Abstract
Intestinal epithelial tissue is constantly regenerated as a means to maintain proper tissue function. Previous studies have demonstrated that denervation of the parasympathetic or sympathetic nervous system to the intestine alters this process. However, results are inconsistent between studies, showing both increases and decreases in proliferation after denervation of the parasympathetic or sympathetic. The effect appears to correlate with (1) the timing post-denervation, (2) denervation-induced changes in food intake, (3) the denervation technique used, and (4) which intestinal segment is investigated. Thus, we proposed that parasympathetic or sympathetic denervation does not have an effect on intestinal epithelial regeneration when you (1) evaluate denervation after long-term denervation, (2) control for post-surgical changes in food intake, (3) use minimally invasive surgical techniques and (4) include a segmental analysis. To test this, adult male Sprague Dawley rats underwent parasympathetic denervation via subdiaphragmatic vagotomy, sympathetic denervation via celiacomesenteric ganglionectomy, a parasympathetic denervation sham surgery, or a sympathetic denervation sham surgery. Sham surgery ad libitum-fed groups and sham surgery pair-fed groups were used to control for surgically induced changes in food intake. Three weeks post-surgery, animals were sacrificed and tissue from the duodenum, jejunum, and ileum was excised and immunohistochemically processed to visualize indicators of proliferation (bromodeoxyuridine-positive cells) and apoptosis (caspase-3-positive cells). Results showed no differences between groups in proliferation, apoptosis, or total cell number in any intestinal segment. These results suggest that parasympathetic or sympathetic denervation does not have a significant long-term effect on intestinal epithelial turnover. Thus, intestinal epithelial regeneration is able to recover after autonomic nervous system injury.
Impact statement
This study investigates the long-term effect of autonomic denervation on intestinal epithelial cell turnover, as measured by proliferation, apoptosis, and total cell number. Although previous research has established that autonomic denervation can alter intestinal epithelial turnover under short-term conditions, here we establish for the first time that these changes do not persist long-term when you control for surgical-induced changes in food intake and use targeted denervation procedures. These findings add to the base of knowledge on autonomic control of tissue turnover, highlight the ability of the intestinal epithelium to recover after autonomic injury and reveal possible implications of the use of ANS denervation for disease treatment in humans.
Keywords: Intestinal epithelium, sympathetic, parasympathetic, proliferation, apoptosis, regeneration
Introduction
The autonomic nervous system (ANS) is implicated in the control of tissue regeneration. A focus of prior research has been on the ANS control of intestinal epithelial cell turnover, as it is among the most rapidly regenerating tissues of the body.1 Ablation of either branch of the ANS, the parasympathetic (PNS) or sympathetic (SNS), alters intestinal epithelial cell proliferation as indicated by a change in the mitotic index2–7 and length of the cell cycle.6 These data, though, are inconsistent across studies. After PNS denervation, the direction of fluctuation in proliferation appears to be time dependent. At early time points post-denervation, intestinal epithelial cell proliferation is decreased.2,3 The early decrease in proliferation may be due to a concomitant decrease in food intake, which is known to decrease intestinal epithelial cell proliferation independent of an ANS influence.8 At later time points post-PNS denervation, proliferation has been shown to be increased4,5 or equal to that of control animals,2,9 perhaps demonstrating a recovery over time.2 Thus, these changes may be indirectly driven by changes in food intake after PNS denervation, rather than a direct influence of the loss of the PNS. Sympathetic denervation also exhibits varied results for regeneration of this tissue. The effect on intestinal epithelial proliferation appears to correlate with the extent of denervation induced by specific SNS denervation techniques and which intestinal segment (duodenum, jejunum or ileum) is investigated. A long-term decrease in proliferation is seen after extreme techniques (e.g. chemical sympathectomy).6,7 However, a more focused surgical denervation targeting the celiacomesenteric nerves that innervate the intestine results in no change in proliferation along most of the proximal to distal axis of the intestine.2–4,6 Thus, we propose that PNS or SNS denervation does not have a long-term effect on intestinal epithelial regeneration when you control for post-surgical changes in food intake and use minimally invasive surgical techniques. A recovery of function after long-term ANS denervation is seen in processes related to intestinal function, including motility,10 food intake11,12 and blood flow.13 Recovery is also seen in aspects of tissue regeneration in other organs, including the liver14,15 and the pancreas.16 Thus, it is possible that intestinal epithelial turnover exhibits this same pattern of recovery after a long-term loss of ANS input. In order to test this, male Sprague Dawley rats were subjected to PNS denervation or SNS denervation. To control for surgical effects, we included sham surgical control conditions for each surgical procedure. To control for the change in food intake that often occurs post-ANS denervation, we included a pair-fed sham surgical group for both the PNS and the SNS denervation. We sacrificed the animals three weeks post-surgery because this is generally regarded to be a minimum time for recovery of other intestinal physiological processes after ANS denervation.10–13 We have defined this three-week time point as “long-term” based on the timeline of these other recovery processes. We collected intestinal tissue from three intestinal segments (duodenum, jejunum, and ileum) and immunohistochemically processed the tissue to measure levels of proliferation, apoptosis, and total crypt and villus cell numbers.
