Abstract
2.1 Introduction
WHIM syndrome is a rare combined primary immunodeficiency disorder caused by autosomal dominant gain-of-function mutations in the chemokine receptor CXCR4. It is the only Mendelian condition known to be caused by mutation of a chemokine or chemokine receptor. As such, it provides a scientific opportunity to understand chemokine-dependent immunoregulation in humans and a medical opportunity to develop mechanism-based treatment and cure strategies.
2.2 Areas covered
This review covers the clinical features, genetics, immunopathogenesis and clinical management of WHIM syndrome. Clinical trials of targeted therapeutic agents and potential cure strategies are also included.
2.3 Expert opinion
WHIM syndrome may be particularly amenable to mechanism-based therapeutics for three reasons: 1) CXCR4 has been validated as the molecular target in the disease by Mendelian genetics; 2) the biochemical abnormality is excessive CXCR4 signaling; and 3) antagonists selective for CXCR4 have been developed. Plerixafor is FDA-approved for hematopoietic stem cell (HSC) mobilization and has shown preliminary safety and efficacy in phase I clinical trials in WHIM syndrome. Gene editing may represent a viable cure strategy, since chromothriptic deletion of the disease allele in HSCs resulted in clinical cure of a patient and because CXCR4 haploinsufficiency enhances engraftment of transplanted HSCs in mice.
Keywords: CXCR4, G-CSF, myelokathexis, plerixafor/AMD3100, WHIM syndrome, X4P-001/AMD11070
4.1 Introduction
WHIM syndrome is a rare primary immunodeficiency disorder characterized by four main clinical manifestations: warts, hypogammaglobulinemia, recurrent infections, and myelokathexis, that together make up the acronym WHIM. Myelokathexis is a Greek neologism meaning ‘bone marrow retention’ that was coined in 1964 by Zuelzer to convey a mechanistic explanation for severe congenital neutropenia despite full maturation of mobilizable myeloid cells in the bone marrow of a 9-year-old girl [1]. We now recognize that this patient, designated WHIM-09 in our cohort of WHIM patients at the National Institutes of Health (NIH) [2], was the first WHIM patient described in the biomedical literature. Myelokathexis in WHIM syndrome provides strong evidence in humans that one of the physiological functions of CXCR4 is to control neutrophil egress from bone marrow to the blood.
A series of landmark findings in the study of immunodeficiency, HIV and leukocyte trafficking has led to our current understanding of WHIM syndrome and approaches to its treatment (Figure 1). The acronym WHIM was coined in 1990 by Wetzler and colleagues, who described a pedigree with three affected individuals, each of whom had all four acronymic features, which had also been reported in the 10 previously identified myelokathexis patients at the time [3]. It is now appreciated that the clinical presentation of WHIM syndrome can be quite variable and may overlap with other immunodeficiency disorders. The most common clinical presentation is recurrent bacterial oto-sino-pulmonary and skin infections associated with severe, chronic, non-cyclic neutropenia that often begin during infancy or early childhood. Diagnosis can be delayed for years or decades for four main reasons: 1) the disease is extremely rare, and therefore not considered in the differential diagnosis of patients that present with it; 2) infections are usually not life-threatening and may vary greatly in frequency; thus, the disease may take a “benign” course;3) warts typically occur long after the onset of recurrent infections;and 4) severe neutropenia may be absent during infections when patients often come to medical attention. Myelokathexis is the key to diagnosis but may not be recognized unless a bone marrow biopsy has been performed. The differential diagnosis of myelokathexis includes at least one other congenital condition: G6PC3 deficiency, which is characterized by pure neutropenia and other syndromic features [4].
Figure 1.
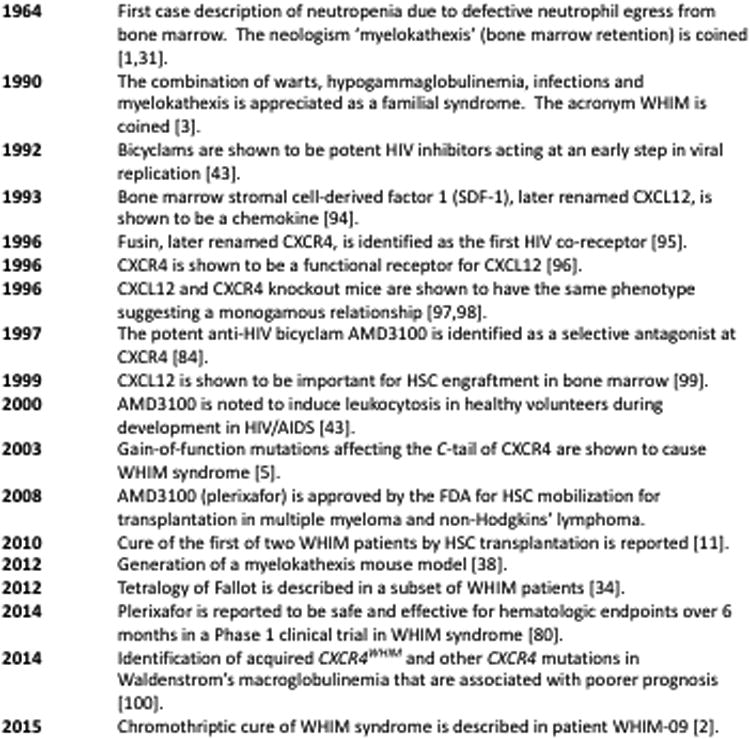
Landmark findings on the path from HIV to CXCR4 to WHIM syndrome.
Most WHIM patients present with panleukopenia and as a result have combined innate and adaptive immunodeficiency. Nevertheless, the susceptibility of WHIM patients to infections is surprisingly restricted to extracellular bacteria and human papillomavirus (HPV), the signature pathogen in the disease.
Almost all cases of WHIM syndrome are caused by autosomal dominant gain-of-function mutations affecting the C-tail of the chemokine receptor CXCR4 (Figure 2), first discovered in 2003 [5]. The C-tail is a regulatory domain, and WHIM mutations prevent normal receptor downregulation and desensitization, causing excessive signaling. Thus, WHIM mutations exaggerate the normal function of CXCR4, leading to the retention of neutrophils and other leukocyte subsets in the bone marrow [6]. The cells appear to otherwise function normally and can be released to the blood by infection and other stresses. This may be the most likely explanation for why WHIM patients are able to survive into adulthood and, in many cases, lead relatively normal lives despite absolute neutrophil counts (ANCs) that may be chronically less than 100/μL. Nevertheless, as reviewed by Beaussant-Cohen et al. [7], chronic morbidity due to repeated infection of the oto-sino-pulmonary tract is common in adults with WHIM syndrome, and premature death due to infection or cancer may occur [3,7–9]. Thus, despite its relatively benign clinical course in many patients, effective treatment for WHIM syndrome still represents an important unmet medical need. At the present time, treatment is not standardized but mainly includes wart removal, HPV vaccination, granulocyte-colony stimulating factor (G-CSF) for neutropenia, antibiotics and antibody replacement.
Figure 2.

CXCR4 evolution and mutations. The amino acid sequence of the C-tail region of wild type CXCR4 is aligned with the corresponding sequence from variants found in patients with WHIM syndrome. The circle locates the single amino acid substitution found in mutation E343K. The underlined sequence denotes de novo sequence imposed by frame shifts.
Current research is directed towards developing therapies targeting CXCR4 specifically with antagonist drugs and gene therapy. Three patients have been cured to date: two by bone marrow transplantation and one by chromothripsis (chromosome shattering) involving fortuitous deletion of the disease allele in an HSC that gained a relative growth advantage [2,10,11]. Two CXCR4 antagonists, plerixafor (AMD3100, trade name Mozobil marketed by Sanofi) given subcutaneously and X4P-001-LD (under development by X4-Pharma) given orally, are currently undergoing clinical trials.
4.2 Epidemiology & Clinical Features
4.2.1 Epidemiology
WHIM syndrome is exceedingly rare with approximately 90 cases reported worldwide since 1964. An estimate based on the French national cohort of WHIM patients suggested an incidence of 0.23 cases per million births [7]. Given the heterogeneity of presentation, many cases may go undiagnosed and unreported. WHIM syndrome appears to be slightly more common in women than in men. Almost all cases have been reported from Europe and the United States; however, the disease has also been described in several Asians. Literature reports do not consistently report race. In familial cases, WHIM syndrome follows an autosomal dominant inheritance pattern [7,12]. Sporadic de novo cases have also been frequently reported [7,13].
4.2.2 Warts
Like many other primary immunodeficiency diseases [14], WHIM patients are at high risk for extensive, persistent and treatment-refractory verrucosis. Nevertheless, wart burden, distribution, duration, age of onset, complications and response to therapy can be quite variable [9].Warts can resolve spontaneously or in response to standard destructive therapy (e.g., cryoablation, surgery) or immunomodulatory therapy (e.g., G-CSF, imiquimod) in some patients [15,16], but may never occur in others [7,17]. Cutaneous warts typically develop in early childhood and mostly affect the hands and feet, whereas genital warts usually emerge in young adulthood after sexual debut. Morphology may range from flat warts to classic verruca to anogenital condylomata accuminata.
HPV infection of the genital tract and oropharynx, but not the skin, may progress to intraepithelial dysplasia and neoplasia. Indeed, cervical and vulvar cancers have been reported in a small subset of WHIM patients [3,7,12,13,18]. In the limited studies that have examined the prevalence of specific HPV subtypes in WHIM patients, both high- (16, 18, 31, 33, 45, 52, and 58) and low-risk (6, 11) subtypes have been detected [19]. In one case, subtypes 2, 5 and 23 were isolated from cutaneous lesions from a WHIM patient who later developed Epstein-Barr virus (EBV)-negative cutaneous T cell lymphoma [7]. HPV 6 alone was also identified in one female patient with an invasive vulvar carcinoma that ultimately proved fatal despite extensive surgery [7].
4.2.3 Hypogammaglobulinemia
Hypogammaglobulinemia is the least penetrant feature of the syndrome [17], and its precise impact on infection susceptibility has not been clearly established. When it occurs, it may be associated with IgA and/or IgM deficiency. It has been reported in two cases that IgG was normal despite selective IgM deficiency [20] or combined IgA and IgM deficiency [13]. B lymphopenia, including reduced memory B cells, is a common feature in WHIM patients [3,10,21–24]; however, a precise pathophysiologic mechanism linking this with a particular immunoglobulin subtype deficiency is lacking.
Anecdotal evidence suggests that WHIM patients may have defective antibody responses and vaccination failure in association with hypogammaglobulinemia. Both a father and daughter with WHIM syndrome who received three doses of live trivalent poliovirus vaccine failed to generate complement-fixing serum antibodies [25]. However, antibody responses to the diphtheria-tetanus vaccine in the daughter were normal. In another WHIM patient, antibody titers for H. influenzae and S. pneumoniae were non-protective initially and weakly responsive only to H. influenzae after vaccination [13]. Two unrelated WHIM patients exhibited normal protective antibody responses to tetanus toxoid vaccine initially, but protective antibody was no longer detectable after one year [9]. In a separate study, anti-tetanus and anti-H. influenzae b antibodies were undetectable six months after vaccination in one patient [7];anti-polio antibodies were absent, anti-tetanus antibodies were low, and anti-pneumococcal antibodies were normal in another patient ten years after vaccination [7]. Two patients developed virologically-confirmed influenza despite receiving the flu vaccine, and one of these also had childhood measles despite vaccination [23]. Prospective studies are needed to validate these anecdotal observations and to define mechanisms.
