Abstract
Purpose
Many patients with high-risk non–muscle-invasive bladder cancer (NMIBC) are either refractory to bacillus Calmette-Guerin (BCG) treatment or may experience disease relapse. We assessed the efficacy and safety of recombinant adenovirus interferon alfa with Syn3 (rAd–IFNα/Syn3), a replication-deficient recombinant adenovirus gene transfer vector, for patients with high-grade (HG) BCG-refractory or relapsed NMIBC.
Methods
In this open-label, multicenter (n = 13), parallel-arm, phase II study (ClinicalTrials.gov identifier: NCT01687244), 43 patients with HG BCG-refractory or relapsed NMIBC received intravesical rAd–IFNα/Syn3 (randomly assigned 1:1 to 1 × 1011 viral particles (vp)/mL or 3 × 1011 vp/mL). Patients who responded at months 3, 6, and 9 were retreated at months 4, 7, and 10. The primary end point was 12-month HG recurrence-free survival (RFS). All patients who received at least one dose were included in efficacy and safety analyses.
Results
Forty patients received rAd–IFNα/Syn3 (1 × 1011 vp/mL, n = 21; 3 × 1011 vp/mL, n = 19) between November 5, 2012, and April 8, 2015. Fourteen patients (35.0%; 90% CI, 22.6% to 49.2%) remained free of HG recurrence 12 months after initial treatment. Comparable 12-month HG RFS was noted for both doses. Of these 14 patients, two experienced recurrence at 21 and 28 months, respectively, after treatment initiation, and one died as a result of an upper tract tumor at 17 months without a recurrence. rAd–IFNα/Syn3 was well tolerated; no grade four or five adverse events (AEs) occurred, and no patient discontinued treatment because of an adverse event. The most frequently reported drug-related AEs were micturition urgency (n = 16; 40%), dysuria (n = 16; 40%), fatigue (n = 13; 32.5%), pollakiuria (n = 11; 28%), and hematuria and nocturia (n = 10 each; 25%).
Conclusion
rAd—IFNα/Syn3 was well tolerated. It demonstrated promising efficacy for patients with HG NMIBC after BCG therapy who were unable or unwilling to undergo radical cystectomy.
INTRODUCTION
Non–muscle-invasive bladder cancer (NMIBC) represents the most common disease state for patients with newly diagnosed bladder cancer.1 Those with high-grade (HG) tumors are at significant risk for both recurrence and progression.2,3 Bacillus Calmette-Guerin (BCG) represents the current preferred management.4-6 Nonetheless, approximately 30% of patients will not respond to BCG; among those who demonstrate an initial response, more than 50% will experience recurrence and progression during long-term follow-up.7
The optimal management of patients with persistent or recurrent tumor after BCG remains controversial.8 Although radical cystectomy provides cancer eradication,9 many patients are elderly, have significant comorbidities with an attendant diminished performance status, and often are unwilling to undergo radical extirpative surgery. Nonextirpative treatment options are available, but studies to date have included relatively small patient numbers and used varied definitions of treatment success8,10-16 Indeed, the US Food and Drug Administration (FDA) and genitourinary oncology community agree that scant progress has been made in the management of this disease since the initial approval of BCG.17-19 Thus an effective alternative to radical cystectomy for patients with disease recurrence after BCG treatment remains an important unmet clinical need.17
Recombinant intravesical interferon alfa-2b protein (IFNα-2b; Intron A; Merck, Kenilworth, NJ) demonstrated promising initial clinical results in NMIBC.20,21 Intravesical IFNα-2b gene delivery offers a novel approach and increases the duration of exposure to IFNα-2b. Recombinant adenovirus (rAd)–IFNα-2b is a replication-deficient adenovirus-based gene transfer vector that encodes the human IFNα-2b gene.22-24 Syn3, a polyamide surfactant, is incorporated into the drug formulation (rAd–IFNα/Syn3; Instiladrin, FKD Therapies Oy, Kuopio, Finland)25 to enhance adenoviral transduction of the bladder lining. Dramatic enrichment of rAd–IFNα gene transfer and expression has been shown with Syn3 in both normal urothelium and human urothelial carcinoma that grows in mice.22-25 rAd–IFNα-2b gene therapy mimics the physiologic events associated with viral infection, which results in local rather than systemic IFNα-2b production and subsequent tumor regression.22
A phase I dose-ascending study of rAd–IFNα/Syn3 was performed for patients with BCG-refractory and relapsing NMIBC.26 Dose-dependent adenoviral gene transfer and urine concentrations of IFNα-2b were confirmed. Of 14 patients treated with dose levels of rAd–IFNα/Syn3 that resulted in measurable urine IFNα, six (43%) were free from recurrence at 3 months and had no dose-limiting toxicity, and two patients remained disease free at 29 and 39 months.26 These provocative findings, predominantly at the two highest doses, prompted this phase II study, designed to evaluate the efficacy and safety of intravesical rAd–IFNα/Syn3 for patients with HG NMIBC refractory to, or with relapse after, BCG.
METHODS
Study Design
This randomized, open-label, parallel-arm study was conducted across 13 centers in the United States between November 5, 2012, and April 8, 2015. The protocol, administrative oversight, and accrual timelines were designed and conducted by the Society of Urologic Oncology Clinical Trials Consortium. The study protocol and informed consent form were reviewed and approved by the respective responsible site institutional review boards and biosafety committees.
