.6% versus 3.0%).
Table 1.
Baseline patient characteristics
| Characteristic | Docetaxel + doxercalciferol | Docetaxel + placebo | P | ||
|---|---|---|---|---|---|
|
|
|
||||
| n | n | ||||
| Age, median and range (y) | 37 | 72.0 (56.0–85.0) | 33 | 70.0 (52.0–82.0) | 0.181 |
| ECOG performance status | 36* | 33 | 0.402 | ||
| 0 | 16 (44.4%) | 10 (30.3%) | |||
| 1 | 18 (50.0%) | 22 (66.7%) | |||
| 2 | 2 (5.6%) | 1 (3.0%) | |||
| Race | 36* | 33 | 0.494 | ||
| Caucasian | 32 (88.9%) | 33 (100%) | |||
| African-American | 2 (5.6%) | 0 (0%) | |||
| Other | 2 (5.6%) | 0 (0%) | |||
| Metastatic sites | 36* | 33 | |||
| Bone | 34 (94.4%) | 31 (93.9%) | |||
| Lymph node | 13 (36.1%) | 19 (57.6%) | |||
| Other | 3 (8.3%) | 5 (15.2%) | |||
| Gleason score, median and range | 36† | 7 (4–10) | 32‡ | 8 (3–10) | 0.288 |
| PSA, median and range (ng/mL) | 36* | 78.9 (10.0–1400) | 33 | 82.4 (2.7–1622) | 0.773 |
| Hemoglobin, median and range (g/dL) | 37 | 13.1 (9.7–15.5) | 33 | 12.4 (10.1–15.0) | 0.516 |
| LDH, median and range (unit/L) | 35*,§ | 216 (139–2486) | 32§ | 195 (130–1003) | 0.534 |
| Alkaline phosphatase, median and range (IU/L) | 36* | 124 (23–1610) | 33 | 136 (43–2760) | 0.760 |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; LDH, lactate dehydrogenase.
Limited information available on one patient randomized but not treated.
Prostate cancer diagnosis based on bone marrow biopsy.
Gleason score unknown.
Lactate dehydrogenase was not recorded for one patient in each arm.
Drug administration and dose modifications
Docetaxel and doxercalciferol arm
Two patients randomized to the doxercalciferol arm did not receive treatment. The remaining 35 men received a total of 219 cycles of docetaxel plus doxercalciferol (median, 6 cycles; range, 1–15 cycles). One patient required dose reduction of doxercalciferol on two occasions: the first was as a result of a grade 4 hypercalcemia and the second was due to a grade 2 hypercalcemia. Only one other patient had a dose reduction of doxercalciferol in this arm (grade 2 hypercalcemia). A third patient with a grade 2 hypercalcemia qualified for but did not have a dose reduction of doxercalciferol due to discontinuation of treatment for other reasons (i.e., urinary obstruction from tumor requiring placement of nephrostomy tubes). The most common reasons a dose of docetaxel was held or reduced in this arm were infection (three patients), pain (three), peripheral neuropathy (two), nail bed changes (three), and diarrhea (three).
Docetaxel and placebo arm
Thirty-three patients received a total of 209 cycles of docetaxel with placebo (median, 6 cycles; range, 1–12 cycles). Two patients had the dose of placebo reduced. One was for a grade 1 creatinine at the treating physician’s discretion. The second was in a different patient for a grade 2 creatinine. The most common reasons a dose of docetaxel was held or reduced in the placebo arm were neutropenia (eight patients), peripheral neuropathy (two), hyperlacrimation (two), and infection (three).
Toxicity
Thirty-five patients in the doxercalciferol arm and 33 patients in the placebo arm were treated and therefore evaluable for toxicity. Table 2 lists the highest-grade toxicity deemed possibly, probably, or definitely related to the study drugs observed per patient. One patient in the placebo arm and no patients in the doxercalciferol arm had a grade ≥2 creatinine. Three patients in the doxercalciferol arm and no patients in the placebo arm had a grade ≥2 calcium. There were three patients who, during the course of treatment, were found to have nephrolithiasis: two patients on the placebo arm and one patient on the treatment arm. The latter patient had a history of nephrolithiasis ≥10 years before study entry.
Table 2.