4.2.4 Infections
Repeated severe infections, often beginning in infancy, significantly affect the quality of life of WHIM patients and may lead to life-threatening chronic complications. The most commonly affected sites include the ear, skin, oral cavity and sino-pulmonary tract. Non-HPV infections are usually caused by common extracellular bacterial pathogens (Table 1). Given the severity of panleukopenia that is often seen in WHIM patients, the relatively limited range of infectious agents that causes clinical problems is surprising. The CD4 count may be extremely low, yet opportunistic infections occur rarely. No cases of Pneumocystis carinii or Kaposi's sarcoma, which occur commonly in AIDS patients, have been reported in WHIM syndrome. Likewise, IgG levels may be low, yet patients do not typically develop invasive infections with encapsulated bacteria. The number of neutrophils is typically below 500 cells/μL and not uncommonly below 100 cells/μL, yet invasive bacterial infections occur infrequently and fungal infections are rare. This may be because WHIM neutrophil function appears to be normal and because neutropenia may not be as severe as in other neutropenic conditions that are associated with invasive fungal infection. Moreover, WHIM neutrophils can be mobilized to increase the absolute neutrophil count during infection, which may provide a protective valve.
Table 1.
Non-HPV pathogens identified in patients with WHIM syndrome.
| Organism | Patient Designation | Site | Reference | Evidence | |
|---|---|---|---|---|---|
| Gram Negative Bacteria | *P. mirabilis | Patient 1 | Respiratory infection | 3 | Sputum culture |
| 5592 | Axillary abscess | 7 | Wound culture | ||
| 5446 | Pneumonia | 7 | Culture | ||
|
| |||||
| *H. influenzae | Pneumonia | 92 | Throat culture | ||
| Patient 1 | Respiratory infection | 3 | Sputum culture | ||
| Patient 2 | Pulmonary infection | 3 | Sputum culture | ||
| J.A. | Meningitis | 21 | CSF culture | ||
| Patient 1 | Respiratory infection | 30 | Sputum culture | ||
| Patient 2 | Respiratory infection | 30 | Sputum culture | ||
| 5592 | Sepsis | 7 | Culture | ||
| 5231 | Pneumonia | 7 | Culture | ||
| 5446 | Pneumonia | 7 | Culture | ||
|
| |||||
| *P. aeruginosa | P1 | Chronic airway colonization | 80 | Culture | |
| P3 | Chronic airway colonization | 80 | Culture | ||
| Septicemia | 93 | Culture | |||
| 5231 | Pneumonia, sepsis, gastrointestinal infection | 7 | Culture | ||
|
| |||||
| E. coli | Patient 2 | Cystitis | 3 | Culture | |
| 5546 | Cystitis | 7 | Culture | ||
| 5231 | Respiratory infection | 7 | Culture | ||
|
| |||||
| ˆM. morganii | 5446 | Pneumonia | 7 | Culture | |
|
| |||||
| ˆB. cartarrhalis | 5446 | Pneumonia | 7 | Culture | |
|
| |||||
| S. typhimurium | 5780 | Gastrointestinal infection | 7 | Culture | |
|
| |||||
| C. jejuni | 5231 | Gastrointestinal infection | 7 | Culture | |
|
| |||||
| Gram Positive Bacteria | *S. aureus | Respiratory infection | 31 | Epiglottic culture | |
| Patient 1 | Respiratory infection | 3 | Sputum culture | ||
| Patient 2 | Respiratory infection | 3 | Sputum culture | ||
| 5231 | Pneumonia, gastrointestinal, infection | 7 | Culture | ||
| 5446 | Pneumonia | 7 | Culture | ||
| 5449 | Adenophlegmon | 7 | Wound culture | ||
| 5780 | Osteoarthritis | 7 | Culture | ||
| WHIM-12 | Skin lesions | 24 | Wound culture | ||
|
| |||||
| *S. pneumoniae | Pneumonia | 92 | Sputum culture | ||
| Patient 1 | Respiratory infection | 30 | Sputum culture | ||
| Patient 2 | Respiratory infection | 30 | Sputum culture | ||
| 5592 | Sepsis | 7 | Blood culture | ||
| 5231 | Pneumonia | 7 | Culture | ||
| Pneumonia | 11 | Culture | |||
| 5446 | Pneumonia, pericarditis | 7 | Culture | ||
| Pneumonia, bacteremia | 13 | Blood culture | |||
|
| |||||
| S. pyogenes | Streptococcal pharyngitis | 92 | Throat culture | ||
|
| |||||
| C. perfringens | Septicemia | 93 | Blood culture | ||
|
| |||||
| Myco- bacteria | Nontypable | 5231 | Chronic skin granuloma | 7 | Skin biopsy |
|
| |||||
| M. gordonae | 5446 | Hepatitis, respiratory infection | 7 | Sputum culture | |
|
| |||||
| Fungi | A. glocus | 5592 | Sinusitis, mastoiditis | 7 | Culture |
|
| |||||
| C. albicans | 5231 | Respiratory infection, gastrointestinal infection | 7 | Cultures | |
| 5446 | Pneumonia | 7 | Culture | ||
|
| |||||
| Viruses | Rubella | Disseminated | 31 | Clinical impression | |
| S.S. | Disseminated | 25 | Clinical impression | ||
| P3 | Disseminated | 23 | Clinical impression | ||
|
| |||||
| Rubeola | Disseminated | 31 | Clinical impression | ||
|
| |||||
| Varicella zoster | Skin lesions | 92 | Clinical impression | ||
| Skin lesions | 31 | Clinical impression | |||
| S.S. | Skin lesions | 25 | Clinical impression | ||
| J.A. | Severe chickenpox | 21 | Clinical impression | ||
| Patient 2 | Severe chickenpox | 32 | Clinical impression | ||
| P10 | Skin lesions | 9 | Clinical impression | ||
| P3 | Dermatomal infection (left calf) | 80 | Polymerase chain reaction | ||
| Unidermatomal thoracic zoster | 13 | Clinical impression | |||
|
| |||||
| Herpes simplex | Herpes labialis | 31 | Clinical impression | ||
| Herpes labialis | 8 | Clinical impression | |||
| 5592 | Stomatitis (HSV1) | 7 | Clinical impression | ||
| 5446 | Stomatitis (HSV1) | 7 | Clinical impression | ||
| Recurrent cutaneous infections | 10 | Clinical impression | |||
| Severe perioral infection | 13 | Clinical impression | |||
| 5231 | Genital lesions (HSV2) | 7 | Clinical impression | ||
|
| |||||
| Epstein-Barr | B lymphoma | 26 | Polymerase chain reaction | ||
| Hemophagocytic lymphohistiocytosis, B lymphoma | 8 | In situ hybridization, immunohistochemistry | |||
|
| |||||
| Molluscum contagiosum | Skin lesions | 8 | Clinical impression | ||
| 5446 | Skin lesions | 7 | Clinical impression | ||
|
| |||||
| Cytomegalovirus | 5231 | Sepsis | 7 | Polymerase chain reaction | |
|
| |||||
| Influenza | P1 | Blood | 23 | Virologic analysis | |
| P2 | Blood | 23 | Virologic analysis | ||
Selected cases include patients with WHIM syndrome, defined as myelokathexis and/or CXCR4 C-tail mutations.
Opportunist
Encapsulated organism
Most infections are treated on an outpatient basis, and the causative agent is usually not pursued or isolated. Nevertheless, where data are available, most infectious agents isolated are extracellular bacteria (Table 1). Severe herpesvirus infections have also been reported in a few patients, including varicella zoster virus (VZV) and recurrent oral herpes simplex virus (HSV) (Table 1). In addition, EBV+ B cell lymphoma has been reported in two patients [8,26].
Bacterial infections in WHIM patients typically resolve in response to oral antibiotics without the need for hospitalization, and prophylactic measures such as G-CSF, intravenous immunoglobulin (IVIg) and antibiotics are used to reduce infection incidence and severity [7].Nevertheless, no consensus approach has been developed through clinical trials for treating WHIM patients. Moreover, the natural history of WHIM syndrome has not been rigorously assessed.
Absence from school or work due to repeated hospitalization for severe infections may weaken psychosocial functioning in WHIM patients. Additionally, sequelae associated with repeated infections may significantly affect quality of life. For example, bronchiectasis may cause WHIM patients to be dependent on supplemental oxygen [3,7], and repeated ear infections can lead to hearing loss and delayed speech development in children [23,24].
4.2.5 Myelokathexis
Myelokathexis refers to neutropenia caused by retention of neutrophils in the bone marrow. Thus, it can only be diagnosed by combined blood analysis and bone marrow biopsy or aspirate. In WHIM syndrome, the bone marrow is hypercellular with an elevated myeloid:erythroid ratio and “shift to the right,” i.e., increased ratio of mature neutrophils to bands. Bone marrow neutrophils often have a distinct morphology with hypersegmentation, unusually long strands connecting the nuclear lobes and vacuolization [1,12,27–29]. These features give bone marrow neutrophils an unusual pyknotic appearance typical of cells undergoing apoptosis, and an increased frequency of apoptotic neutrophils has been documented in a few patients with WHIM syndrome [30–32]. Interestingly, in addition to correcting neutropenia, granulocyte-macrophage (GM)-CSF has demonstrated the ability to normalize neutrophil morphology [29].
4.2.6 Other phenotypes associated with WHIM syndrome
Knowledge of the full range of pathology in WHIM syndrome is limited by the absence of published autopsy data and the paucity of histopathologic studies reported in the disease. Nevertheless, patients appear to have increased risk of several developmental defects. Of the approximately 90 cases of WHIM syndrome reported to date, 3 had Tetralogy of Fallot (ToF), a rare congenital heart defect found in only ∼3/10,000 live births in the general population [33]. Other developmental abnormalities include double aortic arch [7], ventricular septal defect with pulmonary atresia [30], skeletal malformations [34,35], and angioma [36]. Both lymphoid follicular hyperplasia [1] and hypoplasia [25] have been described in the only two descriptions of lymph node histology from WHIM patients in the literature. No descriptions of splenic architecture have been published. Interestingly, the Cxcr4 knockout mouse also has multiple developmental phenotypes apart from those affecting the immune system, which include cardiovascular defects (ventricular septal defect, abnormal gastric vascularization) and cerebellar defects [37], but non-hematopoietic developmental defects have not been reported in the Cxcr41013 ‘WHIM’ model mouse [38].
4.3 Immunopathogenesis
4.3.1 Myelokathexis & Hypogammaglobulinemia
Although myelokathexis in WHIM patients appears to result from enhanced CXCR4 signaling, the precise mechanism responsible for this has not yet been elucidated. One major source of increased signal strength may be defective internalization of CXCR4 WHIM receptors after binding ligand [9,39]. Calcium flux signaling, which is an immediate response to receptor ligation that precedes receptor internalization, is also increased for WHIM receptors, indicating that the intrinsic signaling potential of the mutant receptor is increased independently of effects from impaired receptor downregulation. Further complicating the picture is the fact that the disease has autosomal dominant inheritance, so that each CXCR4+ cell from WHIM patients should express both wild type and WHIM variants of CXCR4. To date, no WHIM receptor-specific antibodies have been developed. Thus, the precise stoichiometry of the two forms of the receptor in primary cells and the extent of homo- and heterodimerization of the two forms are not known. In this regard, the crystal structure of wild type CXCR4 bound to a small molecule antagonist has been solved and shown to resolve as a homodimer [40].
