Abstract
Loss of muscle mass and strength represents one of the most significant contributors to impaired function in older adults. Convenient and non-invasive biomarkers are needed that can readily identify and track age-related muscle change. Previous data has suggested electrical impedance myography (EIM) has the potential to serve in this capacity. In this study we investigated how changes in EIM compared with other standard measures of muscle structure and function in aged compared with young mice. A total of 19 male mice aged approximately 25 months and 19 male mice aged 3 months underwent surface multifrequency EIM of the right gastrocnemius muscle using standard methods. Fore and hind limb grip strength, sciatic compound muscle action potential amplitude, and in-situ force of the gastrocnemius were also measured; after sacrifice, gastrocnemius myofiber size was assessed using standard histology. Spearman correlation coefficients were calculated to investigate the association between EIM and muscle characteristics. EIM in aged animals demonstrated significantly lower 50 kHz impedance phase (p<0.001) and reactance (p<0.01) values as well as reduced multifrequency parameters. In contrast, absolute gastrocnemius muscle mass was no different between young and aged mice (p = 0.58) but was reduced in aged mice after normalization to body mass (p<0.001). Median myofiber size in the aged mice was not different from that of young mice (p = 0.72). Aged mice showed reduced muscle function on the basis of normalized fore limb (p<0.001) and normalized hind limb (p<0.001) grip strength, as well as normalized gastrocnemius twitch (p<0.001) and normalized maximal isometric force (p<0.001). Sciatic compound muscle action potential amplitude was reduced in aged mice (p<0.05). EIM parameters showed good correlation with reduced standard physiological and electrophysiological measures of muscle health. Our study suggests that EIM is sensitive to aged-related muscle change and may represent a convenient and valuable method of quantifying loss of muscle health.
Introduction
Loss of muscle mass and strength, or sarcopenia, is an increasingly important public health problem that results as a consequence of aging in a large proportion of the older adults. There are no convenient, well-accepted diagnostic methods for the assessment of sarcopenia in at-risk individuals [1]. Sarcopenia was originally defined as loss of muscle mass [2, 3], but it has become increasingly apparent that loss of muscle strength is a more important indicator of muscle status during aging [4–7]. Thus, the definition of sarcopenia has changed to describe both loss of muscle strength, sometimes referred to as dynapenia, as well as loss of muscle mass itself [8, 9]. As such, methods for assessing sarcopenia and muscle status have also evolved over time and include measures of physical performance, imaging modalities to analyze muscle size and composition, quantitative strength testing, and molecular markers [1]. The concept of “muscle quality” has also been employed to describe loss of muscle strength out of proportion to loss of muscle mass that occurs in sarcopenia [5–7, 10–12]. Indeed, histological studies have demonstrated a number of alterations in aged muscle, including mild myofiber atrophy, increased connective tissue and fat deposition, and conversion of some muscle fibers from type 2 to type 1 [5–7, 13, 14]. Yet, a simple-to-use, non-invasive tool for sarcopenia assessment that provides information about muscle quality has not been identified.
Electrical impedance myography (EIM) is an emerging technology for the assessment of neuromuscular health that has already shown significant value in a variety of disorders affecting both muscle and nerve, including amyotrophic lateral sclerosis [15, 16], spinal muscular atrophy [17, 18], and Duchenne muscular dystrophy [19]. Whereas prominent pathological change, as occur in these disorders, is readily identified by EIM, the technique also appears sensitive to subtler disorders. For example, EIM alterations have been identified in disuse, both in an animal models and in humans [20, 21]; it has also been shown to be sensitive to the effects of microgravity on muscle [22]. Accordingly, we have also previously assessed EIM in older individuals and identified lower phase values compared with younger individuals [23]. In addition, 4 healthy older adults were also studied longitudinally, and reductions in EIM values were identified [23]. One additional study also identified changes in EIM values in paraspinal muscles of older individuals, consistent with sarcopenic change [24]. However, in these studies the underlying histological and functional status of the muscle and its association with EIM values were not investigated. Therefore, in this study, we evaluated EIM in aged and young mice to identify the technique’s relationship to standard functional and histological measures of muscle condition.
Methods
Ethics statement and experimental animals
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. Our animal protocol was approved by the Beth Israel Deaconess Medical Center Institutional Animal Care and Use Committee (IACUC) (Protocol Number 087–2016), and all efforts were made to minimize any animal suffering. C57BL/6 male mice were obtained from the National Institutes on Aging Animal Program at approximately 3 and 25 months (n = 19 per group). Animals were allowed to acclimate at least 72 hours prior to testing and were fed a standard diet ad libitum. Impedance and in situ force experiments were performed under 1–2% inhaled isoflurane anesthesia. At the conclusion of all studies, the animals were euthanized with carbon dioxide.
Body mass and grip strength assessment
Animals were weighed with an analytical balance. Fore limb and hind limb grip strength were measured in grams by a grip strength meter with a sensor range of 0–1000 grams and accuracy of 0.25% of the full scale with standard pull bar (CAT #1027CSM, Columbus Instruments, Columbus, OH). For both, the animal was first allowed to grip the pull bar; the investigator, grasping the lower back of the animal then pulled it away from the bar until the mouse lost its grip. The maximum force recorded out of 5 trials was recorded. This test was performed separately on the fore and hind limbs.
Animal preparation for EIM/CMAP and in situ force
Impedance and in situ force experiments were performed under 1–2% inhaled isoflurane anesthesia delivered by nose cone with body and muscle temperature being maintained by a heating pad (37°C). A depilatory agent was applied to the right hind limb to remove fur, and the skin was cleaned with 0.9% saline solution. Both legs were taped to the measuring surface at an approximately 45° angle extending out from the body in preparation for measurements.
Compound muscle action potential (CMAP) amplitude
Sciatic CMAP amplitudes were recorded from the sciatic innervated triceps surae muscle of the right hindlimb using a TECA Synergy T2 EMG Monitor System (Viasys, Inc, Madison, WI) as previously described [25–27]. Briefly, the sciatic nerve was supramaximally stimulated at the sciatic notch. CMAP amplitude was recorded using two ring electrodes (Catalogue # 9013S0312, Natus Neurology, Middleton, Wisconsin, USA) placed on the proximal leg over the posterior and anterior compartment muscle groups (active electrode, E1) and on the mid-metatarsal region of the foot (reference electrode, E2). A ground electrode was placed on the left hind paw. CMAP amplitudes were measured peak-to-peak.