Maximal grade of toxicities per patient and type
| Docetaxel + doxercalciferol (n = 35) | Docetaxel + placebo (n = 33) | P* | |||
|---|---|---|---|---|---|
|
|
|
||||
| Grade† | |||||
|
| |||||
| 3 | 4 | 3 | 4 | ||
| Abdominal cramping | 1 | 0.485 | |||
| Aspartate transaminase | 1 | 0.485 | |||
| Cataract | 1 | 1 | 0.999 | ||
| Constipation | 1 | 0.485 | |||
| Diarrhea | 6 | 0.025 | |||
| Dyspnea | 2 | 1 | 0.240 | ||
| Edema | 2 | 0.493 | |||
| Fatigue | 1 | 0.485 | |||
| Flushing | 1 | 0.485 | |||
| Hematuria | 1 | 0.485 | |||
| Hypercalcemia | 1 | 0.999 | |||
| Hyperglycemia | 3 | 2 | 0.999 | ||
| Hypersensitivity reaction | 1 | 0.485 | |||
| Hypophosphatemia | 1 | 0.485 | |||
| Infection without neutropenia | 1 | 0.999 | |||
| Injection site reaction | 1 | 0.999 | |||
| Lacrimation | 1 | 1 | 0.999 | ||
| Lactase dehydrogenase | 1 | 0.485 | |||
| Leukopenia | 3 | 0.109 | |||
| Nail change | 1 | 0.999 | |||
| Nausea | 1 | 0.999 | |||
| Neutropenia | 1 | 8 | 0.012 | ||
| Febrile neutropenia | 1 | 0.999 | |||
| Pleural effusion | 1 | 1 | 0.999 | ||
| Rash | 1 | 0.485 | |||
| Thrombocytopenia | 1 | 0.999 | |||
| Thrombosis/embolism | 1 | 1 | 0.999 | ||
| Total | 23 | 3 | 24 | 3 | |
NOTE: Rate of grade ≥3 toxicity was 46% in the doxercalciferol arm and 42% with placebo (P = 0.785). Toxicities listed were possibly, probably, or definitely related to the study drugs.
Fisher’s exact test.
National Cancer Institute Common Toxicity Criteria version 2.0.
Serum calcium and 24-h urinary calcium
The median serum calcium at baseline was 9.2 ± 0.45 mg/dL (range, 8.1–10.2 mg/dL) in the doxercalciferol arm and 9.2 ± 0.40 mg/dL (range, 8.2–10.2 mg/dL) in the placebo arm. On day 1 of cycle 2, median serum calcium at baseline was 9.4 ± 0.61 mg/dL (range, 8.4–11.5 mg/dL) in the doxercalciferol arm and 9.1 ±0.44 mg/dL (range, 8.1–9.8 mg/dL) in the placebo arm.
Five patients in the treatment arm developed 24-h urinary calcium levels >500 mg; three of these were noted on day 1 of cycle 2. Conversely, no patient in the placebo arm had a value ≥500 mg/24 h. The median 24-h urinary calcium at baseline was 116.1 ± 116.3 mg (range, 2.0–425.0 mg) in the doxercalciferol arm and 109.0 ±108.0 mg (range, 2.0–349.0 mg) in the placebo arm. The median 24-h urinary calcium on day 1 of cycle 2 in the treatment arm was 171.0 ±203.0 mg (range, 5.0–812.0 mg) and 57.0 ± 101.9 mg (range, 1.0–378.0 mg) in the placebo arm.
Patient outcome
Thirty and 33 patients were evaluable for response in the doxercalciferol and placebo arms, respectively. Reasons patients randomized to the doxercalciferol arm were not evaluable for response included (a) elevated serum testosterone at baseline discovered before treatment received (one patient), (b) use of digoxin discovered before treatment began (one), (c) received treatment and discovered to have an elevated baseline serum testosterone level (one), and (d) withdrawn before first post-baseline imaging studies (four patients: three withdrew consent and one discontinued treatment due to toxicity). No patient died on study.
For the 35 patients treated on the doxercalciferol arm, the following methods were used to determine the date of PD: serial PSA measurements (18 patients), serial imaging (12 patients), date of last contact before starting other treatment off study (4 patients), and date of last contact before being lost to follow-up (1 patient). Likewise, for the 33 patients treated on the placebo arm, PD was determined in the following ways: serial PSA measurements (8 patients), serial imaging (20 patients), date of last contact before starting other treatment off study (2 patients), date of last contact before being lost to follow-up (1 patient), and clinical discretion (1 patient). One patient in the placebo arm has not yet experienced PD as of April 2007 and is alive 37.9 months from the date of enrollment.