Patients
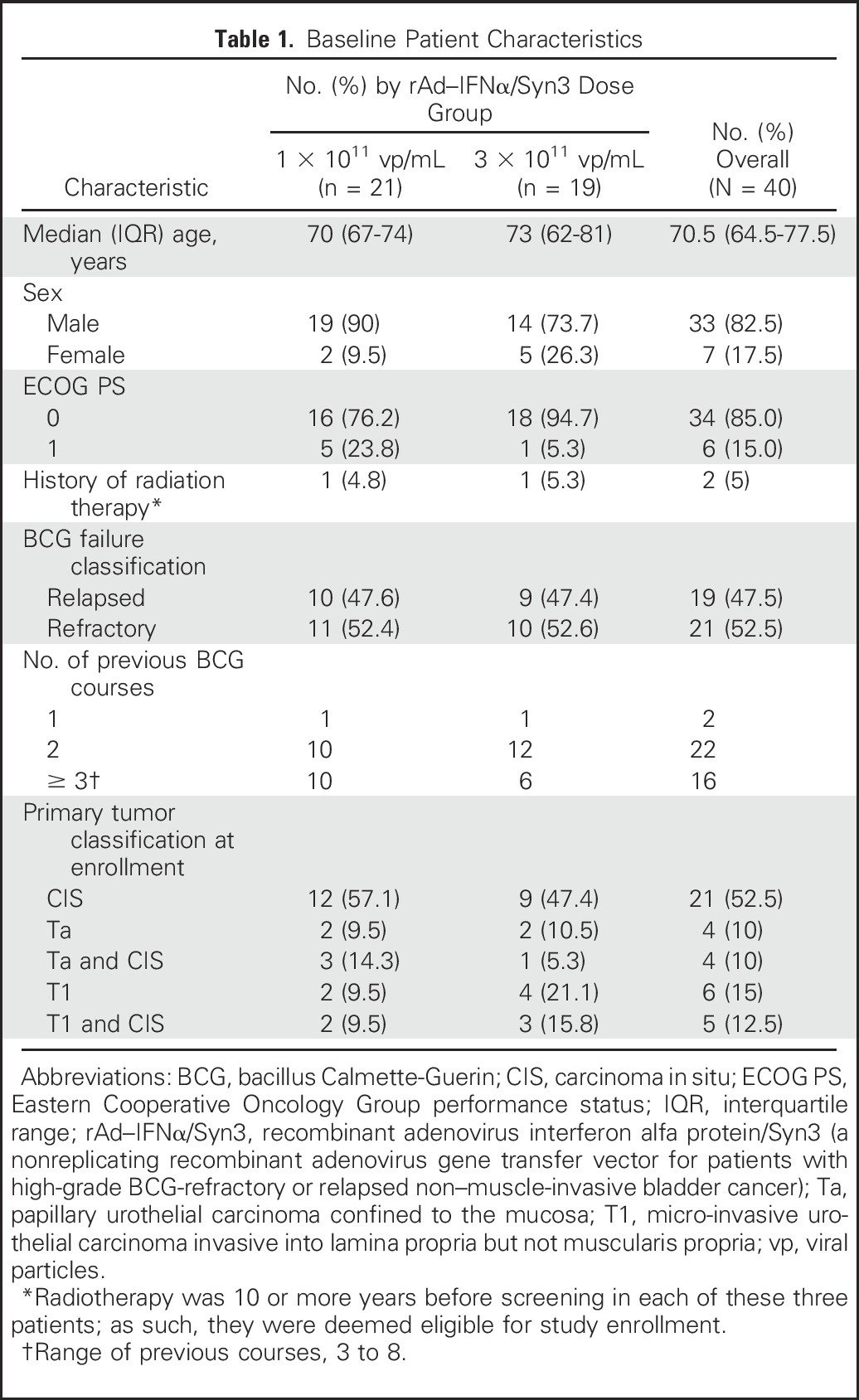
The trial was designed to enroll 40 patients unable or unwilling to undergo radical cystectomy, and there were two dosage groups of 20 patients each. Eligible patients were 18 years or older and had HG BCG-refractory or relapsed NMIBC, including papillary NMIBC alone (Ta or T1), carcinoma in situ (CIS) alone, or a combination of CIS and papillary disease. BCG-refractory disease was defined as the inability to achieve a disease-free state at 6 months after adequate induction BCG therapy with either maintenance or reinduction at 3 months. Adequate induction was defined as a minimum of five of six treatments, and adequate maintenance was defined as a minimum of two of three treatments. BCG relapse was defined as recurrence within 1 year after a complete response to adequate BCG treatment (at least five and two instillations). Patients were required to have undergone visually complete resection of papillary lesions by transurethral resection of bladder tumors. Patients could not have received intravesical therapy within 3 months before beginning study treatment, with the exception of cytotoxic agents when administered as a single instillation immediately after a transurethral resection. All participants who entered the study provided written or oral informed consent.
Random Assignment and Masking
Patients were assigned by computer-generated random assignment, with a constrained 1:1 sequence, to receive either low-dose (1 × 1011 viral particles [vp]/mL) or high-dose (3 × 1011 vp/mL) rAd–IFNα/Syn3. These doses were the most promising observed in the phase I study. The total doses administered were 7.5 × 1012 vp in the low-dose group and 2.25 × 1013 vp in the high-dose group. Treatment allocation was performed centrally with a block size of two for all patients who had successfully completed screening, with the constraint that the first four patients at each site were balanced between cohorts.
Procedures
rAd–IFNα/Syn3 in 75 mL was administered intravesically through a urethral catheter, with a planned retention time of 1 hour; an anticholinergic treatment was allowed to relieve urinary urgency and permit adequate retention. Patients without recurrence of HG disease at months 3, 6, and 9, as evaluated by cytology, cystoscopy, and biopsy (if clinically indicated) were then retreated at months 4, 7, and 10. At 12 months, a final efficacy evaluation was performed. This evaluation included a protocol-mandated biopsy from the site of the index tumor and at least five random biopsies, including the bladder dome, trigone, right and left lateral wall, posterior wall, and prostatic urethra in men with positive cytology or prior disease in this region.
During the study, patients were contacted weekly by phone for the first month after each treatment on days 7, 14 (of months 7 and 10 only), 21, and 28 (± 1 day) to provide information about adverse events (AEs) and concomitant medication use. Assessments for treatment failure were made between 14 and 7 days before retreatment. Patients who were withdrawn from treatment before study completion underwent a safety assessment at least 30 days after last administration of the study drug. All patients are being monitored in a 3-year long-term follow-up period to (1) determine recurrence of HG disease in those patients with a complete response and (2) to assess the long-term impact of treatment with rAd–IFNα/Syn3.
End Points
The primary end point was freedom from HG disease recurrence at 12 months, defined by a negative for cause or end of study biopsy. Secondary end points included response to treatment, defined as no evidence of recurrence of HG disease at 3, 6, and 9 months; incidence and time to cystectomy; and concentration of IFNα-2b in the urine. Safety assessments included physical examination, monitoring of vital signs, ECG, and standard clinical chemistry, hematology, and urinalysis assessments (performed by local laboratories). Safety end points include type, incidence, relatedness, and severity of AEs and severe (≥ grade 3) AEs (SAEs), as assessed by National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.03).
Statistical Analyses
We determined that a cohort of 20 patients would be sufficient to give an 80% probability of rejection of a HG recurrence-free survival (RFS) rate of 10% with an exact 5% one-sided test when the true HG RFS rate was 35%. The operating characteristics for this Fleming design were calculated exactly with the binomial distribution described by A’Hern.27 The hypothesis—that the response rate was equal to or less than the reference rate—was rejected if five or more of the 20 patients achieved HG RFS at 12 months. The proportion of patients who achieved HG RFS at 3, 6, 9, and 12 months was reported for each dose group, together with an exact 90% CI for the proportion. The time to HG recurrence or death was summarized with the Kaplan-Meier method. Analyses were performed with SAS (version 9 or later; SAS Institute, Cary, NC). Both the safety and efficacy (modified intention-to-treat) analysis sets included all patients who received at least one dose of rAd–IFNα/Syn3. A data monitoring committee oversaw the study according to the data monitoring plan.
Analytical Assays and Sample Testing
All analytical assays were developed and validated. Samples were tested according to good laboratory practices methods at Covance Laboratories Ltd (Harrogate, United Kingdom). Description of the assays and the results of sample testing are presented in the Appendix (online only).
