Abstract
Background
Tungiasis is a neglected tropical disease caused by female sand fleas (Tunga penetrans) embedded in the skin. The disease is associated with important morbidity. Tungiasis is endemic along the Coast of Kenya with a prevalence ranging from 11% to 50% in school-age children. Hitherto, studies on epidemiological characteristics of tungiasis in Africa are scanty.
Methods
In a cross-sectional study 1,086 individuals from 233 households in eight villages located in Kakuyuni and Malanga Sub-locations, Kilifi County, on the Kenyan Coast, were investigated. Study participants were examined systematically and the presence and severity of tungiasis were determined using standard methods. Demographic, socio-economic, environmental and behavioral risk factors of tungiasis were assessed using a structured questionnaire. Data were analyzed using bivariate and multivariate regression analysis.
Results
The overall prevalence of tungiasis was 25.0% (95% CI 22.4–27.5%). Age-specific prevalence followed an S-shaped curve, peaking in the under-15 year old group. In 42.5% of the households at least one individual had tungiasis. 15.1% of patients were severely infected (≥ 30 lesions). In the bivariate analysis no specific animal species was identified as a risk factor for tungiasis. Multivariate analysis showed that the occurrence of tungiasis was related to living in a house with poor construction characteristics, such as mud walls (OR 3.35; 95% CI 1.71–6.58), sleeping directly on the floor (OR 1.68; 95% CI 1.03–2.74), the number of people per sleeping room (OR = 1.77; 95% CI 1.07–2.93) and washing the body without soap (OR = 7.36; 95% CI 3.08–17.62). The odds of having severe tungiasis were high in males (OR 2.29; 95% CI 1.18–44.6) and were very high when only mud puddles were available as a water source and lack of water permitted washing only once a day (OR 25.48 (95% CI 3.50–185.67) and OR 2.23 (95% CI 1.11–4.51), respectively).
Conclusions
The results of this study show that in rural Kenya characteristics of poverty determine the occurrence and the severity of tungiasis. Intra-domiciliary transmission seems to occur regularly.
Author summary
Tungiasis (sand flea disease) is an ectoparasitic skin disease and belongs to the group of NTDs (Neglected Tropical Diseases). It is caused by sand fleas penetrating into the skin of the feet, causing an inflammatory reaction with pain and itching. Attempts to remove the flea with inappropriate sharp tools are painful and cause bacterial superinfection, eventually leading to restricted mobility. In resource-poor communities without access to health care, prevention is the most valuable control measure. In this study we identified important risk factors for the occurrence of tungiasis and sever disease. The most relevant risk factors were poor hygiene practices and poor housing conditions. Simple control interventions such as having solid walls and floors in the house, improved access to water and washing with soap could reduce the disease burden considerably.
Introduction
Tungiasis (sand flea disease) is a parasitic skin disease caused by female sand fleas (Tunga penetrans) penetrated into the skin of human or animal hosts. Tungiasis belongs to the family of neglected tropical diseases (NTDs) [1,2]. It is prevalent in resource-poor rural communities in sub-Saharan Africa, the Caribbean and South America [3–7]. Children between 5 and 14 years and the elderly bear the highest disease burden with prevalences up to 85% [7]. While the great majority of patients harbours less than 10 embedded sand fleas, single individuals may have hundreds of parasites [8,9]. Once embedded in the skin, typically of the toes, the soles and the heels [10], the flea matures. Within the period of up to five weeks it grows until it reaches the size of a pea, produces and releases eggs and finally dies [11]. Morbidity is related to an intense inflammatory response triggered by the development of sand fleas embedded in the epidermis [10,12,13]. Bacterial superinfection is common and intensifies the inflammation. Inflammation and mutilation of the feet eventually lead to impairment of mobility [12]. Main risk factors found in previous studies in Brazil and Nigeria are poor housing and the presence of animals on the compound [14,15]. Awareness of the public health importance of tungiasis has been growing in Kenya in recent years, but valid data on epidemiological characteristics do not exist. In order to develop a sustainable control program for tungiasis in resource-poor communities along the Kenyan Coast, two population-based studies were performed: one in households and the other in schools. Here, we report the results of the household-based study.
Materials and methods
Ethics statement
The study was approved by the Ethics Review Committee at Pwani University, Kilifi County, Kenya; approval number ERC/PhD/010/2014. The custodians and their protégés were informed about the objectives and procedures of the study in their mother language (Giriama or Swahili) by a Community Health Worker (CHW). The right to deny participation and withdraw consent at any given time was clearly explained.
The informed consent form was read out loud word by word in Giriama or Swahili and explained further when required, before any interviews were conducted. Questions of the custodian and the children were discussed and answered by a CHW. Consent was obtained via fingerprint or signature from the legal guardian. The examination was performed in a protected surrounding to guarantee the privacy of the patient. Children and adolescents were only examined in the presence of their caregiver.
Any individuals found to have tungiasis were referred to the local CHWs for treatment and follow up according to their standard protocols which have been approved for use by the Ministry of Health at national and county level. For other illnesses requiring treatment a referral form was prepared by a CHW, and patients were referred to the nearest Health Facility. Washing and treatment were also made available for compound members with tungiasis who did not participate in the study.
The information provided to the households verbally is included as supplementary electronic information along with the consent form which was to be signed (S1 Appendix).
Study area and study population
The study was performed in eight villages located in Kakuyuni and Malanga Sub-locations of Malindi Sub-county, Kilifi County, eastern Kenya, in the dry season from August to October 2014. In the area tungiasis is endemic with prevalences ranging from 30 to 85% in school age children (S2 Appendix).
In Malindi Sub-county rural communities are small and consist of clusters of two to five houses separated by bush or farm land. The area is divided into two ecological zones: Kakuyuni Sub-location, a very densely populated area in the coastal strip with homesteads located side by side. It has a tropical climate with an average annual rainfall of 1,200 mm, temperatures ranging from 28–34°C and high humidity most of the year. Malanga Sub-location is located inland and is much drier with average annual rainfall of 400 mm. Homesteads are located about 100 m from each other in this area. There are two rainy seasons: one between March and May and the other between October and November, interspersed with dry seasons.
Malindi Sub-county has a population of 272,000 with 42.3% being under 15 years of age. The population included in the survey are entirely of the Giriama tribe. While 55% of households have access to piped water and 60% to improved sanitation, only 17% have access to electricity (Malindi Public Health Office 2015). Many of the people live in mud-walled houses with a thatch roof and sandy floor (First Kilifi County Integrated Development Plan 2013–2017). For Kilifi County as whole the poverty rate (i.e. < 1 US$ per day) is 71.4% (http://www.crakenya.org/county/kilifi/). The majority of the population in the study area practice subsistence agriculture, charcoal burning and small scale businesses. The main foodstuffs cultivated are maize, cassava, coconuts, and mangoes.
Study design
The study was a cross-sectional survey of a random sample of households in Kakuyuni and Malanga Sub-locations, Kilifi County, Kenya. These sublocations were selected because no intervention against tungiasis had been performed so far.
For this study a household was classified as a single structure/house. Since most people live in homesteads of extended families, sharing eating, washing and sanitation facilities, we selected one structure/house per homestead in a standardized manner, always choosing the first house on the left when entering the compound.
Individuals of any age and sex were eligible for participation as long as they had spent at least 4 nights per week in the selected household for the last three months. To be included, a household needed to have someone over the age of 18 present at the time of the visit to sign the consent forms and respond to the interview questions.
During the preparation phase contact was made with the County and District leadership in both the Ministry of Health and the Ministry of Education, the Zonal Education Officer and the Community Health Officers to obtain their approvals and support for the study. We held meetings with all CHWs in each Sub-location, gave specific training on tungiasis and explained the aims and procedures of the study, emphasizing that participation was completely voluntary and subjects had the opportunity to withdraw from the study at any point of time.
The study was carried out between August 13 and October 5, 2014, i. e. during a dry season. A total of 1,086 individuals from 233 households in eight villages were included in the study.
