Abstract
Background
Vascular calcification is associated with structural and functional abnormality of the heart and blood vessels. We investigated the relationship between intradialytic hypotension (IDH) and vascular calcification in hemodialysis (HD) patients, and their impacts on cardiovascular events (CVEs).
Method
We enrolled 191 maintenance HD patients who underwent plain abdomen radiography for abdominal aortic calcification score (AACS). A nadir systolic blood pressure (BP) < 90 mm Hg or the requirement of bolus fluid administration was required to quantify the hypotension diagnosis. IDH was defined as > 2 hypotension episodes during 10 HD treatments.
Results
Among the 191 patients, IDH occurred in 32. AACS was higher in the IDH group compared with the no-IDH group (8.4 ± 6.0 vs. 4.9 ± 5.2, respectively; P = 0.001). High AACS was an independent risk factor after adjustment for age, diabetes mellitus, ultrafiltration, diastolic BP, and calcium level (odds ratio (OR) = 1.09, 95% CI = 1.01–1.18; P = 0.03). Patients with both IDH and AACS ≧ 4 had the highest cumulative CVE rate (27.9%, P = 0.008) compared with 11.2%, 12.5%, and 6% for those with AACS ≧ 4 only, with IDH only, and neither, respectively. In multivariate analysis, the presence of both IDH and AACS ≧ 4 was a significant predictor of CVE (hazard ratio (HR) = 2.84, 95% CI = 1.04–7.74, P = 0.04).
Conclusion
IDH is associated with abdominal aortic calcification and is an independent risk factor for IDH. Both IDH and high AACS were significant predictors of CVE.
Introduction
Intradialytic hypotension (IDH) is a common complication during hemodialysis (HD). The National Kidney Foundation Kidney Disease Outcomes Quality Initiative (KDOQI) defines IDH as “a decrease in systolic BP by ≥ 20 mm Hg or a decrease in MAP [mean arterial pressure] by 10 mm Hg associated with symptoms that include: abdominal discomfort; yawning; sighing; nausea; vomiting; muscle cramps; restlessness; dizziness or fainting; and anxiety” [1]. On the basis of the KDOQI definition, IDH occurs in approximately 20%–30% of HD sessions [2].
IDH continues to be a leading problem, especially in the cardiovascularly compromised patients. This predominance can be explained by the fact that structural and functional abnormalities of the heart and blood vessels increase patient sensitivity to changes in fluid status [3]. Vascular calcification is common in end-stage renal disease and is associated with cardiac changes [4]. It induces stiffening of the vessel wall and reduces vascular compliance, which has been found to predict cardiovascular mortality. Previous studies have reported that diastolic left ventricular (LV) dysfunction may be induced by increased vascular calcification in chronic HD patients [5].
IDH not only causes discomfort, but also increases patient mortality and cardiovascular events (CVEs) [6–8]. Vascular calcification induces a reduction in vascular compliance and diastolic LV dysfunction. Therefore, IDH may be associated with vascular calcification in HD patients.
This study aimed to determine the relationship between IDH and abdominal aortic calcification evaluated by plain abdomen radiography in HD patients. In addition, the clinical impact on CVEs was evaluated.
Methods
Study population
This study was conducted at Kangdong Sacred Heart Hospital, Kangnam Sacred Heart Hospital, and Chuncheon Sacred Heart Hospital. One hundred and ninety-one maintenance HD patients (49 men, 142 women) were eligible between April 2014 and April 2016. HD was performed thrice weekly (for 4 hours/day). Subjects had each received > 3 months of outpatient HD at one of the participating centers and underwent plain abdominal radiography for abdominal aortic vascular calcification. This prospective, observational study was approved by the hospitals’ Institutional Review Boards. Written informed consent was obtained from all patients before enrollment.