RESULTS
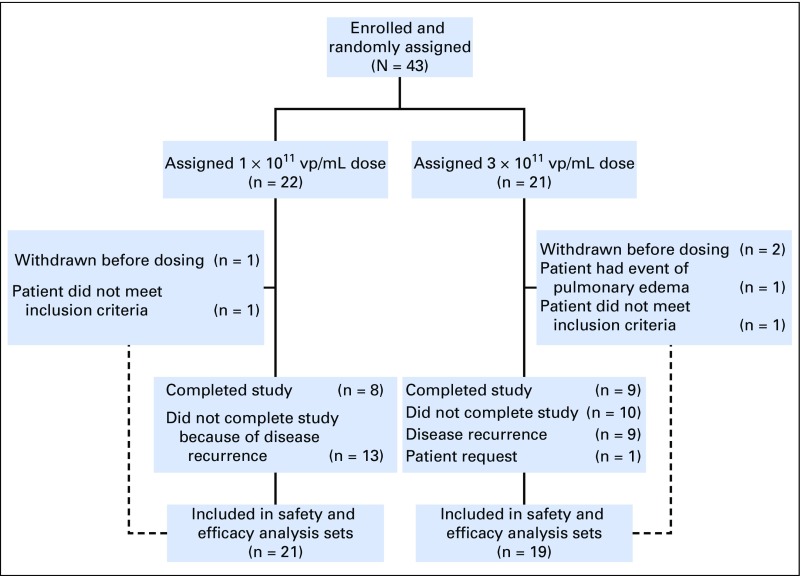
Patient disposition is shown in Figure 1. Baseline characteristics are listed in Table 1.
Fig 1.
Patient dispositions. vp, viral particles.
Table 1.
Baseline Patient Characteristics
Primary End Point: HG RFS
The12-month HG RFS rate was comparable between the two dose groups, with 33.3% of patients (7 of 21; 90% CI, 16.8 to 53.6) in the low-dose group and 36.8% (7 of 19; CI, 18.8 to 58.2) in the high-dose group alive and free of HG disease at 12 months. Overall, 35.0% of patients (14 of 40; 90% CI, 22.6% to 49.2%) remained free of HG recurrence at 12 months after the initiation of rAd–IFNα/Syn3 treatment (Table 2). Off-schedule disease assessments did not affect findings (Appendix, online only). The median time to HG recurrence or death was 6.5 months (90% CI, 3.52 to 12.78 months); the median time to HG recurrence was 3.52 months (90% CI, 3.02 to 12.78 months) for the low-dose group and was 11.73 months (90% CI, 5.88 months to not evaluable) for the high-dose group.
Table 2.
Incidence of HG RFS at 3, 6, 9, and 12 Months
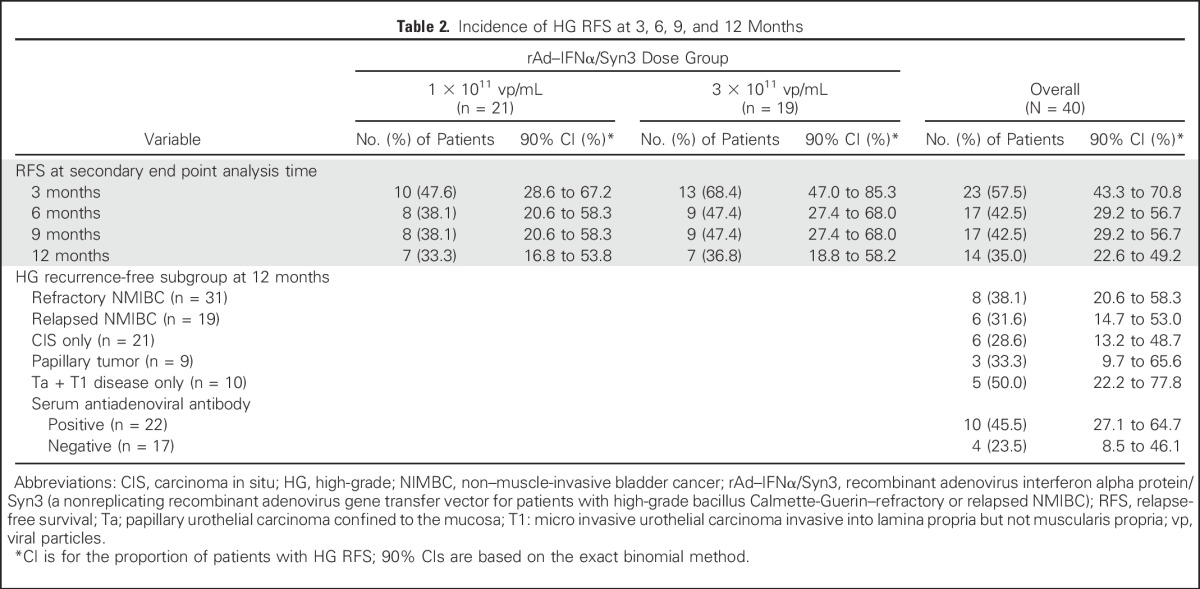
When patient subgroups and secondary end points were considered in exploratory analyses, the 12-month HG RFS rates were broadly similar for men and women, for younger and older patients, for refractory or relapsed NMIBC, for CIS only or papillary tumors and CIS, and for patients with Ta and T1 disease only (Table 2). Interestingly, of the 14 patients who were recurrence free at 12 months, 10 (71%) of the 14 had an antiadenovirus antibody response (defined as four times the predose titer), compared with 11 (24%) of 25 who experienced recurrence.
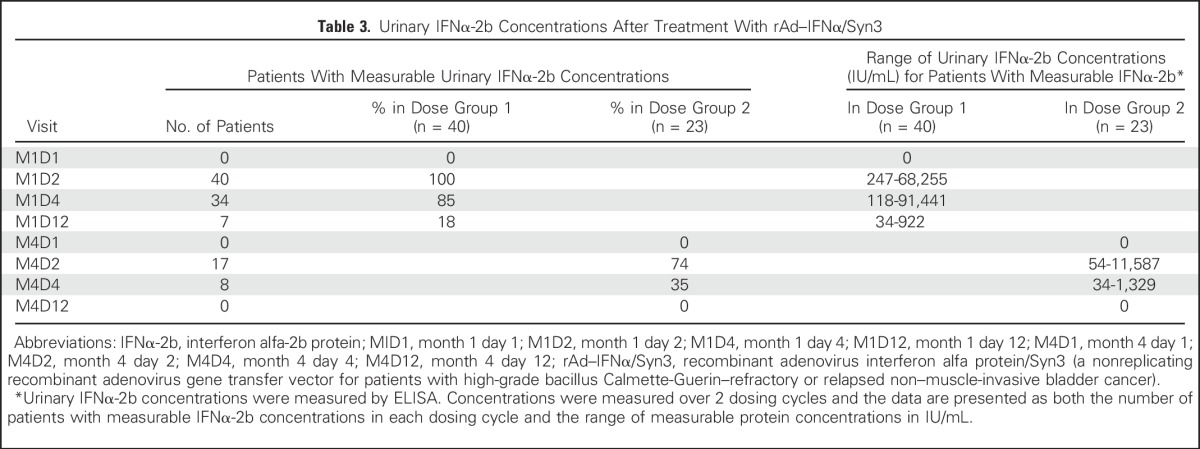
Significant levels of urine IFNα-2b were measurable in all patients in month 1 at days 2, 4, and 12 (Table 3). Of those patients who received a second dose, measurable IFNα-2b urine concentrations were noted in month 4 on days 2 and 4 after drug administration. Urine IFNα-2b concentrations did not appear to correlate with dose or clinical response.
Table 3.
