Abstract
Background:
Seizures after ischemic stroke have not been well-studied. We aim to determine the frequency, determinants, and significance of early seizures after thrombolysis for acute ischemic stroke.
Methods:
Data are from the Enhanced Control of Hypertension and Thrombolysis Stroke Study (ENCHANTED), an international, multicenter, randomized controlled trial where patients with acute ischemic stroke were randomized to low-dose (0.6 mg/kg) or standard-dose (0.9 mg/kg) IV alteplase. The protocol prespecified prospective data collection on in-hospital seizures over 7 days postrandomization. Logistic regression models were used to determine variables associated with seizures and their significance on poor outcomes of death or disability (modified Rankin scale scores 3–6), symptomatic intracerebral hemorrhage (sICH), and European Quality of Life 5-Dimensions questionnaire [EQ-5D] over 90 days.
Results:
Data were available for 3,139 acute ischemic stroke participants, of whom 42 (1.3%) had seizures at a median 22.7 hours after the onset of symptoms. Baseline variables associated with seizures were male sex (odds ratio [OR] 2.19, 95% confidence interval [CI] 1.07–4.50), severe neurologic impairment (NIH Stroke Scale score ≥10; OR 2.16, 95% CI 1.06–4.40), and fever (OR 4.55, 95% CI 2.37–8.71). Seizures independently predicted poor recovery: death or major disability (OR 2.88, 95% CI 1.28–6.47), unfavorable ordinal shift of mRS scores (OR 1.94, 95% CI 1.10–3.39), and lower than median EQ-5D health utility index score (OR 3.50, 95% CI 1.37–8.91). There was no association of seizures with sICH in adjusted analysis.
Conclusions:
In thrombolysis-treated patients with acute ischemic stroke, seizures are uncommon, occur early, and predict poor recovery.
Clinicaltrials.gov identifier:
Seizures occur in 2%–18% of stroke patients,1–5 but the frequency may vary according to various factors: the type, location, and severity of stroke; complications such as delirium, hyponatremia, pneumonia, and fever; time period of observation; and by sex, age, and use of thrombolysis.6 Although seizures that occur early after the onset of acute ischemic stroke represent only a small proportion of poststroke seizures,1,4 they appear to have adverse effects on recovery by increasing risks of death.1,7 However, the strength of association is inconsistent, with some studies reporting an increase in mortality after adjustment for confounding variables,1 while other studies have shown no association with mortality at 6 months poststroke.8 Findings are also contradictory concerning the association between seizures and symptomatic intracerebral hemorrhage (sICH) after acute ischemic stroke.9,10 We sought to provide a reliable assessment of the frequency, determinants, and effects of early seizures among thrombolysis-treated ischemic stroke patients who participated in the large-scale Enhanced Control of Hypertension and Thrombolysis Stroke Study (ENCHANTED).
METHODS
Overview
ENCHANTED was an international, multicenter, prospective, open-label, quasifactorial, randomized controlled trial with blinded outcome evaluation, as outlined in detail elsewhere.11 A total of 3,310 patients with acute ischemic stroke who fulfilled standard eligibility criteria for thrombolytic treatment (median age, 67 years; 63% Asian) participated in the alteplase dose arm of the trial. They were randomly assigned to receive low-dose (0.6 mg per kilogram of body weight) or standard-dose (0.9 mg per kilogram) IV alteplase within 4.5 hours after onset of symptoms.
Standard protocol approvals, registrations, and patient consents
The trial protocol12 was approved by appropriate regulatory authorities and ethics committees at participating centers, and written informed consent was obtained from patients or, where appropriate, approved surrogates. The study is registered at ClinicalTrials.gov (NCT01422616).
Measures
Data on seizures (i.e., onset time, syndrome type, and number; use of antiepilepsy treatment) during 7 days (or to death or at hospital discharge, if sooner) was prespecified to be collected as part of the study protocol. Seizures were observed by trial investigators (mainly neurologists) and classified according to International League Against Epilepsy criteria.13 Baseline severity of neurologic impairment was measured using the NIH Stroke Scale (NIHSS; scores range from 0 to 42, higher scores indicate greater severity). Management details (i.e., fever occurrence, time from stroke onset to alteplase treatment, and stroke unit and intensive care unit admission) were collected to 7 days after randomization. The primary endpoint of functional outcome was assessed on the modified Rankin Scale (mRS)14 at 90 days, by trained researchers who were blind to the treatment assignment and management. The mRS is a global 7-level measure of functioning in which scores of 0–2 indicate a good outcome with no/minor neurologic symptoms or slight disability, 3–5 indicate major disability with grades of dependency, and 6 indicates death. A secondary outcome was sICH, defined according to various standard criteria (appendix e-1 at Neurology.org/cp), with the key measure being from the Safe Implementation of Thrombolysis in Stroke-Monitoring Study (SITS-MOST).15 Health-related quality of life was measured using the European Quality of Life group 5-Dimension self-report questionnaire (EQ-5D),16 where scores range from −0.594 (worse than death) to 1 (optimal health).
