Abstract
The development of resources for clinical interpretation of cancer-associated genetic alterations has significantly lagged behind the technical developments enabling their detection in a time-and cost-efficient manner. The lack of scientific and informatics decision support for oncologists can lead to no action being taken or suboptimal therapeutic choices being made, which could affect the clinical outcome of a patient as well as convoluting research findings from clinical trials. In this article, we describe the precision oncology decision support (PODS) platform developed within The Sheikh Khalifa Bin Zayed Al Nahyan Institute for Personalized Cancer Therapy (IPCT) at MD Anderson Cancer Center; the platform aims to bridge the gap between molecular alteration detection and identification of appropriate treatments.
Keywords: Precision oncology, decision support, genomics, personalized medicine
Introduction
Precision oncology, or personalized cancer medicine, is based on the observation that cancers, even within the same disease site, are driven by a multitude of different molecular aberrations. Under this paradigm, physicians seek to define these alterations and tailor therapy to target the functional consequences of the aberrations specifically. Clinical trials currently exploring whether targeted therapy is more advantageous than standard-of-care across tumor types include the SHIVA clinical trial at the Institut Curie [1], the IMPACT 2 clinical trial at MD Anderson Cancer Center and NCI-MPACT (Molecular Profiling-Based Assignment of Cancer Therapy for Patients with Advanced Solid Tumors). However, precision oncology has already been applied and proven beneficial in several specific tumor types. For example, it is standard of care to treat HER2+ metastatic breast cancers with an FDA-approved HER2-targeting agent [2], because HER2 amplification and overexpression are proven to be predictive of response [3,4]. However, there is not an FDA-approved therapy indicated for the vast majority of molecular alterations. Alternatively, physicians might choose to treat the patient in an experimental setting, but several questions must be addressed beforehand. (i) Is the specific alteration actionable? (ii) If actionable, what are the genotype-matched therapeutic options targeting the aberration or the pathway activated by the aberration? (iii) What is the level of evidence for each drug in the context of the patient’s tumor type? (iv) Are the agents available and where are they from? There are tremendous challenges associated with each of these questions as a result of physician time constraints and limitations in genomic knowledge that are not realistic for a practicing oncologist to overcome without ongoing support from geneticists, molecular biologists, computational scientists, computer programmers and bioinformaticians [5]. Thus, decision-support platforms, such as the precision oncology decision support (PODS) team within the Sheikh Khalifa Bin Zayed Al Nahyan Institute for Personalized Cancer Therapy (IPCT) at the MD Anderson Cancer Center, and others [6], are being developed to support this approach. In this paper, we present the PODS approach for discerning which genomic alterations are actionable, in which tumor types, for which drugs and how this information is communicated to oncologists. There are several different types of biomarkers applicable for decision support. However, next-generation sequencing (NGS) is most widely used because it is timely and cost-efficient [7,8]. Thus, the PODS team has first focused efforts on decision-support related to NGS.
Determining whether a specific alteration is actionable
Once a physician receives a molecular testing report from a Clinical Laboratory Improvement Amendments (CLIA)-certified laboratory, he or she must decide if a detected alteration is actionable (i.e. could actions based on the aberration benefit the patient). If DNA is sequenced from a matched normal sample, a mutation can be confidently determined as somatic or germline. Identified germline alterations should be reviewed as there are different considerations for determining the actionability of these alterations and return of these results with the support of genetic counseling. In this paper, we focus our discussion on the actionability of somatic mutations. Alterations can be considered actionable because they are diagnostic, prognostic or predictive of drug response; however, the primary objective of the PODS team is to determine whether alterations are actionable with regard to therapeutic drug response. The PODS team reviewed over 524 genes routinely tested in clinical samples to determine whether there is a clinically available drug that directly or indirectly targets alterations in the gene; and whether there is (as a basic minimum) preclinical evidence showing that genomic alterations in the gene are drivers of tumorigenesis and confer sensitivity to targeted agents. Genes that meet both of these criteria and/or are used as selection criteria for clinical trial enrolment qualify as actionable – a full list is available from Meric-Bernstam et al. [9]. Our list is comparable with the recently published TARGET actionable gene list [10] for those genes deemed therapeutically actionable. However, we supplemented our list with genes where alterations are being selected for in clinical trials and have not focused on genes actionable for diagnostic or prognostic purposes only. In the PODS actionable list, we provide the types of alterations that are actionable for each gene. For example, fibroblast growth factor receptor (FGFR)1 is actionable for activating mutations, gene amplification and activating gene fusions [11,12]. However, there is only evidence to support actionability of MLL gene fusions [13–15], not mutations or copy number changes, at this time.