Urinary IFNα-2b Concentrations After Treatment With rAd–IFNα/Syn3
In long-term follow-up, seven patients (18%) who withdrew from the study because of HG disease recurrence within the 12-month study period died at a median of 16 months (range, 2 to 26 months) after the withdrawal date. There is no indication that these deaths were treatment related. The cause of death was unknown in four patients, whereas two died as a result of progressive bladder cancer and one died as a result of liver failure unrelated to treatment 17 months after withdrawal from the study. The four patients for whom the cause of death is unknown were being observed locally after they completed their end-of-study evaluation. Fourteen patients (35%) who experienced an HG recurrence within the first year underwent a radical cystectomy at a median of 9 months (range, 4 to 28 months) from day 1 of month 1.
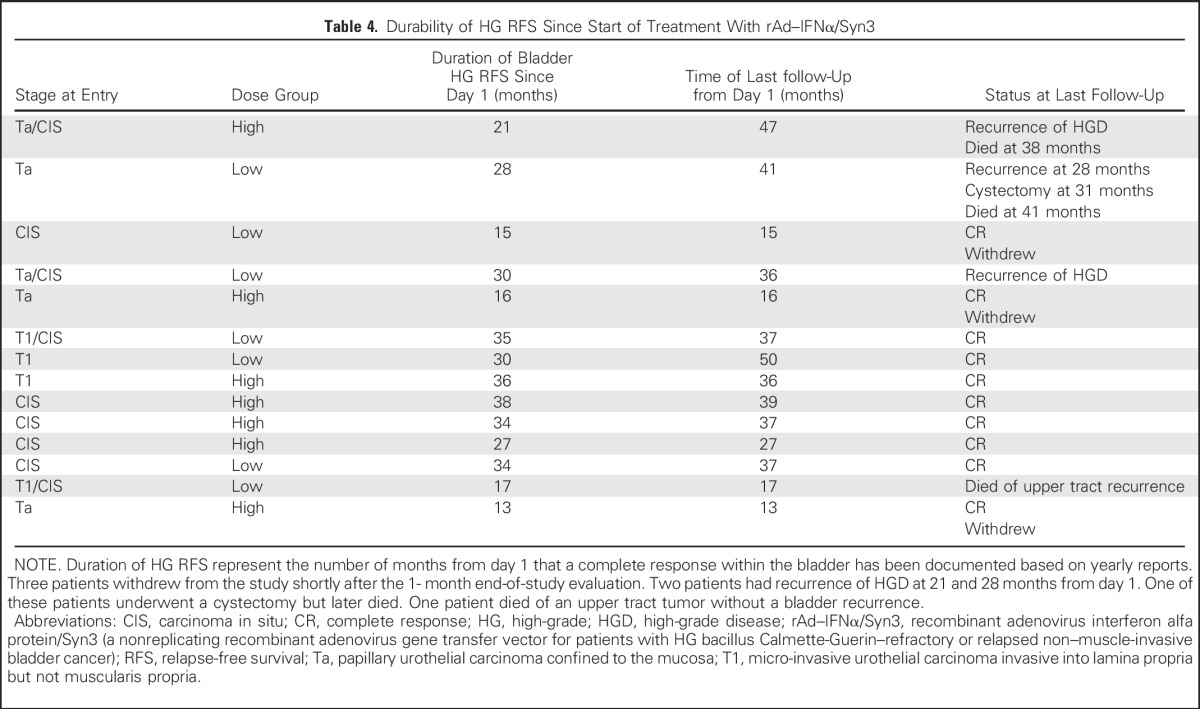
Patients are being monitored for 3 years to collect long-term follow-up data. Of the 14 patients who remained disease free at 12 months, additional follow-up data are being collected for 11; 3 withdrew from the study. Nine of these 11 patients are alive, and eight remained disease-free during a period of 15 to more than 36 months (Table 4). Two patients experienced HG recurrence at 21 and 28 months, respectively, from the start of treatment. One of these patients who experienced progression to muscle invasion underwent a radical cystectomy 31 months after the initiation of treatment and later died at 41 months. The other, who experienced recurrence at 21 months, remained alive and free from distant recurrence at 36 months. One patient free from bladder recurrence at 12 months died as a result of an upper tract tumor at 17 months.
Table 4.
Durability of HG RFS Since Start of Treatment With rAd–IFNα/Syn3
Safety End Points
Overall, 39 patients (97.5%) experienced AEs during the study; 20 patients (95%) were in the low-dose arm, and 19 patients (100%) were in the high-dose arm (Data Supplement). In 34 of these patients (85%), at least one AE was considered to be drug related;: in 18 (87.5%) of 21 patients in the low-dose arm and in 16 (84.2%) of 19 patients in the high-dose arm. The most frequently reported drug-related AEs were micturition urgency in 16 patients (40%), dysuria in 16 patients (40%), fatigue in 13 patients (32.5%), pollakiuria in 11 patients (28%), hematuria and nocturia in 10 patients each (25% each). Notably, for the majority of patients (78%), the AEs were transient and classified as either grade 1 or 2. Nine patients (22%) reported a total of 19 grade 3 AEs: 12 in the low-dose arm (coronary artery occlusion, diarrhea, sepsis, arthralgia, renal neoplasm, transitional cell carcinoma, carotid artery occlusion, syncope, renal failure, nephroureterectomy, COPD, and hypotension) and seven in the high-dose arm (abdominal pain, back pain, fracture, syncope, dysuria [n = 2], and acute renal failure; Data Supplement). Although coded as A’s according to convention, the renal neoplasm, transitional cell carcinoma, and nephroureterectomy reports in the low-dose arm reflect the diagnosis and treatment of a separate upper tract urothelial carcinoma in one patient. All grade 3 AEs occurred only once. There were no grade 4 or 5 events.
Overall, five patients exhibited a total of 10 SAEs: three patients had a total of eight SAEs in the low-dose group, and two patients had a total of two SAEs in the high-dose group. Of these, one episode of diarrhea (low-dose group; treated with 1,000 mL of 0.9% sodium chloride intravenously) and one episode of acute renal failure (high-dose group; urine culture, 59,000 to 99,000 colony-forming units/mL of Klebsiella pneumoniae; treated with antibiotics) were considered related to the study drug. Both resolved with medical therapy. There was no significant difference in the initial occurrence of AEs in those who received the low or high dose of rAd–IFNα/Syn3.
DISCUSSION
We report the results from a completed phase II trial of intravesical rAd–IFNα/Syn3 for patients with recurrent NMIBC after BCG. Several important findings emerge from the resultant data set, including a 12-month HG RFS of 35% by intention-to-treat analysis of all patients dosed. Notably, responses were durable: the majority remained disease-free for close to 24 months. We noted a 30% durable complete response for patients with any element of CIS and a 50% RFS for patients with papillary disease only at study entry. Likewise, the 12-month RFS in heavily pretreated patients was 31%. rAd–IFNα/Syn3 treatment was well tolerated; there were no grade 4 or 5 events, and no patients discontinued treatment because of drug-related AEs. Analytical assays indicated that IFNα-2b was measurable in the urine of all patients, which provided evidence for effective adenoviral-mediated gene transfer. Bioassays revealed no evidence for rAd–IFNα DNA in the blood, which provided additional reassurance for biosafety.