Electrical impedance myography (EIM)
A fixed rigid 4-electrode impedance-measuring array was applied over the left gastrocnemius muscle. EIM measurements were performed with the EIM1103 System (Myolex, Inc, San Francisco, CA), which obtains impedance data at 41 frequencies from 1 kHz to 10 MHz as previously described [25, 28]. The 50 kHz phase, resistance, and reactance values were extracted from the frequency set as were the modeled multifrequency Cole parameters. The three Cole parameters of interest included the center frequency, fc, the dispersion coefficient alpha,α, and the cell density, R0/Rinf [29, 30]. The meaning of each of these is described in more detail below.
In situ muscle physiology
After the CMAP and EIM measurements, a non-survival surgery was performed to expose the left gastrocnemius muscle and calcaneal tendon [31]. The soleus muscle and underlying fascia were dissected away from the calcaneal tendon; the tendon was then connected to a force lever arm (described in more detail below) and the leg stabilized by inserting a disposable monopolar needle (902-DMF37-S, Natus neurology, Middleton, Wisconsin, USA) through the knee joint. Twitch and tetanic force were recorded following stimulation of the sciatic nerve with 200 ms square pulses via insulated monopolar needles (F-E2M-48, Grass Technologies, Warwick, Rhode Island, USA). A high-speed servomotor-based apparatus (Model 305C, Aurora Scientific, Aurora, Ontario, Canada) was used to measure force output. The output signals from the lever system were interfaced to a PC-platform integrating a PXIe-8135 quad-core processor based embedded controller and a two-channel acquisition board PXI-4461 from National Instruments (Austin, Texas, USA). A custom program controlled the lever arm movement and output of a biphasic pulses current muscle stimulator (Model 701, Aurora Scientific). Stimulation current and resting tension were adjusted to maximize twitch force produced by a single stimulus pulse. Optimal length was measured with digital calipers as the distance between the knee and the calcaneal tendon. All subsequent isometric tetanic force data were collected at this stimulation current and resting tension. Isometric force frequency relationship was recorded after stimulation by a train of square wave stimuli at 100Hz, 110Hz, 120Hz, and 200Hz and the maximum isometric tetanic force was recorded. At the conclusion of all studies, the animals were euthanized with carbon dioxide.
Muscle histology
Wet muscle mass of the excised gastrocnemius muscle was measured with a standard analytical balance. The right gastrocnemius was harvested from a subset of animals (9 mice aged 3-month and 9 mice aged 26-month-old animals) and placed in 10% formalin. Samples were then embedded in paraffin blocks, sectioned into 10 μm slices and stained with anti-collagen VI antibody (Abcam ab6588). Sections were subsequently imaged at 20x with a Zeiss AxioImager M1 epifluorescence microscope and fiber area was measured using Volocity® software (PerkinElmer, Akron, Ohio, USA).
Data analysis
Impedance data was processed with MATLAB (The Mathworks, Natick, MA) to extract the Cole parameters. Statistical analysis on the raw impedance values and Cole parameters was then performed using GraphPad Prism (GraphPad Software, Inc. La Jolla, CA); p < 0.05, two-tailed was considered significance for all tests. All mass, grip strength, force, and 50 kHz EIM data are reported as median with upper and lower 95% confidence intervals. We compared older and younger animals using Mann-Whitney tests. For correlation analyses, Spearman correlation coefficients were calculated. We analyzed the EIM parameters with two-way ANOVA to explore the effect of age and electrode orientation (longitudinal versus transverse orientation with the direction of the muscle fibers).
Results
Body mass, muscle mass, grip strength, and CMAP
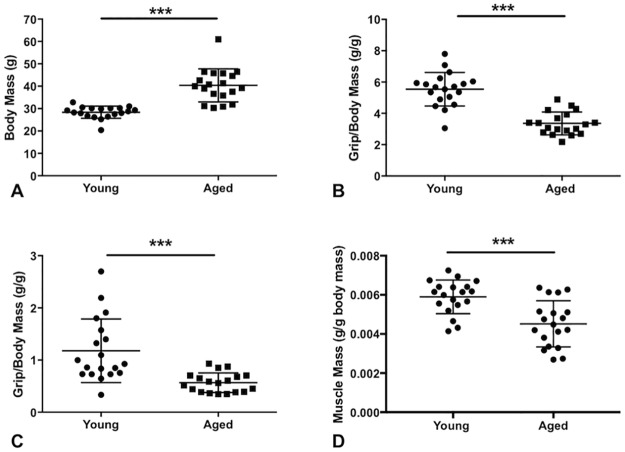
Body mass, wet muscle mass, and grip strength are summarized in Fig 1 and Table 1. The aged mice were considerably heavier but absolute muscle mass remained virtually identical. Interestingly, despite having similar absolute muscle mass, both absolute and normalized fore limb and hind limb grip strength were significantly reduced in older animals. The fore limb strength data were more consistent, which is likely related to the relative technical ease of obtaining fore limb strength measurements as compared to hind limb. CMAP also demonstrated reduced amplitude in aged mice (52.7mV, 37.1 to 75.7mV) compared with young mice (62.2mv, 56.6 to 78.9mV) (p<0.05) (Data not shown in Fig 1).
Fig 1. Mean body mass, muscle mass, and grip testing.
Aged mice demonstrated increased body mass (A) reduced normalized fore limb grip (B), reduced normalized hind limb grip strength (C), and reduced normalized wet muscle mass (D) compared with young mice. ***, p<0.001. Data shown as mean ± standard deviation in Fig 1. Aged mice also demonstrated significant reduction in absolute forelimb (young = 160.0g, 138.0 to 174.0 g versus aged = 127.0g, 119.0 to 152.0g; p<0.001) and absolute hindlimb (young = 26.0g, 22.0 to 47.0g versus aged = 21.0g,16.0 to 29.0g; p<0.05) strength (not shown). In contrast, absolute gastrocnemius muscle mass was similar between aged and young mice (young = 0.167g, 0.137to 0.194g) versus aged mice = 0.186g, 0.156 to 0.194g); p = 0.58) (not shown).