Data were collected through a door-to-door survey of the selected households with the help of local CHWs. Eligible patients were explained the procedure and were asked for consent. In case of minors a caregiver (usually the mother) was asked to provide informed consent. If household members were not present during our first visit, we returned to the house on one further occasion. Individuals who could not be reached at home during the second visit were invited to come to the local health facility within the next days. Household members who could not be examined on any occasion were not included in the study.
In order to identify risk factors for the occurrence of tungiasis and severe disease, we requested information about demographic, socio-economic, environmental and behavioural characteristics of the individuals and the household. Structured interviews were conducted with the head of household (usually the mother) using a pre-tested questionnaire in Giriama or Swahili. Environmental, socioeconomic and some behavioural risk factors were assessed at the household level, other risk factors were assessed on the individual level.
Since cash flow does not correctly indicate the economic status of a household in low-income communities [16,17], we used an asset score similar to the one previously established for cutaneous larva migrans, another neglected tropical skin disease associated with poverty [18]. The score is composed of the following assets:
Presence in the household of a radio (2 points), television (5 points), fridge (5 points), gas/solar lamp (1 point); possession of at least one mobile phone (1 point), bicycle (3 points) and motor bike (10 points). The score can vary between 0 and 27 points.
For the diagnosis of tungiasis, the feet of the patients were carefully washed with soap in a basin. Each individual was examined for tungiasis based on a standardized procedure [3]. Since a high number of lesions at the feet frequently coincides with the presence of ectopic lesions at the hands [19], we also systematically examined the hands of the patients. Patients were also asked whether they had tungiasis lesions in other regions of the body. Lesions were staged according to the Fortaleza classification and counted [11]:
stage I: penetrating sand flea
stage II: brownish/black dot with a diameter of 1–2 mm surrounded or not by an erythema
stage III: circular yellow-white watch glass-like patch with a diameter of 3–10 mm and with a central black dot
stage IV: brownish-black crust with or without surrounding necrosis
Stage I to III are viable sand fleas; in stage IV the parasite is dying or already dead [11] Lesions manipulated with a sharp instrument (by the patient or their caregiver) with the intention to remove the embedded parasite were documented as manipulated lesions. Based on the number of lesions present, the intensity of tungiasis was classified as light (1–5 lesions), moderate (6–30 lesions) or high (>30 lesions) [14].
Statistical analysis
The data were entered into an Excel database (Excel Version 2013, Microsoft, Redmont, Washington, USA), checked for errors which might have occurred during data entry and then transferred to SPSS (PASW Statistics 18.0, SPSS Inc., Chicago, IL, USA). The data analysis was carried out using the Analysis ToolPack Add-In (Microsoft, Redmont, Washington, USA). Graphs were created with the PowerPivot Add-In (Microsoft, Redmont, Washington, USA). Relative frequencies were compared with the Chi-square test and Fisher’s exact test. The Spearman rank correlation coefficient was calculated to determine the significance of correlations. Odds ratios together with their 95% confidence interval (CI) were calculated first in a bivariate analysis. In a second step, variables which were significantly (p < 0.05) related to the occurrence of tungiasis and/or severe disease were entered in a multivariate logistic regression model with stepwise forward inclusion of variables to identify independent exposure variables. Factors which showed up as significant in the bivariate analysis but were assessed only in individuals older than 18 were not included in the logistic regression model. For risk factors suitable for an intervention, population attributable fractions (PAF) were calculated. The PAF, calculated as % exposed among cases x attributable risk (AR), is the fraction of cases which would not have occurred if an exposure had been avoided, assuming the exposure is causal and the other risk factors in the population remain unchanged. AR is calculated as (OR– 1)/OR and is the risk of tungiasis in the exposed group due to the exposure. The sample size of this study was estimated based on field studies performed in Brazil and Nigeria and contained the following assumptions: control-case-ratio 1:3; hypothetical proportion of controls with exposure 30%; least detectable odds ratio 1.75; power of the test 0.90; confidence level 0.95. This would require 205 cases and 610 controls. To account for uncertainties and drop out we attempted to include a sample of 1000 individuals.
Results
Characteristics of the study population
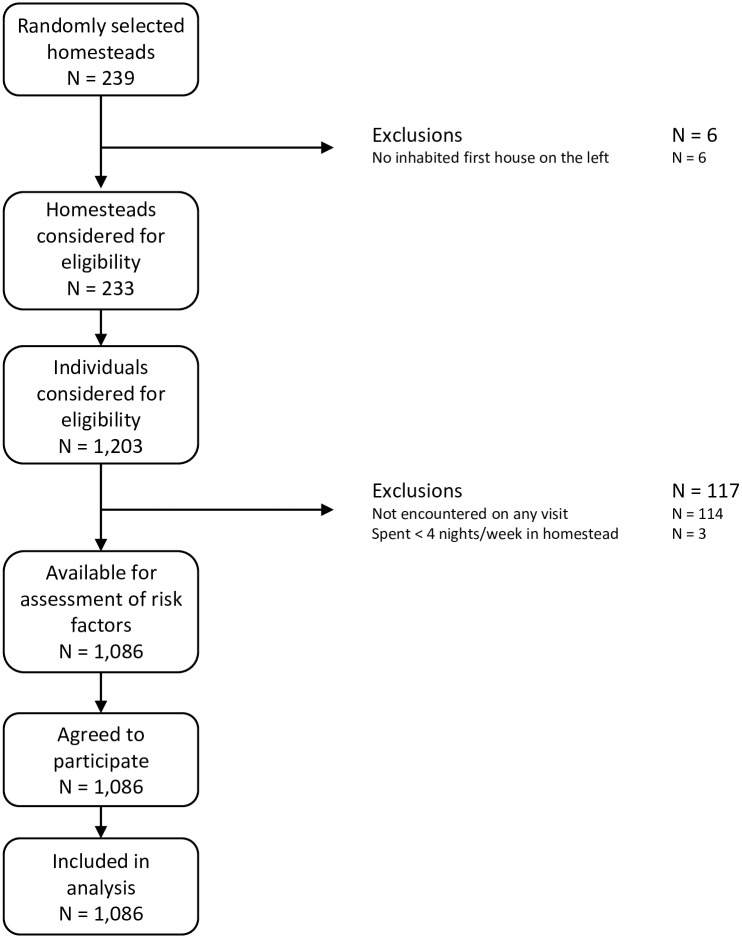
Of the 239 homesteads visited 233 fulfilled the criterion of having an inhabited first house on the left. Of the 1,203 individuals living in these households, 114 (72 males and 42 females) were not encountered on any of the visits, reducing the study population to 1,089. Of these, three did not fulfil the inclusion criterion of having spent at least four nights per week in the selected homestead during the last three months. Thus, the number of individuals available for the assessment of risk factors was 1,086, all of which agreed to being interviewed and examined (Fig 1). Three hundred and twenty four patients (70 households) were recruited from Kakuyuni, 221 (41) from Goshi, and 172 (43) from Vihingoni community in Kakuyuni Sub-location; 116 (24) from Mtoroni, 27 (5) from Yembe, 133 (28) from Kadzitsoni, 76 (18) from Chembe and 17 (4) from Bahati community in Malanga Sub-Location.
Fig 1. Flow chart of study population.
The study population comprised 57.3% females, and 58.6% under the age of 15 years. Of those over 18 years, 54.1% reported being Christians while 19.6% were Muslims, 31.4% were illiterate and a further 34% had not completed primary school education. The majority of houses (89%) had dirt floors and mud walls (84.5%), did not have improved latrines (56.7% used the bush, 32.6% used traditional latrines) and shared community taps for their source of water (83.7%) (Tables 1 and 2).
