Abstract
Purpose
To determine whether the pattern of lung nodules in children with metastatic hepatoblastoma (HB) correlates with outcome.
Methods
Thirty-two patients with metastatic HB were enrolled on Children’s Oncology Group Protocol AHEP0731 and treated with vincristine and irinotecan (VI). Responders to VI received two additional cycles of VI intermixed with six cycles of cisplatin/fluorouracil/vincristine/doxorubicin (C5VD), and nonresponders received six cycles of C5VD alone. Patients were imaged after every two cycles and at the conclusion of therapy. All computed tomography scans and pathology reports were centrally reviewed, and information was collected regarding lung nodule number, size, laterality, timing of resolution, and pulmonary surgery.
Results
Among the 29 evaluable patients, only 31% met Response Evaluation Criteria in Solid Tumors (RECIST) for measurable metastatic disease. The presence of measurable disease by RECIST, the sum of nodule diameters greater than or equal to the cumulative cohort median size, bilateral disease, and ≥ 10 nodules were each associated with an increased risk for an event-free survival event (P = .48, P = .08, P = .065, P = .03, respectively), with nodule number meeting statistical significance. Ten patients underwent pulmonary resection/metastasectomy at various time points, the benefit of which could not be determined because of small patient numbers.
Conclusion
Children with metastatic HB have a poor prognosis. Overall tumor burden may be an important prognostic factor for these patients. Lesions that fail to meet RECIST size criteria (ie, those < 10 mm) at diagnosis may contain viable tumor, whereas residual lesions at the end of therapy may constitute eradicated tumor/scar tissue. Patients may benefit from risk stratification on the basis of the burden of lung metastatic disease at diagnosis.
INTRODUCTION
Hepatoblastoma (HB) is the most common pediatric liver malignancy.1,2 Prognosis is based on surgical resectability of the primary tumor, presence of metastatic disease, and tumor histology, but only one third of patients have resectable disease at diagnosis. Neoadjuvant chemotherapy that is primarily cisplatin and doxorubicin based has enhanced resectability and overall survival.3 Approximately 10% to 20% of patients present with lung metastases, and overall survival of these children has ranged between 25% and 50%.4,5 The Societé Internationale d’Oncologie Pediatrique-Epithelial Liver Tumor Study Group (SIOPEL) 4 trial recently demonstrated improved survival for these high-risk children.4-6 Although surgical resection of the primary tumor is the basis for curative therapy, the role and benefit of pulmonary nodule resection remains incompletely evaluated.7,8 The management of pulmonary metastases is particularly relevant for approximately 20% to 30% of patients in whom orthotopic liver transplantation (OLT) may be necessary to excise the primary tumor. Because extrahepatic disease is a contraindication to OLT, the resolution of pulmonary disease is required to render a patient a candidate for transplantation.9
Computed tomography (CT) scans are the primary modality for diagnosing and assessing the response of pulmonary metastases. However, the radiologic definition of lung involvement is sometimes challenging. Although the image resolution of CT is on the order of tenths of centimeters, CT cannot definitively distinguish malignant disease from scar tissue, vascular structures, inflammation, or infection and also cannot delineate whether residual radiographic abnormalities denote persistent viable disease.10,11 The Response Evaluation Criteria in Solid Tumors (RECIST) has been used as a guideline to define measurable disease in patients with solid tumors.12 By original RECIST definition, measurable lung lesions must have a longest diameter of ≥ 10 mm on CT scan. Therefore, many patients with newly diagnosed disease may have abnormal lesions on chest CT scan that are considered nonmeasurable metastatic disease. There have been no prospective studies that have specifically characterized lung metastases in pediatric patients with HB. Given the paucity of data and the limitations inherent to making a diagnosis on the basis of imaging alone, there are not currently evidence-based data on the optimal treatment of patients with pulmonary metastatic disease.
The Children’s Oncology Group (COG) Protocol for Treatment of Children with All Stages of Hepatoblastoma (AHEP0731) was designed for all patients with newly diagnosed HB and included a specific stratum for patients with metastatic disease.13 A detailed report on the treatment and outcome of patients with metastatic disease is published elsewhere. The objective of this paper is to report the study findings regarding the surgical management of pulmonary nodules, to review radiologic considerations for evaluating pulmonary metastatic disease, and to determine prognostic factors yielding an evidence-based approach for the treatment of these patients. We describe the relationship between lung nodule number, size, laterality (unilateral v bilateral), and total nodule burden, with chemotherapy response, pulmonary resection, and survival.
METHODS
Patients
AHEP0731 opened in September of 2009. Patients were stratified based on COG stage, tumor histology, initial alpha-fetoprotein (AFP) level, and the presence of metastases as determined by the institutional investigators. Patients were eligible for the high-risk stratum if they had either metastatic disease or a low diagnostic AFP level < 100 ng/mL, regardless of initial extent of disease. Additional eligibility criteria included age younger than 21 years, newly diagnosed biopsy-proven HB (except in emergent cases), and normal hematologic, hepatic, renal, and cardiac function. The study protocol was approved by the National Cancer Institute, the Pediatric Central Institutional Review Board, and the institutional review boards of participating institutions. Informed consent was obtained before the treatment of all patients.
