Abstract
Background:
Interest in and use of marijuana by persons with multiple sclerosis (MS) has increased. While potential benefits have been reported, so have concerns about potential risks. Few large studies have been conducted about the perceptions and current usage of marijuana and medical cannabinoids in persons with MS.
Methods:
Participants in the North American Research Committee on Multiple Sclerosis (NARCOMS) registry were surveyed in 2014 regarding legality and history of marijuana usage, both before and after diagnosis with MS.
Results:
A total of 5,481 participants responded, with 78.2% female, 90% relapsing disease at onset, and a current mean age of 55.5 (10.2) years. Sixty-four percent had tried marijuana prior to their MS diagnosis, 47% have considered using for their MS, 26% have used for their MS, 20% have spoken with their physician about use, and 16% are currently using marijuana. Ninety-one percent think marijuana should be legal in some form. Men, those with higher disability, current and past nicotine smokers, and younger age were associated with a higher likelihood of current use.
Conclusions:
The majority of responders favor legalization and report high interest in the use of marijuana for treatment of MS symptoms, but may be reluctant to discuss this with health care providers. Health care providers should systematically inquire about use of marijuana.
Interest in the use of cannabinoids by persons with multiple sclerosis (MS) has increased over time as potential symptomatic benefits for pain and spasticity have been reported in clinical trials.1–9 While benefits have been noted, safety concerns were also raised in a systematic review by the American Academy of Neurology.10 Access to medical marijuana has also become easier. In December 2014, the US federal government ended restrictions on the use of medical marijuana in those states where it had been approved before May 2014 (figure e-1 at Neurology.org/cp). The District of Columbia and 28 states have laws allowing at a minimum medical marijuana usage, while 9 states allow low tetrahydrocannabinol (THC), high cannabidiol (CBD) marijuana with restrictions.
Outside of the United States, approximately 40% of people with MS report having used cannabis at some point in their lives for any reason, while 14%–18% report regular use, mainly for MS symptom relief.11,12 Within the United States, a survey of 125 persons with MS found that 18% had used marijuana for pain management.13 Given the emerging data supporting the potential benefit of cannabinoids, and recent changes in access to medical marijuana in the United States, we aimed to assess the opinions and usage history of marijuana in a large population of persons living with MS.
METHODS
NARCOMS registry
The North American Research Committee on Multiple Sclerosis (NARCOMS) registry is a voluntary longitudinal registry capturing health-related information from persons with MS at enrollment and semiannually thereafter. Between July 31 and September 1, 2014, all participants who had completed a NARCOMS semiannual update survey in the prior 2 years were invited to complete a survey online regarding marijuana usage as delineated further below. To reduce potential nonresponse bias due to the potential sensitivity of the topic, the survey was anonymous and not linked with longitudinal NARCOMS data.
Standard protocol approvals, registrations, and patient consents
Participant consent was obtained prior to the start of the survey and the study was approved by the institutional review board at the University of Alabama at Birmingham.
Participant characteristics
Participants reported their gender, current age, annual household income, and age at diagnosis. They reported tobacco smoking status (cigarettes, electronic cigarettes, or cigars) as current, every day; current, some days; former; or never. Annual household income was captured as less than $15,000; $15,001–$30,000; $30,001–$50,000; $50,001–$100,000; over $100,000; and I do not wish to answer (those preferring not to answer were excluded for covariate statistical models described below). Current MS course was captured as relapsing active, “I have had a relapse in the last 2 years” (relapsing-remitting MS [RRMS–A]); relapsing stable, “I have not had a relapse in at least 2 years” (RRMS-S); progressive, but “I used to have relapses” (secondary progressive MS [SPMS]); or progressive, “I have never had a relapse” (primary progressive MS [PPMS]). Disability level was reported using the Patient-Determined Disease Steps (PDDS), a validated measure of self-reported disability (overall) that correlates highly