ANS denervation is currently used in patients to treat a variety of diseases. PNS denervation is used for the treatment of gastric ulcers17 and may occur as a consequence of gastric bypass surgery.18 SNS denervation is currently used to treat hyperhidrosis,19 facial blushing,20 and a variety of other conditions,21 New treatments involving autonomic denervation may also be implemented in the future for other diseases involving autonomic imbalance, such as rheumatoid arthritis22 or chronic pain.23 Identifying whether intestinal regeneration is chronically disrupted after these procedures is important because alterations are known to lead to aberrant tissue function. A chronic decrease in proliferation can lead to mucosal atrophy, malabsorption, and diarrhea.24 In contrast, a chronic increase in proliferation may lead to increased epithelial cell numbers, excessive nutrient absorption,25,26 and the development of tumors.27 Thus, identifying whether intestinal epithelial regeneration is able to recover chronically after ANS denervation is important for determining whether these surgeries should be used in medicine.
Materials and methods
Animals
All procedures were approved by the Institutional Animal Care and Use Committee at the University of Illinois at Urbana-Champaign. Male Sprague-Dawley rats (n = 56; Harlan, Hsd:Sprague Dawley® SD®; 225–275 g) were single-housed in tub cages with ad libitum access to a chow diet (Harlan 8640, Harlan Sprague Dawley Inc, Indianapolis, IN), tap water, and a ceramic bowl for enrichment. They were maintained on a 12:12 light:dark cycle (lights on 0700 h, lights off 1900 h). Food intake and body weight were measured daily at 1500 h. The animals acclimated to these conditions for one week prior to surgery.
Surgical procedures
Animals underwent an overnight fast prior to undergoing surgery. Animals were given an anesthesia mixture (0.1 mL/100g) intramuscularly containing the following: 100 mg/mL ketamine (Henry Schlein Animal Health, Dublin OH), 100 mg/mL xylazine (Akorn, Decatur, IL), 10 mg/mL Acepromazine Maleate (Boehringer Ingelheim, St. Joseph, MO) in 0.9% sterile saline (Henry Schlein Animal Health, Dublin, OH). PNS denervation, or vagotomy (n = 12), and sham surgeries (n = 15) were performed as previously described.28–32 Briefly, the dorsal and ventral branches of the subdiaphragmatic vagus were located by blunt dissection of the overlying tissues, isolated, and ligated above the hepatic, celiac, and accessory celiac branches. Each nerve trunk was cut distal to the ligature and above the vagal branches, producing total vagal transection. For sham vagotomy surgeries, the esophagus, stomach, dorsal and ventral branches of the subdiaphragmatic vagus were manipulated, but the vagi were left intact. Sympathetic denervation, or celiacomesenteric ganglionectomy (n = 11), and sham surgeries (n = 14) were performed as previously described.28,29,33 Briefly, the left kidney, cranial mesenteric artery and celiac artery were exposed by blunt dissection of the overlying tissues, and the celiac and mesenteric ganglia were located between the arteries and were removed. Sham celiacomesenteric ganglionectomy surgeries were performed by manipulating the tissue without removal of the celiac or mesenteric ganglia. Before closure of the surgical sites, 0.25% bupivacaine (2 mg/kg; Hospira, Lake Forest, IL) was applied to the incision site. Rimadyl (5 mg/kg; Pfizer, New York, NY) was given subcutaneously while still under anesthesia and for two days after surgery.
Feeding protocol
Animals that underwent PNS or SNS surgical denervation had ad libitum access to chow diet for three weeks. PNS or SNS sham surgery animals were divided into two groups based on average body weight: ad libitum access to chow or pair-fed chow in equal average kcal to that eaten by the denervated group for three weeks. The pair-fed animals were fed in the middle of the light cycle on a one day delayed scheduled from their paired denervated group. Thus, the six groups were (1) PNS denervated, (2) PNS pair-fed sham, (3) PNS ad lib-fed sham, (4) SNS denervated, (5) SNS pair-fed sham and (6) SNS ad lib-fed sham.
Sacrifice
After the three-week feeding period, animals had their food removed at the beginning of the light cycle and were sacrificed at the beginning of the dark cycle. This 12 h fast was sufficient to minimize the amount of food present in the intestine at the time of sacrifice. At 6 h prior to sacrifice, 5-bromo-2′-deoxyuridine (BrdU; Sigma-Aldrich, St. Louis, MO) injections were administered intraperitoneally (50 mg/kg) in a volume of 1.5 mL sterile dH2O. Animals were then sacrificed under isoflurane (Henry Schlein Animal Health, Dublin, OH) anesthesia by decapitation. Tissue was collected from the duodenum, jejunum, and ileum.
Tissue processing
Intestinal segments were flushed with 1 × phosphate-buffered saline (PBS) and filled with 4% paraformaldehyde (PFA; Sigma-Aldrich, St. Louis, MO) for 12 h at 4℃ and then stored in 1 × PBS at 4℃ prior to paraffin embedding. Paraffin embedding was done in a Leica ASP300 Tissue Processor (Leica, Wetzlar Germany) and embedded using Paraplast tissue embedding media (Fisher Scientific, West Lawn, NJ). For slide preparation, sections were cut on a microtome at 5 µm and then mounted on Superfrost™ Plus Microscope Slides (Fisher Scientific, Pittsburgh, PA).