Operative reports and central pathologic review of specimens acquired at any time during treatment were required. Central review of radiographic studies (CT scans, magnetic resonance imaging, and abdominal ultrasounds obtained at any time before tumor resection) was performed by the study radiologists (A.J.T., M.B.M.) after de-identification at the Imaging and Radiation Oncology Core (IROC-RI, formerly QARC). When appropriate, response was classified according to RECIST criteria.14 Pretreatment Extent of Disease (PRETEXT) staging was assessed by central review.15
Treatment
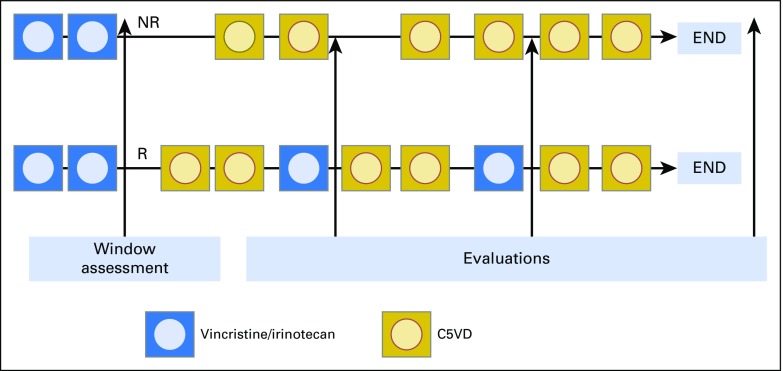
Patients with high-risk disease received two cycles of vincristine and irinotecan (VI) as up-front window therapy. Responders to VI were to receive two additional cycles of VI intermixed with six cycles of cisplatin/fluorouracil/vincristine/doxorubicin (C5VD), and nonresponders were to receive six cycles of C5VD alone (Appendix Fig A1, online only).
Patients could have their primary tumor removed whenever feasible, either by subtotal hepatectomy or via complete hepatectomy with OLT, optimally after cycle 7 in responders or after cycle 6 in nonresponders, to allow for postsurgical chemotherapy. Surgical approach to metastatic lesions was not mandated by the protocol and was at the discretion of the treating physicians. If the metastases disappeared with chemotherapy, no pulmonary surgical intervention was performed. Removal of presumed metastases was to be considered at any time to render a patient free of extrahepatic disease and a suitable OLT candidate.
Evaluation of Response
Overall response to therapy was assessed using RECIST criteria as well as decline of AFP (Appendix, online only). Patients who had complete resolution of all radiographic disease and a normal AFP at end of therapy and during follow-up without new disease were deemed to have achieved a complete remission.
For the purposes of this study, one study radiologist (A.J.T.) reviewed all chest CT scans. For each patient, the diameter of visualized lung nodules, of any size, were recorded to a maximum of 10 nodules per patient. For evaluation of these nodules, RECIST criteria were applied only when discriminating target lesions. Nodules of all sizes (up to a maximum of 10 and including those < 10 mm) were used to define disease burden and were tracked on serial images to assess response to therapy, reduction in size, and resolution. Detailed information was collected regarding total nodule number, individual and cumulative nodule diameters, laterality, timing of nodule resolution, and size of individual residual lesions at the end of therapy. Operative and pathology reports were evaluated for all patients undergoing nodule resection, and the timing of resection, number of nodules removed, and tumor viability for resected nodules were recorded.