with a physician-scored Expanded Disability Status Scale (EDSS).14 The PDDS is an ordinal scale from 0 (normal) to 8 (bedridden), where a score of 0 is similar to an EDSS score of 0; a PDDS score of 3 represents early gait disability without needing an assistive device and is similar to an EDSS score of 4.0–4.5; and scores of 4, 5, and 6 are similar to EDSS scores of 6–6.5, requiring use of an assistive device.15 Disability in the domains of spasticity and cognition were captured using Performance Scales. The Performance Scales are ordinal scales with 6 levels: 0 (normal), 1 (minimal), 2 (mild), 3 (moderate), 4 (severe), and 5 (total disability).16,17 The NARCOMS depression scale and the tremor and coordination scales were also collected; they use a similar ordinal scale to Performance Scales and have been validated for use in MS.17,18
Marijuana
While cannabis is often categorized as organically grown or as cannabis-based pharmaceutically developed medications (e.g., Sativex; GW Pharmaceuticals, Cambridge, UK), the general public, including persons with MS, may have difficulty distinguishing between the two. Therefore, participants were directed to consider marijuana (cannabis) in any form when responding to questions. Specifically, they were advised that “‘Marijuana’ refers to smoking, eating, ingesting, or using an oil, spray, or other controlled medication derived from marijuana or synthetic marijuana (Sativex, Dronabinol/Marinol, Nabilone/Cesamet).” Participants were asked if they had ever used marijuana (even once) before their MS diagnosis and how old they were when they first used marijuana. They reported whether they had ever considered using marijuana to treat their MS symptoms, if they had used it to treat their symptoms, if they were currently using it, and if so, how often in the prior 30 days (current use, yes/no; if yes, how often in the past 30 days did you use marijuana? [0–30 days]). They were also asked if they lived in a state or country where medical marijuana or marijuana was legal. Participants were also asked if they thought medical marijuana or medications similar to marijuana should be approved to treat MS and under what circumstances (yes, with a prescription only; yes, with no restrictions; or no, it should not be legal). If marijuana were legal, participants were asked the preferred way to use marijuana or a medication derived from marijuana, where they could choose multiple options: smoke, oral as an oil, oral as a pill, nasal spray, topical as a patch or cream, no preference, or would not use it even if it was legal. Participants were also asked which general symptoms they thought marijuana may improve and if they had experienced any of those symptoms: muscle spasms/cramps/spasticity, tremor, overactive bladder/bladder spasms, pain or numbness, migraine/headaches, anxiety, insomnia, eye disorders, nausea/gastrointestinal symptoms, other, or none. Participants were not provided definitions or examples for the types of symptoms in these categories.
Statistical analysis
Descriptive measures are presented as number, percent, mean, and SD, as applicable. Unadjusted categorical group comparisons were made for categorical outcomes using likelihood ratio χ2, Fisher exact test, and for continuous outcomes with analysis of variance or Wilcoxon tests, as applicable. Covariate nominal logistic regression models were used to determine associations with opinion on legality (yes/no) and current disability status as measured by the PDDS MS course, tobacco smoking status, annual household income (as defined above), current age (years), and gender (male/female). The same covariates plus marijuana usage before MS diagnosis (yes/no) were also used in a covariate-adjusted nominal logistic regression for associations with current usage of marijuana.
Statistical analyses were performed using SAS V9.4 and JMP Pro Version 12 (SAS Institute Inc., Cary, NC); p values ≤ 0.05 were considered statistically significant.
RESULTS
Participants
Of the 12,260 participants invited to complete the survey, 5,665 (46.2%) responded. Of these, 5,481 (96.8%) reported gender, age, MS type, disability (as measured by the PDDS), and at least one response to a marijuana-related question and were included in the analysis. Due to the anonymous nature of the survey, characteristics of the nonresponders could not be assessed independently of the characteristics of those invited. However, for respondents and invitees, nearly 80% were women (78.2% responders, 79.4% of invitees), and most reported their clinical course to be relapsing at onset (90.3% of responders, 88.5% of invitees; table e-1).