Thirty patients in the doxercalciferol arm and 33 patients in the placebo arm were evaluable for PSA response. There was no difference between treatment arms in the rate of PSA response (i.e., 46.7% in the doxercalciferol arm and 39.4% in the placebo arms; P = 0.560). Additionally, there was no difference in the median time to PSA response between those treated with doxercalciferol treatment (3.7 months; range, 2.8–6.5 months) and those who were not (3.7 months; range, 2.8–4.6 months). Twenty-four patients in the treatment arm and 23 patients in the placebo arm were considered evaluable for objective tumor response per WHO criteria. No complete responses were observed. Partial objective response rate was 12.5% (three patients) with doxercalciferol versus 8.7% (two patients) with placebo (P = 0.672). Stable disease rate was 70.8% (17 patients) with doxercalciferol versus 60.9% (14 patients) with placebo (P = 0.471). PD as a best response was seen in 16.7% (four patients) on the doxercalciferol arm versus 30.4% (seven patients) on the placebo arm (P = 0.265).
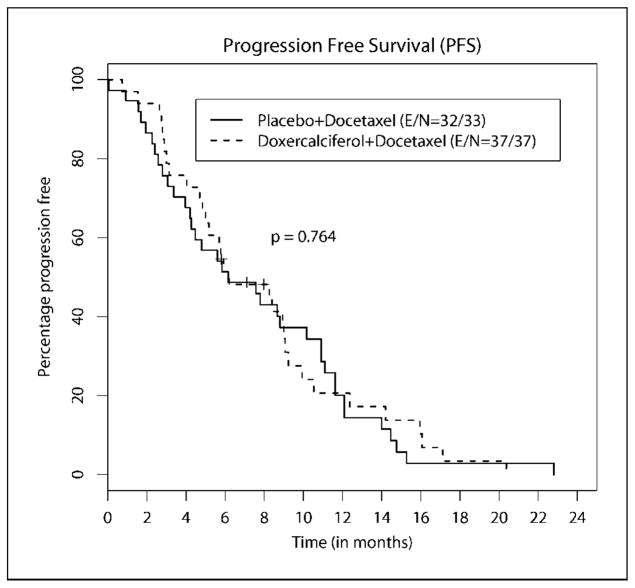
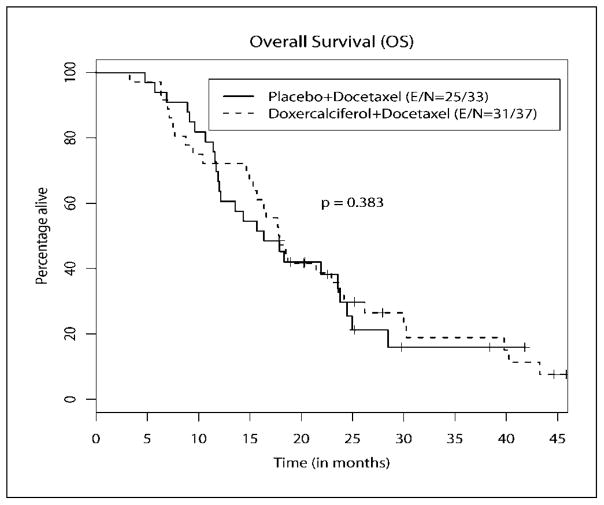
Thirty-seven patients in the doxercalciferol arm and 33 in the placebo arm (i.e., all randomized patients) were evaluable for survival (i.e., intent-to-treat analysis). Figures 1 and 2 show the distributions of PFS and OS. With a median follow-up of 17.6 months (range, 3.3–45.2 months), 31 and 25 patients have died in the doxercalciferol and placebo arms, respectively. The median PFS of patients was 6.17 months (95% confidence interval, 4.20–10.17 months) in the doxercalciferol arm and 6.20 months (95% confidence interval, 4.83–9.07 months) in the placebo arm (P = 0.764). The median OS of patients was 17.8 months (95% confidence interval, 14.9–23.6 months) in the doxercalciferol arm and 16.4 months (95% confidence interval, 11.9–23.8 months) in the placebo arm (P = 0.383).