Table 1. Body mass, wet muscle mass, and grip strength.
| Young | Aged | p value | |
|---|---|---|---|
| Body mass (g) | 28.79 (26.7, 30.15) | 39.98 (35.87, 45.75) | <0.001 |
| Absolute muscle mass (g) | 0.167 (0.137, 0.194) | 0.186 (0.156, 0.194) | 0.58 |
| Normalized muscle mass (g/g) | 0.006142 (0.005484, 0.006411) | 0.004484 (0.003358, 0.005148) | <0.001 |
| Forelimb grip strength (g) | 160.0 (138.0, 174.0) | 127.0 (119.0, 152.0) | <0.001 |
| Hindlimb grip strength (g) | 26.0 (22.0, 47.0) | 21.0 (16.0, 29.0) | <0.05 |
| Normalized forelimb grip strength (g/g) | 5.675 (4.894, 6.041) | 3.293 (2.776, 3.927) | <0.001 |
| Normalized hindlimb grip strength (g/g) | 0.93 (0.73, 1.57) | 0.56 (0.39, 0.70) | <0.001 |
| CMAP amplitude (mV) | 62.2 (56.6, 78.9) | 52.7 (37.1, 75.7) | <0.05 |
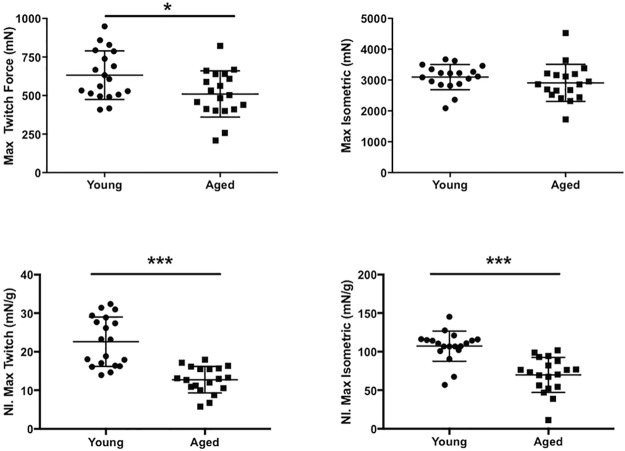
| Twitch force (mN) | 607.0 (506.0, 787.0) | 503.0 (410.0, 639.0) | <0.05 |
| Isometric force (mN) | 3120 (2846, 3352) | 2860 (2445, 3198) | 0.14 |
| Optimal length (mm) | 17.97 (17.17, 18.33) | 18.16 (17.90, 18.45) | 0.34 |
| Normalized twitch (mN/g) | 23.17 (16.37, 28.87) | 12.98 (10.56, 15.70) | <0.001 |
| Normalized maximum isometric (mN/g) | 110.60 (102.10, 116.00) | 73.21 (54.43, 87.78) | <0.001 |
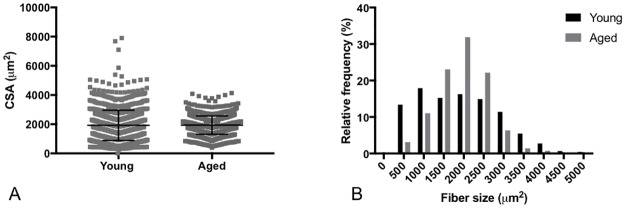
| Myofiber CSA (μm2) | 1845 (1426, 2730) | 1929 (1779, 2146) | 0.72 |
For all group comparisons, a total of 19 young and 19 aged mice were analyzed excluding muscle fiber cross-sectional area (CSA), whereas young = 9 mice (1433 muscle fibers) and aged mice = 9 mice (893 muscle fibers). Grip strength and muscle force were normalized to body mass. Data shown as median with upper and lower 95% confidence intervals.
Muscle physiology and muscle morphometrics
In situ muscle force data for the gastrocnemius is shown in Fig 2 and in Table 1. For the absolute values, twitch force shows a significant reduction in the aged mice whereas the maximal isometric force is not significantly different between the young and aged mice. When normalized to body mass, there is a significant reduction (p<0.001) in both twitch and maximal isometric force. Fig 3 shows the mean muscle fiber size and muscle fiber size distributions between groups. When comparing muscle fiber size between 9 young and 9 aged mice, cross sectional areas (CSA), grouped and averaged by each individual mouse, show no significant difference between groups (p = 0.72). The coefficient of variation (standard deviation/mean) of the CSA of the young mice (0.46 ± 0.08) was significantly increased (p<0.01) compared with that of aged mice (0.31± 0.08). This decrease in muscle fiber size heterogeneity in the aged mice can be seen in the narrowing of the frequency distribution histogram (Fig 3).
Fig 2. Muscle force testing.
In situ testing of gastrocnemius muscle force demonstrates (A) reduced absolute twitch force in aged (n = 19) compared with young mice (n = 19) (p<0.05) and (B) similar absolute maximal tetanic isometric force measurements (p = 0.14). Both (C) normalized twitch (p<0.001) and (D) maximum isometric force are significantly reduced in the aged mice (p<0.001). Data shown as mean ± standard deviation.
Fig 3. Muscle fiber cross-section area and frequency distribution.
(A) There was no difference in myofiber CSA between the groups (p = 0.72). Young (n = 9 mice, 1433 muscle fibers measured) and aged mice (n = 9 mice, 893 muscle fibers measured). Data shown as mean ± standard deviation. (B) However, there is a decrease in muscle fiber size heterogeneity in the aged mice as compared to the young animals as evidenced by a narrowing of the frequency distribution for aged mice.
