Abstract
Purpose
To assess the relative risk of Alzheimer’s disease (AD) among patients with prostate cancer who received androgen deprivation therapy (ADT), after adjustment for other cancer therapies.
Methods
Data from demographics, survival, diagnoses codes, procedure codes, and other information about beneficiaries age 67 years or older in the Medicare claims database was assessed to determine the unadjusted and adjusted risks of AD and of dementia from ADT. The prespecified survival analysis method was competing risk regression.
Results
Of the 1.2 million fee-for-service Medicare beneficiaries who developed prostate cancer in 2001 to 2014, 35% received ADT. Of these, 109,815 (8.9%) and 223,765 (18.8%) developed AD and dementia, respectively, and 26% to 33% died without either outcome. Unadjusted rates of AD and all-cause mortality per 1,000 patient-years were higher among ADT recipients; the unadjusted rates of AD were 17.0 and 15.5 per 1,000 person-years in recipients and nonrecipients, respectively, and the unadjusted rates of all-cause mortality were 73.0 and 51.6 per 1,000 person-years, respectively. The unadjusted rates for dementia in ADT recipients versus nonrecipients were 38.5 and 32.9, respectively, and the unadjusted rates of mortality were 60.2 versus 40.4, respectively. However, after analysis was adjusted for other cancer therapies and other covariates, patients with ADT treatment had no increased risk of AD (subdistribution hazard ratio [SHR], 0.98; 95% CI, 0.97 to 0.99) and had only a miniscule (1%) risk of dementia (SHR, 1.01; 95% CI, 1.01 to 1.02); patients treated with ADT were more likely to die before progression to AD (SHR, 1.24; 95% CI, 1.23 to 1.24) or dementia (SHR, 1.26; 95% CI, 1.25 to 1.26). The risks of AD and dementia were not associated with duration of ADT (ie, no dose effect). Other secondary analyses confirmed these results.
Conclusion
These data suggest that ADT treatment has no hazard for AD and no meaningful hazard for dementia among men age 67 years or older who are enrolled in Medicare.
INTRODUCTION
Prostate cancer (PCa) is the most common cancer in men; it accounted for 21% of all male cancer occurrences in 2016 and for 8% of all male cancer deaths.1 PCa has surgical, radiation therapy, and medical treatment options.
Nead et al2,3 reported a strong association between one treatment option, chemical androgen deprivation therapy (ADT), and the risk of both Alzheimer’s disease (AD; hazard ratio [HR], 1.88) and dementia (HR, 2.17), and they noted a dose effect. Their results were consistent with decreased levels of testosterone as a risk factor for AD.4,5 However, these two prior studies were based on data from only two institutions, observed few events (n = 125 patients with AD and n = 314 patients with dementia), and did not consider other cancer therapies (eg, chemotherapy, radiation therapy, prostatectomy) in the analyses.
The Virtual Research Data Center (VRDC) of the Centers for Medicare and Medicaid Services (CMS) carries information about more than a million patients with PCa, including their medical treatments and outcomes, such as AD, dementia, and death.6 We used the VRDC as a source of de-identified big data for an observational cohort study to assess the association between use of ADT and risk of AD or dementia, and all major PCa therapies were included as covariates. Here, we report the results of that study.
METHODS
Study Population
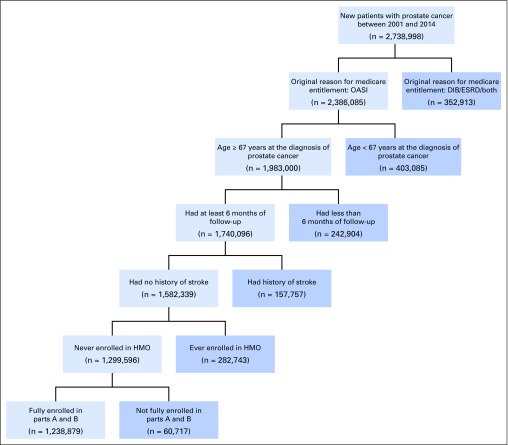
The initial study cohort came from the set of Medicare beneficiaries fully enrolled in Parts A and B during the entire study period of 2001 to 2014. From this population, the subselected beneficiaries were men who were eligible for Medicare on the basis of old age (≥ 65 years) and whose first diagnosis of PCa was reported on or after 2001 as well as at age 67 years or older. This 67-year age condition provided a 2-year washout period to filter out prevalent occurrences of PCa that existed before Medicare enrollment.7 Then, the following patients were excluded: those who had been enrolled in Medicare Advantage at any time during the study (because CMS has no claim records for such patients); those with AD, dementia, or stroke diagnosed before the PCa diagnosis; and those with fewer than 6 months of follow-up after PCa diagnosis (which eliminated PCa occurrences first reported after July 30, 2014; Fig 1). The data were exempted from human subject review by the Office of Human Research Protection at the National Institutes of Health.
Fig 1.
Deposition of study population: cohort selection. DIB, disability insurance benefits; ESRD, end-stage renal disease; HMO, health maintenance organization; OASI, old age and survivors insurance.
Source of Diagnosis and Treatment Information and Other Covariates
The Medicare Master Beneficiary Summary File (MBSF) includes the date of first occurrence for each of 27 common chronic conditions (from January 1, 1999 to the present).8,9 The MBSF data were used to define the first onset of PCa, AD, dementia, and each of 22 nonfemale disease covariates (Data Supplement).
