Abstract
Paulownia tomentosa is a fast-growing tree species with multiple uses. It is grown worldwide, but is native to China, where it is widely cultivated in saline regions. We previously confirmed that autotetraploid P. tomentosa plants are more stress-tolerant than the diploid plants. However, the molecular mechanism underlying P. tomentosa salinity tolerance has not been fully characterized. Using the complete Paulownia fortunei genome as a reference, we applied next-generation RNA-sequencing technology to analyze the effects of salt stress on diploid and autotetraploid P. tomentosa plants. We generated 175 million clean reads and identified 15,873 differentially expressed genes (DEGs) from four P. tomentosa libraries (two diploid and two autotetraploid). Functional annotations of the differentially expressed genes using the Gene Ontology and Kyoto Encyclopedia of Genes and Genomes databases revealed that plant hormone signal transduction and photosynthetic activities are vital for plant responses to high-salt conditions. We also identified several transcription factors, including members of the AP2/EREBP, bHLH, MYB, and NAC families. Quantitative real-time PCR analysis validated the expression patterns of eight differentially expressed genes. Our findings and the generated transcriptome data may help to accelerate the genetic improvement of cultivated P. tomentosa and other plant species for enhanced growth in saline soils.
Introduction
Plants, as sessile organisms, must cope with a multitude of biotic and abiotic stresses throughout their life cycle. Among the abiotic stresses (e.g., high salinity, extreme temperatures, drought, heavy metal contamination, and nutrient deficiency), salt stress is the most serious threat to sustainable agricultural productivity worldwide, but especially in developing countries [1]. High salinity affects more than 800 million ha of land, which represents more than 6% of the total global land area. This area includes 45 million ha (i.e., approximately 20%) of the 230 million ha of irrigated land [2]. Soil salinity affects plants in two ways: One, high salt concentrations make it difficult for roots to take up enough water; and two, high intracellular salt concentrations can be toxic to plants.
Paulownia tomentosa is indigenous to China, where it grows in the plains and at altitudes up to 2000 m [3]. This tree species has been introduced to Japan and Southeast Asia, Australia, Brazil, Europe, and North and Central America. P. tomentosa is an economically important tree species in the family Scrophulariaceae. It is used to make aircraft parts, toys, musical instruments, plywood, furniture, and medicinal compounds [4]. Paulownia species are also useful as fertilizers and fodder [3]. Its benefits to the environment are partly based on the fact it can grow in nutrient-poor soil and has a deep root system [5, 6]. These characteristics make P. tomentosa potentially useful for reforestation of areas with nutrient-poor soils [7]. P. tomentosa trees can adapt to different soil conditions and climates [8, 9], and are planted mainly in salinized soil, saline-alkali soil [10], or in regions with limited irrigation water [11]. This suggests that this tree species may be genetically adapted to saline conditions and other stresses. Thus, the molecular mechanisms regulating the adaptation of P. tomentosa to salt stress should be characterized.
An autotetraploid P. tomentosa line has been generated from a diploid line using colchicines [12]. Compared with diploid P. tomentosa plants, the autotetraploid plants exhibit higher net photosynthetic rates and better wood physical properties [13, 14]. We previously analyzed the morphology and physiology of P. tomentosa trees, and confirmed that autotetraploid P. tomentosa trees are more stress resistant than the diploid trees [15–17].
Before the Paulownia fortunei genome was sequenced, several studies of the P. tomentosa transcriptome [9, 18–20], miRNAs [21–23], and proteome were conducted [24]. These studies were focused mainly on diploid and autotetraploid P. tomentosa, Paulownia witches’ broom disease, and drought, and salt stress against the background of the transcriptome data. The genomes of many plant species have now been sequenced, including Phoenix dactyliferaL. [25], Malus × domesticaBorkh [26], and Populustrichocarpa [27], and the increasing availability of sequenced plant genomes has encouraged researchers to analyze non-model plants using high-throughput sequencing techniques. Sequenced genomes also improve the accuracy of gene annotations. However, until now, there have been no published investigations focused on Paulownia species, including P. tomentosa, based on the P. fortunei genome. Additionally, there has been very little research into the effects of salt stress on P. tomentosa [20], and the mechanism regulating salt tolerance has not been elucidated.
In this study, we used an RNA-sequencing (RNA-Seq) technique based on the P. fortunei genome to analyze the transcript profiles of salt-stressed and control diploid and autotetraploid P. tomentosa lines. The diploid and autotetraploid P. Tomentosa trees were acquired from the same tissue culture, and may represent biological replicates. The autotetraploid P. tomentosa line was considered to be more appropriate for investigating plant responses to salt stress than the natural diploid line. The Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) databases were used to analyze the differentially expressed genes (DEGs) among four pairwise comparisons. The expression patterns of eight DEGs were confirmed with a quantitative real-time polymerase chain reaction (qRT-PCR) assay. Additionally, bioinformatics analyses helped to elucidate plant hormone signal transduction pathways (abscisic acid and cytokinin) and photosynthetic activities. Furthermore, some transcription factors (TFs), such as bHLH, MYB, and NAC, were observed to be associated with salt stress responses or ploidy levels. To the best of our knowledge, this is the first report comparing the diploid and autotetraploid P. tomentosa lines based on the whole genome sequence of P. fortunei. The genetic basis of P. tomentosa salt tolerance was also investigated.
Materials and methods
Plant materials and salt treatments
All plant materials used in this study were obtained from the Institute of Paulownia, Henan Agricultural University, Zhengzhou, Henan Province, China. The tissue-cultured diploid and autotetraploid P. tomentosa seedlings were grown for 30 days at 25 ± 2°C on half-strength Murashige and Skoogmedium [28] with a 16-h photoperiod (light intensity: 130 μmol m−2 s−1). The test-tube plantlets were then transferred outdoors into nutritive bowls containing normal garden soil supplemented with 0.11% (quality score) NaCl solution, and grown for another 30 days. Consistently growing healthy seedlings were transferred to larger nutritive bowls with trays underneath. After 50 days, the uniformly growing seedlings were irrigated with 0% (control) or 0.4% (quality score) NaCl solutions. For the salt treatment, NaCl was weighed and divided into three equal parts, which were dissolved in water and poured into the bowls. The water that accumulated in the trays below the bowls was poured back into the bowls. The seedlings were treated with the control or salt solution every 3 days. After all of the salt water was added back into the bowls, the plants were watered every 2 days to maintain the soil moisture content at 75%. After 15 days, the second pair of leaves (fully expanded leaves) from the apex shoot of the control and salt-treated diploid and autotetraploid P. tomentosa plants were collected and immediately frozen in liquid nitrogen. The samples were stored at −80 ± 2°C. The control diploid and autotetraploid P. tomentosa samples were named PT2 and PT4, respectively, while the salt-treated diploid and autotetraploid samples were named PT2S and PT4S, respectively.
RNA extraction, cDNA library preparation, and sequencing
Total RNA was extracted from PT2, PT4, PT2S, and PT4S leaf samples using the Plant RNA Isolation Kit (AutoLab, Beijing, China) and concentrated using an RNeasy MinElute Cleanup Kit (Qiagen, Valencia, CA, USA). The RNA was then treated with DNase I to eliminate any contaminating genomic DNA. Oligo-d(T) magnetic beads were used to isolate mRNA from the purified total RNA. The mRNA was fragmented in fragmentation buffer (Life Technologies, Beijing, China), and then used as the template for cDNA synthesis. Short fragments were purified and resolved with ethidium bromide buffer for the subsequent end-repair and A (adenine)-tailing. The short fragments were linked to adapters, and analyzed by agarose gel electrophoresis. The suitable fragments were selected as templates for qRT-PCR. The quality and quantity of the sample libraries were analyzed using an Agilent 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA, USA). The qRT-PCRs were conducted using an ABI StepOnePlus Real-Time PCR System (ABI, New York, NY, USA). Finally, the libraries were sequenced on an IlluminaHiSeq™ 2000 platform.
Quality control and alignment/mapping of clean reads
The original image data were transformed into sequence data via base calling. These raw reads were filtered using the SOAPnuke program, as previously described [20], and the remaining “clean” reads were used in the downstream bioinformatics analyses. The quality of the clean reads data was assessed using base composition and quality distribution charts. The remaining reads were aligned to the P. fortunei genome and gene sequences using BWA and Bowtie, respectively. The statistics of alignment results will be presented for each reference. During the RNA-sequencing experiment, transcripts were chemically fragmented and then sequenced. The distribution of reads on the genes was used to evaluate the randomness of the RNA fragmentation associated with the constructed libraries [29].
Gene expression analysis
Gene expression levels were determined with the RSEM (RNA-Seq by Expectation Maximization) software package. The FPKM method (fragments per kb per million fragments) was used to calculate expression levels and to identify DEGs [30]. In multiple tests, the false discovery rate (FDR) was applied to determine the threshold p-value [31]. FDR ≤ 0.001 and absolute log2 ratio ≥ 1 were used as the threshold to evaluate the significance of gene expression differences.
Correlations between two samples were assessed based on the FPKM result. Ideally, the squared correlation value should be ≥ 0.92 according to the standard recommended by the ENCODE. The distances between expressed genes were calculated according to the Euclidean method. The sum of squared deviations algorithm was used to calculate the distance between samples so that a cluster tree could be built. The DEGs between two libraries were identified using the Audic-Claverie statistic [32]. The DEGs were functionally annotated based on GO terms and KEGG pathways.
Analysis of differentially expressed genes
The DEGs were first mapped to GO terms in the GO database, and the number of genes for each term was calculated. Then, the hypergeometric test was used to identify significantly enriched GO terms (http://www.geneontology.org/). The calculated p-values were adjusted using the Bonferroni correction [33], with a corrected p-value ≤ 0.05 used as the threshold. GO terms that fulfilled, this condition were defined as significantly enriched, and were considered to describe the main biological functions of the DEGs. The WEGO program [34] was used to functionally classify the DEGs and to assess the distribution of gene functions in P. tomentosa at a macro level. The KEGG database was used to identify enriched pathways associated with the DEGs [33].
