Abstract
Purpose
Little is known about the patterns and predictors of the use of end-of-life health care among patients with acute myeloid leukemia (AML). End-of-life care is particularly relevant for older adults with AML because of their poor prognosis.
Methods
We performed a population-based, retrospective cohort study of patients with AML who were ≥ 66 years of age at diagnosis and diagnosed during the period from 1999 to 2011 and died before December 31, 2012. Medicare claims were used to assess patterns of hospice care and use of aggressive treatment. Predictors of these end points were evaluated using multivariable logistic regression analyses.
Results
In the overall cohort (N = 13,156), hospice care after AML diagnosis increased from 31.3% in 1999 to 56.4% in 2012, but the increase was primarily driven by late hospice enrollment that occurred in the last 7 days of life. Among the 5,847 patients who enrolled in hospice, 47.4% and 28.8% started their first hospice enrollment in the last 7 and 3 days of life, respectively. Among patients who transferred in and out of hospice care, 62% received transfusions outside hospice. Additionally, the use of chemotherapy within the last 14 days of life increased from 7.7% in 1999 to 18.8% in 2012. Patients who were male and nonwhite were less likely to enroll in hospice and more likely to receive chemotherapy or be admitted to intensive care units at the end of life. Conversely, older patients were less likely to receive chemotherapy or have intensive care unit admission at the end of life, and were more likely to enroll in hospice.
Conclusion
End-of-life care for older patients with AML is suboptimal. Additional research is warranted to identify reasons for their low use of hospice services and strategies to enhance end-of-life care for these patients.
INTRODUCTION
The prognosis of older patients with acute myeloid leukemia (AML) is poor and has remained unchanged over the past several decades.1 The median survival for patients with AML ≥ 65 years of age is approximately 2 months and worsens with advancing age to as low as 1 month for patients ≥ 85 years of age.2-5 Hence, end-of-life care is particularly relevant for this patient population.
Adequate use of hospice care and cautious use of intensive treatment have been proposed and well established as core measures for quality of end-of-life care among patients with cancer.6-8 Although these measures are accepted by most hematologists,9 patients with hematologic malignancies often underuse hospice and receive more end-of-life intensive care.10-15 Patients with hematologic malignancies face special challenges in choosing end-of-life care, such as the need for transfusion among patients with AML, which typically is not provided in the hospice setting.16
Fragmented end-of-life care may impose an additional burden on patients and their families. For instance, transitions in and out of hospice care are not a rare phenomenon with 8.8% of hospice users in Medicare disenrolled in the last 6 months of life,17 and 6.6% of hospice users having more than one transition in care after hospice enrollment.18 Of hospice users with transitions, 53.4% were admitted to hospitals.18 However, little is known about hospice transitions in patients with AML.
El-Jawahri et al12 recently published an intriguing study assessing health care use among older patients with AML treated at two tertiary cancer hospitals. They found that 23.1% of patients were admitted to hospice and only 11.3% stayed in hospice for > 7 days.12 In contrast, 84.5% of patients were hospitalized within 30 days of death and 61% died in the hospital.12 These findings inspired us to evaluate the patterns of end-of-life care in a larger, population-based cohort of patients with AML, because hematologic oncologists at tertiary care centers may differ substantially from providers in community settings in their practice of end-of-life discussions with patients who have hematologic malignanices.19 In addition, by constructing a retrospective cohort of > 13,000 older patients with AML nationwide, we extended the current literature by comprehensively assessing the patterns, trends, and factors associated with their end-of-life health care use, specifically hospice enrollment and use of aggressive treatments.
METHODS
Data Source
We used the Surveillance, Epidemiology, and End Results (SEER) Medicare linked database to assemble a population-based cohort of older adults with AML. The SEER registries account for approximately 28% of the US population.20,21 The SEER-Medicare database links patient-level information on incident cancer diagnoses reported to the SEER registries with a master file of Medicare enrollment and claims for inpatient, outpatient, and physician services.22 The Yale human investigation committee determined this study did not directly involve human subjects.
Study Population
Patients included in our study fulfilled the following eligibility criteria: diagnosed with AML between 1999 and 2011 at ≥ 66 years of age; had known month of diagnosis; was not reported from autopsy or death certificate only; died before December 31, 2012; had continuous Medicare fee-for-service coverage (parts A and B) and were not enrolled in health maintenance organizations from 12 months before diagnosis through death; and had information on type of residential area (urban/rural) and census tract.
Outcomes Measures
We focused on three well-established quality measures of end-of-life care in oncology that can be assessed through claims: hospice enrollment, intensive care unit (ICU) admission, and chemotherapy administration. Hospice enrollment was considered an indicator of appropriate end-of-life care, whereas ICU admission and chemotherapy were indicators of potentially aggressive end-of-life care. For this analysis, we defined late hospice enrollment as one that occurred within the last 7 days of life and aggressive treatment as ICU admission within the last 30 days of life, chemotherapy within the last 14 days of life, or both.
Covariates
We used Medicare claims to identify treatments for AML, including chemotherapy and transfusion. We examined patient characteristics that might influence hospice enrollment and end-of-life health care use, including age at death, sex, race, marital status, comorbidity, state buy-in status within 12 months before death (ie, state payment of part or all of the patient's Medicare Part B premium or the patient is in the Medicaid program), type of residential area (big metropolitan, metropolitan or urban, less urban or rural), SEER region (Northeast, Midwest, South, or West), median household income at the census-tract level (in quintiles), and year of death ( 1999 to 2003, 2004 to 2007, or 2008 to 2012). We used inpatient, outpatient, and carrier claims within 12 months before death to calculate a modified Elixhauser comorbidity score.23,24 Census-tract level median household income and state buy-in status were used as proxies for neighborhood- and individual-level socioeconomic status, respectively.
