Abstract
This study aimed to compare the effects of oral midazolam and chloral hydrate in pre-echocardiography sedation of children. In this double-blind clinical trial, 68 children were randomly assigned to midazolam (0.2 mg/kg) or chloral hydrate (50 mg/kg). The intensity, duration, and onset of the drugs’ effects were assessed. Data were analyzed using the χ2 and Mann-Whitney tests (P ≤ .05). The average onset and duration of sedation in the children assigned to midazolam was shorter than in those assigned chloral hydrate (6.35 ± 3.65 and 19.14 ± 5.86 minutes, P = .0001, and 27.64 ± 8.34 and 48.97 ± 14.81 minutes, P = .0001). Gastrointestinal side effects were more frequent in the chloral hydrate group (23.5% against 0%, P = .003). According to the results of the present study, chloral hydrate and midazolam can be appropriate choices for pre-echocardiography sedation of patients without cardiovascular risk factors. Considering the similar effectiveness, more rapid onset, and shorter duration of sedation, besides less side effects in the midazolam group, researchers recommend the routine use of this drug.
Keywords: oral midazolam, oral chloral hydrate, pediatric sedation, echocardiography
Introduction
Echocardiography is a very helpful and accurate tool in evaluating the normal and abnormal cardiac anatomy. Echocardiography is efficient in the assessment of congenital cardiac anomalies, cardiac muscular strength, and particularly the ability of the left ventricle and heart valve problems.1
One of the major difficulties in pediatric echocardiography is anxiety and fear in children and problems in separating them from their parents while being transferred to the echocardiography room, and therefore, most of them need premedication, regarding their medical status and background, duration of echocardiography, optimal induction of anesthesia, and psychological state of the child and parents.2 Premedication is associated with improved quality of echocardiography and decrease in medical errors.3
The psychological and pharmacological preparation before echocardiography is known as premedication. Premedication drugs are administered orally, intramuscularly, intravenously, rectally, sublingually, or nasally, of which oral premedication is the most appropriate and acceptable in children. Premedication is necessary for children after the age of 10 to 21 months because separation anxiety begins at this age. Midazolam and chloral hydrate are the most common drugs used in the premedication of children.4,5
The drugs usually prescribed in premedication of children in echocardiography are midazolam and chloral hydrate. Previously, chloral hydrate was considered as a therapeutic standard in pre-echocardiography sedation.6 Previous studies have indicated the safety and high efficiency of this drug in completing echocardiography.7 In spite of the clinical benefits, the use of this drug has become limited due to decreased production of the drug due to commercial reasons.8 Therefore, more attention has been paid to other drugs from this group such as midazolam. Midazolam is a fast-acting benzodiazepine that provides short-term sedation with minimal side effects, by inhibitory effects on the central nervous system.9 The efficiency and side effects of these 2 drugs have been evaluated in various studies. In the study by Wheeler et al, no significant difference was found in the onset of the sedation effect of these 2 drugs, although recovery time in the midazolam group was significantly lower than in the chloral hydrate group; also children had deeper sedation in the chloral hydrate group compared with the midazolam group.10 In the study by Layangool et al, it was shown that children in the chloral hydrate group required a repeated dose for an effective sedation and the onset and duration of the drug effect was significantly shorter in the midazolam group. According to the findings of this study, midazolam had higher efficacy and better results in echocardiography of children (pediatric echocardiography).11
We performed this clinical trial with the aim of comparing the efficacy in sedation of children before undergoing echocardiography.
Method
In this randomized double-blind (patient and observer) clinical trial, 34 children received oral midazolam and 34 children received oral chloral hydrate.
Patients
In this study, children below 10 years of age who were referred to Birjand city’s Valiasr Pediatric Hospital and Heart Clinic, and were indicated with echocardiography during the year 2015, took part. Inclusion criteria were the following: (1) full consciousness, (2) lack of respiratory distress, and (3) lack of hypotonia; exclusion criteria included the following: (1) not completing echocardiography due to restlessness of the child, (2) lack of parents’ cooperation because of the drug’s side effects, (3) instability of patient’s vital signs before receiving the drug, and (4) requiring intensive care unit care.
Groups
Patients were randomly divided into 2 therapeutic groups using 1:1 blocks. In both groups, the children were awake for at least 2 hours before receiving the drug. Oral midazolam (2.0 mg/kg) or oral chloral hydrate syrup (50 mg/kg) was indicated for the first and second groups, respectively (the dose of these 2 drugs was determined according to the latest articles on pediatric sedation before noninvasive procedures; a 10% concentration of the chloral hydrate syrup was provided by Birjand’s Valiasr Hospital pharmacy, and for oral midazolam the Darou Pakhsh 5 mg/mL midazolam syrup was used). After the onset of sedative effects and appropriate cooperation of the child the procedure was started, and after the end of echocardiography, the children were followed-up every 10 minutes till complete recovery (consciousness).