TFs generally contain a DNA-binding domain and a trans-acting functional domain [35]. We used the hmmsearch program to search the HMMS database for domain characteristics, and to predict if a gene encoded a TF. Additionally, the TopHat program was used [36] to analyze the various types of alternative splicing (AS)-related clean reads mapped to the P. fortunei reference genome in the four samples. The AS events that were identified in both replicates were considered as stable events.
Comparison of gene expression profiles among different samples
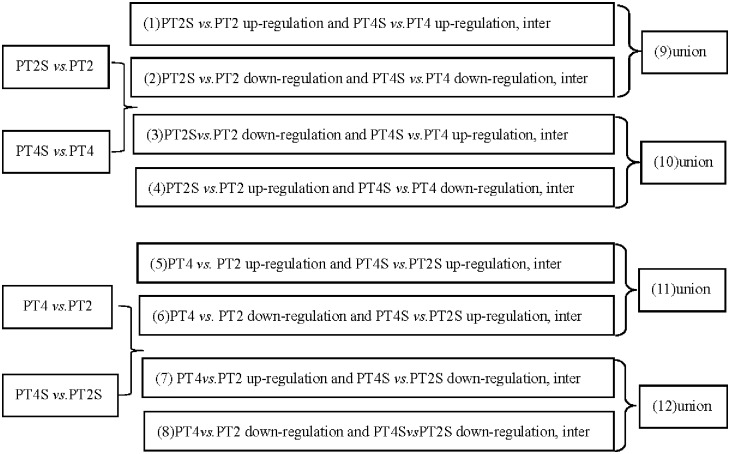
The following pairwise comparisons of DEGs were evaluated (Fig 1): Comparison A: co-up- and co-down-regulation in PT2S vs. PT2 and PT4S vs. PT4; Comparison B: down-regulation in PT2S vs. PT2, and up-regulation in PT4S vs. PT4 as well as up-regulation in PT2S vs. PT2, and down-regulation in PT4S vs. PT4; Comparison C: up-regulation in PT4 vs. PT2 and PT4S vs. PT2S as well as down-regulation in PT4 vs. PT2 and up-regulation in PT4S vs. PT2S; and Comparison D: up-regulation in PT4 vs. PT2, and down-regulation in PT4S vs. PT2S as well as down-regulation in PT4 vs. PT2 and PT4S vs. PT2S.
Fig 1. Comparison schemes of the four samples.
PT2 and PT4 represent the diploid and autotetraploid P. tomentosa with salt-untreated, PT2S and PT4S represents the diploid and autotetraploid P. tomentosa with salt-treated (0.4% Nacl).(1) Differentially expressed genes (DEGs) were co-up-regulation between the PT2S vs. PT2 and PT4S vs. PT4 comparison to screen for gene from both diploid and autotetraploid P. tomentosa after salt treatment were strengthened. (2) DEGs co-down-regulation in the PT2S vs. PT2 and PT4S vs. PT4 comparison. (3) DEGs down-regulation in the PT2S vs. PT2 comparison, but up-regulation in the PT4S vs. PT4 comparison. (4) DEGs up-regulation in the PT2S vs. PT2 comparison, but down-regulation in the PT4S vs. PT4 comparison. (5) DEGs were co-up-regulation between the PT4 vs. PT2 and PT4S vs. PT2S comparison. (6) DEGs down-regulation in the PT4 vs. PT2 comparison but up-regulation in the PT4S vs. PT2S comparison. (7) DEGs up-regulation in the PT4 vs. PT2 comparison but down-regulation in the PT4S vs. PT2S comparison. (8)DEGs co-down-regulation in the PT4 vs. PT2 and PT4S vs. PT2S comparison. Comparison A: (1) and (2). Comparison B: (3) and (4). Comparison C: (5) and (6). Comparison: from (7) and (8).
Quantitative real-time polymerase chain reaction analysis of differentially expressed genes
Total RNA that was extracted from the PT2 and PT4 leaf samples as described above. Three independent biological samples of each were employed in this analysis. The SsoAdvanced™ SYBR® Green Supermix (Bio-Rad, Hercules, CA, US) was used for the qRT-PCRs. The amplification reactions were performed as follows: 95°C for 3 min, followed by 40 cycles of 95°C for 10 s and 55°C for 30 s, and derived melting curves were obtained. The reactions were performed on a CFX96™ Real-Time System (Boi-Rad, Hercules, CA, USA) and all reactions were run in triplicate. 18S rRNA was used as an internal reference gene. The relative expression levels were calculated using the 2-ΔΔCt method. The primers used for the qPCR reactions were listed in Table 1.
Table 1. Primers of qRT-PCR for validation of the selected genes.
| Gene ID | Description | Primers (5’-3’) |
|---|---|---|
| PAU016338.1 | predicted protein | F: GCTAACAAGGAACTGAAT: AATTGAACTGTGTATGCT |
| PAU023935.1 | phosphoinositide phospholipase C 2 isoform 1 | F: GCCACAATTCTTATCTGA: CAATAACTCTTACACCTCTA |
| PAU024013.1 | zinc finger A20 and AN1 domain-containing | F: CTATGAAGCAAGAACAAG: ATAACGATAGCAACATCT |
| PAU022560.1 | fructose 1,6 bisphosphate aldolase class 1 | F: GATATTATTGGATGGTGAACA: CTCAGCAAGGTAGAAGAA |
| PAU015218.1 | probable serine/threonine-protein kinase Cx32 | F: TTGCTGTTAAGAAGTTGAA: CACCAGATTAGGATGAGA |
| PAU018076.1 | hypothetical protein PRUPE_ppa005569mg | F: TTGAGGATGGAAGTGATA: AATACTGACCTTATGCTTAG |
| PAU000558.1 | putative receptor-like serine-threonine protein kinase | F: GCTGCTTCTTATTCTTAT: AATGATTCCAACTATTCC |
| PAU011992.1 | hypothetical protein PRUPE_ppa005598mg | F: AGCCAAGAAGGTTATTATTAC: GAGAGTAGTCCTGTTCATT |
Results
Sequencing of mRNA and alignments with the reference genes and genome
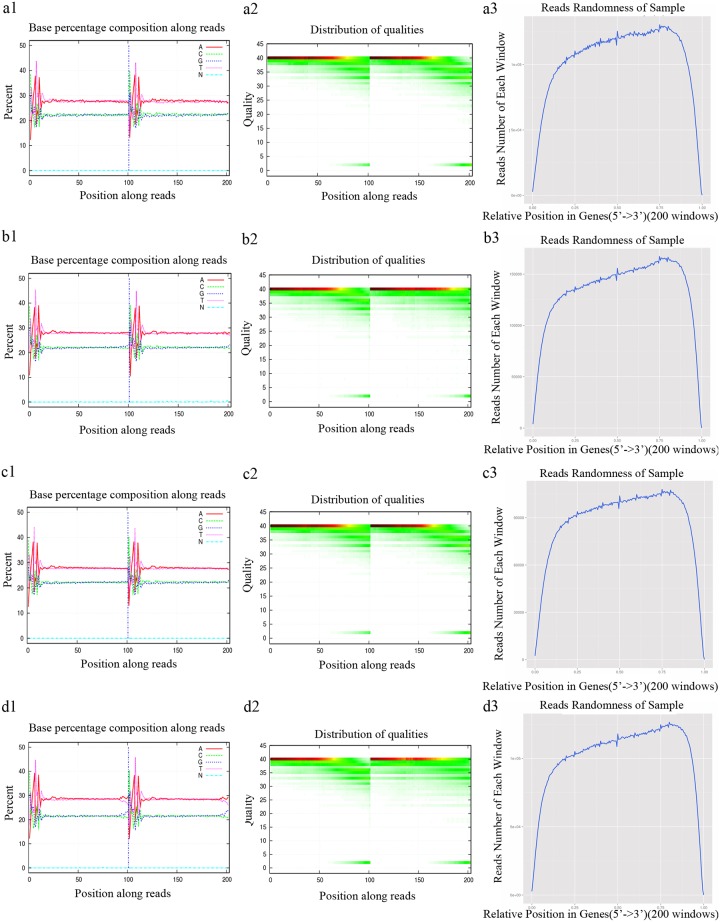
Approximately 187 million raw reads were generated for the four sequenced libraries (PT2, PT2S, PT4, and PT4S). Low-quality reads as well as reads with adapter sequences or several unknown bases were eliminated. The quality of the remaining clean reads was assessed using base composition and quality distribution charts. We observed a balanced base composition, with a T curve that was in accordance with the A curve, as well as a satisfactory base composition, high quality sequences, and base ratios that were mostly > 20 (Fig 2). The clean reads were evenly distributed on the genes as indicated by the assessment of the randomness. A total of 175 million clean reads (43,592,404 from PT2, 52,337,180 from PT2S, 33,909,164 from PT4, and 45,725,468 from PT4S) were generated with Q20 of 98.2% (PT2), 96.86% (PT2S), 98.17% (PT4), and 97.24% (PT4S), and Q30 of 95.1% (PT2), 93.12% (PT2S), 95.16% (PT4), and 93.17% (PT4S). The GC content was 44.7% (PT2), 45.15% (PT2S), 44.68% (PT4), and 43.29% (PT4S). These results suggested that high-quality sequencing data were obtained. The clean reads were mapped to the P. fortunei genes and genome with mapping means of 48.92% and 71.63% respectively (Table 2). We observed that 8.95–17.62% of the total mapped clean reads were aligned to two or more positions, and were considered multi-position matches. Multi-position matches can be problematic during transcriptome-level studies, because they are affected by read complexity and length, Therefore, we excluded the multi-position reads from subsequent analyses to decrease the error rate. The unmapped reads may represent novel genes.