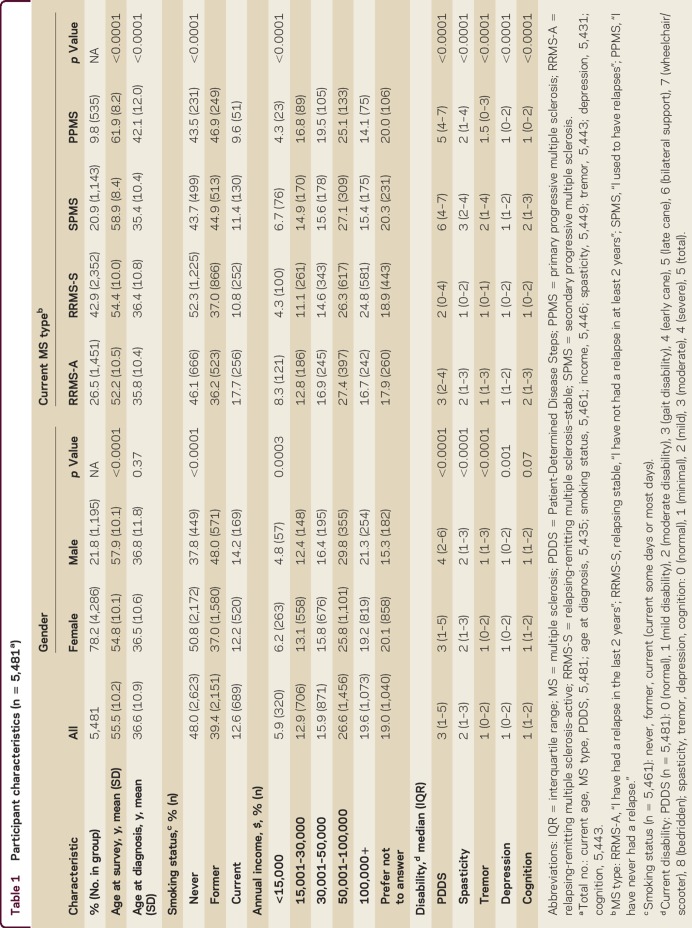
The median PDDS was 3 (gait disability) but was higher for men compared to women (p < 0.0001; table 1). Men reported greater disability than women in all domains, except cognition. Persons with SPMS also reported higher PDDS scores compared to all other types of MS (all p < 0.0001), and higher levels of spasticity, tremor, depression, and cognition compared to other types of MS.
Table 1.
Participant characteristics (n = 5,481a)
Marijuana legality
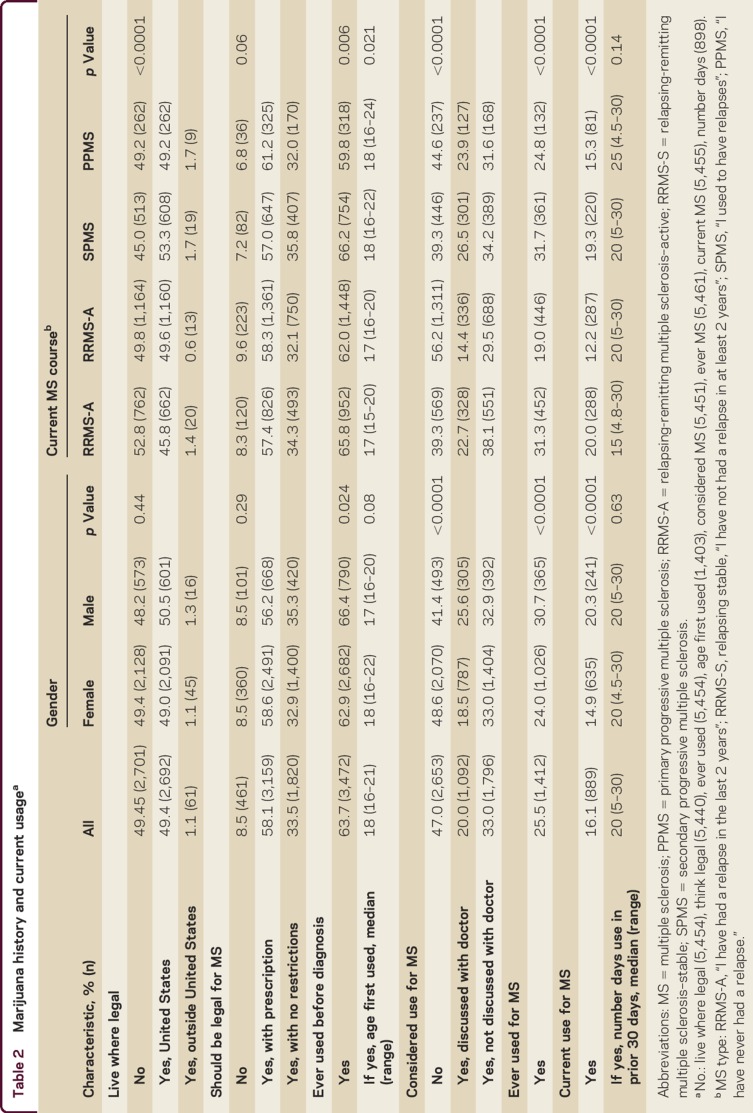
Half of responders lived where marijuana was legal in some form, including 1.1% outside the United States. Overall, 4,979 (91.5%) respondents thought marijuana should be legal for MS, of whom 3,159 (63.4%) thought this should require a prescription; the remainder did not think restrictions should exist (table 2). These findings did not differ by gender or by type of MS. Current tobacco smokers slightly favored legalization (95.9%) over former smokers (93.5%) and never smokers (88.8%; p < 0.0001). If legal, the preferred routes of administration were oral as a pill (47.5%), topical as a patch or lotion (28.5%), oral as an oil (22.4%), smoked (22.0%), or as a spray (18.1%); 21.9% had no preference, and 17.6% indicated they would not use even if legal. Of those who indicated they would not use even if legal, 63.2% of them think marijuana should be legal in some form.