Electrical impedance myography (EIM)
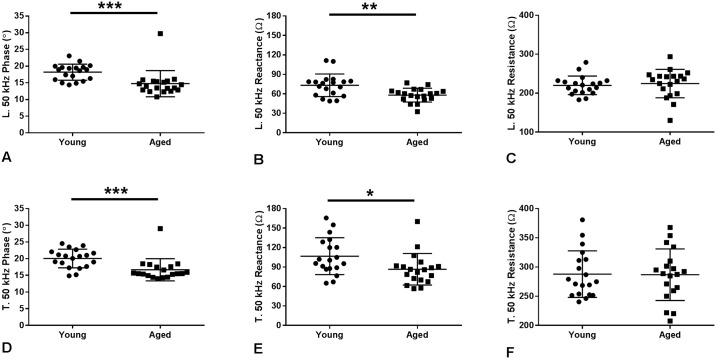
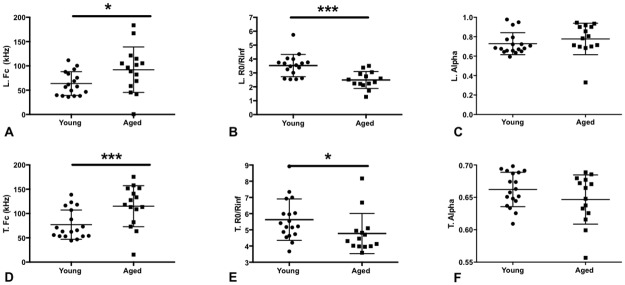
We evaluated single frequency impedance parameters at 50 kHz (Fig 4 and Table 2) that have previously been found to hold value in both human and animal studies. Cole multifrequency parameters, which attempt to provide insight into pathological changes in muscle on the basis of basic impedance theory, were also derived (Fig 5 and Table 2) [29]. The 50 kHz phase and reactance values both in the longitudinal and transverse directions showed major reductions, with differences in the phase being most pronounced. However, there was no significant change in the resistance values for either direction of measurement. Two-way ANOVA was also performed to examine the effects age and electrode orientation (longitudinal versus transverse) concurrently on EIM results. This analysis demonstrated significant effects on 50 kHz Phase by both age (p<0.001) and electrode orientation (p<0.05). Similarly, reactance was affected by both age (p<0.001) and electrode arrangement (p<0.001). In contrast, resistance was not affected by age (p>0.05) but is affected by electrode orientation (p<0.001). The Cole parameter of central frequency (fc) demonstrated significant differences between groups for both the longitudinal and transverse directions. The fc, which is inversely related to muscle fiber size, may be expected to be elevated in the older mice in which some subtle atrophy, perhaps not identified on histology, may be present. While the alpha value (a measure of cell-size variation within the animal’s muscle), failed to reveal a difference, the R0/Rinf, a measure of cell density, showed a significantly higher value for the younger mice, consistent with the presence of more cells/unit area. Stated another way, the reduced R0/Rinf in older animals could suggest an increase in non-cellular components (e.g., increased connective tissue or fat).
Fig 4. Electrical impedance myography (EIM) characteristics at 50 kHz.
Longitudinal 50 kHz (A) phase (p<0.001) and (B) reactance (p<0.01) are reduced in aged mice, but (C) resistance is unchanged (p = 0.27). Similarly, transverse 50 kHz (D) phase (p<0.001) and (E) reactance (p<0.01) are reduced in aged mice but (F) resistance is unchanged (p = 0.84). Data shown as mean ± standard deviation.
Table 2. 50 kHz and cole parameter results of longitudinal and transverse electrical impedance myography measurements of the left gastrocnemius.
| Young | Aged | p value | ||
|---|---|---|---|---|
| Longitudinal | 50 kHz phase | 18.9 (16.0, 19.6) n = 19 | 13.9 (12.8, 15.5) n = 19 | <0.001 |
| 50 kHz reactance | 76.2 (57.9, 79.6) n = 19 | 60.2 (52.0, 64.3) n = 19 | <0.01 | |
| 50 kHz resistance | 220.6 (201.7, 232.0) n = 19 | 234.7 (198.7, 243.6) n = 19 | 0.27 | |
| Transverse | 50 kHz phase | 20.7 (17.7, 22.1) n = 19 | 15.5 (14.9, 17.6) n = 19 | <0.001 |
| 50 kHz reactance | 100.6 (87.9, 128.7) n = 19 | 85.8 (70.1, 91.9) n = 19 | <0.05 | |
| 50 kHz resistance | 274.9 (252.6, 313.0) n = 19 | 286.9 (259.4, 310.2) n = 19 | 0.84 | |
| Longitudinal | fc (kHz) | 57.5 (39.7, 83.8) n = 17 | 99.3 (58.7, 121.3) n = 14 | <0.01 |
| R0/Rinf | 3.56 (3.01, 3.76) n = 17 | 2.43 (2.12, 3.05) n = 14 | <0.001 | |
| alpha | 0.682 (0.656, 0.772) n = 18 | 0.794 (0.697, 0.918) n = 14 | 0.084 | |
| Transverse | fc (kHz) | 64.3 (53.4, 107.1) n = 18 | 122.9 (82.1 152.0) n = 14 | <0.001 |
| R0/Rinf | 5.31 (4.74, 6.03) n = 18 | 4.38 (3.98, 5.10) n = 14 | <0.05 | |
| alpha | 0.6603 (0.644, 0.689) n = 18 | 0.656 (0.616, 0.680) n = 14 | 0.24 |
Phase shown in degrees, and reactance and resistance are shown in Ohms. Data presented as median with upper and lower 95% confidence intervals.
Fig 5. Electrical impedance cole parameters.
A. Longitudinal fc is increased in aged mice (p<0.05). B. Longitudinal R0/Rinf is diminished in aged mice (p<0.001). C. In contrast, longitudinal alpha is unchanged (p = 0.084). D. Similarly, transverse fc is increased in aged mice (p<0.01). E. Transverse R0/Rinf is decreased in aged mice (p<0.05), and (F.) transverse alpha is again unchanged (p = 0.24). Data shown as mean ± standard deviation.
