Abstract
The present study aimed at examining beneficial effects of intermittent hypoxia training (IHT) under prediabetic conditions. We investigate the effects of three-week IHT on blood glucose level, tolerance to acute hypoxia, and leukocyte mRNA expression of hypoxia inducible factor 1α (HIF-1α) and its target genes, i.e. insulin receptor, facilitated glucose transporter–solute carrier family-2, and potassium voltage-gated channel subfamily J. Seven healthy and 11 prediabetic men and women (44–70 years of age) were examined before, next day and one month after three-week IHT (3 sessions per week, each session consisting 4 cycles of 5-min 12% O2 and 5-min room air breathing). We found that IHT afforded beneficial effects on glucose homeostasis in patients with prediabetes reducing fasting glucose and during standard oral glucose tolerance test. The most pronounced positive effects were observed at one month after IHT termination. IHT also significantly increased the tolerance to acute hypoxia (i.e. SaO2 level at 20th min of breathing with 12% O2) and improved functional parameters of respiratory and cardiovascular systems. IHT stimulated HIF-1α mRNA expression in blood leukocytes in healthy and prediabetic subjects, but in prediabetes patients the maximum increase was lagged. The greatest changes in mRNA expression of HIF-1α target genes occurred a month after IHT and coincided with the largest decrease in blood glucose levels. The higher expression of HIF-1α was positively associated with higher tolerance to hypoxia and better glucose homeostasis. In conclusion, our results suggest that IHT may be useful for preventing the development of type 2 diabetes.
Impact statement
The present study investigated the beneficial effects of intermittent hypoxia training (IHT) in humans under prediabetic conditions. We found that three-week moderate IHT induced higher HIF-1α mRNA expressions as well as its target genes, which were positively correlated with higher tolerance to acute hypoxia and better glucose homeostasis in both middle-aged healthy and prediabetic subjects. This small clinical trial has provided new data suggesting a potential utility of IHT for management of prediabetes patients.
Keywords: Intermittent hypoxia, diabetes, hypoxia inducible factor-1, hypoxia inducible factor-1-regulated genes, adaptation, hyperglycemia
Introduction
The method of intermittent hypoxia training (IHT) is an emerging therapeutic modality for treatment and prevention of various human diseases and has gained increasing attention. The mechanisms underlying the beneficial effects of IHT have been investigated at the multiple biological levels, from systemic physiological reactions to genomic regulation.1–5 The potential therapeutic uses of IHT in treating cerebrovascular and cardiovascular disorders have been the focal areas of extensive research.6,7
Despite these advances, the effects of IHT on diabetes mellitus, especially type 2 diabetes, one of the most prevalent pathological conditions in the current world population, are much less investigated.8 In the mid-1990s, Ukrainian scientists first demonstrated in diabetic animals that IHT could reduce vascular risk factors and increases blood insulin levels via inhibition of the islet destruction and promotion of new beta-cell formation in acinar tissue.9 These authors recently confirmed that two-week IHT led to an increase in the area of pancreatic islets and the number of β-cells in diabetic rats, mainly due to a significant reduction of β-endocrine cells apoptosis. The positive effect was maintained for at least 10 days.10 These findings in preclinical animal studies suggested possible utilization of IHT in control or treatment of type 2 diabetes and its associated insulin resistance. It is notable that the favorable effects of IHT on glucose metabolism were also suggested.11,12 In particular, it was shown that hypoxic training increased glycolytic enzyme activities, enhanced the number of mitochondria in skeletal muscles, and improved insulin sensitivity as well.13,14
Glucose-lowering effects of IHT were also previously reported in diabetic patients15,16 and the beneficial effect is particularly important in elderly population with higher risks in developing diabetes.
Moderate levels of intermittent hypoxia mobilize genome that in turn activates a cascade of intracellular signaling transduction, which involves various receptors, mitochondrial respiratory chain, key intracellular regulatory systems, early genes, superfamilies of the inducible and activation transcription factors, which are sequentially engaged in the processes of initiation and induction of hypoxic tolerance. One of the key regulators of oxygen homeostasis under hypoxic conditions is hypoxia inducible factor (HIF), which initiates transcriptional activation of numerous target genes to improve oxygen delivery and utilization17 as well as glucose homeostasis.12,18,19 Delicate balance exists between HIF-1 level and optimal metabolic functions.20,21 Malfunction of these relations leads to hyperglycemia and type 2 diabetes. Based on these results, we have suggested that the use of IHT for treatment of patients with prediabetic abnormalities can improve carbohydrate metabolism and lead to prevention of diabetes development.
Among the HIF-1α target genes, energy-independent facilitative glucose transporter-1 (GLUT-1; encoded by solute carrier family-2 gene SLC2A1) is one of most important for regulating glucose metabolism which predominates in many types of human cells22 and is the only vehicle that transports glucose into the brain.23 GLUT-1 mediates glucose uptake increasing intracellular glucose levels to be used by glycolysis and other metabolic pathways.24,25 GLUT-1 is upregulated under hypoxia, and its activity depends of the severity of hypoxic impact.26,27
Another HIF-1α target gene important for glucose homeostasis is insulin receptor (INSR). Insulin exerts its physiological effects through this member of tyrosine kinase family of transmembrane signaling proteins encoded by a single gene INSR.28 Increase of INSR level could alleviate insulin resistance. Recent studies indicated that INSR is expressed at higher levels under hypoxic stress29 and overexpression of INSR improves obese and diabetic phenotypes in mice.30
ATP-sensitive potassium (KATP) channels are also involved in the regulation of insulin secretion in the β-cells of pancreas. It couples cell metabolism to electrical activity of the plasma membrane by regulating membrane K+ fluxes.31,32 KATP channels also play important role in adaptation to intermittent hypoxia.33,34 One of the pore forming subunits of KATP channels is encoded as KCNJ8 (potassium inwardly-rectifying channel, subfamily J), also known as KIR6.1.35
Under the context, the present study was designed to investigate the effects of a three-week session of IHT on blood glucose and hypoxic tolerance in healthy humans and patients with prediabetes. Furthermore, we focused on the effects of IHT on mRNA expression of HIF-1α and its targeted genes, such as INSR, SLC2A1, and KCNJ8.
Materials and methods
Characteristics of participants
Seven healthy volunteers (44–68 years, 3 males and 4 females) and 11 prediabetic patients (48–70 years, 5 males and 6 females) participated in the current study. The prediabetes patients were diagnosed using the criteria issued by the American Diabetes Association. We included patients who had an elevated fasting glucose level (5.6 to 6.9 mmol/L), impaired glucose tolerance (i.e. plasma glucose level of 7.8 to 11.0 mmol/L 2 h after an oral dose of 75 g glucose challenge), or their combination. Subjects in the healthy control group had no cardiovascular, respiratory, endocrine or central nervous system disorders and their fasting glucose concentration was less than 5.6 mmol/L and less than 7.8 mmol/L 2 h after a standard glucose tolerance test.
This clinical study was conducted under the state laws of Ukraine and the ethical principles of the 1964 Declaration of Helsinki. The research protocols, patient health information and informed consent forms were approved by the Ethics Committee of Chebotarev Institute of Gerontology, Kiev, Ukraine. All subjects received detailed information of the study process and a written informed consent was obtained from each of the participated subjects. All participants were nonsmokers and did not take any medication two weeks prior to and during the sessions of the study. They had no infections during the past month and no major cardiovascular or respiratory complications. All subjects underwent measurements of several anthropometric variables (Table 1), which indicated no significant difference in age and height between healthy and prediabetes groups, whereas the body weight, body mass index, and waist circumference were higher in the prediabetes patients than those of healthy control subjects. All participants were informed about the strict observance of lifestyle one month prior to, during, and one month after IHT (including levels of physical activity, caloric content of daily diet, consumption of coffee and tea, abstinence of alcohol, etc.). They kept diaries in which they noted any lifestyle changes, if occurred. Any violation of the regime by the subjects resulted in exclusion from the study.
