Abstract
Background:
The monitoring of the effects of direct oral anticoagulants may be beneficial during emergencies and adverse events. We aimed to explore direct oral anticoagulant monitoring in “real-world” settings, in which monitoring methods are limited and loading time can be estimated based on only patient reports.
Methods:
In 164 patients, plasma anti-Xa activity was assessed using a STA®-Liquid Anti-Xa reagent (Diagnostica Stago, Asnieres, France), and prothrombin time was measured using HemosIL® RecombiPlasTin 2G (Instrumentation Laboratory, Bedford, MA, USA). The loading time was calculated according to the previous dosing time reported by the patient. In the clinic setting, rivaroxaban and apixaban were administered to 103 patients with atrial fibrillation and a blood sample was tested once during a clinic visit. In the hospitalization setting, edoxaban was administered to 61 patients undergoing arthroplasty for prophylaxis of a venous thrombosis and blood samples were tested 3 and 18 h after the last intake.
Results:
Plasma Xa activity in the clinical setting ranged widely (rivaroxaban: 1.1–424.4 ng/mL, apixaban: 15.4–469.2 ng/mL) during the 11.7 ± 7.0 h following the previous dose. The values varied over a wide range (up to a factor of 2) at the same loading time, especially around the peak period. The plasma anti-Xa activity of rivaroxaban and apixaban showed linear correlations with prothrombin time (R2 = 0.828 and 0.717, respectively). Edoxaban administration prolonged the prothrombin time by only 1.6 ± 1.1 s from the trough to the peak, to a degree that was negatively correlated with age, but not with plasma creatinine level, creatinine clearance, or body mass index.
Conclusion:
In real-world settings, plasma anti-Xa monitoring should be interpreted considering the wide variations in data, reflecting the variability in patient-reported loading time and interpatient variability.
Keywords: Direct oral anticoagulants, anti-Xa activity, prothrombin time
Introduction
One of the advantages of using direct oral anticoagulants (DOACs) is that they do not need monitoring. However, an estimation of the drug concentration may be required in an emergency situation, such as active bleeding, in order to determine the effect of anticoagulation.1 Among the various methods that are currently available for monitoring the effectiveness of DOACs, liquid chromatography is generally regarded as the gold standard. However, because it requires more expertise, is less widely available, and requires longer assay times compared with other modalities,2 it is typically not performed as a routine test. The prothrombin time (PT) and international normalized ratio of prothrombin time (PT-INR) have been utilized as the routine measurements for warfarin and are also known to be prolonged by the use of DOACs. However, the degree of prolongation varies among agents and drugs.3 For DOACs, direct measurements of anti-activated factor X (Xa) activity may provide a proxy scale for anti-Xa agents.4 Unfortunately, measurement techniques that are both accurate and precise for any DOACs are not widely available.5 Given that the anti-Xa assay is used only for research purposes in Japan and it is not covered by insurance, commercial calibrators are not always available. In our facility, the STA®-Liquid Anti-Xa assay (Diagnostica Stago, Asnieres, France)6 with rivaroxaban and apixaban calibrators was the only assay available during the study.
More fundamentally, measuring a drug’s loading time is difficult in “real-world” clinical settings, unlike in clinical trials for pharmacokinetics. Physicians have no choice but to estimate the drug intake time based solely on patient feedback. The scheduled visit and blood sampling cannot always be performed as punctually as they should be, especially in large Japanese hospitals, which are often overcrowded.
In ideal pharmacological studies, participants are instructed to take medicine at precise times and blood sampling is performed with knowledge of the precise loading time.7–9 However, in the clinical “real-world” settings, in which the loading time is calculated solely based on the patient’s reports and where access to anti-Xa assays is limited, these conditions may affect the patient’s parameters. Therefore, the aim of this study was to elucidate the clinical role of anti-Xa monitoring in “real-world” settings and to raise awareness of the underlying discrepancies compared to ideal conditions, as they have been previously reported. We evaluated the characteristics of the “real world” under two scenarios. The first scenario was a clinic setting, where blood samples were tested once during a clinic visit for patients with atrial fibrillation (AF) under rivaroxaban or apixaban. The timing of the last dose taken was recorded at the time of blood sampling. The second scenario was a hospitalization setting, where blood samples from arthroplasty patients taking edoxaban were analyzed to obtain peak and trough values. In addition to plasma anti-Xa activity, measurements of PT, activated partial thromboplastin time (APTT), Factor Xa, and other coagulation tests were performed to evaluate the feasibility of DOAC monitoring as a part of routine medical care. Thus, we aimed to elucidate the correlations between plasma anti-Xa activity and other confounding factors in “real-world” monitoring.
Methods and materials
This cross-sectional observational study was approved by our institutional review board based on the ethical guidelines of the Declaration of Helsinki. All patients gave their informed consent for the blood sampling and tests.