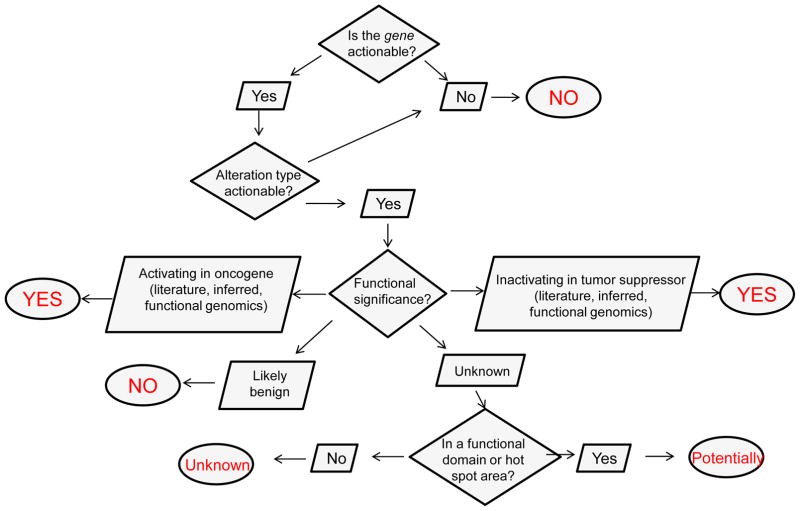
Once the gene and alteration type are determined to be actionable, the variant (i.e. specific mutation or fusion event) itself must be considered. To facilitate clinical decision making, we provide a granular classification of variant actionability that takes into consideration what is known in the published literature, our in-house functional genomics platform, as well as information about the localization of the mutation within the gene, which might be suggestive of actionability. Thus, we have created the following classifications for variant actionability: ‘yes –literature based’, ‘yes – functional genomics’, ‘yes – inferred’, ‘potentially’, ‘unknown’ and ‘no’ (Box 1). The actionable variant calls are directly related to the functional significance of the variant (Figure 1). A variant is functionally significant if it results in a change in the activity, expression or stability of the expressed protein. The PODS team systematically categorizes the functional significance of each variant as ‘activating’, ‘inferred activating’, ‘inactivating’, ‘inferred ‘inactivating’, ‘likely benign’ or ‘unknown’, based on manually curated information (Box 2). A variant is classified as actionable if the functional effect is activating or inferred activating in an oncogene or inactivating or inferred inactivating in a tumor suppressor. Variants with published evidence that they probably do not alter function (i.e. likely benign) are considered not actionable. The IPCT also has an experimental arm, which tests for an alteration’s effect on the growth and/or viability of BaF3 and MCF10A cells. However, there are no data currently available regarding the functional significance of a specific aberration within the published literature or IPCT functional genomics platform. Nevertheless, knowledge of the variant location within the gene and relationship to known functional aberrations can provide some insight. We utilize this information to separate those mutations of unknown functional significance into two categories of actionability: ‘potentially’ or ‘unknown’. Currently, functional predictions based on computational tools are not used to define actionability owing to limitations in accuracy and agreement across currently available platforms [16]. We are iteratively building, validating and improving algorithms in house, however, these are not sufficiently robust for patient management [17].
