Abstract
Purpose
To determine the sensitivity and specificity of optical coherence tomography angiography (OCTA) in the detection of choroidal neovascularization (CNV) in age-related macular degeneration (AMD).
Design
Prospective case series.
Subjects
Prospective series of seventy-two eyes were studied, which included eyes with treatment-naive CNV due to AMD, non-neovascular AMD, and normal controls.
Methods
All eyes underwent OCTA with a spectral domain (SD) OCT (Optovue, Inc.). The 3D angiogram was segmented into separate en face views including the inner retinal angiogram, outer retinal angiogram, and choriocapillaris angiogram. Detection of abnormal flow in the outer retina served as candidate CNV with OCTA. Masked graders reviewed structural OCT alone, en face OCTA alone, and en face OCTA combined with cross-sectional OCTA for the presence of CNV.
Main Outcome Measure
The sensitivity and specificity of CNV detection compared to the gold standard of fluorescein angiography (FA) and OCT was determined for structural SD-OCT alone, en face OCTA alone, and with en face OCTA combined with cross-sectional OCTA.
Results
Of 32 eyes with CNV, both graders identified 26 true positives with en face OCTA alone, resulting in a sensitivity of 81.3%. Four of the 6 false negatives had large subretinal hemorrhage (SRH) and sensitivity improved to 94% for both graders if eyes with SRH were excluded. The addition of cross-sectional OCTA along with en face OCTA improved the sensitivity to 100% for both graders. Structural OCT alone also had a sensitivity of 100%. The specificity of en face OCTA alone was 92.5% for grader A and 97.5% for grader B. The specificity of structural OCT alone was 97.5% for grader A and 85% for grader B. Cross-sectional OCTA combined with en face OCTA had a specificity of 97.5% for grader A and 100% for grader B.
Conclusions
Sensitivity and specificity for CNV detection with en face OCTA combined with cross-sectional OCTA approaches that of the gold standard of FA with OCT, and it is better than en face OCTA alone. Structural OCT alone has excellent sensitivity for CNV detection. False positives from structural OCT can be mitigated with the addition of flow information with OCTA.
Introduction
Age-related macular degeneration (AMD) is a leading cause of irreversible vision loss in the United States.1 The majority of vision loss in AMD is due to the development of choroidal neovascularization (CNV), characterized by exudation of blood, lipid, or fluid into the sub-retinal or sub-retinal pigment epithelium space.2–3 The current gold standard for the diagnosis of neovascular AMD is the presence of CNV demonstrated by dye leakage on fluorescein angiography (FA).4–5 Nearly universally in clinical practice today, FA is combined with structural optical coherence tomography (OCT) to diagnose neovascular AMD.6–7 FA identifies only non-specific leakage rather than the choroidal neovascular lesion itself. Indocyanine green angiography (ICGA) is another useful imaging modality; however, both ICGA and FA are invasive and may be associated with nausea, urticaria, and rarely anaphylaxis.8–10
With the recent advent of optical coherence tomography angiography (OCTA), non-invasive, safe, and rapid image acquisition has become possible, creating detailed three-dimensional angiograms of retinal and choroidal blood vessels.11–12 With OCTA, CNV is detected as pathologic flow in the outer retinal slab, between Bruch’s membrane (BM) and the outer border of the outer plexiform layer (OPL).13–14 Previous studies have shown sensitivity of CNV detection with OCTA ranging from 50–86.5% and specificity ranging from 67.6–100%.15–20 Of these studies that evaluated active CNV, sensitivity and specificity was determined with en face OCTA only.15–19 Detecting CNV with en face OCTA only is dependent on accurate segmentation, and errors may lead to false positives and false negatives. Cross-sectional images of the volumetric angiogram allow for more accurate depth discrimination of flow and is not dependent on segmentation. Additionally, cross-sectional OCTA can help with differentiating projection artifact from true flow. Projection artifact refers to moving red blood cells in the superficial retinal vessels casting fluctuating shadows onto deeper structures that are misinterpreted as true flow. Projection artifact is strongest on bright structures on OCT, such as retinal pigment epithelium and can result in false positive CNV detection.21–22 Additionally, projection artifact is easily identified with cross-sectional OCTA as flow in deeper retina that has corresponding flow signal in superficial vessels.
In this study, we report the sensitivity and specificity of CNV detection in AMD (treatment-naïve neovascular AMD and non-neovascular AMD) with structural SD-OCT alone, en face OCTA alone, and with both en face OCTA and cross-sectional OCTA overlaid on structural OCT together.
Methods
Study Population
In this prospective case series, consecutive subjects were recruited from the retina clinics at the Casey Eye Institute (Oregon Health and Science University, Portland, OR) from September 2014 to September 2015. Informed consent was obtained in accordance with the Institutional Review Board/Ethics Committee of the Oregon Health and Science University and in compliance with the Declaration of Helsinki. HIPAA compliance was ensured for all work. At the time of the study, OCTA algorithms were not FDA-approved. Study participants had to have treatment-naïve neovascular AMD, including the presence of drusen and no previous history of CNV diagnosis or treatment. Presence of CNV required signs of subretinal neovascularization with leakage on FA within a 3×3 mm area centered on the fovea and the presence of intra-retinal fluid (IRF), subretinal fluid (SRF), or sub retinal pigment epithelium (RPE) fluid on structural OCT. Eyes without CNV consisted of a combination of two groups: healthy normal eyes and eyes with non-neovascular AMD. Patients with non-neovascular AMD required drusen and pigment changes without fluid, lipid, or hemorrhage on clinical examination and fundus photos as well as no fluid on structural OCT. Normal age-matched controls had normal clinical examination and normal fundus photos. Exclusion criteria included poor quality images due to media opacity or motion artifact.