Since normal CXCR4 signaling is thought to be physiologically important for neutrophil homing to and retention in the bone marrow, increased CXCR4 signaling in WHIM neutrophils is thought to exaggerate this normal response and thereby to cause pathologic retention of large numbers of neutrophils in the bone marrow and peripheral neutropenia. This model is supported by animal studies showing that AMD3100/plerixafor mobilizes leukocytes, including neutrophils, to the blood mainly from bone marrow [41]. In contrast to this model, direct in vivo imaging studies in mice have suggested that the increase in the blood neutrophil count in response to AMD3100 treatment is caused by release of marginated neutrophils from the lung, as well as by inhibition of homing of aged neutrophils from blood to bone marrow [42]. Whether this mechanism explains neutropenia in WHIM patients is not known. WHIM neutrophils in the blood have high levels of CXCR4 and high levels of apoptosis markers and are therefore primed for efficient homing back to the bone marrow, potentially enhancing neutropenia.
The mechanisms accounting for lymphopenia in WHIM patients have not been clearly delineated. Although studies in WHIM mice suggest that development of lymphoid precursor cells in the bone marrow may be impaired, indirect data suggest trafficking of lymphocytes from primary immune organs to secondary immune organs and blood may also be defective [22,41,43]. In addition, recent studies have provided new insight into the impact of the WHIM mutation on lymphocyte function. Normally, T cells expressing CXCR4 are recruited to form an immunological synapse with dendritic cells in lymph node [44]. However, T cells expressing CXCR4 WHIM receptors show impaired synapse formation due to competing migratory signals from exogenous CXCL12 in lymph node [44]. This results in defective T cell activation that further affects immunoglobulin class switching and other T cell-dependent B cell responses [45]. This may explain why there is a delay in production of IgG-switched antibodies in WHIM syndrome [46]. In agreement with this, a lack of isotype switched memory B cells was identified in two WHIM patients, perhaps illustrating an important role for CXCR4 in germinal center organization and function. Impairment of immunoglobulin class switch recombination has also been reported in other rare immunodeficiency disorders such as hyper IgM syndrome [47], common variable immune deficiency [48], and X-linked lymphoproliferative syndrome [49].
In the CXCR41013 mouse model of WHIM syndrome, lymph node architecture is abnormal, including an absence of B cell follicles, an expansion of T cell zones, and an overall increase in cellularity in the lymph node [38]. In addition, both lymphoid follicular hyperplasia and hypoplasia have been observed in WHIM patients [1,25]. Moreover, follicular hypoplasia was detected in the spleen of the mouse model of WHIM syndrome [38]. However, the WHIM mouse model has limitations, since the CXCR41013 knock-in recapitulates only myelokathexis, not hypogammaglobulinemia or spontaneous bacterial infections, and mice are not susceptible to HPV [50].
4.3.2 HPV in WHIM syndrome
WHIM mutations are thought to affect HPV-infected keratinocytes directly or through effects on the immune response to the virus. With regard to the former mechanism, CXCL12 has been reported to be expressed in HPV-infected keratinocytes of patients with and without WHIM syndrome, but not in uninfected keratinocytes or in keratinocytes in the context of other dermatological conditions [20]. Moreover, the HPV oncogenes E6 and E7 are able to induce expression of both CXCL12 and its receptors CXCR4 and ACKR3/CXCR7 in HPV-18 immortalized keratinocytes [51]. Importantly, HPV-mediated keratinocyte transformation required HPV oncogene E6- and E7-enabled signaling through the CXCL12-CXCR4WHIM axis. Conversely, a recent study indicated that CXCR4WHIM promotes stabilization of E6 and E7 in differentiated keratinocytes [52]. While CXCR4 itself did not promote keratinocyte proliferation, HPV-infected keratinocytes expressing CXCR4 WHIM receptors demonstrated increased proliferation compared to infected cells with wild type CXCR4. Accordingly, CXCR4 blockade decreased oncogene expression.
With regard to the antiviral immune response, numerous CXCR4+ immune cell types, including monocytes, dendritic cells, cytotoxic T lymphocytes (CTLs), and natural killer (NK) cells, all play a role in HPV immunity [53]. Monocytopenia, T lymphopenia and NK cell deficiency [8,23,24] have been reported in WHIM patients, and the CXCR4 WHIM receptor is associated with impaired T cell immunological synapse formation [45]. However, mechanistic studies of specific roles these cells play in WHIM-associated HPV pathogenesis are lacking. The remarkable spontaneous resolution of warts, myelokathexis, and monocytopenia, but not lymphopenia, in patient WHIM-09 after chromothriptic deletion of the WHIM allele of CXCR4 and 163 other genes in the myeloid compartment suggests a role for myeloid cells, such as monocytes or monocyte-derived cells including dendritic cells in WHIM-associated HPV pathogenesis [2]. Plasmacytoid dendritic cells (pDCs) are an important source of interferon-alpha (IFNɑ) in response to viral infections [54] and are markedly decreased in some WHIM patients [9,24], possibly due to high CXCR4 expression and signal strength [55]. Consistent with this observation, peripheral blood mononuclear cells (PBMCs) from WHIM patients produced significantly less IFNɑ than PBMCs from healthy donors in response to stimulation with both HSV-1 and CpG, a Toll-like-receptor (TLR)-9 agonist [56]. Furthermore, WHIM-associated warts were negative for dermal pDCs and MxA, while warts from healthy subjects were positive for both.
Although the precise mechanisms by which HPV immunity occurs in immunocompetent populations are unknown, three successful HPV vaccines containing virus-like particles comprised of the major capsid protein L1 have been developed. HPV infects a relatively immune privileged site, the epidermis, and only slowly generates viral proteins there, which partially explains the delayed adaptive immune responses to the virus. In contrast, the vaccine is injected intramuscularly, generating a robust immune response in immunocompetent vaccinees. At present, only one report has described in detail the immunogenicity of HPV vaccination in the context of WHIM syndrome [57]. A twelve-year-old female WHIM patient without warts received three injections of the quadrivalent vaccine for HPV-6, -11, -16, and -18 according to the licensed protocol, and cellular and humoral responses were evaluated and compared to immunocompetent subjects over eight months. Two months after the third dose, serum HPV-specific antibody titers of 100-400 were detected in the patient, compared to titers of 6,400-102,400 in the controls. The patient's immune serum was capable of neutralizing HPV at a titer of 50-400, while control immune sera had significantly greater neutralizing capacity (1,600–25,600). Interestingly, ex vivo lymphoproliferative responses to Gardasil were detectable four months after the second injection for the WHIM patient versus two months after the first injection for control patients. This finding suggests adaptive immunity in response to HPV vaccination may be present but might be delayed and weaker in WHIM patients. Further investigation is necessary to confirm this and to extend our understanding of natural immune responses to HPV, as well as whether vaccination confers lasting protection against HPV in WHIM patients.
4.4 Molecular Genetics
CXCR4 is the only G protein-coupled receptor (GPCR) that binds to the chemokine CXCL12, also known as stromal cell-derived factor-1 (SDF-1). Conversely, CXCL12 is the only chemokine that binds to CXCR4, which triggers a conformational change [58] and coupling to members of the Gi family of heterotrimeric G proteins [59]. Ligand-activated Gαi inhibits adenylyl cyclase in addition to activating Src family kinases [60]. At the same time, the Gβγ subunit activates PLC-β and PI3K and induces ERK phosphorylation [61], inducing gene transcription, chemotaxis, adhesion, proliferation and apoptosis, among other functions [62].
It should be noted that while it is highly selective and specific for the chemokine CXCL12, CXCR4 has other non-chemokine ligands, including the pro-inflammatory chromatin-associated protein HMGB1 [63]. Likewise, although CXCL12 is selective and specific for CXCR4 among all other GPCRs, it is also a functional ligand for the atypical chemokine receptor 3 (ACKR3, also known as CXCR7), which signals through the β-arrestin pathway rather than G proteins [64]. How CXCR4 WHIM receptors affect ACKR3 signaling and vice versa have not been defined and are only relevant in a few cell types that co-express the two receptors, such as marginal zone B cells [65–68].
WHIM mutations that truncate the C-tail cause an obligate loss of serine and threonine residues that are normally phosphorylated in activated receptors by GRK (G protein-coupled receptor kinase). The loss of phosphorylation prevents β-arrestin recruitment and receptor downregulation, providing one mechanism for increased and prolonged signaling. There is also evidence that the third intracellular loop of CXCR4 is critical in downregulation and endocytosis of the receptor [69].Although the basal levels of CXCR4 expression may not be increased in WHIM syndrome and in some patients may be low [23], downstream chemotactic signaling may still be elevated.
Like all GPCRs, CXCR4 consists of seven transmembrane domains with an extracellular N-terminus and an intracellular C-terminus. All known mutations that cause WHIM syndrome occur at the C-terminus. Nine mutations have been previously reported, the most common of which is CXCR4R334X [24,70]. Of these nine mutations, four are truncation mutations, four are frameshift mutations, and only one is a point mutation (Figure 2).
The point mutation CXCR4E343K is a charge-changing substitution of lysine for glutamic acid reported in a single multigenerational family with five affected members [23]. The clinical manifestations are relatively mild and heterogeneous in this family, and the mutant receptor exhibits a smaller defect in ligand-induced downregulation than the most common WHIM variant CXCR4R334X. Nevertheless, calcium flux and chemotactic signaling are not different. Another interesting WHIM mutation is CXCR4L329fs, a de novo mutation found in a 10-month-old boy that coincidentally has also been reported as a somatic mutation in tumor cells from patients with Waldenström's macroglobulinemia [24]. Again, typical WHIM pathology was found in the patient, and, like CXCR4R334X, CXCR4L329fs produced similar calcium flux responses to CXCL12, but showed less receptor downregulation than CXCR4R334X in patient PBMCs. Interestingly, many other CXCR4 mutations have been identified in a large subset of patients with Waldenstrom's macroglobulinemia, some of which are identical to other WHIM mutations, including R334X [71]. Waldenstrom's patients with CXCR4 mutations have been reported to have a poorer prognosis than those without. The convergence of a plethora of CXCR4 mutations, some even identical, in both an inherited and an acquired disease of blood leukocytes, suggests that the C-tail of CXCR4 may be a mutation hotspot.CXCR4 is a highly conserved gene from man to fish, and the C-tail is even more highly conserved than the protein as a whole. The last 19 amino acids, where most WHIM mutations are found, are 100% identical from human to birds and differ at only one position in zebrafish, suggesting that each of these amino acids may be functionally critical.
Other mechanisms that increase CXCR4 signaling might also be predicted to cause WHIM syndrome or a variant of it. In this regard, patients with G6PC3 deficiency, a disorder caused by complete glucose-6-phosphatase deficiency in neutrophils, present with severe congenital neutropenia that was shown in two siblings to be associated with increased CXCR4 expression on neutrophils, most likely the result of metabolic stress. Not surprisingly, a myelokathexis-like picture has also been reported for a subset of these patients [4].
Two other unrelated patients with full-blown WHIM syndrome have been reported whose leukocytes exhibit increased CXCR4 signaling despite normal receptor expression and lack of a CXCR4 mutation [72]. The mechanism appears to involve GRK3 protein deficiency, although no GRK3 mutations were found. Consistent with this, knocking-down GRK3 in control cells results in increased chemotactic responses to CXCL12 as well as impaired CXCR4 desensitization, phenocopying leukocytes from WHIM patients with CXCR4 mutations. Conversely, overexpressing GRK3 in control cells decreases chemotactic responses to CXCL12. In addition, leukocytes from GRK3 knockout mice exhibit decreased CXCR4 desensitization and increased chemotaxis when stimulated with CXCL12, and the mice exhibit myelokathexis and partial hypogammaglobulinemia [73]. GRK6 has also been shown to interact with the wild type CXCL12-CXCR4 signaling axis but failed to associate with the CXCR4 WHIM receptor [74]. Neutrophils in GRK6 knockout mice demonstrated both increased chemotaxis towards CXCL12 and a lack of desensitization of CXCR4 [75].