Immunohistochemistry: Proliferation
Tissue sections were deparaffinized in xylenes followed by a series of graded ethanols, then rinsed in deionized water. Antigen retrieval was performed by immersing the slides in sodium citrate buffer (Sodium Citrate Hydrochloride, Fisher Scientific and Tween 20, Sigma- Aldrich, Germany, pH 6.0) for 20 min in a water bath at 95℃, then allowed to cool while still immersed in the sodium citrate buffer solution for 30 min at RT. Slides were rinsed between each step in 1 × PBS. Slides were incubated in blocking solution containing 0.3% Triton-X (Sigma-Aldrich, St. Louis, MO) and 3% Normal Donkey Serum (JacksonImmunoResearch, West Grove, PA) diluted in 1 × PBS at RT for 1 h. Sections were then incubated overnight in an anti-BrdU primary antibody (1:1000, ab1893, Abcam, San Francisco, CA) diluted in the blocking solution. Sections were incubated in a donkey anti-sheep IgG H&L secondary antibody (1:500, ab6899, Abcam, San Francisco, CA) diluted in 1 × PBS for 1 h at RT. Sections were then incubated in an avidin–biotin complex (Vectastain Elite reagents, Vector Labs, Burlingame, CA) for 1 h at RT. A diaminobenzidine-hydrogen peroxidase reaction was performed using a substrate kit (SK-4100, Vector Laboratories, Burlingame, CA). Sections were counterstained in hematoxylin (Fisher HealthCare, Houston, TX) and dehydrated in a series of graded ethanols followed by xylenes. Slides were coverslipped with Permount mounting media (Fisher Scientific, Fair Lawn, NJ) and stored at RT.
Immunohistochemistry: Apoptosis
Slides were processed as described above with the following exceptions: Slides were incubated in the blocking solution for 1.5 h at RT, PB was used for washes, the primary antibody was anti-caspase-3 antibody (1:2500, cat. no. 9662, Cell Signaling Technologies, Danvers, MA), and the secondary was donkey anti-rabbit IgG (711-065-152, Jackson ImmunoResearch, West Grove, PA).
Quantification
Intestinal tissue sections were visualized using a NanoZoomer Digital Pathology System (Hamamatsu, Hamamatsu City, Japan) using a 40× objective and NDP Scan software. Quantification was performed by visual inspection of the images on a desktop computer using NDP View 2 software by two individuals that were blinded to the treatments. Nine crypts or villi per section were chosen based on intact morphology of the intestine. Total crypt cell number, BrdU-positive crypt cell number, proliferation ratio (defined as BrdU-positive crypt cell number divided by total crypt cell number), and crypt depth were determined from slides immunohistochemically processed for BrdU. Total villus cell number, caspase-3-positive villus cell number, apoptotic ratio (defined as caspase-3-positive villus cell number divided by total villus cell number), and villus height were determined from slides immunohistochemically processed for caspase-3.
Statistical analysis
All variables were analyzed using Number Crunching Statistical Software (NCSS LLC, Kaysville, UT). Food intake and body weight were analyzed using separate two-way repeated measures analyses of variance (ANOVA; 3 × 13; treatment group × day) for PNS and SNS experiments. Measures of proliferation and apoptosis were analyzed using separate one-way ANOVA (treatment group) for each intestinal segment (duodenum, jejunum or ileum). Tukey–Kramer post hoc analyses were utilized where appropriate. Differences among groups were considered statistically significant if P < 0.05.
Results
Food intake
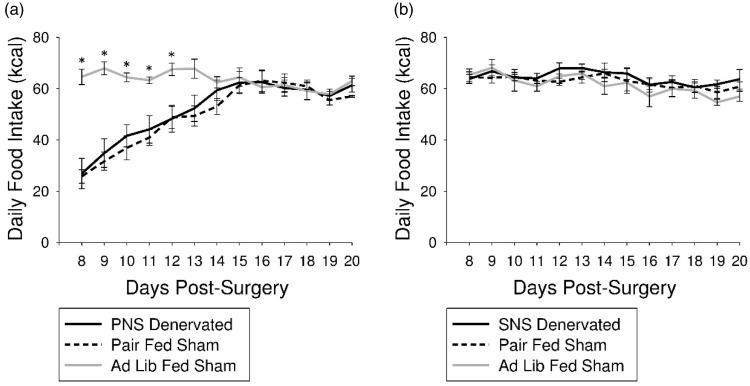
Average 24 h food intake (kcal) was decreased in PNS denervated and pair-fed sham groups compared with ad lib-fed sham animals on days 8 through 12 post-surgery (P < 0.05; Figure 1(a)). There were no differences in average 24 h food intake (kcal) between SNS denervated, ad lib-fed sham, or pair-fed sham animals at any time point (Figure 1(b)).
Figure 1.
Influence of PNS or SNS denervation on daily food intake. (a) Average daily food intake was decreased in PNS denervated animals and pair-fed sham animals compared with ad lib-fed sham surgical controls on days 8 through 12 post-surgery (P < 0.05; *). (b) SNS denervation did not significantly alter average daily food intake
Body weight
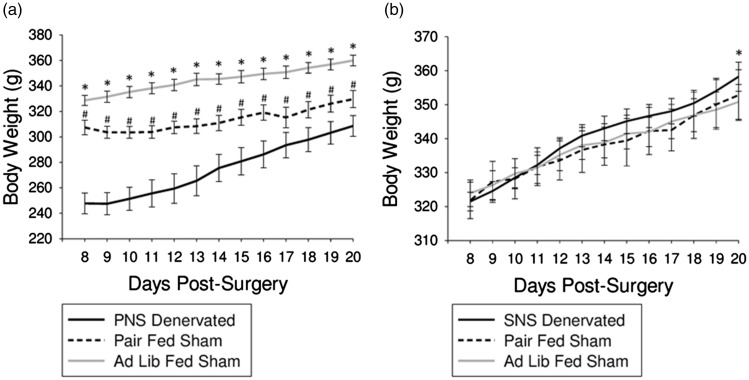
Body weight was significantly different between all three PNS groups across all time points, with the lowest body weight seen in the PNS denervated animals and the highest in the ad lib-fed sham animals (P < 0.05; Figure 2(a)). Body weight was increased in the SNS denervated animals compared with the ad lib-fed sham animals only on day 20 post-surgery (P < 0.05; Figure 2(b)).