Table 1. Demographic characteristics of the study population (n = 1,086 individuals).
| Characteristic | Frequency | (%) |
|---|---|---|
| Sex | ||
| Female | 622 | 57.3 |
| Male | 464 | 42.7 |
| Age (years) | ||
| 0–4 | 211 | 19.4 |
| 5–9 | 245 | 22.6 |
| 10–14 | 180 | 16.6 |
| 15–19 | 80 | 7.4 |
| 20–39 | 189 | 17.4 |
| 40–59 | 114 | 10.5 |
| ≥ 60 | 64 | 5.9 |
| n.k. | 3 | 0.3 |
| Village | ||
| Kakuyuni | 324 | 29.8 |
| Goshi | 221 | 20.3 |
| Vihingoni | 172 | 15.8 |
| Mtoroni | 116 | 10.7 |
| Yembe | 27 | 2.5 |
| Kadzitsoni | 133 | 12.2 |
| Chembe | 76 | 7.0 |
| Bahati | 17 | 1.6 |
| Religiona | ||
| None | 19 | 4.9 |
| Muslim | 76 | 19.6 |
| Christian | 210 | 54.1 |
| Traditionist | 76 | 19.6 |
| n.k. | 7 | 1.8 |
| Educationa | ||
| Illiterate | 122 | 31.4 |
| Primary school not completed | 132 | 34.0 |
| Primary school completed | 133 | 34.3 |
| n.k. | 1 | 0.3 |
| Occupationa | ||
| Unemployed | 89 | 22.9 |
| Farmer | 179 | 46.1 |
| Other occupation | 113 | 29.1 |
| n.k. | 7 | 1.8 |
a information on religion, education and occupation were only collected for adults ≥ 18 years (n = 381)
n.k. = not known
Table 2. Socio-economic characteristics of the study population (n = 233 households).
| Characteristic | Frequency | (%) |
|---|---|---|
| Housing | ||
| Type of floor material | ||
| Cement/stone | 26 | 11.2 |
| Smeared mud | 136 | 58.4 |
| Sand/dust | 70 | 30.0 |
| Mixed mud and sand | 1 | 0.4 |
| Type of wall material | ||
| Stone | 31 | 13.3 |
| Mud | 197 | 84.5 |
| Mixed stone and mud | 5 | 2.1 |
| Type of roof material | ||
| Makutia | 112 | 48.1 |
| Mabatib | 118 | 50.6 |
| Mixed makuti and mabati | 1 | 0.4 |
| Tiles | 2 | 0.9 |
| Sanitation | ||
| Toilet | ||
| Flush toilet | 10 | 4.3 |
| Ventilated pit latrine | 15 | 6.4 |
| Traditional latrine | 76 | 32.6 |
| Bush | 132 | 56.7 |
| Waste disposal | ||
| Pit | 85 | 36.5 |
| Pile | 100 | 42.9 |
| Spread | 47 | 20.2 |
| Compost | 1 | 0.4 |
| Water source | ||
| Tap on compound | 36 | 15.5 |
| Shared community tap | 195 | 83.7 |
| Mud puddles | 2 | 0.9 |
| Time to reach water source (min) | ||
| 0–4 | 73 | 31.3 |
| 5–9 | 53 | 22.7 |
| 10–14 | 42 | 18.0 |
| 15–19 | 20 | 8.6 |
| 20–29 | 12 | 5.2 |
| ≥ 30 | 33 | 14.2 |
| Healthcare | ||
| Time to reach next health facility (min) | ||
| 0–9 | 16 | 6.9 |
| 10–19 | 40 | 17.2 |
| 20–29 | 43 | 18.5 |
| 30–39 | 70 | 30.0 |
| 40–49 | 16 | 6.9 |
| 50–59 | 2 | 0.9 |
| ≥ 60 | 46 | 19.7 |
| Economic status | ||
| Monthly income per household (KSh)c | ||
| 0–4850 | 87 | 37.3 |
| > 4850 | 40 | 17.2 |
| n.k. | 106 | 45.5 |
| Number of meals per day | ||
| 1 | 6 | 2.6 |
| 2 | 62 | 26.6 |
| > 2 | 165 | 70.8 |
| Land ownership | ||
| Own | 228 | 97.9 |
| Rent | 3 | 1.3 |
| Squatt | 2 | 0.9 |
| Domestic animals | ||
| Animals on compound | ||
| Any animal | 205 | 88.0 |
| Dogs | 59 | 25.3 |
| Cats | 59 | 25.3 |
| Goats | 140 | 60.1 |
| Cows | 70 | 30.0 |
| Chicken | 172 | 73.8 |
| Ducks | 42 | 18.0 |
a palm leaves
b corrugated iron sheets
c KSh 4850 correspond to the minimum wage in Kenya for an unskilled worker in agricultural industry at the time of the survey and is equivalent to ~ 55 USD
n.k. = not known
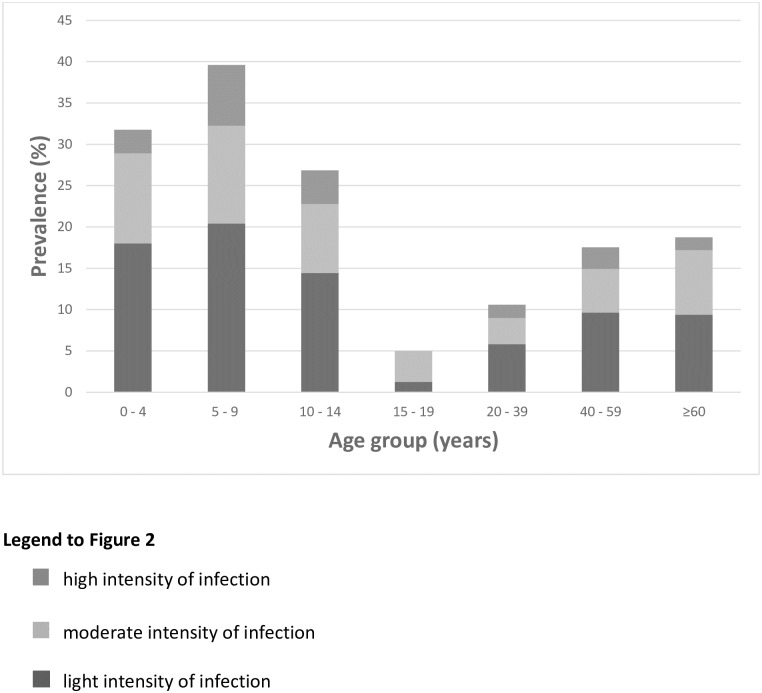
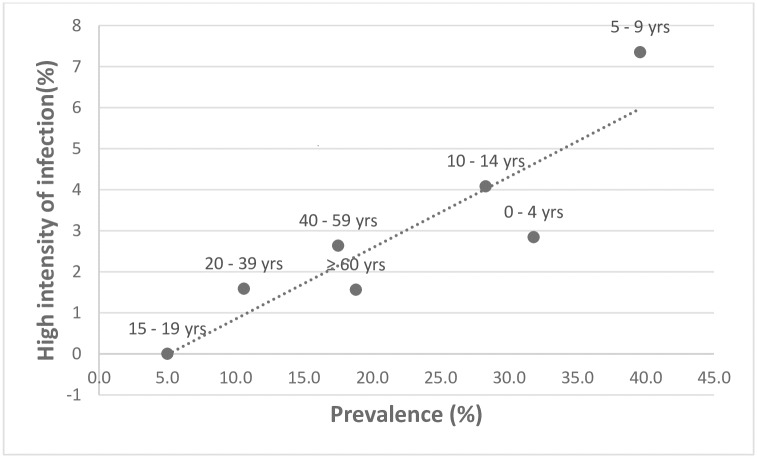
The overall prevalence of tungiasis in the study population was 25.0% (95% CI 22.4–27.5%), but in 42.5% of the households at least one individual had tungiasis. Of those with tungiasis, 52.8% had a light (1 to 5 lesions), 32.1% a moderate (6 to 30 lesions) and 15.1% a high intensity of infection (>30 lesions). Five percent of the patients had ectopic lesions, almost exclusively on the hands. Age-specific prevalences and intensity of infection are shown in Fig 2. There was a tendency of higher occurrence of tungiasis in elderly individuals living alone, although it was not significant (p = 0.2111). In 14 single-person households there were two adults < 40 years without tungiasis, six 40 to 59 year olds of whom 2 had tungiasis and six > 60 year olds of whom 4 had a mild to severe tungiasis. The prevalence of infection and high intensity of infection correlated significantly (Fig 3) (rho = 0.90, p = 0.0059), with the highest prevalence being in the under 15 year olds and over 40 years. The youngest patient was four months old, 4 patients were younger than one year, while the oldest patient was 80 years old.