Correlation of EIM with other measures
In addition to seeking to identify differences between the groups, we also sought to evaluate the relationship between the different impedance parameters and the more standard metrics we obtained. Table 3 shows the associations between longitudinal and transverse impedance values at 50 kHz combining both the young and aged mice. Both phase and reactance show good correlation with multiple measures of muscle function when measured in both longitudinal and transverse orientations. In contrast, 50 kHz transverse resistance correlated only with muscle mass and normalized tetanic force, and there were no significant correlations for longitudinal 50 kHz resistance.
Table 3. Correlations between 50 kHz electrical impedance myography parameters, grip strength normalized to body mass, muscle mass, normalized twitch force, and normalized tetanic force.
| Normalized forelimb grip | Normalized hindlimb grip | Muscle mass | Normalized twitch force | Normalized maximum force | ||
|---|---|---|---|---|---|---|
| Longitudinal | Phase | r = 0.68 p<0.001 | r = 0.50 p<0.01 | r = -0.39 p<0.05 | r = 0.34 p<0.05 | r = 0.42 p<0.05 |
| Reactance | r = 0.47 p<0.01 | r = 0.38 p<0.05 | r = -0.48 p<0.01 | r = 0.39 p<0.05 | r = 0.40 p<0.05 | |
| Resistance | r = -0.18 p = 0.28 | r = -0.13 p = 0.45 | r = -0.14 p = 0.41 | r = 0.08 p = 0.64 | r = 0.14 p = 0.43 | |
| Transverse | Phase | r = 0.6 p<0.001 | r = 0.46 p<0.01 | r = -0.46 p<0.01 | r = 0.37 p<0.05 | r = 0.47 p<0.01 |
| Reactance | r = 0.34 p<0.05 | r = 0.33 p<0.05 | r = -0.48 p<0.01 | r = 0.39 p<0.05 | r = 0.61 p<0.001 | |
| Resistance | r = -0.08 p = 0.66 | r = 0.01 p = 0.96 | r = -0.38 p<0.05 | r = 0.27 p = 0.11 | r = 0.46 p<0.01 |
A total of 38 mice (19 young and 19 aged) were included for each comparison excluding tetanic specific force (n = 36, 18 young and 18 aged mice). Spearman correlation coefficients (r) are shown.
Discussion
This study demonstrates that EIM is sensitive to age-associated alterations in mouse muscle, supporting the potential of EIM to serve as a simple, non-invasive approach for assessing sarcopenic change in older men and women. A key finding in this study includes the fact that differences between old and young mice using standard assessment tools were fairly modest. This was particularly the case for comparison of myofiber CSA and absolute muscle mass, which suggested minimal differences between the young and aged mice. Other investigators have also observed that C57BL/6 mice show relatively mild changes in muscle at 24 months [32, 33]. Interestingly, despite the lack of difference in absolute gastrocnemius muscle mass and findings of similar muscle fiber size, the aged mice demonstrated reduced muscle function on grip and during in situ twitch force testing. Similarly, the electrophysiological status of muscle was altered in aged mice as revealed with reduced CMAP amplitude. The loss of CMAP amplitude, which represents the total electrophysiological response of a muscle or group of muscles following supramaximal stimulation of the innervating motor nerve, has also been noted previously in both prior clinical and preclinical studies of aging [34, 35]. Our findings of relatively preserved muscle mass but loss of force and CMAP amplitude are aligned with prior clinical studies demonstrating that loss of muscle strength is more rapid as compared with loss of lean mass in aging individuals [5–7]. Furthermore, this supports the fact that more recent consensus definitions of sarcopenia have been expanded to include not only loss of muscle mass but also reductions in muscle function [36].
What do the alterations in EIM values identified in this study suggest? First, it is important to point out that these impedance effects are unlikely to be related simply to increasing fat percentage of body composition with age. If that were the case, there would be an expectation of increasing resistance values (the impedance value most readily impacted by fat) in concert with the reductions in phase and reactance. However, resistance values were virtually identical in our studies, suggesting the phase and reactance are impacted by other factors. Generally, reactance, and consequently phase, would be expected to decrease if there were reductions in myofiber CSA. Similarly, the Cole parameter fc demonstrated a significant increase in the older animals, which would also be observed in the setting of myofiber atrophy. Interestingly while the 50 kHz reactance and phase are significantly reduced and fc is increased in the aged mice, standard morphometric analysis of muscle fiber CSA did not reveal any significant difference in muscle fiber size. The conflicting results between EIM and histology could reflect the fact that certain cell populations (e.g. severely atrophied myofibers due to axon dropout) were simply ignored when measuring fiber size using light microscopy. The reduction in R0/Rinf is also consistent with this possibility, since it suggests a decrease in cell density in the older mice (any small atrophic fibers behaving bioelectrically more as extracellular debris rather than actual cells). The Cole parameter alpha, α, is supposed to provide a measure of cell size distribution. In contrast to the sensitivity of most of the impedance parameters, α was not different between the young and old mice, despite the fact that the measured coefficient of variation in myofiber size revealed less variation in the aged mice.
Decreased muscle quality has been shown to be a good predictor of functional decline, morbidity, and mortality in aging individuals [7, 37]. Loss of muscle quality has been attributed to a number of underlying pathological and pathophysiological changes [38]. One potential cause includes the deposition of intramuscular fat with aging leading to impaired muscle contractility [12, 39–42]. Additionally, both the loss of motor neurons and the loss of motor unit connectivity at the neuromuscular junction have been implicated as demonstrated in a number of preclinical and clinical studies [43–50]. Regardless of the major factors that drive loss of muscle quality, EIM appears to be sensitive to these alterations as is apparent in the striking EIM differences between young and aged mice as well as EIM’s consistent correlations with muscle strength, mass, and function. In our view the relationship between obtained EIM values and the measured force generating capability of the muscle is the most compelling outcome from this study. Stated another way, it may be that EIM values can serve as a surrogate measures to actual force measurements and muscle quality. We have previously shown in both clinical and animal studies that, in a number of neuromuscular conditions, EIM parameters are related to force and function [15, 51–53]. Moreover, we have recently identified a relationship between lower extremity force and impedance values in healthy older adults [54]. This work provides further support for that relationship.