Fig 2. Sequencing data assessment.
Base composition of clean data in PT2 (a1), PT2S (b1), PT (c1), and PT4S (d1); base quality of clean data in PT2 (a2), PT2S (b2), PT4 (c2), and PT4S (d2); reads distribution on PT2 (a3), PT2S (b3), PT4 (c3), and PT4S (d3) genes.
Table 2. Statistics of alignment (map to reference gene and genome) from the four libraries (exposed to salt stress for 15 days).
| Mapping Type | PT2 | PT2S | PT4 | PT4S | ||||
|---|---|---|---|---|---|---|---|---|
| map to reference gene | map to reference genome | map to reference gene | map to reference genome | map to reference gene | map to reference genome | map to reference gene | map to reference genome | |
| Total clean Reads | 43592404 | 43592404 | 52337180 | 52337180 | 33909164 | 33909164 | 45725468 | 45725468 |
| Total BasePairs | 4402832804 | 4402832804 | 5286055180 | 5286055180 | 3424825564 | 3424825564 | 4618272268 | 4618272268 |
| Total Mapped Reads | 20876540 (47.89%) |
33862719 (77.68%) |
26809022 (51.22%) |
36218429 (69.20%) |
17561062 (51.79%) |
25551420 (75.35%) |
20476884 (44.78%) |
29388276 (64.27%) |
| Perfect Match | 11357231 (26.05%) |
18314073 (42.01%) |
13019282 (24.88%) |
17207067 (32.88%) |
9174493 (27.06%) |
13318604 (39.28%) |
9426154 (20.61%) |
12806562 (28.01%) |
| Mismatch | 9519309 (21.84%) |
15548646 (35.67%) |
13789740 (26.35%) |
19011362 (36.32%) |
8386569 (24.73%) |
12232816 (36.08%) |
11050730 (24.17%) |
16581714 (36.26%) |
| Unique Match | 13702658 (31.43%) |
28050402 (64.35%) |
17735298 (33.89%) |
31131225 (59.48%) |
11585642 (34.17%) |
22045482 (65.01%) |
13482456 (29.49%) |
25295986 (55.32%) |
| Multi-position Match | 7173882 (16.46%) |
5812317 (13.33%) |
9073724 (17.34%) |
5087204 (9.72%) |
5975420 (17.62%) |
3505938 (10.34%) |
6994428 (15.30%) |
4092290 (8.95%) |
| Total Unmapped Reads | 22715862 (52.11%) |
9729685 (22.32%) |
25528156 (48.78%) |
16118751 (30.80%) |
16348100 (48.21%) |
8357744 (24.65%) |
25248582 (55.22%) |
16337192 (35.73%) |
Overall analysis of gene expression
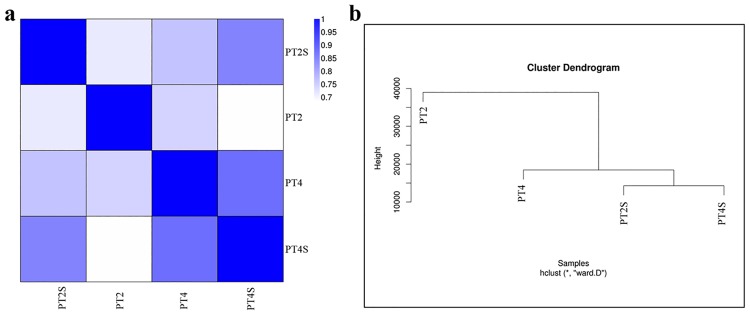
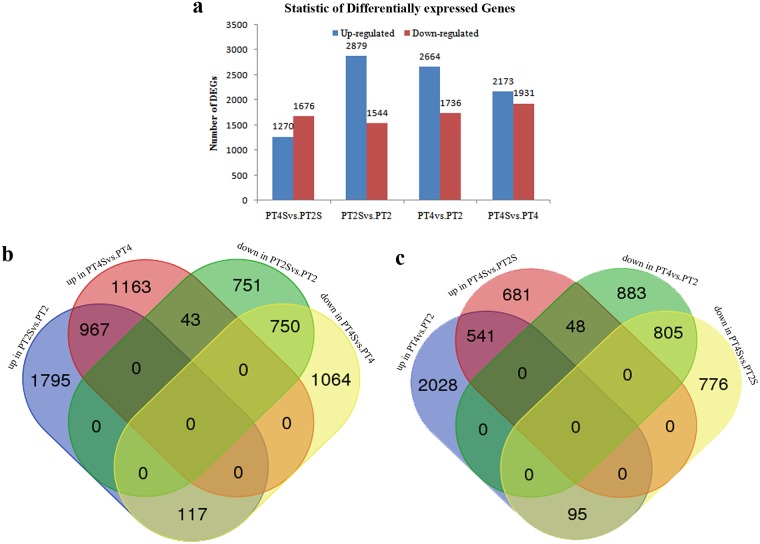
The correlation value for PT4 and PT4S was > 0.86, indicating the molecular factors responsive to salt stress partially overlapped. The correlations among the other samples are indicated in Fig 3A and S1 Table. The correlation values were close to 1 for the same samples in almost every experiment, indicating that our sequencing data were reliable and the selected samples were reasonable. The clustering of the expression profiles for the salt stress and control treatments of the four samples is presented in Fig 3B. The dendrogram revealed that PT2S and PT4S sequences tended to cluster together. We detected 4,423 DEGs between PT2S and PT2, and 4,104 DEGs between PT4S and PT4. Additionally, 2,946 and 4,400 DEGs were observed for the comparisons between PT4S and PT2S and between PT4 and PT2, respectively (Fig 4A, S2 Table).
Fig 3. Heatmap of all correlation values (a) and cluster tree of the four accessions (PT2, PT2S, PT4, and PT4S) (b).
Fig 4.
(a) The differently expressed genes in PT4S vs. PT2S, PT2S vs. PT2, PT4 vs. PT2, and PT4S vs. PT4. (b, c) Details of the comparison schemes, up: up-regulated, down: down-regulated.
In the pairwise comparisons of specific sets of DEGs, we detected 1,717 DEGs for Comparison A, which may be related to P. tomentosa responses to saline conditions (Fig 4B, S3 Table); and 160 DEGs for Comparison B, which may be relevant to the differences in the salt-induced responses of PT2 and PT4. Furthermore, Comparisons C and D revealed 589 (17.50%) and 900 (26.74%) DEGs, respectively (Fig 4C, S3 Table), which may help to explain why PT4 is more salt-tolerant than PT2.
Analysis of differentially expressed genes using the GO and KEGG databases
The DEGs detected in Comparisons A, B, C, and D (Fig 1) were categorized into the three main GO categories using the Blast2GO program. Comparisons A, B, C, and D comprised 1,117, 116, 395, and 614 DEGs classified into 40, 27, 36, and 36 functional groups, accounting for 65.02%, 72.50%, 66.95%, and 68.15% of the total DEGs, respectively (S1 Fig, S3 and S4 Tables). Under the biological process category, metabolic process consisted of 561, 64, 179, and 283 DEGs, and cellular process included 467, 50, 146, and 235 DEGs for Comparisons A, B, C, and D, respectively. Under the cellular component category, cell and cell part each consisted of 504, 47, 204, and 299 DEGs for Comparisons A, B, C, and D, respectively. Under the molecular function category,catalytic activity and binding contained the highest numbers of DEGs.
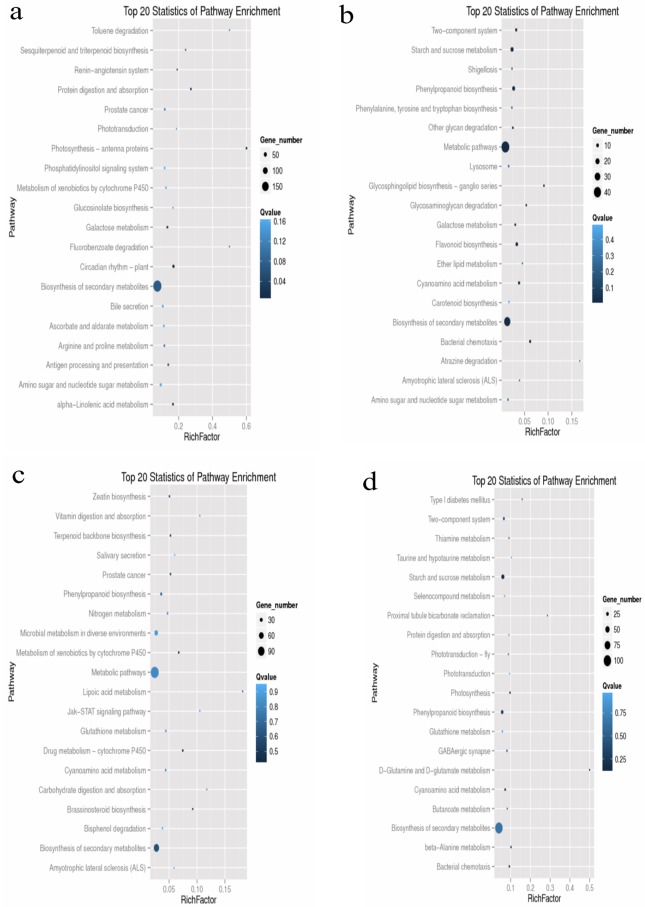
The DEGs detected in Comparisons A, B, C, and D were analyzed using the KEGG database to identify associated pathways. A KEGG enrichment analysis of DEGs from Comparisons A, B, C, and D involved 1,062, 103, 364, and 581 DEGs, accounting for 61.82%, 63.98%, 61.69%, and 64.48% of the total DEGs, respectively. The enriched pathways for the top 20 DEGs from Comparisons A, B, C, and D are provided in Fig 5. The top 20 DEGs were associated mainly with metabolic pathways (ko01100) and the biosynthesis of secondary metabolites (ko01110), as well as plant hormone signal transduction (ko04075), photosynthesis (ko00195), isoflavonoid biosynthesis (ko00943), and nitrogen metabolism (ko00910).