Fig 2. Age-specific prevalence and intensity of tungiasis in the study area.
Column heights indicate overall prevalence in age groups.
Fig 3. Correlation between age-specific prevalence and age-specific frequency of high intensity of infection (> 30 lesions); rho = 0.90, p = 0.006.
Risk factor analysis
Prevalence and severity of tungiasis varied considerably between the villages with Yembe and Bahati having a prevalence of 59.3% and 64.7% respectively, while Mtoroni and Vihingoni had prevalences of 7.8% and 13.4% (Table 3). Residence in Yembe and Bahati was a significant risk factor for tungiasis (OR 17.3 and 21.8 respectively, p<0.0001) and in Kakuyuni for both occurrence of tungiasis (OR 6.5, p<0001) and severe tungiasis (OR 9.2, p<0.05). Tables 3 to 5 show demographic, socio-economic, behavioral, environmental and geographic risk factors in the bivariate analysis.
Table 3. Bivariate analysis of geographic risk factors (n = 1,086 individuals).
| Exposure variable | n | Frequency of tungiasis | Presence of tungiasis | Presence of severe Tungiasis (> 30 lesions) |
||||
|---|---|---|---|---|---|---|---|---|
| (%) any | (%) heavy | OR (95% CI) | P value | OR (95% CI) | P value | |||
| Location | ||||||||
| Malanga Sublocationa | Mtoroni | 116 | 7.8 | 0.9 | Reference | |||
| Yembe | 27 | 59.3 | 3.7 | 17.29 (6.20–48.23) | <0.0001 | 4.42 (0.27–73.05) | 0.2987 | |
| Kadzitsoni | 133 | 33.8 | 3.8 | 6.08 (2.82–13.12) | <0.0001 | 4.49 (0.52–30.02) | 0.1732 | |
| Chembe | 76 | 10.5 | 0.0 | 1.40 (0.51–3.80) | 0.5106 | 0.50 (0.02–12.52) | 0.6754 | |
| Bahati | 17 | 64.7 | 0.0 | 21.80 (6.53–72.74) | <0.0001 | 2.20 (0.09–56.17) | 0.6334 | |
| Kakuyuni Sublocation | Kakuyuni | 324 | 35.5 | 7.4 | 6.54 (3.19–13.40) | <0.0001 | 9.20 (1.23–68.80) | 0.0306 |
| Goshi | 221 | 19.9 | 2.7 | 2.96 (1.39–6.30) | 0.0050 | 3.21 (0.38–26.98) | 0.2831 | |
| Vihingoni | 172 | 13.4 | 2.3 | 1.84 (0.82–4.12) | 0.1416 | 2.74 (0.30–24.81) | 0.3704 | |
a The village with the lowest prevalence was used as reference.
Table 5. Bivariate analysis of educational, occupational and environmental risk factors (n = 1,086).
| Exposure variables | n | Frequency of tungiasis | Presence of tungiasis | Presence of severe tungiasis (> 30 lesions) |
||||
|---|---|---|---|---|---|---|---|---|
| % (any) | (%) heavy | OR (95% CI) | p-value | OR (95% CI) | p-value | |||
| Educationa | Primary school completed | 133 | 6.8 | 0.8 | Reference | |||
| Primary school not completed | 132 | 15.9 | 3.8 | 2.61 (1.15–5.93) | 0.02 | 5.20 (0.60–45.10) | 0.14 | |
| Illiterate | 122 | 19.7 | 0.8 | 3.37 (1.50–7.59) | 0.003 | 1.09 (0.07–17.63) | 0.95 | |
| Occupationa | Other occupation | 113 | 8.8 | 0.9 | Reference | |||
| Farmer | 179 | 15.6 | 1.1 | 1.91 (0.89–4.10) | 0.10 | 1.27 (0.11–14.12) | 0.85 | |
| Unemployed | 89 | 15.7 | 4.6 | 1.92 (0.81–4.56) | 0.14 | 5.27 (0.58–48.02) | 0.14 | |
| Religiona | None | 19 | 26.3 | 0.0 | Reference | |||
| Muslim | 76 | 5.3 | 0.0 | 0.16 (0.04–0.65) | 0.01 | 0.25 (0.00–13.26) | 0.50 | |
| Christian | 210 | 13.3 | 1.0 | 0.43 (0.14–1.29) | 0.13 | 0.47 (0.02–10.09) | 0.63 | |
| Traditionist | 76 | 21.1 | 6.6 | 0.75 (0.23–2.38) | 0.62 | 3.00 (0.16–56.64) | 0.46 | |
| Presence of domestic animals on compound | Dogs Yes | 300 | 26.3 | 4.3 | 1.11 (0.82–1.50) | 0.52 | 1.23 (0.63–2.40) | 0.55 |
| No | 786 | 24.4 | 3.6 | Reference | ||||
| Cats Yes | 260 | 26.5 | 3.5 | 1.12 (0.81–1.53) | 0.50 | 0.89 (0.42–1.89) | 0.76 | |
| No | 826 | 24.5 | 3.9 | Reference | ||||
| Goats Yes | 633 | 28.3 | 3.9 | 1.55 (1.16–2.06) | 0.003 | 1.12 (0.59–2.13) | 0.72 | |
| No | 453 | 20.3 | 3.5 | Reference | ||||
| Cows Yes | 339 | 28.3 | 3.8 | 1.29 (0.97–1.73) | 0.08 | 1.02 (0.52–2.00) | 0.94 | |
| No | 747 | 23.4 | 3.7 | Reference | ||||
| Chicken Yes | 801 | 24.3 | 3.9 | 0.88 (0.65–1.20) | 0.44 | 1.11 (0.54–2.29) | 0.79 | |
| No | 285 | 26.7 | 3.5 | Reference | ||||
| Ducks Yes | 215 | 25.6 | 4.7 | 1.04 (0.74–1.47) | 0.81 | 1.32 (0.64–2.74) | 0.45 | |
| No | 871 | 24.8 | 3.6 | Reference | ||||
| Access to health care | ||||||||
| Time to reach the nearest health facility (min) | 0–9 | 70 | 37.1 | 11.4 | Reference | |||
| 10–19 | 192 | 21.9 | 2.1 | 0.47 (0.26–0.86) | 0.01 | 0.16 (0.05–0.57) | 0.004 | |
| 20–29 | 184 | 28.8 | 7.6 | 0.68 (0.38–1.22) | 0.20 | 0.64 (0.26–1.60) | 0.33 | |
| 30–59 | 436 | 23.9 | 2.1 | 0.53 (0.31–0.90) | 0.02 | 0.16 (0.06–0.44) | 0.001 | |
| ≥60 | 204 | 22.5 | 2.9 | 0.49 (0.27–0.88) | 0.02 | 0.23 (0.08–0.70) | 0.01 | |
a calculated for individuals ≥18 years (n = 388)
The bivariate analyses identified many risk factors for tungiasis (Table 4). These included being of male sex (OR = 1.59, p = 0.001) and age < 15 and ≥ 40 years (OR between 4.04 and 12.45, p<0.001 and p<0.01, respectively). Living in a house with a floor of sand/earth (OR = 4.31, p < 0.0001) and mud walls (OR = 4.11, p < 0.0001) were significantly related to the occurrence of tungiasis. Other significant risk factors were: using a traditional latrine or bush as a toilet; spreading waste on the compound or disposing waste on a pile; using mud puddles as a water source (all p < 0.05); a low frequency of washing (only once a day, OR = 1.99, p<0.0001) and not using soap (OR = 3.81, p<0.001); living in crowded houses (4–6 persons per household, OR = 1.69, p < 0.05); sleeping together with many other persons in a room (p < 0.001) or children sleeping on the floor (OR = 1.89, p < 0.001). In individuals 18 years or older, not completing primary school or never having attended primary school at all increased the odds of being affected by tungiasis by a factor of three (OR = 3.37, p<0.05, Table 5).