Several agents have been evaluated as second-line treatment after BCG; however, none (to date) have provided robust and durable responses. Valrubicin (Valstar; Endo Pharmaceuticals, Malvern, PA), the only agent currently approved by the FDA for the treatment of BCG-refractory CIS, provided a complete response rate of 18% at 6 months and a 1-year disease-free survival rate of approximately 10%.16 Promising results from early-phase trials have been reported for intravesical taxane and gemcitabine.10-14 Joudi et al15 reported the final results from a national multicenter phase II trial of BCG plus IFNα-2b and noted that 45% of patients with BCG failure were free from recurrence at 2 years. However, only 44% were treated for an HG recurrence, and 61% received only one prior course of BCG.15 A recent retrospective analysis of BCG and IFNα-2b reported a 38.6% RFS at 12 months. Again, many of these patients (20 of 44) received only one prior course of BCG, and 16 patients experienced relapse after 12 months.27 Overall, the limited number of patients studied in previous trials, as well as the modest RFS with treatment despite a less stringently defined eligibility, illustrates the unmet need for effective and evidence-based second-line therapy for patients with BCG-unresponsive disease that improves disease-specific patient outcomes and avoids cystectomy.
We recognize that this study is limited by its relatively small sample size and the lack of a comparative treatment arm. However, the trial was designed to determine optimal dosing and to provide preliminary efficacy to develop a definitive single-arm registration study.12 Few agents have actually gone beyond phase I and II development, so it is readily apparent that the traditional pathway for drug registration does not work for NMIBC. This concern was addressed through deliberations among the Society of Urologic Oncology, American Urological Association, and FDA, with the consensus that a single-arm trial with a mixed population of papillary disease and CIS was appropriate for the BCG-unresponsive population, given that a minimal threshold of patients who had some component of CIS was met.17
Although the clinical impact of rAd–IFNα is encouraging, the mechanisms that mediate its antitumor activity remain undefined. In preclinical studies, IFNα and rAd–IFN beta inhibited angiogenesis,28,29 and IFNα directly induced apoptosis in human bladder cancer cells by inducing autocrine tumor necrosis factor–related apoptosis-inducing ligand production.30 Furthermore, rAd–IFNα overcame resistance to the IFNα protein in vitro and in animal models.22 It is now well established that IFNα controls dendritic cell maturation and antigen presentation and promotes tumor recognition by T cells and natural killer cells, and that these effects likely play more important roles in tumor growth inhibition than the direct effects of IFNα on tumor cells.31-33 Like IFN gamma, IFNα induces programmed death ligand 1 expression,34 which may limit tumor immune recognition and almost certainly inhibits T-cell activation; this may explain the resistance to rAd–IFNα by some of the bladder cancers treated in this study. Combination therapy with IFNα and an anti–programmed death 1 inhibitor was more efficacious in preclinical studies than either agent alone at inhibition of melanoma tumor growth, and combination trials in NMIBC are under consideration.34 Finally, studies have demonstrated that local delivery of IFNα is better than systemic delivery to enhance tumor immune recognition, and viral transduction itself provides an important signal for kickstarting the immune system. Thus, in addition to serving as a bioreactor for sustained IFNα production (in contrast to the transient levels measured after intravesical instillation of the IFNα protein),24 IFNα gene therapy should produce unique, desirable effects on antitumor immunity through local (as opposed to systemic) IFNα production and viral activation of intracellular pattern receptors. Thus, there are multiple reasons to explain the enhanced efficacy of rAd–IFNα compared with IFNα-2b in the treatment of refractory NMIBC.35
In summary, rAd–IFNα/Syn3 was well tolerated and demonstrated promising efficacy for patients with HG NMIBC after BCG therapy. A phase III trial of high-dose rAd–IFNα/Syn3, which provided longer median HG RFS and equivalent biosafety, is ongoing.
ACKNOWLEDGMENT
We thank Ruth B. Murray for critical comments and assistance in preparation of this manuscript for publication. This research was conducted through the Society of Urologic Oncology-Clinical Trials Consortium (SUO-CTC), and we thank Joan Chiaviello, MS, of the SUO-CTC, who provided coordination and operational support for the study.
Appendix
Supplemental Methods
Role of the funding source.
FKD Therapies Oy (Kuopio, Finland) provided funding to the investigators for study design, conduct, treatment administration, and data collection. The study database was held by the funder. All authors had unrestricted access to the raw and final study data and were responsible for data interpretation, the preparation of the report, and the decision to submit for publication.
Recombinant Adenovirus Interferon Alfa Protein/Syn3 production.
First-generation replication-deficient serotype 5 adenovirus vector, which expressed human interferon alfa-2b (IFNα-2b) cDNA under a cytomegalovirus promoter, was produced under good manufacturing practice conditions in 293 cells, as previously described,1 with slight modifications of the process. It was tested to be free of endotoxin, microbiologic contaminants, and other impurities. The structure of the vector was verified by sequencing. Production of recombinant IFNα-2b was verified from each production lot with immunologic methods. The excipient Syn3 is a polyamide surfactant that enhances adenoviral gene transfer to the bladder epithelium.2,3
Analytical Assays
Sample collections and assay methods.
Whole blood and urine samples were collected on days 1 (predose), 2, 4, and 12 of months 1 and 4 for measurement of recombinant adenovirus IFNα-2b (rAd–IFNα-2b) DNA and IFNα-2b concentrations (urine only). Serum samples for IFNα-2b protein concentration measurements also were collected on days 1 (predose), 2, 4, and 12 of months 1 and 4. Serum samples for antibody assays were collected before dosing on day 1 of months 1, 4, 7, and 10, as well as at the month-13/withdrawal visit.
Urine samples for rAd–IFNα-2b DNA, IFNα-2b, and exploratory assays were collected into a sterile container and stabilized with the addition of buffer that contained 10% bovine serum albumin and 50 mM of HEPES (pH, 7.4). Two mL of buffer was added to each 20-mL sample of urine as soon as possible after collection of the urine sample. After addition of the stabilization buffer, aliquots were transferred into 2-mL cryotubes by using sterile pipette tips and were put on ice. Whole-blood samples for determination of rAd–IFN DNA by polymerase chain reaction (PCR) were collected into EDTA-containing tubes. Blood samples were collected at the required time points, were divided into sterile polypropylene cryotubes with sterile pipette tips, and were frozen at −70°C until shipment for analysis. Whole-blood samples for serum IFNα-2b measurements and for determination of antiadenoviral and anti–IFNα-2b antibodies were drawn at the required time points. The samples were drawn into red top Vacutainer (Becton, Dickinson, and Co., Franklin Lakes, NJ) tubes and allowed to clot at room temperature for 30 minutes. The samples were then centrifuged at 4°C, × 1,500 g, for 15 minutes, and the serum was separated into cryovials. All samples for all assays were frozen at −70°C within 5 hours of collection and were stored for shipment and analysis.
IFNα-2b protein concentration assay.
Measurement of IFNα-2b concentrations in urine and serum samples was done by ELISA with a MesoScale discovery platform (Meso Scale Diagnostics, Bethesda, MD). Samples were incubated with a master mix to allow IFNα-2b to bind to biotinylated-anti–IFNα antibodies and sulfo-tagged (sTag)–anti-IFNα antibodies to form an antibody-bridge complex. After incubation, samples were added to the streptavidin-coated plate. The biotinylated–anti-IFNα antibodies bound to the streptavidin-coated plate, which allowed any unbound material to be washed away. Read buffer that contained tripolyamine was added. The sTag associated with anti-IFNα antibodies produced a chemiluminescent signal when an electrical voltage was applied. The concentration of IFNα-2b in samples was then back-calculated from a calibration curve. The method had a lower level of quantification of 31 IU/mL and an upper level of quantification of 2,000 IU/mL.