A 10% sample of patients with Part D drug records was obtained from mid-2006 (the onset of Part D) to identify prescription medications for PCa dispensed from community pharmacies. The list of Parts B and D drugs for PCa by category are available in the Data Supplement; however, no Part D data were used in the primary analysis because Part B records (in-office dispensing) captured almost all of the patients who ever received such treatments, and because Part D data were unavailable for 95% of the available PCa occurrences.
Outcome and Event Status
The CMS first occurrence of PCa diagnosis was used as the starting time for all of the survival analyses. Individuals were observed until the first of the following occurrences: Dec 31, 2014; the first documentation of AD; or the Medicare-reported date of death. In the first set of analyses, the occurrence of AD as coded by CMS, (which others have validated10) was used; in the second set, the CMS coding for dementia was used.
Descriptive Statistics
Secular trends occurred during the 14 year-long observation window. They are reported in the Results.
Statistical Model
The analysis was adjusted for covariates. For age, the age at occurrence of PCa was used; for ethnicity, the name-based algorithms of the Research Triangle Institute were used, because the Institute classifies ethnicity better than Medicare’s demographic ethnicity field does.11 As a surrogate for socioeconomic status, degree of Medicaid eligibility (fully dual, partially dual, or nondual)12 and rural residence indicator were used. The year of the PCa diagnosis was used to adjust for trends in treatment choices. For health conditions of patients, each of 22 male-relevant chronic conditions reported in the MBSF file before the end of follow-up was used as binary covariates; chemical/surgical ADT, radiation therapy, total prostatectomy, and chemotherapy were included as separate binary covariates. A propensity score was used to mitigate selection bias toward ADT assignment. First, logistic regression was performed to calculate the propensity score of receipt of ADT as a function of all the covariates, and the covariates were considered only if they began before the PCa diagnosis. Then, the score was used to generate the inverse probability of treatment weights. Conditional on the propensity score, observed baseline covariates should be similar between treated and untreated patients,13,14 although such adjustment may not be necessary with large sample sizes.15 A competing risk is an outcome beyond the primary outcome that precludes the occurrence of the primary outcome. The standard Cox model overestimates the risk of the primary outcome when the risk of death exceeds that of the primary outcome and/or the duration of follow-up is greater than 5 years.16 Both criteria apply to these data; so, the competing risk regression was used as the prespecified survival analysis model. To display the event dynamics,17 Cause-specific HRs (CSHRs) that used standard Cox data, as well as subdistribution hazard ratios (SHRs) that used competing risk regression, were presented side by side for both AD (dementia) and mortality (Data Supplement).
The CMS pools data from thousands of sites. Because, in some context, such pooling could result in spurious null association,18 a secondary analysis was performed, which was stratified by the state of residence of the beneficiary for both outcomes, and another secondary analysis was performed to identify dose effects by comparison of the hazards of ADT use for 1 year or less versus use for greater than 1 year.
To be sure that any differences in the outcomes would not be attributed to differences in the analysis, a third analysis, which followed the approach of Nead et al,2,3 was performed to the study the start time for patients with and without ADT. This analysis included most of the smaller set of covariates in both the standard Cox model (the method of Nead et al2,3) and the more appropriate competing risk regression.
Time by covariate interaction terms were included as predictors in an analysis to test the proportional hazard assumption.17 For covariates that failed this test, the corresponding interaction term in the model was retained to deal with its nonproportionality.17
Finally, to assess ascertainment, patients with PCa were identified from one of the two institutions in the studies by Nead et al2,3—Stanford Health—by using the Stanford National Provider Identifiers (Data Supplement). Then, the number of AD occurrences reported for those patients on any Medicare claim was compared with the number identified only on Stanford claims. This study only included patients diagnosed with PCa after October 2006, when the National Provider Identifier registry began.
RESULTS
Study Population and Secular Trends
Of the 1,238,879 patients in the study who met all of the inclusion criteria, 35% were ever treated with either chemical or surgical ADT. Patients were observed for an average of 5.5 years (total, 6,839,877 person-years). During the observation period, the percentage of patients enrolled in Medicare Advantage programs increased from 16.0% in 2001% to 31.3% in 2014, whereas the proportion in fee-for-service decreased inversely. After adjustment for this decrease, the annual incidence of PCa during these 14 years also declined, from 1.7% in 2001% to 0.9% in 2014; these trends have been reported previously.19,20
Distribution and Trends in Types of PCa Treatments
Radiation therapy and chemical ADT were used by 37.4% and 34.8% of all patients, respectively. Of patients who received radiation therapy, 84% received it as external-beam radiation or neutron beam therapy; 28.6% received brachytherapy; and 1.5% received proton-beam therapy. Chemotherapy, total prostatectomy, and orchiectomy were used less frequently: 11.7%, 10.2%, and 1.1%, respectively. Of all patients, 37.0% received no active treatment, and 27.0% received more than one kind of treatment (eg, 54.3% of patients who received radiation therapy also received ADT therapy).
During the 8 years when Part D records were available, the distribution of medications among patients who received any ADT drug was as follows: gonadotropin-releasing hormone (GnRH) agonists, 94.2%; androgen receptor blockers, 47.3%; GnRH antagonists, 8.5%; androgen production blockers (ie, CYP17 inhibitors), 6.7%; and estrogen, 0.7%. Among a small subset of the 27,115 patients with PCa whose Parts A, B, and D records were available, 8,158 (30%) had at least one Part B or Part D ADT record, and Part B claims identified ADT use in all but 517 (< 2% of patients who had both Parts B and D records).
