Abstract
Programmed death-1 (PD-1) receptor system represents a part of recently reported immunoregulatory pathway. PD-1 is an immune checkpoint molecule, which plays an important role in downregulating the immune system proinflammatory activity. Until recently, PD-1 expression was not established on immune cells of the preterm infants. The study objectives were to confirm expression of the PD-1 receptors on the monocytes isolated from very low birth weight newborns (VLBW), and to analyze their expression during the first week of life and late-onset sepsis. Peripheral blood mononuclear cells were isolated from 76 VLBW patients without early-onset sepsis on their 5th day of life (DOL). PD-1 expression was determined on the monocyte subsets (classical, intermediate, non-classical) by flow cytometry. In case of late-onset sepsis (LOS), the same analysis was performed. Our results demonstrated that on the 5th DOL, PD-1 receptors were present in all the monocyte subsets. Children, whose mothers had received antenatal steroids, presented higher absolute numbers of non-classical monocytes with PD-1 expression. Infants born extremely preterm who later developed LOS, initially showed a lower percentage of PD-1 receptor-positive intermediate monocytes in comparison to neonates born very preterm. During LOS, we observed a rise in the percentage of classical monocytes with PD-1 expression. In case of septic shock or fatal outcome, there was a higher percentage and absolute count of intermediate monocytes with PD-1 expression in comparison to children without these complications. In conclusion, monocytes from VLBW children express PD-1 receptors. Antenatal steroid administration seems to induce PD-1 receptor expression in the non-classical monocytes. PD-1 might play a role in immunosuppressive phase of sepsis in the prematurely born children with septic shock and fatal outcome.
Introduction
Neonates, especially those born preterm, are highly susceptible to systemic infections [1]. Developmental functional immaturity of the immune system is regarded as one of the reasons for the high rate of sepsis in the prematurely born patients [2]. Circulating monocytes (MO) are important elements of the innate immunity. MO in the peripheral blood can be divided into three subsets according to the CD14 and CD16 antigen surface expression: CD14++CD16- (classical, CL MO), CD14++CD16+ (intermediate, IM MO) and CD14+CD16++ (non-classical, NC MO) [3]. The major population are CL MO, which constitute about 90% of the entire monocyte pool, whereas the IM and NC MO subsets account for about 10% under physiological conditions in adults [4]. CL MO show high phagocytic activity. The CD16+ MO population (IM and NC) can significantly increase during infection and inflammation [5], which was documented both in adult [6], as well as neonatal [7] patients with bacterial sepsis.
High rates of morbidity and mortality in sepsis result from a prolonged immunosuppression after a rapid proinflammatory response; it is associated with attenuated pathogen clearance and/or susceptibility to superinfection [8]. The programmed death receptor-1 (PD-1) pathway seems to play a role in sepsis-induced immune suppression [9]. PD-1 (CD279) is a cell surface receptor that belongs to the immunoglobulin superfamily, first described by Ishida and colleagues in 1992 [10]. It is one of the key negative regulators of the immune response, maintains immune tolerance [11], prevents development of autoimmune diseases [12], and controls extend of healthy tissue damage during infection [13]. It is an inducible molecule expressed on the surface of activated cells, mainly T cells, B cells, NK cells, but also monocytes and dendritic cells [14–15]. A negative signal transmitted from the activated PD-1 receptor leads to decreased activity of the immune system by inhibition of the TCR or BCR connected signaling pathways, decreased production of the cytokines and proteins promoting immune cell survival (e.g. Bcl-2), and increased synthesis of IL-10, which inhibits the immune response [13].
MO from adult subjects during sepsis display increased expression of PD-1 simultaneously with decreased HLA-DR expression. Inhibition of PD-1 in the pre-clinical studies appears to restore defects of the immune functions and to improve survival in sepsis [16–18].
To our knowledge, expression of the PD-1 receptors on the circulating monocytes has never been investigated in prematurely born infants. Here, we would like to present results of a prospective cohort study designed to determine expression changes of PD-1 receptors on the monocyte subpopulations in the very low birth weight (VLBW) preterm children soon after birth and in the subgroup with late-onset sepsis (LOS).
Materials and methods
Study design and setting
This was a prospective observational study performed at a 30-bed Neonatal Intensive Care Unit (NICU), Department of Paediatrics, Institute of Paediatrics, Faculty of Medicine, Jagiellonian University Medical College, Krakow, Poland, between 2014 and 2016. Protocol of the study was approved by the Jagiellonian University Ethic Committee.
Patient selection
The newborns enrolled to this study were recruited from the patients consecutively admitted to NICU.
Inclusion criteria were:
Signed informed consent by the parents,
Birthweight < 1500g,
Gestational age (GA) ≤ 32 weeks,
Age ≤ 72 hours at the time of enrolment.
Exclusion criteria were:
Early-onset sepsis (defined as a blood culture-proven clinical sepsis occurring up to 3 days of life),
Multiple congenital malformations.
Monitoring during hospitalization
All the subjects enrolled in the study underwent careful clinical monitoring for symptoms of LOS. LOS was defined as a blood culture-proven clinical sepsis occurring ≥ 72 hours of age. Clinical symptoms of LOS were first identified by an attending neonatologist, and then verified with a positive blood culture. Septic shock definition was based on this mentioned in the Surviving Sepsis Campaign, adjusted for age range based on the criteria of Goldstein as determined by the consensus [19–20].