Box 1. Categories of actionable variant.
| YES: literature based | There are peer-reviewed published data that shows the alteration is activating and occurs in an oncogene or is inactivating and occurs in a tumor suppressor. |
| YES: inferred | The functional significance is inferred to be activating in an oncogene or inferred to be inactivating in a tumor suppressor. Additionally, gene amplification that has not been proven in the published literature to result in increased protein expression and signaling in tumors is categorized as inferred activating. |
| YES: functional genomics | The genetic alteration shows a growth or viability advantage when compared with cells expressing the wild-type counterpart of the gene, as assessed in a functional genomics platform utilizing cells such as BaF3 or MCF10A. |
| Potentially | The alteration is of unknown functional significance but is potentially actionable because it: (i) is located within a functional domain; (ii) occurs in a hot-spot area of known oncogenic function; or (iii) other alterations of the same codon are oncogenic. |
| Unknown | The functional significance is unknown and the alteration is not located within a functional domain or in near proximity to other functional alterations. |
| No | (i) The gene is not defined as actionable; (ii) the functional significance is determined to be ‘likely benign’; (iii) peer-reviewed published literature shows the alteration does not have an effect on the function or tumorigenic properties of the protein; or (iv) there is peer-reviewed published literature showing that the alteration has an inactivating effect on an oncogene. |
Figure 1.
Flowchart for deciphering whether the genetic variant is actionable. This flowchart represents the questions asked (diamonds) and possible information determined (parallelograms) to reach an answer (circles) regarding whether a genetic alteration or variant is actionable.
Box 2. Categories of functional significance.
| Activating | There is peer-reviewed published evidence or evidence from a functional genomics platform that the genetic alteration increases the activity, expression or stability of the encoded protein. If the gene is an oncogene, evidence that the mutation results in increased tumorigenic properties (enhanced proliferation, survival, etc.) is also sufficient. |
| Inferred activating | The alteration results in the loss or mistranslation of an inhibitory domain, and there is peer-reviewed published evidence that loss of the domain increases the activity of the protein and/or results in increased tumorigenic properties. Additionally, gene amplification that has not been proven in the published literature to result in increased protein expression and signaling in tumors is categorized as inferred activating. |
| Inactivating | There is peer-reviewed published evidence or evidence from a functional genomics platform that the mutation decreases the activity, expression or stability of the encoded protein. If the gene is a tumor suppressor, evidence that the mutation results in increased oncogenic activity is also sufficient. |
| Inferred inactivating | The alteration results in loss or mistranslation of functionally significant domains, and there is peer-reviewed published evidence that loss of these domain(s) results in loss of the expression or activity of the encoded protein and/or increased tumorigenic properties. |
| Likely benign | An alteration is characterized as likely benign if: (i) it is reported as a germline polymorphism in population studies, and there are no available data indicating this variant may be tumor-promoting; or (ii) there is peer-reviewed published evidence that the alteration has no effect on the function of the protein or any tumorigenic properties. Because not all aspects of protein function can possibly be determined, the functional significance is ‘likely benign’. |
| Unknown | There is no peer-reviewed published evidence or no evidence from a functional genomics platform that the mutation affects the activity or stability of the encoded protein or has a direct role in promoting tumorigenesis. |
Providing clinicians with information regarding variant actionability is extremely useful for clinical trial patient selection. For example, the interpretation of results derived from a trial designed to test the hypothesis that FGFR inhibitors are effective for tumors expressing alterations in FGFR genes will be complicated by the inclusion of patients with mutations known or predicted to not result in activation of the FGFR pathway. Although there are public databases that provide variant-level information about published drug associations, such as My Cancer Genome (http://www.mycancergenome.org/) or Targeted Cancer Care (https://targetedcancercare.massgeneral.org/), information about the functional effect of an alteration is often lacking and is limited to more-frequently detected alterations. In the absence of decision support, clinicians would be left to filter through millions of abstracts available on PubMed or publicly available literature accessible through internet browsers, via keyword searches that are often inefficient at procuring a complete set of literature of high relevance for their review. Thus, the PODS platform provides support to the physician by extracting, curating and communicating information about a variant’s actionability back to the physician.