The 14 years of patient observations were divided into nine 1.5-year intervals, which were based on PCa onset date and which assessed trends in treatment choices accordingly (data not shown). The proportion of patients with newly diagnosed PCa who were treated with active surveillance increased from 33.9% for patients who entered the study during the first 1.5-year interval to 53.0% during the last 1.5 years. Conversely, chemical ADT use decreased from 42.7% to 23.3%; radiation therapy, from 36.9% to 26.7%; chemotherapy, from 14.7% to 4.4%; and orchiectomy, from 1.8% to 0.3%. Only prostatectomy increased, slightly, from 7.7% to 9.8% between the first and last intervals. Observation of people earlier in the disease course in later epochs might account for some of these differences.
Differences in Patient Characteristics and Outcomes
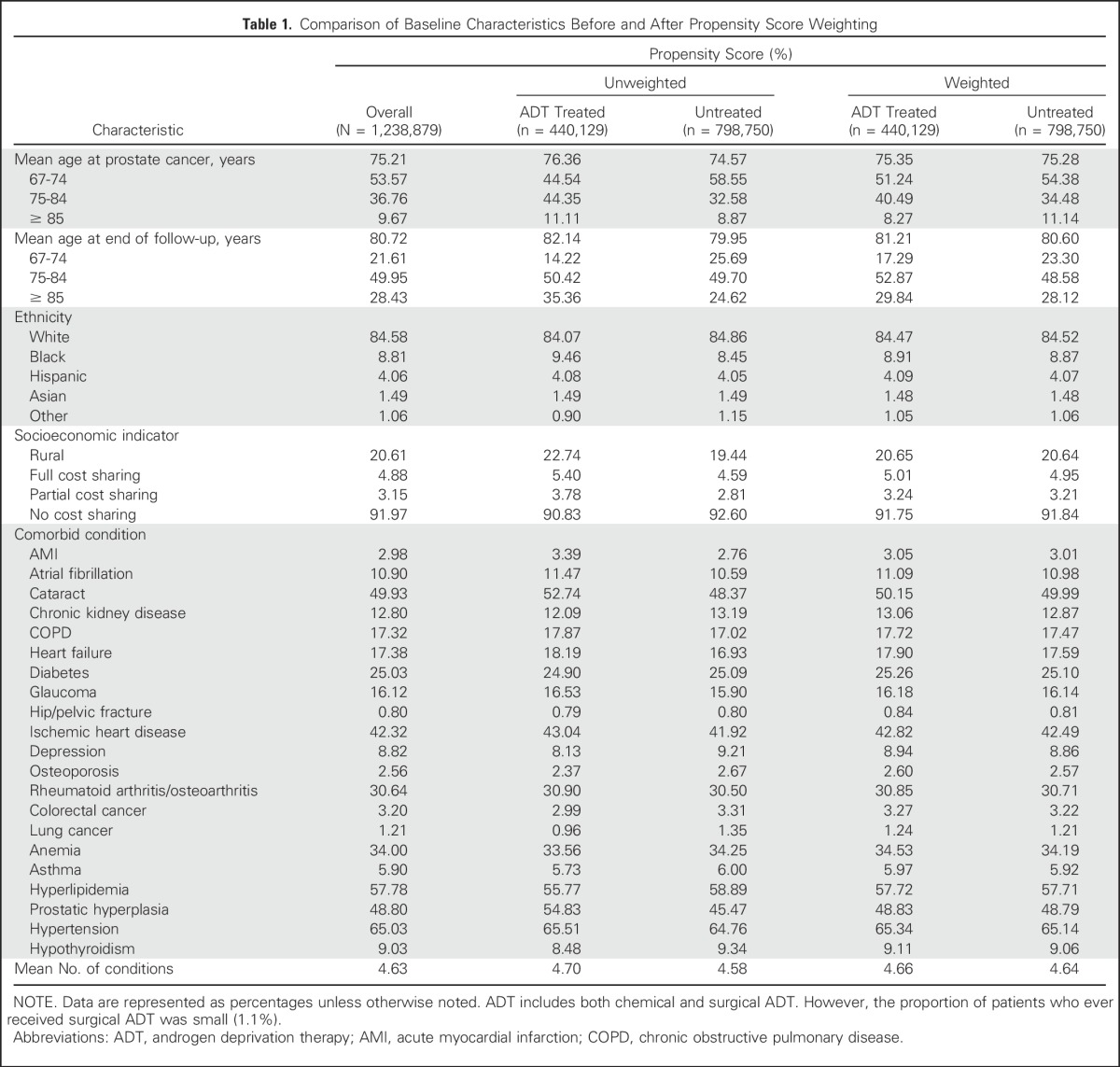
Table 1 compares underlying patient characteristics between ADT-treated and -untreated groups before and after propensity score weighting. Before such weighting, ADT-treated patients were, on average, 2 years older at PCa onset (76.4 v 74.6 years) and were more likely to be black (9.5% v 8.5%), to be dual eligible (9.2% v 7.4%), and to reside in rural areas (22.7% v 19.4%) than untreated counterparts. Propensity score weighting diminished these differences.
Table 1.