Data collection
Data regarding baby’s perinatal history, hospitalization course, laboratory results and other LOS pertaining data were collected in our hospital database.
Blood collection/Division into groups
First sample of blood was drawn from all the study participants on the 5th day of life (DOL). The blood sample (500 μl) was collected into EDTA-containing tubes (Vacutainer System®, Becton Dickinson Biosciences, San Jose) and processed in the laboratory at Department of Clinical Immunology, Institute of Pediatrics, Faculty of Medicine, Jagiellonian University Medical College, Krakow, within 2 hours from the draw. For this study, the samples were retrospectively categorized into 2 groups depending on whether the patients did or did not develop LOS during their hospitalization:
Non-LOS group—samples collected on the 5th DOL from newborns, who did not develop LOS during hospitalization in the NICU
Before-LOS group—samples collected on the 5th DOL from newborns, who developed the episode of culture-proven LOS during hospitalization in the NICU.
Children, who were diagnosed with LOS during hospitalization, had their second blood sample collected within 24 hours from the onset of LOS symptoms. Once LOS was confirmed by a positive blood culture, these samples formally formed a third, LOS-group.
Flow cytometry
Undiluted whole blood samples were washed by addition of 3 ml of 0.9% NaCl in polypropylene round-bottom tubes (BD Biosciences) and centrifuged (1000 × g). Then, blood sample was put into a TruCOUNT™ Tube (BD Biosciences, San Jose, USA) and stained on ice for 30 min with: anti-CD45-APC, anti- HLA-DR-PerCP, anti-CD14-FITC, anti-CD16-PE (BD Biosciences) and anti-PD1-PE-Cy7 (eBioscience) monoclonal antibodies (mAbs). The samples were then treated with FACS Lysing Solution (BD) until erythrocytes were lysed, and the cells were immediately processed in the FACSCanto flow cytometer (Becton Dickinson, San Jose, USA) along with 10.000 of beads per tube. The absolute numbers of CD14++CD16-, CD14++CD16+ and CD14+CD16++ monocytes were calculated with reference to the bead count. The percentage and the absolute numbers of PD-1- positive cells were estimated in each of the monocyte subset. Flow cytometric data were analyzed using FlowJo software (Tree Star, Inc, Ashland, OR). The gating strategy and analysis of MO subsets was previously described by us [21] and others [22] and is presented on Fig 1.
Fig 1. Gating strategy for studying monocyte subsets and differential expression of PD-1 in monocyte subsets.
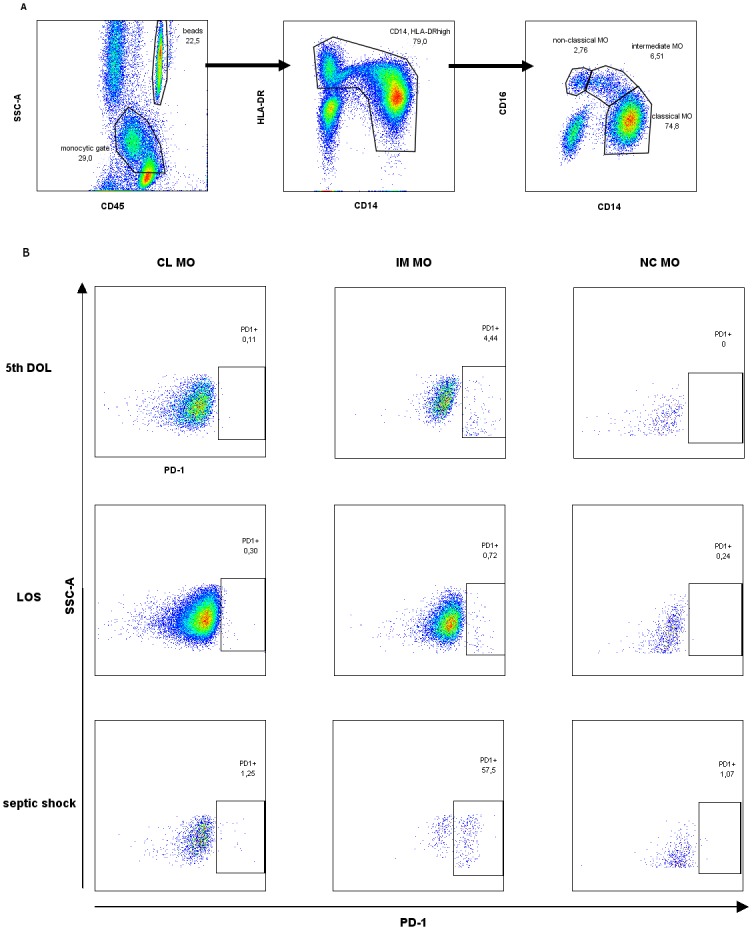
(A) Gating strategy of monocyte subsets. The CD45-positive monocytes (“monocytic gate”) together with adjacent lymphocytes were further analyzed. The cells were then gated to exclude CD14-HLA-DR- NK cells and finally divided into CD14++CD16- (classical), CD14++CD16+ (intermediate) and CD14+CD16++ (non-classical) monocytes. The absolute numbers of monocyte subsets were estimated based on acquired bead count (“beads gate” on plot no 1). (B) PD-1 expression on monocyte subsets. PD-1 expression pattern is shown on each of the monocyte subsets (classical, intermediate and non-classical subsets) in a patient analyzed on 5th DOL, a patient that developed LOS, and a patient with septic shock.