Table 2.
Marijuana history and current usagea
A covariate-adjusted logistic model was used to consider sociodemographic characteristics as predictors of favoring legality of marijuana (yes/no). Age (p = 0.16), gender (p = 0.76), and current PDDS (p = 0.45) were not associated with a positive opinion on legality. Current MS type (p < 0.0001), smoking status (p < 0.0001), and current annual household income (excluding those who preferred not to answer; p = 0.005) were associated with opinion on legality. Higher income levels were associated with favoring legalization (table e-2). Current and former smokers were more likely to favor legality compared to never smokers. Respondents with PPMS were more likely to favor legality compared to RRMS-S and RRMS-A. Respondents with SPMS were more likely to favor legality compared to RRMS-S and RRMS-A. No difference existed between PPMS and SPMS or RRMS-S and RRMS-A.
Marijuana usage
The majority of respondents had tried marijuana before their MS diagnosis. A higher proportion of men reported prior use than women (table 2). Responders who had ever smoked were more likely to have tried marijuana before their MS diagnosis compared to those who had never smoked (odds ratio [OR] 4.3 [95% confidence interval (CI) 3.9–4.9]), after adjusting for age at diagnosis (p = 0.20) and gender (p = 0.67). Those with SPMS reported higher prior use compared to other types of MS. The median age at first use was 18 years, which did not differ by gender. Those with RRMS began using marijuana a median of 1 year earlier than those with progressive forms of MS.
Slightly more than half of respondents had considered using marijuana to manage their MS (n = 2,888; 53.0%), most of whom had not discussed use with their doctors (62.2%; table 2). A higher proportion of men had considered use for their MS compared to women (p < 0.0001). Of all types of MS, those reporting RRMS-S disease were least likely to have considered use for their MS. Those who had used marijuana before their MS diagnosis were more likely to have considered marijuana usage for their MS compared to those who had not (OR 3.8 [95% CI 3.4, 4.3]; p < 0.0001).
Overall, 16.1% of respondents reported that they currently used marijuana for their MS. Current users were more likely to favor legalization (97.8% vs 90.3%; p < 0.0001) and live where marijuana is legal (59.2% vs 48.8%; p < 0.0001). As PDDS scores increased, use of marijuana increased until a moderate level of disability was reached, after which use declined with increasing disability (table e-3). Findings were similar for spasticity, cognition, tremor, and depression.
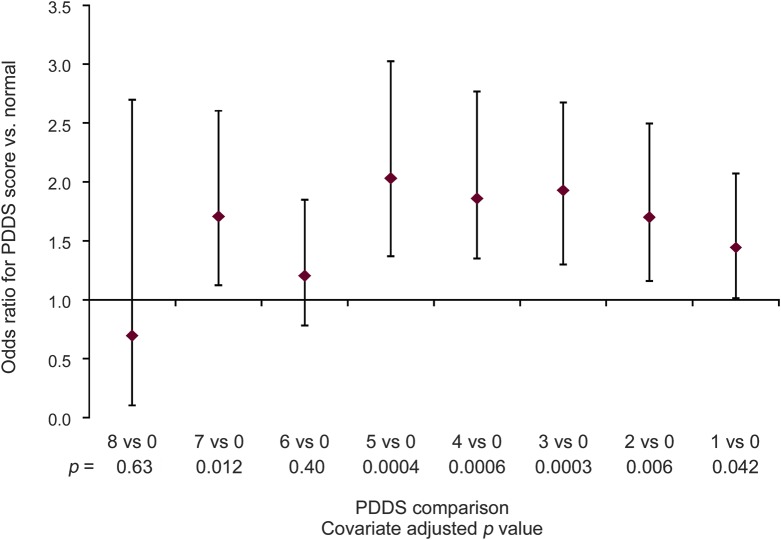
In a logistic model, gender, age, smoking status, income, usage before MS, MS course, and current disability were associated with current marijuana usage. Men were more likely than women to be current users (OR 1.5 [95% CI 1.2–1.8]). Older age was associated with lower odds of current use (OR 0.95 [95% CI 0.95–1.1]). Current and former smokers were more likely to be current users compared to never smokers (table e-2) and current smokers were also more likely than former smokers to be current users. Respondents with lower levels of income were more likely to be current users compared to those with higher income levels. Prior marijuana users were more likely than those who had not used marijuana before their MS diagnosis to be current users. Respondents with SPMS were more likely to be current users compared to those with RRMS-S and RRMS-A but those with RRMS-S were less likely to be current users compared to those with RRMS-A (OR 0.8 [95% CI 0.6–1.0]). Respondents with higher levels of disability were more likely to be current users compared to those with normal/no disability (figure 1). Findings were similar for spasticity, cognition, tremor, and depression (figure e-2, A–D).