Comparison of Baseline Characteristics Before and After Propensity Score Weighting
Crude Analysis: Death and AD
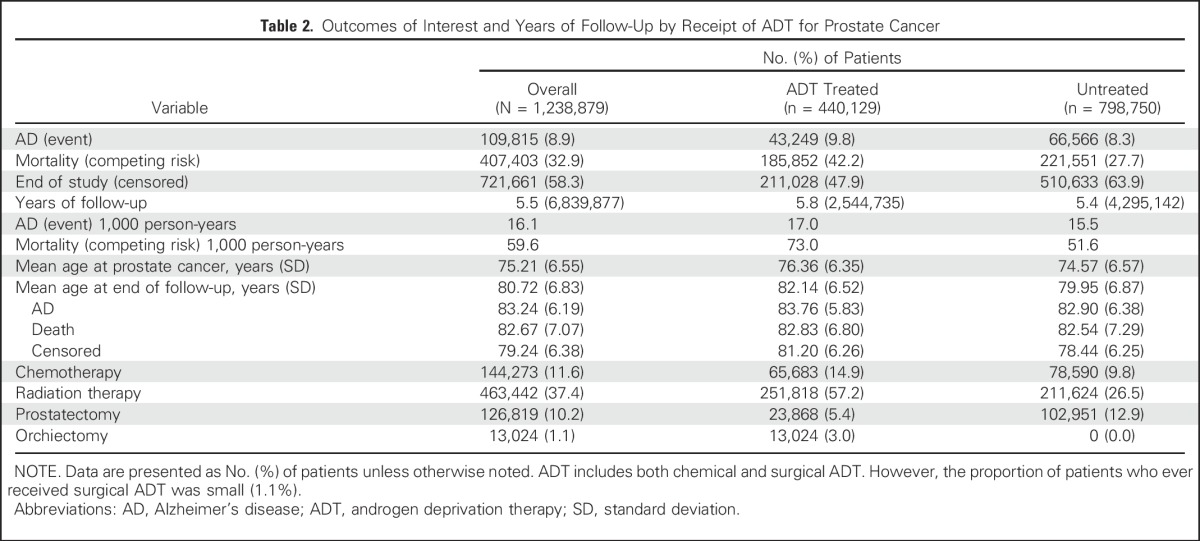
Of all the patients (N = 1,238,879), 8.9% developed AD, and almost four times as many, 32.9%, died during nearly 7 million total years of follow-up. This corresponds to crude rates of 16.1 ADs and 59.6 deaths per 1,000 person-years. ADT-treated patients incurred a higher unadjusted rate of AD (17.0 v 15.5) and of all-cause mortality (73.0 v 51.6) per 1,000 patient-years than untreated patients (Table 2).
Table 2.
Outcomes of Interest and Years of Follow-Up by Receipt of ADT for Prostate Cancer
Primary Analysis
First, all covariates and time-by-covariate interaction terms were used to test the proportional hazard assumption. The proportional hazard assumptions for AD (P = .798) and for dementia (P = .687) were met for ADT. The primary analyses included all covariates and the interaction terms (not shown) for covariates that failed the test. (Tables 3-5)
Table 3.
Hazard Ratios of AD and All-Cause Mortality Presented Side by Side for Competing Risk Regression
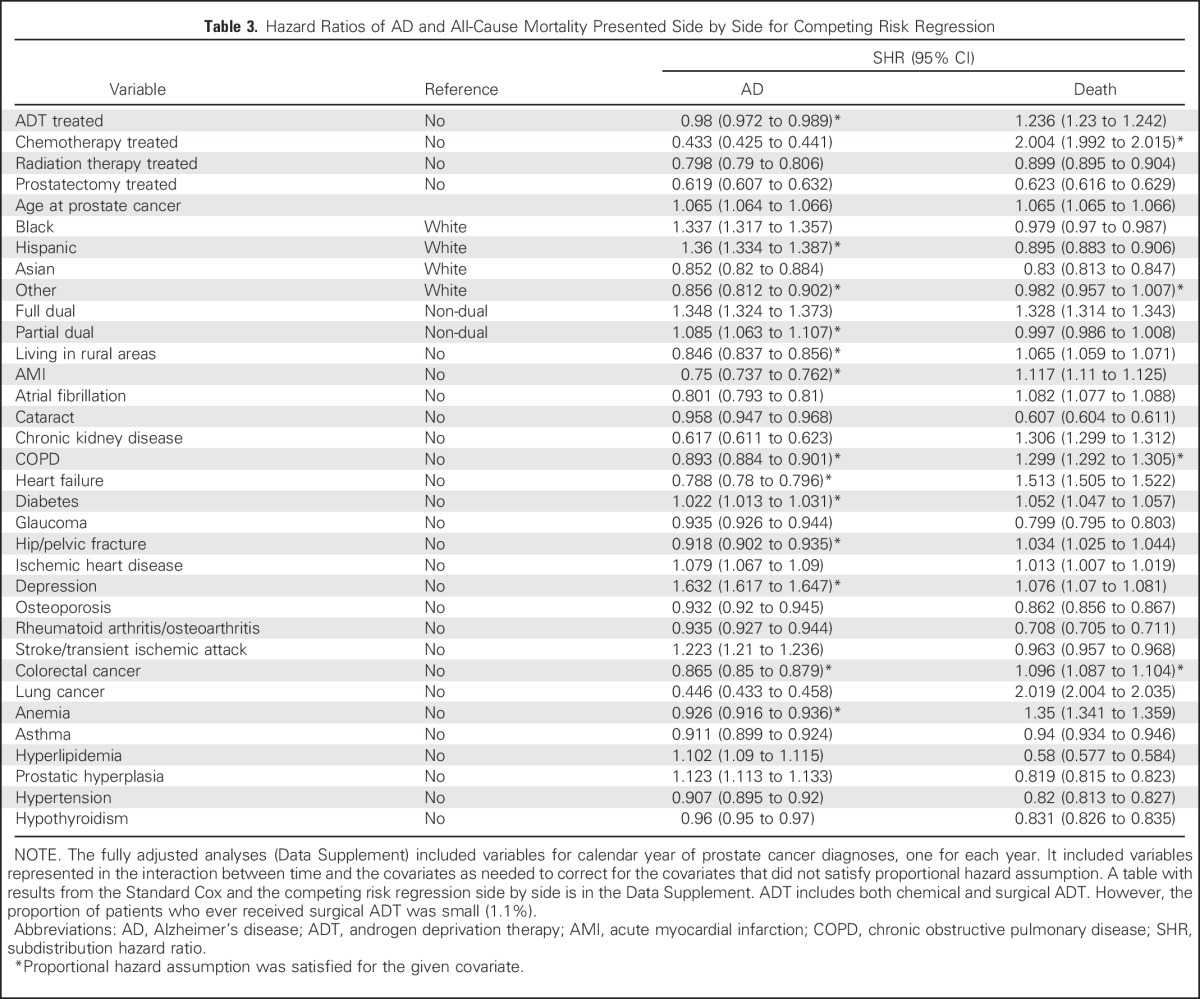
Table 5.