Table 1.
Anthropometric characteristics of the participantsa
| Groups | Gender (female/male) | Age (year) | Height (cm) | Weight (kg) | BMI (kg/m2) | Waist (cm) |
|---|---|---|---|---|---|---|
| Healthy | 5/2 | 58.7 ± 11.8 | 170 ± 15 | 76.1 ± 17.3 | 27.2 ± 6.4 | 93.7 ± 9.2 |
| Prediabetes | 7/4 | 66.4 ± 5.2 | 167 ± 10 | 90.2 ± 9.9 | 33.2 ± 5.6 | 99.7 ± 8.9 |
| Healthy vs. prediabetes | NS | NS | P < 0.05 | P < 0.05 | P = 0.05 |
BMI: body mass index; waist: waist measurements; NS: no significant difference.
Data are mean ± SD. Student’s t-test was used to evaluate the statistical significance of the differences between healthy and prediabetes groups.
Experiment protocols
All sessions of the present study were conducted in a quiet room at a temperature of 22–23oC within a clinical research center of the Chebotarev Institute of Gerontology. The logistic plan and timetable of the studies are summarized in Table 2. Measurement sessions were performed during two days before IHT course, one day and one month after the termination of IHT. For determination of HIF-1α mRNA expression and its target genes in blood leukocytes, venous blood samples were collected again next day after one-week IHT course. Patient examination included: (1) anthropometric measurements; (2) determination of HIF1α mRNA and its target genes; (3) standard oral glucose tolerance test (OGTT) with plasma glucose determination; and (4) acute hypoxic test (AHT) with measurements of routine cardiovascular parameters.
Table 2.
Experimental timetable of the procedures, sample collections, and functional tests before, during, and after the IHT sessions
| Date of investigation | Procedures and tests |
||||
|---|---|---|---|---|---|
| Venous blood sampling for mRNA assays | Fasting blood glucose and OGTT | Cardio-vascular parameters | Acute hypoxic test | IHT sessions | |
| Monday (week 1, 2 days before IHT start) | + | + | |||
| Tuesday (week 1, 1 day before IHT start) | + | + | |||
| Wednesday (week 1), Friday (week 1), Monday (week 2) | +++ | ||||
| Tuesday (week 2, 24 hours after IHT) | + | ||||
| Wednesday, Friday (week 2), Monday, Wednesday, Friday (week 3), Monday (Week 4) | ++++++ | ||||
| Tuesday (week 4, 24 hours after IHT) | + | + | |||
| Wednesday (week 4) | + | + | |||
| Tuesday (1 month after IHT) | + | + | |||
| Wednesday (1 month after IHT) | + | + | |||
IHT: intermittent hypoxic training; OGTT: oral glucose tolerance test.
In the morning of the first experiment day, after three-day routine hospital diet (250–300 g carbohydrates) and normal physical activity, a venous blood sample was drawn under fasting condition from the median antecubital vein for measurement of fasting glucose level as well as genetic analysis. Thereafter, a standard OGTT was conducted according to Ryden et al.,36 which used 75 g of glucose mixed in 250 mL of water. Venous blood samples were drawn at 120 min after the oral glucose ingestion. Plasma glucose concentrations were analyzed by glucose oxidase method in normoxic conditions on semi biochemical analyzer BTS-330 using reagents “Glucose”, Bio LATEST Lachema Diagnostica.
Next day, after a light breakfast, the baseline cardiovascular parameters of the subjects were measured in a relaxing sitting position with spontaneous breathing of room air. Arterial blood oxygen saturation (SaO2) and heart rate (HR) were recorded using a patient vital sign monitor UM 300-12 (UTAS, Ukraine, http://www.utasco.com). Systolic (SBP) and diastolic (DBP) blood pressure values were measured on brachial artery with a mercury sphygmomanometer (Erkameter 3000, Germany). After all the baseline tests, the participants were connected to an open breathing circuit through a mask to perform an AHT37: breathing a gas mixture with 12% O2 for 20 min while monitoring the changes in the subject’s cardiovascular parameters and SaO2. This study analyzed the indices at the 20th min of the test.
From the next morning, after a light breakfast, all participated subjects received the sessions of IHT three times a week for the subsequent three weeks, i.e. each subject received total of nine sessions of IHT. Each session consisted of four cycles of 5-min hypoxia (12% inspired O2) followed by 5-min normoxia (room air breathing). The normobaric hypoxia was administered to the subjects in sitting position, using a hypoxic apparatus—Hypotron® (Kiev Polytechnic Institute, National Technical University of Ukraine). The subjects’ SBP, DBP, HR, and SaO2 were continuously monitored and recorded. Next day and one month after the end of three-week IHT, the post-test examinations were conducted in the same manner as the pre-test ones (Table 2).
Determination of gene expression
mRNA expression of HIF-1α, INSR, SLC2A1, and KCNJ8 was determined in circulating blood leukocytes collected in various time points using real-time polymerase chain reaction (RT-PCR) assay. Blood leukocytes were obtained by centrifuging the blood samples at 1500 g for 1.5 min. After centrifugation, supernatant with interphase fraction was collected and transferred in new tube. After a secondary centrifugation (3000 g for 3 min) the supernatant was removed, the precipitate was used for RNA isolation using phenol–chloroform extraction after homogenization with guanidine isothiocyanate (Trizol RNA Prep 100 Kit, Russian Federation). Total RNA concentration was determined with a spectrophotometer ND1000 (NanoDrop Technologies Inc., USA). cDNA was synthesized from 5 µg of total RNA by reverse transcription with 10 mmol/L Tris-HCl (pH 9.0), 5 mmol/L MgCl2; 1 mmol/L dNTPs; 20 U Ribo-Lock, Random hexamer primers (0.5 µg·µL−1) and 200 U RevertAid H Minus M-MuLV Reverse Transcriptase. PCR was performed using an Applied Biosystems 2700 (PerkinElmer, USA).
Gene expression of HIF-1α (Assay ID: Hs00153153_m1), SLC2A1 (Hs00892681_m1), INSR (Hs00961554_m1), and KCNJ8 (Hs00958961_m1) was determined using TaqMan® Gene Expression Assay (Applied Biosystems, USA). The pairs of forward and reverse primers for genes above mentioned and the TaqMan® probes for the target mRNAs were designed by Applied Biosystems based on the human mRNA sequence. Gene expression in each probe was normalized with β-actin, using a TaqMan® human β-actin control reagent. The thermal cycles of PCR amplification consisted of initial denaturation step at 95℃ for 20 s, followed by treatment at 95℃ for 3 s, and at 60℃ for 30 s and for 50 cycles using a 7500 Fast Real-time PCR equipment (Applied Biosystems). The cycle threshold is defined as the number of cycles required for the fluorescence signal to exceed the detection threshold. The expression level of each target gene was calculated relative to the housekeeping gene (β-actin) as the difference between the threshold values of the two genes. Each PCR step was performed in duplicate and the calculations were done using the 7500 Fast System SDS software (Applied Biosystems).
Statistical analysis
All data were analyzed using SPSS software version 21.0 (SPSS Inc., USA). Student’s t-test was used to test anthropometric differences between healthy and prediabetes groups (Table 1). To evaluate the changes of blood glucose concentration over time in both groups (Table 3), two-way analysis of variance (ANOVA) with repeated measures was used followed by Bonferroni post hoc test to determine both group main effect (healthy vs. prediabetes) and time effect (for 3 time-points: Pre-IHT baseline, 1 day after IHT, 1 month after IHT). To assess the differences between physiological parameters at 20th min of acute hypoxia test before and after IHT (Table 4), three-way ANOVA with repeated measures and Bonferroni post hoc test was used to analyze both the group main effect, time effect, and hypoxia effect for each of dependent variables. Pearson product–moment correlation coefficient (r) was calculated to show the degree of linear relationship between variables. The level of statistical significance was set at P < 0.05. Data are expressed as mean ± SD.