Figure 1. Likelihood of current use (yes vs no) by disability level (odds ratio with 95% confidence interval).
Covariate-adjusted multinomial logistic model: gender, current age, use prior to multiple sclerosis (MS) diagnosis, MS course, current smoking status, current annual income, current disability/Patient-Determined Disease Steps (PDDS) level (no. in level): 0: normal (667), 1: mild disability (624), 2: moderate disability (425), 3: gait disability (571), 4: early cane (652), 5: late cane (455), 6: bilateral support (467), 7: wheelchair/scooter (504), 8: bedridden (23).
Perceived effectiveness
The proportion of responders currently experiencing the symptoms queried are shown in table e-4, overall and stratified by type of MS and current marijuana use. The most common symptoms were muscle spasms/cramps/spasticity, followed by pain and bladder symptoms. Generally, these symptoms were least often present in those with RRMS-S. A higher proportion of current users reported having all of the symptoms queried compared to nonusers, except for overactive bladder (p = 0.13) and eye disorders (p = 0.06).
Among all respondents, the perceived effectiveness of marijuana was highest for spasticity (75.5%), followed by pain (63.1%), and lowest for overactive bladder (20.3%) and eye disorders (14.2%). A higher proportion of current users thought marijuana was effective in treating all symptoms queried compared to nonusers (all p < 0.0001). Fewer than 1% of current users indicated that marijuana would not be effective in treating any symptoms, compared to 8% of nonusers (p < 0.0001); this did not differ by type of MS (p = 0.22).
For those experiencing a symptom, perceived effectiveness varied by type of MS for pain/numbness, insomnia, headaches, and eye disorders (table e-3). Generally, respondents with RRMS-A reported a higher perceived efficacy. Those currently using marijuana reported higher perceived efficacy than nonusers regarding all symptoms queried.
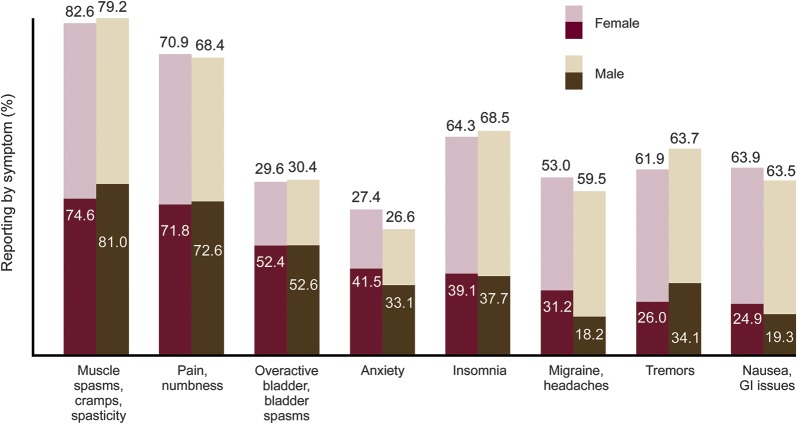
A higher proportion of men reported muscle spasms/cramps/spasticity and tremors (both p < 0.0001) compared to women. However, a lower proportion of men with spasticity thought marijuana was effective in treating spasticity compared to women (p = 0.021; figure 2). Perceived effectiveness in treating tremor did not differ by gender (p = 0.52). A higher proportion of women reported migraines/headaches, anxiety, and nausea/gastrointestinal issues (all p < 0.0001) compared to men; however, there was no difference in the proportion of those having the symptoms or in perceived efficacy for those symptoms by gender. A higher proportion of men reported that marijuana was not effective in improving any symptoms (9.4% vs 5.9%; p < 0.0001). Differences persisted when adjusted for type of MS, age, duration of disease, and current marijuana usage.