Patient population
A total of 164 patients who were taking DOACs for anticoagulation were included in this study. Among 103 patients with AF, rivaroxaban was administered in 66 (15 mg: 56 patients; 10 mg: 10 patients) and apixaban in 37 (10 mg: 29 patients; 5 mg: 8 patients). Edoxaban (30 mg) was administered in 61 patients for prophylaxis of venous thrombosis after total hip or knee arthroplasty. All dosage regimens were based upon the guidelines of the Japanese Pharmaceuticals and Medical Devices Agency, and no inappropriate dose reductions were implemented. No patient had cirrhosis or a hematological disorder.
Blood sampling
In the clinic scenario, blood samples were tested once for each patient during a clinic visit at the Keio University Hospital. Patients were instructed to take rivaroxaban or apixaban once or twice daily, as prescribed, for more than 7 days. At the next clinic visit, a blood sample was obtained and the time of the last dose was noted. The loading time was defined as the interval between the time of the last dose based on the patient’s report and the blood sampling time.
In the hospitalization scenario, blood sampling was performed twice in patients scheduled in standard clinical practice for arthroplasty at the Sonoda Joint Replacement and Sports Medical Center. Edoxaban was started 2 days after the surgery. Blood samples were drawn for testing on postoperative day 14 at around 3 h (a peak sample) and 18 h (a trough sample) after the last dose was delivered to the patients.
Plasma anti-Xa activity was analyzed using a STA-Liquid Anti-Xa reagent (Diagnostica Stago) and calibrated by a STA®-Rivaroxaban Calibrator kit (Diagnostica Stago, working range: 60–500 ng/mL) for all blood samples. Besides the discordance of the calibrators, all tests were performed according to the recommendations of the manufacturers. In samples from patients taking apixaban, a STA®-Apixaban Calibrator kit (Diagnostica Stago, working range: 20–500 ng/mL) was also used. Coagulation testing included APTT, using a HemosIL® SynthASil® kit (Instrumentation Laboratory, Bedford, MA, USA; normal range: 25.4–38.4 s, accuracy: ±10%); PT, using HemosIL® RecombiPlasTin 2G (Instrumentation Laboratory; normal range: 9.1–12.1 s, accuracy: ±15%); protein C, using HemosIL® Protein C (Instrumentation Laboratory; normal range: 69%–144%, accuracy: ±15%); prothrombin fragment 1 + 2 (F1 + 2), using an Enzygnost® F1 + 2 monoclonal kit (Siemens Healthcare K.K., Tokyo, Japan; normal range: 69–229 pmol/L, accuracy: ±20%); D-dimer, using LPIA-ACE D-dimerII (LSI Medience Corporation, Tokyo, Japan; normal range: <1.0 µg/mL, accuracy: ±15%); and Factor X, using HemosIL® Factor X (Instrumentation Laboratory; normal range: 77%–131%, accuracy: ±15%). Each sample was centrifuged and plasma samples were prepared according to the recommendation of the Clinical and Laboratory Standards Institute. Samples were kept at –80°C until assay. All assays were performed by the LSI Medience Corporation according to the manufacturer’s instructions.
Data analysis
Previous medical histories, drugs, and patient backgrounds, including the parameters required for calculating creatinine clearance (Ccr), were collected using the Cockcroft–Gault equation. The relationships between plasma anti-Xa activity, loading time, and other coagulation tests were analyzed. In the blood samples from patients taking apixaban, the difference between the values obtained using a rivaroxaban and an apixaban calibrator kit was analyzed. In blood samples from patients taking edoxaban, the degree of the PT prolongation between the trough and peak was assessed.
Statistical methods
Data are presented as mean ± standard deviation, percentage, or number of subjects. Differences between the groups were analyzed using the Mann–Whitney U test or analysis of variance. A p value of less than 0.05 was considered significant. The odds ratio (OR) and the corresponding 95% confidence interval (95% CI) were calculated. Pearson correlation coefficients were derived for associations between plasma anti-Xa activity and the other coagulation markers. Data are displayed in scatter plots and were assessed using linear or exponential approximation. A Bland–Altman and linear regression analyses were performed to compare the difference between the calibrators. All statistical analyses were performed using the SPSS statistical software (version 23.0; SPSS Inc., Chicago, IL, USA).
Results
Patient backgrounds
A total of 164 patients (age: 68 ± 10 years, male: n = 88, paroxysmal AF: n = 67, serum creatinine level: 0.8 ± 0.3 mg/dL) were investigated. The patient characteristics are shown in Table 1 (66 AF patients taking rivaroxaban, 37 AF patients taking apixaban, and 61 arthroplasty patients taking edoxaban). There was no significant difference among AF patients in age (p = 0.286), body mass index (p = 0.119), CHADS2 score (p = 0.988), or CHA2DS2-VASc score (p = 0.564). The loading time was more heterogeneous in patients receiving apixaban compared with patients receiving rivaroxaban. Neither the serum creatinine nor the Ccr levels differed (p = 0.934 and p = 0.084, respectively). The prevalence of female gender, gastric ulcer, or cancer did not differ between the groups. Only one of the patients who underwent arthroplasty had AF.