Determining clinically available therapeutic options
If a variant is deemed actionable or potentially actionable, genotype-matched therapies for consideration are presented to the treating oncologist. To accomplish this, drugs are annotated for their direct targets and relevant genes. Direct targets are proteins directly bound and inhibited by the drug (IC50<1 μM), and the genes encoding them are annotated as relevant for the drug. Additionally, indirect targets are considered if there is published evidence that mutations in the gene sensitize cells to downstream targeting drugs. For example, there is evidence that mutations in the phosphatase and tensin homolog (PTEN) gene sensitize cells to mammalian target of rapamycin (mTOR) inhibitors [18,19]. Thus, mTOR is an indirect target of PTEN mutations, and PTEN is annotated as a relevant gene for all mTOR inhibitors. Information is retrieved by searching the published literature, as well as drug–gene interaction databases, such as the Druggable Genome Interaction Database (http://dgidb.genome.wustl.edu/) and other publicly available sources, such as NCI Drug Dictionary (http://www.cancer.gov/drugdictionary). As with other literature searches, these are extensively manually curated prior to use by PODS.
Determining the level of evidence for use
Once potential therapeutic options are identified, the level of evidence for use in an alteration-and tumor-type-specific context is considered. There is an extremely limited number of genotypes associated with an FDA-approved indication for treatment [20]. If there is no FDA-approved drug indicated for the patient’s tumor type and specific alteration, then the physician could choose to: (i) use an FDA-approved drug off-label (targeting the same alteration approved in another tumor type or to target a different variant thought to have the same functional effect; (ii) use an FDA-approved drug within the context of a clinical trial designed to test the use and efficacy of the drug in different tumor types or on other variants of the same gene; or (iii) use experimental therapies through clinical trials. Off-label use is generally discouraged except in scenarios where there are strong clinical data showing that the alteration sensitizes cells of diverse tumor types to the drug [20]. We have advocated that all off-label use and responses should be captured in a registry to increase the utility of the process. Ultimately, creation of larger international data-sharing initiatives could facilitate sharing of clinical outcome information for drugs used in experimental settings in an international database. To facilitate decision making, the PODS team developed a three-tiered scale that considers the level of evidence for use of a drug in the context of a biomarker and tumor type, as described by Meric-Bernstam et al. [9]. Level 1A associations are for drugs with an FDA-approved indication to treat tumors with a specific alteration within specific tumor types. The lower levels of evidence are based on experimental (clinical or preclinical) data. The PODS level of evidence scheme is complimentary to other proposed schemes [10,16,21–23]. It takes into account whether the utility of the biomarker was assessed prospectively or retrospectively, as a primary or secondary objective, and separates clinical data in adequately powered studies from case studies and preclinical data. However, our scheme is primarily focused on the predictive value of the alteration to the therapy or effectiveness of a drug within a biomarker-selected cohort, as opposed to prognostic or diagnostic use of biomarkers, and it provides fine granularity of the evidence for links between specific aberrations and experimental therapies. The level of evidence is important for review when considering multiple drugs for a particular alteration and when considering which from multiple alterations to target. Additionally, our scheme considers genotypes that confer resistance to a therapy. For example, KRAS mutations confer resistance to anti-epidermal growth factor receptor (EGFR) therapies in colon cancer [24,25], thus colon cancer patients harboring activating KRAS mutations should not be treated with these therapies outside of clinical trials assessing ways to bypass the effects of KRAS. Thus, it is crucial to consider the therapeutic implications of alterations in the context of one another.
Often there is no evidence at the level of a specific variant. For example, a single-patient case study shows a non-small-cell carcinoma (NSCLC) with a HER2 A775_G776insYVMA mutation responds to afatinib therapy (level 3A evidence) [26]; however, there are no studies showing sensitivity of NSCLC tumors harboring another activating HER2 mutation, D769H [27], specifically to afatinib. Thus, to capture these differences in specificity of evidence, the PODS annotation scheme captures four tiers of evidence: gene, functional class (activating or inactivating), alteration type (mutations, copy number changes and gene fusions) and variant.