Table 3.
Glucose blood serum concentration during oral glucose tolerance test (OGTT) before and after the three-week sessions of IHTa
| Healthy (n = 7) | Prediabetes (n = 11) | Group main effect healthy vs. prediabetes | Time effect 3 time-points | Group + time effect | |
|---|---|---|---|---|---|
| Fasting glucose (mmol/L) | |||||
| Pre-IHT baseline | 4.6 ± 0.4 | 5.6 ± 0.6 | F = 25.967 | F = 0.845 | F = 1.084 |
| 1 day after IHT | 4.5 ± 0.3 | 5.4 ± 0.7 | P = 0.000 | P = 0.434 | P = 0.348 |
| 1 month after IHT | 4.6 ± 0.5 | 5.2 ± 0.4* | |||
| 2 h post-OGTT glucose (mmol/L) | |||||
| Pre-IHT baseline | 5.3 ± 1.5 | 7.9 ± 1.5 | F = 25.757 | F = 2.590 | F = 9.570 |
| 1 day after IHT | 5.0 ± 1.2 | 7.0 ± 1.9* | P = 0.000 | P = 0.102 | P = 0.007 |
| 1 month after IHT | 4.9 ± 1.2 | 6.4 ± 1.0** |
IHT: intermittent hypoxic training; SaO2: blood oxygen saturation; HR: heart rate; SBP: systolic blood pressure.
Data are mean ± SD and were analyzed with two-way ANOVA with repeated measures.
P < 0.05 versus pre-IHT baseline; **P < 0.01 versus pre-IHT baseline.
Table 4.
Arterial blood oxygen saturation and cardiovascular indices at 20th min of acute hypoxia test (12% inspired O2) before and after the three-week sessions of IHTa
| Healthy |
Prediabetes |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Normoxia | Hypoxia | Normoxia | Hypoxia | Group main effect | Time effect | Hypoxia effect | Time + Hypoxia Effect | Group + hypoxia + time effect | |
| SaO2 (%) | |||||||||
| Pre-IHT baseline | 98.8 ± 0.6 | 80.9 ± 3.1 | 98.6 ± 0.5 | 80.3 ± 3.0 | F = 0.030 | F = 20.247 | F = 1536.695 | F = 28.454 | F = 0.038 |
| 1 day after IHT | 98.9 ± 0.5 | 86.1 ± 3.3* | 98.7 ± 0.5 | 85.0 ± 2.8* | P = 0.834 | P = 0.000 | P = 0.000 | P = 0.000 | P = 0.848 |
| 1 month after IHT | 98.8 ± 0.6 | 84.5 ± 3.2* | 98.8 ± 0.6 | 83.8 ± 2.5* | |||||
| HR (beat per min) | |||||||||
| Pre-IHT baseline | 65.4 ± 3.9 | 74.1 ± 12.2 | 69.8 ± 3.4 | 75.5 ± 4.6 | F = 1.354 | F = 5.098 | F = 15.328 | F = 0.466 | F = 0.018 |
| 1 day after IHT | 63.0 ± 2.9 | 68.4 ± 8.2* | 66.4 ± 3.4 | 68.1 ± 7.6* | P = 0.262 | P = 0.020 | P = 0.001 | P = 0.666 | P = 0.982 |
| 1 month after IHT | 64.5 ± 4.2 | 68.9 ± 7.4* | 67.4 ± 8.4 | 69.4 ± 10.4* | |||||
| SBP (mmHg) | |||||||||
| Pre-IHT baseline | 128 ± 21 | 146 ± 18 | 132 ± 20 | 154 ± 18 | F = 0.932 | F = 17.264 | F = 31.718 | F = 26.126 | F = 0.360 |
| 1 day after IHT | 124 ± 16 | 134 ± 19* | 123 ± 14* | 136 ± 15* | P = 0.350 | P = 0.001 | P = 0.000 | P = 0.000 | P = 0.704 |
| 1 month after IHT | 125 ± 20 | 138 ± 21 | 128 ± 13 | 140 ± 17 | |||||
IHT: intermittent hypoxic training; SaO2: arterial blood oxygen saturation; HR: heart rate; SBP: systolic blood pressure.
Data are mean ± SD and were analyzed with three-way ANOVA with repeated measures.
P < 0.05 vs. pre-IHT baseline.
Results
All subjects well tolerated the entire process of medical examination and IHT sessions. No subjective discomforts and/or any other adverse effects were reported.
Blood glucose level
Table 3 demonstrates the effects of IHT on blood glucose level in healthy subjects and prediabetic patients. Prior to IHT, the fasting glucose level was within the normal range in both groups, but in healthy group it was significantly lower (∼18%) than prediabetes group. The results of OGTT indicated that 2 h after 75 g glucose ingestion the plasma glucose concentration increased by 15% in the healthy subjects, but by 41% in the prediabetic patients (P < 0.05).
Two-way ANOVA results indicated that main group effect (healthy vs. prediabetes) was significant (P < 0.01). On the other hand, although the general time effect was not significant due to the absence of IHT influence on healthy subjects, a separate Bonferroni post-hoc analysis in the prediabetic patients showed a significant difference between fasting glucose pre-IHT baseline and one month after IHT (P < 0.05), between 2-h post-OGTT glucose pre-IHT baseline and one day after IHT (P < 0.05) as well as one month after IHT (P < 0.01). Both group main effect and group + time effect were statistically significant for 2-h post-OGTT glucose (Table 3).
Tolerance to acute hypoxia
Table 4 demonstrates the values of blood oxygen saturation and cardiovascular indices during AHT before and after the three-week sessions of IHT. Under acute hypoxia (12% inspired O2 for 20 min), SaO2 (a non-invasive indicator of hypoxic tolerance) dropped at the initial stage by 18% in healthy group and 19% in prediabetes group. No statistical difference between the two groups was observed (group main effect F = 0.030, P = 0.834). At the end of three-week IHT, SaO2 fell much less (by 13% or 14%, respectively) suggesting the body has gained an increased tolerance to hypoxia. This effect maintained at one month after IHT completion (time effect F = 20.247, P = 0.001; time + hypoxia effect F = 28.454, P = 0.001). The indices of cardiovascular response to acute hypoxia also showed an increased tolerance to acute hypoxia. For examples, HR was significantly lower during 20-min hypoxic load in both groups at the end of or one month after the three-week IHT sessions (time effect F = 5.098, P = 0.02; hypoxia effect F = 15.328; P = 0.001). Similarly, the hypoxia-triggered increases in SBP were reduced right after the end of IHT (time effect F = 17.264, P = 0.001; hypoxia effect F = 31.718, P = 0.001; time + hypoxia effect F = 26.126, P = 0.001). Three-way ANOVA test did not show significant common effect group + hypoxia + time effect, which demonstrates the absence of differences between groups in adaptive reactions of cardiovascular indices to hypoxia.