Statistical Analysis
Frequencies and percentages were used to describe health care use at the end of life for all patients with AML. Pearson’s χ2 tests were used to compare patterns of use between patients with different characteristics. Locally weighted scatterplot smoothing regression was used to evaluate the prevalence of hospice enrollment and end-of-life aggressive treatment by year. The Cochran-Armitage test was used to test for trend over time. Multivariable logistic regression models were used to identify factors associated with hospice enrollment and use of aggressive end-of-life treatment. In addition, among patients who enrolled in hospice, we assessed factors associated with late enrollment. All significance tests were two sided with an α-level of .05. All analyses were conducted using SAS (version 9.4; SAS Institute, Cary, NC).
RESULTS
Sample Characteristics
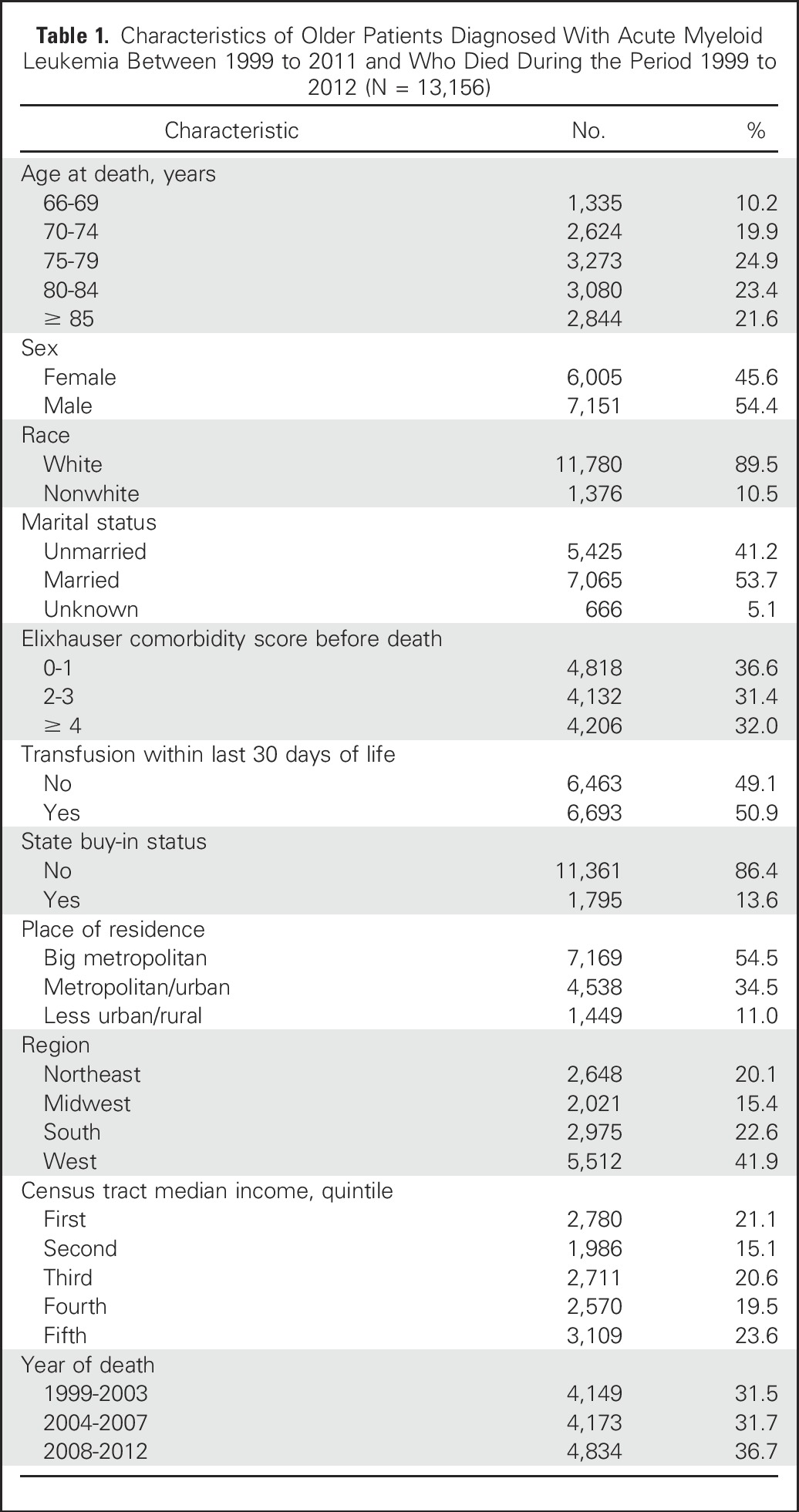
Our study population included 13,156 patients with AML. Most were white, and more than half were male, married, and resided in big metropolitan areas (Table 1). Median survival was 2.4 months (interquartile range, 1.12-7.16 months). Among the 13,156 patients, 5,847 (44.4%) enrolled in hospice and 5,816 (44.2%) received chemotherapy after their AML diagnosis. A total of 5,662 patients (43.0%) died in hospital and 5,322 (40.5%) died in hospice care.
Table 1.