Another aspect that clinicians could consider when deciding whether to act on a variant is its frequency among mutations detected in cancer. Informatics tools developed by PODS automatically extract frequency information for all variants reported in cBIO (http://www.cbioportal.org/), Catalogue of Somatic Mutations in Cancer (COSMIC; http://cancer.sanger.ac.uk/cosmic/) or MD Anderson sequencing platforms. A more frequently reported alteration might be considered more likely to be a driver of tumorigenesis [28]. Additionally, the frequency of alleles within the tumor sample sequenced (allelic frequency) could also be used to determine the likelihood that an alteration is a driver event and for prioritizing which of multiple alterations to pursue [29,30]. If a particular drug can target multiple alterations detected in a patient’s tumor this strengthens the logic for the use of that therapeutic agent.
Determining the availability of genotype-matched therapies
If there is no FDA-approved drug indicated for the patient’s alteration and tumor type then a physician could consider an experimental therapy within a clinical trial setting. It can be difficult and time consuming to identify all of the genotype-matched clinical trials appropriate for a patient. This is partly because there is no structured format for recording genotypes that are being selected for or that are relevant within registered clinical trial documents. Thus, the PODS team systematically curates clinical trials for their relevance to specific genotypes. We categorize clinical trials in two broad categories: genotype-selected and genotype-relevant. Genotype-selected trials require detection of a particular biomarker for patient enrolment. Genotype-relevant trials use drugs that are annotated by PODS as relevant for alterations in specific genes. Because the vast majority of clinical trials are not genotype-selected trials, this process dramatically increases the number of trials for consideration compared with a keyword search for a gene within clinical trial registries. Additionally, we are currently in the process of creating an interactive clinical trial portal where dynamic logistical data, such as trial status, number of open slots, currently accepted tumor types and granular eligibility criteria (performance status, brain/CNS metastasis allowance, etc.) are captured, in addition to manually curated, genotype-relevant information. The portal is queryable by clinicians for the purpose of identifying open clinical trials that best match a patient’s molecular and clinical profile.
Communicating information between decision support and clinicians
The PODS team is developing several methods for communicating curated information to clinicians. Our clinical trial portal is one means by which physicians can proactively search for trials that are a genotype match for their patients. Additionally, we provide proactive email alerts of genotype-matched clinical trials available at MD Anderson Cancer Center to physicians that receive molecular testing for their patients through IPCT. These methods facilitate identification of clinical trials once it is determined that an alteration is actionable. To supply clinicians with a convenient means of requesting annotations of alterations for the purpose of determining actionability, we have created a web-based form where requests are entered in a standardized manner. Annotations are provided that include the functional significance, actionable gene and actionable variant calls previously described in addition to a detailed descriptive annotation of the information, with references, that was used to make those decisions. More detailed reports, including curated drug information and level of evidence, are being designed.
After annotations are provided, a clinical decision follow-up questionnaire is sent to the requester, enabling the PODS team to capture which alterations were indeed acted on by the physician and the therapy chosen. This is powerful information for retrospective studies analyzing the functional effect of an alteration and patient response to a particular therapy. Additionally, the PODS team routinely submits any alteration requested by a clinician for annotation and deemed ‘unknown’ for functional significance to the functional genomics platform. When results are available, the information is communicated to the requesting clinician. Lastly, we have created an open-access Website: Personalized Cancer Therapy (https://pct.mdanderson.org/), which currently provides information regarding over 24 actionable genes. Each gene page discusses genetic alterations deemed functionally or therapeutically significant, relevant drugs, level of evidence associated with each drug in genotype and tumor type-context, and genotype-matched clinical trials available at MD Anderson and elsewhere. Importantly the data underlying the portal are extensively curated by domain experts as well as by clinicians actively involved in the management of appropriate clinical trials. This is an expanding website, with new gene pages added on a continuous basis and information routinely updated.