HIF-1α mRNA expression
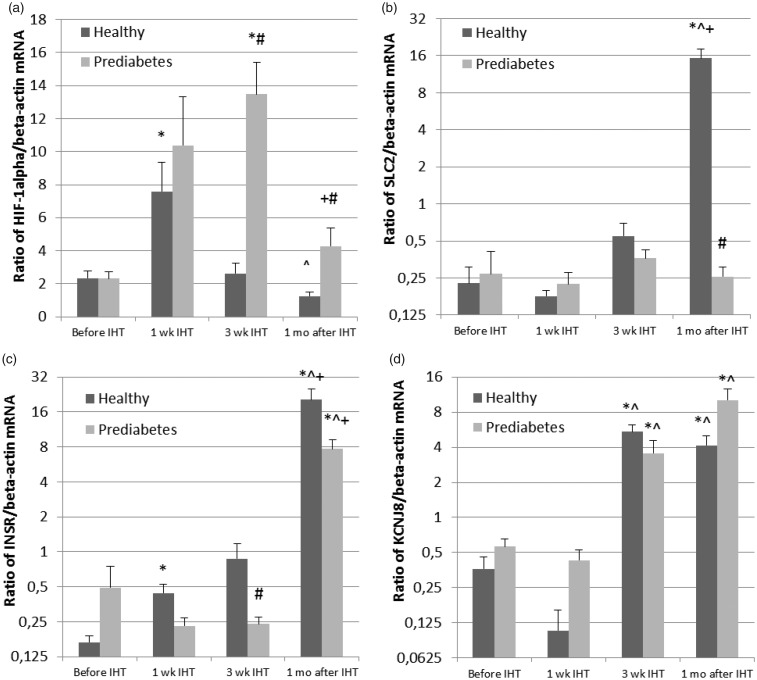
Initial level of HIF-1α mRNA expression was comparable in the healthy group and prediabetes group (Figure 1(a)). IHT resulted in approximately four-fold (Healthy) and five-fold (Prediabetes) increase during the first week of IHT. In the next two weeks HIF-1α expression returned to the baseline level in the healthy subjects. However, in the prediabetic patients HIF-1α expression continued to increase, exceeding the initial level for 6.5 times and remained two-fold higher one month after the end of IHT.
Figure 1.
Effect of IHT on mRNA expression of hypoxia inducible factor 1α (HIF-1α) (a), facilitated glucose transporter–solute carrier family-2 (SLC2) (b), insulin receptor (INSR) (c), and potassium voltage-gated channel subfamily J (KCNJ8) (d) in healthy subjects and prediabetic patients. Data are presented as mean ± SD. *P < 0.05 vs. pre-IHT baseline; ^P < 0.05 vs. 1 wk IHT; +P < 0.05 vs. 3 wk IHT; and #P < 0.05 vs. healthy group. IHT: intermittent hypoxia training
SLC2 mRNA expression
mRNA expression of SLC2—the insulin-independent glucose transporter (Figure 1(b)) was not different significantly between the two groups prior to IHT. At the end of three-week IHT sessions, SLC2 mRNA expression increased significantly in the healthy participants with subsequent 80-fold augmentation in a month after IHT termination, but no changes were observed throughout the test period in prediabetes group, indicating a prediabetes-related defect in this transporter in response to intermittent hypoxia.
INSR mRNA expression
Basal INSR mRNA expression was not statistically different between the two groups, mainly due to large individual variance among the prediabetes patients, from 0.1 to 12 units (Figure 1(c)). During IHT this parameter gradually increased in the healthy subjects reaching a five-fold increase by the end of IHT and continued to increase in the coming month, exceeding more than 100 times above the baseline value. Meanwhile, in the prediabetic patients, IHT caused slight decrease in INSR mRNA expression during training period, and only in a month after IHT termination this parameter increased to a level of 16-folds above the baseline, suggesting a remarkable delay in response to IHT.
KCNJ8 mRNA expression
The pre-IHT basal mRNA expression of KCNJ8 potassium channels was identical in both groups (Figure 1(d)). During the first week of IHT no significant changes was observed, but at the end of three-week IHT sessions KCNJ8 increased about 15-folds in healthy group and six-folds in prediabetes group. Interestingly, one month after IHT termination, the augmented level of KCNJ8 was maintained in healthy group, but showed more pronounced increase in prediabetes group.
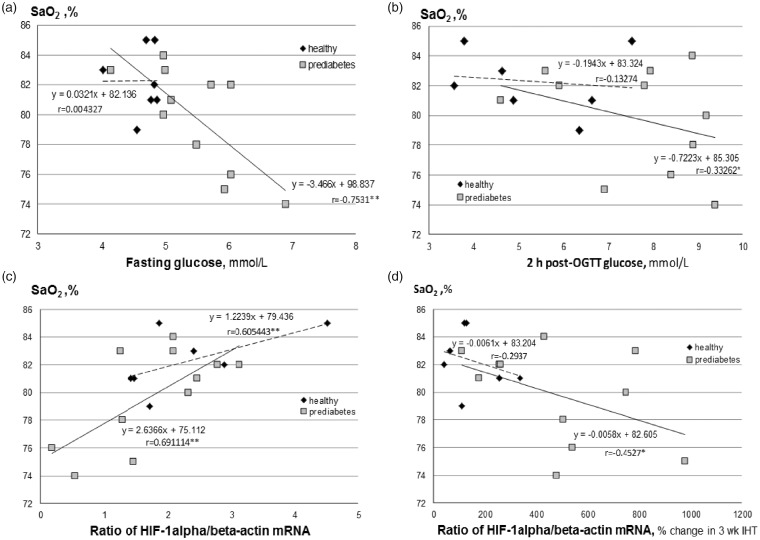
Correlation analysis
Figure 2 demonstrates the relationship between SaO2 at 20th minute of acute hypoxic test (the marker of tolerance to acute hypoxia) and several measured parameters. Although no significant correlation was found between the levels of SaO2 and baseline fasting blood glucose (Figure 2(a)) or 2-h post-OGTT blood glucose (Figure 2(b)) in healthy group, a strong negative correlation was identified in prediabetes group. In these prediabetes patients, the lower SaO2 under acute hypoxic test, the higher fasting glucose (Figure 2(a), r = −0.75; P < 0.01) and 2-h post-OGTT glucose level (Figure 2(b), r = −0.33, P < 0.05). In addition, we also found a significant positive correlation between SaO2 and baseline HIF-1α mRNA expression in both healthy (r = 0.61; P < 0.01) and prediabetes groups (r = 0.69; P < 0.01) (Figure 2(c)). Besides, a negative correlation between SaO2 and % changes in mRNA expression of HIF-1α between baseline and the post-IHT examination in prediabetes group (Figure 2(d), r = −0.42, P < 0.05). These data indicate that the subjects with lower tolerance to hypoxia had initially higher blood glucose level and greater increase in HIF-1α mRNA expression under IHT.
Figure 2.
Relationships between baseline SaO2 at 20th minute of acute hypoxic test (AHT, breathing with 12% of oxygen) and baseline fasting glucose (a), baseline 2 h post-OGTT glucose (b), baseline mRNA expression of HIF-1α (c), and % changes in mRNA expression of HIF-1α between the pre-IHT baseline and post-IHT values (d). HIF: hypoxia inducible factor; OGTT: oral glucose tolerance test. Symbols of correlation coefficient (*P < 0.05 or **P < 0.01) indicate a significant degree of linear relationship between the variables
Discussion
The present study revealed the following salient findings: (1) three-week sessions of IHT afforded beneficial effects on glucose homeostasis in patients with prediabetes (Table 3), particularly IHT significantly reduced fasting and 2-h post-OGTT blood glucose levels with the most pronounced beneficial effects observed at one month after the end of IHT; (2) IHT significantly increased the body’s tolerance to acute hypoxia and improved cardiovascular function under hypoxia in both healthy and prediabetic individuals (Table 4); (3) subjects with higher initial level of blood glucose had lower tolerance to hypoxia (Figure 2(a) and (b)); (4) IHT stimulated HIF-1α mRNA expression in blood leukocytes in a bi-phasic manner, which showed early activation during the first week of IHT and subsequently returned to the pre-IHT basal level in healthy men, but in prediabetic patients the maximum response to IHT was observed in a delayed manner at the end of IHT (Figure 1(a)); (5) HIF-1α regulated genes such as INSR, SLC2, and KCNJ8 were differentially affected by IHT in the healthy and prediabetic individuals; the greatest changes in mRNA expression of the target genes occurred one month after termination of IHT and coincided with the largest decrease in blood glucose levels, both fasting and under hyperglycemia load (Figure 1(b) to (d)); and (6) the higher expression of HIF-1α was positively associated with higher tolerance to hypoxia and better glucose homeostasis in both healthy and prediabetes subjects and interestingly the greater increase in HIF-1α mRNA expression under IHT was observed in the subjects with lower resistance to hypoxia.