Fig 5. Scatter plot of KEGG pathway enrichment statistics of the comparison schemes.
Top 20 statistics of pathway enrichment for Comparison A (a), Comparison B (b), Comparison C (c), Comparison D (d), respectively.
Differentially expressed genes encoding transcription factors
The important roles of TFs in plant defenses against abiotic stresses in various species have been reported [37, 38]. In this study, we detected 202, 315, 273, and 364 DEGs encoding 237, 371, 323, and 446 TFs in the PT4S vs. PT2S, PT2S vs. PT2, PT4 vs. PT2, and PT4S vs. PT4 comparisons, respectively (S5 Table). These TFs were classified into 36, 42, 44, and 44 TF families in the four comparisons, including the AP2-EREBP, bHLH, GRAS, MYB, NAC, and WRKY TF families (Table 3). The PT4S vs. PT4 and PT2S vs. PT2 comparisons had the highest numbers of TFs and TF families. Interestingly, S1Fa-like and TIG, which may be salt-sensitive TFs, were detected only in the PT4S vs. PT4 and PT2S vs. PT2 comparisons. Additionally, GRF and BSD, which may be salt-tolerant TFs in the autotetraploid P. tomentosa, were identified only in the PT4S vs. PT2S and PT4 vs. PT2 comparisons.
Table 3. The number of DEGs in transcription factor families in response to salt stress treatments.
| TF family | Number of genes | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PT4S vs.PT2S | PT2S vs.PT2 | PT4 vs.PT2 | PT4S vs.PT4 | |||||||||
| Number | Up | Down | Number | Up | Down | Number | Up | Down | Number | Up | Down | |
| ABI3VP1 | 2 | 0 | 2 | 3 | 2 | 1 | 3 | 0 | 3 | 6 | 3 | 3 |
| Alfin-like | 0 | 0 | 0 | 2 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| AP2-EREBP | 24 | 7 | 17 | 33 | 7 | 26 | 32 | 9 | 23 | 38 | 14 | 24 |
| ARF | 7 | 3 | 4 | 5 | 5 | 0 | 12 | 8 | 4 | 5 | 4 | 1 |
| ARR-B | 3 | 1 | 2 | 4 | 2 | 2 | 2 | 0 | 2 | 3 | 3 | 0 |
| BES1 | 0 | 0 | 0 | 3 | 3 | 0 | 1 | 0 | 1 | 2 | 2 | 0 |
| bHLH | 15 | 11 | 4 | 34 | 21 | 13 | 19 | 14 | 5 | 27 | 16 | 11 |
| BSD | 1 | 0 | 1 | 0 | 0 | 0 | 2 | 1 | 1 | 0 | 0 | 0 |
| bZIP | 0 | 0 | 0 | 6 | 4 | 2 | 3 | 1 | 2 | 6 | 4 | 2 |
| C2C2-CO-like | 4 | 3 | 1 | 7 | 6 | 1 | 7 | 3 | 4 | 8 | 7 | 1 |
| C2C2-Dof | 6 | 5 | 1 | 6 | 4 | 2 | 2 | 1 | 1 | 9 | 8 | 1 |
| C2C2-GATA | 1 | 0 | 1 | 5 | 4 | 1 | 5 | 4 | 1 | 4 | 1 | 3 |
| C2C2-YABBY | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 1 | 1 |
| C2H2 | 8 | 5 | 3 | 11 | 11 | 0 | 10 | 3 | 7 | 15 | 14 | 1 |
| C3H | 6 | 4 | 2 | 9 | 6 | 3 | 11 | 4 | 7 | 14 | 11 | 3 |
| CAMTA | 0 | 0 | 0 | 6 | 5 | 1 | 1 | 1 | 0 | 3 | 0 | 3 |
| CPP | 1 | 1 | 0 | 2 | 2 | 0 | 1 | 1 | 0 | 1 | 1 | 0 |
| DBP | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 |
| E2F-DP | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| EIL | 0 | 0 | 0 | 2 | 0 | 2 | 3 | 0 | 3 | 0 | 0 | 0 |
| FAR1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 5 | 5 | 0 | |
| FHA | 1 | 0 | 1 | 4 | 3 | 1 | 3 | 2 | 1 | 2 | 1 | 1 |
| G2-like | 7 | 5 | 2 | 12 | 7 | 5 | 7 | 2 | 5 | 15 | 11 | 4 |
| GeBP | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 2 | 0 |
| GRAS | 13 | 8 | 5 | 15 | 8 | 7 | 11 | 4 | 7 | 23 | 19 | 4 |
| GRF | 2 | 2 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 |
| HB | 0 | 0 | 0 | 2 | 2 | 0 | 1 | 0 | 1 | 4 | 4 | 0 |
| HSF | 5 | 4 | 1 | 14 | 2 | 12 | 8 | 1 | 7 | 12 | 7 | 5 |
| LIM | 1 | 1 | 0 | 3 | 1 | 2 | 3 | 3 | 0 | 3 | 1 | 2 |
| LOB | 4 | 0 | 4 | 5 | 3 | 2 | 5 | 0 | 5 | 4 | 4 | 0 |
| MADS | 1 | 0 | 1 | 3 | 3 | 0 | 2 | 1 | 1 | 2 | 2 | 0 |
| mTERF | 6 | 3 | 3 | 5 | 5 | 0 | 8 | 8 | 0 | 1 | 0 | 1 |
| MYB | 33 | 18 | 15 | 52 | 29 | 23 | 48 | 32 | 16 | 77 | 40 | 37 |
| MYB-related | 26 | 12 | 14 | 35 | 18 | 17 | 40 | 29 | 11 | 57 | 25 | 32 |
| NAC | 22 | 11 | 11 | 18 | 12 | 6 | 19 | 4 | 15 | 19 | 17 | 2 |
| PBF-2-like | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 |
| PLATZ | 1 | 0 | 1 | 2 | 1 | 1 | 2 | 0 | 2 | 2 | 2 | 0 |
| PWP-PK | 4 | 3 | 1 | 3 | 1 | 2 | 2 | 0 | 2 | 5 | 5 | 0 |
| S1Fa-like | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 |
| SBP | 2 | 1 | 1 | 3 | 2 | 1 | 1 | 1 | 0 | 3 | 2 | 1 |
| sigma70-like | 1 | 1 | 0 | 6 | 2 | 4 | 4 | 3 | 1 | 3 | 0 | 3 |
| SRS | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 |
| TA2 | 2 | 1 | 1 | 4 | 1 | 3 | 1 | 0 | 1 | 3 | 1 | 2 |
| TCP | 2 | 0 | 2 | 2 | 1 | 1 | 3 | 0 | 3 | 4 | 3 | 1 |
| Tify | 1 | 0 | 1 | 5 | 0 | 5 | 3 | 2 | 1 | 5 | 1 | 4 |
| TIG | 0 | 0 | 0 | 4 | 3 | 1 | 0 | 0 | 0 | 3 | 3 | 0 |
| Trihelix | 3 | 3 | 0 | 8 | 7 | 1 | 7 | 3 | 4 | 12 | 11 | 1 |
| TUB | 0 | 0 | 0 | 2 | 1 | 1 | 3 | 1 | 2 | 3 | 3 | 0 |
| VOZ | 2 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 2 | 2 | 0 |
| WRKY | 17 | 7 | 10 | 22 | 8 | 14 | 22 | 3 | 19 | 28 | 16 | 12 |
| zf-HD | 2 | 2 | 0 | 2 | 2 | 0 | 1 | 1 | 0 | 2 | 2 | 0 |
| Total | 237 | 124 | 113 | 371 | 207 | 164 | 323 | 153 | 170 | 446 | 279 | 167 |
We detected 159, 8, 29, and 51 DEGs encoding 193, 9, 34, and 57 TFs from 37, 6, 18, and 21 families in Comparisons A, B, C, and D, respectively (S6 Table). The four common TF super-families among these comparisons were AP2/EREBP, bHLH, MYB, and NAC. Of these, the bHLH, MYB, and NAC TFs were mostly up-regulated in response to increased salt stress and ploidy level, whereas AP2/EREBP was mostly down-regulated.