Characteristics of Older Patients Diagnosed With Acute Myeloid Leukemia Between 1999 to 2011 and Who Died During the Period 1999 to 2012 (N = 13,156)
Hospice Enrollment
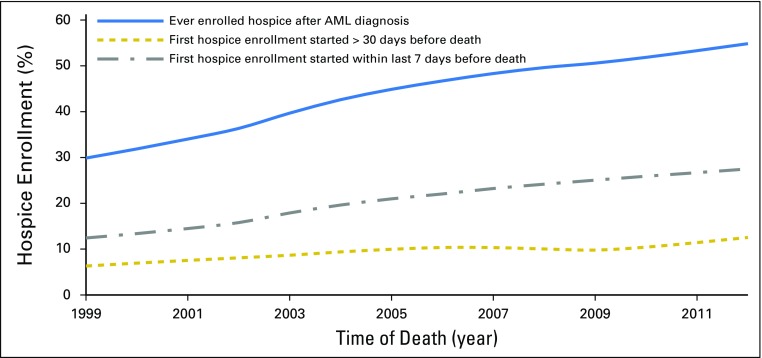
Among 5,847 patients who enrolled in hospice at any point between AML diagnosis and death, 47.3% and 28.8% started their first hospice enrollment in the last 7 and 3 days of life, respectively. Although the percentage of patients receiving hospice care increased consistently from 31.3% in 1999 to 56.4% in 2012 (P for trend < .01), the increase was mainly due to enrollment that began within the last 7 days before death (Fig 1). Compared with patients who died within 30 days after diagnosis, patients with longer survival were more likely to enroll in hospice (48.1% v 30.7%; P < .01). Among those enrolled in hospice, nearly half (51.2%) of patients who died within 30 days started their first hospice enrollment in the last 3 days of life; in contrast, the percentage among those survived longer was 24.9%.
Fig 1.
Hospice care from 1999 to 2012 at the end of life in older patients with acute myeloid leukemia (AML).
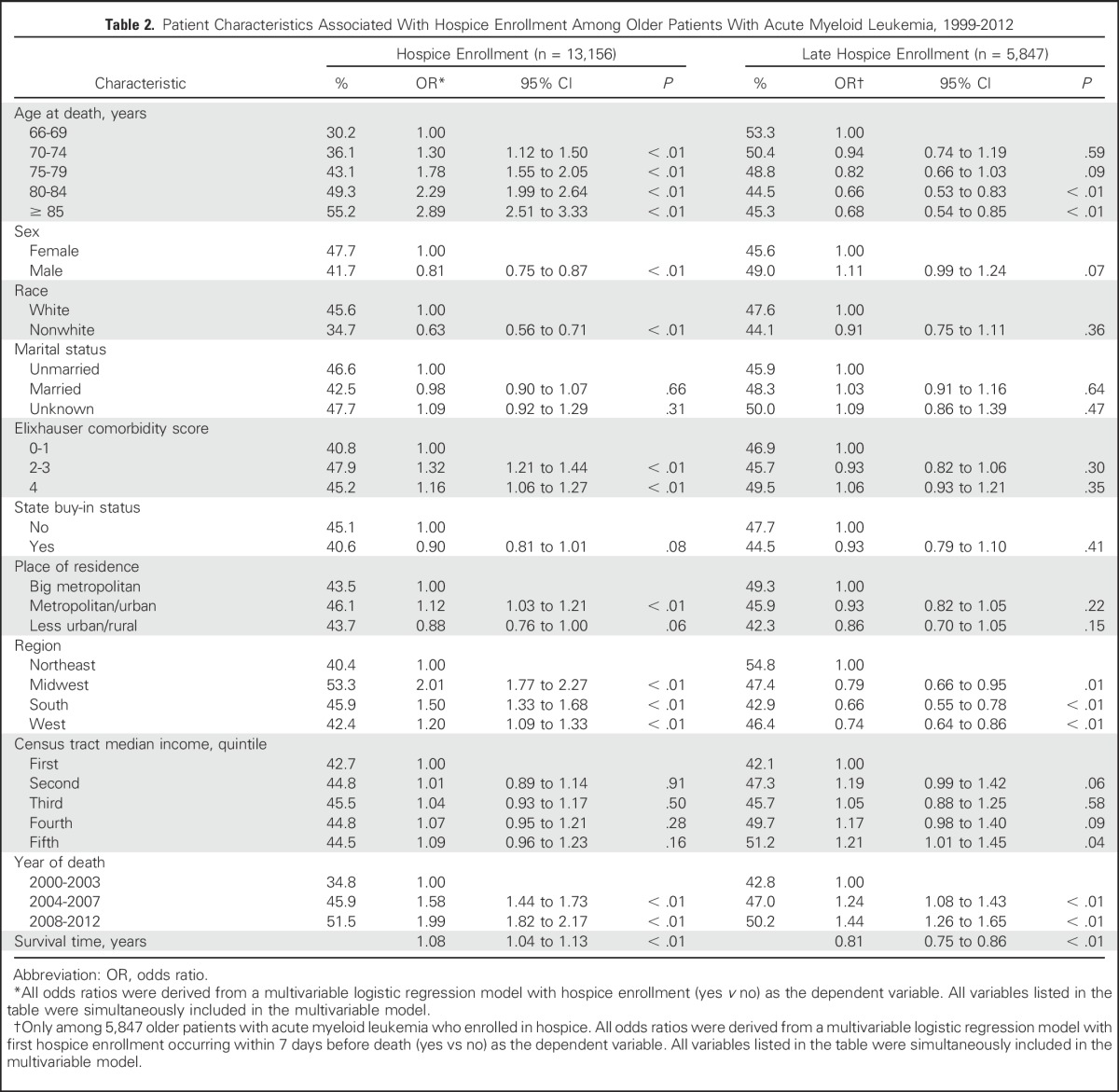
Patients who were older, female, white, had more comorbidities, resided in regions other than the Northeast or metropolitan/urban areas, died in more recent years, or had longer survival, were more likely to enroll in hospice (P < .01 for all; Table 2). Among patients who enrolled in hospice, we further assessed factors that might be related to late hospice enrollment (ie, in the last 7 days before death). Patients who were older, resided in regions other than the Northeast, or survived longer were less likely to have late enrollment, whereas patients who died in more recent years were more likely to have late enrollment (P < .05 for all; Table 2).