Hazard Ratios of Dementia Disease and All-Cause Mortality Presented Side by Side for Competing Risk Regression
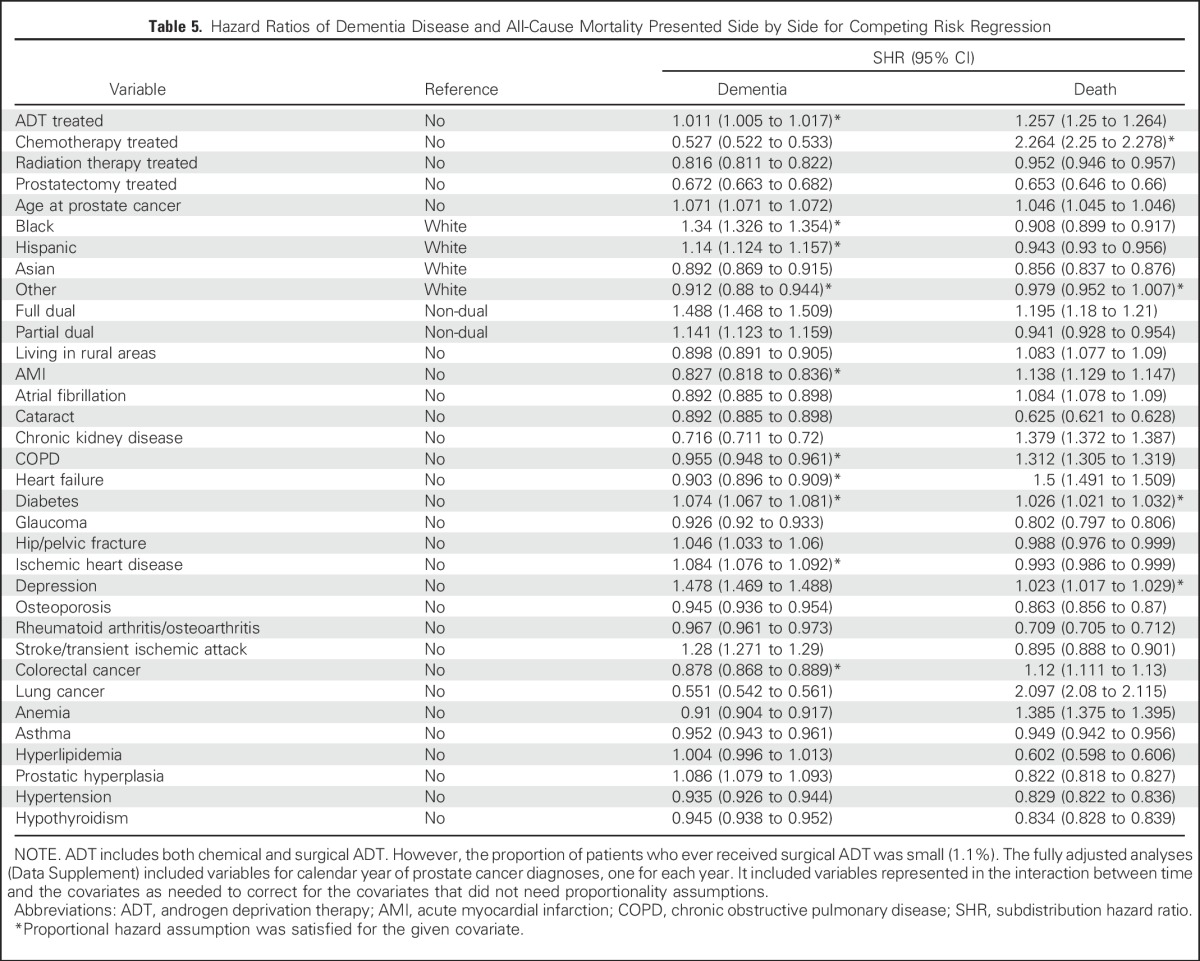
Table 3 lists the adjusted competing risk HRs for AD and death side by side. ADT was associated with a small (2%) decreased AD rate (SHR, 0.98; 95% CI, 0.97 to 0.99) and an increased death rate (SHR, 1.24; 95% CI, 1.23 to 1.24). Among other PCa treatments, chemotherapy was associated with a large increase in death rate (SHR, 2.00) and a corresponding reduction in AD rate (SHR, 0.43). Radiation therapy and prostatectomy were associated with younger age ranges and, correspondingly, lower rates for both AD and death. Asian and “other” ethnicity categories had smaller hazards for AD than the white ethnicity category (SHR, 0.85 and 0.86, respectively); black and Hispanic ethnicity categories had larger hazards (SHR, 1.34 and 1.36, respectively) than the white category. The socioeconomic indicator also was predictive. SHRs ranged from 1.35 to 1.09 for those who were fully or partially eligible for both Medicare and Medicaid, respectively, and the indicator results were directionally similar to reports regarding the general populations.21,22 Among 22 chronic diseases, the presence of diabetes, ischemic heart disease, depression, stroke and transient ischemic attack, hyperlipidemia, and prostatic hyperplasia significantly increased hazards of AD. Diabetes, ischemic heart disease, depression, and stroke and transient ischemic attack have been reported as risks and could serve as positive controls.18 Likewise, cataracts and glaucoma were slightly protective and could serve as negative controls. Nine of 12 diseases associated with an increased death risk also had a reduced AD rate, as might be expected.