Statistical Analysis
Event-free survival (EFS) was measured from the time of patient enrollment until the last follow-up or an analytic event was observed, whichever occurred first (Appendix).16 The effects of pulmonary resection on the risk of EFS event and death were estimated by time-dependent covariates methodology. A resection accomplished variable was calculated for every patient as 0 at the time of enrollment, and this was changed to 1 on the day the pulmonary surgical resection was accomplished and remained 1 throughout the patient’s subsequent follow-up. The relative hazard rate for pulmonary resection was estimated by partial likelihood, and the Wald test was used to obtain the P value for the hypothesis that pulmonary resection did not affect subsequent risk for EFS event.16
Hypotheses regarding the equality of risk of EFS event or death across groups defined by other patient characteristics determined at enrollment were assessed by means of the log-rank statistic.17 P values for all statistical tests are two sided, and P values ≤ .05 were considered statistically significant for this analysis.
RESULTS
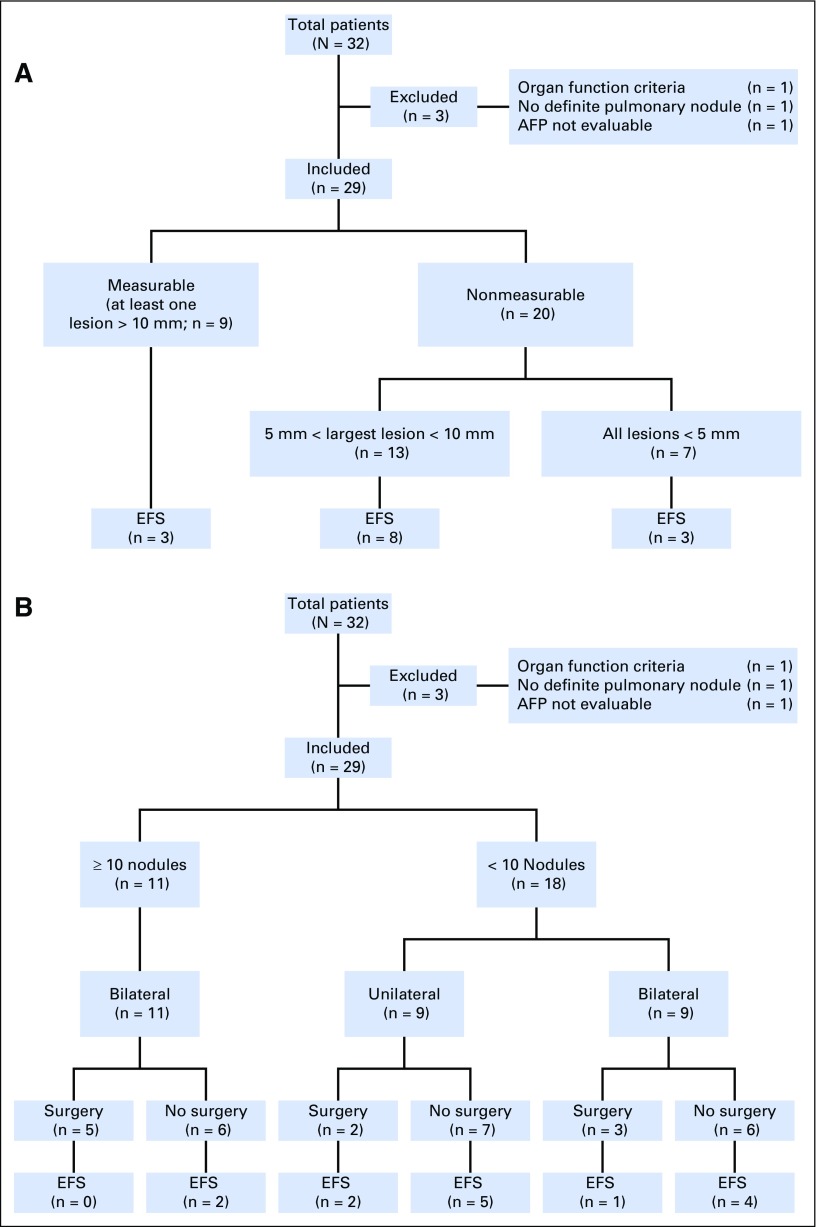
Thirty-two patients, all with metastatic disease, were enrolled in the high-risk arm of AHEP0731 between September 2009 and February 2012. Outcome current to June 30, 2014 was used in this analysis. Three of the 32 patients enrolled were excluded from this analysis: one patient was ineligible because of failure to meet organ function criteria, one patient was not evaluable because of a technical error in obtaining the initial AFP level, and one patient did not have confirmation of nodules on central review. The demographic and treatment data of the evaluation cohort can be found in Table 1.
Table 1.
Characteristics of Patients With High-Risk Metastatic Hepatoblastoma
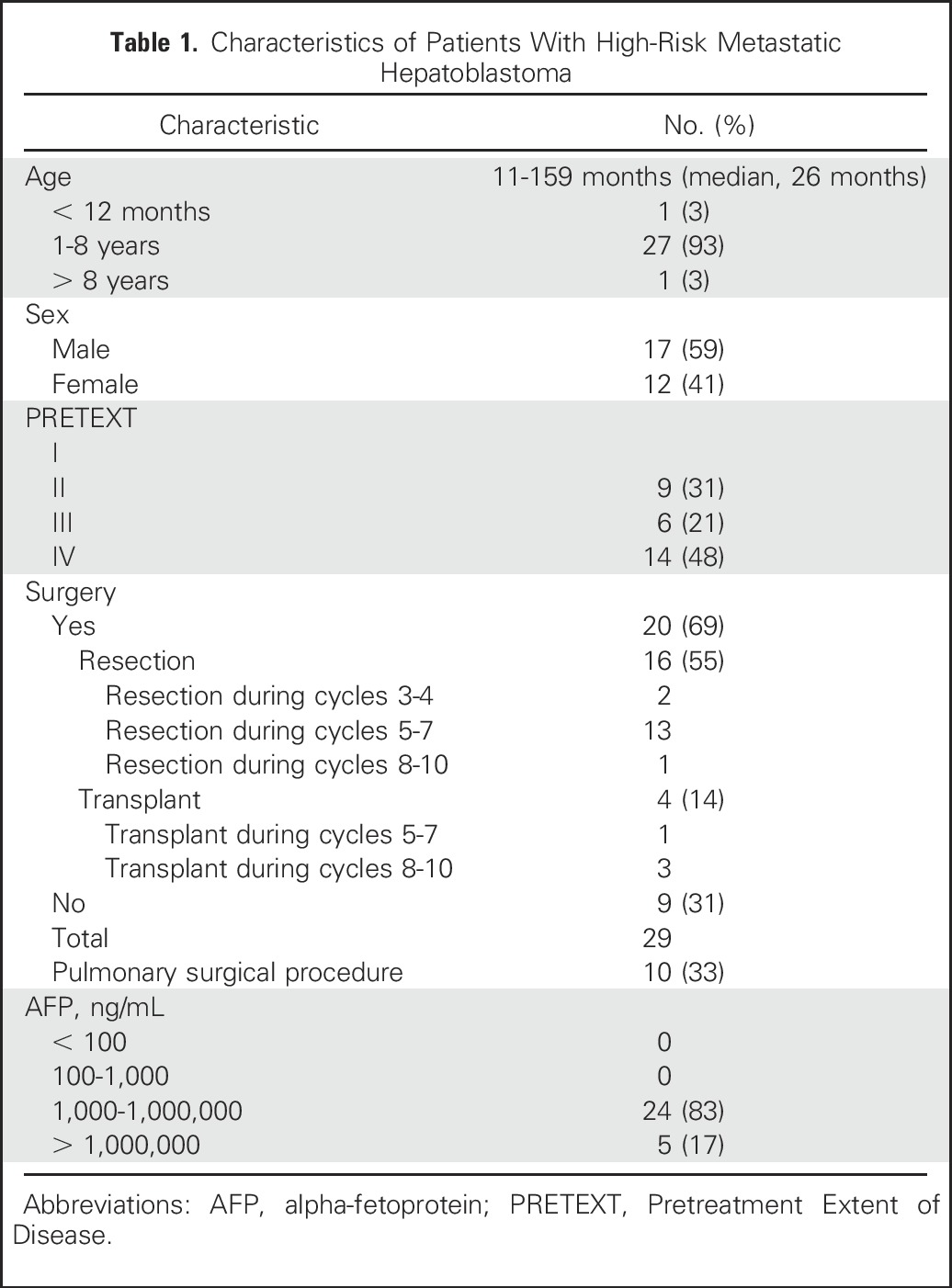
Only nine of the 29 patients (31%) had measurable pulmonary disease at diagnosis by RECIST criteria (nodules ≥ 10 mm), and seven (24%) had all nodules measuring < 5 mm. Accounting for nodules of any size, the mean and median number of nodules per patient was six and seven, respectively (range, 1 to 10). The cohort median nodule size, generated from the cumulative sum diameters of all nodules for each individual, was 22 mm (range, 2 to 209 mm). Twenty patients (69%) had bilateral disease and nine patients (31%) had unilateral disease (Table 2). There was no significant difference in the number of nodules, laterality, or total size when stratified by sex or age.