Fig. 1.
Progression-free survival.
Fig. 2.
Overall survival.
Early study closure and futility calculation
This study was closed early after meeting accrual numbers for the planned interim analysis. This decision was due initially to a change in the sponsor’s interest in pursuing an oncologic indication for doxercalciferol. Consequently, a futility calculation was conducted at the planned interim analysis. The conditional power given the observed PSA response rates at the interim analysis to detect a 20% difference in the PSA response rate between treatment arms at a one-sided 10% significance level, under the assumption that the targeted accrual goal of 132 patients will be met for the anticipated difference of 0.60 versus 0.40, is 0.35. The conditional power under the best scenario (0.65 versus 0.35) is only 0.66. Therefore, based on the low conditional power levels achieved at the interim analysis, there was justification to stop the trial early due to futility.
Discussion
In this randomized, double-blinded study, we combined doxercalciferol, a vitamin D2 analogue, with docetaxel for the treatment of metastatic AIPC. We hypothesized that sustained vitamin D receptor binding to the metabolites of doxercalciferol afforded by daily administration of this relatively less calcemic analogue may be necessary for optimal anticancer activity. This study was closed prematurely at planned interim analysis. Doxercalciferol did not augment PSA response attributed to docetaxel alone and there was no difference between arms in the median time to achieve PSA response. Similarly, PFS and OS, secondary end points, were similar between arms. Doxercalciferol was well tolerated in that a difference in the cumulative rate of grade ≥3 toxicity was not seen and hypercalcemia was not prevalent.
Since our trial ended, the results of the AIPC Study of Calcitriol Enhancing Taxotere (ASCENT) have been published (31). ASCENT randomized patients with AIPC to weekly docetaxel with placebo or a high-dose formulation of calcitriol, DN-101 (36). Contrary to our study, calcitriol was given intermittently and as a pulse dose. Although the primary end point of PSA response was similar between arms, median OS, a secondary end point, was estimated to be superior with DN-101 (24.5 versus 16.3 months). The incidence of grade ≥3 non-hematologic toxicity was more favorable in the DN-101 arm leading to conjecture that vitamin D analogues may be modulating the toxicity of chemotherapy, thus allowing for improved dose intensity. In contrast, for reasons that are unclear, grade ≥3 neutropenia was more prevalent in the placebo arm in our study.
Strengths of our study include its randomization of patients and use of a double-blinded placebo, treatment of chemotherapy-naive patients who provide a less confounding evaluation of clinical efficacy, multicenter accrual spanning academic and community settings, and its novelty as the first phase II assessment of doxercalciferol with chemotherapy to treat AIPC. We now recognize, based on current data, that a weakness of this study was the use of PSA as a criterion for response, which may have confounded the results. For instance, since the initiation of this study, TAX327 showed that PSA response alone did not predict survival (2, 37). Additionally, a survival advantage in the treatment arm was suggested in ASCENT, although there was no difference in PSA response between arms. Lastly, vitamin D analogues induce the release of markers of differentiation, such as PSA, in vitro (22, 38) while simultaneously inducing growth inhibition (39). These issues reflect on our study where treatment was discontinued for 31 patients (i.e., 47.0%) due to PD determined using serial PSA values.
Why were differences in response rate and survival not seen in this study? We propose several variables that, acting alone or together, may account for this. First, the study was powered to assess differences in PSA response and not survival. Therefore, the fact that no difference was seen can be interpreted to mean that doxercalciferol in combination with docetaxel was not active or that the study was simply underpowered to show a difference. We observed a physiologic effect from doxercalciferol as evidenced by increased urinary calcium. However, we do not know whether a pharmacodynamic change occurred at the level of the tumor with this regimen of doxercalciferol. Second, doxercalciferol may be more efficacious at a dose higher or more frequent than 10 μg daily in combination with docetaxel. We were conservative with the doxercalciferol regimen due to concerns over clinically insignificant mild calcium elevations in prior studies with doxercalciferol (23). Third, the study was closed prematurely, which could at first glance be interpreted as a possible reason why a difference in the end points was not seen between arms. However, our futility calculation showed that continuing the study to full accrual would not have changed the outcome. Fourth, baseline vitamin D levels were not assessed in this study. To our knowledge, there are no prospective clinical studies evaluating the effect of pretreatment vitamin D levels on outcome in AIPC. Given the controversy surrounding the role of vitamin D in the pathogenesis of prostate cancer, this analysis in future studies may prove informative.