Figure 2.
Influence of PNS or SNS denervation on body weight. (a) Average body weight was decreased in PNS denervated animals and pair-fed sham animals compared with ad lib-fed sham animals across all time points (P < 0.05; *). Average body weight was decreased in PNS denervated animals compared with pair-fed sham days across all time points (P < 0.05; #). (b) Average body weight of SNS denervated animals was increased compared with ad lib-fed sham animals on day 20 post-surgery (P < 0.05; *)
Effect of PNS denervation on proliferation
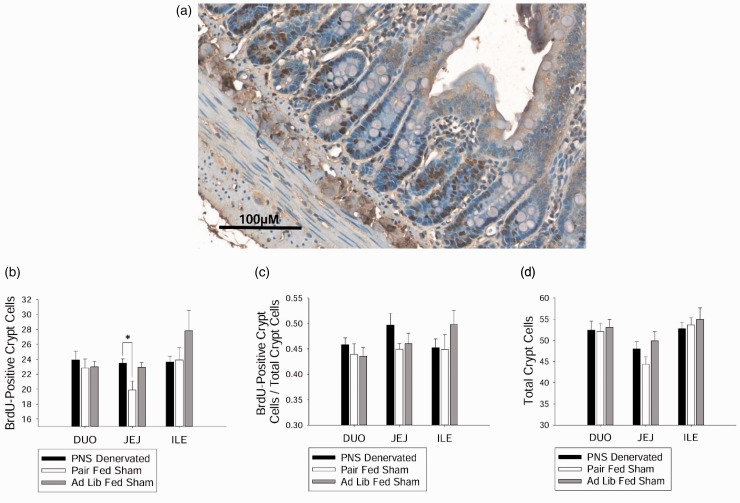
BrdU-positive cells (representative image, Figure 3(a)) in the jejunum were increased in the PNS denervated animals compared with pair-fed sham animals (P < 0.05, Figure 3(b)), but PNS denervated and ad lib-fed sham were not different from each other. There were no differences in the number of BrdU-positive cells between the groups when the duodenum or ileum were analyzed (Figure 3(b)). Proliferation ratio and total number of crypt cells were not different between the groups across all segments of the intestine (Figure 3(c) and (d)).
Figure 3.
Effect of PNS denervation on intestinal epithelial crypt cell proliferation. (a) Intestinal tissue immunohistochemically processed with an anti-BrdU antibody to visualize intestinal epithelial crypt cell proliferation. Tissue counterstained with hematoxylin. (b) Average proliferating crypt cell number in the jejunum was increased in PNS denervated animals compared with pair-fed sham animals (P < 0.05). (c) There were no differences between groups in average proliferation ratio in the duodenum, jejunum, or ileum. (d) There were no differences between groups in average total crypt cell number in the duodenum, jejunum, or ileum
Effect of SNS denervation on proliferation
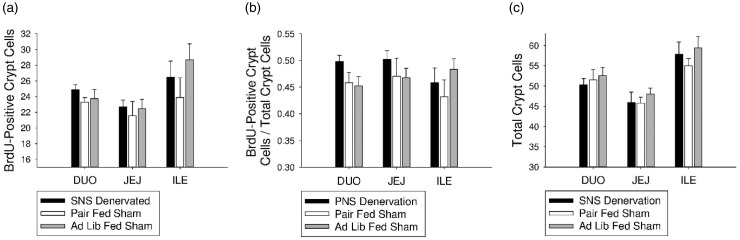
There were no differences between the groups in BrdU-positive cell number, proliferation ratio, or total crypt cell number in the duodenum, jejunum, or ileum (Figure 4(a) to (c)).
Figure 4.
Effect of SNS denervation on intestinal epithelial crypt cell proliferation. (a) There were no differences between groups in average apoptotic villus cell number in the duodenum, jejunum, or ileum. (b) There were no differences between groups in average apoptotic ratio in the duodenum, jejunum, or ileum. (c) There were no differences between groups in average total crypt cell number in the duodenum, jejunum, or ileum
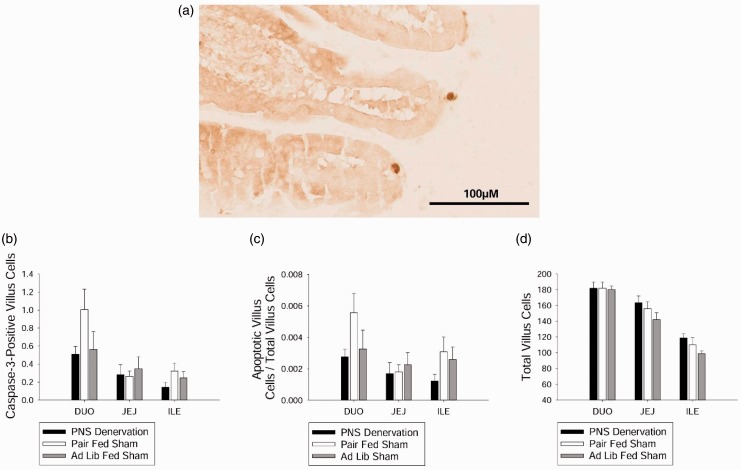
Effect of PNS denervation on apoptosis
There were no differences between groups in caspase-3-positive (representative image, Figure 5(a)) villus cell number, apoptotic ratio, or total number of villus cells (Figure 5(b) to (d)) in the duodenum, jejunum, or ileum.