Table 1.
Patient characteristics.
| AF patients |
Arthroplasty patients |
|||||
|---|---|---|---|---|---|---|
| Rivaroxaban | Apixaban | p | OR | 95% CI | Edoxaban | |
| Age (years) | 67.5 ± 9.6 | 69.8 ± 8.9 | 0.286 | N/A | N/A | 67.8 ± 10.3 |
| Body mass index | 24.4 ± 3.1 | 23.2 ± 2.5 | 0.119 | N/A | N/A | 25.1 ± 4.8 |
| CHADS2 | 1.1 ± 1.1 | 1.1 ± 1 | 0.988 | N/A | N/A | 1.1 ± 1.1 |
| CHA2DS2-VASc | 2.1 ± 1.5 | 2.2 ± 1.3 | 0.564 | N/A | N/A | 2.6 ± 1.4 |
| Cr (mg/dL) | 0.9 ± 0.2 | 0.9 ± 0.3 | 0.934 | N/A | N/A | 0.6 ± 0.2 |
| Ccr (mL/min) | 77.7 ± 26.9 | 67.8 ± 23.1 | 0.084 | N/A | N/A | 86.2 ± 30.7 |
| BNP (pg/mL) | 84.3 ± 78.8 | 93.5 ± 65.2 | 0.237 | N/A | N/A | 35.3 ± 35.6 |
| Dosing period (days) | 146.1 ± 90 | 80.4 ± 55.7 | 0.001 | N/A | N/A | 12.1 ± 0.3 |
| Loading time (h) | 9.1 ± 6.3 | 6.4 ± 6.5 | 0.027 | N/A | N/A | 10.6 ± 7.6 |
| LVEF (%) | 59.2 ± 7.8 | 57.7 ± 8.4 | 0.396 | N/A | N/A | N/A |
| Left atrial size (cm) | 4.1 ± 0.6 | 4 ± 0.6 | 0.897 | N/A | N/A | N/A |
| LAA flow (cm/s) | 52.1 ± 14.5 | 64.1 ± 19.4 | 0.073 | N/A | N/A | N/A |
| Heart failure | 1 (1.5%) | 4 (10.8%) | 0.035 | 7.879 | 0.846–73.344 | 0 (0%) |
| Hypertension | 33 (50%) | 17 (45.9%) | 0.693 | 0.850 | 0.379–1.905 | 34 (55.7%) |
| Age > 65 years | 45 (68.2%) | 27 (73%) | 0.611 | 1.260 | 0.517–3.073 | 39 (63.9%) |
| Age > 75 years | 17 (25.8%) | 12 (32.4%) | 0.470 | 1.384 | 0.573–3.343 | 17 (27.9%) |
| Diabetes | 10 (15.2%) | 7 (18.9%) | 0.621 | 1.307 | 0.451–3.782 | 15 (24.6%) |
| Stroke | 6 (9.1%) | 0 (0%) | 0.059 | 0.619 | 0.529–0.723 | 2 (3.3%) |
| Vascular disease | 6 (9.1%) | 0 (0%) | 0.059 | N/A | N/A | 3 (4.9%) |
| Female | 13 (19.7%) | 13 (35.1%) | 0.084 | 2.208 | 0.891–5.472 | 50 (82%) |
| AF | 66 (100%) | 37 (100%) | N/A | N/A | N/A | 1 (1.6%) |
| Gastric ulcer | 4 (6.1%) | 1 (2.7%) | 0.447 | 0.431 | 0.046–4.002 | |
| Cancer | 6 (9.1%) | 6 (16.2%) | 0.280 | 1.935 | 0.576–6.502 | |
| Aspirin use | 2 (3%) | 1 (2.7%) | 0.924 | 0.889 | 0.078–10.147 | 16 (26.2%) |
AF: atrial fibrillation; BNP: brain natriuretic peptide; Cr: creatinine; Ccr: creatinine clearance; dosing time: days after initiating anticoagulation; LAA flow: flow velocity of the left atrial appendage measured using transesophageal echocardiography; loading time: hours from the last dose to the blood sampling test; LVEF: left ventricular ejection fraction.
Values are presented as mean ± standard deviation or number of patients (percentage). Values are compared among AF patients and all patients.