The abovementioned findings are conceptually supportive to the notion of anti-diabetes effect of IHT, which was first demonstrated by Kolesnyk et al. in rats9 and subsequently confirmed in other animal and human studies using different IHT models13,38–42 as well as during the high altitude hypoxia adaptation.43,44 However, it is notable that severe intermittent hypoxia, such as those found in patients with obstructive sleep apnea (OSA) may cause various negative effects, including the suggested association between OSA and type 2 diabetes.45–47 It is increasingly realized that different patterns of intermittent hypoxia could result in divergent effects on metabolic function21,48–52 and the specific mode of hypoxia, including depth, duration, and cyclic frequency, can be critical for determining the healing or harmful results of intermittent hypoxia.2,53 Our present investigation provided direct evidence suggesting a moderate and non-invasive protocol of four short cycles of 5-min hypoxic breathing (12% O2) and 5-min normoxic breathing (room air), three times a week for three weeks is sufficient to reduce fasting and 2-h post-OGTT hyperglycemia in prediabetic patients, while increasing their resistance to acute hypoxia with improved cardiovascular functional parameters under hypoxia.
Hypoxia is well known to initiate an adaptive gene transcription program via HIFs, among which HIF-1 triggers hypoxia-dependent gene expression in regulating many metabolic processes for the improvement of O2 transport capacity.11,17,20,21,54 Reduced levels of HIF-1α have been found in the cells or tissues collected from diabetic animals or patients, indicating an inhibitory effect of hyperglycemia on HIF-1α expression.18,55–59 This diabetes-blunted HIF-1α response to hypoxic conditions may result in impaired angiogenesis and inability to upregulate glycolytic ATP generation in the type 2 diabetic heart.60
The selection of leukocytes to be studied on cellular response to hypoxia had several rationales. The primary rationale to study leukocytes is their cellular lifespan (2–10 days), which allows observing the cell phenotypic changes under intermittent hypoxia sessions. In fact, leukocytes are the only nucleated fraction in blood cells, in which the changes in gene expression under hypoxic stimuli can be non-invasively quantified and it has been thought to reflect better the processes of genetic activation in cells than the mRNAs extracted from blood plasma. For example, a previous study by Tissot van Patot et al.61 using leukocytes demonstrated that HIF-1 DNA binding activity was enhanced in vivo in response to acute hypoxia in 14 men exposed to hypobaric hypoxia (4300 m or equivalent to 12% O2) in a hypoxic chamber for 8 h, both HIF-1 DNA binding and HIF-1α protein levels in leukocytes were elevated, in association with plasma and urinary markers of hypoxic stress. To our best knowledge this is the first time to show HIF-1α mRNA increased following IHT sessions. The present study also showed that the maximum increase in HIF-1α expression induced by IHT occurred much earlier in healthy people than those in prediabetic patients, which may indicate the inhibitory effect of higher blood glucose levels on HIF-1α response to IHT in the prediabetic patients. In supporting this notion, recent study by Xiao et al.59 demonstrated that whereas hyperglycemia upregulates HIF-1α signaling in some cell types, high glucose can also inhibit HIF-1α and its target genes. Regarding the mechanisms of HIF-1α impairment under diabetic conditions, the negative effects of various diabetes-associated factors include overproduction of reactive oxygen species, increased sensitivity to Von Hippel-Lindau (VHL) machinery, and altered osmolarity and proteasome activity, which could deactivate HIF-1α.
Our present study further investigated mRNA expression of several main target genes of HIF-1α, which are likely participating in insulin reception (INSR), facilitated glucose transport (SLC2A1), and regulation of insulin secretion in the β-cells (KCNJ8). It is known that as a part of adaptive response to hypoxia, there is upregulated expression of several genes encoding glycolytic enzymes.27 However, to our best knowledge, an IHT-induced change in mRNA expression of these genes has not been reported prior to our current study. Our present results showed that whereas no significant difference in baseline expression of the examined genes between prediabetes and healthy subjects, the changes in HIF-1α-regulated gene expression in response to IHT was subsequent to those of HIF-1α mRNA in the healthy group and the maximum response was observed one month after the end of IHT (Figure 1(b) to (d)). Notably the prediabetes group exhibited different temporal profiles.
It is well recognized that physiological effects of insulin implemented mainly through INSR by binding to α subunit of INSR and stimulating the intrinsic kinase activity of β subunit of INSR.28 Increased levels of INSR could alleviate insulin resistance, because overexpression of INSR improved obese and diabetic phenotypes in rodents.62 KCNJ8 is one of the subunits of KATP channels and presents in many tissues, including pancreatic islet cells, therefore it is considered as metabolic sensors via coupling cellular metabolic status to cell membrane potential.32,35,63 Functional and structural defects in KATP channels impair insulin secretion leading to the onset of diabetes.64 Our present study demonstrated an increased KCNJ8 mRNA expression at the end of IHT in both healthy and prediabetic subjects and more pronounced effect at one month after IHT in prediabetic patients. Based on the observed similarity in KCNJ8 mRNA expression among healthy and prediabetes subjects under IHT, we postulate that KCNJ8 channels may be less vulnerable during the development of diabetes. Such a hyperactive KCNJ8 following IHT may lead to the increase in insulin secretion by β-cells. Previous laboratory animal studies suggested an organ-protective role of vascular KATP channel under diabetic condition, via suppressing diabetic oxidative stress.32 Cardioprotective and antiarrhythmic effect of adaptation to intermittent hypoxia is also mediated via activation of KATP channels.34
GLUT-1 is one of the key components of the HIF-1α-mediated hypoxia response.24,65 It is responsible for basal glucose uptake and expressed in virtually all tissues under normal conditions.66 HIF-1α accelerates the expression and activation of GLUT-1 and induces glucose uptake and glycolysis,26 which in turn induces HIF-1α degradation67 and GLUT-1 mutations that reduce its function are associated with reduced glucose uptake.68 HIF-1α and GLUT-1 levels increased significantly in the cells exposed to chronic intermittent hypoxia, suggesting a transcriptional activation and adaptive response to intermittent hypoxia.24,65 Alterations in glucose transporter genes are also associated with major pathologies, e.g. Alzheimer’s disease.69 GLUT-1 itself is a relatively stable protein as compared with HIF-1α70 and therefore its changes in response to hypoxia-reoxygenation should have a more sustained profile. In our present study, IHT elicited no change in SCL2A1 mRNA expression in the prediabetes group. The exact molecular mechanism underlying the loss of ability of IHT to upregulate GLUT-1 in prediabetic patients requires further investigation.
Notably, the most significant changes in mRNA expression of HIF-1α target genes were observed a month after IHT termination and were coincided with the largest decrease in fasting blood glucose and 2-hour post-OGTT glucose levels in the prediabetic patients (Table 3). Such long-term cause-and-effect relationships are not fully understood. Our previous studies in healthy people have also shown that the most pronounced changes in circulating hematopoietic stem and progenitor cell counts were observed not during IHT but two weeks after the completion of IHT.71 Mechanism underlying the much delayed augmentation mRNA expression of HIF-1α target genes by IHT remains unclear and should be elucidated in future studies.