Figure 2. Current symptoms and perceived effectiveness of marijuana.
Light portion of bar: of those with symptom, % think marijuana is effective for symptom; dark portion of bar: % with symptom. GI = gastrointestinal.
DISCUSSION
We surveyed a large population of persons with MS regarding their use of and attitudes toward marijuana. Over 90% of respondents thought that access to marijuana should be legalized for MS, and it was perceived as effective for a broad range of symptoms. Although more than one-half of respondents had considered using marijuana for MS, fewer than 20% did so. Consistent with prior surveys, marijuana users were more likely to be male, to be tobacco users, and to have higher self-reported disability.11,12 We also found that marijuana use before MS diagnosis, younger age, and lower income levels were associated with greater odds of current marijuana use. However, high income as compared to lower income levels was associated with favoring legalization of marijuana.
The proportion of respondents who reported ever using marijuana was higher than reported in other, smaller surveys of persons with MS in the United States. However, it was similar to rates of use reported in the general US population of similar age.11–13,19 Similar to studies outside of the United States, 16% of respondents reported current use of marijuana,11,12 and more than half have considered using marijuana for their MS. Median monthly use was 20 out of the prior 30 days, similar to prior reports of 5–6 d/wk.12 Usage at this level would indicate a considerable financial cost, though a higher proportion of those with lower income reported higher current usage. It is unknown whether this reflects lack of access to other, more costly prescription therapies.
Respondents perceived that marijuana would be most effective for spasticity and pain, whether they were or were not using marijuana currently. Prior clinical trial results have indicated modest effectiveness for treatment of spasticity and pain but evidence to support treatment of other symptoms such as tremor is limited and conflicting.10 We did not address perceived adverse effects of marijuana; however, concerns have arisen regarding the potential adverse effect of smoked marijuana on processing speed and memory, supported by altered cerebral activation on MRI.20,21 These findings suggest that further evaluation of medical marijuana for managing a broader range of symptoms, while carefully evaluating adverse effects, is warranted.
The high proportion of responders who have considered use for MS but have not discussed it with their physicians indicates that the topic is either not being addressed by health care providers or that patients may be unwilling to discuss the issue or admit they have considered using marijuana. Persons with MS frequently use complementary and alternative therapies, and a large proportion do not share this information with their providers. Given that the Food and Drug Administration has not approved medical marijuana for use in the United States, the levels of active ingredients (THC or CBD) are not regulated, and may vary by source of the product obtained and by batch. Lack of regulation may lead to variable effectiveness and variable adverse effects.22,23 Potential drug interactions or exacerbated comorbidities may arise when providers are not fully aware of therapies that their patients are using.24,25 Health care providers should actively inquire about use of marijuana, especially in states where marijuana is legal and accessible without prescription.
The cross-sectional nature of the survey limits the ability to draw a causal relationship between current usage and disability status. The anonymous nature of the survey precluded linkage with longitudinal registry data. The NARCOMS registry participants who completed the routine Fall 2014 Update Survey were similar to respondents to this survey.
Persons with MS exhibit high interest in the use of marijuana for treatment of MS and perceive it to be highly effective for several symptoms. This high interest in considering marijuana to treat MS and high perception of efficacy would likely support recruitment into randomized clinical trials of controlled medical marijuana to determine effectiveness to treat symptoms that have not been adequately studied in this population. Health care providers should be aware of this interest, and the potential unwillingness to initiate the discussion with them.
AUTHOR CONTRIBUTIONS
S.S. Cofield: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, acquisition of data, statistical analysis, study supervision. A.R. Salter: drafting/revising the manuscript. T. Tyry: drafting/revising the manuscript. C. Crowe: drafting/revising the manuscript. G.R. Cutter: study concept or design, analysis or interpretation of data, obtaining funding. R.J. Fox: drafting/revising the manuscript, study concept or design, analysis or interpretation of data. R.A. Marrie: drafting/revising the manuscript, analysis or interpretation of data, scientific director of NARCOMS Registry.
Supplementary Material
ACKNOWLEDGMENT
NARCOMS is supported in part by the Consortium of Multiple Sclerosis Centers (CMSC) and The Foundation of the CMSC. Performance Scales Questions spacticity and cognition, Copyright Registration Number/Date: TXu000743629/1996-04-04; assigned to DeltaQuest Foundation, Inc., effective October 1, 2005. US Copyright law governs terms of use.