Concluding remarks
The PODS platform provides clinical decision support for precision oncologists within MD Anderson by providing them with easily accessible means to obtain scientifically curated information about the functional effects of genetic alterations, genotype-matched therapeutics and genotype-matched clinical trials that are relevant for their patients. Personalized Cancer Therapy (https://pct.mdanderson.org/) makes aspects of the process available to all physicians and indeed is being used to drive clinical trial designs elsewhere. We believe the future implementation of an electronic health record system with structured fields for detected molecular alterations as well as enforcement of clinical trial registries with the same structured information is necessary to enable a routine delivery of precision oncology. Delivering on the promise of precision oncology will require a strong, user friendly and rapidly adapting decision support process. Precision oncology is truly a team approach that must incorporate expert information from clinicians, computational scientists, bioinformaticians, computer programmers and biological scientists [5,9,16,31,32]. The PODS platform brings these experts together to improve patient care and enhance research efforts.
Highlights.
Precision oncology matches tumor molecular alterations with appropriate therapies
Decision support platforms are crucial for optimal and scalable implementation
Actionability of specific variants, as well as specific genes, should be assessed
Level of evidence should be considered for each alteration and therapy considered
Clinical outcome registries for drugs used in experimental settings are suggested
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Le Tourneau C, et al. Randomised proof-of-concept Phase II trial comparing targeted therapy based on tumour molecular profiling vs conventional therapy in patients with refractory cancer: results of the feasibility part of the SHIVA trial. Br J Cancer. 2014;111:17–24. doi: 10.1038/bjc.2014.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wolff AC, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol. 2013;31:997–4013. doi: 10.1200/JCO.2013.50.9984. [DOI] [PubMed] [Google Scholar]
- 3.Smith I, et al. 2-year follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer: a randomised controlled trial. Lancet. 2007;369:29–36. doi: 10.1016/S0140-6736(07)60028-2. [DOI] [PubMed] [Google Scholar]
- 4.Slamon DJ, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 5.Welch BM, Kawamoto K. The need for clinical decision support integrated with the electronic health record for the clinical application of whole genome sequencing information. J Pers Med. 2013;3:306–325. doi: 10.3390/jpm3040306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Servant N, et al. Bioinformatics for precision medicine in oncology: principles and application to the SHIVA clinical trial. Front Genet. 2014;5:152. doi: 10.3389/fgene.2014.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sboner A, et al. The real cost of sequencing: higher than you think! Genome Biol. 2011;12:125. doi: 10.1186/gb-2011-12-8-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tran B, et al. Cancer genomics: technology, discovery, and translation. J Clin Oncol. 2012;30:647–660. doi: 10.1200/JCO.2011.39.2316. [DOI] [PubMed] [Google Scholar]
- 9.Meric-Bernstam F, et al. A decision support framework for genomically-informed investigational cancer therapy. J Natl Cancer Inst. 2015:107. doi: 10.1093/jnci/djv098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Allen EM, et al. Whole-exome sequencing and clinical interpretation of formalin-fixed, paraffin-embedded tumor samples to guide precision cancer medicine. Nat Med. 2014;20:682–688. doi: 10.1038/nm.3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greulich H, Pollock PM. Targeting mutant fibroblast growth factor receptors in cancer. Trends Mol Med. 2011;17:283–292. doi: 10.1016/j.molmed.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Turner N, Grose R. Fibroblast growth factor signalling: from development to cancer. Nat Rev Cancer. 2010;10:116–129. doi: 10.1038/nrc2780. [DOI] [PubMed] [Google Scholar]