There are a number of limitations to this study that need to be highlighted. First, we obtained histological data only in a subset of animals due to a technical problem with our histological processing of the first sets of young and old animals studied. Second, it would have been very helpful to have saved additional tissue for analysis of connective tissue and fat deposition as we had done in some earlier work, which was not performed here. Increases in these components would have added insight into the observed impedance alterations [55]. Also, in this analysis, we only performed surface EIM measurements and no ex vivo studies, post-mortem. However, as we have seen in other work, the ex vivo measurements often simply reflect the in vivo findings, but are considerably more challenging to acquire [56].
This study raises a number of questions for future study. First, an additional dedicated study of the relationship between EIM alterations in aging muscle is needed. One next step may be to evaluate additional rodent models of accelerated aging [57]. Alternatively, investigation in human subjects, potentially including muscle biopsy to fully evaluate the tissue and relationship to impedance change, may be required. Regardless, the results presented here add to a growing body of literature supporting the potential application of EIM in the assessment of age-associated muscle deterioration.
Supporting information
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was supported by the National Institute of Health/National Institute of Neurological Disorders and Stroke (https://www.ninds.nih.gov/) grant R01NS055099 to SBR. WDA received funding from the National Institute on Aging/National Institute of Health (https://www.nia.nih.gov/) (Grant R03AG050877). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Cesari M, Fielding RA, Pahor M, Goodpaster B, Hellerstein M, Van Kan GA, et al. Biomarkers of sarcopenia in clinical trials—recommendations from the International Working Group on Sarcopenia. Journal of Cachexia, Sarcopenia and Muscle. 2012;3(3):181–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosenberg IH. Summary comments. American Journal of Clinical Nutrition. 1989;50(5 SUPPL.):1231–3. [Google Scholar]
- 3.Rosenberg IH. Sarcopenia: origins and clinical relevance. J Nutr. 1997;127. [DOI] [PubMed] [Google Scholar]
- 4.Goodpaster BH, Carlson CL, Visser M, Kelley DE, Scherzinger A, Harris TB, et al. Attenuation of skeletal muscle and strength in the elderly: the Health ABC Study. J Appl Physiol. 2001;90. [DOI] [PubMed] [Google Scholar]
- 5.Newman AB, Haggerty CL, Goodpaster B, Harris T, Kritchevsky S, Nevitt M, et al. Strength and muscle quality in a well-functioning cohort of older adults: the Health, Aging and Body Composition Study. J Am Geriatr Soc. 2003;51(3):323–30. . [DOI] [PubMed] [Google Scholar]
- 6.Goodpaster BH, Park SW, Harris TB, Kritchevsky SB, Nevitt M, Schwartz AV, et al. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. The journals of gerontology Series A, Biological sciences and medical sciences. 2006;61(10):1059–64. Epub 2006/11/02. . [DOI] [PubMed] [Google Scholar]
- 7.Newman AB, Kupelian V, Visser M, Simonsick EM, Goodpaster BH, Kritchevsky SB, et al. Strength, but not muscle mass, is associated with mortality in the health, aging and body composition study cohort. The journals of gerontology Series A, Biological sciences and medical sciences. 2006;61(1):72–7. Epub 2006/02/04. . [DOI] [PubMed] [Google Scholar]
- 8.Clark BC, Manini TM. Sarcopenia = / = dynapenia. The journals of gerontology Series A, Biological sciences and medical sciences. 2008;63(8):829–34. Epub 2008/09/06. . [DOI] [PubMed] [Google Scholar]
- 9.Cruz-Jentoft A, Baeyens J, Bauer J, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39(4):412–23. doi: 10.1093/ageing/afq034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lynch NA, Metter EJ, Lindle RS, Fozard JL, Tobin JD, Roy TA, et al. Muscle quality. I. Age-associated differences between arm and leg muscle groups. Journal of Applied Physiology. 1999;86(1):188–94. [DOI] [PubMed] [Google Scholar]
- 11.Goodpaster BH, Kelley DE, Thaete FL, He J, Ross R. Skeletal muscle attenuation determined by computed tomography is associated with skeletal muscle lipid content. J Appl Physiol. 2000;89. [DOI] [PubMed] [Google Scholar]
- 12.Goodpaster BH, Carlson CL, Visser M, Kelley DE, Scherzinger A, Harris TB, et al. Attenuation of skeletal muscle and strength in the elderly: The Health ABC Study. J Appl Physiol. 2001;90(6):2157–65. . [DOI] [PubMed] [Google Scholar]
- 13.Lexell J, Taylor CC, Sjostrom M. What is the cause of the ageing atrophy? Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15- to 83-year-old men. Journal of the neurological sciences. 1988;84(2–3):275–94. Epub 1988/04/01. . [DOI] [PubMed] [Google Scholar]
- 14.Brooks SV, Faulkner JA. Skeletal muscle weakness in old age: underlying mechanisms. Medicine and science in sports and exercise. 1994;26(4):432–9. Epub 1994/04/01. . [PubMed] [Google Scholar]
- 15.Rutkove SB, Caress JB, Cartwright MS, Burns TM, Warder J, David WS, et al. Electrical impedance myography correlates with standard measures of ALS severity. Muscle & nerve. 2014;49(3):441–3. Epub 2013/11/26. doi: 10.1002/mus.24128 . [DOI] [PubMed] [Google Scholar]
- 16.Rutkove SB, Caress JB, Cartwright MS, Burns TM, Warder J, David WS, et al. Electrical impedance myography as a biomarker to assess ALS progression. Amyotrophic lateral sclerosis: official publication of the World Federation of Neurology Research Group on Motor Neuron Diseases. 2012;13(5):439–45. doi: 10.3109/17482968.2012.688837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rutkove SB, Shefner JM, Gregas M, Butler H, Caracciolo J, Lin C, et al. Characterizing spinal muscular atrophy with electrical impedance myography. Muscle & nerve. 2010;42(6):915–21. doi: 10.1002/mus.21784 [DOI] [PubMed] [Google Scholar]