Table 4. Bivariate analysis of demographic, housing, economic and behavioral risk factors (n = 1,086).
| Exposure variable | n | Frequency of tungiasis | Presence of tungiasisa | Presence of severe tungiasis (> 30 lesions) |
||||
|---|---|---|---|---|---|---|---|---|
| (%)any | (%)heavy | OR (95% CI) | P value | OR (95% CI) | P value | |||
| Demographic characteristics | ||||||||
| Sex | Female | 622 | 21.2 | 2.4 | Reference | |||
| Male | 464 | 30.0 | 5.6 | 1.59 (1.20–2.09) | 0.001 | 2.40 (1.26–4.59) | 0.008 | |
| Age group (years) | 0–4 | 211 | 31.8 | 2.8 | 8.84 (3.10–25.17) | <0.001 | 5.09 (0.28–91.45) | 0.27 |
| 5–9 | 245 | 39.6 | 7.3 | 12.45 (4.41–35.15) | <0.001 | 13.09 (0.78–219.77) | 0.07 | |
| 10–14 | 180 | 28.3 | 5.6 | 7.51 (2.61–21.60) | <0.001 | 9.92 (0.57–171.31) | 0.11 | |
| 15–19 | 80 | 5.0 | 0.0 | Reference | ||||
| 20–39 | 189 | 10.6 | 1.6 | 2.25 (0.74–6.80) | 0.15 | 3.02 (0.15–59.17) | 0.47 | |
| 40–59 | 114 | 17.5 | 2.6 | 4.04 (1.33–12.33) | 0.01 | 5.05 (0.26–99.21) | 0.29 | |
| ≥60 | 64 | 18.8 | 1.6 | 4.38 (1.34–14.35) | 0.01 | 3.80 (0.15–94.95) | 0.42 | |
| Persons per household | 1–3 | 148 | 18.2 | 4.7 | Reference | |||
| 4–6 | 453 | 27.4 | 4.2 | 1.69 (1.06–2.69) | 0.03 | 0.88 (0.36–2.14) | 0.78 | |
| ≥7 | 485 | 24.7 | 3.1 | 1.47 (0.93–2.35) | 0.10 | 0.64 (0.26–1.61) | 0.34 | |
| Children/household | 0–3 | 454 | 23.6 | 2.9 | Reference | |||
| 4–5 | 324 | 24.4 | 4.9 | 1.05 (0.75–1.46) | 0.79 | 1.76 (0.84–3.72) | 0.13 | |
| ≥6 | 308 | 27.6 | 3.9 | 1.24 (0.89–1.72) | 0.21 | 1.38 (0.62–3.06) | 0.43 | |
| Adults/household | 0–1 | 224 | 29.9 | 6.3 | 1.65 (0.89–3.07) | 0.11 | 10.81 (0.64–183.47) | 0.09 |
| 2–3 | 784 | 24.0 | 3.4 | 1.22 (0.69–2.17) | 0.49 | 5.70 (0.34–94.35) | 0.22 | |
| ≥4 | 78 | 20.5 | 0.0 | Reference | ||||
| Housing | ||||||||
| Type of floor material | Cement/stone | 129 | 11.6 | 0.0 | Reference | |||
| Smeared mud | 661 | 22.7 | 4.2 | 2.23 (1.26–3.94) | 0.005 | 11.65 (0.71–192.08) | 0.08 | |
| Sand/dust | 293 | 36.2 | 4.4 | 4.31 (2.39–7.76) | <0.0001 | 12.47 (0.74–211.31) | 0.08 | |
| Type of wall material | Stone | 153 | 8.5 | 0.0 | Reference | |||
| Mud | 901 | 27.6 | 4.6 | 4.11 (2.29–7.40) | <0.0001 | 14.81 (0.91–241.97) | 0.06 | |
| Mixed | 32 | 28.1 | 0.0 | 4.21 (1.62–10.98) | 0.003 | 4.72 (0.09–242.42) | 0.43 | |
| Type of roof material | Mabati | 553 | 21.9 | 1.4 | Reference | |||
| Makuti | 519 | 28.7 | 6.4 | 1.44 (1.09–1.90) | 0.01 | 4.36 (2.12–10.11) | 0.0001 | |
| Both | 7 | 0.0 | 0.0 | 0.24 (0.01–4.18) | 0.32 | 4.38 (0.23–81.08) | 0.33 | |
| Other | 7 | 14.3 | 0.0 | 0.60 (0.07–4.99) | 0.63 | 4.38 (0.23–81.08) | 0.33 | |
| Location of kitchen | Outside the house | 757 | 25.8 | 3.6 | Reference | |||
| Inside the house | 329 | 23.1 | 4.3 | 0.87 (0.64–1.17) | 0.35 | 1.20 (0.62–2.32) | 0.58 | |
| Number of sleeping rooms | ≥4 | 68 | 13.2 | 0.0 | Reference | |||
| 3 | 179 | 20.7 | 2.8 | 1.71 (0.78–3.76) | 0.18 | 4.32 (0.24–79.15) | 0.32 | |
| 2 | 435 | 23.2 | 2.3 | 1.98 (0.95–4.14) | 0.07 | 3.38 (0.20–58.36) | 0.40 | |
| 1 | 404 | 30.7 | 6.4 | 2.90 (1.40–6.04) | 0.004 | 9.59 (0.58–159.27) | 0.11 | |
| Persons/sleeping room | <3 | 403 | 18.4 | 2.2 | Reference | |||
| 3–4 | 366 | 24.3 | 1.9 | 1.43 (1.01–2.02) | 0.04 | 0.85 (0.31–2.32) | 0.75 | |
| 4,5–6 | 195 | 33.8 | 8.7 | 2.27 (1.54–3.36) | <0.0001 | 4.18 (1.83–9.56) | <0.001 | |
| ≥7 | 122 | 34.4 | 6.6 | 2.33 (1.49–3.66) | <0.001 | 3.07 (1.16–8.14) | 0.02 | |
| Sleeping situation of children | Raised beda | 910 | 23.2 | 2.9 | Reference | |||
| Floor | 146 | 36.3 | 8.2 | 1.89 (1.30–2.74) | <0.001 | 3.04 (1.50–6.18) | 0.002 | |
| Taking turns | 30 | 23.3 | 10.0 | 1.01 (0.43–2.38) | 0.98 | 3.78 (1.08–13.25) | 0.04 | |
| Sanitation | ||||||||
| Water source | Tap on compound | 159 | 22.0 | 3.8 | Reference | |||
| Shared community tap | 918 | 24.9 | 3.6 | 1.18 (0.79–1.76) | 0.42 | 0.95 (0.39–2.31) | 0.91 | |
| Mud puddles | 9 | 77.8 | 22.2 | 12.40 (2.46–62.39) | 0.002 | 7.29 (1.24–42.80) | 0.003 | |
| Toilet | Flush toilet | 36 | 5.6 | 0.0 | Reference | |||
| Ventilated pit latrine | 88 | 17.0 | 0.0 | 3.49 (0.76–16.14) | 0.11 | 0.41 (0.01–21.18) | 0.65 | |
| Traditional latrine | 314 | 25.8 | 4.8 | 5.91 (1.39–25.15) | 0.01 | 3.78 (0.22–64.48) | 0.35 | |
| Bush | 648 | 26.7 | 4.0 | 6.19 (1.47–26.05) | 0.01 | 3.11 (0.19–52.02) | 0.43 | |
| Waste disposal | Pit | 415 | 24.1 | 1.9 | Reference | |||
| Pile | 465 | 23.7 | 4.7 | 0.98 (0.72–1.33) | 0.87 | 2.53 (1.11–5.74) | 0.03 | |
| Spread | 199 | 30.7 | 5.5 | 1.39 (0.96–2.03) | 0.08 | 2.98 (1.18–7.52) | 0.03 | |
| Compost | 7 | 0.0 | 0.0 | 0.21 (0.01–3.70) | 0.28 | 3.20 (0.19–60.59) | 0.43 | |
| Time to reach water source (min) | 0–4 | 341 | 25.2 | 4.4 | Reference | |||
| 5–9 | 272 | 29.8 | 5.5 | 1.26 (0.88–1.80) | 0.21 | 1.27 (0.61–2.64) | 0.53 | |
| 10–14 | 202 | 21.8 | 3.0 | 0.83 (0.55–1.25) | 0.36 | 0.67 (0.25–1.74) | 0.41 | |
| 15–19 | 79 | 19.0 | 0.0 | 0.69 (0.38–1.28) | 0.24 | 0.13 (0.01–2.24) | 0.16 | |
| 20–29 | 46 | 23.9 | 8.7 | 0.93 (0.45–1.92) | 0.85 | 2.07 (0.66–6.53) | 0.21 | |
| ≥30 | 146 | 23.3 | 0.7 | 0.90 (0.57–1.42) | 0.65 | 0.15 (0.02–1.15) | 0.07 | |
| Frequency of washing | Twice a day | 828 | 21.7 | 3.0 | Reference | |||
| Once a day | 236 | 35.6 | 6.4 | 1.99 (1.45–2.72) | <0.0001 | 2.18 (1.13–4.21) | 0.02 | |
| Less often | 22 | 31.8 | 4.5 | 1.68 (0.67–4.18) | 0.27 | 1.53 (0.20–11.82) | 0.68 | |
| Use of soap | Always | 566 | 22.8 | 3.2 | Reference | |||