Figure 5.
Effect of PNS denervation on intestinal epithelial cell apoptosis. (a) Intestinal tissue immunohistochemically processed with an anti-caspase 3 antibody to visualize intestinal epithelial cells undergoing apoptosis. (b) There were no differences between groups in average apoptotic villus cell number in the duodenum, jejunum, or ileum. (c) There were no differences between groups in average apoptotic ratio in the duodenum, jejunum, or ileum. (d) There were no differences between groups in average total villus cell number in the duodenum, jejunum, or ileum
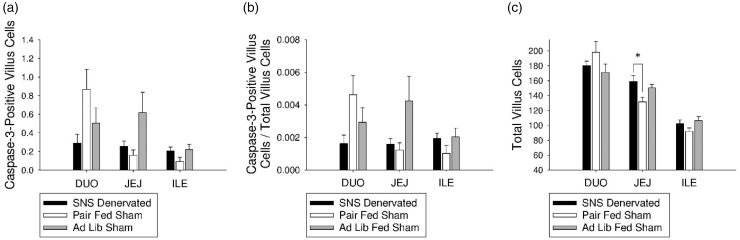
Effect of SNS denervation on apoptosis
There were no differences between groups in caspase-3-positive villus cell number or proliferation ratio in the duodenum, jejunum, or ileum (Figure 6(a) and (b)). However, total crypt cell number was increased in the SNS denervated animals compared with pair-fed sham animals (P < 0.05; Figure 6(c)).
Figure 6.
Effect of SNS denervation on intestinal epithelial cell apoptosis. (a) There were no differences between groups in average apoptotic villus cell number in the duodenum, jejunum, or ileum. (b) There were no differences between groups in average apoptotic ratio in the duodenum, jejunum, or ileum. (c) Average total villus cell number was increased in SNS denervated animals compared with pair-fed sham animals (P < 0.05)
Discussion
We found no differences in proliferation, apoptosis, crypt or villus cell number in the intestinal epithelium after long-term PNS or SNS denervation (three weeks) compared to the sham control groups. These results coincide with those of others when the denervated groups are compared with sham surgical control animals at later time points post-denervation2,3 and has been found in humans post-ANS denervation.9,34 It appears that the mechanisms controlling proliferation and apoptosis are able to recover post-denervation as has been found in other processes related to intestinal function (motility,10 food intake11,12 and blood flow13) and in aspects of tissue regeneration in other organs (liver14,15 and the pancreas16). Because cell turnover is vital in maintaining proper tissue function, these results are important in order to reveal possible implications of the use of ANS denervation in human medicine.
Previous research supports the idea that the direction of fluctuation in proliferation after PNS denervation appears to be time dependent. Within the first few days after PNS denervation, intestinal epithelial proliferation decreases, as measured in the rat ileum at four different post-denervation time points, including 6 h, 1 day, 2 days, and 3 days.2,3 Several groups demonstrate that proliferation increases one week post-PNS denervation in the jejunum4,5 and the ileum in rats.4 However, others suggest that there is no difference in the ileum compared to sham controls at 6 or 10 days post-denervation.2 Despite these discrepancies in proliferation at early time points, human jejunal biopsies post-PNS denervation have suggested that there is no change in intestinal proliferation three and six weeks post-surgery.9 Long-term studies in the rat did not measure proliferation specifically, but no histological changes were seen in the jejunum or ileum from 12 to 32 weeks post-PNS denervation.35 Together, these studies suggest changes in proliferation at early time points after PNS denervation followed by eventual stabilization of proliferation. However, these studies did not control for surgically driven decreases in food intake, a known influence on intestinal epithelial cell proliferation.8 The present study accounted for surgically induced changes in food intake and still demonstrated recovery of intestinal epithelial proliferation after PNS denervation in all three intestinal segments.
The effect of SNS denervation on intestinal epithelial proliferation appears to correlate with the extent of denervation induced by specific denervation techniques. Chemical denervation techniques, which ablate sympathetic nerves globally, produce a long lasting decrease in proliferation.6,7 However, more precise surgical denervations produce a short-term effect in the jejunum4,6 or no effect in the ileum.2,3 As surgical rather than chemical denervation of the SNS is used in human medicine, we were interested in the effect of surgical SNS denervation (i.e. celiacomesenteric ganglionectomy) on intestinal epithelial turnover in each segment of the intestine. The present results revealed no differences in intestinal epithelial proliferation or apoptosis in the duodenum, jejunum or the ileum three weeks post-SNS denervation. These results are similar to those of others using the same surgical approach.2,3 Thus, it appears that intestinal epithelial turnover may also recover after minimally-invasive surgical SNS denervation.