Differentially expressed genes involved in alternative splicing
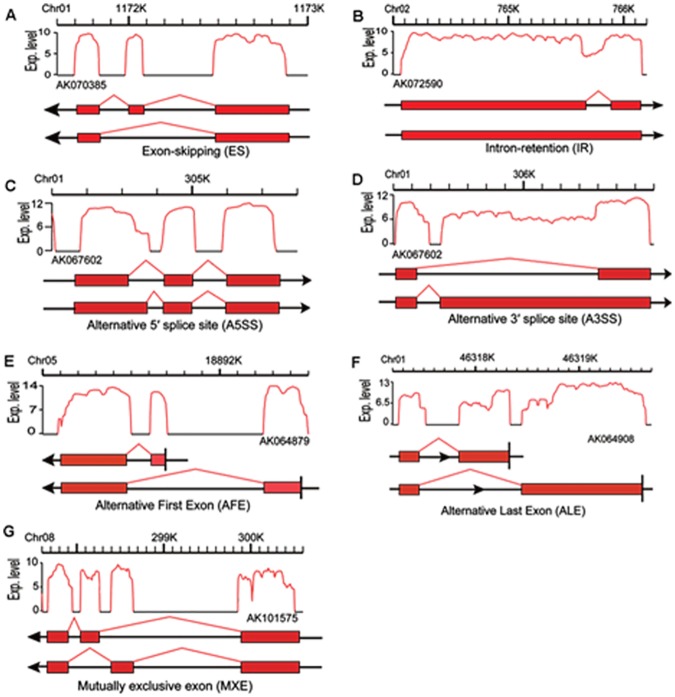
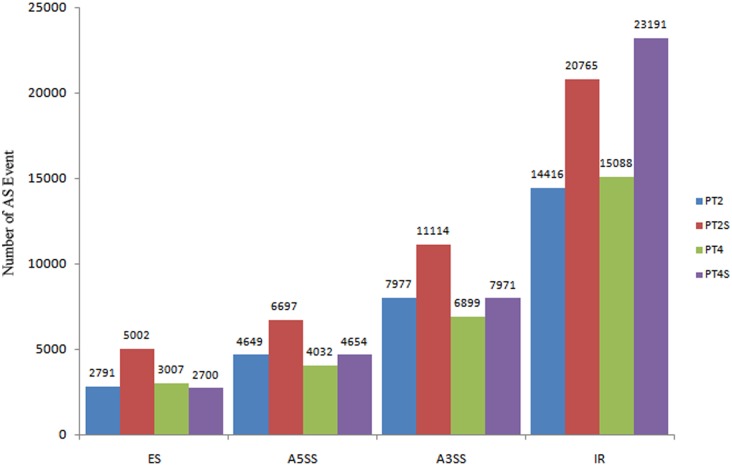
AS affects many plant physiological processes, including responses to abiotic and biotic stresses [39]. There are seven main types of AS: Exon skipping (ES), Intron retention (IR), Alternative 5′ splice site (A5SS), and Alternative 3′ splice site (A3SS), Alternative first exon (AFE), Alternative last exon (ALE), and Mutually exclusive exon (MEX) (Fig 6). The AS of eukaryotic genes mainly involves the first four types. The last three types were excluded in this study because of the potential for considerable false-positive results. Among the four included AS categories, we observed that IR was the most common (73,460), followed by A3SS (33,961), A5SS (20032), and ES (13500) (Fig 7). That IR was the primary AS category was consistent with the findings of previous studies [40, 41]. Of the four main AS types, A5SS, A3SS, and IR were significantly enhanced in response to saline conditions. Furthermore, IR was especially enhanced in the PT4S vs. PT2S and PT4 vs. PT2 comparisons. These results implied that IR is influenced by the ploidy level.
Fig 6. Alternative splicing events “Exp. Level” of Y-axis equals to log2 (Reads number).
Fig 7. Classification of the four main AS events in this study.
Differentially expressed genes involved in plant hormone signal transduction
Plant hormone signal transduction is crucial for plant responses to adverse environmental conditions. We detected 157 DEGs related to plant hormone signal transduction. In Comparison A, 90 DEGs were related to signal transductions involving several hormones, including ABA, GA, IAA, CK, ET, JA, SA, and BR, and in Comparisons B, C, and D 7, 20, and 40 DEGs were related to plant hormone signal transduction, respectively (S7 Table).
Differentially expressed genes involved in photosynthesis
Exposure to high-salt conditions impairs osmotic homeostasis as well as the cellular ionic and redox balance, and also inhibits photosynthetic activities. In this study, the “photosynthesis, light reaction”, “photosynthetic membrane”, “chloroplast”, and “chloroplast part” GO terms were enriched in Comparisons A, B, C, and D. The GO analyses indicated that 228 DEGs were associated with “photosynthesis”. The KEGG pathway analyses revealed that photosystem II P680 reaction center D1 protein (psbA), photosystem II oxygen-evolving enhancer protein 3 (psbQ), photosystem I P700 chlorophyll a apoprotein A1 (psaA), photosystem I subunit PsaN (psaN), ferredoxin (petF), and ferredoxin-NADP+reductase (petH) were significantly enriched in the four comparisons (S8 Table).
Validation of differentially expressed genes by quantitative real-time polymerase chain analysis
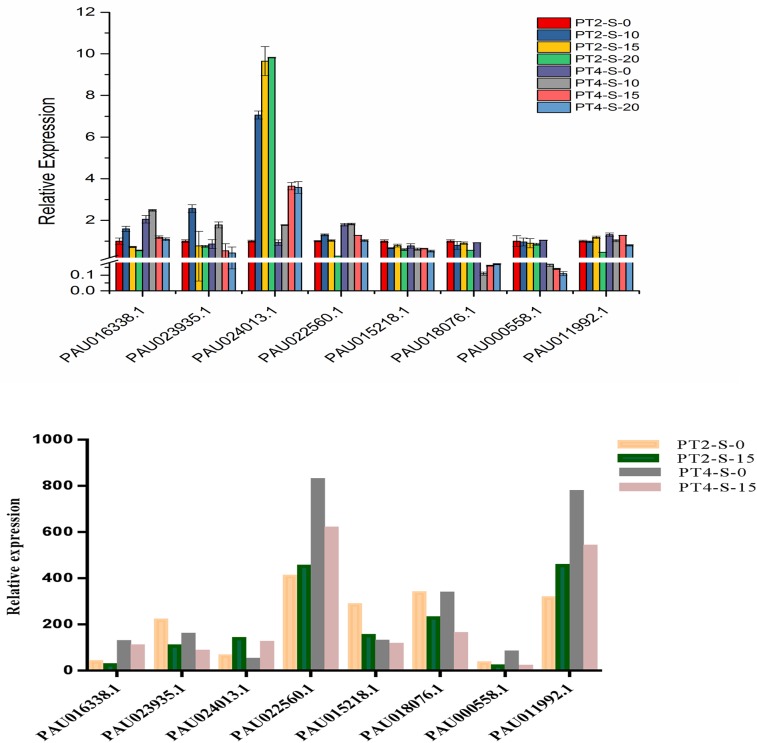
To further validate the reliability and reproducibility of the gene expression results from the RNA-Seq data, eight DEGs from the four libraries were selected for qRT-PCR analysis. The qRT-PCR results for the eight DEGs reflected the same trends (up- and down-regulation trends) as those acquired from the RNA-Seq data using the FPKM method (Fig 8A). The disaccord between the transcriptome and qRT-PCR results is likely because transcriptome data better reflect small changes in gene expression and better detection of low-abundant than qRT-PCR, as reported previously [42]. Overall, the qRT-PCR results support the reliability of the relative values provided by the RNA-Seq analysis.
Fig 8. Expression pattern confirmation of selected genes by qRT-PCR.
(A) Quantitative Real-Time PCR (qRT-PCR) analysis of 8 selected differentially expressed genes. 18S rRNA was used as the internal reference gene. For each group, the PT2 (S-0) expressed level was considered as 1.00, and other samples were normalized accordingly. Standard error of the mean for three technical replicates is represented by the error bars. S-0, S-10, S-15, and S-20 (0, 10, 15, and 20 days), 0.4% NaCl salt-treated for PT2 and PT4, respectively. (B) Changes in the relative expression levels of 8 selected genes as determined by RNA-Seq.
The qRT-PCR results are related to the DEG data. The expression trends of all 8 genes from RNA-Seq and qRT-PCR analyses were largely consistent, demonstrating the correlation analysis of DEGs under salt stress based on RNA-Seq and qRT-PCR data (Fig 8B).
Discussion
Salinity is a major environmental stressor that constrains tree growth in arid and semi-arid regions [43]. High-salt conditions can induce morphological and physiological changes in P. tomentosa leaves [20]. Currently, only limited information is available regarding the mechanism underlying the salt tolerance of P. tomentosa. In this study, the second pairs of leaves from the apex shoot of PT4S, PT4, PT2S, and PT2 seedlings were analyzed by RNA-Seq to elucidate the complex mechanisms that regulate the responses of diploid and autotetraploid P. tomentosa to salt-stress conditions. The transcriptome data yielded 175 million clean reads, many of which were differentially expressed. Overall, the control plants had fewer total mapped reads than the salt-treated plants (i.e., PT2 < PT2S and PT4 < PT4S) (Table 2). Additionally, some overlapping DEGs were detected. A comparison of the control and salt-treated P. tomentosa plants of different ploidy levels decreased the number of DEGs. This increased the likelihood that the identified genes were related to plant responses and adaptations to saline conditions. “Plant hormone signal transduction” (ko07075) and “Photosynthesis” (ko00195) were considerably enriched metabolic pathways. The enriched TFs may be useful for clarifying the molecular mechanism underlying the salt tolerance of the diploid and autotetraploid P. tomentosa lines.
Transcription factors involved in salt stress responses
Transcription factors are important for the acclimation of plants to extreme environmental stresses, including high-salt conditions. In this study, members of the bHLH, MYB, NAC, GRAS, WRKY, and AP2/EREBP families were the most abundant stress- or ploidy-related TFs. Some calcium-dependent TFs are regulated by the direct binding of a Ca2+ ion or the Ca2+/calmodulin (CaM) complex or by post-translation modifications mediated by Ca2+ or the Ca2+/CaM complex [44]. AtNIG1 (Arabidopsis thaliana NaCl-inducible gene 1), which is a basic helix–loop–helix (bHLH) TF, was the first identified salt stress-responsive Ca2+-binding TF involved in salt stress signaling in plants according to a suppression subtractive hybridization analysis [45]. In the current study, we identified 62 up-regulated and 33 down-regulated bHLH genes that were induced by salt stress, which is consistent with the results of a previous study in A.thaliana [46]. The MYB super family, which includes MYB and MYB-related TFs, was the largest TF family in our study. MYB TFs have regulatory roles in plant developmental processes and defense responses [47]. A two-repeat MYB (i.e., MYB2), which contains a Ca2+-dependent CaM-binding domain, regulates the expression of dehydration- and salt-responsive genes in A. thaliana [48]. Additionally, the over-expression of OsMYB3R-2 in A. thaliana [49] and OsMYB48-1 in rice [50] reportedly enhanced tolerance to high-salt conditions. We detected 203 up-regulated and 165 down-regulated MYB genes in our transcriptome data. The NAC TFs, which form one of the largest plant-specific TF families, are essential for responses to various abiotic stresses. A NAC domain-containing TF was identified as a CaM-binding protein. Analyses of transcriptional regulation mediated by a CaM-binding NAC protein helped characterize the function of the Ca2+/CaM complex [51]. The over-expression of TaNAC2 in A. thaliana plants led to increased tolerance to salt, drought, and freezing stresses [52]. We observed that NAC expression levels were mostly up-regulated in salt-stressed P. tomentosa plants. This is consistent with the results of a previous study in cotton [53]. These DEGs may be relevant for future studies of P. tomentosa salt tolerance.