| Sometimes | 486 | 25.5 | 4.3 | 1.16 (0.87–1.54) | 0.30 | 1.37 (0.72–2.61) | 0.33 | |
| Never | 34 | 52.9 | 5.9 | 3.81 (1.89–7.69) | <0.001 | 1.90 (0.42–8.56) | 0.40 | |
| Always | 566 | 22.8 | 3.2 | |||||
| Economic status | ||||||||
| Income per capita (KSh/month)b | >3400 | 25 | 12.0 | 0.0 | Reference | |||
| 1000–3400 | 166 | 18.1 | 1.2 | 1.62 (0.45–5.76) | 0.46 | 0.78 (0.04–16.61) | 0.87 | |
| <1000 | 414 | 27.1 | 2.9 | 2.72 (0.80–9.26) | 0.11 | 1.58 (0.09–27.52) | 0.75 | |
| n.k. | 481 | 26.2 | 5.6 | 2.60 (0.77–8.85) | 0.13 | 3.09 (0.18–52.04) | 0.43 | |
| Asset score | 0–4 | 761 | 27.2 | 4.3 | 7.85 (1.05–58.71) | 0.04 | 2.07 (0.12–34.85) | 0.61 |
| 5–15 | 303 | 20.8 | 2.6 | 5.51 (0.73–41.77) | 0.10 | 1.29 (0.07–23.16) | 0.86 | |
| ≥16 | 22 | 4.5 | 0.0 | Reference |
||||
| Number of meals/day | >2 | 773 | 23.7 | 3.5 | Reference | |||
| 2 | 291 | 27.8 | 4.1 | 1.24 (0.92–1.69) | 0.16 | 1.19 (0.59–2.38) | 0.63 | |
| 1 | 22 | 31.8 | 9.1 | 1.50 (0.60–3.75) | 0.38 | 2.76 (0.61–12.43) | 0.19 | |
a Bed height was not assessed systematically, but was approximately 45 cm above the ground (personal observation)
b KSh 4850 correspond to the minimum wage in Kenya for an unskilled worker in agricultural industry at the time of the survey and is equivalent to ~ 55 USD
n.k. = not known
On conducting the multivariate analyses, only the demographic exposure variables male sex and age under 15 remained highly significant (Table 6). Exposure variables indicating a low economic status such as poor construction characteristics of the house, direct sleeping on the floor, many people sleeping in a single room and restricted access to water also remained as significant factors.
Table 6. Risk factors of tungiasis/severe tungiasis after multivariate analysis.
| Presence of tungiasis | Presence of severe tungiasis (> 30 lesions) |
|||
|---|---|---|---|---|
| Adjusted OR (95% CI) | P value | Adjusted OR (95% CI) | P value | |
| Being of male sex | 2.29 (1.18–4.46) | 0.01 | ||
| Age | ||||
| 0–4 | 8.90 (2.94–26.89) | <0.0001 | ||
| 5–9 | 12.88 (4.31–38.54) | <0.0001 | ||
| 10–14 | 7.23 (2.37–22.02) | <0.0001 | ||
| 40–59 | 3.49 (1.07–11.39) | 0.04 | ||
| ≥ 60 | 5.32 (1.50–18.85) | 0.01 | ||
| Using mud puddles as water source | 25.48 (3.50–185.67) | 0.001 | ||
| Washing only once a day | 2.23 (1.11–4.51) | 0.03 | ||
| Using soap when washing: | ||||
| sometimes | 1.57 (1.09–2.28) | 0.02 | ||
| never | 7.36 (3.08–17.62) | <0.0001 | ||
| Staying in a house with: | ||||
| 4.5–6 persons/sleeping room | 1.77 (1.07–2.93) | 0.03 | ||
| children sleeping on the floor | 1.68 (1.03–2.74) | 0.04 | ||
| Time to health facility 10–19 min | 0.47 (0.23–0.95) | 0.04 | 0.20 (0.06–0.69) | 0.01 |
| 30–59 min | 0.12 (0.04–0.34) | <0.0001 | ||
| ≥ 60 min | 0.22 (0.07–0.68) | 0.009 | ||
| Living in a house with mud walls | 3.35 (1.71–6.58) | <0.0001 | ||
Population Attributable Fractions were calculated for those variables which are amenable to modification (Table 7). The PAF for living in a house with mud walls was 64.45%, for washing without soap 16.61% and washing only once a day 20.18%.
Table 7. Population attributable fractions for exposure variables amenable to modification.
| AR (%) |
% exposed among cases | PAF (%) |
|
|---|---|---|---|
| Washing only once a day | 55.16 | 36.6 | 20.18 |
| Not using soap when washing | 36.31 | 45.8 | 16.61 |
| Staying in a house with mud walls | 70.15 | 91.9 | 64.45 |
PAF is the fraction of cases which would not have occurred if an exposure had been avoided and is calculated as % exposed among cases x attributable risk (AR).
AR is the risk of tungiasis in the exposed group due to the exposure and is calculated as (OR– 1)/OR.
Discussion
Tungiasis is a NTD prevalent in resource-poor communities in South America, the Caribbean and sub-Saharan Africa [3–7]. Although the disease is associated with important morbidity, it is neglected by health care providers globally [2,20–23]. Widespread control has never been attempted, only isolated efforts to treat infected individuals, often by non-governmental organizations. In East Africa, this is largely due to the lack of data on prevalence and severity of disease and hitherto risk factors have only been investigated in restricted age groups.
This study showed a prevalence of 25% in the overall study population and 33.8% in children under 15 years. The overall prevalence is similar to that found in a community-based study in Central Uganda (where the median prevalence in humans was 22%, but only animal keeping households were included), but considerably lower than prevalences observed in rural and urban resource-poor communities in Brazil and Nigeria (with prevalences up to 45%) [6,7,20,24,25]. Age-specific prevalence followed an S-shape curve, peaking in the 5 to 9 year age group and the elderly, an unusual epidemiological characteristic which seems to be true for all geographic areas and independent of the overall prevalence [6,7,15,21]. This may be due to certain age-specific behavioural patterns associated with different degrees of exposure, e.g. young children playing on the ground, as suggested by Muehlen et al. [6] and the elderly spending large amounts of time lying on the ground. Other hypotheses are a protecting effect of the increasing corneal layer of the feet [26,27], a higher level of practice and dexterity in taking out embedded sandfleas with increasing age [7] and more attention given to personal hygiene.