- 18.Rutkove SB, Gregas MC, Darras BT. Electrical impedance myography in spinal muscular atrophy: A longitudinal study. Muscle & nerve. 2012;45(5):642–7. doi: 10.1002/mus.23233 [DOI] [PubMed] [Google Scholar]
- 19.Zaidman CM, Wang LL, Connolly AM, Florence J, Wong BL, Parsons JA, et al. Electrical impedance myography in Duchenne muscular dystrophy and healthy controls: A multicenter study of reliability and validity. Muscle & nerve. 2015;52(4):592–7. doi: 10.1002/mus.24611 . [DOI] [PubMed] [Google Scholar]
- 20.Tarulli AW, Duggal N, Esper GJ, Garmirian LP, Fogerson PM, Lin CH, et al. Electrical impedance myography in the assessment of disuse atrophy. Arch Phys Med Rehabil. 2009;90(10):1806–10. Epub 2009/10/06. doi: 10.1016/j.apmr.2009.04.007 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li J, Spieker AJ, Rosen GD, Rutkove SB. Electrical impedance alterations in the rat hind limb with unloading. Journal of musculoskeletal & neuronal interactions. 2013;13(1):37–44. Epub 2013/03/01. . [PMC free article] [PubMed] [Google Scholar]
- 22.Sung M, Li J, Spieker AJ, Spatz J, Ellman R, Ferguson VL, et al. Spaceflight and hind limb unloading induce similar changes in electrical impedance characteristics of mouse gastrocnemius muscle. Journal of musculoskeletal & neuronal interactions. 2013;13(4):405–11. [PMC free article] [PubMed] [Google Scholar]
- 23.Aaron R, Esper GJ, Shiffman CA, Bradonjic K, Lee KS, Rutkove SB. Effects of age on muscle as measured by electrical impedance myography. Physiological measurement. 2006;27(10):953–9. Epub 2006/09/05. doi: 10.1088/0967-3334/27/10/002 . [DOI] [PubMed] [Google Scholar]
- 24.Anderson DE, Bean JF, Holt NE, Keel JC, Bouxsein ML. Computed tomography-based muscle attenuation and electrical impedance myography as indicators of trunk muscle strength independent of muscle size in older adults. American journal of physical medicine & rehabilitation / Association of Academic Physiatrists. 2014;93(7):553–61. Epub 2014/02/11. doi: 10.1097/phm.0000000000000059 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li J, Geisbush TR, Arnold WD, Rosen GD, Zaworski PG, Rutkove SB. A comparison of three electrophysiological methods for the assessment of disease status in a mild spinal muscular atrophy mouse model. PloS one. 2014;9(10):e111428 Epub 2014/10/28. doi: 10.1371/journal.pone.0111428 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arnold WD, Sheth KA, Wier CG, Kissel JT, Burghes AH, Kolb SJ. Electrophysiological Motor Unit Number Estimation (MUNE) Measuring Compound Muscle Action Potential (CMAP) in Mouse Hindlimb Muscles. J Vis Exp. 2015;(103). Epub 2015/10/06. doi: 10.3791/52899 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arnold WD, Porensky PN, McGovern VL, Iyer C, Duque S, Li X, et al. Electrophysiological Biomarkers in Spinal Muscular Atrophy: Preclinical Proof of Concept. Annals of Clinical and Translational Neurology. 2014;1(1):34–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li J, Staats WL, Spieker A, Sung M, Rutkove SB. A Technique for Performing Electrical Impedance Myography in the Mouse Hind Limb: Data in Normal and ALS SOD1 G93A Animals. PloS one. 2012;7(9):e45004 doi: 10.1371/journal.pone.0045004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grimnes S, Martinsen OG. Bioimpedance and Bioelectricity Basics. Second ed London: Academic press; 2008. [Google Scholar]
- 30.Sanchez B, Bandarenka AS, Vandersteen G, Schoukens J, Bragos R. Novel approach of processing electrical bioimpedance data using differential impedance analysis. Medical engineering & physics. 2013;35(9):1349–57. Epub 2013/04/23. doi: 10.1016/j.medengphy.2013.03.006 . [DOI] [PubMed] [Google Scholar]
- 31.Sanchez B, Li J, Yim S, Pacheck A, Widrick JJ, Rutkove SB. Evaluation of Electrical Impedance as a Biomarker of Myostatin Inhibition in Wild Type and Muscular Dystrophy Mice. PloS one. 2015;10(10):e0140521 doi: 10.1371/journal.pone.0140521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Graber TG, Kim J-H, Grange RW, McLoon LK, Thompson LV. C57BL/6 life span study: age-related declines in muscle power production and contractile velocity. Age. 2015;37(3):36 doi: 10.1007/s11357-015-9773-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krishnan VS, White Z, McMahon CD, Hodgetts SI, Fitzgerald M, Shavlakadze T, et al. A Neurogenic Perspective of Sarcopenia: Time Course Study of Sciatic Nerves From Aging Mice. Journal of Neuropathology & Experimental Neurology. 2016;75(5):464–78. doi: 10.1093/jnen/nlw019 [DOI] [PubMed] [Google Scholar]
- 34.Walsh ME, Sloane LB, Fischer KE, Austad SN, Richardson A, Van Remmen H. Use of Nerve Conduction Velocity to Assess Peripheral Nerve Health in Aging Mice. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2015;70(11):1312–9. doi: 10.1093/gerona/glu208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ward RE, Boudreau RM, Caserotti P, Harris TB, Zivkovic S, Goodpaster BH, et al. Sensory and motor peripheral nerve function and longitudinal changes in quadriceps strength. The journals of gerontology Series A, Biological sciences and medical sciences. 2015;70(4):464–70. Epub 2014/10/17. doi: 10.1093/gerona/glu183 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cruz-Jentoft A, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, Schneider SM, Topinková E, Vandewoude M, Zamboni M; European Working Group on Sarcopenia in Older People. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Aging. 2010;39:412–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fragala MS, Kenny AM, Kuchel GA. Muscle quality in aging: a multi-dimensional approach to muscle functioning with applications for treatment. Sports medicine (Auckland, NZ). 2015;45(5):641–58. Epub 2015/02/07. doi: 10.1007/s40279-015-0305-z . [DOI] [PubMed] [Google Scholar]