Optical Coherence Tomography Angiography
OCTA was performed with a commercial 70 kHz spectral domain (SD) OCT system (RTVue-XR Avanti, Optovue, Inc.) with an axial resolution of 5 μm in tissue. Each OCTA scan covered a 3 × 3 mm macular area and contained a volumetric horizontal priority (x-fast) and a volumetric vertical priority (y-fast) raster scan. Each volumetric scan had 304 A-scans per B-scan and two consecutive B-scans at 304 locations. Flow was detected using the split-spectrum amplitude decorrelation angiography (SSADA) algorithm.23 The orthogonal scans were then registered and merged through Optovue’s MCT software to correct for motion from microsaccades. The merged volumetric angiograms were then exported for custom processing using the Center for Ophthalmic Optics & Lasers-Angiography Reading Toolkit (COOL-ART) software. These custom programs were developed at the Casey Eye Institute using the MATLAB programming language. Specifically, automated segmentation was used to identify the inner limiting membrane, outer boundary of the outer plexiform layer, and Bruch’s membrane.24 A trained grader (SSG) masked to diagnosis then reviewed the segmentation and performed manual correction as needed in COOL-ART software.24 Next, maximum flow projection and mean reflectance projection were used to generate en face angiograms and structural OCT images of different tissue slabs.24 We defined tissue slabs as follows: superficial retina was between the inner limiting membrane and outer boundary of the OPL; outer retina was between OPL and BM; and the choriocapillaris was a 10 μm slab beneath BM. To reduce projection artifacts, a slab subtraction technique was employed, whereby the superficial retinal angiogram was subtracted from the outer retinal angiogram.25 Composite cross-sectional OCTA included flow presented in false color over structural OCT in standard gray color. The entire volumetric angiogram was available as a folder with 304 tiff files that could be individually viewed with Windows Photo Viewer.
Grading
Medical retina specialists (AF and KVB) underwent training for CNV detection with OCTA. A series of training slides on PowerPoint (Microsoft®, Seattle, WA) was assembled by expert graders (STB and SSG) highlighting examples of CNV, as well as motion artifact, projection artifact, and segmentation errors that may result in CNV misdiagnosis (Fig. 1). Following training, graders were tasked with diagnosing CNV in the test cases. Graders were masked to the diagnosis, study participant identity, and scan date. All cases were de-identified and randomized prior to grading. At the first time point, each grader was presented with PowerPoint slides containing en face structural OCT of the whole scan and the RPE for identifying any irregularities in the reflectance pattern and en face OCTA of the whole scan, superficial retina, outer retina, outer retina after projection removal through slab subtraction, and choriocapillaris for assessing CNV. In order to be counted as a positive for CNV detection, graders had to correctly encircle the CNV using PowerPoint drawing tool. If graders encircled the wrong region and missed the CNV, this was counted as a false negative. Non-blinded expert graders (STB and SSG) reviewed the results to ensure true CNV was encircled and resolved disagreements between the two specialists.
Figure 1.
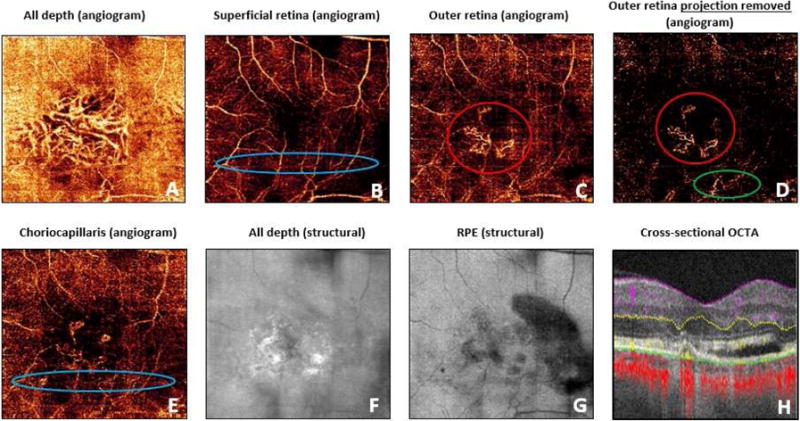
An example of training slide. En face angiograms included: (A) all depth angiogram, (B) superficial retinal slab, (C) outer retinal slab, (D) outer retinal slab with projection removal, and (E) choriocapillaris slab. (F) En face structural OCT and (G) en face structural OCT segmented at retinal pigment epithelium was provided to allow graders to identify reflectance abnormalities. (H) Cross-sectional OCT angiogram provides both flow and structural information. Examples of true choroidal neovascularization (red circles), projection artifact from superficial retinal blood vessels (green circle), and motion artifact (blue circles) were provided.
At a second time point, graders evaluated both en face OCTA and cross-sectional OCTA. Graders were able to scroll through the entire volumetric angiogram. To be counted as candidate CNV, graders needed to identify flow in the outer retinal slab that was not attributable to projection artifact. Sensitivity and specificity was calculated for each grader with both en face OCTA only and with en face OCTA combined with cross-sectional OCTA.
At a third time point, graders (KVB and CJF) reviewed structural SD-OCT alone for the presence of CNV. The graders were able to review the entire volumetric scan. CNV was defined as the presence of subretinal hyperreflective material, irregularly elevated pigment epithelial detachment (PED), or PED distinct from drusen.26
Results
Seventy-four study participants were enrolled, of which 50% were female, and mean age was 76.7 years (range 51–95, with a standard deviation of 8.9 years). The mean age for normal, non-neovascular AMD, and neovascular AMD subjects was 70.3 +/− 8.6, 81.7 +/− 5.4, and 76.7 +/− 9.1 years, respectively. Seventy-four OCT angiograms were obtained. All graders reviewed scans independently, and disagreements were reviewed by expert graders (STB, SSG, and YJ). Two cases were excluded due to poor image quality in the study eye. In both cases, excessive motion artifact led to uninterpretable scans. Of 72 eyes, 32 had treatment-naïve active CNV by dye leakage on FA and fluid on structural OCT. Forty eyes (20 normal and 20 non-neovascular AMD) had no CNV by structural OCT, clinical examination, and color photos.