Statistical analysis
Complete case analysis was used. Univariate comparisons of characteristics between participants with and without seizures were conducted using χ2 test for categorical variables and 2-sample t test for continuous variables. Other than age, only variables having an association (p < 0.2) with seizures were considered for inclusion in multivariate models. Multivariate logistic regression was used to identify independent predictors of seizures with stepwise removal of nonsignificant covariates identified using the Wald test until all the remaining variables were statistically significant (p < 0.05). The association between seizures and clinical outcomes was identified through multivariate logistic regression with adjustment for minimization and key prognostic covariates.11,12 Data were reported with odds ratios (OR) and 95% confidence intervals (CI). All analyses were undertaken using SAS Enterprise version 7.1 (SAS Institute, Cary, NC).
RESULTS
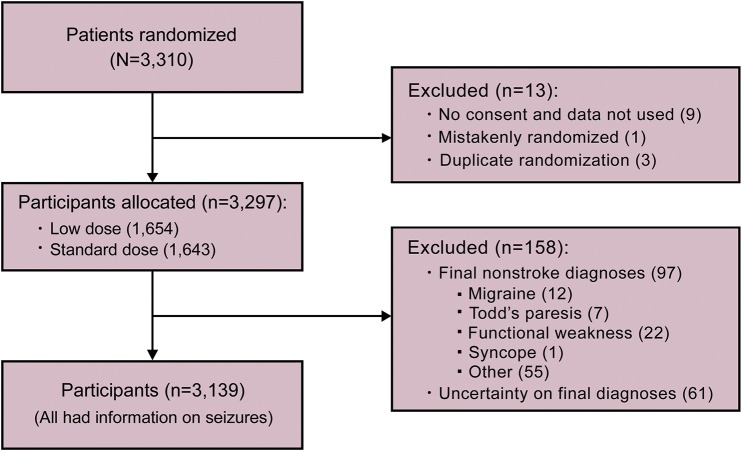
Of the 3,310 participants in the alteplase dose arm of ENCHANTED, 3,139 had a final diagnosis of ischemic stroke and information on seizures (figure).
Figure. Flow of participants.
Seizures occurred in 42 participants (1.3%): simple partial in 16 (38%), complex partial in 11 (26%), partial with secondary generalization in 2 (5%), primary generalized without an observable focal onset in 11 (26%), and undetermined type in 2 (5%). Antiepilepsy drugs were used in in 37 (88%). Most (27 [64%]) patients had a single seizure (interquartile range [IQR] 1–2). A total of 22 (52%) patients had seizures within 24 hours, including 3 at the time of presentation. The median time from stroke onset to first seizure was 22.7 (IQR 3.5–48.3) hours.
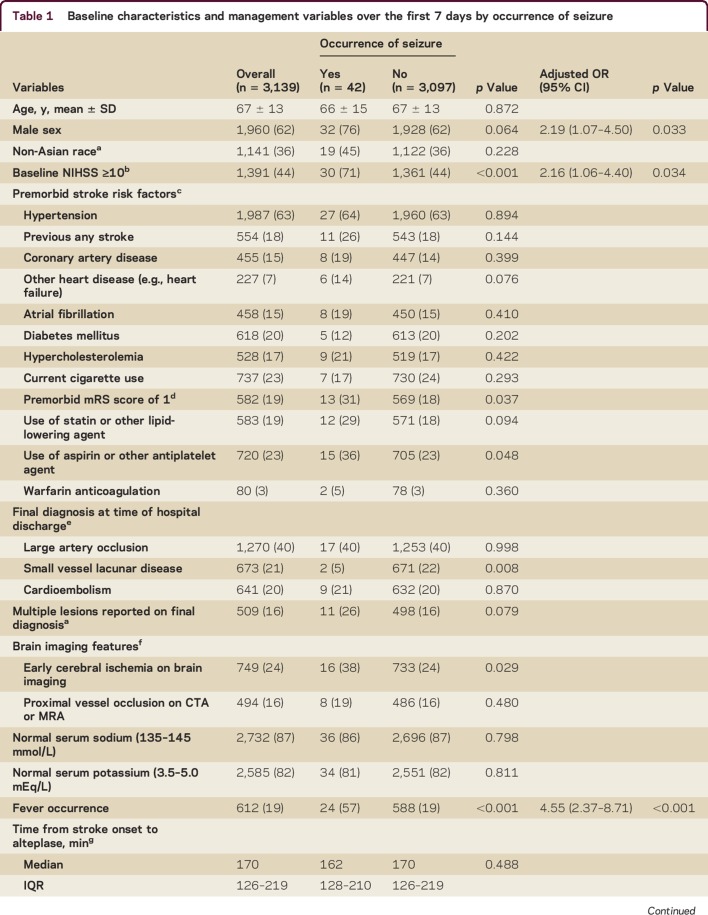
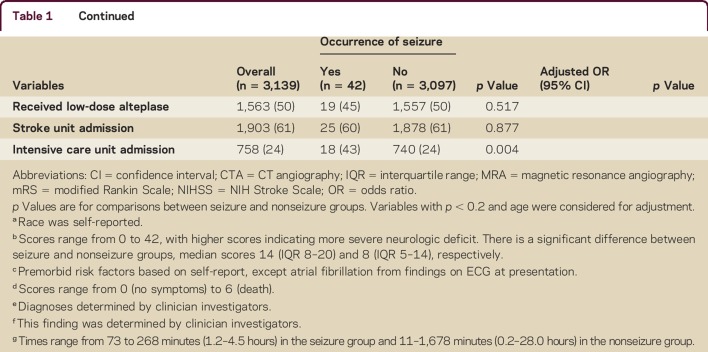
Table 1 shows that patients with seizures had more severe neurologic deficit than others (median NIHSS scores 14 vs 8), premorbid symptoms (mRS score of 1), signs of early cerebral ischemia, nonlacunar type pathology, fever, intensive care unit admission, and prior use of aspirin or other antiplatelet agent. Multivariable analysis indicates the following baseline or management characteristics were independently associated with early seizures: being male (OR 2.19, 95% CI 1.07–4.50), greater neurologic impairment (NIHSS ≥10; OR 2.16, 95% CI 1.06–4.40), and fever (OR 4.55, 95% CI 2.37–8.71).