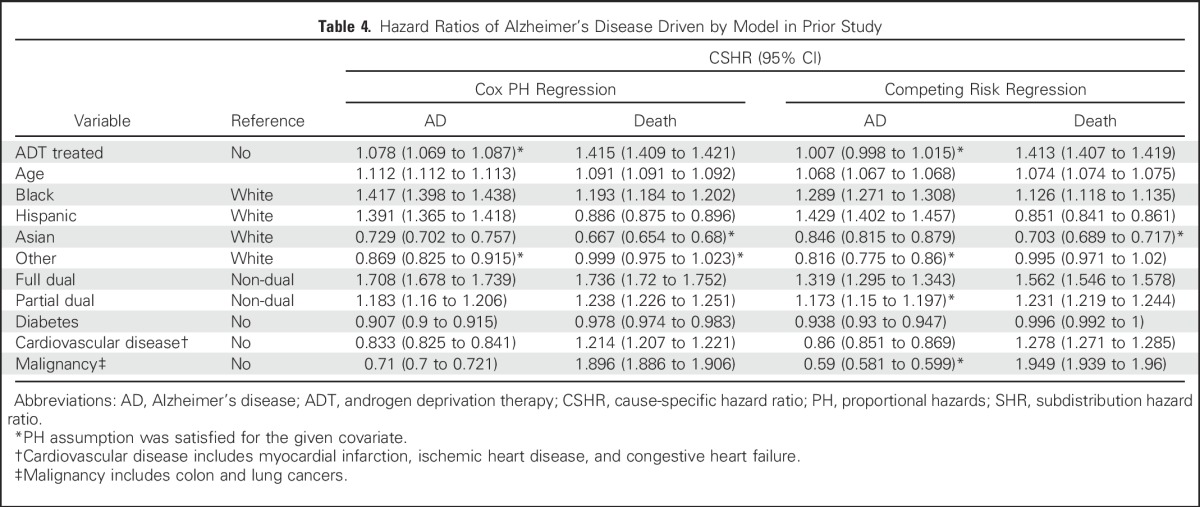
Stratification by state of residence at time of enrollment (50 states plus DC) was added to the primary analysis to show that the pooling of data by Medicare from many institutions did not distort the results, and no added risk of AD from ADT was observed (SHR, 0.99; 95% CI, 0.98 to 0.99; Data Supplement). Also, no significant effect of ADT on the rate of AD was noted in an analysis tailored more closely to the design of the study by Nead et al2 (SHR, 1.01; 95% CI, 0.99 to 1.02; Table 4). Finally, dose effects of ADT on the risk of AD were assessed in the 214,528 patients who received ADT for less than a year compared with the 225,601 patients who received ADT for more than a year. The hazard ratio was larger for the shorter versus the longer periods of treatment (SHR, 1.12 and 0.89, respectively; Data Supplement).
Table 4.
Hazard Ratios of Alzheimer’s Disease Driven by Model in Prior Study
The overall prevalence of AD in the prior study by Nead et al2 was substantially lower (0.7%) than the prevalence in this study (8.9%). The patients from Stanford also were much younger than the patients in this study (67.3 v 76.4 years), but the AD prevalence differences persisted in the older-age strata (75 to 84 years: 32.7% v 50.0% for Stanford v Medicare enrollees; > 85 years: 17.8% v 35.7% for Stanford v Medicare enrollees). Moreover, Stanford Medicare claims carried only half of the post-PCa AD diagnoses found in the whole Medicare dataset for the patients with PCa in the Medicare Stanford PCa program, which suggests under-ascertainment.