Using en face OCTA only, each grader correctly detected 26 of 32 eyes with CNV, resulting in a sensitivity of 81.3% (Table). Both graders had the same 6 false negative cases. Four of the false negatives were associated with large subretinal hemorrhage (SRH). Excluding the four cases with SRH cases, the sensitivity rose to 93.8% for both graders. The remaining false negatives occurred in a case with a large PED and a small retinal angiomatous proliferation (RAP) lesion that are described in detail below.
Table.
Sensitivity and specificity of choroidal neovascularization detection for graders.
| Grader A | Grader B | |
|---|---|---|
| Structural SD-OCT alone | ||
| Sensitivity | 100% | 100% |
| Specificity | 97.5% | 85% |
| En face OCTA alone | ||
| Sensitivity | 81.3% | 81.3% |
| Specificity | 92.5% | 97.5% |
| En face OCTA with cross-sectional OCTA | ||
| Sensitivity | 100% | 100% |
| Specificity | 97.5% | 100% |
Of 40 eyes without CNV, grader A correctly identified 37 true negatives for a specificity of 92.5% (Table). Two of the false positives were due to the grader erroneously encircling motion or projection artifact in eyes with non-neovascular AMD. Grader A’s third false positive was associated with a small region of flow evident in outer retinal slab in a region of geographic atrophy (GA). Grader B correctly identified 39 of 40 true negatives for a specificity of 97.5%. The lone false negative was due to a region of motion artifact that was encircled in a healthy control eye.
Combining the en face OCTA with cross-sectional OCTA, the sensitivity improved to 100% for both graders. The addition of the cross-sectional OCTA improved the specificity of grader A to 97.5% and the specificity of grader B to 100% (Table).
Using structural SD-OCT alone in the detection of CNV, both graders had a sensitivity of 100%. Specificity for Grader A was 97.5% and for grader B was 85%, respectively (Table).
Case Descriptions
Examples of False Negatives
In 4 eyes with neovascular AMD, both graders could not identify CNV due to the presence of large SRH. The CNV signal is attenuated on both en face OCTA and cross-sectional OCTA in all of the eyes with SRH. An example of this is illustrated in Figure 2. On FA, dye leakage is visualized within and superior to the SRH (Fig. 2E). On the en face OCTA outer retinal slab, flow is visible on the outer retinal slab, but no clear vascular structures are visualized (Fig. 2B). Cross-sectional OCTA shows flow present in the outer retina distinct from projection artifact and subretinal fluid is clearly visible (Fig. 2C). Both graders changed their assessment from false negative to true positive after reviewing cross-sectional OCTA.
Figure 2.
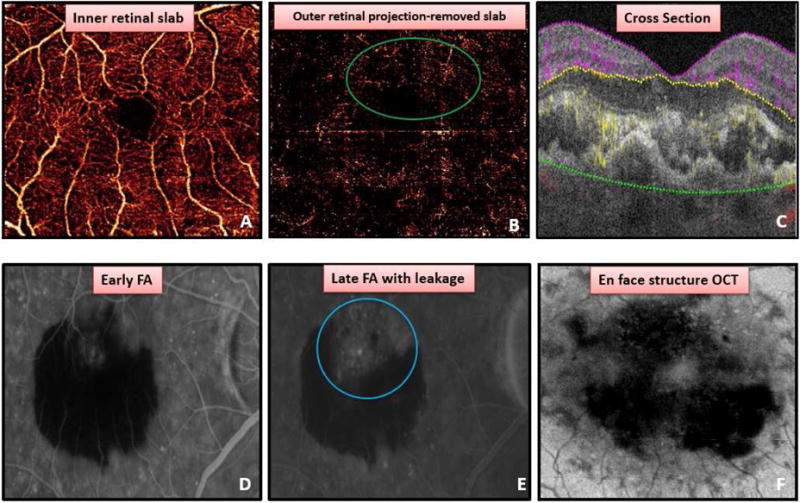
Detection of choroidal neovascularization (CNV) in a case with subretinal hemorrhage (SRH). (A) Normal retinal vessels present in inner retinal slab. (B) Outer retinal projection-removed angiogram with flow signal, but no clear vascular structures (green circle) due to SRH. (C) Cross-sectional OCT angiogram displaying both fluid and flow suggestive of CNV (yellow) present in outer retina. Early (D) and late (E) fluorescein angiography showing leakage (blue circle). (F) En face structural OCT showing loss of OCT signal due to SRH.
Both graders failed to identify a CNV lesion with en face OCTA in an eye with a large retinal PED (Fig. 3). In this case, image quality was low, and significant motion artifact was present (Fig 3A,B). It is likely that OCT signal was scattered by elevated RPE preventing visualization of definitive vascular structures beneath the RPE. Other studies have reported similar difficulty visualizing CNV in the setting of a large PED.18 With the addition of cross-sectional OCTA, there is projection artifact on the RPE; however, some flow just underneath RPE did not appear to have corresponding flow above it, suggesting possible CNV (Fig. 3C). Additionally, SRF could be visualized, allowing both graders to classify the eye as positive for CNV.
Figure 3.