Table 1.
Baseline characteristics and management variables over the first 7 days by occurrence of seizure
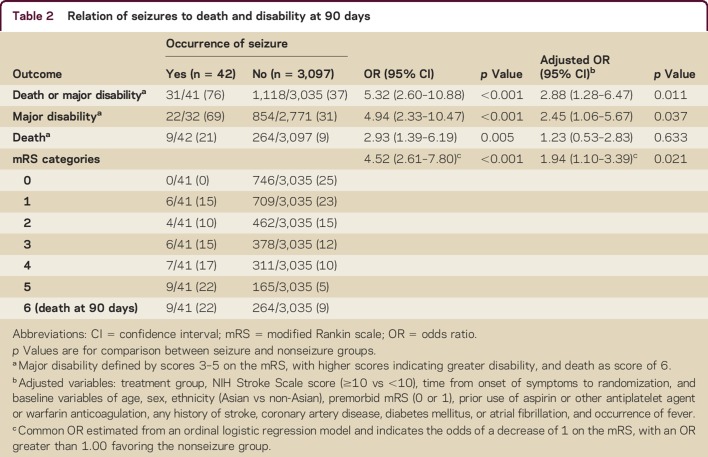
Death or major disability (mRS 3–6) occurred in 31 of 41 participants (76%) with seizures compared with 1,118 of 3,035 participants (37%) without seizures (p < 0.001) (table 2). After adjustment, seizures remained an independent predictor of poor outcome: death or major disability (OR 2.88, 95% CI 1.28–6.47) and major disability alone (OR 2.45, 95% CI 1.06–5.67), but not with death (OR 1.23, 95% CI 0.53–2.83). Moreover, adjusted ordinal analysis showed that seizures were associated with an unfavorable ordinal shift on the mRS (OR 1.94, 95% CI 1.10–3.39).
Table 2.
Relation of seizures to death and disability at 90 days
For the other outcomes, sICH tended to be more frequent in those with seizures, but this was not significant in multivariable analysis (table e-1). However, after adjustment, participants with seizures were more likely to have lower EQ-5D scores (OR 3.50, 95% CI 1.37–8.91) (table e-1).
DISCUSSION
Early seizures are uncommon in patients who receive lytic treatment for acute ischemic stroke and are associated with being male, more severe neurologic deficit (NIHSS score ≥10), and fever in the first 7 days. Yet seizures are a serious complication of this illness/management, as they independently predict an adverse functional outcome and poor quality of life.
Despite our dataset being derived from a clinical trial that tended to include patients with relatively mild severity of ischemic stroke who were managed according to standardized protocols, the frequency of seizures in our study is consistent with other studies reporting occurrences of 2.5% (10/400)10 and 1.2% (28/2,327)17 in the first 7 days after the onset of symptoms, where all10 or some of17 the patients were treated with IV alteplase. In the longer term, however, the frequency approaches 10% in a broader range of patients.2,3 The most common seizure type in our cohort—simple or complex partial—is also consistent with other studies2,18 and likely relates to the underlying focal ischemic lesion.
The greater frequency of postischemic stroke seizures in men was also noted in a Chinese study,19 and gives some support for a protective effect of estrogen, although most female participants in our study were postmenopausal. Low testosterone has been shown to enhance ictal activity.20 As shown in previous studies, stroke severity, whether measured clinically2,5,21 or by the size and location of lesion,22 is related to seizures. The relevance of fever to seizures could be related to infection1,23,24 or inflammation after ischemic stroke; further studies are required to investigate the role of comorbid stress or tissue necrosis in triggering seizures. Thrombolytic treatment can increase the risk of hemorrhagic transformation of cerebral infarction,25 but there are conflicting animal and human data regarding the risk of seizures after thrombolysis.17,26 Our data add to the literature that dose (i.e., low vs standard) of alteplase treatment did not appear to have a major effect on the risk of early seizures in acute ischemic stroke.