A parallel analysis was conducted with dementia as the event of interest (Table 5). As in the AD analyses, unadjusted rates of dementia (38.5 v 32.9) and all-cause mortality (60.2 v 40.4) per 1,000 patient-years were higher among ADT recipients than nonrecipients. In the competing risk model, ADT had a significant—but tiny (1%)—effect on the dementia rate (SHR, 1.01; 95% CI, 1.01 to 1.02). The effects were slightly larger for dementia in the corresponding analyses by state (SHR, 1.01; 95% CI, 1.01 to 1.02), by dose effect (≤ 1-year SHR, 1.14; > 1-year SHR, 0.92), and like the analysis by Nead et al2 (SHR, 1.04; 95% CI, 1.04 to 1.05).
The results of the standard Cox regression, along with the competing risk analysis, are available in the Data Supplement. Because the death rates are much greater than the event rates (42.9% v 9.8% for AD and 33.6% v 21.5% for dementia) the standard Cox model exaggerated the effect sizes of ADT by a few percentage points compared with the more appropriate competing risk model16—up to a risk of 2% for AD (CSHR, 1.02; 95% CI, 1 to 1.03) and of 6% for dementia (CSHR, 1.06; 95% CI, 1.05 to 1.07).
DISCUSSION
In this study, we sought to assess the degree to which ADT is associated with AD by using data from 1,238,879 patients with PCa who were observed during 14 years of Medicare claims. The average observation time was 5.5 years (total, 6,839,877 years), during which 8.9% developed AD, 32.9% died, and 58.3% reached the end of the study period (Dec 31, 2014) without experiencing either outcome.
With the prespecified model of a competing risk, no hazard of AD from ADT was observed in any of the analyses, which included all of the major PCa treatment modalities, underlying patient characteristics, 22 chronic diseases, and both age and year of PCa diagnosis as covariates. The competing risk model actually showed a slight (2%) decrease in the AD rate, which possibly was attributable to the high death rate (SHR, 1.24). A parallel set of analyses was conducted with dementia as the outcome. ADT had significant, but miniscule (1%), effect on the dementia rate in the competing risk model (SHR, 1.01).
Sensitivity analyses were conducted to evaluate the effects of pooling heterogeneous data, and another analysis replicated the study by Nead et al.2,3 These analyses found no risk of AD and only minuscule increases in the risk of dementia, not the nearly two-fold increase reported in the previous studies.2,3 Finally, no ADT dose effect on AD or dementia was observed.
The differences in conclusions between this study and the study by Nead et al2,3 could be a consequence of different choices of methods, definitions, and data source that are described by Madigan et al.18 However, this study had a larger patient samples (1.2 million v 17,000), longer follow-up time (median, 4.9 v 2.7 years), and more complete ascertainment than the study by Nead et al.2,3 Furthermore, the findings of this study are consistent with those of a number of other studies that addressed the risk of dementia or cognitive impairment in patients with PCa who were treated with ADT.23-28 Three of these studies with smaller sample sizes reported nonsignificant HRs that were > 1,23,24,26,27 and one study showed a nonsignificant HR that was < 128; most of the studies were too underpowered to be conclusive.
The patients with PCa in this study had a much higher rate of AD than those in the study by Nead et al2,3 (8.9% v 0.7%), in part because of their younger mean age (67.3 v 75.2 years) and in part because of likely ascertainment (50%) problems in the study by Nead et al,2,3 which assumed that the occurrence of AD or dementia would be reported at the index care systems. However, some patients would have moved to a different care system by the time these disorders revealed themselves.29
These findings are subject to a number of limitations. First, to take advantage of 100% of the Medicare Parts A and B data for fee-for-service beneficiaries between 2001 and 2014, we did not account for use of antiandrogens from Part D data, but this was a small slice of data. Second, the propensity score approach does not mitigate differences in unobserved factors. Third, we lacked information about many important AD and dementia risk factors, such as family history; smoking habits; and measurements like blood pressure, PCa staging information, and biomarkers. Fourth, we did not adjust for the use of routine medications that patients may have taken for problems other than PCa.
In summary, these data suggest that ADT presents no hazard of AD to men age 67 years or older who are enrolled in Medicare. Indeed, ADT appears slightly protective. Also, the hazard for dementia associated with ADT use is miniscule and not clinically important.
Medicare data have been discounted as just billing data, but the VRDC now carries information about all billed in-office and pharmacy-dispensed medications, vital statuses, and records for all encounter diagnoses and billed procedures as an alternative or a complement to observational data from single institutions. Size matters, and the large and relatively complete Medicare database should be used more widely in observational studies of older patients.
Footnotes
Supported by the Intramural Research Program of the National Library of Medicine, National Institutes of Health.
See accompanying Editorial on page 3380
AUTHOR CONTRIBUTIONS
Conception and design: All authors
Collection and assembly of data: Seo Hyon Baik, Clement Joseph McDonald
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Risk of Alzheimer’s Disease Among Senior Medicare Beneficiaries Treated With Androgen Deprivation Therapy for Prostate Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Seo Hyon Baik
No relationship to disclose
Fabricio Sampaio Peres Kury
No relationship to disclose
Clement Joseph McDonald
No relationship to disclose
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A: Cancer statistics, 2016. CA Cancer J Clin 66:7-30, 2016 [DOI] [PubMed] [Google Scholar]
- 2.Nead KT, Gaskin G, Chester C, et al. : Androgen deprivation therapy and future Alzheimer’s disease risk. J Clin Oncol 34:566-571, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nead KT, Gaskin G, Chester C, et al. : Association between androgen deprivation therapy and risk of dementia. JAMA Oncol 3:49-55, 2017 [DOI] [PubMed] [Google Scholar]
- 4.Nelson CJ, Lee JS, Gamboa MC, et al. : Cognitive effects of hormone therapy in men with prostate cancer: A review. Cancer 113:1097-1106, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hogervorst E, Bandelow S, Combrinck M, et al. : Low free testosterone is an independent risk factor for Alzheimer’s disease. Exp Gerontol 39:1633-1639, 2004 [DOI] [PubMed] [Google Scholar]
- 6. Centers for Medicare and Medicaid Services: CMS announces new data sharing tool. https://www.cms.gov/Newsroom/MediaReleaseDatabase/Press-releases/2013-Press-releases-items/2013-11-12.html.