Anti-Xa activity versus loading time
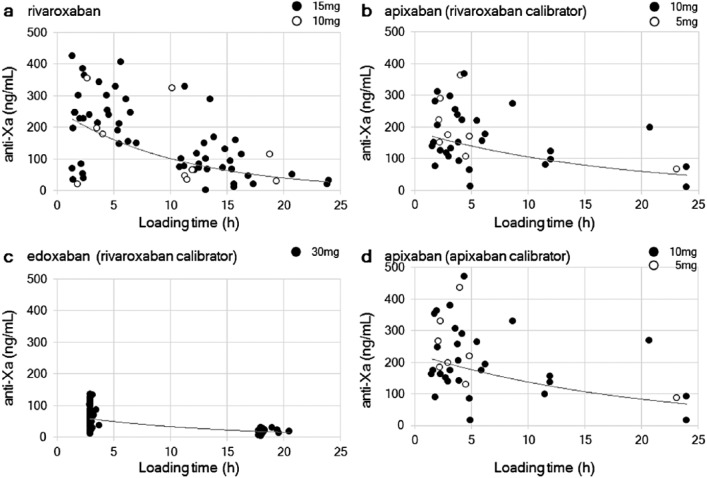
The values of plasma anti-Xa activity for each DOAC, measured using the rivaroxaban calibrator, are shown in Figure 1(a)–(c). The plasma anti-Xa activity decreased with the loading time for all DOACs. The values varied over a wide range (up to a factor of 2) at the same loading time, especially around the peak period. The dosing regimen did not appear to affect the plasma anti-Xa activity. Age, body mass index, and Ccr did not differ among the top and bottom five outliers that were selected from the scatter plot. The peak value of plasma anti-Xa activity did not differ according to dosing regimen, either in patients taking rivaroxaban (15 vs 10 mg, 214.8 ± 113.8 ng/mL (n = 23) vs 187.2 ± 136.9 ng/mL (n = 4), p = 0.489) or in those taking apixaban (10 vs 5 mg, 179.8 ± 96.1 ng/mL (n = 20) vs 209.9 ± 88.8 ng/mL (n = 7), p = 0.514).
Figure 1.
Plasma anti-Xa activity versus loading time. The plasma anti-Xa activity of (a) rivaroxaban, (b) apixaban, and (c) edoxaban using a rivaroxaban calibrator and (d) apixaban using an apixaban calibrator is shown. The dosage regimens are indicated by black and white dots. The estimated formula with an exponential approximation and the coefficient of determination (R2) is shown.
Comparison of calibrators
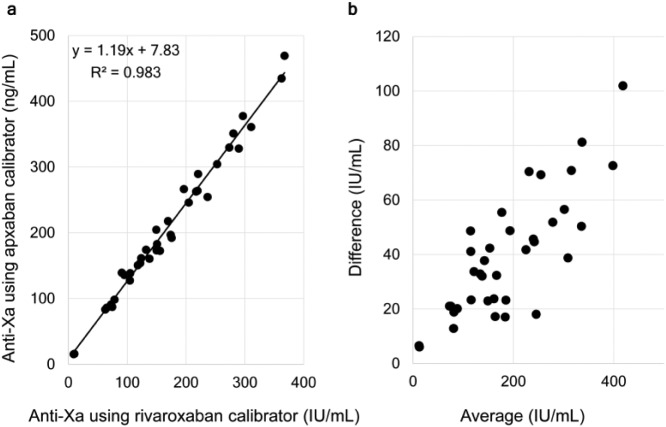
The blood samples of patients taking apixaban were also evaluated using the apixaban calibrator (Figure 1(d)). The correlation between measurements of plasma anti-Xa activity obtained using the rivaroxaban calibrator and the apixaban calibrator is shown in Figure 2. There was a strong coefficient of determination (R2 = 0.983), showing a linear correlation with an approximately 20% deviation between the two calibrators (value of apixaban calibrator =1.1859 × value of rivaroxaban calibrator + 7.826; Figure 2(a)). The Bland–Altman analysis showed a significant difference between the calibrators (p < 0.001; Figure 2(b)).
Figure 2.
Difference between calibrators. The relationship between the plasma anti-Xa activity of apixaban using a rivaroxaban and an apixaban calibrator is shown. (a) The blood samples as analyzed by both calibrators and the values are compared and (b) Bland–Altman analysis was also performed.
Correlation among coagulation tests
The correlations between plasma anti-Xa activity measured using a rivaroxaban calibrator and the other coagulation tests are shown in Table 2. There was a strong and significant correlation with PT values (rivaroxaban: r = 0.910, apixaban: r = 0.849, edoxaban: r = 0.759). The correlation with APTT values was also significant, but milder than that of PT values (rivaroxaban: r = 0.673, apixaban: r = 0.344, and edoxaban: r = 0.535). Factor X activity was moderately and negatively correlated with the plasma anti-Xa activity only in rivaroxaban (r = –0.561). The levels of protein C were significantly correlated only with the peak anti-Xa activity in edoxaban (r = 0.404). The levels of D-dimer and F1 + 2 were not significantly correlated. In patients taking edoxaban, only the peak values showed a positive collinearity with the PT and APTT (r = 0.659 and 0.539, respectively), although the trough values did not. The peak value was also mildly and negatively correlated with the Ccr value (r = –0.277), and the trough value was mildly and negatively correlated with age (r = –0.390).
Table 2.