Even though there was no difference in the proliferation or apoptosis of the intestinal epithelium in the denervated compared with the sham surgical control groups in any segment of the intestine, there was an effect of pair feeding on the proliferation of the intestinal epithelium within the jejunum post-PNS denervation. The decrease in proliferation in the pair-fed group was seen even though at the time of sacrifice, all three PNS groups were eating equal kilocalories of food. This may be the result of how these animals were fed each day. They were provided the same amount of food consumed by the corresponding denervated group at the same time every day and ate all of the food within a few hours, instead of freely feeding throughout the day/night cycle. This may have conditioned the animals to anticipate and ingest food at regularly scheduled meals and set a circadian rhythm. We found that pair-feeding within the jejunum decreased intestinal epithelial proliferation and that PNS denervation attenuated this decrease in proliferation. This difference is seen even though at the time of sacrifice, all three groups were eating similar kilocalories of food. Meal feeding has been shown to alter the circadian rhythm of intestinal epithelial proliferation.36 Given that this only occurred in the PNS pair-fed group and not the SNS pair-fed group, there was likely an interaction between meal feeding and the amount (in kcals) provided over days or weeks. This is because the PNS denervated, and not the SNS denervated group, decreased their food intake post-surgery. Thus, only the PNS pair-fed group was fed lesser kcals than they may have eaten ad libitum and less than the SNS pair-fed group was eating. These data support the idea that the kcal amount of food alters intestinal epithelial proliferation and that there can be lasting effects of the amount of food eaten on the proliferative capacity of the intestinal epithelium.8,37,38
Compensatory mechanisms occur after ANS denervation in order to reestablish intestinal function under a variety of conditions and include (1) an upregulation of gastrointestinal (GI) receptor expression, (2) compensation by the non-denervated ANS branch and (3) enteric nervous system modulation. For example, PNS denervation initially decreases intestinal motility,39 a process which is heavily mediated by serotonin.40 PNS denervation has also been shown to reduce the amount of 5-HT-3 positive serotonergic nerve fibers in the GI tract.41 As gene expression of the serotonin receptor 5-HT-3 is upregulated in the intestinal epithelium, motility recovers.42 This restored motility can be blocked with administration of a 5-HT3 antagonist.42 Therefore, upregulation of serotonin receptors in the epithelium is thought to be driving the recovery of intestinal motility. Since serotonin receptors have also been found to be involved in intestinal epithelial proliferation, upregulation of the receptors after PNS denervation may contribute to the recovery of cell turnover.43,44 Changes in the specific primary neurotransmitter receptors of the denervated ANS branch are also likely to undergo changes to facilitate recovery of the intestinal epithelial turnover. PNS denervation upregulates the amount of muscarinic acetycholinergic gene expression in the stomach45 and downregulates the expression in the small intestine46 The intact branch of the ANS may also participate in the compensation to recover function after denervation. PNS denervation decreases the concentration of norepinephrine, the main SNS neurotransmitter, in the stomach.47,48 Therefore, changes in aspects of both the denervated and intact branches of the ANS may be driving the recovery of function after ANS denervation. The enteric nervous system (ENS), a network of neurons intrinsic to the GI tract, modulates specific intestinal epithelial progenitors to control regeneration.49 Given that the ENS makes synaptic connections to both the PNS and the SNS,50 it is likely that the ENS may also contribute to the recovery of tissue regeneration after the loss of ANS input as it does in other intestinal functions.51 Taken together, varied compensatory mechanisms after ANS denervation may collectively contribute to the recovery of intestinal function, including proliferation and apoptosis.
We found that the regenerative capacity of the intestinal epithelium is not chronically disrupted by denervation of either branch of the ANS and that these data support the narrative that compensatory mechanisms recover a variety of physiological functions to homeostatic levels after autonomic injury. This finding is important because the regeneration process is integral to maintain epithelial function and ANS denervation is currently used in patients to treat a variety of diseases.
Acknowledgements
The authors are grateful to Weinan Zhou, Kate Blackmore, and Mary Kate Feldner for technical support. This research was supported by USDA Hatch ILLU-538-926 to MJD.
Authors’ contributions
EAD, MCW, AIS, and MJD participated in the design of the studies; EAD, MCW, and HP participated in surgical procedures; EAD and EY conducted the bench work and data quantification; EAD and MJD analyzed the data; EAD wrote the manuscript; AIS and MJD edited the manuscript.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.van der Flier LG, Clevers H. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Ann Rev Physiol 2009; 71: 241–60. [DOI] [PubMed] [Google Scholar]