Follow-up
After administration of the drugs, duration and onset of their sedative effects and gastrointestinal, respiratory, and neurologic side effects were evaluated and compared between the 2 groups every 2 minutes.
Sedation was compared between the 2 groups according to the Richmond Agitation-Sedation Scale (RASS) standards.
Ethical Considerations
This research was approved by Birjand University of Medical Science’s Institutional Ethics Committee, with the following subject: “Comparing the effects of oral midazolam and chloral hydrate in sedation of children before echocardiography” (Code Ir.bums.1395.247) and was registered in Iran’s Clinical Trial Registration Center on May 4, 2017, coded IRCT2017042317756N13.
Statistical Methods
Sample size was considered according to the study by Layangool et al,11 and the average duration of sedation was 54.6 (26.8) and 30.5 (29.4) minutes in the chloral hydrate and midazolam groups, respectively. For reaching a statistical power of 90% and a type 1 error of 0.05, the sample size required was 29, which became 34 due to 15% missing. Thus, the total sample size in both groups was 68 children.
After entering the data into SPSS software (version 23), descriptive data were reported using the appropriate charts, center indicators (measures of central tendency), and dispersion. After comparison of variables in the 2 groups, quantitative variables were assessed using the Kolmogorov-Smirov test, regarding normal distribution, and results showed that the mean RASS (P = .0001), mean age (P = .0001), and average onset and duration of drug efficacy (P = .001) did not have normal distribution. In order to compare side effects in the studied groups, the χ2 (or exact Fisher’s) test was done, and the Mann-Whitney test was used to evaluate quantitative variables with significance level of .05.
Results
In total, 68 children entered the study of whom 34 were in the midazolam group and 34 in the chloral hydrate group. The 2 groups were similar with regard to age and gender (Table 1). Comparing the frequency of sedation scores based on the RASS in the 2 groups, results showed that frequency of light and moderate sedation in the midazolam group was 55.9% and 11.8%, respectively, and in the chloral hydrate group it was 50% and 20.6%, respectively, showing a significant difference (P = .5; Table 2).
Table 1.
Comparison of the Demographic Data of the Midazolam and Chloral Hydrate Groups.
| Variable | Midazolam (n = 34) | Chloral-Hydrate (n = 34) | |
|---|---|---|---|
| Age (mean) | 15.15 ± 18.92 months | 14.28 ± 14.25 months | Z = −0.153a, P = .8 |
| ≤1 year old, n (%) | 21 (61.8%) | 17 (50%) | χ2 = 0.9 |
| >1 year old, n (%) | 13 (38.2%) | 17 (50%) | P = .3 |
| Gender | |||
| Male, n (%) | 21 (61.8%) | 22 (64.7%) | χ2 = 0.06b |
| Female, n (%) | 13 (38.2%) | 12 (35.3%) | P = .8 |
The χ2 test was used for the comparison of the 2 groups.
The Mann-Whitney test was for the comparison of the 2 groups.
Table 2.
Comparison of the frequency of the Richmond Agitation-Sedation Scale Sedation Scores in the 2 Groups.a
| Score | Midazolam (n = 34) | Chloral Hydrate (n = 34) | |
|---|---|---|---|
| Alert and calm | 6 (17.6%) | 3 (8.8%) | |
| Drowsy | 5 (14.7%) | 7 (20.6%) | Phi = 0.1 |
| Light sedation | 19 (55.9%) | 17 (50%) | P = .5 |
| Moderate sedation | 4 (11.8%) | 7 (20.6%) |
Data are presented as n (%). The Fisher’s exact test was used to compare the 2 groups.
The average onset time of sedation in the midazolam and chloral hydrate groups was 6.35 ± 3.65 and 19.14 ± 5.86 minutes, respectively (P = .0001). Mean duration of sedation in the midazolam and chloral- hydrate groups was 27.64 ± 8.34 and 48.97 ± 14.81 minutes, respectively (P = .0001; see Figures 1 and 2).
Figure 1.