Analytical assays for rAd–IFNα DNA in blood and urine.
To assess systemic exposure and urinary concentrations of rAd–IFNα vector DNA, a sensitive and specific quantitative PCR (qPCR) assay for the vector DNA was developed and validated. In both assay matrices, amplification was detected in all replicates of the standard curve (1 × 109 viral particles [vp]/225 μl to 1 × 103 vp/225 µl for all valid runs), and the correlation coefficient of the dilutions (R2) was greater than or equal to 0.98 for all qPCRs performed. An assessment of the specificity of the qPCR assay was made with human and Escherichia coli DNA. No cross reactivity with either matrix was observed when 1-, 0.5-, and 0.1-µg templates were present in the qPCR assay. To determine if either human or E. coli DNA could interfere with the accuracy of the qPCR assay, a spike of 2 × 103 vp/2 µl of rAd–IFNα DNA (derived from the appropriate matrix matched standard) was spiked into a background of each concentration of genomic DNA.
Anti-IFN α antibody assay.
Assessment of anti-IFNα antibody concentrations was done with a validated human anti-IFNα platinum ELISA from Affymetrix eBioscience (product code BMS217TEN; Thermo Fisher Scientific, Waltham, MA) with a mouse monoclonal anti-IFNα antibody as the positive control. The assay paradigm was a quasi-quantitative assay sequence that consisted of a screening assay to determine whether a positive signal existed, a competitive inhibition confirmation assay of the positive signal, and a titration assay that used serially diluted samples in buffer.
Antiadenovirus type 5 antibody assay.
Antiadenovirus type 5 antibody concentrations were measured in serum from each patient with an ELISA-based assay. Serum samples from a predose dilution series were assessed for antiadenovirus antibodies to establish the baseline titer for each patient. Serum was diluted to the predose titer, and then 1:2 and 1:4 dilutions of the predose titer dilution were made for sample testing at each time point. Antibodies then were measured at the predose titer and in each dilution. In this quasi-quantitative assay, antibody titer results greater than twice the predose titer were considered significant.
Supplemental Results
Sensitivity analysis of primary end point.
To assess the impact of off-schedule disease assessments on the primary efficacy end point, a sensitivity analysis was conducted in which 12 months was defined according to the assessment date as opposed to the nominal month-13 assessment. Results for the sensitivity analysis were identical to the primary efficacy end point: 14 of 40 patients (35%) overall showed high-grade recurrence free survival at 12 months and experienced comparable incidences for the dose groups (low-dose: n = 7 of 21 [33%]; high-dose: n = 7 of 19 [37%]).
IFNα-2b serum concentrations.
Serum IFNα-2b concentrations were low. At day 2 of month 1, 31 of the 40 patients had concentrations less than 31 IU/mL (limit of assay quantification). Six patients had concentrations greater than 31 IU/mL but less than 50 IU/mL, and two patients had concentrations greater than 50 IU/mL but less than 160 IU/mL.
Blood and Urine rAd–IFNα DNA measurements.
Median blood and urine concentrations of rAd DNA were measured with a qPCR assay that had a level of detection of 1 × 103 vp/225 μL. Importantly, no measurable rAd–IFNα DNA was detected in blood after the initial dosing. Of the 23 patients who received a second dose at month 4, only one patient (4.3%), randomly assigned to the 3 × 1011 vp/mL dose group, had a positive test result for a low level of virus detected at day 2 of month 1 (7.7 × 103 vp/225 μL), which was not measurable by day 4 of month 1.
As expected, all 40 patients had significant copies of rAd DNA in their urine at day 2 of month 1; the median value was 1.13 × 106 vp/225 μL. Thirty-nine patients had measurable concentrations of rAd DNA copies at day 4 of month 1. However, these were approximately three orders of magnitude lower; the median value was 8.08 × 104 vp/225 μL. Thirty-three patients (85%) had measurable concentrations at day 12 of month 1, and the median value was 2.3 × 104 vp/225 μL. In the 23 patients who received a second dose of rAd–IFN, 22 had measurable concentrations of rAd DNA at day 2 of month 4 and a median value of 5.13 × 105 vp/225 μL, and 20 patients had measurable concentrations of approximately eight times the level of detection at day 4 of month 4 and a median value of 8.45 × 103 vp/225 μL. By day 12 of month 4, only six patients (29%) had measurable copies of rAd–IFNα DNA in the urine. Results for the two dose cohorts were comparable.
Anti-IFNα antibody and antiadenovirus antibody concentrations.
Anti–IFNα-2b antibody concentrations in serum were measured in serum from each patient. With the sole exception of one patient who had a weak 1:20 titer at day 12 of month 1, no other patient at any time point had measurable anti–IFNα-2b antibodies. Antiadenovirus type 5 antibody concentrations were measured in serum from each patient with a quasi-quantitative assay (see Covance Laboratories, Harrogate, UK for details).
Antibody data were collected at days 1 and 12 of month 1, day 1 of month 7, day 1 of month 10, and at the month-13/withdrawal assessment. The data demonstrated that 22 patients (55.0%) had a significant antiadenovirus antibody response (defined as four times the predose titer). Of the 14 patients who experienced a complete response, 10 (71%) had a significant antiadenovirus antibody response, and four (29%) did not demonstrate a significant response. These data suggest that a significant antiadenovirus vector antibody response does not appear to correlate with lack of efficacy. A definitive antibody titer for any of the positive patients was not determined.
Safety
A summary of all treatment-emergent adverse events is provided in the Data Supplement.
Footnotes
Supported by FKD Therapies Oy (Kuopio, Finland), by GU SPORE in Bladder Cancer Grant No. CA091846, and by Cancer Center Support Grant No. CA016672.
Clinical trial information: NCT01687244.