Table 2.
Patient Characteristics Associated With Hospice Enrollment Among Older Patients With Acute Myeloid Leukemia, 1999-2012
A total of 341 patients were discharged from hospice before death; 89 patients of these were last discharged before the last 30 days of life. Additionally, 199 patients had more than one hospice claim, representing transition in and out of hospice enrollment (range, 1 to 5) after AML diagnosis. When patients were in gaps between hospice enrollments, a higher proportion of them received transfusion than chemotherapy (Table 3).
Table 3.
Patients’ Receipt of Transfusion or Chemotherapy Outside Hospice in the Last 30 Days of Life
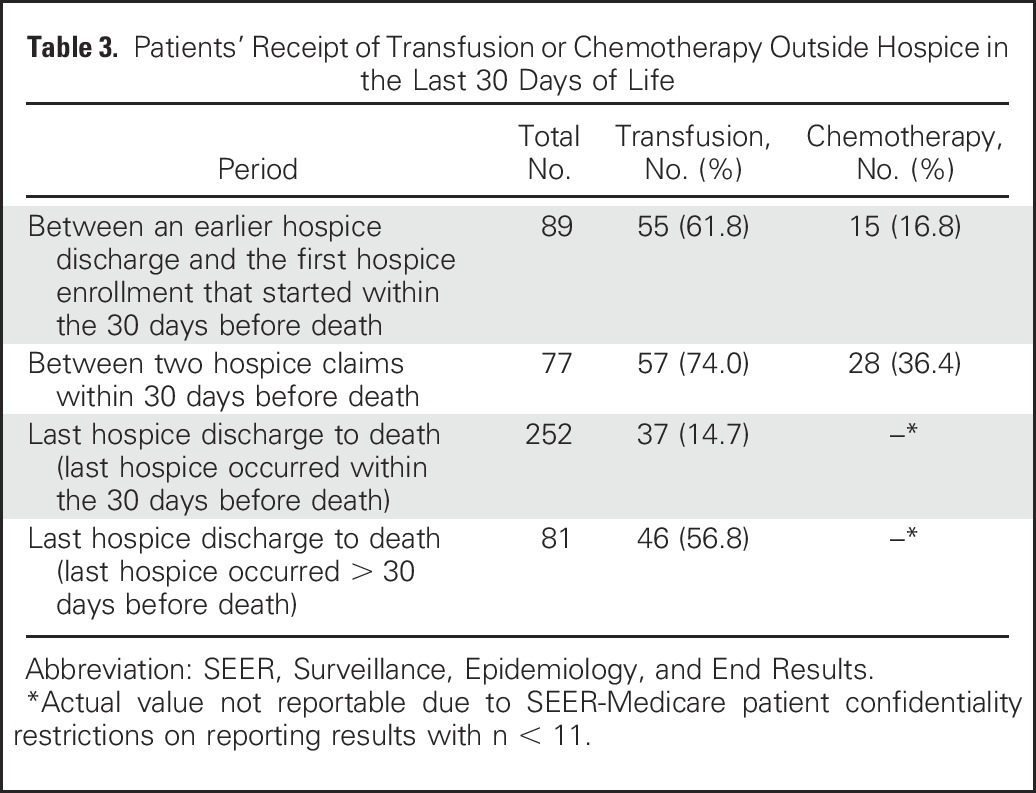
In a sensitivity analysis, we removed patients who survived < 1 month after diagnosis, reducing our sample size from 13,156 to 10,376 and increasing the observed survival from 2.4 to 3.7 months. In this subcohort, over the period of 1999 to 2012, the percentage of patients with hospice enrollment increased from 44.4% to 48.1%; among patients who were enrolled in hospice, the percentage of late enrollment decreased from 47.4% to 42.5%. We also evaluated the pattern of hospice use by chemotherapy status after AML diagnosis. Compared with patients with AML who had undergone some type of chemotherapy (n = 5,475), those who had never received chemotherapy (n = 4,901) were more likely to receive hospice care (56.1% v 41.0%; P < .01) and start hospice care > 30 days before death (16.2% v 7.8%; P < .01).
Chemotherapy at the End of Life
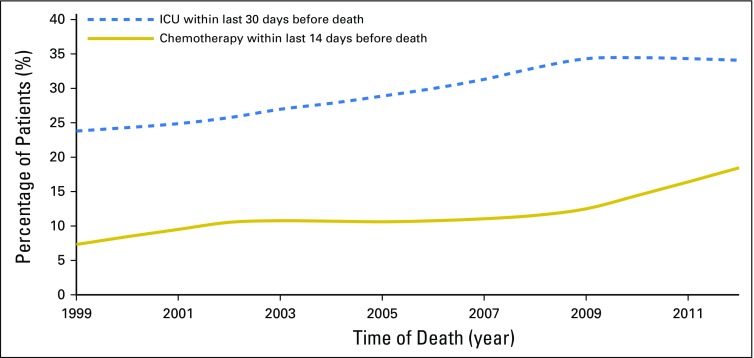
Overall, 1,528 (11.6%) patients underwent chemotherapy within 14 days before death; the percentage increased from 7.7% in 1999 to 18.8% in 2012 (P for trend < .01; Fig 2). Compared with patients who did not receive chemotherapy within 14 days before death, those who did were more likely to have ICU care in the last 30 days of life (43.0% v 28.4%; P < .01) but less likely to enroll in hospice (22.1% v 47.4%; P < .01).
Fig 2.
Intensive care unit (ICU) admission and chemotherapy from 1999 to 2012 at the end of life in older patients with acute myeloid leukemia from 1999 to 2012.