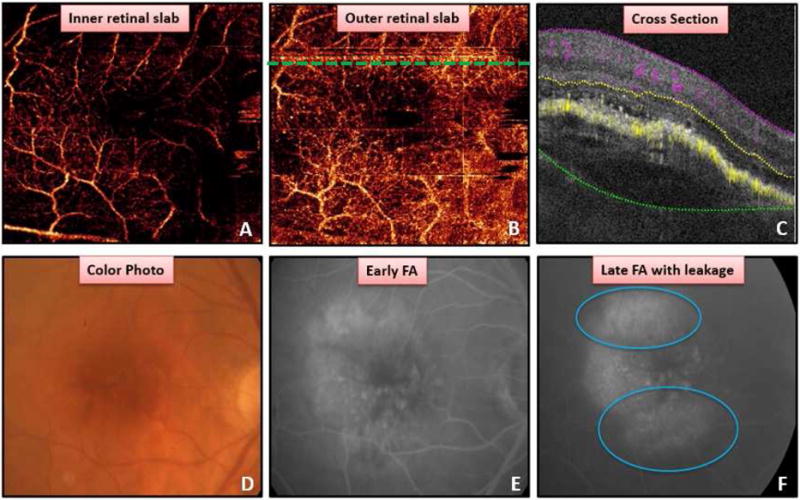
Detection of choroidal neovascularization (CNV) associated with large pigment epithelial detachment (PED). (A) Poorly focused superficial retinal angiogram. (B) Outer retinal angiogram revealing no definitive vasculature to suggest CNV. Dashed line corresponds to cross-sectional OCT angiogram (OCTA) in C. Cross-sectional OCTA (C) with projection artifact on retinal pigment epithelium (RPE) and some areas of flow beneath RPE without definitive flow from retinal vessels above, in addition to subretinal fluid and PED. Color photo (D) showing PED. Early (E) and late (F) fluorescein angiogram revealing pooling and leakage (blue circle) associated with PED.
In another case, both graders failed to detect a small RAP lesion with outer retinal en face OCTA (Fig. 4). A small region of dye leakage inferior to the fovea was present with FA (Fig. 4D,E). Grader A mistakenly classified the case as no CNV. Grader B erroneously encircled motion artifact (Fig. 4B – blue circle) and failed to encircle the location of the RAP lesion (Fig. 4B – green circle). Expert graders counted this as a false negative. Given the predominantly vertical (or axial) direction of the RAP vasculature in the outer retina, it appears as only a small dot with en face OCTA and is difficult to distinguish from residual projection artifact, even with projection artifact removal (Fig. 4C). With the addition of cross-sectional OCTA (Fig. 4F), both graders were able to detect flow in the outer retina along with IRF and correctly diagnosed the presence of CNV.
Figure 4.
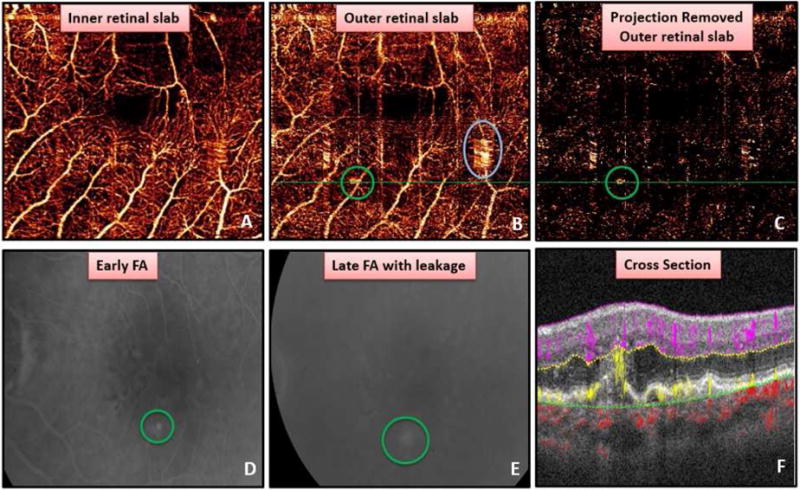
Detection of a small retinal angiomatous proliferation (RAP) lesion. (A) Superficial retinal angiogram. (B) Outer retinal angiogram showing the true choroidal neovascularization (green circle) and region of motion artifact erroneously circled by a grader (blue circle). (C) Projection artifact removed outer retinal slab with RAP (green circle) lesion unable to be distinguished from residual projection artifact. Early (D) and late (E) fluorescein angiogram showing leakage from RAP lesion. (F) Cross-sectional OCT angiogram demonstrating flow extending from deep retinal capillaries to retinal pigment epithelial detachment as well as intra-retinal fluid.
Examples of False Positives
Grader A incorrectly identified CNV in an eye with non-neovascular AMD. On color photo, there are regions of GA and RPE mottling (Fig. 5). On the outer retinal and projection-removed outer retinal en face angiograms, Grader A encircled in blue what was believed to be CNV (Fig. 5C,D). Experts determined the area circled was due to motion and projection artifact. Grader A correctly determined absence of CNV when cross-sectional OCTA was available, which allowed her to conclude apparent flow in the outer retina was artifact and not true CNV (Fig 5F).
Figure 5.
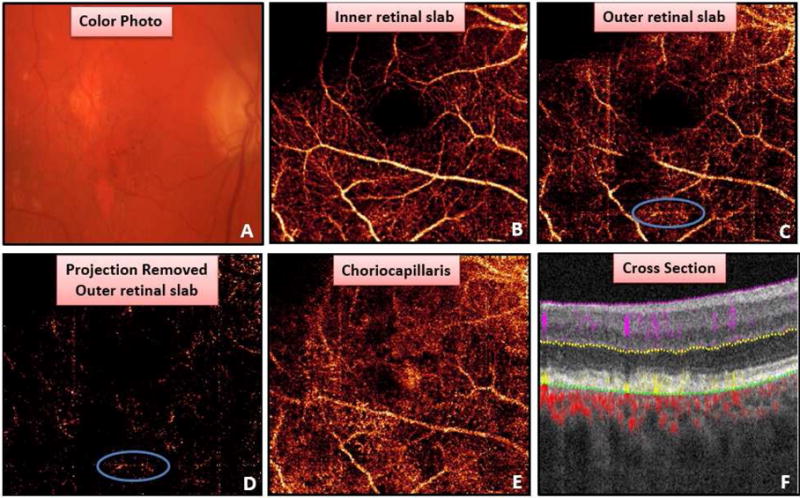
Example of false positive due to projection artifact and motion artifact. (A) Color photo with drusen, retinal pigment epithelium mottling, and geographic atrophy. (B) Superficial retinal angiogram. A region of motion (blue circle) and projection artifact identified by grader in outer retinal slab (D) and projection removed outer retinal slab (D). (E) Choriocapillaris slab. Cross-sectional OCT angiogram (F) reveals all flow in outer retina is due to projection artifact allowing grader to correctly classify as choroidal neovascularization not present.