In addition, it was suggested that glucose sensing in the carotid bodies may play a role in metabolism.72,73 We previously described in prediabetic patients the relationships between the tolerance to hypoxia and cardiorespiratory response to acute hypoxia as well as the severity of glucose metabolism disorder.37 Our current study also found that prediabetic patients with impaired glucose homeostasis had lower tolerance to hypoxia (Table 4, Figure 2). The higher expression of HIF-1α was positively associated with higher tolerance to hypoxia and better glucose homeostasis in both healthy and prediabetes subjects. Besides, the greatest increase in HIF-1α mRNA expression under IHT was observed in the subjects with lower tolerance to hypoxia (Figure 2(d)). This observation is in accordance with previous findings from the rats with high or low resistance to hypoxia,74,75 which reported higher increase in HIF-1 mRNA in the brain tissue of low tolerant animals following adaptation to intermittent hypoxia.
Taken together, the close relationships between the IHT-reduced blood glucose levels and the IHT-enhanced tolerance to hypoxia, which are also associated with the IHT-enhanced HIF-1α expression and its target genes, clearly suggested a better normalization of carbohydrate metabolism during IHT.
Nevertheless, several limitations of the present study include that we only investigated the transcription of HIF-1α as a possible mechanism of potentiation of the “weak links” of the HIF-1α-mediated responses in diabetic patients. At the same time, the obtained results about the induction of target genes indirectly indicate the effectiveness of HIF-1α protein stabilization and its transcriptional activity. Undoubtedly, future studies focusing on posttranslational modifications of HIF-1α protein would be highly warranted and informative.
Conclusion
The present study elucidated the poorly understood molecular mechanism underlying the beneficial effects of IHT under prediabetic conditions. We found that three-week moderate IHT induced higher HIF-1α and its target genes mRNA expressions, which were positively correlated with higher tolerance to acute hypoxia and better glucose homeostasis in both middle-aged healthy and prediabetic subjects. Our results suggest a potential utility of IHT as an effective non-pharmacologic preventive therapy for management of prediabetes patients.
Authors' contributions
All authors participated in the design and interpretation of the studies, data analysis, review, and final approval of the manuscript. TVS designed the study and wrote the manuscript. TVS, AGP, and VBS elaborated the study protocols. AGP and TID performed and interpreted genetic analyses. VIP, EE, AVG, SN, VC, and VBS conducted physiological investigations and performed statistical analyses of the results. LX critically edited the manuscript. VBS provided the enrollment and clinical examination of the subjects as well as general research management.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Xi L, Serebrovskaya TV. Intermittent hypoxia: from molecular mechanisms to clinical applications, 1st ed New York, NY: Nova Science Publishers, Inc., 2009. [Google Scholar]
- 2.Xi L, Serebrovskaya TV. Intermittent hypoxia and human diseases, 1st ed London: Springer, 2012. [Google Scholar]
- 3.Navarrete-Opazo A, Mitchell GS. Therapeutic potential of intermittent hypoxia: a matter of dose. Am J Physiol Regul Integr Comp Physiol 2014; 307: R1181–R1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mateika JH, El-Chami M, Shaheen D, Ivers B. Intermittent hypoxia: a low-risk research tool with therapeutic value in humans. J Appl Physiol 2015; 118: 520–32. [DOI] [PubMed] [Google Scholar]
- 5.Dempsey JA, Morgan BJ. Humans in hypoxia: a conspiracy of maladaptation? Physiology 2015; 30: 304–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Manukhina EB, Downey HF, Shi X, Mallet RT. Intermittent hypoxia training protects cerebrovascular function in Alzheimer's disease. Exp Biol Med 2016; 241: 1351–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Serebrovskaya TV, Xi L. Intermittent hypoxia training as non-pharmacologic therapy for cardiovascular diseases: practical analysis on methods and equipment. Exp Biol Med 2016; 241: 1708–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xi L, Chow CM, Kong X. Role of tissue and systemic hypoxia in obesity and type 2 diabetes. J Diabetes Res 2016; 2016: 1527852–1527852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kolesnyk IU, Orestenko I, Seredenko MM, Abramov AV. The effect of intermittent hypoxic training on pancreatic endocrine function in animals with diabetes mellitus. Fiziol Zh 1994; 40: 87–95. [PubMed] [Google Scholar]
- 10.Kolesnyk I, Kadzharian I, Abramov AV. The influence of the intermittent hypoxia trainings on the functional status of corticoliberin- and beta-endorphin-synthesizing neurons of the paraventricular nucleus hypothalamus in rats. Fiziol Zh 2013; 59: 25–9. [PubMed] [Google Scholar]
- 11.Tekin D, Dursun AD, Xi L. Hypoxia inducible factor 1 (HIF-1) and cardioprotection. Acta Pharmacol Sin 2010; 31: 1085–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen X, Zhao T, Huang X, Wu L, Wu K, Fan M, Zhu L. Intermittent hypoxia maintains glycemia in streptozotocin-induced diabetic rats. Cell Stress Chaperones 2016; 21: 515–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mackenzie R, Maxwell N, Castle P, Elliott B, Brickley G, Watt P. Intermittent exercise with and without hypoxia improves insulin sensitivity in individuals with type 2 diabetes. J Clin Endocrinol Metab 2012; 97: E546–E555. [DOI] [PubMed] [Google Scholar]
- 14.Tian YM, Liu Y, Wang S, Dong Y, Su T, Ma HJ, Zhang Y. Anti-diabetes effect of chronic intermittent hypobaric hypoxia through improving liver insulin resistance in diabetic rats. Life Sci 2016; 150: 1–7. [DOI] [PubMed] [Google Scholar]
- 15.Morishima T, Hasegawa Y, Sasaki H, Kurihara T, Hamaoka T, Goto K. Effects of different periods of hypoxic training on glucose metabolism and insulin sensitivity. Clin Physiol Funct Imaging 2015; 35: 104–9. [DOI] [PubMed] [Google Scholar]
- 16.Kolchinskaia AZ. Medical use of stepwise adaptation to hypoxia. Vestn Ross Akad Med Nauk 1997; 5: 12–9. [PubMed] [Google Scholar]
- 17.Semenza GL. O2-regulated gene expression: transcriptional control of cardiorespiratory physiology by HIF-1. J Appl Physiol 2004; 96: 1173–7. [DOI] [PubMed] [Google Scholar]
- 18.Portnichenko VI, Nosar VI, Sydorenko AM, Portnichenko AH, Mankovska IM. Continuous adaptation of rats to hypobaric hypoxia prevents stressor hyperglycemia and optimizes mitochondrial respiration under acute hypoxia. Fiziol Zh 2012; 58: 56–64. [PubMed] [Google Scholar]
- 19.Han X, Sun S, Zhao M, Cheng X, Chen G, Lin S, Guan Y, Yu X. Celastrol stimulates hypoxia-inducible factor-1 activity in tumor cells by initiating the ROS/Akt/p70S6K signaling pathway and enhancing hypoxia-inducible factor-1alpha protein synthesis. PLoS One 2014; 9: e112470–e112470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Girgis CM, Cheng K, Scott CH, Gunton JE. Novel links between HIFs, type 2 diabetes, and metabolic syndrome. Trends Endocrinol Metab 2012; 23: 372–80. [DOI] [PubMed] [Google Scholar]