4.5 Treatment
Treatment for WHIM patients is not standardized but aims to mitigate hematologic defects and clinical symptoms associated with the disease [76]. It is controversial whether the main driver for susceptibility to infection is leukopenia versus hypogammaglobulinemia or the combination of the two. Neutropenia, hypogammaglobulinemia and bacterial infections are the focus of current treatment strategies [76] and clinical trials (ClinicalTrials.gov NCT00967785, NCT02231879, NCT03005327). There are no pharmacologic agents that have a demonstrated ability to prevent or treat warts in WHIM patients. The HPV vaccine is limited to a small subset of the most highly cancer-associated strains. Successful treatment of warts in WHIM patients is typically restricted to destructive therapies.
Current therapies for neutropenia and infections in WHIM patients include G-CSF and IVIg [77,78]. G-CSF selectively increases ANC by multiple mechanisms including induction of CXCL12 degradation in bone marrow to promote neutrophil egress. It has no effect on other leukocyte subsets. Pooled IVIg provides passive antibody replacement for patients who are deficient or low in serum immunoglobulin levels, but obviously is not useful for novel pathogens or pathogens originating from a different geographic distribution from the donors [77,78]. G-CSF and IVIg are given empirically as no clinical trials have been conducted to determine the efficacy of either treatment specifically for WHIM patients [79,80]. There is anecdotal evidence attesting both to benefit [23,24,30] as well as to lack of benefit [10,81] in WHIM patients. WHIM syndrome involves a special type of neutropenia, since patients have a large reservoir of neutrophils with normal function that can be mobilized by appropriate stresses. It should be noted that G-CSF can cause significant side effects, including bone pain [16,32], which is common and can be disabling, and myelofibrosis and leukemia [82], which appear to be rare outcomes.
While G-CSF is preferred, GM-CSF has also been used in the past to treat neutropenia in WHIM syndrome [29]. The functional differences between G-CSF and GM-CSF may be due to differences in their signaling pathways and receptor expression patterns [83]. GM-CSF is FDA approved for stem cell mobilization and treatment of neutropenia related to stem cell transplantation. Similar to G-CSF, GM-CSF is given empirically. It seems to be less well-tolerated than G-CSF, particularly with regard to bone pain and fever, and is rarely used now. Together, these limitations indicate a need for other treatment options.
Plerixafor, also known as AMD3100, is a bicyclam small molecule that is currently being studied for the treatment of WHIM syndrome (Figure 3) (ClinicalTrials.gov NCT00967785, NCT02231879). It was originally discovered in a screen for HIV entry inhibitors and later found to block HIV entry by binding to CXCR4, a major HIV entry factor [43,84]. It was withdrawn from clinical development because most HIV strains preferentially use CCR5 rather than CXCR4 for entry into CD4+T cells and because of side effects that occurred at the high doses needed to maximize reduction of HIV burden. It was noted serendipitously during the AIDS trials to cause leukocytosis involving all major leukocyte subsets as well as CD34+hematopoietic stem and progenitor cells (HSPCs) [85,86]. Therefore, plerixafor was next developed as an HSC mobilizing agent and approved by the FDA for that indication in concert with G-CSF to mobilize patients in preparation for autologous stem cell transplantation after chemotherapy, specifically in multiple myeloma and non-Hodgkin's lymphoma [87,88].AMD3100 has poor oral bioavailability and is given subcutaneously. It is rapidly distributed to the blood where concentrations peak at one hour after dosing. The peak for leukocyte mobilization to the blood depends on the subset type but is ∼3 hours for neutrophils. It is not metabolized but has a short half-life of ∼4 hours due to rapid renal clearance, and it is completely cleared from the circulation by 24 hours.AMD3100 is also a weak ACKR3/CXCR7 agonist, but this is unlikely to be relevant at the low doses used in treating WHIM patients [89].
Figure 3.
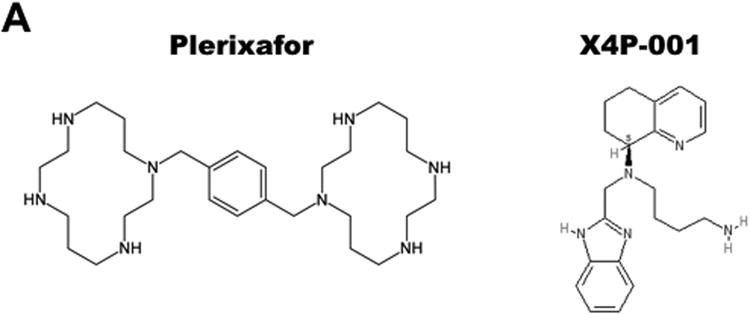
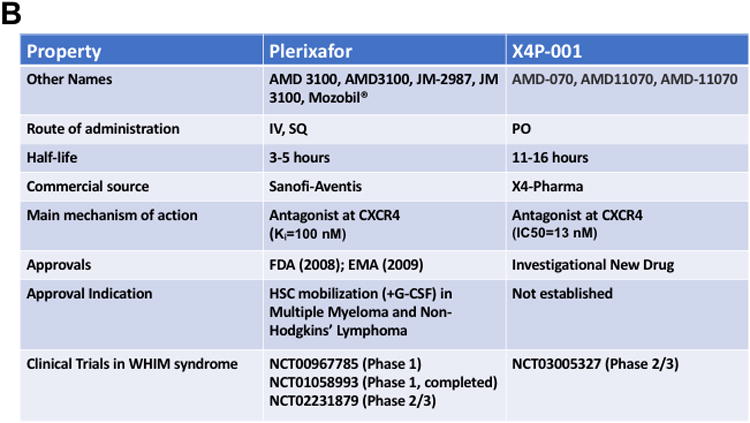
Selective CXCR4 antagonists in clinical trials for WHIM syndrome. A) Structures. B) Properties.
In two phase I dose escalation trials [79,90], plerixafor significantly increased the WBC, mainly due to increased lymphocytes. The absolute neutrophil and monocyte counts were also increased, and the fold increases for all three major subsets were similar [79]. No significant side effects were observed. Importantly, the ANC could be elevated over the critical infection susceptibility threshold of 500 cells/μL by extremely low doses of plerixafor (0.02 mg/kg).In a 6-month follow-up trial in 3 patients with a history of recurrent infections, the frequency of infections appeared to be reduced by plerixafor 0.01 mg/kg sq BiD dosing; in 2 patients, no infections occurred while on plerixafor [79]. There was also evidence of reduced wart burden while on drug. Thus, preliminary data support continued investigation of daily low dose plerixafor for treating panleukopenia and preventing bacterial infections in the context of WHIM syndrome.A phase II/III double-blind crossover trial is currently underway to evaluate the safety and efficacy of plerixafor compared to G-CSF (NCT02231879).
Other CXCR4 antagonists include AMD11070, which is currently being studied by X4-Pharma in a phase II/III trial for the treatment of WHIM syndrome (NCT03005327). Also known as X4P-001LD, AMD11070 was developed as a potential anti-HIV drug like plerixafor (Figure 3) [91]. In a preliminary study, no adverse side effects were observed after patients were given 200 mg twice a day for ten days [91]. It is also being tested in cancer (NCT02823405 and others). Whether this AMD3100 analog performs better than plerixafor or G-CSF in the treatment of WHIM syndrome is unknown. One obvious potential advantage is that it has oral bioavailability and is being tested as a low dose oral formulation in WHIM patients.
To date, three patients have been clinically cured of WHIM syndrome. Two were cured by allogeneic stem cell transplantation [10,11], and one was cured by chromothripsis, a naturally-occurring, spontaneous and in this case highly fortuitous shattering and rearrangement of one copy of chromosome 2, which selectively deleted the WHIM allele as well as one copy of 163 other genes in HSCs and myeloid but not lymphoid cells (Figure 4) [2]. The molecular mechanisms leading to chromothripsis have not yet been defined. This case indicates that having one copy of CXCR4 in the myeloid lineage is not lethal [2] and that myeloid expression of the mutation is required for the main clinical manifestations of the disease. Extending this, Cxcr4+/o hemizygous mice, which lack one copy of Cxcr4 in all nucleated cells, appear healthy. Murine stem cells hemizygous for Cxcr4 were found to have an engraftment advantage in competitive transplantation studies, which may explain at least in part the patient's cure mechanism [2]. This finding suggests that inducing CXCR4 haploinsufficiency by gene editing may facilitate stem cell engraftment in gene therapy as well as in other stem cell transplantation applications. Unlike loss of function mutations, WHIM syndrome cannot be cured by gene replacement outside of the disease gene locus. The gain-of-function mutant allele must be silenced or corrected in situ. Accomplishing this in patient HSCs could conceivably eliminate the disease-causing mutation and facilitate engraftment in one step, thereby promoting cure.
Figure 4.
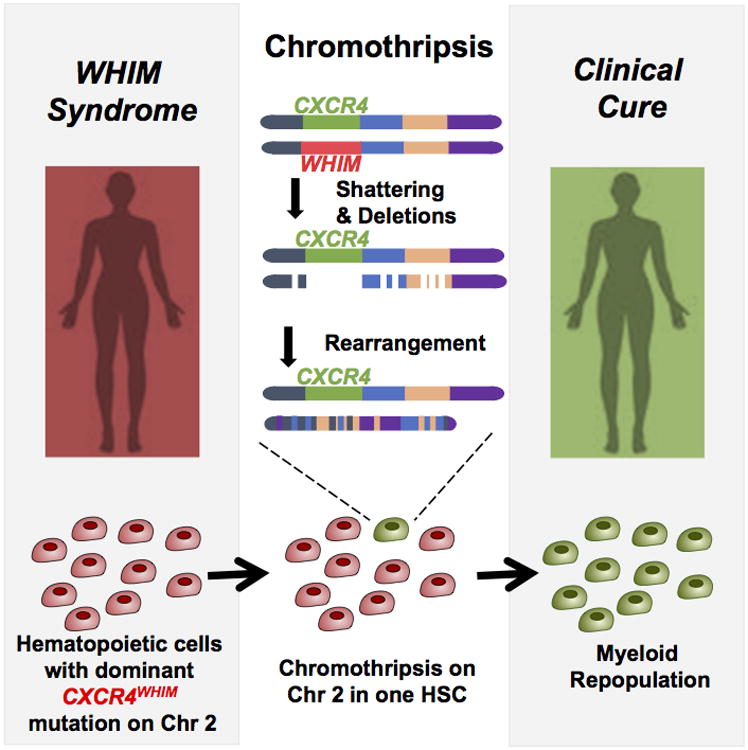
Chromothriptic cure of WHIM syndrome in patient WHIM-09. This patient was the first ever reported with WHIM syndrome. As an adult, neutropenia, warts and susceptibility to recurrent infection spontaneously resolved. Her HSCs and the entire myeloid lineage but not the lymphoid lineage was found to be a clonal derivative of a cell that had undergone chromothripsis on chromosome 2. In particular, one copy of chromosome 2 was found to contain 17 gaps, deleting 164 genes including the WHIM allele of CXCR4. Mouse HSCs lacking one copy of wild type CXCR4 were found to have an engraftment advantage, identifying a potential cure mechanism for the patient.