Footnotes
Supplemental data at Neurology.org/cp
STUDY FUNDING
NARCOMS is a project of the Consortium of Multiple Sclerosis Centers (CMSC) and receives funding from CMSC and the Foundation of the CMSC. Neither the CMSC nor the Foundation has access to data or are involved in analysis, interpretation, or publication review.
DISCLOSURE
S.S. Cofield has served on scientific advisory boards for MedImmune, Orthotech Biotech, and US Department of Defense; receives publishing royalties from Oxford University Press; serves as a consultant for American Shoulder and Elbow Society; and receives research support from Pfizer, the Consortium of Multiple Sclerosis Centers (CMSC), and the American College of Rheumatology. A.R. Salter, T. Tyry, and C. Crowe report no disclosures. G.R. Cutter serves on scientific advisory boards for AMO Pharmaceuticals, Apotek, Gilead Pharmaceuticals, Horizon Pharmaceuticals, Modigenetech/Prolor, Merck, Merck/Pfizer, Opko Biologics, Sanofi-Aventis, Reata Pharmaceuticals, Receptos/Celgene, Teva Pharmaceuticals, NHLBI (Protocol Review Committee), and NICHD (OPRU oversight committee); has received speaker honoraria from CMSC and Teva; serves on the editorial boards of Multiple Sclerosis and Alzheimer's & Dementia: Translational Research & Clinical Interventions and as statistical consulting reviewer for Journal of the American Society of Nephrology; is President of Pythagoras, Inc., a private consulting company located in Birmingham, Alabama; serves as a consultant for Atara Biotherapeutics, Bioeq GmbH, Cerespir Inc., CMSC (grant), Genzyme, Genentech, Innate Therapeutics, Janssen Pharmaceuticals, Klein-Buendel Incorporated, Medimmune, Medday, Nivalis, Novartis, Opexa Therapeutics, Roche, Savara Inc., Somahlution, Teva Pharmaceuticals, Transparency Life Sciences, and TG Therapeutics; works on studies funded to the CMSC subcontracted for analysis of NARCOMS Registry; receives/has received research support from NIH (NINDS, NIAID, NHLBI, NICHD, NIA, NIAMS, NIDDK), CMSC, US Department of Defense, UAB/UCSD, Children's Hospital (Boston), and Myasthenia Gravis Foundation of America; receives stocks/stock options from Pythagoras, Inc.; and has participated in a medico-legal case. R.J. Fox serves on scientific advisory boards for Biogen Idec and Novartis; serves on the editorial boards of Neurology® and Multiple Sclerosis Journal; receives publishing royalties for Multiple Sclerosis and Related Disorders (Demos Medical, 2013); serves as a consultant for Actelion, Biogen, Genentech, Novartis, Mallinckrodt, MedDay, and Teva; and receives research support from Biogen, Novartis, National MS Society, NIH, and CMSC. R.A. Marrie serves on the editorial boards of Neurology and Multiple Sclerosis Journal and has received research support from Sanofi-Aventis, Canadian Institutes of Health Research, Research Manitoba, MS Society of Canada, the National MS Society, MS Scientific Foundation, CMSC, Rx & D Health Research Foundation, Crohn's and Colitis Canada, and the Waugh Family Chair in Multiple Sclerosis. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.

REFERENCES
- 1.Zajicek J, Fox P, Sanders H, et al. Cannabinoids for treatment of spasticity and other symptoms related to multiple sclerosis (CAMS study): multicentre randomised placebo controlled trial. Lancet 2003;362:1517–1526. [DOI] [PubMed] [Google Scholar]
- 2.Zajicek JP, Sanders HP, Wright DE, et al. Cannabinoids in multiple sclerosis (CAMS) study: safety and efficacy data for 12 months follow up. J Neurol Neurosurg Psychiatry 2005;76:1664–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zajicek JP, Hobart JC, Slade A, Barnes D, Mattison PG; Research Group. Multiple sclerosis and extract of cannabis: results of the MUSEC trial. J Neurol Neurosurg Psychiatry 2012;83:1125–1132. [DOI] [PubMed] [Google Scholar]
- 4.Wade DT, Makela P, Robson P, House H, Bateman C. Do cannabis-based medicinal extracts have general or specific effects on symptoms in multiple sclerosis? A doubleblind, randomized, placebo-controlled study on 160 patients. Mult Scler 2004;10:434–441. [DOI] [PubMed] [Google Scholar]