In another example, Grader A identified CNV in one case based upon the presence of flow in the outer retina with en face OCTA and cross-sectional OCTA within an area of GA (Fig. 6). With the exception of this case, Grader A and B agreed on all other cases when both en face OCTA and cross-sectional OCTA were used to identify CNV. On the color photo and en face structural OCT, a well-defined area of GA can be seen (Fig 6A,B). Within this region of atrophy, flow is visualized on both cross-sectional OCTA (Fig. 6 C) and the en face outer retinal angiogram (Fig. 6E – blue circle). Despite manual correction, some flow from a choroidal vessel is present in the en face outer retinal angiogram, and grader A classified this as positive for CNV. With cross-sectional OCTA, the deep capillary retinal plexus is deeper than usual because of photoreceptor degeneration. Grader A erroneously thought this was pathologic flow from CNV in the outer retina. Expert graders (STB, SG, YJ) adjudicated this case and determined this was not true CNV. FA was not obtained because it was not clinically indicated.
Figure 6.
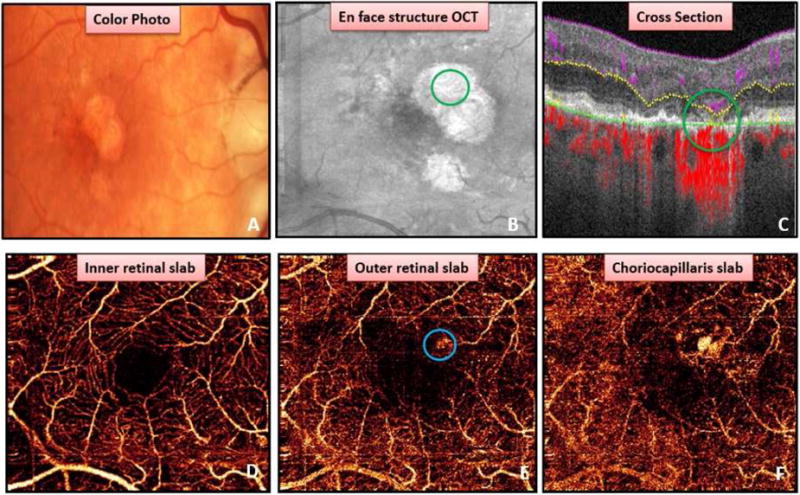
Flow in a region of geographic atrophy (GA). Color photo (A) and en face structural OCT (B) showing GA. (C) Cross-sectional OCT angiogram demonstrating deep capillary plexus flow appearing deeper than usual due to photoreceptor loss (green circle) as well as choroidal vessel adjacent to segmentation line (green line). (D) Inner retinal angiogram. (E) Region of flow on en face outer retinal angiogram (blue circle) is due to segmentation error resulting in erroneously classified as choroidal neovascularization by Grader A. Choroidal vessel present in area of GA in the choriocapillaris angiogram (F).
Discussion
This is the first study we are aware of comparing sensitivity and specificity of structural SD-OCT alone, en face OCTA alone, and en face OCTA combined with cross-sectional OCTA for the detection of treatment-naïve CNV in AMD using FA and OCT as the gold standard. Of the three approaches studied, en face OCTA combined with cross-sectional OCTA performed the best (Table). With cross-sectional OCTA, both flow and structural SD-OCT information are available on the same scan. SD-OCT alone can provide clues suggesting the presence of CNV, such as the presence of SRF, IRF, and subretinal hyperreflective material, and allows identification of areas where the RPE is separated from BM. A large multi-centered study found sensitivity of CNV detection for en face OCTA alone was 66.7%, and this improved to 85.7% by combining en face OCTA with structural OCT.19 In our study, cross-sectional OCTA combined with en face OCTA improved the sensitivity for CNV detection to 100% for both graders. Interestingly, structural SD-OCT alone also had a sensitivity of 100% for both graders, findings similar to other studies.26–28 However, the high sensitivity of structural OCT was associated with a lower specificity in this series, findings also consistent with other studies, which have reported a wide range of specificity at 47%, 81%, and 98% for CNV detection.26–28 Graders in this study could only classify CNV as present if flow was detected in the outer retina that was distinct from projection artifact with cross-sectional OCTA, resulting in improved specificity compared to structural OCT. Structural OCT alone seems to make clinicians suspicious of CNV but less able to rule out CNV.
The addition of cross-sectional OCTA to en face OCTA helped reduce false negatives that were present with en face OCTA alone. In cases with SRH and PED, blockage and scattering of OCTA signal prevented identifiable vascular structures in the outer retinal angiogram with en face OCTA. However, not all OCTA signal is blocked or scattered, and with cross-sectional OCTA, graders were able to detect pathologic flow (CNV) in the outer retina and identify candidate CNV (Fig. 2C and Fig. 3C). In a false negative case with a RAP lesion, vertically oriented vessels in the outer retina could not be distinguished from projection artifact or motion artifact with en face OCTA (Fig. 4C). With cross-sectional OCTA, vessels arising from the deep capillary plexus diving towards a PED were visible, consistent with a RAP lesion (Fig. 4F). In each of these aforementioned cases, both graders were able to change their diagnosis from false negative to true positive after reviewing cross-sectional OCTA.