- 2.Musso F, Lachat J-J, Cruz AR, Gonçalves RP. Effect of denervation on the mitotic index of the intestinal epithelium of the rat. Cell Tissue Res 1975; 163: 395–402. [DOI] [PubMed] [Google Scholar]
- 3.Lachat JJ, Goncalves RP. Influence of autonomic denervation upon the kinetics of the ileal epithelium of the rat. Cell Tissue Res 1978; 192: 285–97. [DOI] [PubMed] [Google Scholar]
- 4.Callaghan BD. The effect of pinealectomy and autonomic denervation on crypt cell proliferation in the rat small intestine. J Pineal Res 1991; 10: 180–5. [DOI] [PubMed] [Google Scholar]
- 5.Tsibulevskii A, Orlova EN. [Physiologic regeneration of jejunal epithelium following bilateral subdiaphragmatic vagotomy in rats]. Biull Eksp Biol Med 1976; 81: 236–7. [PubMed] [Google Scholar]
- 6.Tutton PJ, Helme RD. The influence of adrenoreceptor activity on crypt cell proliferation in the rat jejunum. Cell Tissue Kinet 1974; 7: 125–36. [DOI] [PubMed] [Google Scholar]
- 7.Kennedy MF, Tutton PJ, Barkla DH. Adrenergic factors involved in the control of crypt cell proliferation in jejunum and descending colon of mouse. Clin Exp Pharmacol Physiol 1983; 10: 577–86. [DOI] [PubMed] [Google Scholar]
- 8.Brown HO, Levine ML, Lipkin M. Inhibition of intestinal epithelial cell renewal and migration induced by starvation. Am J Physiol 1963; 205: 868–72. [DOI] [PubMed] [Google Scholar]
- 9.Bejar J, Broitman SA, Zamcheck N. Effect of vagotomy upon the small intestine. Gut 1968; 9: 87–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andrews PL, Bingham S. Adaptation of the mechanisms controlling gastric motility following chronic vagotomy in the ferret. Exp Physiol 1990; 75: 811–25. [DOI] [PubMed] [Google Scholar]
- 11.Schneider K, Rezek M, Novin D. Effects of visceral sympathectomy on 2-deoxy-d-glucose induced eating. Physiol Behav 1976; 16: 55–8. [DOI] [PubMed] [Google Scholar]
- 12.Rezek M, Vanderweele DA, Novin D. Stages in the recovery of feeding following vagotomy in rabbits. Behav Biol 1975; 14: 75–84. [DOI] [PubMed] [Google Scholar]
- 13.Mackie DB, Turner MD. The effect of truncal vagotomy on jejunal and ileal blood flow. J Surg Res 1971; 11: 356–63. [DOI] [PubMed] [Google Scholar]
- 14.Tanaka K, Ohkawa S, Nishino T, Niijima A, Inoue S. Role of the hepatic branch of the vagus nerve in liver regeneration in rats. Am J Physiol 1987; 253(4 Pt 1): G439–44. [DOI] [PubMed] [Google Scholar]
- 15.Kato H, Shimazu T. Effect of autonomic denervation on DNA synthesis during liver regeneration after partial hepatectomy. Eur J Biochem 1983; 134: 473–8. [DOI] [PubMed] [Google Scholar]
- 16.Edvell A, Lindstrom P. Vagotomy in young obese hyperglycemic mice: effects on syndrome development and islet proliferation. Am J Physiol 1998; 274(6 Pt 1): E1034–9. [DOI] [PubMed] [Google Scholar]
- 17.Olbe L. Therapeutic applications of vagotomy. 1994; 67: 153–7. [PMC free article] [PubMed] [Google Scholar]
- 18.Okafor PN, Lien C, Bairdain S, Simonson DC, Halperin F, Vernon AH, Linden BC, Lautz DB. Effect of vagotomy during Roux-en-Y gastric bypass surgery on weight loss outcomes. Obesity Res Clin Pract 2015; 9: 274–80. [DOI] [PubMed] [Google Scholar]
- 19.Dumont P, Denoyer A, Robin P. Long-term results of thoracoscopic sympathectomy for hyperhidrosis. Ann Thorac Surg 2004; 78: 1801–7. [DOI] [PubMed] [Google Scholar]
- 20.Girish G, D'Souza RE, D'Souza P, Lewis MG, Baker DM. Role of surgical thoracic sympathetic interruption in treatment of facial blushing: a systematic review. Postgrad Med 2017; 129: 267–75. [DOI] [PubMed] [Google Scholar]
- 21.Hashmonai M, Cameron AEP, Licht PB, Hensman C, Schick CH. Thoracic sympathectomy: a review of current indications. Surg Endosc 2016; 30: 1255–69. [DOI] [PubMed] [Google Scholar]
- 22.Koopman FA, Stoof SP, Straub RH, Van Maanen MA, Vervoordeldonk MJ, Tak PP. Restoring the balance of the autonomic nervous system as an innovative approach to the treatment of rheumatoid arthritis. Mol Med 2011; 17: 937–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xie W, Chen S, Strong JA, Li AL, Lewkowich IP, Zhang JM. Localized sympathectomy reduces mechanical hypersensitivity by restoring normal immune homeostasis in rat models of inflammatory pain. J Neurosci 2016; 36: 8712–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shaw D, Gohil K, Basson MD. Intestinal mucosal atrophy and adaptation. World J Gastroenterol 2012; 18: 6357–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singh A, Balint JA, Edmonds RH, Rodgers JB. Adaptive changes of the rat small intestine in response to a high fat diet. Biochim Biophys Acta 1972; 260: 708–15. [DOI] [PubMed] [Google Scholar]
- 26.Ferraris RP, Vinnakota RR. Intestinal nutrient transport in genetically obese mice. Am J Clin Nutr 1995; 62: 540–6. [DOI] [PubMed] [Google Scholar]
- 27.Krausova M, Korinek V. Wnt signaling in adult intestinal stem cells and cancer. Cell Signal 2014; 26: 570–9. [DOI] [PubMed] [Google Scholar]
- 28.Brown TA, Washington MC, Metcalf SA, Sayegh AI. The feeding responses evoked by cholecystokinin are mediated by vagus and splanchnic nerves. Peptides 2011; 32: 1581–6. [DOI] [PubMed] [Google Scholar]