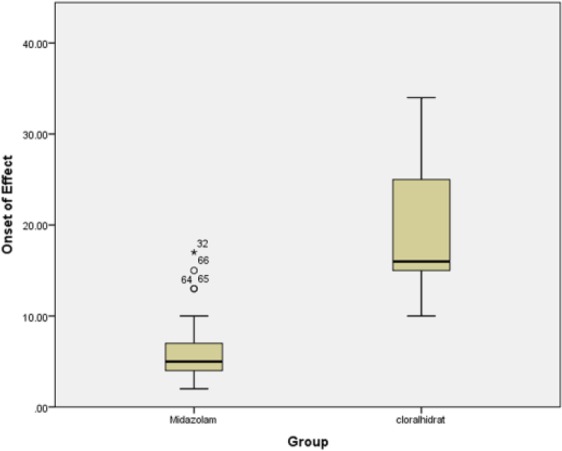
Comparison of the average onset time in the 2 groups.
Figure 2.
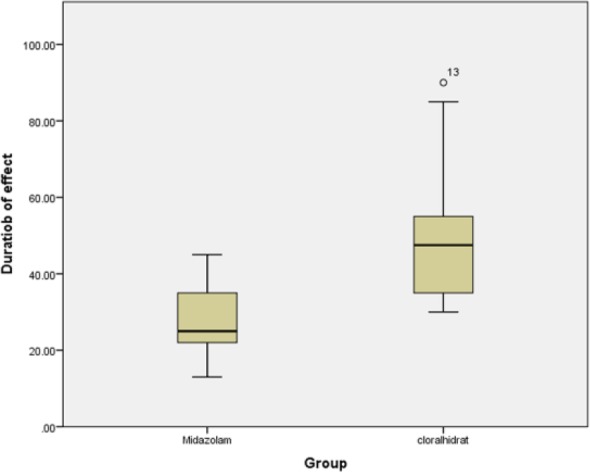
Comparison of the mean duration of drug effectiveness in the 2 groups.
Results of comparing the side effects in the studied groups indicated that no neurologic or respiratory side effects were reported in the 2 groups. With regard to gastrointestinal side effects, the findings showed that they were significantly higher in the chloral hydrate group when compared with the midazolam group (23.5% vs 0%, P = .003).
Discussion
This study was conducted with the aim of comparing the effects of oral midazolam and chloral hydrate in the sedation of children before echocardiography.
Overall, 68 patients entered the study of whom 34 were in the midazolam group and 34 in the chloral hydrate group. The 2 groups were similar in age (P = .8) and gender (P = .8). The average onset time of sedative effects and duration of sedation was significantly shorter in the midazolam group, compared with the chloral hydrate group. Frequency of light and moderate sedation in the midazolam and chloral hydrate groups were 55.9% and 11.8%, and 50% and 20.6%, respectively, which showed no significant difference (P = .5) between the 2 groups. No neurologic or respiratory side effects were reported in any of the groups. Frequency of gastrointestinal side effects in the chloral hydrate group was significantly higher than that in the midazolam group.
Regarding sedation of the 2 methods, findings of the current study showed that onset and duration of sedation were shorter in the midazolam group. Also, in this study no neurologic or respiratory side effects were reported in any of the groups. However, gastrointestinal side effects were significantly higher in the chloral hydrate group than in the midazolam group. In a study by Hasani et al12 in Tehran’s Pediatric Medical Center, the effects of oral premedication using ketamine-midazolam, just ketamine, and just midazolam on presurgery anxiety of children aged 2 to 10 years was evaluated.
Results showed that anxiety was better relieved in the “ketamine-midazolam” group. Anxiolysis and the behavior while separating from parents was more efficient in the ketamine-midazolam group. No specific side effects were reported in the patients being premedicated by midazolam.12 Sajedi et al13 conducted a randomize double-blind clinical trial in which the children were divided into 2 groups: 0.5 mg/kg in the oral midazolam group and another group that combined 0.25 mg/kg oral midazolam and 2.5 mg/kg ketamine. The results showed that onset of sedation was faster in the combined group. There was no difference between the 2 groups in the sedation criteria. Also, there was no difference regarding the child’s behavior, drug acceptance, anxiolysis, child’s behavior while separating from parents, recovery time, and consciousness after recovery. The findings on intravenous line was more acceptable in the combined group. The only side effect observed in the midazolam group was nausea.13 In the study by Kaviani et al study in Esfahan, 60 children aged between 3 and 7 years were randomly divided into 2 groups of 0.5 mg/kg midazolam and 6 mg/kg ketamine. After 15 minutes, the children received a combination of N2O-O2 and the drug each with a 50% concentration. This sedative combination was effective in 90% of patients and there was so statistically significant difference between the groups. Consciousness during the procedure was higher in the midazolam group compared with the ketamine group. Also, recovery time was shorter in the midazolam group. The 2 oral drugs midazolam and ketamine in combination with nitrous oxide have a positive effect in providing sedation and children’s behavior control during dental procedures.14 In the study by Wheeler et al, which aimed to evaluate and compare the sedative effects of chloral hydrate and midazolam, onset of sedation had no significant difference between the 2 groups, but recovery time was significantly shorter in the midazolam group. Also, chloral hydrate provided a deeper sedation than midazolam and no irreversible and considerable side effects were observed in any of the 2 groups.10 The study by Moshiri et al in Arak aimed to determine the effects of propofol (0.4 mg/kg) and midazolam (0.04 mg/kg) as a premedication drug for controlling patient anxiety before anesthesia. Both drugs were more effective than placebo in reducing patient anxiety, and there