- 38.Narici MV, Maffulli N, Maganaris CN. Ageing of human muscles and tendons. Disability and rehabilitation. 2008;30(20–22):1548–54. Epub 2008/07/09. doi: 10.1080/09638280701831058 . [DOI] [PubMed] [Google Scholar]
- 39.Marcus RL, Addison O, Dibble LE, Foreman KB, Morrell G, Lastayo P. Intramuscular adipose tissue, sarcopenia, and mobility function in older individuals. Journal of aging research. 2012;2012:629637 doi: 10.1155/2012/629637 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McGregor RA, Cameron-Smith D, Poppitt SD. It is not just muscle mass: a review of muscle quality, composition and metabolism during ageing as determinants of muscle function and mobility in later life. Longevity & Healthspan. 2014;3(1):9 doi: 10.1186/2046-2395-3-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Therkelsen KE, Pedley A, Hoffmann U, Fox CS, Murabito JM. Intramuscular fat and physical performance at the Framingham Heart Study. Age. 2016;38(2):31 doi: 10.1007/s11357-016-9893-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cawthon PM, Fox KM, Gandra SR, Delmonico MJ, Chiou CF, Anthony MS, et al. Do muscle mass, muscle density, strength, and physical function similarly influence risk of hospitalization in older adults? J Am Geriatr Soc. 2009;57(8):1411–9. Epub 2009/08/18. doi: 10.1111/j.1532-5415.2009.02366.x . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chai RJ, Vukovic J, Dunlop S, Grounds MD, Shavlakadze T. Striking denervation of neuromuscular junctions without lumbar motoneuron loss in geriatric mouse muscle. PloS one. 2011;6(12):e28090 Epub 2011/12/14. doi: 10.1371/journal.pone.0028090 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Delbono O. Neural control of aging skeletal muscle. Aging Cell. 2003;2(1):21–9. doi: 10.1046/j.1474-9728.2003.00011.x [DOI] [PubMed] [Google Scholar]
- 45.Payne AM, Zheng Z, Messi ML, Milligan CE, Gonzalez E, Delbono O. Motor neurone targeting of IGF-1 prevents specific force decline in ageing mouse muscle. The Journal of physiology. 2006;570(Pt 2):283–94. Epub 2005/11/19. doi: 10.1113/jphysiol.2005.100032 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang Z-M, Zheng Z, Messi ML, Delbono O. Extension and magnitude of denervation in skeletal muscle from ageing mice. The Journal of physiology. 2005;565(3):757–64. doi: 10.1113/jphysiol.2005.087601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tomlinson BE, Irving D. The numbers of limb motor neurons in the human lumbosacral cord throughout life. Journal of the neurological sciences. 1977;34(2):213–9. Epub 1977/11/01. . [DOI] [PubMed] [Google Scholar]
- 48.Doherty TJ, Vandervoort AA, Brown WF. Effects of ageing on the motor unit: a brief review. Canadian journal of applied physiology = Revue canadienne de physiologie appliquee. 1993;18(4):331–58. Epub 1993/12/01. . [DOI] [PubMed] [Google Scholar]
- 49.Gilmore KJ, Morat T, Doherty TJ, Rice CL. Motor Unit Number Estimations and Neuromuscular Fidelity in Three Stages of Sarcopenia. Muscle & nerve. 2016. Epub 2016/09/01. . [DOI] [PubMed] [Google Scholar]
- 50.Kaya RD, Nakazawa M, Hoffman RL, Clark BC. Interrelationship between muscle strength, motor units, and aging. Exp Gerontol. 2013;48(9):920–5. Epub 2013/07/09. doi: 10.1016/j.exger.2013.06.008 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li J, Pacheck A, Sanchez B, Rutkove SB. Single and modeled multifrequency electrical impedance myography parameters and their relationship to force production in the ALS SOD1G93A mouse. Amyotroph Lateral Scler Frontotemporal Degener. 2016;17(5–6):397–403. doi: 10.3109/21678421.2016.1165258 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rutkove SB, Shefner JM, Gregas M, Butler H, Caracciolo J, Lin C, et al. Characterizing spinal muscular atrophy with electrical impedance myography. Muscle Nerve. 2010;42(6):915–21. Epub 2010/11/26. doi: 10.1002/mus.21784 . [DOI] [PubMed] [Google Scholar]
- 53.Rutkove SB, Geisbush TR, Mijailovic A, Shklyar I, Pasternak A, Visyak N, et al. Cross-sectional evaluation of electrical impedance myography and quantitative ultrasound for the assessment of Duchenne muscular dystrophy in a clinical trial setting. Pediatric neurology. 2014;51(1):88–92. Epub 2014/05/13. doi: 10.1016/j.pediatrneurol.2014.02.015 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Arnold WD RS, Buck MD, Bohorquez J, Bean JF. Poster 1–29 Electrical impedance myography correlates with muscle mass and function in a cohort at risk for sarcopenia, Abstracts of the 9th International Conference on Cachexia, Sarcopenia and Muscle Wasting, Berlin, Germany, 10–11 December 2016 Journal of Cachexia, Sarcopenia and Muscle. 2016;7(5):626–62. [Google Scholar]
- 55.Ballak SB, Degens H, Buse-Pot T, de Haan A, Jaspers RT. Plantaris muscle weakness in old mice: relative contributions of changes in specific force, muscle mass, myofiber cross-sectional area, and number. Age (Dordr). 2014;36(6):9726 doi: 10.1007/s11357-014-9726-0 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li J, Geisbush TR, Rosen GD, Lachey J, Mulivor A, Rutkove SB. Electrical impedance myography for the in vivo and ex vivo assessment of muscular dystrophy (mdx) mouse muscle. Muscle & nerve. 2014;49(6):829–35. Epub 2014/04/23. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Romanick M, Thompson LV, Brown-Borg HM. Murine models of atrophy, cachexia, and sarcopenia in skeletal muscle. Biochim Biophys Acta. 2013;1832(9):1410–20. doi: 10.1016/j.bbadis.2013.03.011 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.