Contrary to the finding of another study,1 we were unable to show a definite association between seizures and mortality. This may be due to the ENCHANTED patients having better premorbid health (previously independent), and being less likely to die within 24 hours than a broader group of patients with acute ischemic stroke. There is an indication that early seizures increased the propensity for disability as the association with 90-day disability outcomes was consistent across the various approaches to analyze the mRS scores and the overall and individual components (data not shown) of the EQ-5D, independent of the severity of neurologic impairment at presentation. Any association between seizures and sICH could relate to increased tissue excitability within the ischemic penumbra,9 or increased intracranial pressure,7 but we could not find a clear association between these outcomes in multivariable analysis, although the numbers were small.
Strengths of this study include the broad range of well-characterized patients who were recruited from different health care settings with prospective, detailed, and complete data on early seizures. However, there are several limitations: this was a selected clinical trial population and we lack data on the location (cortical involvement or not) and extent of cerebral ischemia at this time, both of which have been associated with seizures.5,7,22,27 Further, we are unable to assess the influence of a history of epilepsy or the use of antiepilepsy agents, although these have not been found to predict the incidence of poststroke seizures.17 Furthermore, we do not have data on the use of specific antiepilepsy agents, their dosage, or duration of treatment. We note a deficiency in the literature on the efficacy of antiepilepsy agents in the prevention or control of seizures or of their influence on stroke recovery. Finally, we do not have any EEG data to confirm the reported diagnoses or to identify subclinical seizures. Of note, stroke severity predicts electrical seizures identified on continuous EEG.28 Most previous studies did not employ EEG, while most of those that did report the use of EEG have not presented any data on the findings in relation to early seizures.2,10,17,18 However, in one study, EEGs were obtained in 10 out of 29 patients with seizures, showing mild nonspecific changes in 3, focal slowing in 6, and paroxysmal features in 1 (generalized status epilepticus).7
Although seizure is an uncommon complication of acute ischemic stroke in patients treated with IV alteplase, it has adverse effects upon recovery. Early recognition of those at high risk and effective management of comorbid factors such as fever may improve outcomes.
AUTHOR CONTRIBUTIONS
Y. Xu: statistical analysis, interpretation of data, first draft, and revision of the manuscript. M.L. Hackett: interpretation of data, supervision, and revision of the manuscript. J. Chalmers: design/conceptualization of the study, interpretation of data, supervision, and revision of the manuscript. R.I. Lindley: revision of the manuscript. X. Wang: check of statistical analysis and interpretation of data. Q. Li: check of statistical analysis and interpretation of data. T. Robinson: design/conceptualization of the study and revision of the manuscript. H. Arima: design/conceptualization of the study and revision of the manuscript. P.M. Lavados: design/conceptualization of the study and revision of the manuscript. C.S. Anderson: design/conceptualization of the study, interpretation of data, supervision, and revision of the manuscript.
Supplementary Material
ACKNOWLEDGMENT
The authors thank the participants and their relatives; the clinical and research teams of the various emergency departments, intensive care units, stroke units, and neurology departments; The George Clinical project staff in Australia and China; Apollo Medical Imaging Technology, Melbourne, Australia; and Beijing MedSept Consulting. Maree Hackett holds a National Heart Foundation Future Leader Fellowship (2014–2017, 100034). Thompson Robinson is a National Institute for Health Research Senior Investigator. Craig Anderson holds a National Health and Medical Research Council (NHMRC) Senior Principal Research Fellowship.