Statistical analysis
Basic demographic data were compared using the Wilcoxon test or two-sided t-test as appropriate. Qualitative values were compared by the chi-square test. Student’s t-test was used to establish differences in the continuous variables with normal distribution between studied groups. For data with distribution other than normal, Wilcoxon test was used. Extreme values defined as lower than Q1-3IQR, or as higher than Q3+3IQR were excluded from the analyses. A probability value of p<0.05 was considered statistically significant. JMP® 13.1.0 (SAS Institute Inc., 2016) was used for statistical analysis.
Results
Study population
A group of 76 preterm-born infants without early-onset sepsis with mean gestational age 27.7 (SD 2.4) weeks and mean birth weight 1042 (SD 257) grams was enrolled to the study. Thirty nine infants developed LOS during hospitalization, mostly caused by Gram-positive bacteria. LOS occurred on 17th DOL (IQR 12–21.5 DOL; range: 8–34 DOL). Five infants diagnosed with LOS developed septic shock, and a total of eight patients from the entire study group died during hospitalization at NICU (Table 1).
Table 1. Comparison of selected demographic variables and hospitalization data of the patients in the two studied groups.
| Group non-LOS n = 37 |
Group before-LOS n = 39 |
P value and statistical test used | |
|---|---|---|---|
| Perinatal history | |||
| Birth weight [g], mean (SD) | 1142 (241) | 948 (237) | 0.0011T |
| Gestational age [weeks], mean (SD) | 28.6 (2.2) | 26.8 (2.3) | 0.0015T |
| Male gender | 21 (57%) | 16 (41%) | 0.169C |
| Vaginal delivery | 13 (35%) | 10 (26%) | 0.407C |
| Antenatal steroids | 9 (24%) | 12 (31%) | 0.577C |
| Hospitalization | |||
| Peripheral lymphocyte count on 5th DOL [cells/μl], median (IQR) | 2640.5 (1256.5–3363.8) | 1964.5 (1420–2350) | 0.350W |
| Late-onset sepsis | - | 39 | |
| Gram-positive | - | 28 (72%) | |
| Gram-negative | - | 7 (18%) | |
| Polymicrobial | - | 4 (10%) | |
| Septic shock | - | 5 (13%) | |
| Outcome | |||
| Death during hospitalization at NICU | 2 (5%) | 6 (15%) | 0.147C |
T–two-sided T test,
C—Chi-square test,
W–Wilcoxon test.
DOL—day of life, NICU—Neonatal Intensive Care Unit
Comparison of median levels of monocyte PD-1 expression on 5th day of life
Analysis of the blood collected on the 5th DOL showed that the majority of the isolated monocytes constituted the CL subtype, whereas IM and NC monocytes were present in smaller counts. There were cells positive for PD-1 receptors’ expression in all the monocyte subsets on the 5th DOL. The IM MO subset showed the highest expression of PD-1 receptor (Table 2).
Table 2. Counts of monocyte subsets, monocyte subsets with PD-1 expression, and a percentage of PD-1+ cells within each monocyte subset in the blood samples from VLBW infants collected on the 5th DOL.
Data presented as median and IQR.
| CL MO | IM MO | NC MO | p | |
|---|---|---|---|---|
| MO count (cell/μl); median [IQR] | 850 [463–1921] | 75 [35–134] | 41 [12–106] | p < 0.0001*, Post-hoc tests**: p < 0.0001 for CL MO vs. IM MO, p < 0.0001 for CL MO vs. NC MO, p = 0.0009 for IM MO vs. NC MO |
| PD-1-positivecount (cell/μl); median[IQR] | 2 [0.2–5.42] | 8.5 [3–15] | 0.33 [0–2] | p < 0.0001*, Post-hoc tests**: p < 0.0001 for CL MO vs. IM MO, p = 0.0011 for CL MO vs. NC MO, p < 0.0001 for IM MO vs. NC MO |
| PD-1-positive (%); median [IQR] | 0.29 [0.05–0.58] | 11.6 [6.34–22.6] | 1.53 [0.17–4.81] | p < 0.0001*, Post-hoc tests**: p < 0.0001 for CL MO vs. IM MO, p < 0.0001 for CL MO vs. NC MO, p < 0.0001 for IM MO vs. NC MO |
*—Kruskal—Wallis test,
**—nonparametric comparisons for each pair using Wilcoxon method
CL MO—classical monocytes, IM MO—intermediate monocytes, NC MO—non-classical monocytes
We did not find significant differences in percentages or numbers of the MO subsets expressing PD-1 receptor in regards to as function of the newborns’ gender or mode of their delivery. Interestingly, even though there was no difference in absolute counts of any MO subset in infants whose mothers had received prenatal steroids (n = 21) compared to those who had not undergo such a treatment, they had a significantly higher absolute count of NC MO expressing PD-1 (1000/ml [0–5000] vs. 110/ml [0–1000]; Wilcoxon, p = 0.0371).