Data collection and definition
Baseline characteristics, including demographics, medical history, laboratory data, and HD data, were collected at the time of IDH assessment. Echocardiography, plain abdominal radiography, and PWV measurement were carried out across one week at the time of IDH assessment. Blood was drawn before starting each dialysis session. Body mass index (BMI) was calculated as the ratio of weight in kilograms divided by the square of height in meters. Mean pre-HD systolic and diastolic blood pressure and ultrafiltration were calculated as the average of 10 values at the time of IDH assessment. CVE was defined as a history of coronary artery disease, arrhythmia, cerebrovascular disease, or peripheral vascular disease; coronary artery disease was defined as a history of angioplasty, coronary artery bypass grafting, myocardial infarction, or angina; cerebrovascular disease was defined as a previous history of transient ischemic attack, stroke, or carotid endarterectomy; and peripheral vascular disease was defined as a history of claudication, ischemic limb loss and/or ulceration, or peripheral revascularization procedure. A nadir SBP < 90 mm Hg or the requirement for bolus fluid administration was required to quantify the hypotension diagnosis. IDH was defined as > 2 episodes hypotension during 10 HD treatments.
Outcome measures
The primary study outcome was the association between IDH and vascular calcification. The secondary outcome was new-onset CVE. Vascular calcification was scored by abdominal aortic calcification score (AACS) as described by Kauppila et al [9]. A lateral radiograph of the abdomen was performed in a standing position and the aorta was identified as the tubular structure coursing in front of the anterior surface of the spine. We used a semi-quantitative scoring system; only the abdominal aorta segments in front of the first to fourth lumbar vertebrae were considered. Points were assigned on a 0–3 scale to areas of calcification identified along the anterior or posterior surface of the aorta (0 = absent; 1 = small; 2 = moderate; 3 = large) according to the length of each calcified plaque with respect to the craniocaudal length of the closet vertebra. Hence, the score could vary from a minimum of 0 to a maximum of 24 points. All radiographs were read by two investigators and a consensus was reached on the interpretation of all films.
Brachial-ankle PWV (baPWV) was measured using a Vascular Profiler 1000 (VP-1000; Colin Co. Ltd., Komaki, Japan). The baPWV was automatically calculated from the distance between two arterial recording sites divided by transit time. Comprehensive echocardiographic measurements were performed using an ultrasound machine (Vivid 7; GE Vingmed ultrasound AS, Horten, Norway) with a 2.5 MHz probe, based on the imaging protocol by the American Society of Echocardiography guideline.
Statistical analysis
The data are expressed as means ± standard deviations. Comparisons of continuous variables were performed using t-tests. Categorical variables were compared using either chi-square or Fisher’s exact test, as appropriate. For the primary analysis, we performed multivariate logistic regression to test for the independent contribution of vascular calcification (VC) to IDH. The cumulative incidence of CVEs was calculated using the Kaplan–Meier method and compared using the log-rank test. Cox proportional hazard model was used to identify the independent variables related to CVEs. Baseline associations were estimated by demographic factors and comorbid conditions. The factors might confound associations with outcomes of t testing and chi-squared testing. Associated confounder and clinically important factors were included in univariate analyses. All variables with p-values less than 0.05 in the univariate analyses were included in the multivariate models. All calculations were performed using SPSS 18.0 (SPSS Inc. Armonk, NY). P < 0.05 was considered statistically significant.
Results
The 191 subjects’ mean age was 60 ± 12 years and 142 (74.3%) were women. One hundred three (53.9%) had diabetes mellitus and 92 (48.2%) had a previous history of CVE. IDH occurred in 32 of the 191 subjects. Their mean ultrafiltration was 2.6 ± 1.0 L and mean AACS score was 5.5 ± 5.5. Demographic and biochemical parameters of all subjects, with and without IDH, are in Table 1.