Footnotes
Supplemental data at Neurology.org/cp
Contributor Information
Collaborators: ENCHANTED Study Group, John Chalmers, Craig Anderson, Richard Lindley, Hisatomi Arima, Yining Huang, Jong Sung Kim, Pablo Lavados, Tsong-Hai Lee, Christopher Levi, Mark Parsons, Sheila Martins, Jeyaraj Pandian, Octavio Pontes-Neto, Tom Robinson, Vijay Sharma, Nguyen Huy Thang, Shoichiro Sato, Christian Stapf, Jiguang Wang, Joanna Wardlaw, Philip Bath, Joseph Broderick, Andrew Demchuk, Geoff Donnan, R. John Simes, Graeme J. Hankey, Peter Sandercock, Marie-Germaine Bousser, K.S. Lawrence Wong, Laurent Billot, Qiang Li, Anish Scaria, Xia Wang, Yoichiro Hirakawa, Mark Woodward, Richard Lindley, Candice Delcourt, Cheryl Carcel, Penny Gordon, Sully Xiomara Fuentes Patarroyo, Dino Benito, Chris Levi, Mark Parsons, Hossein Zareie, Yongjun Cao, Amy Kunchok, Stephen Winters, Shelagh Coutts, Cheryl Carcel, Tom Moullaali, Yongjun Cao, Jie Yang, Guojun Wu, Shihong Zhang, Lili Song, Guofang Chen, Tom Robinson, Lisa Manning, Xiaoying Chen, Joyce Lim, Sharon Tucker, Leibo Liu, Sully Xiomara Fuentes Patarroyo, Penelope Gordon, Sarah Richtering, Xia Wang, Kerry Jenson, Elizabeth Knight, Elizaveta Ivanova, Emma Thembani, Elizabeth Odgers, Elizabeth Sanders, Sabrina Small, Ruchita Vaghasiya, Manuela Armenis, Paul Donnelly, Merza Ahmad Baig, Nick Blacklock, Bala Naidu, Ravinder Singh, Helen Monaghan, Phillipa Smith, Parisa Glass, Zhen Xi, Hua Deng, Buliang Cui, Ying Guo, Lingyu He, Ruolan Jia, Nan Li, Wei Li, Mengxiao Liu, Ziwei Xu, Meng Zhang, Ting Zhang, Yan Zhao, Yunjeong In, Su Jin Kim, Jung Eun Ahn, Sul Hwa Kim, Young Lan Hong, Veronica Olavarria, Francisca Gonzalez, Bernardita Portales, Alejandra Malavera, Magda Carla Ouriques Martins, Ching-Yi Wang, Shan-Jen Ryu, Alice Durham, Hardeep Aujla, Sue Lewin, Tracy Kumar, Hong Ha Uyen, Nguyen Anh Giang, Le Thi My Linh, Le Thi Thuy An, Do Minh Phuong, Pham Vu Bich Ngoc, Nguyen Mai Hang, Nguyen Thi Bich Tran, Ha Thi Thu Hien, Mai Bao Yen, M. Krause, M. Priglinger, S. Day, S. Jala, E. O'Brien, L. Davies, C. Delcourt, C. Carcel, T. Paraskevaidis, C.S. Anderson, T. Wijeratne, S. Celestino, L.Y. Law, G. Ng, K. Nagao, C. Levi, M. Parsons, F. Miteff, N. Spratt, L. Kaauwai, S. Martins, R. Brondani, A. Almeida, G. Weiss, M. Portal, M. Camilo, D. Favoretto, F. Alves, F. Dias, C. Barreira, C. Moro, A. Longo, R. Liberato, R. Barbosa, P. Magalhães, R. Bazan, G. Braga, G. Luvizutto, P. Ribeiro, M. Polin, L. Marques, F. Oliveira, M. Battaglini, F. Lourenço, K. Ferreira, G. Silva, L. Duarte, M. Alves, J. Sousa, M. Uhehara, V. Olavarría, M. Valenzuela, A. Brunser, V. Díaz-Tapia, R. Rivas, C. Klapp, L. Carvallo, P. Carvallo, G. Bustamante, L. Bravo, J. M. Matamala, R. Guerrero, E. Mansilla, J. Flores, M. Alvarado, A. Herrera, C. Reyes, G. Chen, L. Ping, S. Zhou, W. Liu, L. Liu, Y. Tian, Z. Li, Z. Chen, C. Liu, Y. Cao, J. Shi, S. You, X. Zhang, J. Wang, L. Wang, L. Wang, Z. Zhen, S. Zhang, J. Zhang, M. Yan, L. Wang, X. Tao, Q. Zhang, J. Zhang, Y. Li, R. Li, G. Cheng, Q. Chen, Y. Zhang, J. Zhou, J. Yang, Y. Liu, M. Xu, X. Wei, L. Rong, H. Liu, Y. Lv, C. Zhang, X. Zhang, J. Xia, H. Wang, Y. Zhang, X. Li, Y. Wang, X. Li, A. Lu, S. Tan, Y. Huang, Y. Wang, L. Wu, Z. Liu, S. Huang, Y. Gao, L. Wu, H. Sun, J. Zhang, X. Guo, Y. Lian, T. Xu, D. Geng, X. Yan, D. Wang, N. Zhao, J. Liu, Y. Pan, H. Wang, Z. Wang, Y. Niu, Y. Wang, D. Liu, W. Wu, H. Wang, L. Yang, S. Liu, S. Zhang, D. Wang, X. Chang, J. Liu, S. Wang, D. Wang, W. Huang, Y. Yang, A. Song, X. Zhang, M. Zou, B. Hu, L. Zhou, L. Sun, C. Geng, L. Song, H. Zhang, Y. Wu, L. Huang, Y. Hao, H. Yan, B. Qiao, A. Zhang, Y. Yang, Y. Liu, J. Feng, W. He, X. Liu, Q. Huang, Q. Yang, W. Zheng, X. Zhuang, X. Chen, L. Li, H. Nie, X. Wu, T. Wu, B. Bao, Y. Wang, X. Zeng, F. Guo, H. Dai, Y. Xu, Y. Shao, X. Zhang, S. Li, L. Tai, L. Xu, L. Wu, T. Zhang, Y. Zhang, E. Xu, Q. Lin, L. Zhan, J. Du, Y. Lai, Y. Zhou, H. Duan, Q. Cao, Q. Ma, M. Cai, W.H.T. Leung, F. Silva, J.A. Castellanos, M.A. Malavera, Clínica