4.6 Conclusion
Since 1964 when the first patient was described, treatment of WHIM syndrome has progressed from ill-conceived splenectomy to mechanism-based treatment trials of plerixafor and AMD11070 targeting the molecular cause of the disease, giving hope to patients. WHIM syndrome is unusual in being 1) the only Mendelian condition caused by a chemokine receptor; 2) caused by a gain-of-function mutation; and 3) possibly amenable to treatment with a small molecule receptor antagonist. Studies of WHIM syndrome have provided insights of general significance including the role of monocyte-derived cells in control of HPV disease, the role of myeloid cells in control of recurrent infections, and the role of CXCR4 in leukocyte distribution and organ development. They also provide evidence that defective innate immunity plays a dominant role in the pathogenesis of WHIM syndrome. The unique case of WHIM-09 has revealed the importance of CXCR4 copy number in regulating HSC engraftment during transplantation, which may have general applicability to hematological diseases. The story of plerixafor highlights the importance of serendipity and astute observation for unexpected results in science and medicine for the benefit of patients.
5. Expert Opinion
Although discovering the disease gene for a Mendelian condition may bring instant clarity to a complex problem, it is important not to be blinded to new and remaining questions and controversies. There is already some evidence that WHIM syndrome is genetically heterogeneous, but how common exceptions to CXCR4 mutation are in WHIM syndrome and the precise alternative genetic explanations have not been established. For example, no genotype:phenotype correlations or other explanations have been determined. Differential exposure to environmental factors, such as HPV and other infectious agents are obviously relevant. Hematologic heterogeneity is also still a mystery.
In addition, the gain of function in CXCR4 that has been documented for WHIM receptors is not yet understood at the molecular level. Whether the WHIM receptor operates as a monomer or as a homodimer or heterodimer complexed with wild type CXCR4 is not known, nor is the stoichiometry of the two forms defined. WHIM mutations could have multiple combinatorial biochemical effects on CXCR4 signal strength, including effects on downregulation/desensitization, receptor degradation, G protein-coupling and usage of alternative or hijacked signaling pathways. The relative contribution of CXCR4 signaling in keratinocytes versus immunodeficiency to HPV pathogenesis in WHIM syndrome is also poorly understood.
Moreover, hematologic and immunologic mechanisms underlying pathogenesis are also not fully defined. The report that the WHIM mutation can affect immunologic synapse formation suggests that impaired leukocyte trafficking alone may not fully account for WHIM phenotypes. Moreover, the precise step(s) in the multistep model of leukocyte trafficking that are affected by the WHIM mutation have not been defined, especially in the bone marrow where neutrophils are retained. In addition, the factors that enable neutrophils to be released from bone marrow in response to infection and stress and the impacts of WHIM mutations and bone marrow retention on neutrophil survival are need to be examined. Since a WHIM mouse model is available, intravital imaging is being used to study precisely and directly the effects of the mutation on trafficking by specific types of leukocytes in vivo.
Furthermore, WHIM patients are highly susceptible to Tetralogy of Fallot, implying that CXCR4 is critical for cardiovascular development; however, why the penetrance of this phenotype is so low requires explanation. More generally, the natural history of WHIM syndrome has not been defined, and given the availability of treatments for neutropenia and hypogammaglobulinemia, it may no longer be ethical to try.
WHIM syndrome may be particularly amenable to development of targeted mechanism-based therapeutics for three reasons. First, the molecular target, CXCR4, has been validated by Mendelian genetics in humans as well as in three animal models. Second, CXCR4 is a member of a superfamily of highly druggable proteins, the G protein-coupled receptors. Third, WHIM mutations increase CXCR4 function, implying that an antagonist or other blocking agent might be effective. In this regard, the small molecule CXCR4 antagonist plerixafor (AMD3100, Mozobil) has been approved by the FDA for HSC mobilization for transplantation in certain cancers. It has demonstrated safety and efficacy for durably correcting panleukopenia in phase I clinical trials in WHIM syndrome, and it is currently being compared with G-CSF in a phase II/III clinical trial in WHIM syndrome for control of infection frequency. A second phase II/III trial of the low dose orally bioavailable CXCR4 antagonist X4P-001-LD compared to placebo is also enrolling.
WHIM syndrome may be particularly amenable to gene editing as a cure strategy for three reasons. First, only one CXCR4 allele is mutated in the disease. Second, the chromothripsis patient WHIM-09 has provided direct evidence that selectively inactivating the mutant CXCR4 allele in the myeloid lineage can eliminate the main clinical manifestations of the disease. Third, CXCR4 haploinsufficiency, the genetic consequence of inactivating the WHIM allele, fortuitously enhances HSC engraftment.
Article highlight box.
Autosomal dominant gain-of-function mutations in the C-tail of chemokine receptor CXCR4 cause 98% of reported cases of WHIM syndrome. Coincidentally, CXCR4 C-tail mutations are also commonly found in patients with Waldenstrom's macroglobulinemia, suggesting a mutational hotspot, and are associated with poorer prognosis.
The main clinical manifestations of WHIM syndrome are warts, hypogammaglobulinemia, recurrent bacterial oto-sino-pulmonary and skin infections, and myelokathexis. Myelokathexis is neutropenia due to impaired egress of functionally normal neutrophils from the bone marrow. However, many patients have panleukopenia.
Despite combined immunodeficiency, invasive and life-threatening infection is uncommon in WHIM patients, due to the ability of neutrophils to be released from bone marrow during infection.
The molecular pathogenesis of WHIM syndrome involves impaired CXCR4 downregulation and possibly other unknown mechanisms resulting in increased signaling strength. This results in exaggeration of the normal function of CXCR4 to promote neutrophil retention in the bone marrow. Patients may also have impaired vaccine responses possibly due to defects in lymphocyte development and trafficking as well as immune synapse formation.
Targeted mechanism-based treatment strategies using CXCR4 antagonists are currently in clinical trials.
A cure strategy involving gene editing of the mutant allele in HSCs that has a fortuitous side-effect of enhancing HSC engraftment potential has been conceived based on the case of the first patient ever described with WHIM syndrome. WHIM-09 acquired a second mutation through chromothripsis (chromosome shattering) that deleted the WHIM mutation in an HSC, which acquired a selective growth advantage and repopulated her myeloid lineage, resulting in clinical cure.
Acknowledgments
This work was supported by the Division of Intramural Research of the National Institute, of Allergy and Infectious Diseaes, NIH.
Footnotes
Financial and competing interests disclosure: The authors report no conflicts of interest.
Contributor Information
Lauren E. Heusinkveld, Laboratory of Molecular Immunology, Bldg 10, Room 11N113, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD 20892.
Erin Yim, Laboratory of Molecular Immunology, Bldg 10, Room 11N113, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD 20892.
Alexander Yang, Laboratory of Molecular Immunology, Bldg 10, Room 11N113, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD 20892.
Ari B. Azani, Laboratory of Molecular Immunology, Bldg 10, Room 11N113, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD 20892.
Qian Liu, Laboratory of Molecular Immunology, Bldg 10, Room 11N113, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD 20892.
Ji-Liang Gao, Laboratory of Molecular Immunology, Bldg 10, Room 11N113, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD 20892.
David H. McDermott, Laboratory of Molecular Immunology, Bldg 10, Room 11N113, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD 20892.
Philip M. Murphy, Laboratory of Molecular Immunology, Bldg 10, Room 11N113, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD 20892.
Bibliography
- 1**.Zuelzer WW. “Myelokathexis”--A New Form of Chronic Granulocytopenia. Report of A Case. N Engl J Med. 1964 Apr;270:699–704. doi: 10.1056/NEJM196404022701402. First case report of WHIM syndrome and the first description of myelokathexis in the biomedical literature. [DOI] [PubMed] [Google Scholar]
- 2**.McDermott DH, Gao JL, Liu Q, Siwicki M, Martens C, Jacobs P, Velez D, Yim E, Bryke CR, Hsu N, Dai Z, Marquesen MM, Stregevsky E, Kwatemaa N, Theobald N, Long Priel DA, Pittaluga S, Raffeld MA, Calvo KR, Maric I, Desmond R, Holmes KL, Kuhns DB, Balabanian K, Bachelerie F, Porcella SF, Malech HL, Murphy PM. Chromothriptic cure of WHIM syndrome. Cell. 2015 Feb;160:686–699. doi: 10.1016/j.cell.2015.01.014. Chromothriptic deletion of CXCR4 in a WHIM patient resulted in clinical cure and implicates gene editing as a potential cure strategy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3*.Wetzler M, Talpaz M, Kleinerman ES, King A, Huh YO, Gutterman JU, Kurzrock R. A new familial immunodeficiency disorder characterized by severe neutropenia, a defective marrow release mechanism, and hypogammaglobulinemia. Am J Med. 1990 Nov;89:663–672. doi: 10.1016/0002-9343(90)90187-i. Description of a family with WHIM syndrome and first use of the acronym “WHIM”. [DOI] [PubMed] [Google Scholar]