Table 2.
Characteristics of Pulmonary Nodules and Outcome
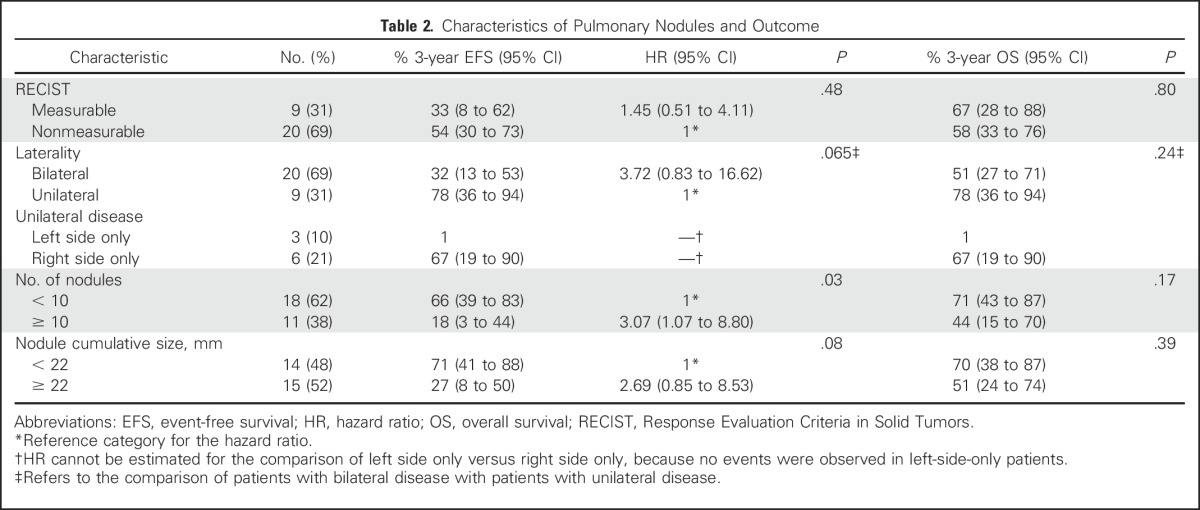
Response
Table 2 describes the differences in EFS and overall survival between patients with different clinical features. There was no statistically significant difference in risk for an event between patients who were initially diagnosed with measurable versus nonmeasurable metastatic disease (hazard ratio [HR], 1.45; P = .48), bilateral disease versus unilateral disease (HR, 3.72; P = .065), or cumulative size < 22 mm versus ≥ 22 mm (HR, 2.69; P = .08; Table 2; Fig 1A). However, patients with bilateral disease and cumulative nodule size greater than the cohort median sum of diameters had a nominally higher risk of event with HRs of 3.72 and 2.69, respectively (Table 2; Fig 1B). Patients with ≥ 10 nodules had significantly higher risk of event than those with < 10 nodules (HR, 3.07; P = .03).
Fig 1.
(A) Nodule size and event-free survival (EFS). (B) Nodule characteristics, pulmonary surgeries, and EFS.
Ten patients had complete radiographic clearance of all pulmonary metastases. Seven of these resolved with chemotherapy; five of these seven remain event free for a median time of 2.9 years (range, 2.4 to 4.2 years) after nodule resolution. The remaining three patients achieved clearance of pulmonary disease with both pulmonary resection/metastasectomy and chemotherapy, and all three remain disease free for a median of 3.6 years (range, 2.6 to 3.7 years) after achieving clearance. Eight of the 10 patients underwent primary tumor resection while on protocol therapy. One patient was removed from protocol therapy and received a liver transplant 2 weeks later. The final patient had resolution of pulmonary disease but local progression in the liver before resection was performed. There were five additional patients with residual radiographic pulmonary lesions ≤ 3 mm on end-of-therapy scans. Only one of these patients relapsed in the liver and died; the remaining four are alive without progression of pulmonary lesions for a median follow-up of 3.3 years (range, 2.7 to 3.8 years) after last assessment.