Table 1. Baseline demographics and biochemical variables in study population according to IDH.
| Variables | IDH | No IDH | p- value |
|---|---|---|---|
| N = 32 | N = 159 | ||
| Demographic data | |||
| Age (years) | 62.1 ± 8.0 | 59.3 ± 12.2 | 0.2 |
| Male, n (%) | 8 (25) | 41 (25.8) | 1.0 |
| Duration of hemodialysis (months) | 65.1 ± 64.9 | 54.4 ± 54.4 | 0.3 |
| Diabetes, n (%) | 24 (75) | 79 (50.6) | 0.009 |
| Smoking, n (%) | 2 (6.3) | 23 (14.5) | 0.2 |
| Previous cardiovascular event | |||
| Coronary artery disease, n (%) | 14 (43.8) | 59 (37.1) | 0.5 |
| Peripheral artery disease, n (%) | 2 (6.5) | 9 (5.7) | 0.9 |
| Cerebrovascular accident, n (%) | 7 (21.9) | 38 (24.2) | 0.7 |
| Mean ultrafiltration | 2.9 ± 0.8 | 2.6 ± 1.0 | 0.03 |
| Mean pre-HD systolic BP | 145.4 ± 28.9 | 152.7 ± 24.5 | 0.1 |
| Mean pre-HD diastolic BP | 72.7 ± 13.8 | 78.9 ± 11.8 | 0.02 |
| Antihypertensive medication | |||
| Beta blocker | 11 (34.4) | 75 (47.2) | 0.2 |
| Calcium channel blocker | 10 (31.3) | 83 (52.2) | 0.03 |
| Renin-angiotensin system blocker | 18 (56.3) | 108 (67.9) | 0.2 |
| Laboratory data | |||
| Hemoglobin (g/dL) | 10.3 ± 1.0 | 10.2 ± 1.3 | 0.6 |
| Albumin (g/dL) | 3.7 ± 0.4 | 3.7 ± 0.4 | 0.5 |
| Calcium (mg/dL) | 8.9 ± 1.0 | 8.5 ± 0.8 | 0.03 |
| Phosphate (mg/dL) | 4.6 ± 1.3 | 4.4 ± 1.4 | 0.3 |
| Cholesterol (mg/dL) | 137.3 ± 34.6 | 136.3 ± 39.3 | 0.9 |
| Triglyceride (mg/dL) | 132 ± 93.2 | 113.3 ± 69.9 | 0.2 |
| High-density lipoprotein (mg/dL) | 42.6 ± 11.9 | 42.6 ± 13.2 | 1.0 |
| Low-density lipoprotein (mg/dL) | 75.9 ± 18.0 | 74.3 ± 25.5 | 0.7 |
| CRP (mg/L) | 4.6 ± 6.5 | 2.9 ± 7.6 | 0.3 |
| Parathyroid hormone (pg/dL) | 265.3 ± 211.4 | 331.5 ± 310.2 | 0.3 |
| Vitamine D use | 10 (31.3) | 83 (52.2) | 0.03 |
| Calcium containing phosphate binder | 21 (65.6) | 112 (70.4) | 0.4 |
| Access type | |||
| Arteriovenous fistula | 26 (81.3) | 133 (83.6) | 0.79 |
| Arteriovenous graft | 6 (18.8) | 22 (13.8) | |
| Catheter | 0 (0) | 4 (2.5) |
Note: values are expressed as median ± SD or median (interquartile range) or number (percentage).
Abbreviations: CRP, C-reactive protein; HD, hemodialysis; BP, blood pressure
IDH occurred in 6.7% of dialysis sessions. No hypotension episode occurred in 129 out of 191 (67.5%) patients while one episode in 30 (15.7%), two episodes in 15 (7.9%), three episodes in 9 (4.7%), four episodes in 3 (16%), five and six episodes in two (1.0%) respectively and seven episodes in one (0.5%) during 10 HD treatments.
Number of hypotensive episodes was linearly associated with vascular calcification (st1306andardized coefficient 0.224, P = 0.002). AACS was higher in the IDH group than the no-IDH group (8.4 ± 6.0 vs. 4.9 ± 5.2, P = 0.001). The median AACS value for all subjects was 4.0. Incidence of AACS ≧ 4 was significantly higher in patients with IDH than in those without IDH (75% vs. 45.9%, respectively; P = 0.003).