- 21.Mackenzie RW, Watt P. A molecular and whole body insight of the mechanisms surrounding glucose disposal and insulin resistance with hypoxic treatment in skeletal muscle. J Diabetes Res 2016; 2016: 6934937–6934937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mueckler M. Facilitative glucose transporters. Eur J Biochem 1994; 219: 713–25. [DOI] [PubMed] [Google Scholar]
- 23.Maher F, Vannucci SJ, Simpson IA. Glucose transporter proteins in brain. FASEB J 1994; 8: 1003–11. [DOI] [PubMed] [Google Scholar]
- 24.Wang B, Wood IS, Trayhurn P. Dysregulation of the expression and secretion of inflammation-related adipokines by hypoxia in human adipocytes. Pflugers Arch 2007; 455: 479–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen C, Pore N, Behrooz A, Ismail-Beigi F, Maity A. Regulation of glut1 mRNA by hypoxia-inducible factor-1. Interaction between H-ras and hypoxia. J Biol Chem 2001; 276: 9519–25. [DOI] [PubMed] [Google Scholar]
- 26.Park HS, Kim JH, Sun BK, Song SU, Suh W, Sung JH. Hypoxia induces glucose uptake and metabolism of adiposederived stem cells. Mol Med Rep 2016; 14: 4706–14. [DOI] [PubMed] [Google Scholar]
- 27.He Q, Yang QC, Zhou Q, Zhu H, Niu WY, Feng J, Wang Y, Cao J, Chen BY. Effects of varying degrees of intermittent hypoxia on proinflammatory cytokines and adipokines in rats and 3T3-L1 adipocytes. PLoS One 2014; 9: e86326–e86326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ebina Y, Ellis L, Jarnagin K, Edery M, Graf L, Clauser E, Ou JH, Masiarz F, Kan YW, Goldfine ID. The human insulin receptor cDNA: the structural basis for hormone-activated transmembrane signalling. Cell 1985; 40: 747–58. [DOI] [PubMed] [Google Scholar]
- 29.Tian Y, Yao J, Liu S, Jiang C, Zhang J, Li Y, Feng J, Liu Z. Identification and expression analysis of 26 oncogenes of the receptor tyrosine kinase family in channel catfish after bacterial infection and hypoxic stress. Comp Biochem Physiol Part D Genomics Proteomics 2015; 14: 16–25. [DOI] [PubMed] [Google Scholar]
- 30.Sasaki T, Kuroko M, Sekine S, Matsui S, Kikuchi O, Susanti VY, Kobayashi M, Tanaka Y, Yuasa T, Kitamura T. Overexpression of insulin receptor partially improves obese and diabetic phenotypes in db/db mice. Endocr J 2015; 62: 787–96. [DOI] [PubMed] [Google Scholar]
- 31.Kanno T, Rorsman P, Gopel SO. Glucose-dependent regulation of rhythmic action potential firing in pancreatic beta-cells by KATP-channel modulation. J Physiol 2002; 545: 501–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li SS, Cui N, Yang Y, Trower TC, Wei YM, Wu Y, Zhang S, Jin X, Jiang C. Impairment of the vascular KATP channel imposes fatal susceptibility to experimental diabetes due to multi-organ injuries. J Cell Physiol 2015; 230: 2915–26. [DOI] [PubMed] [Google Scholar]
- 33.Milano G, Bianciardi P, Corno AF, Raddatz E, Morel S, von Segesser LK, Samaja M. Myocardial impairment in chronic hypoxia is abolished by short aeration episodes: involvement of K+ATP channels. Exp Biol Med 2004; 229: 1196–205. [DOI] [PubMed] [Google Scholar]
- 34.Kolar F, Nekar J, Ostadal B, Maslov LN, Stakheev DL, Tayurskaya AS, Lishmanov YB. Role of ATP-sensitive K+-channels in antiarrhythmic and cardioprotective action of adaptation to intermittent hypobaric hypoxia. Bull Exp Biol Med 2008; 145: 418–21. [DOI] [PubMed] [Google Scholar]
- 35.Seino S. Physiology and pathophysiology of KATP channels in the pancreas and cardiovascular system: a review. J Diabetes Complications 2003; 17(2 Suppl): 2–5. [DOI] [PubMed] [Google Scholar]
- 36.Ryden L, Standl E, Bartnik M, Van den Berghe G, Betteridge J, de Boer MJ, Cosentino F, Jonsson B, Laakso M, Malmberg K, Priori S, Ostergren J, Tuomilehto J, Thrainsdottir I, Vanhorebeek I, Stramba-Badiale M, Lindgren P, Qiao Q, Priori SG, Blanc JJ, Budaj A, Camm J, Dean V, Deckers J, Dickstein K, Lekakis J, McGregor K, Metra M, Morais J, Osterspey A, Tamargo J, Zamorano JL, Deckers JW, Bertrand M, Charbonnel B, Erdmann E, Ferrannini E, Flyvbjerg A, Gohlke H, Juanatey JR, Graham I, Monteiro PF, Parhofer K, Pyorala K, Raz I, Schernthaner G, Volpe M, Wood D. Guidelines on diabetes, pre-diabetes, and cardiovascular diseases: executive summary. The Task Force on Diabetes and Cardiovascular Diseases of the European Society of Cardiology (ESC) and of the European Association for the Study of Diabetes (EASD). Eur Heart J 2007; 28: 88–136. [DOI] [PubMed] [Google Scholar]
- 37.Shatylo VB, Serebrovska TV, Gavalko AV, Egorov E, Korkushko OV. Acute hypoxic test in patients with prediabetes. High Alt Med Biol 2016; 17: 101–7. [DOI] [PubMed] [Google Scholar]
- 38.Gutwenger I, Hofer G, Gutwenger AK, Sandri M, Wiedermann CJ. Pilot study on the effects of a 2-week hiking vacation at moderate versus low altitude on plasma parameters of carbohydrate and lipid metabolism in patients with metabolic syndrome. BMC Res Notes 2015; 8: 103–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guzel D, Dursun AD, Ficicilar H, Tekin D, Tanyeli A, Akat F, Topal CF, Sabuncuoglu B, Bastug M. Effect of intermittent hypoxia on the cardiac HIF-1/VEGF pathway in experimental type 1 diabetes mellitus. Anatol J Cardiol 2016; 16: 76–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kayser B, Verges S. Hypoxia, energy balance and obesity: from pathophysiological mechanisms to new treatment strategies. Obes Rev 2013; 14: 579–92. [DOI] [PubMed] [Google Scholar]
- 41.Mekjavic IB, Amon M, Kolegard R, Kounalakis SN, Simpson L, Eiken O, Keramidas ME, Macdonald IA. The effect of normobaric hypoxic confinement on metabolism, gut hormones, and body composition. Front Physiol 2016; 7: 202–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morishima T, Goto K. Successive exposure to moderate hypoxia does not affect glucose metabolism and substrate oxidation in young healthy men. Springerplus 2014; 3: 370–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.De MP, Fokkert MJ, de Vries ST, de Koning EJ, Dikkeschei BD, Gans RO, Tack CJ, Bilo HJ. Metabolic effects of high altitude trekking in patients with type 2 diabetes. Diabetes Care 2012; 35: 2018–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hurtado A, Escudero E, Pando J, Sharma S, Johnson RJ. Cardiovascular and renal effects of chronic exposure to high altitude. Nephrol Dial Transplant 2012; 27(Suppl 4): iv11–iv16. [DOI] [PubMed] [Google Scholar]