Outcome
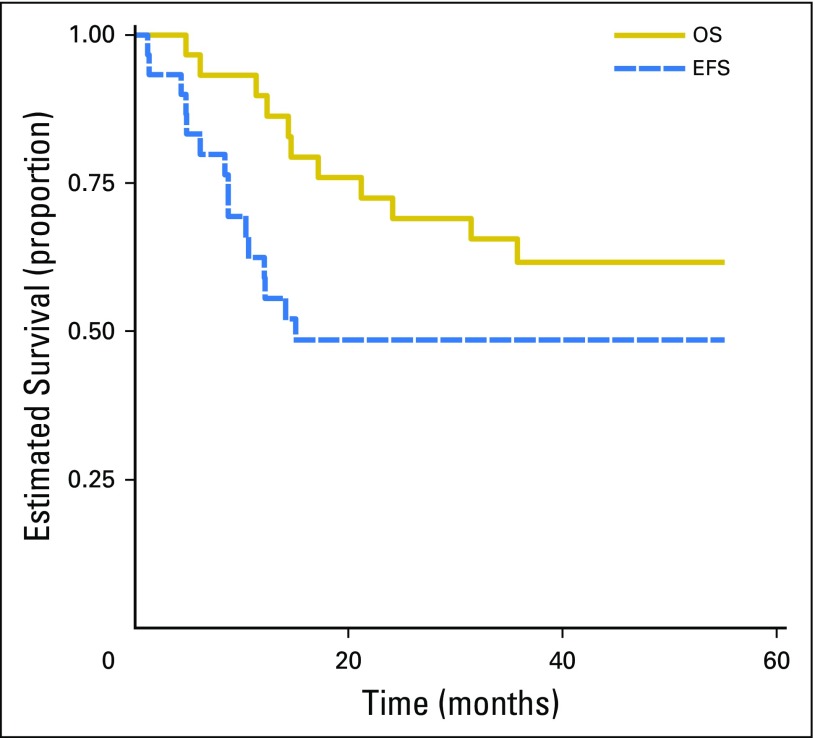
Three-year EFS was 49% (95% CI, 30% to 65%), and overall survival was 62% (95% CI, 42% to 77%; Fig 2). Fifteen patients are alive without progression for a median follow-up of 3.2 years (range, 0.5 to 4.6 years; Table 3). Thirteen patients had recurrent disease. Two patients died without relapse while receiving therapy: one patient had a cardiac arrest after a GI procedure and another died of post-resection complications. Nine patients did not have resection of the primary tumor while receiving protocol therapy. Five of these patients had progressive disease while receiving therapy: three were pulmonary, one combined pulmonary and liver, and one liver alone. Three patients were removed from protocol because of physician choice and still had pulmonary nodules at the time they were removed from protocol therapy, and one additional patient completed therapy but did not have surgery because of persistence of lung nodules.
Fig 2.
Event-free survival (EFS) and overall survival (OS) of patients with metastatic hepatoblastoma.
Table 3.
Event-Free Survival in Patients With High-Risk Hepatoblastoma (AHEP0731)
Pulmonary Resection/Metastasectomy
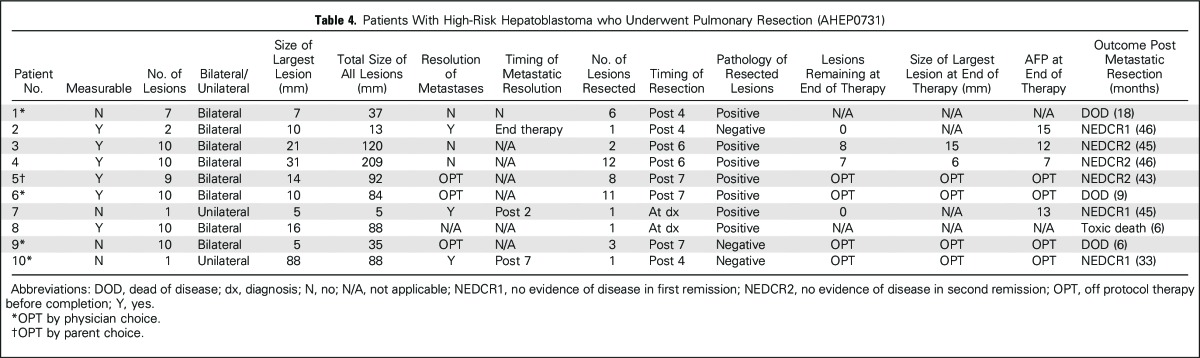
Ten of 29 patients (34%) had resection of pulmonary metastatic disease (Table 4). Of these, two surgeries were performed as diagnostic biopsies and eight occurred later in therapy. There were no reported complications related to pulmonary nodule resection. The median number of metastases removed in these patients was three (range, 1 to 12). Pulmonary resection/metastasectomy was not related significantly to subsequent risk for EFS event or death (P = .11, P = .67, respectively; Table 2). Five of the 11 patients with 10 documented nodules had a pulmonary surgery performed sometime during the course of treatment, and all recurred. Two of these patients remain alive, with follow-up of 45 and 46 months, with a normal AFP, having received no additional chemotherapy. Of the eight patients who had pulmonary resection after initiation of chemotherapy (ie, excluding those who underwent up-front diagnostic biopsy of the pulmonary lesions), pathology demonstrated the presence of viable tumor in five patients; five relapsed and three were alive with follow-up of 43, 45, and 46 months (Table 4). The number of patients is too small to determine the importance of viable tumor and the relationship to timing of resection.
Table 4.
Patients With High-Risk Hepatoblastoma who Underwent Pulmonary Resection (AHEP0731)
DISCUSSION
To our knowledge, this is the largest prospective report detailing characteristics and outcome of children with pulmonary metastatic HB and the first to report that the burden of pulmonary metastatic disease is an adverse prognostic factor. When taking into account nodules of all sizes, a significant increase in risk for an event was associated only with number of nodules (< 10 v ≥ 10). There were nonsignificant increases in risk associated with laterality (bilateral v unilateral) and total burden of metastatic pulmonary disease (< 22 mm v ≥ 22 mm). Although these findings may seem intuitive, they are critically important in assessing current treatment paradigms and considering new algorithms and clinical trial designs, because traditional RECIST criteria may not be the optimal method for evaluating disease response and predicting outcome in HB. Only a third of patients had measurable lesions by RECIST criteria, whereas seven patients had all pulmonary nodules < 5 mm. The potential benefit of pulmonary resection/metastasectomy could not be determined in our series because of small numbers, the variable timing of surgery, and the number of nodules removed. Therefore, in this disease where primary tumor resection is critical for survival, the effect of surgical resection of both limited and extensive pulmonary metastatic disease remains unknown and an important area to be further studied in future trials.