de Marly, M. Muñoz-Collazos, E. Solano, S. Ricci, E. Caterbi, S. Cenciarelli, R. Condurso, E. Gallinella, L. Greco, C. Marando, S. Mastrocola, A. Mattioni, T. Mazzoli, C. Padiglioni, I. Sicilia, F. Corea, A. Guidubaldi, S. Micheli, M. Barbi, K.B. Lee, H.W. Hwang, J. Cha, D.H. Kim, H.W. Nah, J.S. Kim, S.U. Kwon, D.W. Kang, Y.J. Kim, B.J. Kim, J.M. Park, K. Kang, B. Kim, O. Kwon, J.J. Lee, Y.W. Kim, Y.H. Hwang, H.S. Kwon, E.G. Kim, J. Seo, J.H. Jung, J.M. Kim, Z.Y. Kim, J.H. Rha, H.K. Park, C.W. Yoon, J. Koo, K. Lee, T. Kim, A. Ahn, J. Kim, H.J. Song, H.S Jeong, J.G. Lim, S.M. Park, Y.J. Kim, T.J. Song, E.J. Jung, W.K. Seo, K. Oh, H.J. Ji, D.E. Kim, W.S. Ryu, C. Irgens, E. Berge, B. Chan, H.L. Teoh, A. Ahmad, P. Paliwal, J. Ong, T.H. Lee, C.J. Seak, T.Y. Chang, H.L. Po, Y.J. Lin, C.L. Chou, C.W. Liou, T.Y. Tan, C.F. Liu, H.H. Cheng, H.J. Lin, C.M. Yang, H.C. Shen, C.Y. Chang, P.J. Kuo, R.T. Lin ; C.F.Chen, M.N. Wu, C.H. Chen, S.J. Yeh, L.P. Hsieh, J.D. Lee, M. Lee, Y.C. Huang, Y.C. Huang, C.Y. Wu, C.H. Lin, C.C. Yen, Y.T. Chang, Y.T. Hsu, N.C. Suwanwela, A. Chutinet, Y. Likitjaroen, D. Roongpiboonsopit, S. Charnwut, C. Roffe, R. Sanyal, G. Muddegowda, H. Maguire, J. Grocott, K. Finney, L. Kalra, D. Manawadu, N. Sikondari, J. Aeron-Thomas, R. Perry, L. Howaniec, K. Patel, A. Banaras, C. Watchurst, W. Sunman, N. Sprigg, S. Munshi, P.M. Bath, N. Gilzeane, C. Richardson, B. Moynihan, B. Patel, U. Khan, R. Ghatala, B. Clarke, O. Halse, H. Jenkins, P. Bentley, M. Venter, A. Kar, A. Dyker, A. Dixit, M. Omar Hossain, S. Louw, A. Gani, M. Davis, L. Sztriha, J. Teo, T. Ajao, F.K. Chan, M. Alao, J. Kallingal, M. Johnes, L. Harrison, V. O'Loughlin, Z. Naing, T. England, R. Donnelly, J. Scott, M. Maddula, J. Beavan, D. Tryambake, D. Broughton, A. Bergin, L. Dixon, J. Coyle, A. Mistri, K. Musarrat, L. Manning, T. Robinson, D. Eveson, E. Warburton, J. Mitchell, D. Day, N. Church, E. Amis, D. Chadha, P. Anderson, M. Kini, D. Walstow, C. Price, H. Rodgers, N. Weir, M. James, A. Bouring, H. Kingwell, S. Keenan, F. Cappuccio, A. Kenton, B. Dallol, S. Nyabadza, K. Rashed, S. Board, D. Wood, C. Buckley, L. Balian, P. Datta, M. Carpenter, R. Davey, A. Stanners, A. Needle, C. Blank, K. Harkness, A. Ali, E. Richards, K. Stocks, R. Durairaj, C. Athulathmudali, E. Barbon, A. Bibi-Khan, A. Hassan, L. Makawa, E. Veraque, M. Kambafwile, D. Waugh, G. Gunathilagan, D. Hargroves, I. Balogun, T. Webb, L. Cowie, M.J. Macleod, S. Wilkinson, J. Reid, R. Clarke, J. Furnace, S. Andole, N. Gadapa, S. Choudhary, K. Dunne, S. Dima, D. Bruce, G. Rogers, E. Brown, S. Clayton, M. Cooper, M. Abu Nasar, A. Rajapakse, I. Wynter, L. Sekaran, S. Sethuraman, F. Justin, M. Tate, T. Iyngkaran, M. Wani, T. Anjum, M. Krishnan, M. James, A. Bowring, H. Kingwell, S. Keenan, T. Nguyen Huy, A. Truong Le Tuan, L. Dam Thi Cam, M. Ngo Ba, B. Pham Nguyen, H. Nguyen Thi Thu, M. Nguyen Duy, D. Ngo Van, A. Nguyen Dat, T. Mai Duy, C. Nguyen Van, P. Dao Viet, S. Nguyen Hoanh, S. Pham Phuoc, T. Tran Van, B. Doan Thi, H. Hoang Quoc, T. Vo Van, K. Le Kim, T. Bui Ngoc, T. Nguyen Ba, K. Dao Duy, and Q. Pham Thi Ngoc
STUDY FUNDING
The study is funded by the National Health and Medical Research Council (NHMRC) of Australia (project grant 1020462), the Stroke Association of the United Kingdom (reference TSA 2012/01), the National Council for Scientific and Technological Development of Brazil (CNPq grant number 467322/2014-7), and the Ministry for Health, Welfare, and Family Affairs of South Korea.