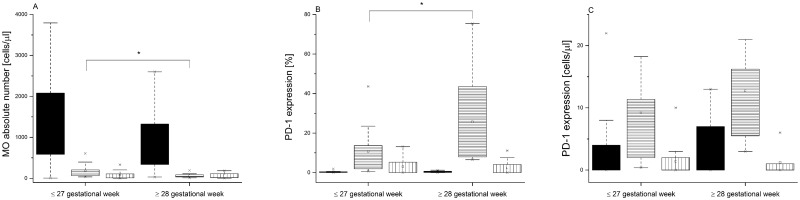
Following LOS diagnosis, the study group was retrospectively divided into two groups with or without sepsis. There was no difference in PD-1 expression on the 5th DOL in the non-LOS and before-LOS populations. There was also no correlation between PD-1 expression and corresponding gestational age on the 5th DOL. However, we noticed that level of maturity at birth affected PD-1 expression in the before-LOS group. The neonates born as extremely preterm (≤ 27 gestational week) presented higher absolute counts of the IM MO (101/μl [58–288] vs. 59/μl [33–89]; Wilcoxon, p = 0.0105) and lower percentages of IM PD-1-positive MO (7.95% [1.8–14] vs. 15.8% [7.7–45.7]; Wilcoxon, p = 0.0136) in comparison to these who were born very preterm (≥ 28 gestational week) (Fig 2).
Fig 2. Monocyte populations and PD-1 expression in the before-LOS group of VLBW infants.
Patients were subdivided into groups based on the level of their gestational age at birth. (A) Absolute numbers of monocyte subsets. (B) Percentages of PD-1 expressing monocytes. (C) Absolute numbers of PD-1 expressing monocytes. Data presented as median and IQR (box), compared with Wilcoxon test. Whiskers—range within 1.5 IQR. Classical monocytes are presented as a black graph, intermediate monocytes are presented as a graph with horizontal lines, whereas non-classical monocytes are presented as a graph with vertical lines. P-value was significant in case of *p<0.05.
PD-1 expression levels in patients with late-onset sepsis
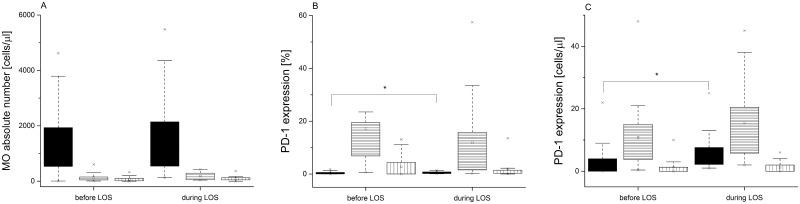
The next step in our study was to analyze the MO subsets and PD-1 expression in children, who developed LOS during hospitalization. We observed increase in absolute count of IM and NC MO during LOS in comparison with the results on the 5th DOL, although the above differences did not reach statistical significance. Moreover, there was an increase in percentage (LOS group vs. before-LOS group: 0.47% [0.017–0.85] vs. 0.23% [0.04–0.61]; Wilcoxon, p = 0.043) and absolute count (LOS group vs. before-LOS group: 3.53/μl [2.1–8.3] vs. 2.24/μl [0–4.25]; Wilcoxon p = 0.0177) of the CL MO with PD-1 expression (Fig 3).
Fig 3. Monocyte subsets and PD-1 expression in the VLBW infants before and during late-onset sepsis.
(A) Absolute numbers of monocyte subsets. (B) Percentages of PD-1 expressing monocytes. (C) Absolute counts of PD-1 positive cells Data presented as median and IQR (box), compared with Wilcoxon test. Whiskers—range within 1.5 IQR. Classical monocytes are presented as a black graph, intermediate monocytes are presented as a graph with horizontal lines, whereas non-classical monocytes are presented as a graph with vertical lines. P-value was significant in case of *p<0.05.
There was no difference in PD-1 expression on the circulating MO during LOS between samples taken from the children with either Gram-positive or Gram-negative systemic infections.
PD-1 expression levels in LOS patients with septic shock and/or fatal outcome
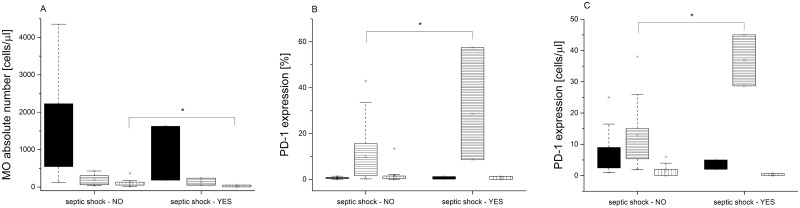
In a LOS-group, we observed significant differences in MO counts and PD-1 expression between patients who developed septic shock (n = 5) vs. those without this complication (n = 34). We observed a lower absolute number of NC subset in septic shock patients (31/μl [2–51] vs. 88/μl [51–134]; Wilcoxon, p = 0.0386). Moreover, they presented with a higher percentage (19.3% [8.59–57.5] vs. 4.44% [1.57–15.6]; Wilcoxon, p = 0.0498) as well as increased absolute number of the IM monocytes with PD-1 expression (36.8 cells/μl [28.7–45] vs. 12 cells/μl [5.2–15.5]; Wilcoxon, p = 0.0335) in comparison to these, whose sepsis was not complicated by septic shock (Fig 4).
Fig 4. Analysis of the monocyte subpopulations in the patients with or without septic shock.
(A) Absolute numbers of monocyte subsets. (B) Percentages of PD-1 positive cells. (C) Absolute numbers of PD-1 positive cells. Data presented as median and IQR (box), compared with Wilcoxon test. Whiskers—range within 1.5 IQR. Classical monocytes are presented as a black graph, intermediate monocytes are presented as a graph with horizontal lines, whereas non-classical monocytes are presented as a graph with vertical lines. P-value was significant in case of *p<0.05.