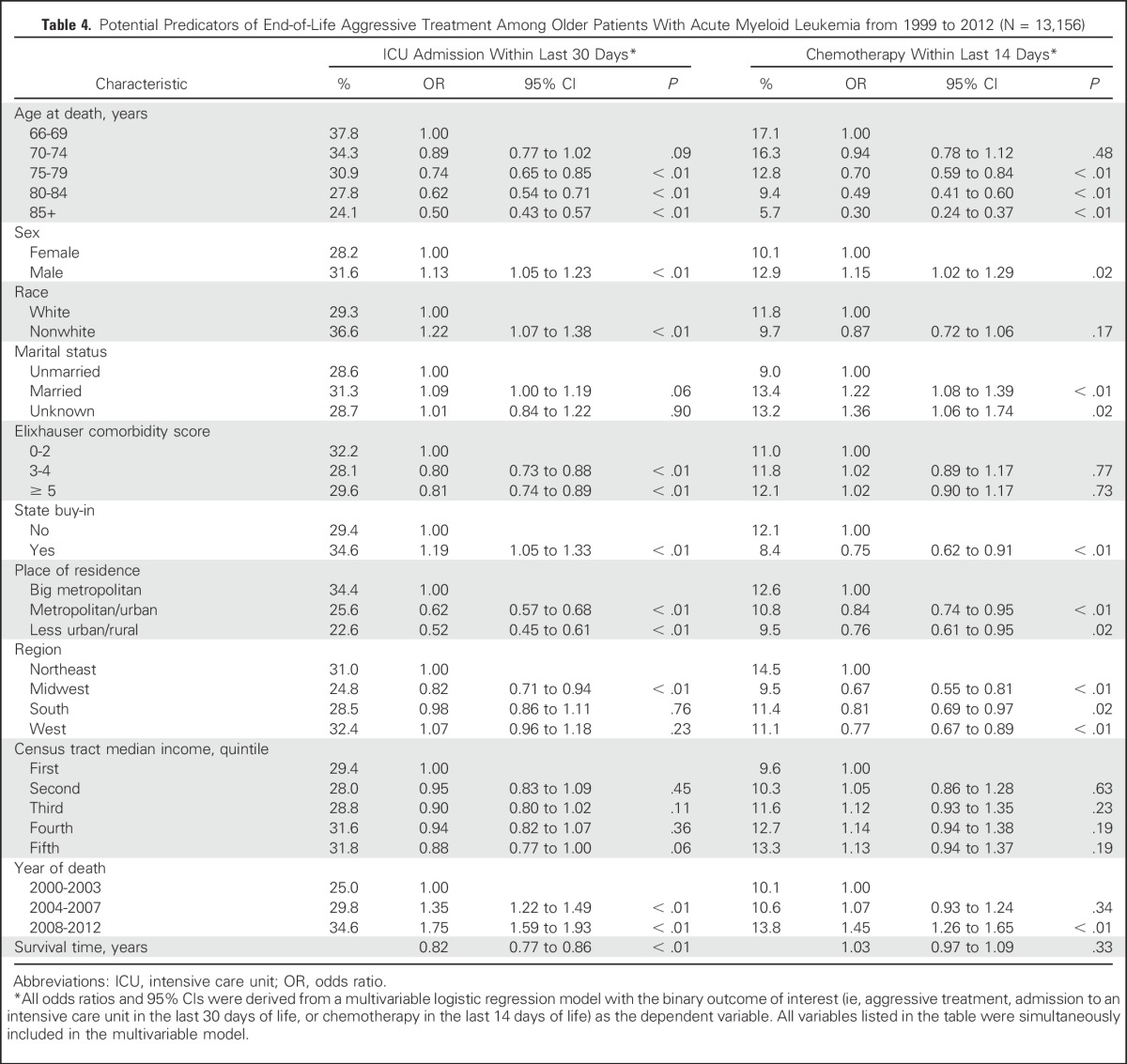
In multivariable analyses, patients who were male, married, or died in more recent years were more likely to receive chemotherapy in the last 14 days of life (Table 4). However, patients less likely to receive chemotherapy at the end of life were older, had state Medicaid buy-in, or did not reside in the Northeast or a big metropolitan area.
Table 4.
Potential Predicators of End-of-Life Aggressive Treatment Among Older Patients With Acute Myeloid Leukemia from 1999 to 2012 (N = 13,156)
ICU Admission at the End of Life
A total of 3,956 patients (30.1%) were admitted to the ICU within 30 days before death, and the percentage increased from 25.2% in 1999 to 31.3% in 2012 (P for < .01; Fig 2). Patients who were admitted to the ICU within 30 days before death were less likely to enroll in hospice than those who were not (26.7% v 52.1%; P < .01). Factors associated with ICU admission were similar to those associated with the receipt of chemotherapy (Table 4), except that patients with state Medicaid buy-in had a higher chance of ICU admission within the last 30 days of life (odds ratio, 1.19; 95% CI, 1.05 to 1.33; P < .01). Additionally, compared with their white counterparts, nonwhite patients were more likely to have an ICU admission (odds ratio, 1.22; 95% CI, 1.07 to 1.38; P < .01).
DISCUSSION
In this large, population-based study examining the end-of-life health care use of older patients with AML, fewer than half of patients were enrolled in hospice and, among these patients, approximately half were enrolled in the last 7 days of life. Although the proportion of patients with hospice enrollment increased continuously between 1999 and 2012, most of the increase was driven by enrollment in the last 7 days before death. During the same period, ICU admission in the last 30 days of life and chemotherapy in the last 14 days of life steadily increased.