The majority of false positives with en face OCTA alone were due to graders identifying motion artifact or projection artifact as candidate CNV. In these cases, graders recognized that no pathologic outer retinal flow was present with cross-sectional OCTA and correctly changed their assessment. One advantage to cross-sectional OCTA that likely contributes to improved sensitivity and specificity is that cross-sectional OCTA is not dependent on segmentation strategies, and clinicians are able to directly view flow in relation to anatomical landmarks when making clinical decisions. Motion and projection artifact have previously been shown to interfere with automatic CNV detection software.24,29–31 It is likely that further advances in projection artifact removal and motion correction will limit these artifacts, thus improving sensitivity and specificity of en face OCTA.32–34
Using en face OCTA only, both graders had a sensitivity of 81.3%. This is similar to other reported sensitivity of CNV detection with en face OCTA ranging from 50–86.5%.15–20 In one study, de Carlo and colleagues imaged patients with SD-OCTA, and two masked and trained readers evaluated 30 OCT angiograms and same-day FA to determine the sensitivity and specificity of CNV detection with OCTA. They reported a sensitivity of 50% (4/8 eyes) and specificity of 91% (20/22 eyes)15. In an unmasked comparative analysis, Moult et al. compared FA/ICGA to OCTA in the identification of CNV in patients known to have neovascular AMD. They initially reported a sensitivity of 84% (16/19 eyes), but after the exclusion of eyes with SRH or inactive CNV, their sensitivity rose to 96%.16 In a single-center retrospective study, two masked graders evaluated OCTA, structural OCT, and FA for presence of CNV in 86 eyes with suspected CNV due to NVAMD; they reported a sensitivity of 86.5% and specificity of 67.6%18. Three out of 7 false negatives were due to large SRH. In our study, SRH was the primary cause of false negatives for en face OCTA alone for CNV detection as well. With the exclusion of these cases, we report that the sensitivity increased to 94%. SRH blocks OCT signal, interfering with the ability to detect CNV in these cases. Practically speaking, this may not be a significant limitation as the presence of SRH in eyes with AMD alone is often associated with the presence of CNV. Lastly, in an observational case series, Carnevali et al. estimated the detection rate for CNV by cross-sectional OCTA in patients with treatment-naïve quiescent CNV due to AMD, which they defined as irregular elevation of the RPE with reflective material in the sub-RPE space, no fluid on SD-OCT, absence of late leakage or pooling on FA, and presence of fibrovascular plaque on late phase of ICGA.20 Patients with non-neovascular AMD, defined by presence of drusenoid PED on SD-OCT and no neovascular network on ICGA, served as negative controls. Two masked readers correctly identified quiescent CNV in 18 of 22 eyes and correctly excluded 22 of 22 eyes without CNV, resulting in a sensitivity of 81.8% and specificity of 100%. Our study yielded a higher sensitivity for detecting CNV using en face and cross-sectional OCTA together but differs from this case series in that we assessed eyes with exudative, or active, CNV.
Specificity was 92.5% and 97.5% for each grader for en face OCTA only. This is on the higher end of previously reported specificity ranging from 67–100%. Our higher specificity may be due in part to the control population we selected, half of which were normal healthy eyes and half were eyes with non-neovascular AMD. Additionally, segmentation is crucially important for accurate en face OCTA interpretation, and all cases underwent manual segmentation when automated segmentation failed. Other studies included previously treated CNV resulting in false positives where flow was detected within a CNV, but anti-VEGF treatment prevented leakage from being present with FA. In our study, we wanted to focus on the role of OCTA to confirm diagnosis of treatment-naïve active CNV, and we did not include cases of CNV under anti-VEGF treatment.
Our case series has a few limitations. In all cases of CNV, SRF or IRF was present on structural OCT. Given this fluid was visible with cross-sectional OCTA, the fluid and not the presence of flow on the OCTA could have biased graders and driven their findings. Graders needed to identify pathologic flow in the outer retina to be counted as CNV. The purpose of this study was to determine if OCTA could differentiate treatment-naïve neovascular AMD eyes from non-neovascular AMD eyes and normal eyes. A more practical clinical study is needed to validate the use of OCTA in differentiating the etiology of fluid on structural OCT from CNV versus the many other possible causes such as diabetic macular edema, retinal vein occlusion macular edema, vitreomacular traction, or atrophic changes associated from geographic atrophy. An additional limitation is the small sample size; 72 angiograms were analyzed, of which 40 eyes did not have CNV and 32 did. This is a relatively small sample size but is one of the larger studies to date evaluating both sensitivity and specificity of CNV detection with OCTA. Participants with non-neovascular AMD or normal eyes did not undergo FA, but with the use of clinical examination, color photos, and structural OCT, it is unlikely CNV was present in these eyes. Our study did not include ICGA, so additional study is needed to determine if OCT angiography is comparable to ICGA in detecting CNV complexes.
In summary, this is the first prospective case series contrasting structural OCT alone with en face OCTA alone and both en face OCTA combined with cross-sectional OCTA in exclusively treatment-naïve neovascular AMD eyes while using non-neovascular AMD eyes with normal age-matched control eyes. Currently, FA is the gold standard to rule in and rule out CNV, and in this study, en face OCTA combined with cross-sectional OCTA compared favorably to FA. Because image acquisition with FA is invasive and time-consuming, it is not ideal for busy clinical practices with elderly patients, while OCTA can be imaged noninvasively, quickly, and safely.14,35 Structural OCT alone was highly sensitive for CNV detection in this series, and OCTA aided mostly with improving specificity. En face OCTA alone can be used detect and follow CNV. However, clinicians will find reviewing the cross-sectional OCTA scans is especially useful to help rule in or out CNV in addition to help distinguish between true pathology and artifact. Additional study with larger sample sizes is needed to further validate the use of OCTA for CNV detection.