- 29.Hunt JV, Washington MC, Sayegh AI. Exenatide and feeding: possible peripheral neuronal pathways. Peptides 2012; 33: 285–90. [DOI] [PubMed] [Google Scholar]
- 30.Sullivan CN, Raboin SJ, Gulley S, Sinzobahamvya NT, Green GM, Reeve JR, Jr., Sayegh AI. Endogenous cholecystokinin reduces food intake and increases Fos-like immunoreactivity in the dorsal vagal complex but not in the myenteric plexus by CCK1 receptor in the adult rat. Am J Physiol Regul Integr Comp Physiol 2007; 292: R1071–80. [DOI] [PubMed] [Google Scholar]
- 31.Kalia M, Sullivan JM. Brainstem projections of sensory and motor components of the vagus nerve in the rat. J Comp Neurol 1982; 211: 248–65. [DOI] [PubMed] [Google Scholar]
- 32.Yox DP, Stokesberry H, Ritter RC. Vagotomy attenuates suppression of sham feeding induced by intestinal nutrients. Am J Physiol 1991; 260(3 Pt 2): R503–8. [DOI] [PubMed] [Google Scholar]
- 33.Wright SA, Washington MC, Garcia C, Sayegh AI. Gastrin releasing peptide-29 requires vagal and splanchnic neurons to evoke satiation and satiety. Peptides 2012; 33: 125–31. [DOI] [PubMed] [Google Scholar]
- 34.Hansen OH, Larsen JK, Svendsen LB. Changes in gastric mucosal cell proliferation after antrectomy or vagotomy in man. Scand J Gastroenterol 1978; 13: 947–52. [DOI] [PubMed] [Google Scholar]
- 35.Ellis H, Pryse-Davies J. Vagotomy in the rat. A study of its effects on stomach and small intestine. Br J Exp Pathol 1967; 48: 135–41. [PMC free article] [PubMed] [Google Scholar]
- 36.Lakatua DJ, White M, Sackett-Lundeen LL, Haus E. Change in phase relations of circadian rhythms in cell proliferation induced by time-limited feeding in BALB/c X DBA/2F1 mice bearing a transplantable Harding-Passey tumor. Cancer Res 1983; 43: 4068–72. [PubMed] [Google Scholar]
- 37.Mah AT, Van Landeghem L, Gavin HE, Magness ST, Lund PK. Impact of diet-induced obesity on intestinal stem cells: hyperproliferation but impaired intrinsic function that requires insulin/IGF1. Endocrinology 2014; 155: 3302–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Beyaz S, Mana MD, Roper J, Kedrin D, Saadatpour A, Hong SJ, Bauer-Rowe KE, Xifaras ME, Akkad A, Arias E, Pinello L, Katz Y, Shinagare S, Abu-Remaileh M, Mihaylova MM, Lamming DW, Dogum R, Guo G, Bell GW, Selig M, Nielsen GP, Gupta, Ferrone CR, Deshpande V, Yuan GC, Orkin SH, Sabatini DM, Yilmaz OH. High-fat diet enhances stemness and tumorigenicity of intestinal progenitors. Nature 2016; 531: 53–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ridolfi TJ, Tong WD, Takahashi T, Kosinski L, Ludwig KA. Sympathetic and parasympathetic regulation of rectal motility in rats. J Gastrointest Surg 2009; 13: 2027–33; discussion 33. [DOI] [PubMed] [Google Scholar]
- 40.Kendig DM, Grider JR. Serotonin and colonic motility. Neurogastroenterol Motil 2015; 27: 899–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Glatzle J, Sternini C, Robin C, Zittel TT, Wong H, Reeve JR, Jr., Raybould HE. Expression of 5-HT3 receptors in the rat gastrointestinal tract. Gastroenterology 2002; 123: 217–26. [DOI] [PubMed] [Google Scholar]
- 42.Tong W, Kamiyama Y, Ridolfi TJ, Zietlow A, Zheng J, Kosinski L, Ludwig K, Takahashi T. The role of 5-HT3 and 5-HT4 receptors in the adaptive mechanism of colonic transit following the parasympathetic denervation in rats. J Surg Res 2011; 171: 510–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gross ER, Gershon MD, Margolis KG, Gertsberg ZV, Li Z, Cowles RA. Neuronal serotonin regulates growth of the intestinal mucosa in mice. Gastroenterology 2012; 143: 408–17.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dong Y, Yang C, Wang Z, Qin Z, Cao J, Chen Y. The injury of serotonin on intestinal epithelium cell renewal of weaned diarrhoea mice. Eur J Histochem 2016; 60: 2689–2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Keshavarzian A, Steck TB, Conway D, Gordon JH, Fields JZ. Canine gastric muscarinic receptors up-regulate after vagotomy. Digest Dis Sci 1990; 35: 449–52. [DOI] [PubMed] [Google Scholar]
- 46.Higuchi H, Murata M, Uchida S, Yoshida H. Changes in density of muscarinic cholinergic receptor by adrenergic denervation with guanethidine. Jpn J Pharmacol 1985; 37: 207–11. [DOI] [PubMed] [Google Scholar]
- 47.Graffner H, Ekelund M, Håkanson R, Rosengren E. Effect of different denervation procedures on catecholamines in the gut. Scand J Gastroenterol 1985; 20: 1276–80. [DOI] [PubMed] [Google Scholar]
- 48.Orloff LA, Orloff MS, Bunnett NW, Walsh JH. Dopamine and norepinephrine in the alimentary tract changes after chemical sympathectomy and surgical vagotomy. Life Sci 1985; 36: 1625–31. [DOI] [PubMed] [Google Scholar]
- 49.Bjerknes M, Cheng H. Modulation of specific intestinal epithelial progenitors by enteric neurons. Proc Natl Acad Sci U S A 2001; 98: 12497–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Costa M, Brookes SJ, Hennig GW. Anatomy and physiology of the enteric nervous system. Gut 2000; 47(Suppl 4): iv15–9. discussion iv26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nakao K, Takahashi T, Utsunomiya J, Owyang C. Extrinsic neural control of nitric oxide synthase expression in the myenteric plexus of rat jejunum. J Physiol 1998; 507(Pt 2): 549–60. [DOI] [PMC free article] [PubMed] [Google Scholar]