AUTHOR CONTRIBUTIONS
Conception and design: Seth P. Lerner, Alan Boyd, F. Peter Treasure, Gillian Gregory, David G. Sawutz, Seppo Yla-Herttuala, Nigel R. Parker
Provision of study materials or patients: All authors
Collection and assembly of data: Daniel J. Canter, Kenneth Ogan, Lawrence I. Karsh, Leonard G. Gomella, Yair Lotan, Trinity J. Bivalacqua, Tracey L. Krupski, Michael E. Woods, Matthew I. Milowsky, Alan Boyd, David G. Sawutz, Nigel R. Parker, Colin P.N. Dinney
Data analysis and interpretation: Neal D. Shore, Stephen A. Boorjian, Tracy M. Downs, Ashish M. Kamat, Robert S. Svatek, Robert L. Grubb III, Seth P. Lerner, Brant A. Inman, Alan Boyd, F. Peter Treasure, David G. Sawutz, Seppo Yla-Herttuala, Nigel R. Parker
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Intravesical rAd–IFNα/Syn3 for Patients With High-Grade, Bacillus Calmette-Guerin–Refractory or Relapsed Non–Muscle-Invasive Bladder Cancer: A Phase II Randomized Study
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Neal D. Shore
Consulting or Advisory Role: Bayer, Janssen Scientific Affairs, Dendreon, Sanofi, Takeda, Tolmar, Ferring, Astellas Medivation
Stephen A. Boorjian
Consulting or Advisory Role: Astellas Medivation
Daniel J. Canter
No relationship to disclose
Kenneth Ogan
Speakers' Bureau: Cook Urologic
Lawrence I. Karsh
Stock or Other Ownership: Swan Valley Medical
Honoraria: Astellas Pharma, Bayer, Dendreon, Janssen, Astellas Medivation
Consulting or Advisory Role: Astellas Pharma, Bayer, Dendreon, Janssen, Astellas Medivation
Speakers' Bureau: Astellas Pharma, Bayer, Dendreon, Janssen, Astellas Medivation, Amgen
Research Funding: Astellas Pharma (Inst), Bayer (Inst), Dendreon (Inst), Janssen (Inst), Astellas Medivation (Inst), Spectrum Pharmaceuticals
Travel, Accommodations, Expenses: Astellas Pharma, Bayer, Dendreon, Spectrum Pharmaceuticals
Tracy M. Downs
Travel, Accommodations, Expenses: Photocure
Leonard G. Gomella
Consulting or Advisory Role: Astellas Pharma, Janssen, Bayer, MDxHealth
Ashish M. Kamat
Honoraria: Pacific Edge
Consulting or Advisory Role: Photocure, Telesta Therapeutics, Sanofi, Merck, Abbott Molecular, Theralase, Heat Biologics, Spectrum Pharmaceuticals, Cepheid
Research Funding: FKD Therapies Oy, Photocure, Merck, Heat Biologics
Yair Lotan
Consulting or Advisory Role: SonaCare Medical, Phyiscal Optics, MDxHealth, Augmenix, BioCancell
Research Funding: Abbott Molecular, Pacific Edge, Cepheid, Metabolon, Danone
Robert S. Svatek
Honoraria: Merck
Research Funding: National Cancer Institute
Travel, Accommodations, Expenses: Merck
Other Relationship: FKD Therapies Oy, GenomeDx
Trinity J. Bivalacqua
No relationship to disclose
Robert L. Grubb III
Employment: MBO Partners (I), Anthem (I)
Honoraria: Argos Therapeutics
Consulting or Advisory Role: Argos Therapeutics
Speakers' Bureau: Blue Earth Diagnostics
Research Funding: Heat Biologics, Argos Therapeutics, FKD Therapies Oy, GlaxoSmithKline
Travel, Accommodations, Expenses: Blue Earth Diagnostics
Tracey L. Krupski
No relationship to disclose
Seth P. Lerner
Consulting or Advisory Role: Urogen Pharma, BioCancell, Incyte, Vaxxion, Nucleix, Ferring
Research Funding: Endo Pharmaceuticals, FKD Therapies Oy, Viventia Biotech
Travel, Accommodations, Expenses: Urogen Pharma, BioCancell, Ferring
Michael E. Woods
No relationship to disclose
Brant A. Inman
Consulting or Advisory Role: Combat Medical, BioCancell, Taris BioMedical, AstraZeneca
Research Funding: Genentech (Inst), Abbott Laboratories (Inst), Nucleix (Inst), FKD Therapies Oy (Inst), Dendreon (Inst)
Matthew I. Milowsky
Research Funding: Merck (Inst), Bristol-Myers Squibb (Inst), Genentech (Inst), Roche (Inst), AstraZeneca (Inst), MedImmune (Inst), Bioclin (Inst), Pfizer (Inst), BIND Therapeutics (Inst), Johnson & Johnson (Inst), Astellas Pharma (Inst), MIrati Therapeutics (InsT), Cerulean Pharma (Inst), Seattle Genetics (Inst), Acerta Pharma (Inst)
Travel, Accommodations, Expenses: Genentech
Alan Boyd
Employment: Boyd Consultants
Leadership: Linear Diagnostics, Celentyx, Genable Technologies
Stock or Other Ownership: Spark Therapeutics, Boyd COnsultants
F. Peter Treasure
Consulting or Advisory Role: Acacia Pharma, Actinogen, Agalimmune, Atlantic, Biocompatibles,Camallergy, Canbex, Cantab Biomanufacturing, CellAct Pharma, Chimerix, Chronos, Dialog Devices, F2G, Finvector, FKD Therapies Oy, F-Star, Genexine, Immunocore, Italfarmaco, MeiraGTX, Mylan, Newtec, Opsona Therapeutics, Origin Sciences, Oxford Biomedica, Pro Bono Bio, ReViral, Shire, Toray Industries, Trizell, USV, Varleigh Diagnostic Consortium, Vaxxas, Orphazyme, Clinigen Group, Sin Poon Pharma
Gillian Gregory
Consulting or Advisory Role: FKD Therapies Oy
David G. Sawutz
Employment: FKD Therapies Oy
Travel, Accommodations, Expenses: FKD Therapies Oy
Seppo Yla-Herttuala
Consulting or Advisory Role: AstraZeneca
Research Funding: AstraZeneca (Inst)
Nigel R. Parker
Leadership: FKD Therapies Oy, Trizell
Consulting or Advisory Role: FKD Therapies Oy, Trizell
Research Funding: FKD Therapies Oy, Trizell
Patents, Royalties, Other Intellectual Property: Various patents in gene therapy
Colin P.N. Dinney
Other Relationship: FKD Therapies Oy, University of Michigan Comprehensive Center
REFERENCES
- 1.Kaufman DS, Shipley WU, Feldman AS: Bladder cancer. Lancet 374:239-249, 2009 [DOI] [PubMed] [Google Scholar]
- 2. Sylvester RJ, van der Meijden AP, Oosterlinck W, et al: Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: A combined analysis of 2596 patients from seven EORTC trials. Eur Urol 49:466-475, 2006. [DOI] [PubMed]
- 3.Fernandez-Gomez J, Madero R, Solsona E, et al. : Predicting nonmuscle invasive bladder cancer recurrence and progression in patients treated with bacillus Calmette-Guerin: The CUETO scoring model. J Urol 182:2195-2203, 2009 [DOI] [PubMed] [Google Scholar]
- 4.Sylvester RJ, van der MEIJDEN AP, Lamm DL: Intravesical bacillus Calmette-Guerin reduces the risk of progression in patients with superficial bladder cancer: A meta-analysis of the published results of randomized clinical trials. J Urol 168:1964-1970, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Sylvester RJ, van der Meijden AP, Witjes JA, et al. : Bacillus Calmette-Guerin versus chemotherapy for the intravesical treatment of patients with carcinoma in situ of the bladder: A meta-analysis of the published results of randomized clinical trials. J Urol 174:86-91, discussion 91-92, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Lamm DL, Blumenstein BA, Crissman JD, et al. : Maintenance bacillus Calmette-Guerin immunotherapy for recurrent TA, T1 and carcinoma in situ transitional cell carcinoma of the bladder: A randomized Southwest Oncology Group Study. J Urol 163:1124-1129, 2000 [PubMed] [Google Scholar]
- 7.Cookson MS, Herr HW, Zhang ZF, et al. : The treated natural history of high risk superficial bladder cancer: 15-year outcome. J Urol 158:62-67, 1997 [DOI] [PubMed] [Google Scholar]