Highlights.
The sensitivity and specificity of choroidal neovascularization detection in treatment-naïve neovascular and non-neovascular age-related macular degeneration with normal control eyes using en face and cross-sectional optical coherence tomography angiography was 100% and 97.5–100%, respectively.
Acknowledgments
Financial Support: Supported by National Institutes of Health (Bethesda, MD) Grants R01 EY024544, DP3 DK104397, R01 EY023285, P30 EY010572 and by unrestricted departmental funding from Research to Prevent Blindness (New York, NY). The sponsor or funding organization had no role in the design or conduct of this research.
Abbreviations
- AMD
age-related macular degeneration
- CNV
choroidal neovascularization
- FA
fluorescein angiography
- OCT
optical coherence tomography
- ICGA
indocyanine green angiography
- OCTA
optical coherence tomography angiography
- BM
Bruch’s membrane
- OPL
outer plexiform layer
- SD
spectral domain
- RPE
retinal pigment epithelium
- SRH
subretinal hemorrhage
- PED
pigment epithelial detachment
- RAP
retinal angiomatous proliferation
- GA
geographic atrophy
- SRF
subretinal fluid
- IRF
intraretinal fluid
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Meeting Presentation: Presented at the Association for Research in Vision and Ophthalmology Annual Meeting, 2016.
Conflict of Interest/Financial Disclosures:
YJ and DH have a financial interest in Optovue, Inc. These potential conflicts of interest are managed by Oregon Health & Science University. DH receives royalties on an OCT patent licensed by the Massachusetts Institute of Technology to Carl Zeiss Meditec and LightLab Imaging. No conflicting relationship exists for any other authors.
References
- 1.Age-Related Eye Disease Study Research Group. Risk Factors Associated with Age-Related Macular Degeneration: A Case-control Study in the Age-Related Eye Disease Study: Age-Related Eye Disease Study Report Number 3. Ophthalmology. 2000;107(12):2224–2232. doi: 10.1016/s0161-6420(00)00409-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wong WL, Su X, Li X, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health. 2014;2:e106–e116. doi: 10.1016/S2214-109X(13)70145-1. [DOI] [PubMed] [Google Scholar]
- 3.Freund KB, Yannuzzi LA, Sorenson JA, et al. Age-related macular degeneration and choroidal neovascularization. Am J Ophthalmol. 1993;115:786–791. doi: 10.1016/s0002-9394(14)73649-9. [DOI] [PubMed] [Google Scholar]
- 4.Watzke RC, Klein ML, Hiner CJ, et al. A comparison of stereoscopic fluorescein angiography with indocyanine green video angiography in age-related macular degeneration. Ophthalmology. 2000;107:1601–1606. doi: 10.1016/s0161-6420(00)00218-9. [DOI] [PubMed] [Google Scholar]
- 5.Do DV, Gower EW, Cassard SD, et al. Detection of new-onset choroidal neovascularization using optical coherence tomography: the AMD DOC Study. Ophthalmology. 2012;119:771–778. doi: 10.1016/j.ophtha.2011.10.019. [DOI] [PubMed] [Google Scholar]
- 6.Freund KB, Zweifel SA, Engelbert M. Do we need a new classification for choroidal neovascularization in age-related macular degeneration? Retina. 2010;30:1333–1349. doi: 10.1097/IAE.0b013e3181e7976b. [DOI] [PubMed] [Google Scholar]
- 7.Hee MR, Baumal CR, Puliafito CA, et al. Optical coherence tomography of age-related macular degeneration and choroidal neovascularization. Ophthalmology. 1996;103:1260–1270. doi: 10.1016/s0161-6420(96)30512-5. [DOI] [PubMed] [Google Scholar]
- 8.Kwiterovich KA, Maguire MG, Murphy RP, et al. Frequency of adverse systemic reactions after fluorescein angiography. Results of a prospective study Ophthalmology. 1991;98:1139–1142. doi: 10.1016/s0161-6420(91)32165-1. [DOI] [PubMed] [Google Scholar]
- 9.Heffner JE. Reactions to fluorescein. JAMA. 1980;243:2029–2030. [PubMed] [Google Scholar]
- 10.Guyer DR, Yannuzzi LA, Slakter JS, et al. Classification of choroidal neovascularization by digital indocyanine green videoangiography. Ophthalmology. 1996;103:2054–2060. doi: 10.1016/s0161-6420(96)30388-6. [DOI] [PubMed] [Google Scholar]
- 11.Jia Y, Bailey ST, Hwang TS, et al. Quantitative optical coherence tomography angiography of vascular abnormalities in the living human eye. Proc Natl Acad Sci U S A. 2015;112(18):E2395–E2402. doi: 10.1073/pnas.1500185112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spaide RF, Klancnik JM, Cooney MJ. Retinal vascular layers imaged by fluorescein angiography and optical coherence tomography angiography. JAMA Ophthalmol. 2015;133(1):45–50. doi: 10.1001/jamaophthalmol.2014.3616. [DOI] [PubMed] [Google Scholar]
- 13.De Carlo TE, Bonini Filho MA, Chin AT, et al. Spectral-domain optical coherence tomogoraphy angiography of choroidal neovascularization. Ophthalmol. 2015;122(6):1228–1238. doi: 10.1016/j.ophtha.2015.01.029. [DOI] [PubMed] [Google Scholar]
- 14.Jia Y, Bailey ST, Wilson DJ, et al. Quantitative optical coherence tomography angiography of choroidal neovascularization in age-related macular degeneration. Ophthalmol. 2014;121(7):1435–1444. doi: 10.1016/j.ophtha.2014.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Carlo TE, Bonini Filho MA, Chin AT, et al. Spectral-domain optical coherence tomography angiography of choroidal neovascularization. Ophthalmology. 2015 Jun;122(6):1228–38. doi: 10.1016/j.ophtha.2015.01.029. [DOI] [PubMed] [Google Scholar]