- 13.Kampen KR, et al. Insights in dynamic kinome reprogramming as a consequence of MEK inhibition in MLL-rearranged AML. Leukemia. 2014;28:589–599. doi: 10.1038/leu.2013.342. [DOI] [PubMed] [Google Scholar]
- 14.Chen YP, et al. CDKN1A-mediated responsiveness of MLL-AF4-positive acute lymphoblastic leukemia to Aurora kinase-A inhibitors. Int J Cancer. 2014;135:751–762. doi: 10.1002/ijc.28708. [DOI] [PubMed] [Google Scholar]
- 15.Daigle SR, et al. Potent inhibition of DOT1L as treatment of MLL-fusion leukemia. Blood. 2013;122:1017–1025. doi: 10.1182/blood-2013-04-497644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dienstmann R, et al. Standardized decision support in next generation sequencing reports of somatic cancer variants. Mol Oncol. 2014;8:859–873. doi: 10.1016/j.molonc.2014.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mao Y, et al. CanDrA: cancer-specific driver missense mutation annotation with optimized features. PLoS One. 2013;8:e77945. doi: 10.1371/journal.pone.0077945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Di Nicolantonio F, et al. Deregulation of the PI3K and KRAS signaling pathways in human cancer cells determines their response to everolimus. J Clin Invest. 2010;120:2858–2866. doi: 10.1172/JCI37539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Templeton AJ, et al. Phase 2 trial of single-agent everolimus in chemotherapy-naive patients with castration-resistant prostate cancer (SAKK 08/08) Eur Urol. 2013;64:150–158. doi: 10.1016/j.eururo.2013.03.040. [DOI] [PubMed] [Google Scholar]
- 20.Bailey AM, et al. Implementation of biomarker-driven cancer therapy: existing tools and remaining gaps. Discov Med. 2014;17:101–114. [PMC free article] [PubMed] [Google Scholar]
- 21.Wagle N, et al. High-throughput detection of actionable genomic alterations in clinical tumor samples by targeted, massively parallel sequencing. Cancer Discov. 2012;2:82–93. doi: 10.1158/2159-8290.CD-11-0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Febbo PG, et al. NCCN Task Force report: evaluating the clinical utility of tumor markers in oncology. J Natl Compr Canc Netw. 2011;9:S1–32. doi: 10.6004/jnccn.2011.0137. [DOI] [PubMed] [Google Scholar]
- 23.MacConaill LE, et al. Prospective enterprise-level molecular genotyping of a cohort of cancer patients. J Mol Diagn. 2014;16:660–672. doi: 10.1016/j.jmoldx.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heinemann V, et al. Clinical relevance of EGFR- and KRAS-status in colorectal cancer patients treated with monoclonal antibodies directed against the EGFR. Cancer Treat Rev. 2009;35:262–271. doi: 10.1016/j.ctrv.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 25.Linardou H, et al. All about KRAS for clinical oncology practice: gene profile, clinical implications and laboratory recommendations for somatic mutational testing in colorectal cancer. Cancer Treat Rev. 2011;37:221–233. doi: 10.1016/j.ctrv.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 26.Mazieres J, et al. Lung cancer that harbors an HER2 mutation: epidemiologic characteristics and therapeutic perspectives. J Clin Oncol. 2013;31:1997–2003. doi: 10.1200/JCO.2012.45.6095. [DOI] [PubMed] [Google Scholar]
- 27.Bose R, et al. Activating HER2 mutations in HER2 gene amplification negative breast cancer. Cancer Discov. 2013;3:224–237. doi: 10.1158/2159-8290.CD-12-0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vandin F, et al. De novo discovery of mutated driver pathways in cancer. Genome Res. 2012;22:375–385. doi: 10.1101/gr.120477.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang L, Wheeler DA. Genomic sequencing for cancer diagnosis and therapy. Annu Rev Med. 2014;65:33–48. doi: 10.1146/annurev-med-120811-171056. [DOI] [PubMed] [Google Scholar]
- 30.Chen K, et al. Clinical actionability enhanced through deep targeted sequencing of solid tumors. Clin Chem. 2015;61:544–553. doi: 10.1373/clinchem.2014.231100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carney PH. Information technology and precision medicine. Semin Oncol Nurs. 2014;30:124–129. doi: 10.1016/j.soncn.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 32.Huser V, et al. Developing genomic knowledge bases and databases to support clinical management: current perspectives. Pharmgenomics Pers Med. 2014;7:275–283. doi: 10.2147/PGPM.S49904. [DOI] [PMC free article] [PubMed] [Google Scholar]