- 4*.McDermott DH, De Ravin SS, Jun HS, Liu Q, Priel DAL, Noel P, Takemoto CM, Ojode T, Paul SM, Dunsmore KP, Hilligoss D, Marquesen M, Ulrick J, Kuhns DB, Chou JY, Malech HL, Murphy PM. Severe congenital neutropenia resulting from G6PC3 deficiency with increased neutrophil CXCR4 expression and myelokathexis. Blood. 2010 Oct;116:2793–2802. doi: 10.1182/blood-2010-01-265942. The authors identified two patients with G6PC3 deficiency who had high expression of CXCR4 on neutrophils. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5*.Hernandez PA, Gorlin RJ, Lukens JN, Taniuchi S, Bohinjec J, Francois F, Klotman ME, Diaz GA. Mutations in the chemokine receptor gene CXCR4 are associated with WHIM syndrome, a combined immunodeficiency disease. Nat Genet. 2003 May;34:70–74. doi: 10.1038/ng1149. Landmark report identifying CXCR4 mutations as the cause of WHIM syndrome. [DOI] [PubMed] [Google Scholar]
- 6.Ma Q, Jones D, Springer TA. The chemokine receptor CXCR4 is required for the retention of B lineage and granulocytic precursors within the bone marrow microenvironment. Immunity. 1999 Apr;10:463–471. doi: 10.1016/s1074-7613(00)80046-1. [DOI] [PubMed] [Google Scholar]
- 7*.Beaussant Cohen S, Fenneteau O, Plouvier E, Rohrlich PS, Daltroff G, Plantier I, Dupuy A, Kerob D, Beaupain B, Bordigoni P, Fouyssac F, Delezoide AL, Devouassoux G, Nicolas JF, Bensaid P, Bertrand Y, Balabanian K, Chantelot CB, Bachelerie F, Donadieu J. Description and outcome of a cohort of 8 patients with WHIM syndrome from the French Severe Chronic Neutropenia Registry. Orphanet J Rare Dis. 2012 Sep;7:71. doi: 10.1186/1750-1172-7-71. Comprehensive description of WHIM patients participating in the French Severe Chronic Neutropenia Registry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Imashuku S, Miyagawa A, Chiyonobu T, Ishida H, Yoshihara T, Teramura T, Kuriyama K, Imamura T, Hibi S, Morimoto A, Todo S. Epstein-Barr virus-associated T-lymphoproliferative disease with hemophagocytic syndrome, followed by fatal intestinal B lymphoma in a young adult female with WHIM syndrome. Warts, hypogammaglobulinemia, infections, and myelokathexis. Ann Hematol. 2002 Aug;81:470–473. doi: 10.1007/s00277-002-0489-9. [DOI] [PubMed] [Google Scholar]
- 9.Tassone L, Notarangelo LD, Bonomi V, Savoldi G, Sensi A, Soresina A, Smith CIE, Porta F, Plebani A, Notarangelo LD, Badolato R. Clinical and genetic diagnosis of warts, hypogammaglobulinemia, infections, and myelokathexis syndrome in 10 patients. Journal of Allergy and Clinical Immunology. 2009 May;123:1170–1173.e3. doi: 10.1016/j.jaci.2008.12.1133. [DOI] [PubMed] [Google Scholar]
- 10*.Moens L, Frans G, Bosch B, Bossuyt X, Verbinnen B, Poppe W, Boeckx N, Slatter M, Brusselmans C, Diaz G, Tousseyn T, Flipts H, Corveleyn A, Dierickx D, Meyts I. Successful hematopoietic stem cell transplantation for myelofibrosis in an adult with warts-hypogammaglobulinemia-immunodeficiency-myelokathexis syndrome. J Allergy Clin Immunol. 2016 Nov;138:1485–1489.e2. doi: 10.1016/j.jaci.2016.04.057. Clinical cure of WHIM syndrome achieved by stem cell transplantation. [DOI] [PubMed] [Google Scholar]
- 11*.Kriván G, Erdos M, Kállay K, Benyó G, Tóth A, Sinkó J, Goda V, Tóth B, Maródi L. Successful umbilical cord blood stem cell transplantation in a child with WHIM syndrome. Eur J Haematol. 2010 Mar;84:274–275. doi: 10.1111/j.1600-0609.2009.01368.x. Clinical cure of WHIM syndrome achieved by stem cell transplantation. [DOI] [PubMed] [Google Scholar]
- 12*.Gorlin RJ, Gelb B, Diaz GA, Lofsness KG, Pittelkow MR, Fenyk JR. WHIM syndrome, an autosomal dominant disorder: clinical, hematological, and molecular studies. Am J Med Genet. 2000 Apr;91:368–376. Description of a family with WHIM syndrome and literature review. [PubMed] [Google Scholar]
- 13.Tarzi MD, Jenner M, Hattotuwa K, Faruqi AZ, Diaz GA, Longhurst HJ. Sporadic case of warts, hypogammaglobulinemia, immunodeficiency, and myelokathexis syndrome. J Allergy Clin Immunol. 2005 Nov;116:1101–1105. doi: 10.1016/j.jaci.2005.08.040. [DOI] [PubMed] [Google Scholar]
- 14.Wieland U, Kreuter A, Pfister H. Human papillomavirus and immunosuppression. Curr Probl Dermatol. 2014 Mar;45:154–165. doi: 10.1159/000357907. [DOI] [PubMed] [Google Scholar]
- 15.Hagan JB, Nguyen PL. WHIM syndrome. Mayo Clin Proc. 2007 Sep;82:1031. doi: 10.4065/82.9.1031. [DOI] [PubMed] [Google Scholar]
- 16.Hord JD, Whitlock JA, Gay JC, Lukens JN. Clinical features of myelokathexis and treatment with hematopoietic cytokines: a case report of two patients and review of the literature. J Pediatr Hematol Oncol. 1997 Oct;19:443–448. doi: 10.1097/00043426-199709000-00007. [DOI] [PubMed] [Google Scholar]
- 17.Diaz GA. CXCR4 mutations in WHIM syndrome: a misguided immune system? Immunol Rev. 2005 Feb;203:235–243. doi: 10.1111/j.0105-2896.2005.00226.x. [DOI] [PubMed] [Google Scholar]
- 18.Cipriani NA, Blair E, Taxy JB. WHIM syndrome and oral squamous cell carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010 Jan;109:105–108. doi: 10.1016/j.tripleo.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 19.Palm MD, Tyring SK, Rady PL, Tharp MD. Human papillomavirus typing of verrucae in a patient with WHIM syndrome. Arch Dermatol. 2010 Aug;146:931–932. doi: 10.1001/archdermatol.2010.184. [DOI] [PubMed] [Google Scholar]
- 20.Balabanian K, Lagane B, Pablos JL, Laurent L, Planchenault T, Verola O, Lebbe C, Kerob D, Dupuy A, Hermine O, Nicolas JF, Latger-Cannard V, Bensoussan D, Bordigoni P, Baleux F, Le Deist F, Virelizier JL, Arenzana-Seisdedos F, Bachelerie F. WHIM syndromes with different genetic anomalies are accounted for by impaired CXCR4 desensitization to CXCL12. Blood. 2005 Mar;105:2449–2457. doi: 10.1182/blood-2004-06-2289. [DOI] [PubMed] [Google Scholar]
- 21.Goddard EA, Hughes EJ, Beatty DW. A case of immunodeficiency characterized by neutropenia, hypogammaglobulinaemia, recurrent infections and warts. Clin Lab Haematol. 1994 Sep;16:297–302. doi: 10.1111/j.1365-2257.1994.tb00425.x. [DOI] [PubMed] [Google Scholar]
- 22.Gulino AV, Moratto D, Sozzani S, Cavadini P, Otero K, Tassone L, Imberti L, Pirovano S, Notarangelo LD, Soresina R, Mazzolari E, Nelson DL, Notarangelo LD, Badolato R. Altered leukocyte response to CXCL12 in patients with warts hypogammaglobulinemia, infections, myelokathexis (WHIM) syndrome. Blood. 2004 Jul;104:444–452. doi: 10.1182/blood-2003-10-3532. [DOI] [PubMed] [Google Scholar]
- 23.Liu Q, Chen H, Ojode T, Gao X, Anaya-O'Brien S, Turner NA, Ulrick J, DeCastro R, Kelly C, Cardones AR, Gold SH, Hwang EI, Wechsler DS, Malech HL, Murphy PM, McDermott DH. WHIM syndrome caused by a single amino acid substitution in the carboxy-tail of chemokine receptor CXCR4. Blood. 2012 Jul;120:181–189. doi: 10.1182/blood-2011-12-395608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu Q, Pan C, Lopez L, Gao J, Velez D, Anaya-O'Brien S, Ulrick J, Littel P, Corns JS, Ellenburg DT, Malech HL, Murphy PM, McDermott DH. WHIM Syndrome Caused by Waldenström's Macroglobulinemia-Associated Mutation CXCR4 (L329fs) J Clin Immunol. 2016 May;36:397–405. doi: 10.1007/s10875-016-0276-3. [DOI] [PubMed] [Google Scholar]
- 25.Mentzer WC, Johnston RB, Baehner RL, Nathan DG. An unusual form of chronic neutropenia in a father and daughter with hypogammaglobulinaemia. Br J Haematol. 1977 Jul;36:313–322. doi: 10.1111/j.1365-2141.1977.tb00654.x. [DOI] [PubMed] [Google Scholar]
- 26.Chae KM, Ertle JO, Tharp MD. B-cell lymphoma in a patient with WHIM syndrome. J Am Acad Dermatol. 2001 Jan;44:124–128. doi: 10.1067/mjd.2001.111337. [DOI] [PubMed] [Google Scholar]
- 27.van Slambrouck CM, Gurbuxani S. On a WHIM. Blood. 2013 Feb;121:875. doi: 10.1182/blood-2012-03-420091. [DOI] [PubMed] [Google Scholar]
- 28.Bassan R, Viero P, Minetti B, Comotti B, Barbui T. Myelokathexis: a rare form of chronic benign granulocytopenia. Br J Haematol. 1984 Sep;58:115–117. doi: 10.1111/j.1365-2141.1984.tb06065.x. [DOI] [PubMed] [Google Scholar]
- 29.Wetzler M, Talpaz M, Kellagher MJ, Gutterman JU, Kurzrock R. Myelokathexis: normalization of neutrophil counts and morphology by GM-CSF. JAMA. 1992 Apr;267:2179–2180. [PubMed] [Google Scholar]
- 30.Taniuchi S, Yamamoto A, Fujiwara T, Hasui M, Tsuji S, Kobayashi Y. Dizygotic twin sisters with myelokathexis: mechanism of its neutropenia. Am J Hematol. 1999 Oct;62:106–111. doi: 10.1002/(sici)1096-8652(199910)62:2<106::aid-ajh8>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 31.Krill CE, Smith HD, Mauer AM. Chronic Idiopathic Granulocytopenia. N Engl J Med. 1964 May;270:973–979. doi: 10.1056/NEJM196405072701902. [DOI] [PubMed] [Google Scholar]
- 32.Aprikyan AA, Liles WC, Park JR, Jonas M, Chi EY, Dale DC. Myelokathexis, a congenital disorder of severe neutropenia characterized by accelerated apoptosis and defective expression of bcl-x in neutrophil precursors. Blood. 2000 Jan;95:320–327. [PubMed] [Google Scholar]
- 33.Apitz C, Webb GD, Redington AN. Tetralogy of Fallot. The Lancet. 2009 Oct;374:1462–1471. doi: 10.1016/S0140-6736(09)60657-7. [DOI] [PubMed] [Google Scholar]
- 34.Badolato R, Dotta L, Tassone L, Amendola G, Porta F, Locatelli F, Notarangelo LD, Bertrand Y, Bachelerie F, Donadieu J. Tetralogy of fallot is an uncommon manifestation of warts, hypogammaglobulinemia, infections, and myelokathexis syndrome. J Pediatr. 2012 Oct;161:763–765. doi: 10.1016/j.jpeds.2012.05.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Plebani A, Cantù-Rajnoldi A, Collo G, Allavena P, Biolchini A, Pirelli A, Schoeller MC, Masarone M. Myelokathexis associated with multiple congenital malformations: Immunological study on phagocytic cells and lymphocytes. Eur J Haematol. 2009 Apr;40:12–17. doi: 10.1111/j.1600-0609.1988.tb00790.x. [DOI] [PubMed] [Google Scholar]
- 36.Siedlar M, Rudzki Z, Strach M, Trzyna E, Pituch-Noworolska A, Błaut-Szlósarczyk A, Bukowska-Strakova K, Lenart M, Grodzicki T, Zembala M. Familial occurrence of warts, hypogammaglobulinemia, infections, and myelokathexis (WHIM) syndrome. Arch Immunol Ther Exp (Warsz) 2008 Dec;56:419–425. doi: 10.1007/s00005-008-0046-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ma Q, Jones D, Borghesani PR, Segal RA, Nagasawa T, Kishimoto T, Bronson RT, Springer TA. Impaired B-lymphopoiesis, myelopoiesis, and derailed cerebellar neuron migration in CXCR4- and SDF-1-deficient mice. Proc Natl Acad Sci U S A. 1998 Aug;95:9448–9453. doi: 10.1073/pnas.95.16.9448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38**.Balabanian K, Brotin E, Biajoux V, Bouchet-Delbos L, Lainey E, Fenneteau O, Bonnet D, Fiette L, Emilie D, Bachelerie F. Proper desensitization of CXCR4 is required for lymphocyte development and peripheral compartmentalization in mice. Blood. 2012 Jun;119:5722–5730. doi: 10.1182/blood-2012-01-403378. This is the first description of a mouse model of WHIM syndrome. [DOI] [PubMed] [Google Scholar]