We performed logistic regression analysis to identify independent risk factors associated with IDH (Table 2). In univariate analysis, diabetes mellitus (odds ratio (OR) = 3.04, 95% confidence interval, CI = 1.29–7.17; P = 0.01), high ultrafiltration (OR = 1.65, 95% CI = 1.05–2.60; P = 0.03), low diastolic BP (OR = 0.96, 95% CI = 0.93–0.99; P = 0.009), high calcium value (OR = 1.57, 95% CI = 1.02–2.43; P = 0.04), calcium channel blocker (OR = 0.41, 95% CI = 0.18–0.93; P = 0.03), vitamin D use (OR = 0.41, 95% CI = 0.19–0.93; P = 0.03) and high AACS (OR = 1.11, 95% CI = 1.04–1.19; P = 0.001) were significantly associated with IDH. High AACS was an independent risk factor after adjustment for age, diabetes mellitus, ultrafiltration, diastolic BP, and calcium level (OR = 1.09, 95% CI = 1.01–1.18; P = 0.03).
Table 2. Logistic models for associating factors of IDH.
| Variables | Univariate | Multivariate | ||
|---|---|---|---|---|
| Odds Ratio (95% C.I.) | p-value | Odds ratio (95% C.I.) | p-value | |
| Age (per year) | 1.0(1.0–1.06) | 0.2 | 1.02 (1.0–1.07) | 0.5 |
| DM (vs. No) | 3.04 (1.29–7.17) | 0.01 | 1.84 (0.7–4.91) | 0.2 |
| Ultrafiltration (per 1L) | 1.65 (1.05–2.60) | 0.03 | 2.06 (1.16–3.66) | 0.01 |
| Diastolic BP (per 10 mmHg) | 0.96 (0.93–0.99) | 0.009 | 0.96 (0.92–1.0) | 0.04 |
| Calcium (per 1 mg/dl) | 1.57 (1.02–2.43) | 0.04 | 1.53 (1.0–2.60) | 0.1 |
| Albumin (per 1 g/dl) | .41 (0.55–3.60) | 0.5 | ||
| Dialysis vintage (per month) | 1.003 (1.0–1.0) | 0.3 | ||
| Calcium channel blocker | 0.41 (0.18–0.93) | 0.03 | 0.32 (0.12–0.83) | 0.02 |
| Vitamin D use | 0.41 (0.19–0.93) | 0.03 | 0.38 (0.15–0.97) | 0.04 |
| AACS | 1.11 (1.04–1.19) | 0.001 | 1.09 (1.01–1.18) | 0.03 |
| Pre-CVE (vs. No) | 2.01 (0.9–4.4) | 0.08 | ||
Abbreviations: DM, diabetes mellitus; BP, blood pressure; AACS, abdominal aortic calcification score; IDH, intradialytic hypotension; CVE, cardiovascular event
The follow-up period was 18.3 ± 6.8 months. During follow-up, new CVE occurred in 18 subjects (9.4%) and 11 subjects (5.8%) died. Coronary artery disease occurred in 10 patients, peripheral vascular disease in 3 patients and cerebrovascular disease in 5 patients. The highest cumulative event rate for new CVE was observed in patients with both IDH and AACS ≧ 4 (27.9%, P = 0.008; Fig 1). Patients with either AACS≧ 4 or IDH had a 11.2%, and 12.5% cumulative CVE rate respectively, but the values were not significantly different from ones of patients with neither AACS ≧ 4 nor IDH (6%; P = 0.65 and P = 0.51, respectively). The cumulative event rate for mortality was not significantly different among the groups.
Fig 1. Cumulative rates of cardiovascular event.