In our cohort, 3-year overall survival of 62% (median follow-up, 38 months) was better than previous COG studies but not as good as the 3-year 79% overall survival (median follow-up, 53 months) reported for metastatic disease in the recent SIOPEL 4 trial.3,6 In this trial, 19 of 39 patients with metastatic disease (49%) achieved a complete chemotherapy response of lung nodules, and 27 (69%) had resection of all lung lesions, with no detail provided on the number of lesions resected in each patient. The role of surgical resection/metastasectomy for HB may be most beneficial in two distinct populations of patients: those whose presenting lung disease fails to clear with chemotherapy, especially to facilitate OLT, and those who achieve a complete response and then develop a pulmonary relapse. Surgical lung resection was evaluated by Meyers et al8 in both of these cohorts of children treated on the COG protocol INT-0098. Of these 38 children, nine underwent pulmonary resection at varying points in therapy, and eight of these nine patients (89%) were long-term survivors. In that same study, there were 20 patients with pulmonary relapse after complete response. Thirteen of these 20 patients underwent pulmonary resection, but only four of these 13 (31%) were long-term survivors.8
There are several retrospective reports in the surgical literature exploring the role of pulmonary resection in other pediatric solid tumors. Many of these studies intuitively conclude that nodule resection is most beneficial across disease types for lesions that persist after chemotherapy and less so for patients with progressive disease.18-20 Some studies conclude that number of metastases, as shown here, and unilaterality are the most significant prognostic factors for survival.21,22 All studies demonstrate that pulmonary resection/metastasectomy is safe and can be performed without significant morbidity.20,21,23,24
Radiographic scans are limited in their ability to determine whether a nodule contains viable tumor. Three of eight patients who had nodule resection performed during protocol therapy had no viable tumor. In addition, four surviving patients had residual radiographic abnormalities at the end of therapy, with biopsy not performed. These findings suggest that treatment decisions cannot depend solely on the presence of radiographic lesions but must take into account total nodule burden, change in size over time, lesion stability, and serum AFP levels (Table 4), with the consideration that residual lesions of stable size may represent eradicated tumor or scar. Biopsy at end of therapy should be entertained for patients in whom serum AFP levels fail to normalize and/or nodules fail to change or diminish appreciably in size.
There is a comprehensive body of literature detailing the limitations inherent to imaging studies in their ability to detect small lesions, particularly lung metastases. For diseases in which thoracotomy and manual lung palpation are routine (ie, pediatric osteosarcoma), it has been demonstrated that the number of intraoperative lesions often far exceeds the number identified by CT scan.18,21 Although this may change over time with the evolution of CT technology, at present, CT is relied on heavily to determine the quantity and size of lung lesions at diagnosis, to assess disease response to therapy, to guide surgical intervention, and to assure retained remission after therapy.25,26 Although the definitive detection of metastases is clearly limited by technology, it remains to be seen whether deviating from RECIST criteria to consider lung lesions of smaller size (ie, ≤ 5 mm) may serve as a better reflection of disease burden, predict the impact of lung metastases on prognosis, and more appropriately guide therapy.
Ultimately, patients with bilateral lung disease and a significant lung tumor burden may be candidates for novel therapies or treatment strategies. The use of maintenance therapy with irinotecan has been described to have success in small numbers of patients.27 Radiotherapy for metastatic disease has been anecdotally reported but never formally studied.28 A more aggressive up-front surgical approach to resect nodules at diagnosis or intensification of therapy for patients unable to clear lung nodules with conventional chemotherapy agents could also be considered. Treatment decisions optimally should be made with some assessment of pathologic response of remaining nodules in conjunction with AFP decline, because persistent radiographic abnormalities may not indicate residual tumor.
Although the recent Children’s Hepatic Tumors International Collaboration (CHIC) publication confirmed metastatic disease to be a poor prognostic factor, it did not incorporate more details regarding pulmonary metastases.29 The upcoming Pediatric Hepatic Malignancy International Therapeutic Trial (PHITT) will allow an opportunity to determine the relevance of these factors prospectively and on a much larger scale. Future trials may help confirm the novel preliminary findings demonstrated here and should use a consistent approach toward lung metastases, with potential risk stratification on the basis of total nodule burden.
ACKNOWLEDGMENT
We thank Fran Laurie, Karina Rossi-Toole, Sandy Kessel, Richard Hanusik, Brandon Davis, and the rest of the staff of the Imaging and Radiation Oncology Core (IROC-RI, formerly QARC) for collection of scans, compilation of data, and facilitation of central review sessions. We also thank Catherine Shannon, Alejandra Miranda, Megan Stahlman, and Judy Everett of the Children’s Oncology Group Operation office for countless hours of support in maintaining the operation of this trial.
Appendix
Treatment
Each cycle of vincristine and irinotecan (VI) consisted of intravenous (IV) irinotecan (50 mg/m2/d or 1.67 mg/kg for patients < 10 kg, IV over 90 minutes, on days 1 to 5) and vincristine (1.5 mg/m2/dose or 0.05 mg/kg for patients < 10 kg, IV push, on days 2, 9, and 16). Patients were then evaluated for response, and those demonstrating at least a partial response to VI were to receive six cycles of cisplatin, fluorouracil, vincristine, and doxorubicin (C5VD) and two additional cycles of VI (inserted in between each two-cycle block of C5VD) for a total of 10 cycles. Those patients who did not respond to VI were to continue with six cycles of C5VD without additional VI. Each course of C5VD included cisplatin (100 mg/m2 or 3.33 mg/kg for patients < 10 kg, IV over 6 hours, on day 1), fluorouracil (600 mg/m2 or 20 mg/kg for patients < 10 kg, IV push, on day 2), vincristine as described above, and doxorubicin (30 mg/m2/d or 1 mg/kg/d for patients < 10 kg, IV over 15 minutes, on days 1 and 2). All patients received dexrazoxane (300 mg/m2 or 10 mg/kg for patients < 10 kg) IV immediately before doxorubicin during the last two cycles of C5VD. All cycles lasted 21 days.