DISCLOSURE
Y. Xu reports no disclosures. M.L. Hackett has received reimbursement for travel expenses from Boehringer Ingelheim; serves/has served as an Associate Editor and Editorial Board member of Cochrane Stroke Group; receives a National Heart Foundation Future Leader Fellowship; and receives research support from Google, National Health and Medical Research Council, Australia Swedish Research Council Framework, Sweden National Institute for Health Research, United Kingdom, National Heart Foundation, Australia Cochrane Chest, and Heart and Stroke, Scotland. J. Chalmers has received funding for travel and speaker honoraria from Servier; serves as Associate Editor for the Journal of Hypertension; and receives research support from Servier and National Health and Medical Research Council of Australia. R.I. Lindley has received speaker honoraria from Pfizer and Covidien; serves as an Associate Editor for the Australasian Journal on Ageing; receives publishing royalties from Stroke: The Facts (Oxford University Press, 2008, 2017) and Understanding Stroke, The British Medical Association Family Doctor Series (Family Doctor Publications Ltd. in association with the British Medical Association, 2000, 2002, 2003, and 2005); and receives research support from National Health and Medical Research Council of Australia. X. Wang and Q. Li report no disclosures. T.G. Robinson serves on scientific advisory boards for Bayer and Daiichi Sankyo; has received speaker honoraria from Bayer and Boehringer Ingelheim; and receives research support from the Stroke Association. H. Arima serves on a scientific advisory board for Kyowa Kirin Pharma; serves on the editorial board of Journal of Hypertension and as an Associate Editor for BMC Public Health and Journal of Epidemiology; serves on the speakers' bureaus for Takeda, Daiichi Sankyo, Asuka, and Bayer; and has received research support from National Health and Medical Research Council of Australia. P. Lavados has received funding for travel and speaker honoraria from Bayer; receives institutional support from Clinica Alemana, Universidad del Desarrollo, to perform stroke administration and research; and receives research support from Boehringer Ingelheim, Astra Zeneca, Bayer, CONICYT, Clinica Alemana-Universiad del Desarrollo, and The George Institute for Global Health. C.S. Anderson serves on scientific advisory boards for Astra Zeneca and Medtronic; has received funding for travel or speaker honoraria from Takeda China and Boehringer Ingelheim; serves on the editorial boards of Stroke, Cerebrovascular Diseases, and International Journal of Stroke; and receives research support from National Health and Medical Research Council of Australia. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.

REFERENCES
- 1.Huang CW, Saposnik G, Fang J, Steven DA, Burneo JG. Influence of seizures on stroke outcomes: a large multicenter study. Neurology 2014;82:768–776. [DOI] [PubMed] [Google Scholar]
- 2.Bladin CF, Alexandrov AV, Bellavance A, et al. . Seizures after stroke: a prospective multicenter study. Arch Neurol 2000;57:1617–1622. [DOI] [PubMed] [Google Scholar]
- 3.Lancman ME, Golimstok A, Norscini J, Granillo R. Risk factors for developing seizures after a stroke. Epilepsia 1993;34:141–143. [DOI] [PubMed] [Google Scholar]
- 4.Burneo JG, Fang J, Saposnik G. Impact of seizures on morbidity and mortality after stroke: a Canadian multi-center cohort study. Eur J Neurol 2010;17:52–58. [DOI] [PubMed] [Google Scholar]
- 5.Goswami RP, Karmakar PS, Ghosh A. Early seizures in first-ever acute stroke patients in India: incidence, predictive factors and impact on early outcome. Eur J Neurol 2012;19:1361–1366. [DOI] [PubMed] [Google Scholar]
- 6.Zhang C, Wang X, Wang Y, et al. . Risk factors for post-stroke seizures: a systematic review and meta-analysis. Epilepsy Res 2014;108:1806–1816. [DOI] [PubMed] [Google Scholar]
- 7.Arboix A, Garcia-Eroles L, Massons JB, Oliveres M, Comes E. Predictive factors of early seizures after acute cerebrovascular disease. Stroke 1997;28:1590–1594. [DOI] [PubMed] [Google Scholar]