- 16.Moult E, Choi W, Waheed NK, et al. Ultrahigh-speed swept-source OCT angiography in exudative AMD. Ophthalmic Surg Lasers Imaging Retina. 2014 Nov-Dec;45(6):496–505. doi: 10.3928/23258160-20141118-03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coscas GJ, Lupidi M, Coscas F. Optical coherence tomography angiography versus traditional multimodal imaging in assessing the activity of exudative age-related macular degeneration: a new diagnostic challenge. Retina. 2014 Nov;35(11):2219–28. doi: 10.1097/IAE.0000000000000766. [DOI] [PubMed] [Google Scholar]
- 18.Gong J, Yu S, Gong Y, et al. The diagnostic accuracy of optical coherence tomography angiography for neovascular age-related macular degeneration: a comparison with fundus fluorescein angiography. J Ophthalmol. 2016;2016:7521478. doi: 10.1155/2016/7521478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Inoue M, Jung JJ, Balaratnasingham C, et al. A comparison between optical coherence tomography angiography and fluorescein angiography for the imaging of type 1 neovascularization. Invest Ophthalmol Vis Sci. 2016 Jul 1;57(9):OCT314–OCT323. doi: 10.1167/iovs.15-18900. [DOI] [PubMed] [Google Scholar]
- 20.Carnevali A, Vittoria M, Querques G, et al. Optical coherence tomography angiography: a useful tool for diagnosis of treatment-naïve quiescent choroidal neovascularization. Am J Ophthalmol. 2016 Jun;169:189–198. doi: 10.1016/j.ajo.2016.06.042. [DOI] [PubMed] [Google Scholar]
- 21.Zheng F, Roisman L, Schall K, et al. Artifactual flow signals within drusen detected by OCT angiography. OSLI Retina. 2016 Jun;47(6):517–522. doi: 10.3928/23258160-20160601-02. [DOI] [PubMed] [Google Scholar]
- 22.Huang D, Jia Y, Gao SS. Interpretation of optical coherence angiography. In: Lumbros HD, Rosenfeld P, Chen C, Rispoli M, Romano A, editors. Clinical Atlas of OCT Angiography. New Dehli: Jaypee Brothers Medical Publishers; 2015. [Google Scholar]
- 23.Jia Y, Tan O, Tokayer J, et al. Split-spectrum amplitude-decorrelation angiography with optical coherence tomography. Opt Express. 2012;20(4):4710–25. doi: 10.1364/OE.20.004710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang M, Wang J, Pechauer AD, et al. Advanced image processing for optical coherence tomographic angiography of macular diseases. Biomed Opt Express. 2015;6(12):4661–4675. doi: 10.1364/BOE.6.004661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu L, Gao SS, Bailey ST, et al. Automated choroidal neovascularization detection algorithm for optical coherence tomography angiography. Biomed Optics Express. 2015;6(9):3564–3596. doi: 10.1364/BOE.6.003564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mokwa NF, Ristau T, Keane PA, et al. Grading of age-related macular degeneration: comparison between color fundus photography, fluorescein angiography, and spectral-domain optical coherence tomography. J Ophthalmol. 2013;2013:385915. doi: 10.1155/2013/385915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khurana RN, Dupas B, Bressler NM. Agreement of time-domain and spectral-domain optical coherence tomography with fluorescein leakage from choroidal neovascularization. Ophthalmol. 2010;117(7):1376–1380. doi: 10.1016/j.ophtha.2009.11.039. [DOI] [PubMed] [Google Scholar]
- 28.Wilde C, Patel M, Lakshmanan A, et al. The diagnostic accuracy of spectral-domain optical coherence tomography for neovascular age-related macular degeneration: a comparison with fundus fluorescein angiography. Eye. 2015;29:602–610. doi: 10.1038/eye.2015.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Camino A, Zhang M, Dongye C, et al. Automated registration and enhanced processing of clinical optical coherence tomography angiography. Quant Imaging in Med and Surg. 2016;6(4):391–401. doi: 10.21037/qims.2016.07.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Camino A, Zhang M, Gao SS, et al. Evaluation of artifact reduction in optical coherence tomography angiography with real-time tracking and motion correction technology. Biomed Opt Express. 2016;7(10):3905–3915. doi: 10.1364/BOE.7.003905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zang P, Liu G, Zhang M, et al. Automated motion correction using parallel-strip registration for wide-field en face OCT angiogram. Biomed Opt Express. 2016;7(7):2823–36. doi: 10.1364/BOE.7.002823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang M, Hwang TS, Campbell JP, et al. Projection-resolved optical coherence tomographic angiography. Biomed Opt Express. 2016;7(3):816–828. doi: 10.1364/BOE.7.000816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang A, Zhang Q, Wang RK. Minimizing projection artifacts for accurate presentation of choroidal neovascularization in OCT micro-angiography. Biomed Opt Express. 2015;6:4130–43. doi: 10.1364/BOE.6.004130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gao SS, Jia Y, Zhang M, et al. Optical coherence tomography angiography. Inves Ophthalmol Vis Sci. 2016;57:OCT27–OCT 36. doi: 10.1167/iovs.15-19043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spaide RF, Klancnik JM, Cooney MJ. Retinal vascular layers imaged by fluorescein angiography and optical coherence tomography angiography. JAMA Ophthalmol. 2015;133:45–50. doi: 10.1001/jamaophthalmol.2014.3616. [DOI] [PubMed] [Google Scholar]