Plant hormone signal transduction pathways involved in salt stress responses
Phytohormones influence plant responses to abiotic stresses. For example, to optimize the chance of survival, phytohormone production may be altered to decrease plant growth, enabling the plant to divert additional resources to maintain responses to environmental stresses [54]. Indeed, the perception of a stress signal causes the phytohormone-related signal transduction cascades in plants to decrease to baseline levels [55, 56].
ABA is a lipophilic plant hormone with key roles in signaling and adaptation to abiotic stresses, such as drought and high salinity [57]. The ABA signal transduction pathway involves the activities of PP2C (Proteinphosphatases 2C), PYR/PYL (abscisic acid receptor PYR/PYL family), SnRK2 (serine/threonine-protein kinase SRK2), and ABF (ABA responsive element binding factor). This pathway is initiated when ABA binds to the pyrabactin resistance-like/regulatory component of ABA receptors (RCAR). Which results in the inactivation of protein phosphatase PP2C and activation of SnRK2-type kinases, ultimately inducing stomatal closure [58]. As a positive regulator of ABA signaling, SnRK2 is crucial for abiotic stress responses in plants [59]. In this study, SnRK2 expression was up-regulated in the PT4 vs. PT2 and PT4S vs. PT4 comparisons (S7 Table), similar to the results of a previous study of maize [60]. There is genetic evidence that A-type PP2Cs are negative regulators of ABA signaling in A.thaliana [61]. Under saline conditions, ABF2 expression is reportedly up-regulated in grape leaves and roots in response to increasing salt concentrations [62]. Thus, the expression of salt-responsive genes may be related to the down-regulation of PP2C and up-regulation of ABF associated with ABA signal transduction. In this study, PP2C expression (PAU005151.1, PAU008363.1, and PAU024054.1) was down-regulated in the PT4 vs. PT2 and PT4S vs. PT2S comparisons. In contrast, ABF (PAU013700.1, PAU023164.1, and PAU013700.1) expression levels increased in salt-stressed PT2S and PT4S plants.
Cytokinins (CKs) are a class of phytohormones involved in various physiological events, including responses to environmental stresses. Recently, CKs were revealed to regulate plant adaptations to salt stress during growth and development [63], and increased CK levels in seeds were observed to increase plant tolerance to salt stress [64]. The CRE1 histidine kinase (the cytokinin response 1), which contains an extracellular domain called CHASE, is a CK receptor. The CK signaling pathway involves several steps [65]. First, CRE1 is activated when CK binds to the CHASE domain and isauto-phosphorylated. Then, histidine-containing phosphotransfer proteins (AHPs) are phosphorylated by the activated CRE1,and migrate from the cytoplasm to the nucleus, where they transfer the phosphate group to (the response regulator proteins) ARRs. The expression of CRE1 (one of the receptor histidine kinases) is induced by various stresses, suggesting that the histidine kinase CK receptors are crucial for CK and stress responses in A. thaliana [66]. In this study, CRE1 expression (PAU010104.1 and PAU010665.1) was up-regulated in the PT2S vs. PT2 and PT4S vs. PT4 comparisons, which is in agreement with the aforementioned results. Furthermore, CRE1 expression (PAU021126.1) was also up-regulated in the PT4 vs. PT2 and PT4S vs. PT2S comparisons, which may be related to differences in ploidy levels. Nishiyama et al. [67] reported that among genes related to CK signaling, the expression of a type-B ARR gene was down-regulated, whereas AHP4 was up-regulated in response to salt treatments. This implies that CK signaling might be inhibited by salt stress. We observed that type-B ARR expression (PAU012990.1) levels were down-regulated in the PT2S vs. PT2 and PT4S vs. PT4 comparisons, which is consistent with the aforementioned findings. Type-B ARR expression (PAU003823.1, PAU001788.1, PAU022082.1, and PAU022609.1) was down-regulated in the PT4 vs. PT2 and PT4S vs. PT2S comparisons, which may be related to the variability in ploidy levels. The expression of AHP (PAU029211.1) was up-regulated in the PT2S vs. PT2 and PT4S vs. PT4 comparisons, which is consistent with the previously described results.
In this study, we identified 157 DEGs related to plant hormone signal transduction. The results of this study along with previously reported findings revealed that autotetraploid P. tomentosa was more salt-tolerant than diploid P. tomentosa. We observed that all the analyzed plant hormones were directly or indirectly involved in regulating plant responses to salt stress. The activities of plant genes and hormones are strongly linked, as indicated by the fact that some plant genes, which are essential for activating plant hormones and other genes, are activated by phytohormones.
Photosynthetic activities involved in salt stress responses
Salt stress decreases plant growth and productivity by disrupting physiological processes [68], especially photosynthetic activities [69]. In this study, several DEGs were involved in photosynthesis, including genes encoding the photosystem II P680 reaction center D1 protein (psbA), photosystem II oxygen-evolving enhancer protein 3 (psbQ), photosystem I P700 chlorophyll a apoprotein A1 (psaA), ferredoxin (petF), and ferredoxin-NADP+ reductase (petH). Thylakoid membrane proteins were influenced by salt stress in Synechococcus sp.PCC 7942 [70]. The photosystem II D1 protein in thylakoid membranes is encoded by a gene in the plastid genome (cpDNA). This protein transforms radiant energy via the oxidation of water and reduction of plastoquinone. In Synechocystis sp., salt stress suppresses the repair of photosystem II by inhibiting the activities of the transcriptional and translational machinery [71]. Additionally, the D1 protein is sensitive to environmental stresses [72]. We observed that the expression of the gene (PAU015578.1), which encoded a D1 protein, was down-regulated in the PT4S vs. PT4 and PT2S vs. PT2 comparisons (S8 Table). This is consistent with the previous observation that D1 levels decreased because of salt stress-induced photoinhibition in Brassica juncea [73]. The psbQ peripheral protein is an important part of the photosystem II complex. It is also the most diverse extrinsic photosystem II protein in higher plants, and can maintain the integrity of photosystem II under high-salt conditions [74]. The psaQ expression level was up-regulated in the PT4 vs. PT2 and PT4S vs. PT2S comparisons, which may be closely related to the differences in ploidy. The psaA gene encodes photosystem I P700 chlorophyll a apoprotein A1, which is one of two large core subunits of photosystem I. It carries a set of cofactors required for a functional electron transport chain through photosystem I. In this study, the abundance of the psaA transcript (PAU029948.1) was up-regulated in the PT2S vs. PT2 comparison. This is in agreement with the results of a previous study of Salicorniabigelovii Torr [75]. Electrons derived from water by the oxygen-evolving complex of photosystem II are transferred to NADP+, leading to the production of NADPH. The electrons are passed along the photosynthetic electron transport chain via the plastoquinone, cytochrome b6f complex, plastocyanin, photosystem I, ferredoxin, and ferredoxin-NADP+oxidoreductase [76].
Conclusions
Here we report for the first time, comparisons of the effects of salt stress on diploid and autotetraploid P. tomentosa plants using Illumina sequencing technology based on the P. fortunei genome as a reference. Overall, 85.7 and 125 million clean reads were mapped to P. fortunei gene and genome sequences, respectively. We identified 15,873 million DEGs among the control and salt-stressed P. tomentosa plants. Some DEGs were functionally associated with plant hormone signal transductions, photosynthesis, and other metabolic pathways. Notably, some TF genes, including NAC, MYB, bHLH, GRAS, WRKY, and AP2/EREBP, were responsive to salt stress and/or ploidy levels. We also identified several DEGs related to AS which were affected by salt stress or ploidy levels. Our results may be useful for future efforts to elucidate the molecular mechanisms underlying salt tolerance as well as the gene regulatory networks of P. tomentosa plants.
Supporting information
(TIF)
(DOCX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLS)
Acknowledgments
We thank Margaret Biswas, PhD, from Liwen Bianji, Edanz Group China (www.liwenbianji.cn/ac), for editing the English text of a draft of this manuscript.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The financial support of this work were the Natural Science Foundation of Henan Province of China (Grant No. 162300410158), the Distinguished Talents Foundation of Henan Province of China (Grant No. 174200510001), Production-study-research cooperation of Henan Province (Grant No. 152107000096), and the National key research and development Program (Grant No. 2016YFD0600106).
References
- 1.Ahmad P, Azooz MM, Prasad MNV. Salt Stress in Plants. New York: Springer New York; 2013. 920–9 p. [Google Scholar]
- 2.FAO. FAO Land and Plant Nutrition Management Service. http://www.fao.org/ag/agl/agll/spush. 2008.