was no significant difference between the 2 drugs in this regard.15 The study by Layanagool et al, which was conducted in 2008 with the aim of comparing oral chloral hydrate (50 mg/kg) and sublingual midazolam (0.3 mg/kg) in pediatric sedation before echocardiography, showed that the onset and duration of sedation in the midazolam group was significantly shorter than the chloral hydrate group.11 However, the chloral hydrate group had a deeper sedation and anesthesia. According to results of this study, midazolam has better results and effectiveness in children’s echocardiography.11 In a study by Moshiri et al in Tehran, on the effectiveness and safety of 2 combinations of drugs, midazolam-ketamine and propofol-alfentanil in providing sedation and analgesia during bone marrow aspiration in children, if was found that the onset of sedation and recovery time was significantly shorter in the propofol-alfentanil group.16 Pariya et al,17 in a study in Shahid Beheshti University of Medical Science, comparing the sedation of oral chloral hydrate (50 mg/kg) and midazolam (0.3 mg/kg) before lumber puncture, concluded that the average sedation degree was higher in the chloral hydrate group. Also, the mean onset time of sedative effects was shorter in the midazolam group. Regarding the side effects, results showed that side effects in the oral midazolam group were higher than in the chloral hydrate group.17 In a clinical trial by Sigari et al18 in Ardabil, which compared the sedation of chloral hydrate and midazolam in children undergoing echocardiography, the findings indicated that onset of the drug’s effects was shorter in the midazolam group. Also, it was observed that sedative effects were deeper in the chloral hydrate group compared with the midazolam group. Results also showed side effects to be significantly lower in the midazolam group.18 In a study by Heistein et al,19 which aimed to assess the effects of chloral hydrate in the sedation of children before echocardiography, a total of 1095 patients were sedated by chloral hydrate and their vital signs were measured and recovered every 5 minutes, and no irreversible side effects or mortalities were reported and the most common side effects were respiratory and gastrointestinal.
Conclusion
According to the results of the current study, chloral hydrate and midazolam can be the right choice in pre-echocardiography sedation, in patients without cardiovascular risk factors.
Considering the similar effectiveness, more rapid onset, and shorter duration of sedation, besides less side effects of midazolam, researchers recommend more use of this drug. The most important problem we faced while performing this study was the scarcity and high cost of the oral forms of midazolam and chloral hydrate. Another limitation was lack of interest in parents due to their fear of the potential side effects of drugs. It is recommended that future studies evaluate the efficacy, effectiveness, and safety of intravenous midazolam.
Author Contributions
FS: Contributed to design; contributed to acquisition, analysis, and interpretation; drafted manuscript; critically revised manuscript; gave final approval; agrees to be accountable for all aspects of work ensuring integrity and accuracy.
HRR: Contributed to conception; contributed to acquisition and analysis; drafted manuscript; critically revised manuscript; gave final approval; agrees to be accountable for all aspects of work ensuring integrity and accuracy.
AE: Contributed to conception and design; contributed to interpretation; drafted manuscript; critically revised manuscript; gave final approval; agrees to be accountable for all aspects of work ensuring integrity and accuracy.
SAJ: Contributed to design; contributed to acquisition; drafted manuscript; critically revised manuscript; gave final approval; agrees to be accountable for all aspects of work ensuring integrity and accuracy.
Acknowledgments
The authors thank all the individuals who participated in this research, especially the echocardiography staff of Valiasr Hospital.
Footnotes
Authors’ Note: This study is the result of the thesis of Ali Ebrahimzadeh as the general physician’s degree (Thesis Number 808) and financial support of the Vice Chancellor for Research and Technology of Birjand University of Medical Sciences.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Financial support was provided by the Vice Chancellor for Research and Technology of Birjand University of Medical Sciences.