More than half of all cases (52.8%) had a low intensity of infection (less than 6 lesions), while 15% had more than 30 lesions. The percentage of patients with severe tungiasis was lower than observed in Brazil [7,15,20,24,25]. However, this is not surprising, taking into account that prevalence and intensity of infection are positively correlated [6,21,28]. The observation that age-specific prevalence significantly correlated to high intensity of infection (rho = 0.90; Fig 3) confirms that children and the elderly bear the highest burden of disease. Anecdotal reports show that elderly individuals without social support structures tend to be infected with tungiasis more frequently [21]. This tendency was confirmed in this study, although it was not significant.
Tungiasis is a zoonosis in which sylvatic, peri-domiciliary and domestic cycles are interlinked in a complex manner [2]. The situation becomes even more intricate when transmission also occurs inside the house, without the involvement of an animal reservoir. Intra-domiciliary transmission indicates that the off-host cycle of T. penetrans is completed inside the house. Usually, this is a room in which family members spend many hours a day, such as the sleeping room. If the floor in this room consists of sand, dried mud or rugged cement with holes and cracks, eggs that have been expelled by embedded female sand fleas overnight and which have fallen on the floor are swept into crevices of the floor or into the cracks between floor and wall, when the room is cleaned with a broom in the morning. Eggs can develop into larvae and pupae in such cracks [29].
That intra-domiciliary transmission occurs in the study area is supported by the finding that direct sleeping on the floor or if walls of the sleeping room consisted of mud remained significant risk factors in the multivariate analysis. The more people slept in a room the higher were the odds of tungiasis in household members.
It is known that different animal species act as reservoirs in different countries [25,30,31]. In our study population, 74% of all households had chicken, 60% had goats, 25% had dogs and 25% had cats. However, no specific animal species was identified as a risk factor for tungiasis in this study. This finding supports the assumption that perhaps in these coastal communities the Tunga penetrans cycle is almost entirely human and does not involve animal reservoirs. It should be noted that animals were not examined for infection in this study, only observed as present in the compound and reported as to where they sleep at night (S3 Appendix). In Northeast Brazil, stray dogs and cats are important reservoirs in urban areas, whereas in rural areas pigs are the most import species [30,31]. Pigs were also identified as the major reservoir of T. penetrans in Nigeria and in Uganda [15,25]. However, pigs were not kept in any of the households in the study area, because a considerable part of the population is Muslim. Actually, being Muslim was identified as a significant protective factor in the bivariate analysis (Table 5), which may be explained by the fact that Muslims wash their feet several times a day before entering the mosque for prayer.
Other risk factors which remained significant after multivariate regression analysis were the limited access to water (water only available from muddy pools), frequency of washing as well as bathing without soap. A similar finding was made in a resource-poor community in Northeast Brazil [14]. It is tempting to speculate that these risk factors are correlated to the reproductive biology of T. penetrans. Female sand fleas are fertilized by males exploring the skin only after females are embedded in the epidermis and have started neosomy [32]. There is circumstantial evidence that males are attracted by odor emitted from the faecal material released by females in regular intervals [12,13]. The faecal material spreads into dermal papillae around the lesion, and since it is very sticky, it needs soap to be washed off. Hence, when soap is not used or unavailability of water prevents any washing at all, more male sand fleas should be attracted to the skin and, hence, more females will be fertilized. Over time, this will lead to a higher intensity of infection.
It has previously been reported that within endemic areas, tungiasis is heterogeneously distributed [2]. This was confirmed in this study: where prevalence varied between villages from 7.8% to 64.7% in the five study villages in Malanga Sub-location, all situated within 4 km of each other and from 13.4% to 35.5% in the three study villages in Kakuyuni Sub-location, within 2 km of each other. Whether the heterogeneity is determined by differences in the predominant type of exposure within a community, such as intra-domiciliary versus peri-domestic could not be clarified in this study.
We found very high Population Attributable Fractions for biologically very plausible variables. Trickling of sand and dust from mud walls creates ideal conditions for the off-host life cycle of sand fleas in cracks of the floor. Building walls of stone or cement would reduce the prevalence of tungiasis by 64 percent. Similar, promoting better hygiene, particularly washing with soap, would reduce the prevalence of tungiasis in the community by 17 and 20%, respectively.
We realize that this study has several limitations. First, there is an overrepresentation of adult females in the study group. The study was conducted during the day on all days of the week, including Saturday and Sunday, in order to encounter school children on the compound. However, since the majority of adult males in our study population worked as farmers and returned only after sunset we could not examine them. Extending our working periods towards the evening was not possible due to insufficient lighting and safety concerns. The distances between the households in Malanga and our time constraints, also meant that there were fewer households included in the study from this area than from Kakuyuni. Ecologically the two areas are quite different.
Taken together, many factors which—by one way or another—are linked to poverty were identified as important risk factors in the bivariate and/or multivariate regression analysis, such as poor construction characteristics of the house, absence of a ventilated pit latrine, no access to drinking water on the compound, a single sleeping room for children and adults, absence of beds and mattresses, unavailability of soap for body wash, an asset score below 5 points and a low level of education among adults. Thus, as seen elsewhere in the world, tungiasis in rural Kenya is a poverty-associated disease in which the poorest of the poor bear the highest burden of disease, but that it can be controlled with simple housing improvements, improved access to water and hygiene practices.
Supporting information
(DOCX)
Prevalence of tungiasis in 5 schools in Kilifi County.
(DOCX)
(DOCX)
(DOC)
Acknowledgments
We would like to thank the Community Health Workers of the Dabaso Tujengane CBO for their assistance in identifying cases and treating the patients. We are particularly in debt to Billy Mwangemi, Kakuyuni Community Health Officer, for his excellent support, Shedrack Mwadai and Wilson Yaa for conducting the interviews. We thank the Kilifi County Departments of Health and Education for their support of the study.
Data Availability
All data are available as an excel file at Harvard Dataverse (doi:10.7910/DVN/SOEBDB).