- 3.Zhu Z, Chao C, Lu X. Paulownia in China: cultivation and utilization. Asian Network for Biological Science and International Development Research Centre, Chinese Academy of Forestry, Beijing: 1986. [Google Scholar]
- 4.Ates S, Ni Y, Akgul M, Tozluoglu A. Characterization and evaluation of Paulownia elongota as a raw material for paper production. 2008;7(22):4153–8. [Google Scholar]
- 5.Doumett S, Lamperi L, Checchini L, Azzarello E, Mugnai S, Mancuso S, et al. Heavy metal distribution between contaminated soil and Paulownia tomentosa, in a pilot-scale assisted phytoremediation study: influence of different complexing agents. Chemosphere. 2008;72(10):1481–90. doi: 10.1016/j.chemosphere.2008.04.083 . [DOI] [PubMed] [Google Scholar]
- 6.Kang KH, Huh H, Kim BK, Lee CK. An antiviral furanoquinone from Paulownia tomentosa steud. Phytotherapy Research. 1999;13(7):624 [DOI] [PubMed] [Google Scholar]
- 7.Melhuish JH Jr, Gentry CE, Beckjord PR. Paulownia tomentosa seedling growth at differing levels of pH, nitrogen, and phosphorus. Journal of Environmental Horticulture. 1990. [Google Scholar]
- 8.Dong Y, Fan G, Zhao Z, Deng M. Compatible solute, transporter protein, transcription factor, and hormone-related gene expression provides an indicator of drought stress in Paulownia fortunei. Funct Integr Genomics. 2014;14(3):479–91. doi: 10.1007/s10142-014-0373-4 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fan G, Wang L, Deng M, Niu S, Zhao Z, Xu E, et al. Transcriptome analysis of the variations between autotetraploid Paulownia tomentosa and its diploid using high-throughput sequencing. Molecular genetics and genomics: MGG. 2015;290(4):1627–38. doi: 10.1007/s00438-015-1023-9 . [DOI] [PubMed] [Google Scholar]
- 10.Jiang J. Cultural science of Paulownia plants. China Forest Press, Beijing: 1993, P,28. [Google Scholar]
- 11.Ipekci Z, Gozukirmizi N. Direct somatic embryogenesis and synthetic seed production from Paulownia elongata. Plant Cell Reports. 2003;22(1):16–24. doi: 10.1007/s00299-003-0650-5 [DOI] [PubMed] [Google Scholar]
- 12.Fan G, Yang Z, Cao Y. Induction of autotetraploid of Paulownia tomentosa (Thunb.) Steud. Plant Physiology Communications. 2007;43(1):109. [Google Scholar]
- 13.Wang Y, Zhang XS, Deng MJ, Zhao ZL, Fan GQ. Study on Photosynthetic Characteristics of Diploid and Tetraploid Paulownia australis. Acta Agriculturae Jiangxi. 2013. [Google Scholar]
- 14.Zhai XQ, Zhang XS, Zhao ZL, Deng MJ, Fan GQ. Study on wood physical properties of tetraploid Paulownia fortunei. Journal of Henan Agricultural University. 2012;46(6):651–0. [Google Scholar]
- 15.Deng M, Zhang X, Fan G, Zhao Z, Dong Y, Wei Z. Comparative studies on physiological responses to salt stress in tetraploid Paulownia plants. Journal of Central South University of Forestry and Technology. 2013;33(11):42–6. [Google Scholar]
- 16.Zhang X, Liu R, Fan G, Zhao Z, Deng M. Study on the physiological response of tetraploid Paulownia to drought. J Henan Agric Univ. 2013;47(5):543–7. [Google Scholar]
- 17.Zhang X, Zhai X, Deng M, Dong Y, Zhao ZL, Fan G. Comparative studies on physiological responses of diploid Paulownia and its tetraploid to drought stress. J Henan Agric Sci. 2013;42:118–23. [Google Scholar]
- 18.Dong Y, Fan G, Deng M, Xu E, Zhao Z. Genome-wide expression profiling of the transcriptomes of four Paulownia tomentosa accessions in response to drought. Genomics. 2014;104(4):295–305. doi: 10.1016/j.ygeno.2014.08.008 . [DOI] [PubMed] [Google Scholar]
- 19.Fan G, Cao X, Zhao Z, Deng M. Transcriptome analysis of the genes related to the morphological changes of Paulownia tomentosa plantlets infected with phytoplasma. Acta Physiologiae Plantarum. 2015;37(10):202 doi: 10.1007/s11738-015-1948-y [Google Scholar]
- 20.Fan G, Wang L, Deng M, Zhao Z, Dong Y, Zhang X, et al. Changes in Transcript Related to Osmosis and Intracellular Ion Homeostasis in Paulownia tomentosa under Salt Stress. Frontiers in plant science. 2016;7:384 doi: 10.3389/fpls.2016.00384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fan G, Zhai X, Niu S, Ren Y. Dynamic expression of novel and conserved microRNAs and their targets in diploid and tetraploid of Paulownia tomentosa. Biochimie. 2014;102:68–77. doi: 10.1016/j.biochi.2014.02.008 . [DOI] [PubMed] [Google Scholar]
- 22.Fan G, Cao Y, Deng M, Zhai X, Zhao Z, Niu S, et al. Identification and dynamic expression profiling of microRNAs and target genes of Paulownia tomentosa in response to Paulownia witches’ broom disease. Acta Physiologiae Plantarum. 2016;39(1). doi: 10.1007/s11738-016-2326-0 [Google Scholar]
- 23.Cao X, Fan G, Cao L, Deng M, Zhao Z, Niu S, et al. Drought stress-induced changes of microRNAs in diploid and autotetraploid Paulownia tomentosa. Genes Genomics. 2017;39(1):77–86. doi: 10.1007/s13258-016-0473-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cao X, Fan G, Dong Y, Zhao Z, Deng M, Wang Z, et al. Proteome Profiling of Paulownia Seedlings Infected with Phytoplasma. Frontiers in plant science. 2017;8 doi: 10.3389/fpls.2017.00342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Al-Mssallem IS, Hu S, Zhang X, Lin Q, Liu W, Tan J, et al. Genome sequence of the date palm Phoenix dactylifera L. Nature communications. 2013;4:2274 doi: 10.1038/ncomms3274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Velasco R, Zharkikh A, Affourtit J, Dhingra A, Cestaro A, Kalyanaraman A, et al. The genome of the domesticated apple (Malus x domestica Borkh.). Nature genetics. 2010;42(10):833–9. doi: 10.1038/ng.654 . [DOI] [PubMed] [Google Scholar]
- 27.Tuskan GA, Difazio S, Jansson S, Bohlmann J, Grigoriev I, Hellsten U, et al. The Genome of Black Cottonwood, Populus trichocarpa (Torr. & Gray). Science. 2006;313(5793):1596–604. doi: 10.1126/science.1128691 [DOI] [PubMed] [Google Scholar]
- 28.Murashige T, Skoog F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiologia Plantarum. 1962;15(3):473–97. doi: 10.1111/j.1399-3054.1962.tb08052.x [Google Scholar]
- 29.Wang Z, Gerstein M, Snyder M. RNA-Seq: a revolutionary tool for transcriptomics. Nature Reviews Genetics. 2009;10(1):57–63. doi: 10.1038/nrg2484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mortazavi A, Williams BA, McCue K, Schaeffer L, Wold B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nature methods. 2008;5(7):621–8. doi: 10.1038/nmeth.1226 [DOI] [PubMed] [Google Scholar]
- 31.Broberg P. A comparative review of estimates of the proportion unchanged genes and the false discovery rate. BMC bioinformatics. 2005;6(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Audic S, Claverie J-M. The significance of digital gene expression profiles. Genome research. 1997;7(10):986–95. doi: 10.1101/gr.7.10.986 [DOI] [PubMed] [Google Scholar]
- 33.Kanehisa M, Araki M, Goto S, Hattori M, Hirakawa M, Itoh M, et al. KEGG for linking genomes to life and the environment. Nucleic acids research. 2008;36(suppl 1):D480–D4. doi: 10.1093/nar/gkm882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ye J, Fang L, Zheng H, Zhang Y, Chen J, Zhang Z, et al. WEGO: a web tool for plotting GO annotations. Nucleic acids research. 2006;34(suppl 2):W293–W7. doi: 10.1093/nar/gkl031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Burdach J, Funnell AP, Mak KS, Artuz CM, Wienert B, Lim WF, et al. Regions outside the DNA-binding domain are critical for proper in vivo specificity of an archetypal zinc finger transcription factor. Nucleic Acids Res. 2014;42(1):276–89. doi: 10.1093/nar/gkt895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Trapnell C, Pachter L, Salzberg SL. TopHat: discovering splice junctions with RNA-Seq. Bioinformatics. 2009;25(9):1105–11. doi: 10.1093/bioinformatics/btp120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang F, Zhu G, Du L, Shang X, Cheng C, Yang B, et al. Genetic regulation of salt stress tolerance revealed by RNA-Seq in cotton diploid wild species, Gossypium davidsonii. Scientific reports. 2016;6. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 38.Lata C, Prasad M. Role of DREBs in regulation of abiotic stress responses in plants. Journal of experimental botany. 2011;62(14):4731–48. doi: 10.1093/jxb/err210 [DOI] [PubMed] [Google Scholar]
- 39.Staiger D, Brown JW. Alternative splicing at the intersection of biological timing, development, and stress responses. The Plant Cell. 2013;25(10):3640–56. doi: 10.1105/tpc.113.113803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marquez Y, Brown JW, Simpson C, Barta A, Kalyna M. Transcriptome survey reveals increased complexity of the alternative splicing landscape in Arabidopsis. Genome research. 2012;22(6):1184–95. doi: 10.1101/gr.134106.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carvalho RF, Feijão CV, Duque P. On the physiological significance of alternative splicing events in higher plants. Protoplasma. 2013;250(3):639–50. doi: 10.1007/s00709-012-0448-9 [DOI] [PubMed] [Google Scholar]