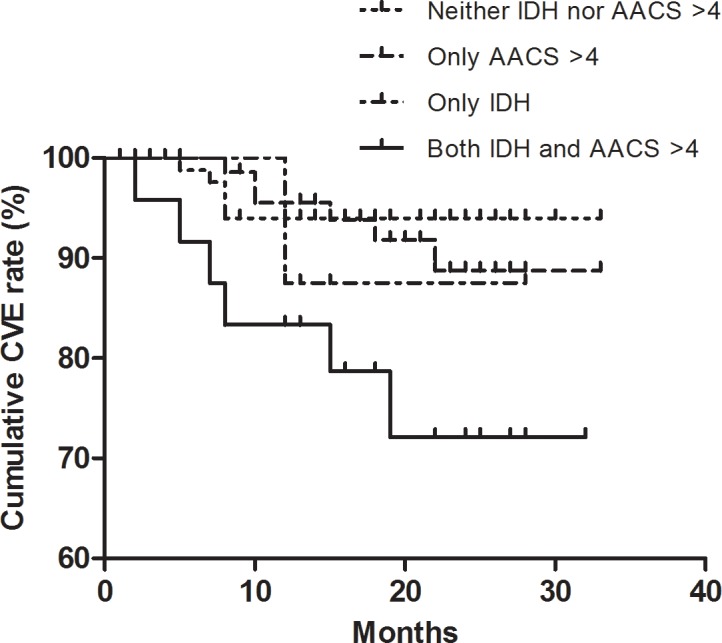
IDH, intradialytic hypotension; AACS, abdominal aortic calcification score.
One hundred forty-three subjects and 159 subjects had PWV measurement and echocardiography, respectively. All subjects were divided into two groups based on the presence or absence of IDH. We also divided them according to AACS score. PWV value and echocardiographic parameters (LV ejection fraction, the peak mitral inflow velocities at the early (E), late (A) diastole, deceleration time (DT), early (E′) and late (A′) diastolic peak velocities, and E/E′ were compared between two groups. When comparing the presence or absence of IDH, only DT differed significantly (245.1 ± 78.2 vs. 210.5 ± 61.4, P = 0.01). Comparison of subjects with AACS ≧ 4 and AACS < 4 showed that PWV (2097 ± 633 vs. 1811 ± 331, P = 0.001), A (93.3 ± 25.8 vs. 85.5 ± 19.6, P = 0.03), E′ (4.4 ± 1.3 vs. 5.1 ± 2.0, P = 0.006), and E/E′ (20.4 ± 8.9 vs. 16.9 ± 7.3, P = 0.005) values were significantly different (Table 3).
Table 3. Pulse wave velocity and echocardiographic index according to vascular calcification.
| Variables | AACS < 4 | AACS ≧ 4 | P- value |
|---|---|---|---|
| (n = 94) | (n = 97) | ||
| Pulse wave velocity | 1811.4 ± 330.6 | 2097.4 ± 633.0 | 0.001 |
| Echocardiac parameter | |||
| LVEF (%) | 56.5 ± 11.1 | 58.6 ± 9.0 | 0.183 |
| LVMI (g/m2) | 135.9 ± 41.6 | 142.4 ± 42.0 | 0.312 |
| E (cm/sec) | 77.1 ± 25.5 | 83.0 ± 26.2 | 0.263 |
| E’ (cm/sec) | 5.1 ± 2.0 | 4.4 ± 1.3 | 0.006 |
| A (cm/sec) | 85.5 ± 19.6 | 93.3 ± 25.8 | 0.031 |
| E/A ratio | 0.9 ± 0.4 | 1.0 ± 0.6 | 0.406 |
| E/E' ratio | 16.9 ± 7.3 | 20.4 ± 8.9 | 0.005 |
| DT (msec) | 214.6 ± 67.5 | 218.7 ± 64.3 | 0.694 |
Note: values are expressed as median ± SD
Abbreviations: LVEF, left ventricular ejection fraction; LVMI, left ventricular mass index; DT, deceleration time; AACS, abdominal aortic calcification score
Cox proportional analysis was used to evaluate risk factors for new CVE (see Table 4). In univariate analysis, age (hazard ratio (HR) = 1.06, 95% CI = 1.02–1.11, P = 0.005), previous CVE history (HR = 4.13, 95% CI = 1.36–12.56, P = 0.01), and IDH (HR = 3.30, 95% CI = 1.28–8.51, P = 0.01) were significantly associated with new CVE. The presence of both IDH and AACS ≧ 4 was a significant predictor for new CVE in univariate (HR = 3.53, 95% CI = 1.32–9.41, P = 0.01) and multivariate analyses (HR = 2.84, 95% CI = 1.04–7.74, P = 0.04). There were no significant predictive factors for mortality.0020
Table 4. Cox proportional hazard models for risk factors of new CVE.