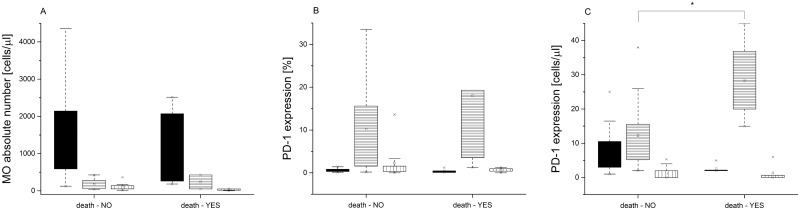
Finally, we demonstrated significant increase in the absolute number of PD-1 positive IM MO in the terminal patients compared to the infants who survived (Fig 5).
Fig 5. Analysis of the monocyte subpopulations in VLBW infants who either did nor did not survived LOS.
(A) Absolute numbers of monocyte subsets. (B) Percentages of PD-1-positive cells. (C) Absolute numbers of PD-1 positive cells. Data presented as median and IQR (box), compared with Wilcoxon test. Whiskers—range within 1.5 IQR. Classical monocytes are presented as a black graph, intermediate monocytes are presented as a graph with horizontal lines, whereas non-classical monocytes are presented as a graph with vertical lines. P-value was significant in case of *p<0.05.
Discussion
In our study, we showed that PD-1 receptor is expressed on the monocytes isolated from prematurely born VLBW children. We analyzed monocyte subsets in the above population on their 5th DOL and during LOS.
The analysis of MO isolated from the VLBW infants five days after birth revealed that the CL subset constituted the majority of the monocytes, whereas IM and NC MO were present in smaller numbers. It was previously reported that the CL MO subset was predominant under stable clinical condition and without ongoing sepsis in adult population [23]. Our results matched also the findings of another study by Wisgrill et al. [24] who showed that the majority of the monocytes from the cord blood of the VLBW babies constituted the CL MO subset.
We would like to emphasize our novel finding which demonstrates that variable PD-1 expression is dependent on the maturation state of the monocytes and that PD-1 receptor is present on the monocytes—cells that should present the ligand.
PD-1 expression is a marker of cell exhaustion [25], but PD-1 holds several other important functions, such as regulation of the immune tolerance phenomenon and normal processes of the immune cells such as their differentiation/maturation and activation [26]. Therefore, PD-1 expression on the immune cells’ surface appeared to be justified from the biological point of view even in the premature infants. Monocytes are a source of tissue macrophages that drive the inflammatory process [27]. It is hypothesized that a PD-1 dependent mechanism can limit the pro-inflammatory activity of these cells within tissues. Furthermore, the PD-1 dependent mechanism may also contribute to the production and release of pro-inflammatory cytokines in microcirculation by CD16+ monocytes.
In our study, infants with a lower gestational age (≤27 gestational weeks) presented higher absolute number of IM MO and had lower percentage of PD-1 positive IM MO in comparison to the cells isolated from the children born ≥28 gestational week. A potential link between lower gestational age and higher count of IM MO was also observed by the previously cited study by Wisgrill et al [24]. However, the reasons of the observed shift towards the IM MO subset in the preterm neonates require further studies. It was suggested that IM MO chemokine receptor pattern indicated their possible role in angiogenesis [28]. On the other hand, since they typically increased in inflammatory state, their higher number might also indicate inflammation that initially caused premature labor.
Taking into consideration our finding that PD-1 expression was diminished on the IM MO in the study population, we suggest the following explanation. According to the exome analysis of the sorted MO subsets, the IM and NC MO display a higher apoptotic potential than the CL MO subset [29], [30]. In concordance with the hypothesis of Zhao at al. [29] this finding may highlight a mechanism used to limit the pro-inflammatory response associated with CD16+ monocytes. As the PD-1 pathway is important in triggering apoptosis [31], our study supports and expands the above mentioned theory. We further propose that the apoptosis of CD16+ MOs is dependent on the level of PD-1 expression. As such, before differentiation into the NC MO, the IM MOs with high PD-1 expression may have already undergone apoptosis, and therein cannot be observed within the NC MO pool. Such a response may be beneficial in neonates who present with a constitutionally lower fraction of PD-1-positive IM MO. These neonates are more likely to have an early proinflammatory response, even to subliminal infectious agents, as the mechanism of elimination of major proinflammatory cytokine manufacturers is less effective. In this context, further research is necessary to evaluate the levels of PD-1-positive IM MO levels in newborns born full term.
We observed that there was higher count of PD-1 positive NC MO subset on the 5th DOL in the infants exposed to antenatal steroid administration. According to Fingerle-Rowson et al. [32], and Dayyani et al. [33], glucocorticoid treatment led to decreased number of NC MO due to a selective induction of their apoptosis. We did not observe any decline in NC MO, however our study differed from the researches cited above in the dosage and timing of steroid administration prior to analysis of MO subsets. There were conflicting studies in regard to steroid effect on the monocyte function. In animal studies by Kramer et al. [34], there was a time-dependent suppression of MO functions, such as phagocytic capacity, hydrogen peroxide production or Il-6 production, in preterm-born lambs after antenatal glucocorticoids administration. In contrast, Kavelaars et al. [35] analyzed cord blood from 38 preterm-born children whose mothers had received full course of betamethasone due to threatened preterm labor and showed lack of effect of the antenatal steroid treatment on Il-6 production by MO. Xing et al. [36], who studied immunosuppressive effects of glucocorticoids in anti-cancer therapy, showed that dexamethasone and hydrocortisone could enhance PD-1 expression both in mouse and human activated T cells in a dose-dependent manner. Further studies are needed to evaluate whether similar mechanisms are present in MO.