Although the rate of hospice use we observed (44.4%) was higher than a previous report (23.2%),12 it is considerably lower than that reported for patients with cancer enrolled in Medicare in general (54.6% for 2003 to 2007 and 61.3% for 2010).25 In our study, male and nonwhite patients were less likely to enroll in hospice, which is consistent with the recognized sex and racial disparities in end-of-life care.26-32 The sex and racial disparities in hospice use may be addressable through physician and patient education, and by improving the provision of culturally sensitive end-of-life care.
Previous studies have also reported that patients with hematologic malignancies have a high rate of late hospice enrollment.12,13,33 At two tertiary-care hospitals only 11.3% of patients with AML enrolled in hospice for > 7 days.12 In a hospice network, 40.3% of patients with leukemia used hospice for < 7 days.13 Patients with hematologic malignancies were more likely to be admitted to hospice in the last 3 days of life than those with solid tumors.34 The high rate of late hospice enrollment may be related to the overall short survival of patients with AML. A sizable proportion of older patients with AML may have an abrupt decline close to the end of life, making it difficult to identify a natural transition to the end-of-life phase35 and hampering timely referral to hospice.11
One medical complication that may not be optimally treated under current hospice models is cytopenia. Transfusion is an important part of supportive care for patients with AML,36 whether or not a patient is at the end of life. However, a national survey of 591 hospices revealed that 40% of hospices did not admit patients with transfusion needs.16 Although many hematologic oncologists acknowledged the importance of hospice care, the lack of availability of transfusions in the hospice setting is an important concern for hospice referral.35 We observed that some patients with AML disenrolled from hospice and then received treatment outside of hospice, consisting most often of transfusion support rather than chemotherapy. Taken together, the transfusion needs of patients with AML may constitute a barrier to timely hospice enrollment and prompt hospice disenrollment. Alternative policies allowing the provision of transfusion in hospice care may better meet the specific needs of older patients with AML, and possibly other patients who may benefit from transfusion near the end of their life. Under current circumstance, physicians likely consider a patient’s need of transfusion when discussing or recommending hospice enrollment.
Our findings suggest that hospice is not being used optimally to provide end-of-life care for older patients with AML. Unrealistic expectations of patients, families, and physicians have been cited as factors contributing to the underuse of hospice in patients with AML,9 but the current hospice model itself may not be well suited to handle the rapid clinical decline and medical complications commonly experienced by older patients with AML approaching the end of life. The increased overall use of hospice with concomitant increase in the proportion admitted within 7 days of death raises the question of whether patients are simply being admitted to hospice to manage death rather than obtaining the benefits of symptom management and palliative support that hospice can provide. Further studies are needed to evaluate the effect of fragmented end-of-life care and develop specific end-of-life quality metrics for patients with hematologic malignancies (eg, metrics related to transfusion or bone marrow assessment).
Between 1999 and 2012, the percentage of patients receiving chemotherapy in the last 14 days of life almost doubled in our study. Other investigators have also observed a high rate of aggressive treatment at the end-of-life in patients with hematologic malignancies.10,12,26,37 The increased use of potentially aggressive treatments, especially chemotherapy, may be partly attributable to the introduction of less-toxic treatments such as the hypomethylating agents azacitidine and decitabine, which are often used off label to treat older patients with AML.38 For inpatient care, SEER-Medicare provides information on whether chemotherapy was administered but does not distinguish chemotherapy given for palliative purposes, which limits our conclusions as to the appropriateness of chemotherapy.
Some patients are willing to receive treatments with much more toxicity for a smaller benefit than will their providers.39 Even among older adults with AML, a fraction of patients who receive intensive therapy may have favorable long-term outcomes.5 This allure of being an outlier in terms of prolonged response may influence patient and provider decisions about therapies right to the end of life. Moreover, there are no clear stopping rules for anticancer treatment.40 Because novel therapies increasingly offer durable clinical responses in a small proportion of patients, improved predictive models and better communication strategies are needed to ensure patients understand the risks and benefits of a given therapy and that end-of-life care remains aligned with the patient’s goals and preferences.
As a claim-based retrospective study, our study has several limitations. First, the cohort only included older patients with AML with Medicare coverage. Thus, our results may not be generalizable to all patients with AML. Second, we measured health care use and treatment on the basis of Medicare claims and did not have information regarding patient preferences or physician recommendations. Third, the diagnostic codes in Medicare claims are not as reliable as the other codes used to identify procedures, tests, therapies, or hospice enrollment, so the reliance on diagnostic codes for the construction of comorbidity as a covariate is a potential limitation. Fourth, we were unable to determine appropriateness of care at the individual level. For example, for a patient who received chemotherapy in the last 14 days of life, we could not tell whether it was used with a palliative intent or for active treatment. Last, we retrospectively assessed the care received by patients from death (ie, starting from death and looking backward in time) and considered a late referral to hospice and/or aggressive treatments shortly before death suboptimal end-of-life care. This approach was subject to attribution bias41,42 because older patients with AML may frequently experience death from early complications of treatment, and intensive treatments may fail to achieve the intended remission. It is easy to assess the appropriateness of care afterward, but much more difficult to do so prospectively.
Our study also has notable strengths. It is the first, to our knowledge, to examine end-of-life health care use of older patients with AML in a large population-based cohort. This allowed us to assess secular trends in hospice enrollment and end-of-life treatment over a 14-year period. In addition, drawing on the longitudinal nature of the linked SEER-Medicare database, we measured health care use for patients starting from their date of AML diagnosis through the date of death. The comprehensive data available also allowed us to adjust for many other factors known or suspected to influence end-of-life health care use in patients with cancer, improving the validity of our findings.
In conclusion, we found that the current end-of-life care for older patients with AML is suboptimal, as reflected by low hospice enrollment and high use of potentially aggressive treatment. Transfer in and out of hospice was associated with the receipt of transfusions. Changes to current hospice services, such as enabling the provision of transfusion support, and improvements in physician-patient communications, may help facilitate better end-of-life care in this patient population.