| Variables | Univariate | Multivariate | ||
|---|---|---|---|---|
| HR (95% C.I.) | p-value | HR (95% C.I.) | p-value | |
| Age (per year) | 1.06 (1.02–1.11) | 0.005 | 1.05 (1.0–1.10) | 0.04 |
| DM (vs. No) | 1.65 (0.62–4.40) | 0.3 | ||
| Pre CVE (vs. No) | 4.13 (1.36–12.56) | 0.01 | 2.42 (0.75–7.88) | 0.1 |
| IDH+AACS>4 (vs. No) | 3.53 (1.32–9.41) | 0.01 | 2.84 (1.04–7.74) | 0.04 |
| Dialysis vintage (per year) | 1.0 (1.0–1.0) | 0.5 | ||
Abbreviations: DM, diabetes mellitus; AACS, abdominal aortic calcification score; IDH, intradialytic hypotension; CVE, cardiovascular event
Discussion
We have demonstrated that IDH is associated with vascular calcification. Patients with IDH had a higher AACS than did patients without IDH. AACS was a significant risk factor for IDH. Cumulative new CVE rate was higher in patients with IDH than without IDH, and highest in patients with both IDH and AACS ≧ 4. In multivariate analysis, having both IDH and AACS ≧ 4 was a significant predictor of new CVE.
Calcification of the coronary arteries and the aorta has recently been recognized as an important risk factor for cardiovascular disease in HD patients [10, 11]. Reduction of large-artery stiffness increases cardiac afterload [4]. Diastolic LV dysfunction is induced by increased afterload in a experimental study [12]. In HD patients, reduced large-artery stiffness may contribute to vascular calcification in the thoracic and abdominal aorta, independent of blood pressure (BP). Therefore, LV diastolic dysfunction may be associated with aortic calcification in HD patients. A study reported that diastolic LV dysfunction may be induced by increased vascular calcification and reduced arterial stiffness in chronic HD patients [5].
IDH is the result of not only cardiovascular system structural abnormalities but also of impaired vascular reactivity [13, 14]. Inadequate cardiovascular response to maintain adequate blood pressure in response to volume stress is a key issue in the pathogenesis of IDH in such patients [2]. HD patients with vascular calcification have a higher degree of arterial stiffness, which is associated with insufficient response to volume stress [4, 15]. Our results suggest an association between IDH with vascular calcification. However, we cannot explain the relationship between systolic and diastolic dysfunction, and IDH. This may be because the pathophysiology of IDH is multifactorial [16]. Meanwhile, vascular calcification was associated with diastolic LV dysfunction and arterial stiffness in this study, which is consistent with previous studies.
HD patients are at greater risk of cardiovascular (CV) disease-related morbidity and mortality compared with the general population [17]. The higher burden of CV disease-related events may be due, in part, to episodic tissue hypoxia resulting from IDH [18]. In this study, we found that presence of IDH was associated with new CVE. Presence of vascular calcification alone was not associated with CVE. However, coexistence of vascular calcification and IDH was an independent predictor of new CVE. Furthermore, the impact was increased in patients with both IDH and vascular calcification. IDH causes frequent ischemic episodes in the heart and arteries [18]. IDH also causes disturbance in achieving an adequate dialysis dose and substantial increases in calcium and phosphate product [19, 20]. Therefore, inadequate dialysis induced by IDH worsens VC in HD patients. In addition, patients with frequent IDH and with atherosclerotic arteries become vulnerable to ischemic injury.