- 39.Kawai T, Choi U, Whiting-Theobald NL, Linton GF, Brenner S, Sechler JMG, Murphy PM, Malech HL. Enhanced function with decreased internalization of carboxy-terminus truncated CXCR4 responsible for WHIM syndrome. Exp Hematol. 2005 Apr;33:460–468. doi: 10.1016/j.exphem.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 40.Wu B, Chien EYT, Mol CD, Fenalti G, Liu W, Katritch V, Abagyan R, Brooun A, Wells P, Bi FC, Hamel DJ, Kuhn P, Handel TM, Cherezov V, Stevens RC. Structures of the CXCR4 chemokine GPCR with small-molecule and cyclic peptide antagonists. Science. 2010 Nov;330:1066–1071. doi: 10.1126/science.1194396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu Q, Li Z, Gao JL, Wan W, Ganesan S, McDermott DH, Murphy PM. CXCR4 antagonist AMD3100 redistributes leukocytes from primary immune organs to secondary immune organs, lung, and blood in mice. Eur J Immunol. 2015 Jun;45:1855–1867. doi: 10.1002/eji.201445245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Devi S, Wang Y, Chew WK, Lima R, A-González N, Mattar CNZ, Chong SZ, Schlitzer A, Bakocevic N, Chew S, Keeble JL, Goh CC, Li JLY, Evrard M, Malleret B, Larbi A, Renia L, Haniffa M, Tan SM, Chan JKY, Balabanian K, Nagasawa T, Bachelerie F, Hidalgo A, Ginhoux F, Kubes P, Ng LG. Neutrophil mobilization via plerixafor-mediated CXCR4 inhibition arises from lung demargination and blockade of neutrophil homing to the bone marrow. J Exp Med. 2013 Oct;210:2321–2336. doi: 10.1084/jem.20130056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.De Clercq E. The bicyclam AMD3100 story. Nat Rev Drug Discov. 2003 Jul;2:581–587. doi: 10.1038/nrd1134. [DOI] [PubMed] [Google Scholar]
- 44.Kallikourdis M, Viola A, Benvenuti F. Human immunodeficiencies related to defective APC/T cell interaction. Front Immunol. 2015 Aug;6:433. doi: 10.3389/fimmu.2015.00433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kallikourdis M, Trovato AE, Anselmi F, Sarukhan A, Roselli G, Tassone L, Badolato R, Viola A. The CXCR4 mutations in WHIM syndrome impair the stability of the T-cell immunologic synapse. Blood. 2013 Aug;122:666–673. doi: 10.1182/blood-2012-10-461830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mc Guire PJ, Cunningham-Rundles C, Ochs H, Diaz GA. Oligoclonality, impaired class switch and B-cell memory responses in WHIM syndrome. Clin Immunol. 2010 Jun;135:412–421. doi: 10.1016/j.clim.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Agematsu K, Nagumo H, Shinozaki K, Hokibara S, Yasui K, Terada K, Kawamura N, Toba T, Nonoyama S, Ochs HD, Komiyama A. Absence of IgD-CD27(+) memory B cell population in X-linked hyper-IgM syndrome. J Clin Invest. 1998 Aug;102:853–860. doi: 10.1172/JCI3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Agematsu K, Futatani T, Hokibara S, Kobayashi N, Takamoto M, Tsukada S, Suzuki H, Koyasu S, Miyawaki T, Sugane K, Komiyama A, Ochs HD. Absence of memory B cells in patients with common variable immunodeficiency. Clin Immunol. 2002 Apr;103:34–42. doi: 10.1006/clim.2001.5197. [DOI] [PubMed] [Google Scholar]
- 49.Ma CS, Pittaluga S, Avery DT, Hare NJ, Maric I, Klion AD, Nichols KE, Tangye SG. Selective generation of functional somatically mutated IgM+CD27+, but not Ig isotype-switched, memory B cells in X-linked lymphoproliferative disease. J Clin Invest. 2006 Feb;116:322–333. doi: 10.1172/JCI25720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Murphy PM, McDermott DH. Unexpected developments in immune organs in WHIM syndrome. Blood. 2012 Jun;119:5610–5612. doi: 10.1182/blood-2012-04-420752. [DOI] [PubMed] [Google Scholar]
- 51.Chow KYC, Brotin É, Ben Khalifa Y, Carthagena L, Teissier S, Danckaert A, Galzi JL, Arenzana-Seisdedos F, Thierry F, Bachelerie F. A pivotal role for CXCL12 signaling in HPV-mediated transformation of keratinocytes: clues to understanding HPV-pathogenesis in WHIM syndrome. Cell Host Microbe. 2010 Dec;8:523–533. doi: 10.1016/j.chom.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 52.Meuris F, Carthagena L, Jaracz-Ros A, Gaudin F, Cutolo P, Deback C, Xue Y, Thierry F, Doorbar J, Bachelerie F. The CXCL12/CXCR4 signaling pathway: A new susceptibility factor in human papillomavirus pathogenesis. PLoS Pathog. 2016 Dec;12:e1006039. doi: 10.1371/journal.ppat.1006039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sasagawa T, Takagi H, Makinoda S. Immune responses against human papillomavirus (HPV) infection and evasion of host defense in cervical cancer. J Infect Chemother. 2012 Dec;18:807–815. doi: 10.1007/s10156-012-0485-5. [DOI] [PubMed] [Google Scholar]
- 54.Cella M, Jarrossay D, Facchetti F, Alebardi O, Nakajima H, Lanzavecchia A, Colonna M. Plasmacytoid monocytes migrate to inflamed lymph nodes and produce large amounts of type I interferon. Nat Med. 1999 Aug;5:919–923. doi: 10.1038/11360. [DOI] [PubMed] [Google Scholar]
- 55.Fitzgerald-Bocarsly P, Dai J, Singh S. Plasmacytoid dendritic cells and type I IFN: 50 years of convergent history. Cytokine Growth Factor Rev. 2008 Feb;19:3–19. doi: 10.1016/j.cytogfr.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tassone L, Moratto D, Vermi W, De Francesco M, Notarangelo LD, Porta F, Lougaris V, Facchetti F, Plebani A, Badolato R. Defect of plasmacytoid dendritic cells in warts, hypogammaglobulinemia, infections, myelokathexis (WHIM) syndrome patients. Blood. 2010 Dec;116:4870–4873. doi: 10.1182/blood-2010-03-272096. [DOI] [PubMed] [Google Scholar]
- 57*.Handisurya A, Schellenbacher C, Reininger B, Koszik F, Vyhnanek P, Heitger A, Kirnbauer R, Förster-Waldl E. A quadrivalent HPV vaccine induces humoral and cellular immune responses in WHIM immunodeficiency syndrome. Vaccine. 2010 Jul;28:4837–4841. doi: 10.1016/j.vaccine.2010.04.057. First and to date only report describing in detail the immune response to HPV vaccination in WHIM syndrome. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Huang X, Shen J, Cui M, Shen L, Luo X, Ling K, Pei G, Jiang H, Chen K. Molecular dynamics simulations on SDF-1alpha: binding with CXCR4 receptor. Biophys J. 2003 Jan;84:171–184. doi: 10.1016/S0006-3495(03)74840-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Busillo JM, Benovic JL. Regulation of CXCR4 signaling. Biochim Biophys Acta. 2007 Apr;1768:952–963. doi: 10.1016/j.bbamem.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cheng M, Huang K, Zhou J, Yan D, Tang YL, Zhao TC, Miller RJ, Kishore R, Losordo DW, Qin G. A critical role of Src family kinase in SDF-1/CXCR4-mediated bone-marrow progenitor cell recruitment to the ischemic heart. J Mol Cell Cardiol. 2015 Apr;81:49–53. doi: 10.1016/j.yjmcc.2015.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cao Y, Hunter ZR, Liu X, Xu L, Yang G, Chen J, Patterson CJ, Tsakmaklis N, Kanan S, Rodig S, Castillo JJ, Treon SP. The WHIM-like CXCR4(S338X) somatic mutation activates AKT and ERK, and promotes resistance to ibrutinib and other agents used in the treatment of Waldenstrom's Macroglobulinemia. Leukemia. 2015 Jan;29:169–176. doi: 10.1038/leu.2014.187. [DOI] [PubMed] [Google Scholar]
- 62.Kucia M, Jankowski K, Reca R, Wysoczynski M, Bandura L, Allendorf DJ, Zhang J, Ratajczak J, Ratajczak MZ. CXCR4-SDF-1 signalling, locomotion, chemotaxis and adhesion. J Mol Histol. 2004 Mar;35:233–245. doi: 10.1023/b:hijo.0000032355.66152.b8. [DOI] [PubMed] [Google Scholar]
- 63.Schiraldi M, Raucci A, Muñoz LM, Livoti E, Celona B, Venereau E, Apuzzo T, De Marchis F, Pedotti M, Bachi A, Thelen M, Varani L, Mellado M, Proudfoot A, Bianchi ME, Uguccioni M. HMGB1 promotes recruitment of inflammatory cells to damaged tissues by forming a complex with CXCL12 and signaling via CXCR4. J Exp Med. 2012 Mar;209:551–563. doi: 10.1084/jem.20111739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rajagopal S, Kim J, Ahn S, Craig S, Lam CM, Gerard NP, Gerard C, Lefkowitz RJ. Beta-arrestin- but not G protein-mediated signaling by the “decoy” receptor CXCR7. Proc Natl Acad Sci U S A. 2010 Jan;107:628–632. doi: 10.1073/pnas.0912852107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Boudot A, Kerdivel G, Habauzit D, Eeckhoute J, Le Dily F, Flouriot G, Samson M, Pakdel F. Differential estrogen-regulation of CXCL12 chemokine receptors, CXCR4 and CXCR7, contributes to the growth effect of estrogens in breast cancer cells. PLoS ONE. 2011 Jun;6:e20898. doi: 10.1371/journal.pone.0020898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Coggins NL, Trakimas D, Chang SL, Ehrlich A, Ray P, Luker KE, Linderman JJ, Luker GD. CXCR7 controls competition for recruitment of β-arrestin 2 in cells expressing both CXCR4 and CXCR7. PLoS ONE. 2014 Jun;9:e98328. doi: 10.1371/journal.pone.0098328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Décaillot FM, Kazmi MA, Lin Y, Ray-Saha S, Sakmar TP, Sachdev P. CXCR7/CXCR4 heterodimer constitutively recruits beta-arrestin to enhance cell migration. J Biol Chem. 2011 Sep;286:32188–32197. doi: 10.1074/jbc.M111.277038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rath D, Chatterjee M, Borst O, Müller K, Stellos K, Mack AF, Bongartz A, Bigalke B, Langer H, Schwab M, Gawaz M, Geisler T. Expression of stromal cell-derived factor-1 receptors CXCR4 and CXCR7 on circulating platelets of patients with acute coronary syndrome and association with left ventricular functional recovery. Eur Heart J. 2014 Feb;35:386–394. doi: 10.1093/eurheartj/eht448. [DOI] [PubMed] [Google Scholar]