Correlation between plasma anti-Xa activity and coagulation assays.
| Anti-Xa activity | Rivaroxaban (n = 66) |
Apixaban (n = 37) |
Edoxaban (n = 122) |
Edoxaban trough (n = 61) |
Edoxaban peak (n = 61) |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | r | p | r | p | |
| PT (s) | 0.910 | <0.001* | 0.849 | <0.001* | 0.759 | <0.001* | 0.113 | 0.385 | 0.659 | <0.001* |
| APTT (s) | 0.673 | <0.001* | 0.344 | 0.037* | 0.535 | <0.001* | 0.053 | 0.685 | 0.539 | <0.001* |
| Factor X (%) | −0.561 | <0.001* | −0.210 | 0.227 | −0.130 | 0.153 | 0.113 | 0.384 | 0.137 | 0.293 |
| D-Dimer (µg/mL) | −0.126 | 0.322 | −0.235 | 0.174 | −0.010 | 0.916 | 0.057 | 0.661 | −0.133 | 0.307 |
| Protein C (%) | 0.026 | 0.839 | 0.260 | 0.132 | 0.116 | 0.205 | 0.001 | 0.996 | 0.404 | 0.001* |
| F1 + 2 (pmol/L) | −0.090 | 0.478 | −0.253 | 0.142 | 0.096 | 0.293 | −0.135 | 0.298 | −0.116 | 0.374 |
| Body mass index | N/A | N/A | N/A | N/A | N/A | N/A | 0.120 | 0.362 | 0.069 | 0.599 |
| Age | N/A | N/A | N/A | N/A | N/A | N/A | −0.390 | 0.002* | 0.234 | 0.069 |
| Ccr (mL/min) | N/A | N/A | N/A | N/A | N/A | N/A | 0.093 | 0.480 | −0.277 | 0.032* |
APTT: activated partial thromboplastin time; Ccr: creatinine clearance; F1 + 2: prothrombin fragment 1 + 2; PT: prothrombin time.
Correlations between the plasma anti-Xa activity of patients taking rivaroxaban, apixaban, and edoxaban (all, peak, and trough values) and the coagulation tests are shown.
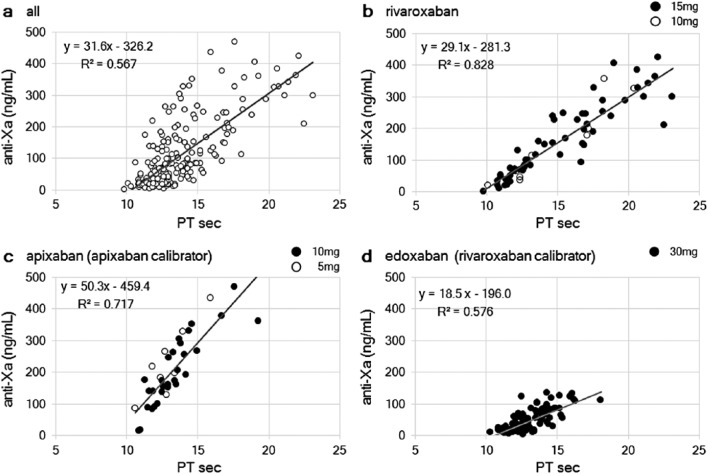
Anti-Xa activity versus PT
The correlation between plasma anti-Xa activity and PT using HemosIL® RecombiPlasTin 2G (Instrumentation Laboratory) is shown in Figure 3. Plasma anti-Xa activity had a positive linear correlation with PT prolongation, though with a weak coefficient of determination (R2 = 0.567; Figure 3(a)). For rivaroxaban, the coefficient of determination was the strongest among the DOACs (R2 = 0.828; Figure 3(b)), although a greater magnitude of PT prolongation was observed with higher anti-Xa activity. For apixaban, there was a sufficient linear correlation (R2 = 0.717; Figure 3(c)); however, the range of PT prolongation was narrower than for rivaroxaban. For edoxaban, a moderate linear correlation with PT prolongation was identified (R2 = 0.576; Figure 3(d)), although the data were regarded as references since they were derived using the rivaroxaban calibrator and a different population. Thus, the sensitivity to PT was the strongest for rivaroxaban among all DOACs. The degree of slope was the largest for apixaban among all DOACs (rivaroxaban vs apixaban vs edoxaban: 29.1 vs 50.3 vs 18.5), suggesting that PT is more sensitive to rivaroxaban and edoxaban than apixaban.
Figure 3.
Plasma anti-Xa activity versus prothrombin time (PT). The relationship between PT and the plasma anti-Xa activity in (a) all samples, (b) rivaroxaban, and (c) apixaban using an apixaban calibrator and (d) edoxaban using a rivaroxaban calibrator is shown. The dosage regimens are indicated by black and white dots. The estimated formula with an exponential approximation and the coefficient of determination (R2) is shown.
Peak versus trough values
For edoxaban, the paired peak and trough PT values were plotted (Figure 4). The mean difference between the peak and the trough PT values was only 1.6 ± 1.1 s (minimum: −0.6, maximum: 4.8). The difference between peak and trough values had a mild negative correlation with age (r = –0.444, p < 0.001), but not with height (p = 0.655), weight (p = 0.793), body mass index (p = 0.672), plasma creatinine level (p = 0.895), or Ccr (p = 0.207).