In our study, we observed a higher count of IM and NC MO subsets in the second blood sample collected within 24 hours after onset of LOS. Similar results were reported by Skrzeczyńska et al. [7] in their studies of the MO subsets changes during neonatal sepsis. Moreover, we observed a significant rise in percentage and absolute number of IM MO with PD-1 expression in a subgroup of patients who developed septic shock. Also, children who died in the course of septic shock had increased absolute number of PD-1 positive IM MO. Our findings were consistent with the report by Huang and colleagues [16] who demonstrated that PD-1 expression on the circulating MO was higher in adult patients with septic shock than in healthy volunteers. Similarly, Guignant et al [37]. in their study of 64 patients with septic shock reported increased expression of PD-1 and its ligands on the MO and CD4+T lymphocytes on days 1–2 and 3–5 after the onset of septic shock. Increased PD-1 and PD-1 ligands’ expression was associated with typical sepsis-related immune dysfunctions, such as decreased monocyte HLA-DR expression, decreased circulating CD4+ T-cell count, increased percentage of the T regulatory cells, as well as increased rates of secondary nosocomial infections and mortality. In contrast, the study by Zhang et al. [38] observed up-regulation of solely ligands for PD-1 (PD-L1), but not PD-1, on CD14+ MO in septic shock adult patients in comparison to healthy volunteers. The only study to evaluate PD-1 expression in neonatal sepsis was performed in PD-1 knockout murine neonates with sepsis caused by cecal slurry [39]; it showed improved survival of the septic PD-1 knockout mice in comparison with the wild-type. In general, it is assumed that increased activity of the PD1/PD-L1 system in sepsis could lead to depletion of the key cells, such as monocytes and T cells, necessary for proper response to infection, therefore impairing essential anti-microbial and regulatory activities [37]. Data from ex-vivo experiments [40], animal experiments [41], as well as from the clinical trials with oncologic patients [42] showed that both antibodies blocking the PD-1 pathway and selected hormones (ghrelin, growth hormone) [43] not only diminished expression of PD-1 related molecules, but also restored function of the MO, neutrophils, T cells, and natural killer cells, which may restore immune function and diminish immunosuppression during sepsis.
We also showed an increase of PD-1-positive CL monocytes. During sepsis, there is an increase in PD-1 expression on the classical MO, and, as they mature, they differentiate into CD16+ (intermediate and non-classical) MO. These MO subsets perpetuate shock mechanisms that develop during sepsis progression. This may be viewed as a physiological “safety mechanism”, such as, less CL MO are able to mature into pro-inflammatory IM and NC MO since they are susceptible to apoptosis at the classical stage.
In summary, the data presented herein demonstrate that 1) MO from VLBW children show expression of PD-1, 2) children whose mothers had received antenatal steroids presented with higher absolute number of PD-1 positive NC MO subset on the 5th DOL, 3) extremely premature infants (born ≤27 gestational week) diagnosed with LOS during their hospitalization showed lower percentage of PD-1 positive IM MO compared to the population born after 28th week of pregnancy 4) there was a rise in the percentage of PD-1 positive CL MO during LOS, 5) prematurely born infants with septic shock or fatal outcome showed a higher percentage and absolute numbers of IM MO with PD-1 expression.
We believe that further research is needed to evaluate changes in expression of PD-1 system on MO subsets during sepsis and septic shock in infant population. Identification of the factors, which may regulate and/or temporarily inhibit PD-1 receptors might provide a valuable tool to fight severe inflammatory diseases, such as neonatal sepsis.
Acknowledgments
We would like to thank Mariola Ożóg and Barbara Hajto for their skillful technical assistance.
Data Availability
All files are available from the Harvard Dataverse database doi:10.7910/DVN/AHUUCD.
Funding Statement
Study supported by National Science Center, Poland: grant number: DEC-2012/07/B/NZ5/01221.