Evaluation of Response
Baseline physical examinations, organ function, alpha-fetoprotein (AFP) levels, and imaging studies, including a computed tomography scan of the chest, were performed before therapy. Additional imaging studies were performed after two cycles of VI and then after every two cycles of C5VD. Assessment of response for study treatment was determined by the treating site. Evaluation of response for the purposes of assessing the efficacy of VI therapy after the first two cycles was based on central imaging review and change in serum AFP level from the time of study enrollment to the completion of two cycles of VI. Overall response to therapy was assessed using Response Evaluation Criteria in Solid Tumors (RECIST) criteria as well as the decline of AFP.
Measurable disease was considered using RECIST to be the presence of at least one target lesion, defined as a lesion having a longest diameter of at least 10 mm on spiral computed tomography scan. A complete response was considered to be the disappearance of all target lesions and a normal AFP level (by institutional standards). A partial response was considered as either at least a 30% decrease in the sum of diameters of target lesions compared with baseline or a decline in serum AFP level of ≥ 90% (1 log10) after two VI cycles, provided the patient did not experience progressive disease during the first two cycles of therapy.
Statistical Analysis
Analytic events for this cohort were progression of disease or occurrence of disease at new sites; treatment failure, defined as the presence of disease after planned chemotherapy; death from any cause before disease progression; or diagnosis of a second malignancy. A patient who did not experience an event before the date of analysis for the trial was considered censored at last patient contact. Overall survival was defined from the time of enrollment until death from any cause or last follow-up, whichever came first. A patient who did not die before the date of analysis for the trial was considered censored at last patient contact.
Fig A1.
Treatment schema. C5VD, cisplatin/fluorouracil/vincristine/doxorubicin; NR, nonresponders; R, responders.
Footnotes
Supported by the Chair’s Grant No. U10 CA98543, the National Clinical Trials Network (NCTN) Operations Center Grant No. U10 CA180886, the NCTN Statistics and Data Center Grant No. U10 CA180899, the Statistics and Data Center Grant No. U10 CA98413 of the Children’s Oncology Group, the Imaging and Radiation Oncology Core (IROC-RI, formerly QARC) Grant No. U10 CA29511 from the National Cancer Institute, and the St Baldrick’s Foundation. A complete listing of grant support for research conducted by Children’s Cancer Group and Pediatric Oncology Group before initiation of the Children’s Oncology Group grant in 2003 is available online at: https://www.childrensoncologygroup.org/index.php/research-funding.
Clinical trial information: NCT00980460.
AUTHOR CONTRIBUTIONS
Conception and design: Allison F. O’Neill, Alexander J. Towbin, Marcio Malogolowkin, Howard M. Katzenstein
Collection and assembly of data: Allison F. O’Neill, Alexander J. Towbin, Mark D. Krailo, Caihong Xia, M. Beth McCarville, Marcio Malogolowkin, Howard M. Katzenstein
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Characterization of Pulmonary Metastases in Children With Hepatoblastoma Treated on Children’s Oncology Group Protocol AHEP0731 (The Treatment of Children With All Stages of Hepatoblastoma): A Report From the Children’s Oncology Group
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Allison F. O'Neill
No relationship to disclose
Alexander J. Towbin
Honoraria: IBM Watson Health
Consulting or Advisory Role: Applied Radiology
Research Funding: Guerbet, Siemens
Patents, Royalties, Other Intellectual Property: Elsevier
Travel, Accommodations, Expenses: IBM Watson Health
Mark D. Krailo
Consulting or Advisory Role: Merck Sharp & Dohme
Caihong Xia
No relationship to disclose
Yun Gao
No relationship to disclose
M. Beth McCarville
No relationship to disclose
Rebecka L. Meyers
No relationship to disclose
Eugene D. McGahren
No relationship to disclose
Greg M. Tiao
No relationship to disclose
Stephen P. Dunn
No relationship to disclose
Max R. Langham Jr
No relationship to disclose
Christopher B. Weldon
No relationship to disclose
Milton J. Finegold
No relationship to disclose
Sarangarajan Ranganathan
No relationship to disclose
Wayne L. Furman
Consulting or Advisory Role: Pfizer
Travel, Accommodations, Expenses: Pfizer
Marcio Malogolowkin
No relationship to disclose
Carlos Rodriguez-Galindo
Honoraria: Novimmune
Howard M. Katzenstein
No relationship to disclose
REFERENCES
- 1. Bulterys M, Goodman MT, Smith MA, et al: Hepatic tumors, in: SEER Pediatric Monograph. 1975-1995. pp 91-99.