ACKNOWLEDGMENT
The authors acknowledge the efforts of the Applied Research Program, National Cancer Institute; the Office of Research, Development and Information, Centers for Medicare and Medicaid Services; Information Management Services; and the SEER Program tumor registries in the creation of the SEER-Medicare database. The interpretation and reporting of the SEER-Medicare data are the sole responsibility of the authors.
Footnotes
Funded by Grant No. R03 CA173810 from the National Cancer Institute. The collection of the California cancer incidence data used in this study was supported by the California Department of Public Health as part of the statewide cancer reporting program mandated by California Health and Safety Code Section 103885; the National Cancer Institute’s SEER Program under contract N01-PC-35136 awarded to the Northern California Cancer Center, contract N01-PC-35139 awarded to the University of Southern California, and contract N02-PC-15105 awarded to the Public Health Institute; and the Centers for Disease Control and Prevention's National Program of Cancer Registries, under agreement #U55/CCR921930-02 awarded to the Public Health Institute.
The ideas and opinions expressed herein are those of the author(s) and endorsement by the State of California, Department of Public Health, the National Cancer Institute, and the Centers for Disease Control and Prevention or their contractors and subcontractors is not intended nor should be inferred.
Listen to the podcast by Dr Abel at ascopubs.org/jco/podcasts
AUTHOR CONTRIBUTIONS
Conception and design: Rong Wang, Xiaomei Ma
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Health Care Use by Older Adults With Acute Myeloid Leukemia at the End of Life
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Rong Wang
No relationship to disclose
Amer M. Zeidan
Consulting or Advisory Role: Celgene, Pfizer, Abbvie, Incyte, Gilead Sciences
Speakers' Bureau: Ariad
Stephanie Halene
No relationship to disclose
Xiao Xu
No relationship to disclose
Amy J. Davidoff
Honoraria: Celgene (I)
Consulting or Advisory Role: Celgene (I)
Research Funding: Celgene (I), Boehringer Ingelheim (I)
Travel, Accommodations, Expenses: Celgene (I)
Other Relationship: Pharmaceutical Research and Manufacturers Association Foundation
Scott F. Huntington
Honoraria: Pharmacyclics
Consulting or Advisory Role: Celgene, Janssen
Travel, Accommodations, Expenses: Celgene
Nikolai A. Podoltsev
Consulting or Advisory Role: Alexion Pharmaceuticals, CTI
Research Funding: Boehringer Ingelheim (Inst), Astellas Pharma (Inst), Daiichi Sankyo (Inst), Sunesis Pharmaceuticals (Inst), Celator (Inst), Pfizer (Inst), Astex Pharmaceuticals (Inst)
Cary P. Gross
Research Funding: 21st Century Oncology, Johnson & Johnson, Pfizer
Steven D. Gore
Consulting or Advisory Role: Celgene, Boehringer Ingelheim, Kyowa Hakko Kirin, Pfizer, Seattle Genetics
Research Funding: Celgene, Boehringer Ingelheim
Xiaomei Ma
Consulting or Advisory Role: Celgene, Incyte
REFERENCES
- 1.Xie Y, Davies SM, Xiang Y, et al. : Trends in leukemia incidence and survival in the United States (1973-1998). Cancer 97:2229-2235, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Pulsoni A, Pagano L, Latagliata R, et al. : Survival of elderly patients with acute myeloid leukemia. Haematologica 89:296-302, 2004 [PubMed] [Google Scholar]
- 3.Lang K, Earle CC, Foster T, et al. : Trends in the treatment of acute myeloid leukaemia in the elderly. Drugs Aging 22:943-955, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Baz R, Rodriguez C, Fu AZ, et al. : Impact of remission induction chemotherapy on survival in older adults with acute myeloid leukemia. Cancer 110:1752-1759, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Oran B, Weisdorf DJ: Survival for older patients with acute myeloid leukemia: A population-based study. Haematologica 97:1916-1924, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Earle CC, Park ER, Lai B, et al. : Identifying potential indicators of the quality of end-of-life cancer care from administrative data. J Clin Oncol 21:1133-1138, 2003 [DOI] [PubMed] [Google Scholar]
- 7.National Quality Forum : National voluntary consensus standards: Palliative care and end-of-life care: A consensus report. https://www.qualityforum.org/Publications/2012/04/Palliative_Care_and_End-of-Life_Care%e2%80%94A_Consensus_Report.aspx
- 8.McNiff KK, Neuss MN, Jacobson JO, et al. : Measuring supportive care in medical oncology practice: Lessons learned from the quality oncology practice initiative. J Clin Oncol 26:3832-3837, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Odejide OO, Cronin AM, Condron NB, et al. : Barriers to quality end-of-life care for patients with blood cancers. J Clin Oncol 34:3126-3132, 2016 [DOI] [PubMed] [Google Scholar]
- 10.Hui D, Didwaniya N, Vidal M, et al. : Quality of end-of-life care in patients with hematologic malignancies: A retrospective cohort study. Cancer 120:1572-1578, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Howell DA, Shellens R, Roman E, et al. : Haematological malignancy: Are patients appropriately referred for specialist palliative and hospice care? A systematic review and meta-analysis of published data. Palliat Med 25:630-641, 2011 [DOI] [PubMed] [Google Scholar]
- 12.El-Jawahri AR, Abel GA, Steensma DP, et al. : Health care utilization and end-of-life care for older patients with acute myeloid leukemia. Cancer 121:2840-2848, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.LeBlanc TW, Abernethy AP, Casarett DJ: What is different about patients with hematologic malignancies? A retrospective cohort study of cancer patients referred to a hospice research network. J Pain Symptom Manage 49:505-512, 2015 [DOI] [PubMed] [Google Scholar]
- 14.LeBlanc TW, Smith JM, Currow DC: Symptom burden of haematological malignancies as death approaches in a community palliative care service: A retrospective cohort study of a consecutive case series. Lancet Haematol 2:e334-e338, 2015 [DOI] [PubMed] [Google Scholar]
- 15.Prigerson HG, Bao Y, Shah MA, et al. : Chemotherapy use, performance status, and quality of life at the end of life. JAMA Oncol 1:778-784, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aldridge Carlson MD, Barry CL, Cherlin EJ, et al. : Hospices’ enrollment policies may contribute to underuse of hospice care in the United States. Health Aff (Millwood) 31:2690-2698, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang SY, Aldridge MD, Gross CP, et al. : Geographic variation of hospice use patterns at the end of life. J Palliat Med 18:771-780, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang SY, Aldridge MD, Gross CP, et al. : Transitions between healthcare settings of hospice enrollees at the end of life. J Am Geriatr Soc 64:314-322, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Odejide OO, Cronin AM, Condron N, et al. : Timeliness of end-of-life discussions for blood cancers: A national survey of hematologic oncologists. JAMA Intern Med 176:263-265, 2016 [DOI] [PubMed] [Google Scholar]
- 20. doi: 10.1097/01.MLR.0000020942.47004.03. Warren JL, Klabunde CN, Schrag D, et al: Overview of the SEER-Medicare data: Content, research applications, and generalizability to the United States elderly population. Med Care 40:IV-3- 18, 2002 (8 Suppl) [DOI] [PubMed] [Google Scholar]
- 21. http://seer.cancer.gov/about/overview.html National Cancer Institute: Overview of the SEER program.