In summary, PSA response was not increased with the addition of doxercalciferol to docetaxel in the setting of advanced AIPC. Interest, however, persists in the clinical evaluation of vitamin D analogues for the treatment of prostate cancer. For example, ASCENT-II is an ongoing international phase III study comparing docetaxel with the DN-101 arm of ASCENT and is powered to detect differences in OS.
Acknowledgments
Grant support: Genzyme and sanofiaventis and NIH grant T32 CA009614 Physician Scientist Training in Cancer Medicine (Dr. Attia).
We thank our patients and their families for their participation in this study and the investigators and their research staff at the following participating research sites, including Dr. Charles Diggs (Dean Medical Center) and Dr. Ajit Divgi (Oncology Alliance), for their dedication.
Footnotes
Note: The results of this study were presented as abstract no.5118 at the 2007 American Society of Clinical Oncology Meeting in Chicago, Illinois.
References
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–12. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 3.Schwartz GG, Hulka BS. Is vitamin D deficiency a risk factor for prostate cancer? (Hypothesis) Anticancer Res. 1990;10:1307–11. [PubMed] [Google Scholar]
- 4.Schwartz GG, Oeler TA, Uskokovic MR, Bahnson RR. Human prostate cancer cells: inhibition of proliferation by vitamin D analogs. Anticancer Res. 1994;14:1077–81. [PubMed] [Google Scholar]
- 5.Skowronski RJ, Peehl DM, Feldman D. Actions of vitamin D3, analogs on human prostate cancer cell lines: comparison with1,25-dihydroxyvitamin D3. Endocrinology. 1995;136:20–6. doi: 10.1210/endo.136.1.7530193. [DOI] [PubMed] [Google Scholar]
- 6.Hedlund TE, Moffatt KA, Uskokovic MR, Miller GJ. Three synthetic vitamin D analogues induce prostate-specific acid phosphatase and prostate-specific antigen while inhibiting the growth of human prostate cancer cells in a vitamin D receptor-dependent fashion. Clin Cancer Res. 1997;3:1331–8. [PubMed] [Google Scholar]
- 7.Guzey M, Kitada S, Reed JC. Apoptosis induction by 1α,25-dihydroxyvitamin D3 in prostate cancer. Mol Cancer Ther. 2002;1:667–77. [PubMed] [Google Scholar]
- 8.Schwartz GG, Wang MH, Zang M, Singh RK, Siegal GP. 1α,25-Dihydroxyvitamin D (calcitriol) inhibits the invasiveness of human prostate cancer cells. Cancer Epidemiol Biomarkers Prev. 1997;6:727–32. [PubMed] [Google Scholar]
- 9.Getzenberg RH, Light BW, Lapco PE, et al. Vitamin D inhibition of prostate adenocarcinoma growth and metastasis in the Dunning rat prostate model system. Urology. 1997;50:999–1006. doi: 10.1016/S0090-4295(97)00408-1. [DOI] [PubMed] [Google Scholar]
- 10.Lokeshwar BL, Schwartz GG, Selzer MG, et al. Inhibition of prostate cancer metastasis in vivo: a comparison of 1,23-dihydroxyvitamin D (calcitriol) and EB1089. Cancer Epidemiol Biomarkers Prev. 1999;8:241–8. [PubMed] [Google Scholar]
- 11.Vegesna V, O’Kelly J, Said J, Uskokovic M, Binderup L, Koeffle HP. Ability of potent vitamin D3 analogs to inhibit growth of prostate cancer cells in vivo. Anti-cancer Res. 2003;23:283–9. [PubMed] [Google Scholar]
- 12.Hedlund TE, Moffatt KA, Miller GJ. Stable expression of the nuclear vitamin D receptor in the human prostatic carcinoma cell line JCA-1: evidence that the antiproliferative effects of 1α,25-dihydroxyvitamin D3 are mediated exclusively through the genomic signaling pathway. Endocrinology. 1996;137:1554–61. doi: 10.1210/endo.137.5.8612485. [DOI] [PubMed] [Google Scholar]