- 8.Yates DR, Brausi MA, Catto JW, et al. : Treatment options available for bacillus Calmette-Guérin failure in non–muscle-invasive bladder cancer. Eur Urol 62:1088-1096, 2012 [DOI] [PubMed] [Google Scholar]
- 9.Herr HW, Sogani PC: Does early cystectomy improve the survival of patients with high-risk superficial bladder tumors? J Urol 166:1296-1299, 2001 [PubMed] [Google Scholar]
- 10.Skinner EC, Goldman B, Sakr WA, et al. : SWOG S0353: Phase II trial of intravesical gemcitabine in patients with nonmuscle invasive bladder cancer and recurrence after 2 prior courses of intravesical bacillus Calmette-Guérin. J Urol 190:1200-1204, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McKiernan JM, Masson P, Murphy AM, et al. : Phase I trial of intravesical docetaxel in the management of superficial bladder cancer refractory to standard intravesical therapy. J Clin Oncol 24:3075-3080, 2006 [DOI] [PubMed] [Google Scholar]
- 12.McKiernan JM, Barlow LJ, Laudano MA, et al. : A phase I trial of intravesical nanoparticle albumin-bound paclitaxel in the treatment of bacillus Calmette-Guérin refractory nonmuscle invasive bladder cancer. J Urol 186:448-451, 2011 [DOI] [PubMed] [Google Scholar]
- 13.Barlow LJ, McKiernan JM, Benson MC: The novel use of intravesical docetaxel for the treatment of non-muscle invasive bladder cancer refractory to BCG therapy: A single institution experience. World J Urol 27:331-335, 2009 [DOI] [PubMed] [Google Scholar]
- 14.Dalbagni G, Russo P, Bochner B, et al. : Phase II trial of intravesical gemcitabine in bacille Calmette-Guérin–refractory transitional cell carcinoma of the bladder. J Clin Oncol 24:2729-2734, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Joudi FN, Smith BJ, O’Donnell MA, et al. : Final results from a national multicenter phase II trial of combination bacillus Calmette-Guérin plus interferon alpha-2b for reducing recurrence of superficial bladder cancer. Urol Oncol 24:344-348, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Dinney CP, Greenberg RE, Steinberg GD: Intravesical valrubicin in patients with bladder carcinoma in situ and contraindication to or failure after bacillus Calmette-Guérin. Urol Oncol 31:1635-1642, 2013 [DOI] [PubMed] [Google Scholar]
- 17.Lerner SP, Dinney C, Kamat A, et al. : Clarification of bladder cancer disease states following treatment of patients with intravesical BCG. Bladder Cancer 1:29-30, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jarow JP, Lerner SP, Kluetz PG, et al. : Clinical trial design for the development of new therapies for nonmuscle-invasive bladder cancer: Report of a Food and Drug Administration and American Urological Association public workshop. Urology 83:262-264, 2014 [DOI] [PubMed] [Google Scholar]
- 19.Kamat AM, Sylvester RJ, Böhle A, et al. : Definitions, end points, and clinical trial designs for non-muscle-invasive bladder cancer: Recommendations from the International Bladder Cancer Group. J Clin Oncol 34:1935-1944, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Belldegrun AS, Franklin JR, O’Donnell MA, et al. : Superficial bladder cancer: The role of interferon-alpha. J Urol 159:1793-1801, 1998 [DOI] [PubMed] [Google Scholar]
- 21.O’Donnell MA, Lilli K, Leopold C, et al. : Interim results from a national multicenter phase II trial of combination bacillus Calmette-Guerin plus interferon alfa-2b for superficial bladder cancer. J Urol 172:888-893, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Benedict WF, Tao Z, Kim CS, et al. : Intravesical Ad-IFN alpha causes marked regression of human bladder cancer growing orthotopically in nude mice and overcomes resistance to IFN alpha protein. Mol Ther 10:525-532, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Tao Z, Connor RJ, Ashoori F, et al. : Efficacy of a single intravesical treatment with Ad-IFN/Syn 3 is dependent on dose and urine IFN concentration obtained: Implications for clinical investigation. Cancer Gene Ther 13:125-130, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Connor RJ, Anderson JM, Machemer T, et al. : Sustained intravesical interferon protein exposure is achieved using an adenoviral-mediated gene delivery system: A study in rats evaluating dosing regimens. Urology 66:224-229, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Yamashita M, Rosser CJ, Zhou JH, et al. : Syn3 provides high levels of intravesical adenoviral-mediated gene transfer for gene therapy of genetically altered urothelium and superficial bladder cancer. Cancer Gene Ther 9:687-691, 2002 [DOI] [PubMed] [Google Scholar]
- 26.Dinney CP, Fisher MB, Navai N, et al. : Phase I trial of intravesical recombinant adenovirus mediated interferon α−2b formulated in Syn3 for Bacillus Calmette-Guérin failures in nonmuscle invasive bladder cancer. J Urol 190:850-856, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. A'Hern RP: Sample size tables for exact single-stage phase II designs. Statist Med 20:859-866, 2001. [DOI] [PubMed]
- 28.Correa AF, Theisen K, Ferroni M, et al. : The role of interferon in the management of BCG refractory nonmuscle invasive bladder cancer. Adv Urol 2015:656918, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dinney CP, Bielenberg DR, Perrotte P, et al. : Inhibition of basic fibroblast growth factor expression, angiogenesis, and growth of human bladder carcinoma in mice by systemic interferon-alpha administration. Cancer Res 58:808-814, 1998 [PubMed] [Google Scholar]
- 30. Izawa JI, Sweeney P, Perrotte P, et al. Inhibition of tumorigenicity and metastasis of human bladder cancer growing in athymic mice by interferon-beta gene therapy results partially from various antiangiogenic effects including endothelial cell apoptosis. Clin Cancer Res 8:1258-1270, 2002. [PubMed]
- 31. Papageorgiou A, Lashinger L, Millikan R, et al: Autocrine TRAIL production mediates interferon-induced apoptosis in human bladder cancer cells. Cancer Res 64:8973-8979, 2005. [DOI] [PubMed]
- 32. Sadler AJ, Williams BR. Interferon-inducible antiviral effectors. Nat Rev Immunol 8:559-568, 2008 . [DOI] [PMC free article] [PubMed]
- 33. Spaapen RM, Leung MY, Fuertes MB, et al. Therapeutic activity of high-dose intratumoral IFN beta requires direct effect on the tumor vasculature. J Immunol 193:4254-4260, 2014. [DOI] [PubMed]
- 34. McCracken MN, Cha AC, Weissman IL. Molecular pathways: Activating T cells after cancer cell phagocytosis from blockade of CD47 “don't eat me” signals. Clin Cancer Res 21:3597-3601, 2015. [DOI] [PMC free article] [PubMed]
- 35.Bald T, Landsberg J, Lopez-Ramos D, et al. : Immune cell-poor melanomas benefit from PD-1 blockade after targeted type I IFN activation. Cancer Discov 4:674-687, 2014 [DOI] [PubMed] [Google Scholar]
- 36.Lamm D, Brausi M, O’Donnell MA, et al. : Interferon alfa in the treatment paradigm for non–muscle-invasive bladder cancer. Urol Oncol 35:21-35, 2014 [DOI] [PubMed] [Google Scholar]