Figure 4.
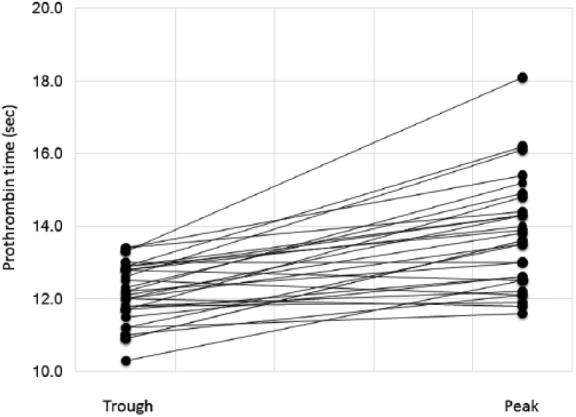
Prothrombin time prolongation after edoxaban administration. The paired values of the plasma anti-Xa activity at the peak (3 h) and trough (18 h) time points are shown.
Discussion
Variability of plasma anti-Xa activity in real-world settings
The real-world monitoring in this study revealed wide variations in data, reflecting the variability of patient-reported drug loading time as well as the inter-individual variability. Because DOACs have a short half-life, knowing the precise loading time may be critical for evaluating the relationship between effect and concentration. In “real-world” monitoring, both in the clinic and hospitalization settings, the correlation of the measured anti-Xa activity was not as clear as that in previous pharmacokinetic studies.10 The anti-Xa activity in one patient could be double that of another patient at the same loading time. The difference might be mainly due to the ambiguous loading time for the short half-life drug. Thus, physicians should be aware of these underlying discrepancies between the “real-world” and ideal pharmacologic studies and recognize the difficulties when interpreting coagulation assays. Based on these results, dose adjustments based solely on “real-world” anti-Xa monitoring are not recommended.
In addition, the drug dose and interpatient variability may have affected the data variations. Previous studies showed that the 10 mg dose of apixaban was significantly higher than the 5 mg dose in AF patients11 and a dose reduction of edoxaban decreased the mean anti-Xa activity by 20%–25%.12 In this study, patients receiving a reduced dose of DOACs did not show reduced plasma anti-Xa activity, although only a small number of patients were on the reduced regimen. All drugs were prescribed strictly according to the package insert, without any intentional dose reduction, which might suggest that the reduced regimen was appropriate in a Japanese population. We also showed that patients with higher anti-Xa activity were not always the elderly, or those with lower a body mass index or lower Ccr. The individual variations in anti-Xa activity need to be recognized.
PT versus anti-Xa activity
PT can be utilized for the routine monitoring of a drug’s effect, as is performed in warfarin management. Our data showed that the PT value obtained using HemosIL RecombiPlasTin 2G (Instrumentation Laboratory) had a linear correlation with plasma anti-Xa activity in patients taking rivaroxaban. However, the PT value using Thromborel S® and Normotest® have been reported to be poorly correlated with rivaroxaban concentrations (R2 = 0.52 and 0.09).13 In the case of apixaban, PT should not be used for estimating its effectiveness,14,15 given that the range of the PT prolongation was as sensitive compared to the other DOACs as was also shown in this study. The concentration of apixaban required in order to double the PT was reported to range from 700 to 3900 μg/L.16 For edoxaban, the mean prolongation was only 1.6 s, based on a comparison of the peak and trough values. This would not allow physicians to measure the drug efficacy in an arthroplasty setting, although it might not be relevant in the patients with AF.12,17 Thus, physicians should reevaluate the PT agents that are used in their facilities and must be aware of the individual characteristics of the PT prolongation for each DOAC.18
Is it worth monitoring plasma anti-Xa activity?
Given the difficulties shown in our real-world survey, monitoring DOACs may or may not be meaningful. First, it is worth monitoring in order to assure adherence, that is, to monitor whether the patient is actually taking the medication, although this can never be fully accurate without continuous monitoring. Second, it might be useful in an emergent situation or perioperative situation.19 The guidelines for emergent surgery propose that the drug plasma concentration should be less than 30 ng/mL for rivaroxaban.20 When a patient with pulmonary thromboembolism received two 150 mg doses of rivaroxaban, APTT was >40 s and PT was >20 s.21 The PT was reported to be prolonged to 46 s after 1400 mg of rivaroxaban.22 When the antidotes to DOACs, such as prothrombin complex fragment,23 Andexanet-alfa,24 and PER977,25 are or will be available, anti-Xa activity might be a valuable marker to determine whether they need to be administered. Third, it might be feasible in complicated cases. In cirrhosis, PT might not be an appropriate marker for the interpretation of anticoagulation, whereas a direct anti-Xa assay has been reported to be useful.26 The assay has also been utilized in patients with end-stage renal failure and a history of heparin-induced thrombocytopenia (HIT) under rivaroxaban in order to prevent major bleeding when treating HIT.27,28 Thus, measurement of the activity might yield independent information in addition to APTT or PT.