- 8.De Herdt V, Dumont F, Henon H, et al. . Early seizures in intracerebral hemorrhage: incidence, associated factors, and outcome. Neurology 2011;77:1794–1800. [DOI] [PubMed] [Google Scholar]
- 9.Alberti A, Paciaroni M, Caso V, Venti M, Palmerini F, Agnelli G. Early seizures in patients with acute stroke: frequency, predictive factors, and effect on clinical outcome. Vasc Health Risk Manag 2008;4:715–720. [PMC free article] [PubMed] [Google Scholar]
- 10.Couillard P, Almekhlafi MA, Irvine A, et al. . Subacute seizure incidence in thrombolysis-treated ischemic stroke patients. Neurocrit Care 2012;16:241–245. [DOI] [PubMed] [Google Scholar]
- 11.Anderson CS, Robinson T, Lindley RI, et al. . Low-dose versus standard-dose intravenous alteplase in acute ischemic stroke. N Engl J Med 2016;374:2313–2323. [DOI] [PubMed] [Google Scholar]
- 12.Anderson CS, Woodward M, Arima H, et al. . Statistical analysis plan for evaluating low- vs. standard-dose alteplase in the Enhanced Control of Hypertension and Thrombolysis Stroke Study (ENCHANTED). Int J Stroke 2015;10:1313–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Proposal for revised clinical and electroencephalographic classification of epileptic seizures. From the commission on classification and terminology of the international league against epilepsy. Epilepsia 1981;22:489–501. [DOI] [PubMed] [Google Scholar]
- 14.Quinn TJ, Dawson J, Walters MR, Lees KR. Functional outcome measures in contemporary stroke trials. Int J Stroke 2009;4:200–205. [DOI] [PubMed] [Google Scholar]
- 15.Wahlgren N, Ahmed N, Davalos A, et al. . Thrombolysis with alteplase for acute ischaemic stroke in the Safe Implementation of Thrombolysis in Stroke-Monitoring Study (SITS-MOST): an observational study. Lancet 2007;369:275–282. [DOI] [PubMed] [Google Scholar]
- 16.Rabin R, de Charro F. EQ-5d: a measure of health status from the EuroQoL group. Ann Med 2001;33:337–343. [DOI] [PubMed] [Google Scholar]
- 17.Alvarez V, Rossetti AO, Papavasileiou V, Michel P. Acute seizures in acute ischemic stroke: does thrombolysis have a role to play? J Neurol 2013;260:55–61. [DOI] [PubMed] [Google Scholar]
- 18.Lamy C, Domigo V, Semah F, et al. . Early and late seizures after cryptogenic ischemic stroke in young adults. Neurology 2003;60:400–404. [DOI] [PubMed] [Google Scholar]
- 19.Cheung CM, Tsoi TH, Au-Yeung M, Tang ASY. Epileptic seizure after stroke in Chinese patients. J Neurol 2003;250:839–843. [DOI] [PubMed] [Google Scholar]
- 20.El-Khayat HA, Shatla HM, Ali GK, Abdulgani MO, Tomoum HY, Attya HA. Physical and hormonal profile of male sexual development in epilepsy. Epilepsia 2003;44:447–452. [DOI] [PubMed] [Google Scholar]
- 21.Reith J, Jorgensen HS, Nakayama H, et al. . Seizures in acute stroke: predictors and prognostic significance: the Copenhagen Stroke Study. Stroke 1997;28:1585–1589. [DOI] [PubMed] [Google Scholar]
- 22.Wang G, Jia H, Chen C, et al. . Analysis of risk factors for first seizure after stroke in Chinese patients. Biomed Res Int 2013;2013:702871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grau AJ, Buggle F, Schnitzler P, Spiel M, Lichy C, Hacke W. Fever and infection early after ischemic stroke. J Neurol Sci 1999;171:115–120. [DOI] [PubMed] [Google Scholar]
- 24.Krakow K, Sitzer M, Rosenow F, Steinmetz H, Foerch C, Arbeitsgruppe Schlaganfall H. Predictors of acute poststroke seizures. Cerebrovasc Dis 2010;30:584–589. [DOI] [PubMed] [Google Scholar]
- 25.Emberson J, Lees KR, Lyden P, et al. . Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. Lancet 2014;384:1929–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tan ML, Ng A, Pandher PS, et al. . Tissue plasminogen activator does not alter development of acquired epilepsy. Epilepsia 2012;53:1998–2004. [DOI] [PubMed] [Google Scholar]
- 27.Labovitz DL, Hauser WA, Sacco RL. Prevalence and predictors of early seizure and status epilepticus after first stroke. Neurology 2001;57:200–206. [DOI] [PubMed] [Google Scholar]
- 28.Carrera E, Michel P, Despland PA, et al. . Continuous assessment of electrical epileptic activity in acute stroke. Neurology 2006;67:99–104. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.