References
- 1. Schechter N, Berde CB, Vaster M, Foley KM. Pain in children with cancer. In: Foley KM, ed. Advances in Pain Research and Therapy. New York, NY: Raven Press; 1990:57-71. [Google Scholar]
- 2. Nicolson SC, Montenegro LM, Cohen MS, et al. A comparison of the efficacy and safety of chloral hydrate versus inhaled anesthesia for sedating infants and toddlers for transthoracic echocardiograms. J Am Soc Echocardiogr. 2010;23:38-42. [DOI] [PubMed] [Google Scholar]
- 3. Stern KW, Gauvreau K, Geva T, Benavidez OJ. The impact of procedural sedation on diagnostic errors in pediatric echocardiography. J Am Soc Echocardiogr. 2014;27:949-955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ljungman G, Kreuger A, Gordh T, Berg T, Sörensen S, Rawal N. Treatment of pain in pediatric oncology: a Swedish nationwide survey. Pain. 1996;68:385-394. [DOI] [PubMed] [Google Scholar]
- 5. Zeltzer LK, Altman A, Cohen D, LeBaron S, Munuksela EL, Schechter NL. American Academy of Pediatrics Report of the Subcommittee on the management of pain associated with procedures in children with cancer. Pediatrics. 1990;86(5 pt 2):826-831. [PubMed] [Google Scholar]
- 6. Hill GD, Walbergh DB, Frommelt PC. Efficacy of reconstituted oral chloral hydrate from crystals for echo sedation. J Am Soc Echocardiogr. 2016;29:337-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Napoli KL, Ingall CG, Martin GR. Safety and efficacy of chloral hydrate sedation in children undergoing echocardiography. J Pediatr. 1996;129:287-291. [DOI] [PubMed] [Google Scholar]
- 8. American Society of Health-System Pharmacists. Discontinued Drug Bulletin. Bethesda, MD: American Society of Health-System Pharmacists; 2012. [Google Scholar]
- 9. Conway A, Rolley J, Sutherland JR. Midazolam for sedation before procedures. Cochrane Database Syst Rev. 2016;CD009491. doi: 10.1002/14651858.CD009491.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wheeler DS, Jensen RA, Poss WB. A randomized, blinded comparison of chloral hydrate and midazolam sedation in children undergoing echocardiography. Clin Pediatr (Phila). 2001;40:381-387. [DOI] [PubMed] [Google Scholar]
- 11. Layangool T, Sangtawesin C, Kirawittaya T, et al. A comparison of oral chloral hydrate and sublingual midazolam sedation for echocardiogram in children. J Med Assoc Thai. 2008;91(suppl 3):S45-S52. [PubMed] [Google Scholar]
- 12. Hasani M, Soltani EA. Comparison of oral premedication with combination of midazolam with ketamine vs midazolam ketamine alone in children, Children Medical Center (year 2000). Tehran Univ Med J. 2002;60:423-428. [Google Scholar]
- 13. Sajedi P, Aghadavoudi O, Salimi-Jazi F. Oral midazolam alone or in combination with ketamine as oral premedication in pediatric ophthalmologic surgeries. J Isfahan Med Sch. 2014;31:1901-1909. [Google Scholar]
- 14. Kaviani N, Eshghi A, Tabibian P. Comparative evaluation of sedative effects of oral midazolam and oral ketamine in combination with inhalation sedation for pediatric behavior control during dental treatment. J Isfahan Dental School. 2008;2:46-51. [Google Scholar]
- 15. Moshiri E, Yazdi B, Khalili M. The comparison effect of midazolam with propofol on pre-operative anxiolysis. Arak Med Univ J. 2009;12:87-94. [Google Scholar]
- 16. Moshiri E, Modir H, Navabi M, Naziri M. Comparison effect of ketamine-propofol versus alfentanil-propofol for creating analgesia and sedation during cystoscopy. Arak Med Univ J. 2014;17:76-83. [Google Scholar]
- 17. Pariya N, Derakhshanfar H. Comparative Evaluation of Sedative Effects of Oral Midazolam and Oral Chloral Hydrate in Sedation for Pediatric During Lumbar Puncture. Tehran, Iran: Shahid Beheshti University of Medical Sciences; 2011. [Google Scholar]
- 18. Sigari S, Ataei M, Emamzadeghan R. Compare of Intra Nasal Midazolam vs Chloral Hydrate in Sedation of Under 5 Years Old Children Before Echocardiography. Ardabil, Iran: Ardabil University of Medical Sciences; 2013. [Google Scholar]
- 19. Heistein LC, Ramaciotti C, Scott WA, Coursey M, Sheeran PW, Lemler MS. Chloral hydrate sedation for pediatric echocardiography: physiologic responses, adverse events, and risk factors. Pediatrics. 2006;117:e434-e441. [DOI] [PubMed] [Google Scholar]