- 5.Wade DT, Makela PM, House H, Bateman C, Robson P. Long-term use of a cannabis-based medicine in the treatment of spasticity and other symptoms in multiple sclerosis. Mult Scler 2006;12:639–645. [DOI] [PubMed] [Google Scholar]
- 6.Rog DJ, Nurmikko TJ, Friede T, et al. Randomized, controlled trial of cannabis-based medicine in central pain in multiple sclerosis. Neurology 2005;65:812–819. [DOI] [PubMed] [Google Scholar]
- 7.Collin C, Davies P, Mutiboko IK, et al. Randomized controlled trial of cannabis-based medicine in spasticity caused by multiple sclerosis. Eur J Neurol 2007;14:290–296. [DOI] [PubMed] [Google Scholar]
- 8.Centonze D, Mori F, Koch G, et al. Lack of effect of cannabis-based treatment on clinical and laboratory measures in multiple sclerosis. Neurol Sci 2009;30:531–534. [DOI] [PubMed] [Google Scholar]
- 9.Kavia RB, De Ridder D, Constantinescu CS, Stott CG, Fowler CJ. Randomized controlled trial of Sativex to treat detrusor overactivity in multiple sclerosis. Mult Scler 2010;16:1349–1359. [DOI] [PubMed] [Google Scholar]
- 10.Yadav V, Bever C Jr, Bowen J, et al. Summary of evidence-based guideline: complementary and alternative medicine in multiple sclerosis: report of the guideline development subcommittee of the American Academy of Neurology. Neurology 2014;82:1083–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chong MS, Wolff K, Wise K, et al. Cannabis use in patients with multiple sclerosis. Mult Scler 2006;12:646–651. [DOI] [PubMed] [Google Scholar]
- 12.Clark AJ, Ware MA, Yazer E, Murray TJ, Lynch ME. Patterns of cannabis use among patients with multiple sclerosis. Neurology 2004;62:2098–2100. [DOI] [PubMed] [Google Scholar]
- 13.Ehde DM, Alschuler KN, Osborne TL, Hanley MA, Jensen MP, Kraft GH. Utilization and patients' perceptions of the effectiveness of pain treatments in multiple sclerosis: a cross-sectional survey. Disabil Health J 2015;8:452–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 1983;33:1444–1452. [DOI] [PubMed] [Google Scholar]
- 15.Marrie RA, Goldman M. Validity of performance scales for disability assessment in multiple sclerosis. Mult Scler 2007;13:1176–1182. [DOI] [PubMed] [Google Scholar]
- 16.Schwartz CE, Vollmer T, Lee H. North American Research Consortium on Multiple Sclerosis Outcomes Study Group. Reliability and validity of two self-report measures of impairment and disability for MS. Neurology 1999;52:63–71. [DOI] [PubMed] [Google Scholar]
- 17.Marrie RA, Cutter CG, Tyry T, Campagnolo D, Volmner T. Validation of NARCOMS depression scale. IJMSC 2008;10:81–84. [Google Scholar]
- 18.Marrie RA, Goldman M. Validation of NARCOMS tremor and coordination scale. IJMSC 2011;13:114–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Azofeifa A, Mattson ME, Schauer G, McAfee T, Grant A, Lyerla R. National estimates of marijuana use and related indicators: national survey on drug use and health, United States, 2002–2014. MMWR Surveill Summ 2016;65:1–25. [DOI] [PubMed] [Google Scholar]
- 20.Pavisian B, Macintosh BJ, Szilagyi G, Staines RW, O'Connor P, Feinstein A. Effects of cannabis on cognition in patients with MS: a psychometric and MRI study. Neurology 2014;82:1879–1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feinstein A, Banwell E, Pavisian B. What to make of cannabis and cognition in MS: in search of clarity amidst the haze. Mult Scler 2015;21:1755–1760. [DOI] [PubMed] [Google Scholar]
- 22.Brunt TM, van Genugten M, Höner-Snoeken K, van de Velde MJ, Niesink RJ. Therapeutic satisfaction and subjective effects of different strains of pharmaceutical-grade cannabis. J Clin Psychopharmacol 2014;34:344–349. [DOI] [PubMed] [Google Scholar]
- 23.Zhang MW, Ho RCM. The cannabis dilemma: a review of its associated risks and clinical efficacy. J Addict 2015;2015:707596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Earleywine M, Barnwell S. Decreased respiratory symptoms in cannabis users who vaporize. Harm Reduct J 2007;4:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seamon MJ, Fass JA, Maniscalco-Feichtl M, Abu-Shraie NA. Medical marijuana and the developing role of the pharmacist. Am J Health Syst Pharm 2007;64:1037–1044. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.