References
- 1.Stoll BJ, Hansen NI, Sánchez PJ, Faix RG, Poindexter BB, Van Meurs KP, et al. Early onset neonatal sepsis: the burden of group B Streptococcal and E. coli disease continues. Pediatrics. 2011. May;127(5):817–26 doi: 10.1542/peds.2010-2217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levy O. Innate immunity of the newborn: basic mechanisms and clinical correlates. Nat Rev Immunol. 2007. May;7(5):379–90 doi: 10.1038/nri2075 [DOI] [PubMed] [Google Scholar]
- 3.Ziegler-Heitbrock L, Ancuta P, Crowe S, Dalod M, Grau V, Hart DN, et al. Nomenclature of monocytes and dendritic cells in blood. Blood. 2010. October 21;116(16):e74–80 doi: 10.1182/blood-2010-02-258558 [DOI] [PubMed] [Google Scholar]
- 4.Ziegler-Heitbrock L. Monocyte subsets in man and other species. Cell Immunol. 2014. May-Jun;289(1–2):135–9 doi: 10.1016/j.cellimm.2014.03.019 [DOI] [PubMed] [Google Scholar]
- 5.Ziegler-Heitbrock L. The CD14+ CD16+ blood monocytes: their role in infection and inflammation. J Leukoc Biol. 2007. March;81(3):584–92 doi: 10.1189/jlb.0806510 [DOI] [PubMed] [Google Scholar]
- 6.Fingerle G, Pforte A, Passlick B, Blumenstein M, Ströbel M, Ziegler-Heitbrock HW. The novel subset of CD14+/CD16+ blood monocytes is expanded in sepsis patients. Blood. 1993. November 15;82(10):3170–6 [PubMed] [Google Scholar]
- 7.Skrzeczyńska J, Kobylarz K, Hartwich Z, Zembala M, Pryjma J. CD14+CD16+ monocytes in the course of sepsis in neonates and small children: monitoring and functional studies. Scand J Immunol. 2002. June;55(6):629–38 [DOI] [PubMed] [Google Scholar]
- 8.Hotchkiss RS, Coopersmith CM, McDunn JE, Ferguson TA. The sepsis seesaw: tilting toward immunosuppression. Nat Med. 2009. May;15(5):496–7 doi: 10.1038/nm0509-496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pickkers P, Kox M. Towards precision medicine for sepsis patients. Crit Care. 2017. January 12;21(1):11 doi: 10.1186/s13054-016-1583-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992. November;11(11):3887–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liang SC, Latchman YE, Buhlmann JE, Tomczak MF, Horwitz BH, Freeman GJ, et al. Regulation of PD-1, PD-L1, and PD-L2 expression during normal and autoimmune responses. Eur J Immunol. 2003. October;33(10):2706–16 doi: 10.1002/eji.200324228 [DOI] [PubMed] [Google Scholar]
- 12.Okazaki T, Honjo T. PD-1 and PD-1 ligands: from discovery to clinical application. Int Immunol. 2007. July;19(7):813–24 doi: 10.1093/intimm/dxm057 [DOI] [PubMed] [Google Scholar]
- 13.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704 doi: 10.1146/annurev.immunol.26.021607.090331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamazaki T, Akiba H, Iwai H, Matsuda H, Aoki M, Tanno Y, et al. Expression of programmed death 1 ligands by murine T cells and APC. J Immunol. 2002. November 15;169(10):5538–45 [DOI] [PubMed] [Google Scholar]
- 15.Riley JL. PD-1 signaling in primary T cells. Immunol Rev. 2009. May;229(1):114–25 doi: 10.1111/j.1600-065X.2009.00767.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang X, Venet F, Wang YL, Lepape A, Yuan Z, Chen Y, et al. PD-1 expression by macrophages plays a pathologic role in altering microbial clearance and the innate inflammatory response to sepsis. Proc Natl Acad Sci U S A. 2009. April 14;106(15):6303–8 doi: 10.1073/pnas.0809422106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brahmamdam P, Inoue S, Unsinger J, Chang KC, McDunn JE, Hotchkiss RS. Delayed administration of anti-PD-1 antibody reverses immune dysfunction and improves survival during sepsis. J Leukoc Biol. 2010. August;88(2):233–40 doi: 10.1189/jlb.0110037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Y, Zhou Y, Lou J, Li J, Bo L, Zhu K, et al. PD-L1 blockade improves survival in experimental sepsis by inhibiting lymphocyte apoptosis and reversing monocyte dysfunction. Crit Care. 2010;14(6):R220 doi: 10.1186/cc9354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013. February;41(2):580–637 doi: 10.1097/CCM.0b013e31827e83af [DOI] [PubMed] [Google Scholar]
- 20.Goldstein B, Giroir B, Randolph A; International Consensus Conference on Pediatric Sepsis. International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med. 2005. January;6(1):2–8 doi: 10.1097/01.PCC.0000149131.72248.E6 [DOI] [PubMed] [Google Scholar]
- 21.Siedlar M, Strach M, Bukowska-Strakova K, Lenart M, Szaflarska A, Węglarczyk K, et al. Preparations of intravenous immunoglobulins diminish the number and proinflammatory response of CD14+CD16++ monocytes in common variable immunodeficiency (CVID) patients. Clin Immunol. 2011. May;139(2):122–32 doi: 10.1016/j.clim.2011.01.002 [DOI] [PubMed] [Google Scholar]
- 22.Heimbeck I, Hofer TP, Eder C, Wright AK, Frankenberger M, Marei A, et al. Standardized single-platform assay for human monocyte subpopulations: Lower CD14+CD16++ monocytes in females. Cytometry A. 2010. September;77(9):823–30 [DOI] [PubMed] [Google Scholar]
- 23.Yang J, Zhang L, Yu C, Yang XF, Wang H. Monocyte and macrophage differentiation: circulation inflammatory monocyte as biomarker for inflammatory diseases. Biomark Res. 2014. January 7;2(1):1 doi: 10.1186/2050-7771-2-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wisgrill L, Groschopf A, Herndl E, Sadeghi K, Spittler A, Berger A, et al. Reduced TNF-α response in preterm neonates is associated with impaired nonclassic monocyte function. J Leukoc Biol. 2016. September;100(3):607–12 doi: 10.1189/jlb.4A0116-001RR [DOI] [PubMed] [Google Scholar]