- 45.Shin MK, Han W, Bevans-Fonti S, Jun JC, Punjabi NM, Polotsky VY. The effect of adrenal medullectomy on metabolic responses to chronic intermittent hypoxia. Respir Physiol Neurobiol 2014; 203: 60–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rajan P, Greenberg H. Obstructive sleep apnea as a risk factor for type 2 diabetes mellitus. Nat Sci Sleep 2015; 7: 113–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang N, Khan SA, Prabhakar NR, Nanduri J. Impairment of pancreatic beta-cell function by chronic intermittent hypoxia. Exp Physiol 2013; 98: 1376–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim NH. Obstructive sleep apnea and abnormal glucose metabolism. Diabetes Metab J 2012; 36: 268–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sharma SK, Ghimire A, Radhakrishnan J, Thapa L, Shrestha NR, Paudel N, Gurung KRM, Budathoki A, Baral N, Brodie D. Prevalence of hypertension, obesity, diabetes, and metabolic syndrome in Nepal. Int J Hypertens 2011; 2011: 821971–821971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mateika JH, Komnenov D. Intermittent hypoxia initiated plasticity in humans: a multipronged therapeutic approach to treat sleep apnea and overlapping co-morbidities. Exp Neurol 2017; 287: 113–29. [DOI] [PubMed] [Google Scholar]
- 51.Carreras A, Kayali F, Zhang J, Hirotsu C, Wang Y, Gozal D. Metabolic effects of intermittent hypoxia in mice: steady versus high-frequency applied hypoxia daily during the rest period. Am J Physiol Regul Integr Comp Physiol 2012; 303: R700–R709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Almendros I, Wang Y, Gozal D. The polymorphic and contradictory aspects of intermittent hypoxia. Am J Physiol Lung Cell Mol Physiol 2014; 307: L129–L140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Serebrovskaya TV, Nosar VI, Bratus LV, Gavenauskas BL, Mankovska IM. Tissue oxygenation and mitochondrial respiration under different modes of intermittent hypoxia. High Alt Med Biol 2013; 14: 280–8. [DOI] [PubMed] [Google Scholar]
- 54.Nanduri J, Vaddi DR, Khan SA, Wang N, Makarenko V, Semenza GL, Prabhakar NR. HIF-1alpha activation by intermittent hypoxia requires NADPH oxidase stimulation by xanthine oxidase. PLoS One 2015; 10: e0119762–e0119762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hosogai N, Fukuhara A, Oshima K, Miyata Y, Tanaka S, Segawa K, Furukawa S, Tochino Y, Komuro R, Matsuda M, Shimomura I. Adipose tissue hypoxia in obesity and its impact on adipocytokine dysregulation. Diabetes 2007; 56: 901–11. [DOI] [PubMed] [Google Scholar]
- 56.Jiang C, Qu A, Matsubara T, Chanturiya T, Jou W, Gavrilova O, Shah YM, Gonzalez FJ. Disruption of hypoxia-inducible factor 1 in adipocytes improves insulin sensitivity and decreases adiposity in high-fat diet-fed mice. Diabetes 2011; 60: 2484–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kihira Y, Miyake M, Hirata M, Hoshina Y, Kato K, Shirakawa H, Sakaue H, Yamano N, Izawa-Ishizawa Y, Ishizawa K, Ikeda Y, Tsuchiya K, Tamaki T, Tomita S. Deletion of hypoxia-inducible factor-1alpha in adipocytes enhances glucagon-like peptide-1 secretion and reduces adipose tissue inflammation. PLoS One 2014; 9: e93856–e93856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Leonska-Duniec A, Ahmetov II, Zmijewski P. Genetic variants influencing effectiveness of exercise training programmes in obesity – an overview of human studies. Biol Sport 2016; 33: 207–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xiao H, Gu Z, Wang G, Zhao T. The possible mechanisms underlying the impairment of HIF-1alpha pathway signaling in hyperglycemia and the beneficial effects of certain therapies. Int J Med Sci 2013; 10: 1412–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Heather LC, Cole MA, Tan JJ, Ambrose LJ, Pope S, Abd-Jamil AH, Carter EE, Dodd MS, Yeoh KK, Schofield CJ, Clarke K. Metabolic adaptation to chronic hypoxia in cardiac mitochondria. Basic Res Cardiol 2012; 107: 268–268. [DOI] [PubMed] [Google Scholar]
- 61.Tissot van Patot MC, Serkova NJ, Haschke M, Kominsky DJ, Roach RC, Christians U, Henthorn TK, Honigman B. Enhanced leukocyte HIF-1alpha and HIF-1 DNA binding in humans after rapid ascent to 4300 m. Free Radic Biol Med 2009; 46: 1551–7. [DOI] [PubMed] [Google Scholar]
- 62.Sasaki M, Fujimoto S, Sato Y, Nishi Y, Mukai E, Yamano G, Sato H, Tahara Y, Ogura K, Nagashima K, Inagaki N. Reduction of reactive oxygen species ameliorates metabolism-secretion coupling in islets of diabetic GK rats by suppressing lactate overproduction. Diabetes 2013; 62: 1996–2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tricarico D, Selvaggi M, Passantino G, De PP, Dario C, Centoducati P, Tateo A, Curci A, Maqoud F, Mele A, Camerino GM, Liantonio A, Imbrici P, Zizzo N. ATP sensitive potassium channels in the skeletal muscle function: involvement of the KCNJ11(Kir6.2) gene in the determination of mechanical Warner Bratzer shear force. Front Physiol 2016; 7: 167–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Velasco M, Diaz-Garcia CM, Larque C, Hiriart M. Modulation of ionic channels and insulin secretion by drugs and hormones in pancreatic beta cells. Mol Pharmacol 2016; 90: 341–57. [DOI] [PubMed] [Google Scholar]
- 65.Chen L, Cao ZL, Han F, Gao ZC, He QY. Chronic intermittent hypoxia from pedo-stage decreases glucose transporter 4 expression in adipose tissue and causes insulin resistance. Chin Med J 2010; 123: 463–70. [PubMed] [Google Scholar]
- 66.Zhao FQ, Keating AF. Functional properties and genomics of glucose transporters. Curr Genomics 2007; 8: 113–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Malhotra R, Tyson DG, Sone H, Aoki K, Kumagai AK, Brosius FC., III Glucose uptake and adenoviral mediated GLUT1 infection decrease hypoxia-induced HIF-1alpha levels in cardiac myocytes. J Mol Cell Cardiol 2002; 34: 1063–73. [DOI] [PubMed] [Google Scholar]
- 68.Thorens B, Mueckler M. Glucose transporters in the 21st Century. Am J Physiol Endocrinol Metab 2010; 298: E141–E145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kaelin WG, Jr., Ratcliffe PJ. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell 2008; 30: 393–402. [DOI] [PubMed] [Google Scholar]
- 70.Cooper C, Liu GY, Niu YL, Santos S, Murphy LC, Watson PH. Intermittent hypoxia induces proteasome-dependent down-regulation of estrogen receptor alpha in human breast carcinoma. Clin Cancer Res 2004; 10: 8720–7. [DOI] [PubMed] [Google Scholar]
- 71.Serebrovskaya TV, Nikolsky IS, Nikolska VV, Mallet RT, Ishchuk VA. Intermittent hypoxia mobilizes hematopoietic progenitors and augments cellular and humoral elements of innate immunity in adult men. High Alt Med Biol 2011; 12: 243–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pokorski M, Pozdzik M, Antosiewicz J, Dymecka A, Mazzatenta A, Di GC. Hypoxic ventilatory reactivity in experimental diabetes. Adv Exp Med Biol 2015; 860: 123–32. [DOI] [PubMed] [Google Scholar]
- 73.Conde SV, Sacramento JF, Guarino MP, Gonzalez C, Obeso A, Diogo LN, Monteiro EC, Ribeiro MJ. Carotid body, insulin, and metabolic diseases: unraveling the links. Front Physiol 2014; 5: 418–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lukyanova LD, Kirova YI. Mitochondria-controlled signaling mechanisms of brain protection in hypoxia. Front Neurosci 2015; 9: 320–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kirova YI, Shakova FM, Germanova EL, Paltsyn AA, Romanova GA, Rybnikova YA, Lukyanova LD. Urgent changes in the expression of hypoxia-inducible factor-1alpha (HIF-1alpha) in the neocortex of rats with different tolerance to acute hypoxia underwent focal ischemic stroke prefrontal cortex. Patol Fiziol Eksp Ter 2014; 3: 9–16. [PubMed] [Google Scholar]