Risk factors for IDH have not been defined clearly from the literature, but several conditions have been held responsible. Patients of advanced age (≥ 65), with diabetic nephropathy, CV disease, or autonomic dysfunction are at risk of developing IDH [16, 21]. Furthermore, clinical features such as low blood pressure levels before an HD session (< 100 mm Hg), poor nutritional status (hypoalbuminemia), and high ultrafiltration rate also predispose chronic dialysis patients to IDH occurrence [16, 21]. Our study results are comparable to the published data and, in this study; vascular calcification is shown to be an important risk factor for IDH in the adjusted multivariate model. Vascular calcification is related to functional disorders of the cardiovascular system including left ventricular dysfunction and peripheral artery malfunction, which may increase the risk of IDH [5, 22].
Our study has some limitations. First, there is the inherent weakness of all studies with a retrospective design; namely, the use of data from past medical records. Second, a number of patients did not undergo PWV measurement and echocardiography. Third, the follow-up period was short; studies of long-term prognostic implications of IDH and vascular calcification on CVE in HD patients are needed. Fourth, quantitative measurement of vascular calcification by computed tomography was not assessed. However, Kaupplia method was able to detect VC and predict CVEs [23]. In addition, our study did not consider the effects of oral medications to treat hypertension.
In conclusion, these results suggest that IDH is associated with abdominal aortic calcification and is an independent risk factor for IDH. Both IDH and high AACS were significant predictors for CVE occurrence. To improve mortality and morbidity in HD patients, a treatment strategy for reducing the frequency of IDH and calcification progression should be considered.
Supporting information
(XLSX)
Acknowledgments
This work was supported by a grant from the Kyowa Kakko Kirin, Korea Co. Ltd. (2014–2016).
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by a grant from the Kyowa Kakko Kirin, Korea Co. Ltd. (2014–2016).
References
- 1.Reilly RF. Attending rounds: A patient with intradialytic hypotension. Clin J Am Soc Nephrol. 2014;9(4):798–803. doi: 10.2215/CJN.09930913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Palmer BF, Henrich WL. Recent advances in the prevention and management of intradialytic hypotension. J Am Soc Nephrol. 2008;19(1):8–11. doi: 10.1681/ASN.2007091006 [DOI] [PubMed] [Google Scholar]
- 3.Converse RL Jr., Jacobsen TN, Jost CM, Toto RD, Grayburn PA, Obregon TM, et al. Paradoxical withdrawal of reflex vasoconstriction as a cause of hemodialysis-induced hypotension. J Clin Invest. 1992;90(5):1657–65. doi: 10.1172/JCI116037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guerin AP, London GM, Marchais SJ, Metivier F. Arterial stiffening and vascular calcifications in end-stage renal disease. Nephrol Dial Transplant. 2000;15(7):1014–21. [DOI] [PubMed] [Google Scholar]
- 5.Fujiu A, Ogawa T, Matsuda N, Ando Y, Nitta K. Aortic arch calcification and arterial stiffness are independent factors for diastolic left ventricular dysfunction in chronic hemodialysis patients. Circ J. 2008;72(11):1768–72. [DOI] [PubMed] [Google Scholar]
- 6.Shoji T, Tsubakihara Y, Fujii M, Imai E. Hemodialysis-associated hypotension as an independent risk factor for two-year mortality in hemodialysis patients. Kidney Int. 2004;66(3):1212–20. doi: 10.1111/j.1523-1755.2004.00812.x [DOI] [PubMed] [Google Scholar]