- 22.Potosky AL, Riley GF, Lubitz JD, et al. : Potential for cancer related health services research using a linked Medicare-tumor registry database. Med Care 31:732-748, 1993 [PubMed] [Google Scholar]
- 23.Elixhauser A, Steiner C, Harris DR, et al. : Comorbidity measures for use with administrative data. Med Care 36:8-27, 1998 [DOI] [PubMed] [Google Scholar]
- 24.Roberts KB, Soulos PR, Herrin J, et al. : The adoption of new adjuvant radiation therapy modalities among Medicare beneficiaries with breast cancer: Clinical correlates and cost implications. Int J Radiat Oncol Biol Phys 85:1186-1192, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.http://www.dartmouthatlas.org/downloads/reports/Cancer_brief_090413.pdf Goodman DC, Morden NE, Chang C, et al: Bronner KK, (eds). Trends in cancer care near the end of life. [Google Scholar]
- 26.Earle CC, Landrum MB, Souza JM, et al. : Aggressiveness of cancer care near the end of life: Is it a quality-of-care issue? J Clin Oncol 26:3860-3866, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haas JS, Earle CC, Orav JE, et al. : Lower use of hospice by cancer patients who live in minority versus white areas. J Gen Intern Med 22:396-399, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miesfeldt S, Murray K, Lucas L, et al. : Association of age, gender, and race with intensity of end-of-life care for Medicare beneficiaries with cancer. J Palliat Med 15:548-554, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hardy D, Chan W, Liu CC, et al. : Racial disparities in the use of hospice services according to geographic residence and socioeconomic status in an elderly cohort with nonsmall cell lung cancer. Cancer 117:1506-1515, 2011 [DOI] [PubMed] [Google Scholar]
- 30.Cohen LL: Racial/ethnic disparities in hospice care: A systematic review. J Palliat Med 11:763-768, 2008 [DOI] [PubMed] [Google Scholar]
- 31.Loggers ET, Maciejewski PK, Paulk E, et al. : Racial differences in predictors of intensive end-of-life care in patients with advanced cancer. J Clin Oncol 27:5559-5564, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barnato AE, Berhane Z, Weissfeld LA, et al. : Racial variation in end-of-life intensive care use: A race or hospital effect? Health Serv Res 41:2219-2237, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sexauer A, Cheng MJ, Knight L, et al. : Patterns of hospice use in patients dying from hematologic malignancies. J Palliat Med 17:195-199, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O’Connor NR, Hu R, Harris PS, et al. : Hospice admissions for cancer in the final days of life: Independent predictors and implications for quality measures. J Clin Oncol 32:3184-3189, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Odejide OO, Salas Coronado DY, Watts CD, et al. : End-of-life care for blood cancers: A series of focus groups with hematologic oncologists. J Oncol Pract 10:e396-e403, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cannas G, Thomas X: Supportive care in patients with acute leukaemia: Historical perspectives. Blood Transfus 13:205-220, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fletcher SA, Cronin AM, Zeidan AM, et al. : Intensity of end-of-life care for patients with myelodysplastic syndromes: Findings from a large national database. Cancer 122:1209-1215, 2016 [DOI] [PubMed] [Google Scholar]
- 38.Walter RB, Estey EH: Management of older or unfit patients with acute myeloid leukemia. Leukemia 29:770-775, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matsuyama R, Reddy S, Smith TJ: Why do patients choose chemotherapy near the end of life? A review of the perspective of those facing death from cancer. J Clin Oncol 24:3490-3496, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Clarke G, Johnston S, Corrie P, et al. : Withdrawal of anticancer therapy in advanced disease: A systematic literature review. BMC Cancer 15:892, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Earle CC, Ayanian JZ: Looking back from death: The value of retrospective studies of end-of-life care. J Clin Oncol 24:838-840, 2006 [DOI] [PubMed] [Google Scholar]
- 42.Bach PB, Schrag D, Begg CB: Resurrecting treatment histories of dead patients: A study design that should be laid to rest. JAMA 292:2765-2770, 2004 [DOI] [PubMed] [Google Scholar]