- 13.Hedlund TE, Moffatt KA, Miller GJ. Vitamin D receptor expression is required for growth modulation by 1α,25-dihydroxyvitamin D3 in the human prostatic carcinoma cell line ALVA-31. J Steroid Biochem Mol Biol. 1996;58:277–88. doi: 10.1016/0960-0760(96)00030-1. [DOI] [PubMed] [Google Scholar]
- 14.Miller GJ, Stapleton GE, Ferrara JA, et al. The human prostatic carcinoma cell line LNCa P expresses biologically active, specific receptors for 1α,25-dihydroxyvi-tamin D3. Cancer Res. 1992;52:515–20. [PubMed] [Google Scholar]
- 15.Miller GJ, Stapleton GE, Hedlund TE, Moffat KA. Vitamin D receptor expression, 24-hydroxylase activity, and inhibition of growth by 1α,25-dihydroxyvita-min D3 in seven human prostatic carcinoma cell lines. Clin Cancer Res. 1995;1:997–1003. [PubMed] [Google Scholar]
- 16.Knutson JC, Hollis BW, LeVan LW, Valliere C, Gould KG, Bishop CW. Metabolism of 1α-hydroxyvi-tamin D2 to activated dihydroxyvitamin D2 metabolites decreases endogenous 1α,25-dihydroxyvitamin D3 in rats and monkeys. Endocrinology. 1995;136:4749–53. doi: 10.1210/endo.136.11.7588202. [DOI] [PubMed] [Google Scholar]
- 17.Strugnell S, Byford V, Makin HL, et al. 1α,24(S)-dihydroxyvitamin D2: a biologically active product of 1α-hydroxyvitamin D2 made in the human hepatoma, Hep3B. Biochem J. 1995;310:233–41. doi: 10.1042/bj3100233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Upton RA, Knutson JC, Bishop CW, LeVan LW. Pharmacokinetics of doxercalciferol, a new vitamin D analogue that lowers parathyroid hormone. Nephrol Dial Transplant. 2003;18:750–8. doi: 10.1093/ndt/gfg030. [DOI] [PubMed] [Google Scholar]
- 19.Bailie GR, Johnson CA. Comparative review of the pharmacokinetics of vitamin D analogues. Semin Dial. 2002;15:352–7. doi: 10.1046/j.1525-139x.2002.00086.x. [DOI] [PubMed] [Google Scholar]
- 20.Sjoden G, Smith C, Lindgren U, DeLuca HF. 1α-Hydroxyvitamin D2 is less toxic than 1α-hydroxyvita-min D3 in the rat. Proc Soc Exp Biol Med. 1985;178:432–6. doi: 10.3181/00379727-178-42028. [DOI] [PubMed] [Google Scholar]
- 21.Gallagher JC, Bishop CW, Knutson JC, Mazess RB, DeLuca HF. Effects of increasing doses of 1α-hydrox-yvitamin D2 on calcium homeostasis in postmenopausal osteopenic women. J Bone Miner Res. 1994;9:607–14. doi: 10.1002/jbmr.5650090504. [DOI] [PubMed] [Google Scholar]
- 22.Bauer JA, Thompson TA, Church DR, Ariazi EA, Wilding G. Growth inhibition and differentiation in human prostate carcinoma cells induced by the vitamin D analog 1α,24-dihydroxyvitamin D2. Prostate. 2003;55:159–67. doi: 10.1002/pros.10219. [DOI] [PubMed] [Google Scholar]
- 23.Liu G, Oettel K, Ripple G, et al. Phase I trial of 1α-hydroxyvitamin D(2) in patients with hormone refractory prostate cancer. Clin Cancer Res. 2002;8:2820–7. [PubMed] [Google Scholar]
- 24.Liu G, Wilding G, Staab MJ, et al. Phase II study of 1α-hydroxyvitamin D(2) in the treatment of advanced androgen-independent prostate cancer. Clin Cancer Res. 2003;9:4077–83. [PubMed] [Google Scholar]
- 25.Hershberger PA, Yu WD, Modzelewski RA, et al. Calcitriol (1,25-dihydroxycholecalciferol) enhances paclitaxel antitumor activity in vitro and in vivo and accelerates paclitaxel-induced apoptosis. Clin Cancer Res. 2001;7:1043–51. [PubMed] [Google Scholar]
- 26.Moffatt KA, Johannes WU, Miller GJ. 1α,25-Dihy-droxyvitamin D3 and platinum drugs act synergistically to inhibit the growth of prostate cancer cell lines. Clin Cancer Res. 1999;5:695–703. [PubMed] [Google Scholar]