- 7.Lin CC, Virgo KS: Diagnosis date agreement between SEER and Medicare claims data: Impact on treatment. Med Care 52:32-37, 2014 [DOI] [PubMed] [Google Scholar]
- 8. Chronic Conditions Data Warehouse: Condition categories. https://www.ccwdata.org/web/guest/condition-categories.
- 9.Gorina Y, Kramarow EA: Identifying chronic conditions in Medicare claims data: Evaluating the Chronic Condition Data Warehouse algorithm. Health Serv Res 46:1610-1627, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taylor DH, Jr, Fillenbaum GG, Ezell ME: The accuracy of Medicare claims data in identifying Alzheimer’s disease. J Clin Epidemiol 55:929-937, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Eicheldinger C, Bonito A: More accurate racial and ethnic codes for Medicare administrative data. Health Care Financ Rev 29:27-42, 2008 [PMC free article] [PubMed] [Google Scholar]
- 12. Data Book: Beneficiaries Dually Eligible for Medicare and Medicaid—January 2016. Washington, DC, MedPAC and MACPAC, 2016. https://www.macpac.gov/wp-content/uploads/2015/01/Jan16_MedPAC_MACPAC_DualsDataBook.pdf.
- 13.Rosenbaum PR, Rubin DB: The central role of the propensity score in observational studies for causal effects. Biometrika 70:41-55, 1983 [Google Scholar]
- 14.Heinze G, Jüni P: An overview of the objectives of and the approaches to propensity score analyses. Eur Heart J 32:1704-1708, 2011 [DOI] [PubMed] [Google Scholar]
- 15.Winkelmayer WC, Kurth T: Propensity scores: Help or hype? Nephrol Dial Transplant 19:1671-1673, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Berry SD, Ngo L, Samelson EJ, et al. : Competing risk of death: An important consideration in studies of older adults. J Am Geriatr Soc 58:783-787, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Latouche A, Allignol A, Beyersmann J, et al. : A competing risks analysis should report results on all cause-specific hazards and cumulative incidence functions. J Clin Epidemiol 66:648-653, 2013 [DOI] [PubMed] [Google Scholar]
- 18.Madigan D, Ryan PB, Schuemie M, et al. : Evaluating the impact of database heterogeneity on observational study results. Am J Epidemiol 178:645-651, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. The Henry J. Kaiser Family Foundation: Medicare Advantage. http://kff.org/medicare/fact-sheet/medicare-advantage/
- 20. National Cancer Institute: Cancer stat facts: Prostate cancer. https://seer.cancer.gov/statfacts/html/prost.html.
- 21.Yaffe K, Falvey C, Harris TB, et al. : Effect of socioeconomic disparities on incidence of dementia among biracial older adults: Prospective study. BMJ 347:f7051, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. doi: 10.1001/archneur.1997.00550230066019. Evans DA, Hebert LE, Beckett LA, et al: Education and other measures of socioeconomic status and risk of incident Alzheimer’s disease in a defined population of older persons. Arch Neurol 54:1399-1405, 1997. [DOI] [PubMed] [Google Scholar]
- 23. doi: 10.4103/1008-682X.179528. Kao LT, Lin HC, Chung SD, et al: No increased risk of dementia in patients receiving androgen deprivation therapy for prostate cancer: A 5-year follow-up study. Asian J Androl 19:414-417, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khosrow-Khavar F, Rej S, Yin H, et al. : Androgen deprivation therapy and the risk of dementia in patients with prostate cancer. J Clin Oncol 35:201-207, 2017 [DOI] [PubMed] [Google Scholar]
- 25.Wiechno PJ, Sadowska M, Kalinowski T, et al. : Does pharmacological castration as adjuvant therapy for prostate cancer after radiotherapy affect anxiety and depression levels, cognitive functions, and quality of life? Psychooncology 22:346-351, 2013 [DOI] [PubMed] [Google Scholar]
- 26.Hershman DL, Unger JM, Wright JD, et al. : Adverse health events following intermittent and continuous androgen deprivation in patients with metastatic prostate cancer. JAMA Oncol 2:453-461, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chung SD, Lin HC, Tsai MC, et al. : Androgen deprivation therapy did not increase the risk of Alzheimer’s and Parkinson’s disease in patients with prostate cancer. Andrology 4:481-485, 2016 [DOI] [PubMed] [Google Scholar]
- 28.Shahinian VB, Kuo Y-F, Freeman JL, et al. : Risk of the “androgen deprivation syndrome” in men receiving androgen deprivation for prostate cancer. Arch Intern Med 166:465-471, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Finnell JT, Overhage JM, Grannis S: All health care is not local: An evaluation of the distribution of Emergency Department care delivered in Indiana. AMIA Annu Symp Proc 2011:409-416, 2011 [PMC free article] [PubMed] [Google Scholar]