Since the concentration might not be equivalent to the effect, plasma anti-Xa activity might not parallel its anticoagulant action. When we consider the risk of thrombogenesis, other coagulation factors should be taken into account. Our data showed a possible relationship between edoxaban and protein C, while a previous study showed a relationship between rivaroxaban and F1 + 2 level.29 Regarding patient backgrounds, the decision to administer DOACs should take into account renal function, age, body weight, and other medications as a general rule; however, the majority of bleeding complications occur in patients with several prevailing risk factors.30 Since the plasma anti-Xa assay directly monitors the target of the DOACs, it might play a unique role in an evaluation of efficacy and risks. A longer follow-up to evaluate its effectiveness and associated bleeding risks is required.
Limitations
First, the number of patients was small. Second, the efficacy and risks were not fully elucidated because of a lack of major bleeding and thrombotic events. Third, the parameters affecting the plasma anti-Xa activity might be different depending on the institution and its facilities. Last, because not all calibrators for the STA-Liquid Anti-Xa reagent were available, the base analysis was performed using the rivaroxaban calibrator in a STA-Liquid Anti-Xa reagent. Therefore, further evaluation is required with the specific calibrators for each DOAC. Alternatively, heparin/low-molecular-weight heparin international standards would be appropriate for comparing the DOACs.
Conclusion
In real-world settings, the results of plasma anti-Xa monitoring should be interpreted considering the wide variations in data, reflecting the variability in patient-reported loading time in addition to interpatient variability.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethics approval: Ethical approval for this study was obtained from Keio University Ethics Committee (Approval No. 20130261).
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Informed consent: Verbal informed consent was obtained from all subjects before the study.
References
- 1. Koscielny J, Rutkauskaite E. Rivaroxaban and hemostasis in emergency care. Emerg Med Int 2014; 2014: 935474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gosselin RC, Adcock D, Hawes EM, et al. Evaluating the use of commercial drug-specific calibrators for determining PT and APTT reagent sensitivity to dabigatran and rivaroxaban. Thromb Haemost 2015; 113: 77–84. [DOI] [PubMed] [Google Scholar]
- 3. Cuker A. Laboratory measurement of the non-vitamin K antagonist oral anticoagulants: selecting the optimal assay based on drug, assay availability, and clinical indication. J Thromb Thrombolysis 2016; 41: 241–247. [DOI] [PubMed] [Google Scholar]
- 4. Lippi G, Ardissino D, Quintavalla R, et al. Urgent monitoring of direct oral anticoagulants in patients with atrial fibrillation: a tentative approach based on routine laboratory tests. J Thromb Thrombolysis 2014; 38: 269–274. [DOI] [PubMed] [Google Scholar]
- 5. Samuelson BT, Cuker A, Siegal DM, et al. Laboratory assessment of the anticoagulant activity of direct oral anticoagulants: a systematic review. Chest 2017; 151: 127–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rathbun S, Tafur A, Grant R, et al. Comparison of methods to determine rivaroxaban anti-factor Xa activity. Thromb Res 2015; 135: 394–397. [DOI] [PubMed] [Google Scholar]
- 7. Bardy G, Fischer F, Appert A, et al. Is anti-factor Xa chromogenic assay for Rivaroxaban appropriate in clinical practice? Advantages and comparative drawbacks. Thromb Res 2015; 136: 396–401. [DOI] [PubMed] [Google Scholar]
- 8. Douxfils J, Tamigniau A, Chatelain B, et al. Comparison of calibrated chromogenic anti-Xa assay and PT tests with LC-MS/MS for the therapeutic monitoring of patients treated with rivaroxaban. Thromb Haemost 2013; 110: 723–731. [DOI] [PubMed] [Google Scholar]
- 9. Samama MM, Contant G, Spiro TE, et al. Evaluation of the anti-factor Xa chromogenic assay for the measurement of rivaroxaban plasma concentrations using calibrators and controls. Thromb Haemost 2012; 107: 379–387. [DOI] [PubMed] [Google Scholar]
- 10. Gosselin R, Grant RP, Adcock DM. Comparison of the effect of the anti-Xa direct oral anticoagulants apixaban, edoxaban, and rivaroxaban on coagulation assays. Int J Lab Hematol 2016; 38: 505–513. [DOI] [PubMed] [Google Scholar]
- 11. Skeppholm M, Al-Aieshy F, Berndtsson M, et al. Clinical evaluation of laboratory methods to monitor apixaban treatment in patients with atrial fibrillation. Thromb Res 2015; 136: 148–153. [DOI] [PubMed] [Google Scholar]