- 69.Gómez-Moutón C, Fischer T, Peregil RM, Jiménez-Baranda S, Stossel TP, Nakamura F, Mañes S. Filamin A interaction with the CXCR4 third intracellular loop regulates endocytosis and signaling of WT and WHIM-like receptors. Blood. 2015 Feb;125:1116–1125. doi: 10.1182/blood-2014-09-601807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kawai T, Malech HL. WHIM syndrome: congenital immune deficiency disease. Curr Opin Hematol. 2009 Jan;16:20–26. doi: 10.1097/MOH.0b013e32831ac557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xu L, Hunter ZR, Tsakmaklis N, Cao Y, Yang G, Chen J, Liu X, Kanan S, Castillo JJ, Tai YT, Zehnder JL, Brown JR, Carrasco RD, Advani R, Sabile JM, Argyropoulos K, Lia Palomba M, Morra E, Trojani A, Greco A, Tedeschi A, Varettoni M, Arcaini L, Munshi NM, Anderson KC, Treon SP. Clonal architecture of CXCR4 WHIM-like mutations in Waldenström Macroglobulinaemia. Br J Haematol. 2016 Mar;172:735–744. doi: 10.1111/bjh.13897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72*.Balabanian K, Levoye A, Klemm L, Lagane B, Hermine O, Harriague J, Baleux F, Arenzana-Seisdedos F, Bachelerie F. Leukocyte analysis from WHIM syndrome patients reveals a pivotal role for GRK3 in CXCR4 signaling. J Clin Invest. 2008 Mar;118:1074–1084. doi: 10.1172/JCI33187. This paper demonstrates the role of GRK3 in CXCR4 signaling by describing two patients with wild-type CXCR4 but symptoms consistent with WHIM syndrome. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tarrant TK, Billard MJ, Timoshchenko RG, McGinnis MW, Serafin DS, Foreman O, Esserman DA, Chao NJ, Lento WE, Lee DM, Patel D, Siderovski DP. G protein-coupled receptor kinase-3-deficient mice exhibit WHIM syndrome features and attenuated inflammatory responses. J Leukoc Biol. 2013 Dec;94:1243–1251. doi: 10.1189/jlb.0213097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.McCormick PJ, Segarra M, Gasperini P, Gulino AV, Tosato G. Impaired recruitment of Grk6 and beta-Arrestin 2 causes delayed internalization and desensitization of a WHIM syndrome-associated CXCR4 mutant receptor. PLoS ONE. 2009 Dec;4:e8102. doi: 10.1371/journal.pone.0008102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vroon A, Heijnen CJ, Raatgever R, Touw IP, Ploemacher RE, Premont RT, Kavelaars A. GRK6 deficiency is associated with enhanced CXCR4-mediated neutrophil chemotaxis in vitro and impaired responsiveness to G-CSF in vivo. J Leukoc Biol. 2004 Apr;75:698–704. doi: 10.1189/jlb.0703320. [DOI] [PubMed] [Google Scholar]
- 76.Pozzobon T, Goldoni G, Viola A, Molon B. CXCR4 signaling in health and disease. Immunol Lett. 2016 Sep;177:6–15. doi: 10.1016/j.imlet.2016.06.006. [DOI] [PubMed] [Google Scholar]
- 77.Dale DC, Bonilla MA, Davis MW, Nakanishi AM, Hammond WP, Kurtzberg J, Wang W, Jakubowski A, Winton E, Lalezari P. A randomized controlled phase III trial of recombinant human granulocyte colony-stimulating factor (filgrastim) for treatment of severe chronic neutropenia. Blood. 1993 May;81:2496–2502. [PMC free article] [PubMed] [Google Scholar]
- 78.Favre O, Leimgruber A, Nicole A, Spertini F. Intravenous immunoglobulin replacement prevents severe and lower respiratory tract infections, but not upper respiratory tract and non-respiratory infections in common variable immune deficiency. Allergy. 2005 Mar;60:385–390. doi: 10.1111/j.1398-9995.2005.00756.x. [DOI] [PubMed] [Google Scholar]
- 79.McDermott DH, Liu Q, Ulrick J, Kwatemaa N, Anaya-O'Brien S, Penzak SR, Filho JO, Priel DAL, Kelly C, Garofalo M, Littel P, Marquesen MM, Hilligoss D, Decastro R, Fleisher TA, Kuhns DB, Malech HL, Murphy PM. The CXCR4 antagonist plerixafor corrects panleukopenia in patients with WHIM syndrome. Blood. 2011 Nov;118:4957–4962. doi: 10.1182/blood-2011-07-368084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80*.McDermott DH, Liu Q, Velez D, Lopez L, Anaya-O'Brien S, Ulrick J, Kwatemaa N, Starling J, Fleisher TA, Priel DAL, Merideth MA, Giuntoli RL, Evbuomwan MO, Littel P, Marquesen MM, Hilligoss D, DeCastro R, Grimes GJ, Hwang ST, Pittaluga S, Calvo KR, Stratton P, Cowen EW, Kuhns DB, Malech HL, Murphy PM. A phase 1 clinical trial of long-term, low-dose treatment of WHIM syndrome with the CXCR4 antagonist plerixafor. Blood. 2014 Apr;123:2308–2316. doi: 10.1182/blood-2013-09-527226. Preliminary data supporting the use of long-term low-dose plerixafor for WHIM syndrome. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bock I, Dugué F, Loppinet E, Bellanné-Chantelot C, Bénet B. [WHIM syndrome: presumptive diagnosis based on myelokathexis on bone marrow smear] Ann Biol Clin (Paris) 2014 Feb;72:111–119. doi: 10.1684/abc.2013.0928. [DOI] [PubMed] [Google Scholar]
- 82.Hess U, Ganser A, Schnürch HG, Seipelt G, Ottmann OG, Falk S, Schulz G, Hoelzer D. Myelokathexis treated with recombinant human granulocyte-macrophage colony-stimulating factor (rhGM-CSF) Br J Haematol. 1992 Feb;80:254–256. doi: 10.1111/j.1365-2141.1992.tb08910.x. [DOI] [PubMed] [Google Scholar]
- 83.Mehta HM, Malandra M, Corey SJ. G-CSF and GM-CSF in Neutropenia. J Immunol. 2015 Aug;195:1341–1349. doi: 10.4049/jimmunol.1500861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84**.Donzella GA, Schols D, Lin SW, Esté JA, Nagashima KA, Maddon PJ, Allaway GP, Sakmar TP, Henson G, De Clercq E, Moore JP. AMD3100, a small molecule inhibitor of HIV-1 entry via the CXCR4 co-receptor. Nat Med. 1998 Jan;4:72–77. doi: 10.1038/nm0198-072. Detailed description of the CXCR4 antagonist AMD3100/plerixafor. [DOI] [PubMed] [Google Scholar]
- 85.Hendrix CW, Flexner C, MacFarland RT, Giandomenico C, Fuchs EJ, Redpath E, Bridger G, Henson GW. Pharmacokinetics and safety of AMD-3100, a novel antagonist of the CXCR-4 chemokine receptor, in human volunteers. Antimicrob Agents Chemother. 2000 Jun;44:1667–1673. doi: 10.1128/aac.44.6.1667-1673.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liles WC, Broxmeyer HE, Rodger E, Wood B, Hübel K, Cooper S, Hangoc G, Bridger GJ, Henson GW, Calandra G, Dale DC. Mobilization of hematopoietic progenitor cells in healthy volunteers by AMD3100, a CXCR4 antagonist. Blood. 2003 Oct;102:2728–2730. doi: 10.1182/blood-2003-02-0663. [DOI] [PubMed] [Google Scholar]
- 87.DiPersio JF, Micallef IN, Stiff PJ, Bolwell BJ, Maziarz RT, Jacobsen E, Nademanee A, McCarty J, Bridger G, Calandra G 3101 Investigators. Phase III prospective randomized double-blind placebo-controlled trial of plerixafor plus granulocyte colony-stimulating factor compared with placebo plus granulocyte colony-stimulating factor for autologous stem-cell mobilization and transplantation for patients with non-Hodgkin's lymphoma. J Clin Oncol. 2009 Oct;27:4767–4773. doi: 10.1200/JCO.2008.20.7209. [DOI] [PubMed] [Google Scholar]
- 88.DiPersio JF, Stadtmauer EA, Nademanee A, Micallef INM, Stiff PJ, Kaufman JL, Maziarz RT, Hosing C, Früehauf S, Horwitz M, Cooper D, Bridger G, Calandra G 3102 Investigators. Plerixafor and G-CSF versus placebo and G-CSF to mobilize hematopoietic stem cells for autologous stem cell transplantation in patients with multiple myeloma. Blood. 2009 Jun;113:5720–5726. doi: 10.1182/blood-2008-08-174946. [DOI] [PubMed] [Google Scholar]
- 89.Kalatskaya I, Berchiche YA, Gravel S, Limberg BJ, Rosenbaum JS, Heveker N. AMD3100 is a CXCR7 ligand with allosteric agonist properties. Mol Pharmacol. 2009 May;75:1240–1247. doi: 10.1124/mol.108.053389. [DOI] [PubMed] [Google Scholar]
- 90.Dale DC, Bolyard AA, Kelley ML, Westrup EC, Makaryan V, Aprikyan A, Wood B, Hsu FJ. The CXCR4 antagonist plerixafor is a potential therapy for myelokathexis, WHIM syndrome. Blood. 2011 Nov;118:4963–4966. doi: 10.1182/blood-2011-06-360586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Moyle G, DeJesus E, Boffito M, Wong RS, Gibney C, Badel K, MacFarland R, Calandra G, Bridger G, Becker S X4 Antagonist Concept Trial Study Team. Proof of activity with AMD11070, an orally bioavailable inhibitor of CXCR4-tropic HIV type 1. Clin Infect Dis. 2009 Mar;48:798–805. doi: 10.1086/597097. [DOI] [PubMed] [Google Scholar]
- 92.O'Regan S, Newman AJ, Graham RC. “Myelokathexis”. Neutropenia with marrow hyperplasia. Am J Dis Child. 1977 Jun;131:655–658. doi: 10.1001/archpedi.1977.02120190049011. [DOI] [PubMed] [Google Scholar]
- 93.Weston B, Axtell RA, Todd RF, Vincent M, Balazovich KJ, Suchard SJ, Boxer LA. Clinical and biologic effects of granulocyte colony stimulating factor in the treatment of myelokathexis. J Pediatr. 1991 Feb;118:229–234. doi: 10.1016/s0022-3476(05)80488-3. [DOI] [PubMed] [Google Scholar]
- 94.Nagasawa T, Kikutani H, Kishimoto T. Molecular cloning and structure of a pre-B-cell growth-stimulating factor. Proc Natl Acad Sci U S A. 1994 Mar;91:2305–2309. doi: 10.1073/pnas.91.6.2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Feng Y, Broder CC, Kennedy PE, Berger EA. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science. 1996 May;272:872–877. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- 96.Bleul CC, Farzan M, Choe H, Parolin C, Clark-Lewis I, Sodroski J, Springer TA. The lymphocyte chemoattractant SDF-1 is a ligand for LESTR/fusin and blocks HIV-1 entry. Nature. 1996 Aug;382:829–833. doi: 10.1038/382829a0. [DOI] [PubMed] [Google Scholar]
- 97.Nagasawa T, Hirota S, Tachibana K, Takakura N, Nishikawa S, Kitamura Y, Yoshida N, Kikutani H, Kishimoto T. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature. 1996 Aug;382:635–638. doi: 10.1038/382635a0. [DOI] [PubMed] [Google Scholar]
- 98.Zou YR, Kottmann AH, Kuroda M, Taniuchi I, Littman DR. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature. 1998 Jun;393:595–599. doi: 10.1038/31269. [DOI] [PubMed] [Google Scholar]
- 99.Peled A, Petit I, Kollet O, Magid M, Ponomaryov T, Byk T, Nagler A, Ben-Hur H, Many A, Shultz L, Lider O, Alon R, Zipori D, Lapidot T. Dependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4. Science. 1999 Feb;283:845–848. doi: 10.1126/science.283.5403.845. [DOI] [PubMed] [Google Scholar]
- 100.Hunter ZR, Xu L, Yang G, Zhou Y, Liu X, Cao Y, Manning RJ, Tripsas C, Patterson CJ, Sheehy P, Treon SP. The genomic landscape of Waldenstrom macroglobulinemia is characterized by highly recurring MYD88 and WHIM-like CXCR4 mutations, and small somatic deletions associated with B-cell lymphomagenesis. Blood. 2014 Mar;123:1637–1646. doi: 10.1182/blood-2013-09-525808. [DOI] [PubMed] [Google Scholar]