- 27.Hershberger PA, McGuire TF, Yu WD, et al. Cisplatin potentiates 1,25-dihydroxyvitamin D3-induced apoptosis in association withincreased mitogen-activated protein kinase kinase kinase 1 (MEKK-1) expression. Mol Cancer Ther. 2002;1:821–9. [PubMed] [Google Scholar]
- 28.Ahmed S, Johnson CS, Rueger RM, Trump DL. Calcitriol (1,25-dihydroxycholecalciferol) potentiates activity of mitoxantrone/dexamethasone in an androgen independent prostate cancer model. J Urol. 2002;168:756–61. [PubMed] [Google Scholar]
- 29.Ting HJ, Hsu J, Bao BY, Lee YF. Docetaxel-induced growth inhibition and apoptosis in androgen independent prostate cancer cells are enhanced by 1α,25-dihydroxyvitamin D3. Cancer Lett. 2007;247:122–9. doi: 10.1016/j.canlet.2006.03.025. [DOI] [PubMed] [Google Scholar]
- 30.Beer TM, Eilers KM, Garzotto M, Egorin MJ, Lowe BA, Henner WD. Weekly high-dose calcitriol and docetaxel in metastatic androgen-independent prostate cancer. J Clin Oncol. 2003;21:123–8. doi: 10.1200/jco.2003.05.117. [DOI] [PubMed] [Google Scholar]
- 31.Beer TM, Ryan CW, Venner PM, et al. Double-blinded randomized study of high-dose calcitriol plus docetaxel compared with placebo plus docetaxel in androgen-independent prostate cancer: a report from the ASCENT Investigators. J Clin Oncol. 2007;25:669–74. doi: 10.1200/JCO.2006.06.8197. [DOI] [PubMed] [Google Scholar]
- 32.Tiffany NM, Ryan CW, Garzotto M, Wersinger EM, Beer TM. High dose pulse calcitriol, docetaxel and estramustine for androgen independent prostate cancer: a phase I/II study. J Urol. 2005;174:888–92. doi: 10.1097/01.ju.0000169261.42298.e6. [DOI] [PubMed] [Google Scholar]
- 33.Bubley GJ, Carducci M, Dahut W, et al. Eligibility and response guidelines for phase II clinical trials in androgen-independent prostate cancer: recommendations from the Prostate-Specific Antigen Working Group. J Clin Oncol. 1999;17:3461–7. doi: 10.1200/JCO.1999.17.11.3461. [DOI] [PubMed] [Google Scholar]
- 34.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–55. [PubMed] [Google Scholar]
- 35.Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer. 1981;47:207–14. doi: 10.1002/1097-0142(19810101)47:1<207::aid-cncr2820470134>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 36.Henner WD, Beer TM. A new formulation of calcitriol (DN-101) for high-dose pulse administration in prostate cancer therapy. Rev Urol. 2003;5(Suppl 3):S38–44. [PMC free article] [PubMed] [Google Scholar]
- 37.Roessner M, de Wit R, Tannock IF. Prostate-specific antigen (PSA) response as a surrogate endpoint for overall survival (OS): analysis of the TAX 327 study comparing docetaxel plus prednisone with mitoxantrone plus prednisone in advanced prostate cancer on behalf of the TAX 327 investigators [abstract 4554] J Clin Oncol. 2004;23:395s. [Google Scholar]
- 38.Esquenet M, Swinnen JV, Heyns W, Verhoeven G. Control of LNCaP proliferation and differentiation: actions and interactions of androgens, 1α,25-dihy-droxycholecalciferol, all-trans retinoic acid, 9-cis retinoic acid, and phenylacetate. Prostate. 1996;28:182–94. doi: 10.1002/(SICI)1097-0045(199603)28:3<182::AID-PROS5>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 39.Feldman D, Skowronski RJ, Peehl DM. Vitamin D and prostate cancer. Adv Exp Med Biol. 1995;375:53–63. doi: 10.1007/978-1-4899-0949-7_5. [DOI] [PubMed] [Google Scholar]