- 7.Kalantar-Zadeh K, Regidor DL, Kovesdy CP, Van Wyck D, Bunnapradist S, Horwich TB, et al. Fluid retention is associated with cardiovascular mortality in patients undergoing long-term hemodialysis. Circulation. 2009;119(5):671–9. doi: 10.1161/CIRCULATIONAHA.108.807362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flythe JE, Inrig JK, Shafi T, Chang TI, Cape K, Dinesh K, et al. Association of intradialytic blood pressure variability with increased all-cause and cardiovascular mortality in patients treated with long-term hemodialysis. Am J Kidney Dis. 2013;61(6):966–74. doi: 10.1053/j.ajkd.2012.12.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kauppila LI, Polak JF, Cupples LA, Hannan MT, Kiel DP, Wilson PW. New indices to classify location, severity and progression of calcific lesions in the abdominal aorta: a 25-year follow-up study. Atherosclerosis. 1997;132(2):245–50. [DOI] [PubMed] [Google Scholar]
- 10.Goodman WG, Goldin J, Kuizon BD, Yoon C, Gales B, Sider D, et al. Coronary-artery calcification in young adults with end-stage renal disease who are undergoing dialysis. N Engl J Med. 2000;342(20):1478–83. doi: 10.1056/NEJM200005183422003 [DOI] [PubMed] [Google Scholar]
- 11.Raggi P, Boulay A, Chasan-Taber S, Amin N, Dillon M, Burke SK, et al. Cardiac calcification in adult hemodialysis patients. A link between end-stage renal disease and cardiovascular disease? J Am Coll Cardiol. 2002;39(4):695–701. [DOI] [PubMed] [Google Scholar]
- 12.Leite-Moreira AF, Correia-Pinto J, Gillebert TC. Afterload induced changes in myocardial relaxation: a mechanism for diastolic dysfunction. Cardiovasc Res. 1999;43(2):344–53. [DOI] [PubMed] [Google Scholar]
- 13.Kooman JP, Gladziwa U, Bocker G, van Bortel LM, van Hooff JP, Leunissen KM. Role of the venous system in hemodynamics during ultrafiltration and bicarbonate dialysis. Kidney Int. 1992;42(3):718–26. [DOI] [PubMed] [Google Scholar]
- 14.London GM, Safar ME, Levenson JA, Simon AC, Temmar MA. Renal filtration fraction, effective vascular compliance, and partition of fluid volumes in sustained essential hypertension. Kidney Int. 1981;20(1):97–103. [DOI] [PubMed] [Google Scholar]
- 15.Blacher J, Demuth K, Guerin AP, Safar ME, Moatti N, London GM. Influence of biochemical alterations on arterial stiffness in patients with end-stage renal disease. Arterioscler Thromb Vasc Biol. 1998;18(4):535–41. [DOI] [PubMed] [Google Scholar]
- 16.Gul A, Miskulin D, Harford A, Zager P. Intradialytic hypotension. Curr Opin Nephrol Hypertens. 2016;25(6):545–50. doi: 10.1097/MNH.0000000000000271 [DOI] [PubMed] [Google Scholar]
- 17.Flythe JE, Kimmel SE, Brunelli SM. Rapid fluid removal during dialysis is associated with cardiovascular morbidity and mortality. Kidney Int. 2011;79(2):250–7. doi: 10.1038/ki.2010.383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chao CT, Huang JW, Yen CJ. Intradialytic hypotension and cardiac remodeling: a vicious cycle. Biomed Res Int. 2015;2015:724147 doi: 10.1155/2015/724147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rocco MV, Burkart JM. Prevalence of missed treatments and early sign-offs in hemodialysis patients. J Am Soc Nephrol. 1993;4(5):1178–83. [DOI] [PubMed] [Google Scholar]
- 20.Sherman RA, Cody RP, Matera JJ, Rogers ME, Solanchick JC. Deficiencies in delivered hemodialysis therapy due to missed and shortened treatments. Am J Kidney Dis. 1994;24(6):921–3. [DOI] [PubMed] [Google Scholar]
- 21.van der Sande FM, Kooman JP, Leunissen KM. Intradialytic hypotension—new concepts on an old problem. Nephrol Dial Transplant. 2000;15(11):1746–8. [DOI] [PubMed] [Google Scholar]
- 22.Vishnu A, Choo J, Wilcox B, Hisamatsu T, Barinas-Mitchell EJ, Fujiyoshi A, et al. Brachial-ankle pulse wave velocity is associated with coronary calcification among 1131 healthy middle-aged men. Int J Cardiol. 2015;189:67–72. doi: 10.1016/j.ijcard.2015.04.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vannini FC, Teixeira AS, Caramori JC, Martin LC, Barretti P. Is Kauppila method able to detect the progression of vascular calcification and predict cardiovascular events in patients undergoing hemodialysis? Clin Nephrol. 2016;85(2):84–91. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.