- 42.Yan X, Dong C, Yu J, Liu W, Jiang C, Liu J, et al. Transcriptome profile analysis of young floral buds of fertile and sterile plants from the self-pollinated offspring of the hybrid between novel restorer line NR1 and Nsa CMS line in Brassica napus. BMC Genomics. 2013;14(1):26 doi: 10.1186/1471-2164-14-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boyer JS. Plant productivity and environment. Science. 1982;218(4571):443–8. doi: 10.1126/science.218.4571.443 [DOI] [PubMed] [Google Scholar]
- 44.Kim MC, Chung WS, Yun DJ, Cho MJ. Calcium and calmodulin-mediated regulation of gene expression in plants. Molecular plant. 2009;2(1):13–21. doi: 10.1093/mp/ssn091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim J, Kim HY. Functional analysis of a calcium-binding transcription factor involved in plant salt stress signaling. FEBS letters. 2006;580(22):5251–6. doi: 10.1016/j.febslet.2006.08.050 . [DOI] [PubMed] [Google Scholar]
- 46.Ahmad A, Niwa Y, Goto S, Ogawa T, Shimizu M, Suzuki A, et al. bHLH106 Integrates Functions of Multiple Genes through Their G-Box to Confer Salt Tolerance on Arabidopsis. Plos One. 2015;10(5):e0126872 doi: 10.1371/journal.pone.0126872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yanhui C, Xiaoyuan Y, Kun H, Meihua L, Jigang L, Zhaofeng G, et al. The MYB transcription factor superfamily of Arabidopsis: expression analysis and phylogenetic comparison with the rice MYB family. Plant molecular biology. 2006;60(1):107–24. doi: 10.1007/s11103-005-2910-y . [DOI] [PubMed] [Google Scholar]
- 48.Yoo JH, Park CY, Kim JC, Heo WD, Cheong MS, Park HC, et al. Direct interaction of a divergent CaM isoform and the transcription factor, MYB2, enhances salt tolerance in arabidopsis. The Journal of biological chemistry. 2005;280(5):3697–706. doi: 10.1074/jbc.M408237200 . [DOI] [PubMed] [Google Scholar]
- 49.Dai X, Xu Y, Ma Q, Xu W, Wang T, Xue Y, et al. Overexpression of an R1R2R3 MYB gene, OsMYB3R-2, increases tolerance to freezing, drought, and salt stress in transgenic Arabidopsis. Plant physiology. 2007;143(4):1739–51. doi: 10.1104/pp.106.094532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xiong H, Li J, Liu P, Duan J, Zhao Y, Guo X, et al. Overexpression of OsMYB48-1, a novel MYB-related transcription factor, enhances drought and salinity tolerance in rice. PLoS One. 2014;9(3):e92913 doi: 10.1371/journal.pone.0092913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim HS, Park BO, Yoo JH, Jung MS, Lee SM, Han HJ, et al. Identification of a calmodulin-binding NAC protein as a transcriptional repressor in Arabidopsis. The Journal of biological chemistry. 2007;282(50):36292–302. doi: 10.1074/jbc.M705217200 . [DOI] [PubMed] [Google Scholar]
- 52.Mao X, Zhang H, Qian X, Li A, Zhao G, Jing R. TaNAC2, a NAC-type wheat transcription factor conferring enhanced multiple abiotic stress tolerances in Arabidopsis. J Exp Bot. 2012;63(8):2933–46. doi: 10.1093/jxb/err462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Peng Z, He S, Gong W, Sun J, Pan Z, Xu F, et al. Comprehensive analysis of differentially expressed genes and transcriptional regulation induced by salt stress in two contrasting cotton genotypes. BMC genomics. 2014;15(1):1 doi: 10.1186/1471-2164-15-760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Skirycz A, Inzé D. More from less: plant growth under limited water. Current Opinion in Biotechnology. 2010;21(2):197–203. doi: 10.1016/j.copbio.2010.03.002 [DOI] [PubMed] [Google Scholar]
- 55.Harrison MA. Cross-talk between phytohormone signaling pathways under both optimal and stressful environmental conditions Phytohormones and Abiotic Stress Tolerance in Plants: Springer; 2012. p. 49–76. [Google Scholar]
- 56.Khan NA, Nazar R, Iqbal N, Anjum NA. Phytohormones and abiotic stress tolerance in plants: Springer Science & Business Media; 2012. [Google Scholar]
- 57.Munns R, Tester M. Mechanisms of salinity tolerance. Annual review of plant biology. 2008;59:651–81. doi: 10.1146/annurev.arplant.59.032607.092911 . [DOI] [PubMed] [Google Scholar]
- 58.Lee SC, Luan S. ABA signal transduction at the crossroad of biotic and abiotic stress responses. Plant, Cell & Environment. 2012;35(1):53–60. doi: 10.1111/j.1365-3040.2011.02426.x [DOI] [PubMed] [Google Scholar]
- 59.Song X, Yu X, Hori C, Demura T, Ohtani M, Zhuge Q. Heterologous Overexpression of Poplar SnRK2 Genes Enhanced Salt Stress Tolerance in Arabidopsis thaliana. Frontiers in plant science. 2016;7:612 doi: 10.3389/fpls.2016.00612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huai J, Wang M, He J, Zheng J, Dong Z, Lv H, et al. Cloning and characterization of the SnRK2 gene family from Zea mays. Plant Cell Rep. 2008;27(12):1861–8. doi: 10.1007/s00299-008-0608-8 . [DOI] [PubMed] [Google Scholar]
- 61.Rubio S, Rodrigues A, Saez A, Dizon M, Galle A, Kim T, et al. Triple loss of function of protein phosphatases type 2C leads to partial constitutive response to endogenous abscisic acid. Plant Physiology. 2009;150(3):1345–55. doi: 10.1104/pp.109.137174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zandkarimi H, Ebadi A, Salami SA, Alizade H, Baisakh N. Analyzing the Expression Profile of AREB/ABF and DREB/CBF Genes under Drought and Salinity Stresses in Grape (Vitis vinifera L.). PloS one. 2015;10(7):e0134288 doi: 10.1371/journal.pone.0134288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hadiarto T, Tran LS. Progress studies of drought-responsive genes in rice. Plant Cell Rep. 2011;30(3):297–310. doi: 10.1007/s00299-010-0956-z . [DOI] [PubMed] [Google Scholar]
- 64.Iqbal M, Ashraf M, Jamil A. Seed enhancement with cytokinins: changes in growth and grain yield in salt stressed wheat plants. Plant growth regulation. 2006;50(1):29–39. doi: 10.1007/s10725-006-9123-5 [Google Scholar]
- 65.Deruère J, Kieber JJ. Molecular mechanisms of cytokinin signaling. Journal of plant growth regulation. 2002;21(1). doi: 10.1007/s003440010045 [DOI] [PubMed] [Google Scholar]
- 66.Tran L-SP, Urao T, Qin F, Maruyama K, Kakimoto T, Shinozaki K, et al. Functional analysis of AHK1/ATHK1 and cytokinin receptor histidine kinases in response to abscisic acid, drought, and salt stress in Arabidopsis. Proceedings of the National Academy of Sciences. 2007;104(51):20623–8. doi: 10.1073/pnas.0706547105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nishiyama R, Le DT, Watanabe Y, Matsui A, Tanaka M, Seki M, et al. Transcriptome analyses of a salt-tolerant cytokinin-deficient mutant reveal differential regulation of salt stress response by cytokinin deficiency. PLoS One. 2012;7(2):e32124 doi: 10.1371/journal.pone.0032124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ashraf M. Some important physiological selection criteria for salt tolerance in plants. Flora-Morphology, Distribution, Functional Ecology of Plants. 2004;199(5):361–76. doi: 10.1078/0367-2530-00165 [Google Scholar]
- 69.Sudhir P, Murthy S. Effects of salt stress on basic processes of photosynthesis. Photosynthetica. 2004;42(2):481–6. doi: 10.1007/S11099-005-0001-6 [Google Scholar]
- 70.Ohnishi N, Murata N. Glycinebetaine counteracts the inhibitory effects of salt stress on the degradation and synthesis of D1 protein during photoinhibition in Synechococcus sp. PCC 7942. Plant physiology. 2006;141(2):758–65. doi: 10.1104/pp.106.076976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Allakhverdiev SI, Nishiyama Y, Miyairi S, Yamamoto H, Inagaki N, Kanesaki Y, et al. Salt stress inhibits the repair of photodamaged photosystem II by suppressing the transcription and translation of psbA genes in synechocystis. Plant Physiol. 2002;130(3):1443–53. doi: 10.1104/pp.011114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Giardi MT, Masojídek J, Godde D. Effects of abiotic stresses on the turnover of the D1 reaction centre II protein. Physiologia Plantarum. 1997;101(3):635–42. [Google Scholar]
- 73.Mittal S, Kumari N, Sharma V. Differential response of salt stress on Brassica juncea: photosynthetic performance, pigment, proline, D1 and antioxidant enzymes. Plant Physiology and Biochemistry. 2012;54:17–26. doi: 10.1016/j.plaphy.2012.02.003 [DOI] [PubMed] [Google Scholar]
- 74.Bricker TM, Burnap RL. The Extrinsic Proteins of Photosystem II: Springer; Netherlands; 2005. 95–120 p. [Google Scholar]
- 75.Zhou F, Hua C, Qiu N, Zheng C, Wang R. Promotion of growth and upregulation of thylakoid membrane proteins in the halophyte Salicornia bigelovii Torr. under saline conditions. Acta Physiologiae Plantarum. 2015;37(2):1–7. doi: 10.1007/s11738-015-1782-2 [Google Scholar]
- 76.Allakhverdiev SI, Kreslavski VD, Thavasi V, Zharmukhamedov SK, Klimov VV, Nagata T, et al. Hydrogen photoproduction by use of photosynthetic organisms and biomimetic systems. Photochemical & Photobiological Sciences. 2009;8(2):148–56. doi: 10.1039/b814932a [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIF)
(DOCX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLS)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.