- 2. doi: 10.1016/s0959-8049(99)00049-0. Perilongo G, Shafford EA: Liver tumours. Eur J Cancer 35:953-958, 1999; discussion 958-959. [DOI] [PubMed] [Google Scholar]
- 3.Ortega JA, Krailo MD, Haas JE, et al. : Effective treatment of unresectable or metastatic hepatoblastoma with cisplatin and continuous infusion doxorubicin chemotherapy: A report from the Children’s Cancer Study Group. J Clin Oncol 9:2167-2176, 1991 [DOI] [PubMed] [Google Scholar]
- 4.Brown J, Perilongo G, Shafford E, et al. : Pretreatment prognostic factors for children with hepatoblastoma-- Results from the International Society of Paediatric Oncology (SIOP) study SIOPEL 1. Eur J Cancer 36:1418-1425, 2000 [DOI] [PubMed] [Google Scholar]
- 5.Perilongo G, Brown J, Shafford E, et al. : Hepatoblastoma presenting with lung metastases: Treatment results of the first cooperative, prospective study of the International Society of Paediatric Oncology on childhood liver tumors. Cancer 89:1845-1853, 2000 [DOI] [PubMed] [Google Scholar]
- 6.Zsiros J, Brugieres L, Brock P, et al. : Dose-dense cisplatin-based chemotherapy and surgery for children with high-risk hepatoblastoma (SIOPEL-4): A prospective, single-arm, feasibility study. Lancet Oncol 14:834-842, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feusner JH, Krailo MD, Haas JE, et al. : Treatment of pulmonary metastases of initial stage I hepatoblastoma in childhood. Report from the Children’s Cancer Group. Cancer 71:859-864, 1993 [DOI] [PubMed] [Google Scholar]
- 8.Meyers RL, Katzenstein HM, Krailo M, et al. : Surgical resection of pulmonary metastatic lesions in children with hepatoblastoma. J Pediatr Surg 42:2050-2056, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Squires RH, Ng V, Romero R, et al. : Evaluation of the pediatric patient for liver transplantation: 2014 practice guideline by the American Association for the Study of Liver Diseases, American Society of Transplantation and the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition. Hepatology 60:362-398, 2014 [DOI] [PubMed] [Google Scholar]
- 10.Heye T, Ley S, Heussel CP, et al. : Detection and size of pulmonary lesions: How accurate is MRI? A prospective comparison of CT and MRI. Acta Radiol 53:153-160, 2012 [DOI] [PubMed] [Google Scholar]
- 11.Biederer J, Hintze C, Fabel M: MRI of pulmonary nodules: Technique and diagnostic value. Cancer Imaging 8:125-130, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Therasse P, Arbuck SG, Eisenhauer EA, et al. : New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92:205-216, 2000 [DOI] [PubMed] [Google Scholar]
- 13. doi: 10.1002/cncr.30591. Katzenstein HM, Furman WL, Malogolowkin MH, et al: Vincristine/irinotecan upfront window treatment of high-risk hepatoblastoma: A Report from the Children’s Oncology Group (COG) AHEP0731 study committee. Cancer 123:2360-2367, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tirkes T, Hollar MA, Tann M, et al. : Response criteria in oncologic imaging: Review of traditional and new criteria. Radiographics 33:1323-1341, 2013 [DOI] [PubMed] [Google Scholar]
- 15.Meyers RL., Tiao G, de Ville de Goyet J, et al. : Hepatoblastoma state of the art: Pre-treatment extent of disease, surgical resection guidelines and the role of liver transplantation. Curr Opin Pediatr 26:29-36, 2014 [DOI] [PubMed] [Google Scholar]
- 16. Kaplan EL, Meier P: Nonparametic estimation from incomplete observations. J Am Stat Assoc 53:457-481, 1958.
- 17.Kalbfleisch JD, Prentice RL. The Statistical Analysis of Failure Time Data. John Wiley and Sons, New York, NY, 2002 [Google Scholar]
- 18. Kayton ML: Pulmonary metastasectomy in pediatric patients. Thorac Surg Clin 16:167-183, 2006. [DOI] [PubMed]
- 19.Warmann SW, Fuchs J: Principles of oncological surgery for lung metastases in paediatric solid tumours [in German]. Zentralbl Chir 134:537-541, 2009 [DOI] [PubMed] [Google Scholar]
- 20.Fuchs J, Seitz G, Handgretinger R, et al. : Surgical treatment of lung metastases in patients with embryonal pediatric solid tumors: An update. Semin Pediatr Surg 21:79-87, 2012 [DOI] [PubMed] [Google Scholar]
- 21.Tronc F, Conter C, Marec-Berard P, et al. : Prognostic factors and long-term results of pulmonary metastasectomy for pediatric histologies. Eur J Cardiothorac Surg 34:1240-1246, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Roth J.A., Putnam JB, Jr, Wesley MN, et al. : Differing determinants of prognosis following resection of pulmonary metastases from osteogenic and soft tissue sarcoma patients. Cancer 55:1361-1366, 1985 [DOI] [PubMed] [Google Scholar]
- 23.Häcker FM, von Schweinitz D, Gambazzi F: The relevance of surgical therapy for bilateral and/or multiple pulmonary metastases in children. Eur J Pediatr Surg 17:84-89, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Di Lorenzo M, Collin PP: Pulmonary metastases in children: Results of surgical treatment. J Pediatr Surg 23:762-765, 1988 [DOI] [PubMed] [Google Scholar]
- 25.McCarville MB, Lederman HM, Santana VM, et al. : Distinguishing benign from malignant pulmonary nodules with helical chest CT in children with malignant solid tumors. Radiology 239:514-520, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Silva CT, Amaral JG, Moineddin R, et al. : CT characteristics of lung nodules present at diagnosis of extrapulmonary malignancy in children. AJR Am J Roentgenol 194:772-778, 2010 [DOI] [PubMed] [Google Scholar]
- 27.Qayed M, Powell C, Morgan ER, et al. : Irinotecan as maintenance therapy in high-risk hepatoblastoma. Pediatr Blood Cancer 54:761-763, 2010 [DOI] [PubMed] [Google Scholar]
- 28.Habrand JL, Nehme D, Kalifa C, et al. : Is there a place for radiation therapy in the management of hepatoblastomas and hepatocellular carcinomas in children? Int J Radiat Oncol Biol Phys 23:525-531, 1992 [DOI] [PubMed] [Google Scholar]
- 29.Meyers RL, Maibach R, Hiyama E, et al. : Risk-stratified staging in paediatric hepatoblastoma: A unified analysis from the Children’s Hepatic tumors International Collaboration. Lancet Oncol 18:122-131, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]