- 12. Ruff CT, Giugliano RP, Braunwald E, et al. Association between edoxaban dose, concentration, anti-Factor Xa activity, and outcomes: an analysis of data from the randomised, double-blind ENGAGE AF-TIMI 48 trial. Lancet 2015; 385: 2288–2295. [DOI] [PubMed] [Google Scholar]
- 13. Konigsbrugge O, Quehenberger P, Belik S, et al. Anti-coagulation assessment with prothrombin time and anti-Xa assays in real-world patients on treatment with rivaroxaban. Ann Hematol 2015; 94: 1463–1471. [DOI] [PubMed] [Google Scholar]
- 14. Douxfils J, Chatelain C, Chatelain B, et al. Impact of apixaban on routine and specific coagulation assays: a practical laboratory guide. Thromb Haemost 2013; 110: 283–294. [DOI] [PubMed] [Google Scholar]
- 15. Gouin-Thibault I, Flaujac C, Delavenne X, et al. Assessment of apixaban plasma levels by laboratory tests: suitability of three anti-Xa assays. A multicentre French GEHT study. Thromb Haemost 2014; 111: 240–248. [DOI] [PubMed] [Google Scholar]
- 16. Hillarp A, Gustafsson KM, Faxalv L, et al. Effects of the oral, direct factor Xa inhibitor apixaban on routine coagulation assays and anti-FXa assays. J Thromb Haemost 2014; 12: 1545–1553. [DOI] [PubMed] [Google Scholar]
- 17. Verhamme P, Wells PS, Segers A, et al. Dose reduction of edoxaban preserves efficacy and safety for the treatment of venous thromboembolism. An analysis of the randomised, double-blind HOKUSAI VTE trial. Thromb Haemost 2016; 116: 747–753. [DOI] [PubMed] [Google Scholar]
- 18. Shimomura D, Nakagawa Y, Kondo H, et al. The influence of assay selection on prothrombin time measured in patients treated with rivaroxaban for nonvalvular atrial fibrillation. J Clin Lab Anal 2016; 30: 941–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lessire S, Douxfils J, Pochet L, et al. Estimation of rivaroxaban plasma concentrations in the perioperative setting in patients with or without heparin bridging. Clin Appl Thromb Hemost 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pernod G, Albaladejo P, Godier A, et al. Management of major bleeding complications and emergency surgery in patients on long-term treatment with direct oral anticoagulants, thrombin or factor-Xa inhibitors: proposals of the working group on perioperative haemostasis (GIHP)–March 2013. Arch Cardiovasc Dis 2013; 106: 382–393. [DOI] [PubMed] [Google Scholar]
- 21. Sajkov D, Gallus A. Accidental rivaroxaban overdose in a patient with pulmonary embolism: some lessons for managing new oral anticoagulants. Clin Med Insights Case Rep 2015; 8: 57–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Linkins LA, Moffat K. Monitoring the anticoagulant effect after a massive rivaroxaban overdose. J Thromb Haemost 2014; 12: 1570–1571. [DOI] [PubMed] [Google Scholar]
- 23. Eerenberg ES, Kamphuisen PW, Sijpkens MK, et al. Reversal of rivaroxaban and dabigatran by prothrombin complex concentrate: a randomized, placebo-controlled, crossover study in healthy subjects. Circulation 2011; 124: 1573–1579. [DOI] [PubMed] [Google Scholar]
- 24. Lu G, DeGuzman FR, Hollenbach SJ, et al. A specific antidote for reversal of anticoagulation by direct and indirect inhibitors of coagulation factor Xa. Nat Med 2013; 19: 446–451. [DOI] [PubMed] [Google Scholar]
- 25. Ansell JE, Bakhru SH, Laulicht BE, et al. Use of PER977 to reverse the anticoagulant effect of edoxaban. N Engl J Med 2014; 371: 2141–2142. [DOI] [PubMed] [Google Scholar]
- 26. Potze W, Arshad F, Adelmeijer J, et al. Routine coagulation assays underestimate levels of antithrombin-dependent drugs but not of direct anticoagulant drugs in plasma from patients with cirrhosis. Br J Haematol 2013; 163: 666–673. [DOI] [PubMed] [Google Scholar]
- 27. Than H, Loh JB, Sum CL, et al. Clinical utility of anti-Xa monitoring and differential sensitivity of prothrombin time assays: an illustrated case report. J Clin Pathol 2015; 68: 318–319. [DOI] [PubMed] [Google Scholar]
- 28. Linkins LA, Warkentin TE, Pai M, et al. Rivaroxaban for treatment of suspected or confirmed heparin-induced thrombocytopenia study. J Thromb Haemost 2016; 14: 1206–1210. [DOI] [PubMed] [Google Scholar]
- 29. Tajiri K, Sato A, Harunari T, et al. Impact of rivaroxaban compared with warfarin on the coagulation status in Japanese patients with non-valvular atrial fibrillation: a preliminary analysis of the prothrombin fragment 1+2 levels. J Cardiol 2015; 65: 191–196. [DOI] [PubMed] [Google Scholar]
- 30. Pfeilschifter W, Luger S, Brunkhorst R, et al. The gap between trial data and clinical practice—an analysis of case reports on bleeding complications occurring under dabigatran and rivaroxaban anticoagulation. Cerebrovasc Dis 2013; 36: 115–119. [DOI] [PubMed] [Google Scholar]