Funding Statement
The study was supported by German Doctors e.V., Bonn, Germany. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Hotez PJ. Forgotten People, Forgotten Diseases: The Neglected Tropical Diseases and Their Impact on Global Health and Development. ASM Press, Washington, DC: 2008 [Google Scholar]
- 2.Feldmeier H, Heukelbach J, Ugbomoiko US, Sentongo E, Mbabazi P, von Samson-Himmelstjerna G, u. a. Tungiasis—A Neglected Disease with Many Challenges for Global Public Health. PLoS Negl Trop Dis. Oktober 2014;8(10):e3133 doi: 10.1371/journal.pntd.0003133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ariza L, Wilcke T, Jackson A, Gomide M, Ugbomoiko US, Feldmeier H, u. a. A simple method for rapid community assessment of tungiasis. Trop Med Int Health TM IH. Juli 2010;15(7):856–64. doi: 10.1111/j.1365-3156.2010.02545.x [DOI] [PubMed] [Google Scholar]
- 4.Chadee DD. Tungiasis among five communities south-western Trinidad, in West Indies. Ann Trop Med Parasitol. 1 Januar 1998;92(1):107–13. [DOI] [PubMed] [Google Scholar]
- 5.Heukelbach J, Franck S, Feldmeier H. High attack rate of Tunga penetrans (Linnaeus 1758) infestation in an impoverished Brazilian community. Trans R Soc Trop Med Hyg. Juli 2004;98(7):431–4. doi: 10.1016/j.trstmh.2003.12.004 [DOI] [PubMed] [Google Scholar]
- 6.Muehlen M, Heukelbach J, Wilcke T, Winter B, Mehlhorn H, Feldmeier H. Investigations on the biology, epidemiology, pathology and control of Tunga penetrans in Brazil. II. Prevalence, parasite load and topographic distribution of lesions in the population of a traditional fishing village. Parasitol Res. August 2003;90(6):449–55. doi: 10.1007/s00436-003-0877-7 [DOI] [PubMed] [Google Scholar]
- 7.Wilcke T, Heukelbach J, César Sabóia Moura R, Regina Sansigolo Kerr-Pontes L, Feldmeier H. High prevalence of tungiasis in a poor neighbourhood in Fortaleza, Northeast Brazil. Acta Trop. September 2002;83(3):255–8. [DOI] [PubMed] [Google Scholar]
- 8.Bezerra SM. Tungiasis—an unusual case of severe infestation. Int J Dermatol. Oktober 1994;33(10):725 [DOI] [PubMed] [Google Scholar]
- 9.Cardoso A. Generalized Tungiasis Treated With Thiabendazole. Arch Dermatol. 1 März 1981;117(3):127. [Google Scholar]
- 10.Feldmeier H, Eisele M, Van Marck E, Mehlhorn H, Ribeiro R, Heukelbach J. Investigations on the biology, epidemiology, pathology and control of Tunga penetrans in Brazil: IV. Clinical and histopathology. Parasitol Res. Oktober 2004;94(4):275–82. doi: 10.1007/s00436-004-1197-2 [DOI] [PubMed] [Google Scholar]
- 11.Eisele M, Heukelbach J, Van Marck E, Mehlhorn H, Meckes O, Franck S, u. a. Investigations on the biology, epidemiology, pathology and control of Tunga penetrans in Brazil: I. Natural history of tungiasis in man. Parasitol Res. Juni 2003;90(2):87–99 [DOI] [PubMed] [Google Scholar]
- 12.Feldmeier H, Eisele M, Sabóia-Moura RC, Heukelbach J. Severe tungiasis in underprivileged communities: case series from Brazil. Emerg Infect Dis. August 2003;9(8):949–55. doi: 10.3201/eid0908.030041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feldmeier H, Heukelbach J, Eisele M, Ribeiro R, Harms G, Mehlhorn H, u. a. Investigations on the biology, epidemiology, pathology and control of Tunga penetrans in Brazil: III. Cytokine levels in peripheral blood of infected humans. Parasitol Res. Oktober 2003;91(4):298–303. doi: 10.1007/s00436-003-0950-2 [DOI] [PubMed] [Google Scholar]
- 14.Muehlen M, Feldmeier H, Wilcke T, Winter B, Heukelbach J. Identifying risk factors for tungiasis and heavy infestation in a resource-poor community in northeast Brazil. Trans R Soc Trop Med Hyg. April 2006;100(4):371–80. doi: 10.1016/j.trstmh.2005.06.033 [DOI] [PubMed] [Google Scholar]
- 15.Ugbomoiko US, Ariza L, Ofoezie IE, Heukelbach J. Risk Factors for Tungiasis in Nigeria: Identification of Targets for Effective Intervention. PLoS Negl Trop Dis [Internet]. 5 Dezember 2007. [zitiert 25. Februar 2017];1(3). Verfügbar unter: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2154384/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Howe LD, Galobardes B, Matijasevich A, Gordon D, Johnston D, Onwujekwe O, u. a. Measuring socio-economic position for epidemiological studies in low- and middle-income countries: a methods of measurement in epidemiology paper. Int J Epidemiol. Juni 2012;41(3):871–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vyas S, Kumaranayake L. Constructing socio-economic status indices: how to use principal components analysis. Health Policy Plan. 1 November 2006;21(6):459–68. doi: 10.1093/heapol/czl029 [DOI] [PubMed] [Google Scholar]
- 18.Reichert F, Pilger D, Schuster A, Lesshafft H, de Oliveira SG, Ignatius R, u. a. Prevalence and Risk Factors of Hookworm-Related Cutaneous Larva Migrans (HrCLM) in a Resource-Poor Community in Manaus, Brazil. PLoS Negl Trop Dis. 24 März 2016;10(3):e0004514 doi: 10.1371/journal.pntd.0004514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heukelbach J, Wilcke T, Eisele M, Feldmeier H. Ectopic localization of tungiasis. Am J Trop Med Hyg. August 2002;67(2):214–6. [DOI] [PubMed] [Google Scholar]
- 20.Ariza L, Seidenschwang M, Buckendahl J, Gomide M, Feldmeier H, Heukelbach J. [Tungiasis: a neglected disease causing severe morbidity in a shantytown in Fortaleza, State of Ceará]. Rev Soc Bras Med Trop. Februar 2007;40(1):63–7. [DOI] [PubMed] [Google Scholar]
- 21.Ugbomoiko US, Ofoezie IE, Heukelbach J. Tungiasis: high prevalence, parasite load, and morbidity in a rural community in Lagos State, Nigeria. Int J Dermatol. Mai 2007;46(5):475–81. doi: 10.1111/j.1365-4632.2007.03245.x [DOI] [PubMed] [Google Scholar]
- 22.Heukelbach J, Ugbomoiko US. Knowledge, attitudes and practices regarding head lice infestations in rural Nigeria. J Infect Dev Ctries. 12 Juli 2011;5(09):652–7. [DOI] [PubMed] [Google Scholar]
- 23.Heukelbach J, Ugbomoiko US. Editorial: Tungiasis in the past and present: A dire need for intervention. Niger J Parasitol. 1 Januar 2007;28(1):1–5. [Google Scholar]
- 24.Jorg Heukelbach AJ. Epidemiology and clinical aspects of tungiasis (sand flea infestation) in Alagoas State, Brazil. J Infect Dev Ctries. 2007; [Google Scholar]
- 25.Mutebi F, Krücken J, Feldmeier H, Waiswa C, Mencke N, Sentongo E, u. a. Animal Reservoirs of Zoonotic Tungiasis in Endemic Rural Villages of Uganda. PLOS Negl Trop Dis. 16 Oktober 2015;9(10):e0004126 doi: 10.1371/journal.pntd.0004126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chadee DD. Distribution patterns of Tunga penetrans within a community in Trinidad, West Indies. J Trop Med Hyg. (97):107–13. [PubMed] [Google Scholar]
- 27.Ade-Serrano MA, Ejezie GC. Prevalence of tungiasis in Oto-Ijanikin village, Badagry, Lagos State, Nigeria. Ann Trop Med Parasitol. August 1981;75(4):471–2. [DOI] [PubMed] [Google Scholar]
- 28.Feldmeier H, Kehr JD, Poggensee G, Heukelbach J. High exposure to Tunga penetrans (Linnaeus, 1758) correlates with intensity of infestation. Mem Inst Oswaldo Cruz. Februar 2006;101(1):65–9 [DOI] [PubMed] [Google Scholar]
- 29.Linardi PM, Calheiros CML, Campelo-Junior EB, Duarte EM, Heukelbach J, Feldmeier H. Occurrence of the off-host life stages of Tunga penetrans (Siphonaptera) in various environments in Brazil. Ann Trop Med Parasitol. Juni 2010;104(4):337–45. doi: 10.1179/136485910X12743554759902 [DOI] [PubMed] [Google Scholar]
- 30.Pilger D, Schwalfenberg S, Heukelbach J, Witt L, Mehlhorn H, Mencke N, u. a. Investigations on the biology, epidemiology, pathology, and control of Tunga penetrans in Brazil: VII. The importance of animal reservoirs for human infestation. Parasitol Res. April 2008;102(5):875–80. doi: 10.1007/s00436-007-0840-0 [DOI] [PubMed] [Google Scholar]
- 31.Heukelbach J, Costa AML, Wilcke T, Mencke N, Feldmeier H. The animal reservoir of Tunga penetrans in severely affected communities of north-east Brazil. Med Vet Entomol. Dezember 2004;18(4):329–35. doi: 10.1111/j.0269-283X.2004.00532.x [DOI] [PubMed] [Google Scholar]
- 32.Thielecke M, Feldmeier H. The fate of the embedded virgin sand flea Tunga penetrans: hypothesis, self-experimentation and photographic sequence. Travel Med Infect Dis. Dezember 2013;11(6):440–3. doi: 10.1016/j.tmaid.2013.10.012 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
Prevalence of tungiasis in 5 schools in Kilifi County.
(DOCX)
(DOCX)
(DOC)
Data Availability Statement
All data are available as an excel file at Harvard Dataverse (doi:10.7910/DVN/SOEBDB).