- 25.Monneret G, Gossez M, Venet F. Sepsis in PD-1 light. Crit Care. 2016. July 5;20(1):186 doi: 10.1186/s13054-016-1370-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fuertes Marraco SA, Neubert NJ, Verdeil G, Speiser DE. Inhibitory Receptors Beyond T Cell Exhaustion. Front Immunol. 2015. June 26;6:310 doi: 10.3389/fimmu.2015.00310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ziegler-Heitbrock L, Hofer TP. Toward a refined definition of monocyte subsets. Front Immunol. 2013. February 4;4:23 doi: 10.3389/fimmu.2013.00023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zawada AM, Rogacev KS, Rotter B, Winter P, Marell RR, Fliser D, et al. SuperSAGE evidence for CD14++CD16+ monocytes as a third monocyte subset. Blood. 2011. September 22;118(12):e50–61 doi: 10.1182/blood-2011-01-326827 [DOI] [PubMed] [Google Scholar]
- 29.Zhao C, Tan YC, Wong WC, Sem X, Zhang H, Han H, et al. The CD14(+/low)CD16(+) monocyte subset is more susceptible to spontaneous and oxidant-induced apoptosis than the CD14(+)CD16(-) subset. Cell Death Dis. 2010. November 4;1:e95 doi: 10.1038/cddis.2010.69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wong KL, Tai JJ, Wong WC, Han H, Sem X, Yeap WH, et al. Gene expression profiling reveals the defining features of the classical, intermediate, and nonclassical human monocyte subsets. Blood. 2011. August 4;118(5):e16–31. doi: 10.1182/blood-2010-12-326355 [DOI] [PubMed] [Google Scholar]
- 31.Roy S, Gupta P, Palit S, Basu M, Ukil A, Das PK. The role of PD-1 in regulation of macrophage apoptosis and its subversion by Leishmania donovani. Clin Transl Immunology. 2017. May 5;6(5):e137 doi: 10.1038/cti.2017.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fingerle-Rowson G, Angstwurm M, Andreesen R, Ziegler-Heitbrock HW. Selective depletion of CD14+ CD16+ monocytes by glucocorticoid therapy. Clin Exp Immunol. 1998. June;112(3):501–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dayyani F, Belge KU, Frankenberger M, Mack M, Berki T, Ziegler-Heitbrock L. Mechanism of glucocorticoid-induced depletion of human CD14+CD16+ monocytes. J Leukoc Biol. 2003. July;74(1):33–9 [DOI] [PubMed] [Google Scholar]
- 34.Kramer BW, Ikegami M, Moss TJ, Nitsos I, Newnham JP, Jobe AH. Antenatal betamethasone changes cord blood monocyte responses to endotoxin in preterm lambs. Pediatr Res. 2004. May;55(5):764–8 doi: 10.1203/01.PDR.0000120678.72485.19 [DOI] [PubMed] [Google Scholar]
- 35.Kavelaars A, van der Pompe G, Bakker JM, van Hasselt PM, Cats B, Visser GH, et al. Altered immune function in human newborns after prenatal administration of betamethasone: enhanced natural killer cell activity and decreased T cell proliferation in cord blood. Pediatr Res. 1999. March;45(3):306–12 doi: 10.1203/00006450-199903000-00003 [DOI] [PubMed] [Google Scholar]
- 36.Xing K, Gu B, Zhang P, Wu X. Dexamethasone enhances programmed cell death 1 (PD-1) expression during T cell activation: an insight into the optimum application of glucocorticoids in anti-cancer therapy. BMC Immunol. 2015. June 26;16:39 doi: 10.1186/s12865-015-0103-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guignant C, Lepape A, Huang X, Kherouf H, Denis L, Poitevin F, et al. Programmed death-1 levels correlate with increased mortality, nosocomial infection and immune dysfunctions in septic shock patients. Crit Care. 2011;15(2):R99 doi: 10.1186/cc10112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang Y, Li J, Lou J, Zhou Y, Bo L, Zhu J, et al. Upregulation of programmed death-1 on T cells and programmed death ligand-1 on monocytes in septic shock patients. Crit Care. 2011;15(1):R70 doi: 10.1186/cc10059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Young WA, Fallon EA, Heffernan DS, Efron PA, Cioffi WG, Ayala A. Improved survival after induction of sepsis by cecal slurry in PD-1 knockout murine neonates. Surgery. 2017. May;161(5):1387–1393 doi: 10.1016/j.surg.2016.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Patera AC, Drewry AM, Chang K, Beiter ER, Osborne D, Hotchkiss RS. Frontline Science: Defects in immune function in patients with sepsis are associated with PD-1 or PD-L1 expression and can be restored by antibodies targeting PD-1 or PD-L1. J Leukoc Biol. 2016. December;100(6):1239–1254 doi: 10.1189/jlb.4HI0616-255R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shindo Y, McDonough JS, Chang KC, Ramachandra M, Sasikumar PG, Hotchkiss RS. Anti-PD-L1 peptide improves survival in sepsis. J Surg Res. 2017. February;208:33–39 doi: 10.1016/j.jss.2016.08.099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell. 2015. April 13;27(4):450–61 doi: 10.1016/j.ccell.2015.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou M, Yang WL, Aziz M, Ma G, Wang P. Therapeutic effect of human ghrelin and growth hormone: Attenuation of immunosuppression in septic aged rats. Biochim Biophys Acta. 2017. January 20 pii: S0925-4439(17)30027-3. [Epub ahead of print] doi: 10.1016/j.bbadis.2017.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All files are available from the Harvard Dataverse database doi:10.7910/DVN/AHUUCD.